Abstract
Bacterial biofilms are difficult to inactivate due to their high antimicrobial resistance. Therefore, new approaches are required for more effective bacterial biofilm inactivation. Airborne acoustic ultrasound improves bactericidal or bacteriostatic activity which is safe and environmentally friendly. While, plasma activated water (PAW) is attracting increasing attention due to its strong antimicrobial properties. This study determined efficacy of combined airborne acoustic ultrasound and plasma activated water from both cold and thermal plasma systems in inactivating Escherichia coli K12 biofilms. The application of airborne acoustic ultrasound (15 min) alone was significantly more effective in reducing E. coli counts in 48 and 72 h biofilms compared to 30 min treatment with PAW. The effect of airborne acoustic ultrasound was more pronounced when used in combination with PAW. Airborne acoustic ultrasound treatment for 15 min of the E. coli biofilm followed by treatment with PAW significantly reduced the bacterial count by 2.2—2.62 Log10 CFU/mL when compared to control biofilm treated with distilled water. This study demonstrates that the synergistic effects of airborne acoustic ultrasound and PAW for enhanced antimicrobial effects. These technologies have the potential to prevent and control biofilm formation in food and bio-medical applications.
Subject terms: Microbiology, Bacteria
Introduction
Biofilms are an assemblage of surface-associated microbial cells that are enclosed in a self-produced extracellular polymeric substance matrix; which is composed of polymeric conglomeration of extracellular polysaccharides, proteins, lipids and DNA1. Due to the complexity of their structure, biofilms exhibit higher antibiotic resistance to disinfection processes than planktonic cells2. Biofilms can form on both biotic and abiotic surfaces and are prevalent throughout industrial environments3,4. Various techniques for removal of the clustered cells exist, mostly involving chlorinated sanitizers, organic acids and other chemical products. However, most of the chemical agents either require high concentration or are ineffective to penetrate into the whole biofilm structure5,6. While, some chemical agents like chlorine based products have resulted in health and environmental issues associated with chemical residues and production of toxic by-products7. Poorly maintained equipment, unhygienic conditions, and improper cleaning procedures in food manufacturing environments may increase the tolerance of microbial biofilms to disinfectants. Bacterial populations are known to carry genes that may protect the bacteria under adverse conditions. For instance, the presence and distribution of qacH and bcrABC genes in Listeria monocytogenes ensure survival and growth of this strain when subjected to sub-lethal levels of disinfectants based on quaternary ammonium compounds8.
Food contact surfaces are a major source of food product microbial contamination during processing, transport and storage. Stainless steel is a common food contact surface for biofilm adhesion in the food industry9. Airborne acoustic ultrasound employs non-contact transducers which are capable of transmitting ultrasonic waves to the product using air as the coupling medium10. In recent years, the design of airborne acoustic ultrasonic devices has been significantly advanced and successfully demonstrated in various food industry applications including; drying, defoaming and decontamination. However, to date, it has not been employed for microbial biofilm disruption.
Plasma is defined as a partially or wholly ionized gas composed of positive and negative ions, electrons, photons, free radicals and neutrons atoms and molecules11. Plasma can be categorised into thermal (equilibrium) and non-thermal (non-equilibrium) plasma12. Thermal plasma has all constituent species, electrons, ions at same temperature, thus in thermodynamic temperature equilibrium state. Whereas, non-thermal plasma is characterised by higher temperature of electrons compared to other active species within plasma i.e. they are in non-equilibrium state13. The cooling effect of ions and uncharged particles are much effective than energy transfer from electrons, therefore the gas still remains in low temperature, therefore they are also referred as cold plasma13. Plasma has been mainly used in bio-medical, textile and polymer industries14–16. However, recent studies have demonstrated potential applications of plasma technology in the food industry17,18. There is a range of different approaches to generate plasma discharges, using different geometry configuration. Typical corona discharge, dielectric barrier discharge (DBD), radio-frequency (RF) discharge, plasma jet (APPJ), microwave discharge are commonly used. Recently, atmospheric plasma jet is widely used plasma discharge, which uses air and special electrode design to prevent arcing. Due to its design and configuration, plasma jet has been applied for biomedical19, surface decontamination20 or food system21. Atmospheric plasma jet is an efficient technology with high chemical activity that inactivates wide range of microorganisms20,22.
There are a range of different methods to apply plasma treatment on the targeted product: direct or indirect mode of plasma exposure23. Several studies have applied atmospheric pressure plasma systems directly to biofilms24. In the last few years, plasma treated liquids have also shown promising effect. Plasma can also be used indirectly via plasma activated water (PAW), whereby the targeted product is not subjected directly to the plasma gas, but is in contact with water which was pre-treated with plasma. Direct treatment has some limitations, including effects on food quality and changes in surface topography. Treating biofilms via PAW is an alternative to direct contact, which can overcome these limitations for water-soluble products25. Replacing the chemical liquid products used in the food industry to remove biofilms with PAW rich in reactive species would be simple to integrate into industrial cleaning protocols. The objective of this study was to investigate the inactivation efficacy of individual and combined airborne acoustic ultrasound and PAW treatments from both cold and thermal plasma systems on Escherichia coli K12 biofilms.
Results and discussion
Effect of airborne acoustic ultrasound on bacterial biofilms
Figure 1 shows the Log10 CFU/mL of biofilms grown for 48 and 72 h on stainless steel coupons and treated using airborne acoustic ultrasound for 15 min. A slightly higher number of adherent cells were observed in case of untreated controls after 72 h of incubation compared to 48 h. Regardless of the higher number of cells, significant reductions of 1.17 ± 0.24 Log10 CFU/mL and 2.19 ± 1.01 Log10 CFU/mL were observed for treatment of samples grown for 48 h and 72 h respectively (p < 0.05). This significant reduction in biofilms after airborne acoustic ultrasound treatment can be attributed to morphological changes occurring in bacterial cell membranes. Previous studies demonstrated fragmented and ruptured bacterial cells with 15 min airborne acoustic ultrasound treatment26. Ultrasound creates stable cavitation and micro-streaming which causes direct damage to the ultrastructure of bacterial cells in biofilms27. However, the acoustic intensity used in this study is relatively much lower compared to contact power ultrasound systems. The microbial inactivation due to airborne acoustic ultrasound can mainly be attributed to physical effects caused by acoustic pressure and partly due to thermal effects and ultrasonic wave stresses28. However, the exact mechanism of microbial inactivation for non-contact type ultrasound is not yet fully understood.
Figure 1.
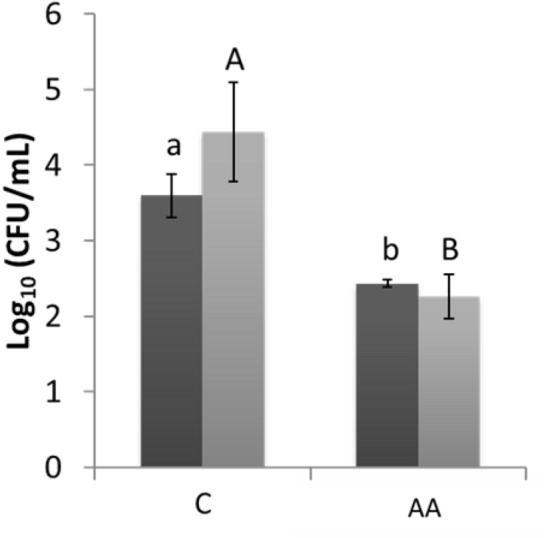
Microbial count after biofilm detachment of ultrasonic-treated samples. “Black square” 48 h biofilm “Grey square” 72 h biofilm. C: control; AA: Airborne acoustic ultrasound 15 min. a,bDifferent letters on column are significantly different (p < 0.05). A, B Different letters on column are significantly different (p < 0.05). Error bars stand for standard deviation.
Antimicrobial efficacy of plasma activated water treatment and synergistic effects with airborne acoustic ultrasound
The effect of PAW alone and in combination with airborne acoustic ultrasound was assessed on 72 h E. coli biofilms formed on the stainless-steel surfaces. Figure 2 presents the Log10 CFU/mL of biofilms grown for 72 h and treated with either cold or thermal PAW alone and in combination with airborne acoustic ultrasound. It can be observed that dipping a biofilm inoculated coupon into non-treated water (CW) significantly reduced the cell population from 4.44 ± 0.65 Log10 CFU/mL to 2.62 ± 0.25 Log10 CFU/mL. The reduction in microbial counts for biofilms washing with water alone is mainly due to the weak biofilm formation of the selected microorganism. The biofilm coupons noted as ‘C’ received no treatment; ‘CW’ were treated with sterile distilled water; ‘cPAW’ were treated with PAW generated from cold plasma; ‘tPAW’ were treated with PAW generated from thermal plasma; ‘AA’ were treated with airborne acoustic ultrasound; ‘AA + cPAW’ were treated with airborne acoustic ultrasound and PAW generated from cold plasma; ‘AA + tPAW’ were treated with airborne acoustic ultrasound and PAW generated from thermal plasma. Biofilms treated with CW, cPAW and tPAW did not show any significant reductions in the count, whereas combined treatment of cold plasma generated PAW with airborne acoustic ultrasound showed a significant reduction of approx. 2.2 ± 0.59 Log10 CFU/mL when compared to control biofilm treated with distilled water. While, E. coli biofilm cells were completely reduced to undetectable levels when treated with AA + tPAW.
Figure 2.
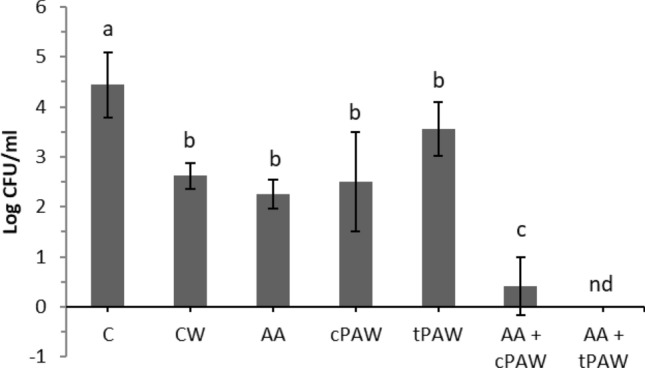
Microbial count after (72 h) biofilm detachment of samples treated with cold and thermal plasma-treated-water alone and in combination with ultrasound treatment. a,b,cDifferent letters on column are significantly different (p < 0.05); ‘nd’ indicates “not detected”; Error bars stand for standard deviation.
The inactivation of biofilms by a combination of PAW and airborne acoustic ultrasound is mainly due to the reactive species in PAW and the physical disruption of the biofilms due to ultrasound. The main biological effects induced due to plasma treatment are attributed to reactive species (reactive oxygen and nitrogen species)29. But biofilms by nature are complex biomaterials, (i) the biofilm architecture and (ii) its composition may limit penetration of reactive species into the biofilm matrix. Many previous studies demonstrated, that increasing treatment time and contact period could improve plasma inactivation efficacy against bacterial biofilm30–33. However, in this study PAW was combined with airborne acoustic ultrasound technology in order to obtain higher inactivation efficacies. An increase in the reduction factor was observed for the combined treatments, where exposure to airborne acoustic ultrasound significantly increased the susceptibility of bacterial biofilms to plasma treatment. These results are in agreement with the study by Yang et al.34, where a significant reduction in mature Candida albicans biofilms was observed when exposed to contact type ultrasound treatment in combination with antifungal agent Amphotericin B. Similarly, Wang et al.35 also demonstrated that low frequency ultrasound can enhance the activity of vancomycin against methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible Staphylococcus aureus (MSSA) biofilms. However, the inactivation efficacy in these studies was influenced by antimicrobial/antifungal agents, drug concentration, holding and irradiation time.
The combined treatment of airborne acoustic ultrasound with PAW enhanced the antimicrobial efficacy of PAW. Regarding the inactivation mechanism, one plausible explanation is that airborne acoustic ultrasound disrupts extracellular polymeric substances, provoking lesions at the surface of the biofilm, which eases the penetration of the reactive species generated in water into biofilm matrix, causing higher inactivation. Recent review on PAW proposed that the antibacterial efficacy of PAW is mainly attributed to the synergistic effects of plasma reactive species, pH and oxidation–reduction potential (ORP)25,36. The exact details and mechanisms for plasma on biofilms are still poorly understood. The antimicrobial effect of PAW on bacterial biofilms are depended on the specifically involved plasma species, its composition, its interactions with biofilm matrix components and its diffusion into the biofilm cells29. Researcher have hypothesised that the interaction of plasma-induced reactive oxygen nitrogen species (RONS) with biofilm matrix components and solutes in the hydrated matrix could cause oxidation reaction as well as phosphodiesterase activity in cells which leads to physiological alterations and reduction in biofilm matrix area36,37. Eventually loss of matrix reduces the adhesiveness and dispersal of biofilm cells. While the biofilm is dispersing the remaining cells are exposed to RONS and become more susceptible to be inactivated by PAW. The reactive species generated in PAW not only affects the cell membrane integrity but also penetrate into membrane channels and provoke reactive oxygen species (ROS) production endogenously which account for the intracellular ROS accumulation inside the cell31,38. Studies by Lukes et al39 and Xu et al31 also demonstrated that RONS interact with intracellular components (DNAs, proteins, lipids) and influence its metabolic responses in microorganisms. These interactions cause oxidative stress on cell components thus initiating lipid and protein peroxidation on the cell membrane, followed by protein and/or DNA damage and cell death36.
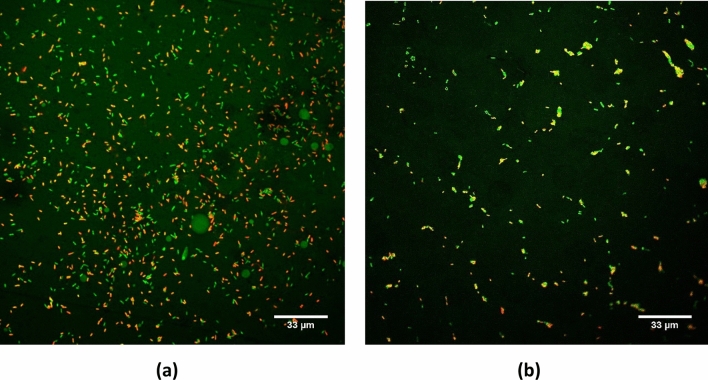
The effect of these treatments on the biofilms was also observed using confocal microscopy. Confocal images of treated and control samples are shown in Fig. 3. The population of biofilms was significantly affected by the airborne acoustic ultrasound treatment. Following the same trend as the plate count method, the images have shown that airborne acoustic ultrasound is an effective tool for biofilm removal. The dead cells (red/yellow-fluorescent stained) increased upon treatment, thus indicating the bactericidal effect of airborne acoustic ultrasound alone or when used in combination with PAW.
Figure 3.
Confocal laser scanning microscope images of E. coli biofilm on glass slide (a) non-treated and following (b) 15 min treatment of airborne ultrasound. The cells were stained with STYO 9 (green fluorescence, live cells)/PI (red/yellow, dead cells). All images were compiled, and noise reduction filter was applied in the Olympus Fluoview FV1000 software (version 4.1.1.5. https://www.olympus-lifescience.com/en/support/downloads/ ).
Physicochemical properties of plasma activated water
Table 1 shows that pH values of the PAW decreased significantly after cold or thermal plasma treatment. The pH of the solution is an important bactericidal property of PAW40; however the reduction in pH is not the sole cause for the bacterial inactivation41. The reason for acidification of PAW is predominantly due to the nitrate and nitrous acids generated by plasma treatment. Nitrates and nitrites in PAW are formed through dissolution of nitrogen oxides (NOX) formed in the air into water36. As demonstrated in Table 1, the concentration of nitrates and nitrites increased with plasma treatment, with higher levels of reactive nitrogen species (RNS) generated in cold PAW than thermal PAW.
Table 1.
Nomenclature of different samples with conditions detailed.
| Samples nomenclature* | Airborne acoustic ultrasound treatment | Cold plasma activated water treatment | Thermal plasma activated water treatment |
|---|---|---|---|
| C | – | – | – |
| CW | – | – | – |
| cPAW | – | 30 min soaking | – |
| tPAW | – | – | 30 min soaking |
| AA | 15 min | – | – |
| AA + cPAW | 15 min | 30 min soaking | – |
| AA + tPAW | 15 min | – | 30 min soaking |
*‘C’ were without any treatment; ‘CW’ were treated with sterile distilled water; ‘cPAW’ treated with cold plasma treated PAW; ‘tPAW’ treated with thermal plasma treated PAW; ‘AA’ treated with airborne acoustic ultrasound, ‘AA + cPAW’ treated with airborne acoustic ultrasound and cold plasma treated PAW; ‘AA + tPAW’ treated with airborne acoustic ultrasound and thermal plasma treated PAW.
The higher the oxidation ability of a solution to take electrons from cell membranes of the bacteria, the higher is the cell instability, which causes damage to the outer and inner cell membranes42. Both plasma treatment methods increased the ORP in the treated PAW in comparison to the control. H2O2 is considered to be a potent anti-microbial agent. Several studies have investigated about the importance of H2O2 to the antimicrobial activity of PAW18,25. In this study, concentration of H2O2 was generated in PAW. In addition, the conductivities of PAW were also recorded in this experiment. As shown in Table 1, the conductivity of PAW after plasma activation was significantly higher compared to the distilled water control. This is due to the generation of active groups (reactive oxygen and nitrate species) formed in water which improves the conductivity. Furthermore, the conductivity of PAW generated from cold plasma jet was higher than that generated from the thermal plasma jet. Overall, no significant difference was observed in the physicochemical properties of PAW generated from cold or thermal plasma, except for RONS concentration, which explains the similar inactivation efficacies observed.
Conclusion
This study demonstrates the individual and combined antimicrobial effects of airborne acoustic ultrasound and PAW for the disruption and inactivation of biofilms. Airborne acoustic ultrasound is an effective tool for biofilm removal and PAW can inactivate cells due to enhanced diffusion of plasma activated water into the matrix. Shortcomings of current treatment approaches together with resistance among bacterial strains against key antimicrobial agents i.e. antibiotics could potentially be addressed through use of these novel technologies. Novel technologies such as plasma and airborne acoustic ultrasound have the potential to effectively eradicate persistent bacterial biofilms with less energy and short treatment times in food processing environments. However, further studies are required to better understand the inactivation mechanisms of these treatments, which affect the physiological and metabolic states of bacteria.
Material and methods
Preparation of coupons for biofilm cultivation
Stainless steel coupons
The surface material investigated in this study was grade 304 stainless steel; coupons (16 mm × 1.2 mm) were obtained from Watermark Engineering (Tallaght, Dublin 24, Ireland). Prior to inoculation, the coupons were placed in Duran bottles and sterilised at 121 °C for 15 min.
Glass slides
For confocal laser scanning microscopic examination, bacterial biofilms were grown on glass slides (1 × 1 cm). The glass slides were washed with acid (1 M HCl) followed by 70% ethanol as described by Fischer et al.43. After washing, the glass slides were air dried in fume hood on Whatman filter paper for 10–15 min before autoclaving at 121 °C for 15 min.
Culture preparation and biofilm formation on coupons
Non-pathogenic Escherichia coli K12 ER2925 was obtained from the microbiological culture stock in Teagasc Food Research Centre (Ashtown, Dublin, Ireland). A loopful of E. coli K12 from the stock culture was transferred into nutrient agar plate (Product code: CM0003B; Oxoid Ireland c/o Fannin Healthcare, Ireland) and incubated at 37 °C for 24 h. A colony was then inoculated in 5 mL of Luria Bertani (noted LB, composed of 10 g of tryptone and 5 g of yeast extract per litre) and incubated for 24 h at 37 °C under shaking conditions (150 rpm). This culture was designated as a working culture and was stored at 4 °C until use, and a new culture was prepared weekly. On the day of the experiment, the optical density (O.D) was measured using EPOCH2C microplate reader (Biotek, Mason Technology Ltd, Dublin, Ireland) and adjusted to 0.15 using fresh LB media which corresponded to approx. 8 Log10 CFU/mL. Sterile coupons were placed in sterile 12-well plates and 1 mL of the OD-adjusted culture was added into each well. The 12-well plates were incubated at 37 °C for 48 and 72 h without medium change or agitation. After incubation, each coupon was washed twice using phosphate buffer saline BR0014 (PBS; Oxoid Limited, Ireland) to remove loosely attached and planktonic cells. The coupons were air dried inside a safety cabinet for 20 min.
Airborne acoustic ultrasound and plasma activated water treatments
Airborne acoustic treatment
All experiments were carried out using an airborne acoustic ultrasonic system (Pusonics S.L., Madrid, Spain) operating at a frequency of 26 kHz with a maximum power output of 170 W. The system consists of an electronic power generator with a dynamic resonance controller, a power amplifier, a high impedance matching box, and a circular stepped-plate transducer. The funnel-shaped acoustic airborne ultrasound construction provided highly focused acoustic field generated by the vibration of a plate transducer44. The biofilm coupons were placed on a stainless-steel plate positioned at a distance of 42.5 cm from the centre of the transducer at a maximum acoustic energy density of 10 W/cm2 (Fig. 4). The biofilm coupons were treated with airborne acoustic ultrasound for 15 min.
Figure 4.
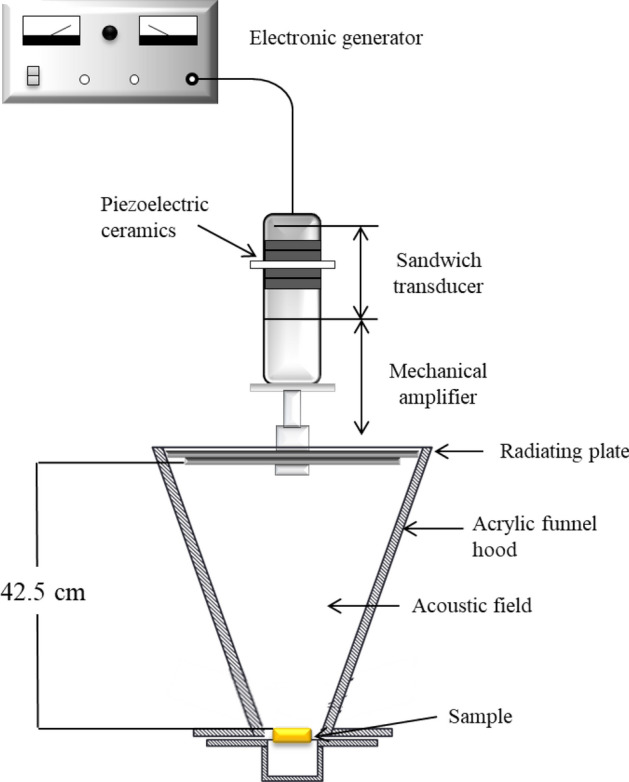
Schematic of the airborne acoustic ultrasound system.
Plasma activated water treatments
Cold plasma treatments were carried out using a system sourced from the National Centre for Plasma Science and Technology at Dublin City University (Glasnevin, Dublin 9, Ireland).This system is elaborately described in previous publication21. Plasma generated within this region exited through a small gap in the ground electrode entering ambient conditions in the plume region where it came into contact with treatment samples (Fig. 5). Plasma was generated at 30 kV using ambient air as the working gas.
Figure 5.
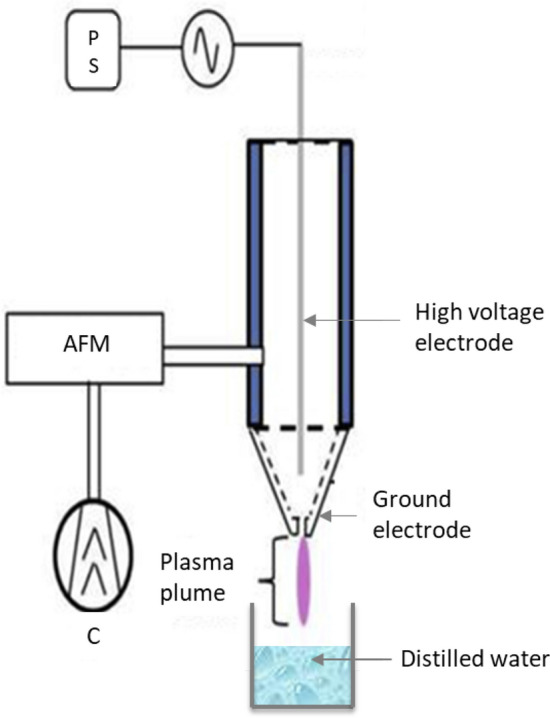
Schematic diagram of PAW generation by cold plasma jet system. (AFM) air flow monitor; (C) gas introduction; (PS) power supply.
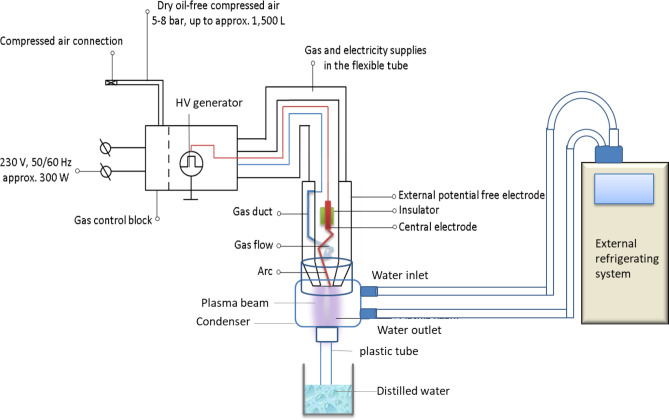
Thermal plasma treatments were carried out using a plasma beam system (Diener electronic GmbH & Co. KG, Ebhausen, Germany) operating at 20 kHz. The system was composed of three main units: a supply unit including a high voltage generator, plasma current and gas control devices; gas and power conductors in a flexible tube; and a plasma torch. The high voltage generator produced a voltage up to 10 kV, which was required to create the electric field to initiate and sustain the electrical plasma discharge. The electrical conductor for the supplied voltage and the process gas tubing were supplied to the plasma torch via a flexible tube, and the plasma particles (ions, electrons, excited atoms and molecules) were generated from the air supplied to the plasma torch. The temperature of the plasma gas would reach up to 200 °C; therefore, a condenser and cooling coil was used to maintain the temperature of the activated gas generated by the plasma at ambient temperature. Cold water (4 ± 0.5 °C) was circulated through the condenser using external refrigerating system (Lauda Ecoline, RE104). The functional principle of this system is shown in Fig. 6.
Figure 6.
Functional principle of thermal atmospheric plasma system. HV: High voltage generator.
For PAW generation from thermal and cold plasma jet, the distance between the nozzle of the jet and water surface was around 7.2 cm. For generation of PAW, a Duran bottle containing 100 mL of sterile deionised water was treated with cold or thermal plasma for 15 min. Individual biofilm coupons were immersed in 10 mL of PAW for 30 min at 4 °C during treatments. Sterile distilled water was used instead of PAW for control treatments.
Combined treatments
To determine the synergistic effects of combined treatment of airborne acoustic ultrasound and PAW, biofilm coupons were first exposed to airborne acoustic ultrasound as described in Sect. 2.3.1 followed by treatment with PAW as described in 2.3.2. Different treatment combinations were carried out as listed in Table 2.
Table 2.
The pH, conductivity, ORP and RONS of PAW compared to untreated controls.
| Parameters | Treatments | ||
|---|---|---|---|
| Control | Cold plasma | Thermal plasma | |
| pH | 6.71 ± 0.03 | 2.85 ± 0.18 | 2.53 ± 0.04 |
| ORP (mV) | 406.07 ± 12.7 | 565.40 ± 1.9 | 510 ± 0.6 |
| Conductivity (µs/cm) | 4.5 ± 0.5 | 333.00 ± 13.44 | 518.0 ± 22 |
| Hydrogen peroxide (µM) | nd | 14.7 ± 3.9 | 13.2 ± 5 |
| Nitrate (µM) | nd | 71.7 ± 5 | 13.09 ± 6 |
| Nitrite (µM) | nd | 33.5 ± 0.7 | 7 ± 0.2 |
Data are expressed as the mean value of three independent experiments, ‘nd’ indicates not detected.
Microbiological analysis
The population of E. coli on control and treated coupons was enumerated using the plate count method. The biofilm cells were detached from coupons using glass beads as described by Vatanyoopaisarn45. The coupon was placed in sterile 10 mL of maximum recovery diluent (MRD, Oxoid Ireland c/o Fannin Healthcare, Ireland) with 10 sterile glass beads (18,406, Sigma-Aldrich, Ireland). After soaking for 10 min, the tube was subjected to vortex-mixing for 1 min at maximum speed, yielding a suspension of E. coli that adhered on the coupon surface. Decimal dilutions were then carried out and spread on nutrient agar plates. After 24 h incubation at 37 °C, the colony forming unit per millilitre (CFU/mL) was calculated.
Confocal laser scanning microscopy
A live-dead BacLight bacterial viability and counting kit L34856 (Thermofisher Scientific, Ireland) was used. This included SYTO 9, a green fluorescent nuclear and chromosome counterstain, and propidium iodide (PI), a red-fluorescent stain which is not permeant to live cells, and thus was used to detect dead cells. The staining procedure was based on the instructions from the kit manufacturer with slight modifications. The working solution of fluorescent stains was prepared by adding 1.5 μL of SYTO 9 stain and 1.5 μL of propidium iodide stain to 1 mL of sterile 0.85% sodium chloride (NaCl). This working solution was prepared and used the same day. Each untreated and treated biofilm slide was covered with 200 µL of prepared staining solution and incubated at room temperature in dark for 15 min. The biofilm slides were carefully washed with 1 mL of NaCl solution twice to remove any dye residues. The biofilm samples were then placed on a 35 mm diameter glass-bottomed dish (ibidi GmbH, Martinsried, Germany) containing one drop of the mounting solution obtained from the live/dead BacLight bacterial viability kit L7007 (Thermofisher Scientific, Ireland). The slides were directly examined on a confocal laser scanning microscope (Olympus Fluoview FV1000) equipped with a 60x / 1.35 NA oil immersion objective. At least 3 randomly chosen microscopic fields were examined for each sample. A noise reduction filter was applied to the images in the Olympus Fluoview FV1000 software.
Physicochemical properties of plasma activated water
The physicochemical properties (including pH, electrical conductivity, ORP, RONS) of PAW were measured immediately after plasma treatment. For these measurements, 30 mL of sterile distilled water was treated with either cold or thermal plasma for 5 min. The pH, electrical conductivity and ORP of each sample was measured using a pH meter (Hanna, pH 213, UK), an electric conductivity meter (Jenway, 4070, UK), and a redox sensitive electrode (Hanna, HI3230B, UK) respectively. Measurements of hydrogen peroxide (H2O2) generated in the PAW were determined by titanium oxysulfate calorimetric method; briefly 10 μL of TiOSO4 (Sigma-Aldrich, Ireland) was added to 100 μL of PAW and then incubated in the dark for 10 min. The titanium sulfonate reagent reacts with hydrogen peroxide present in PAW to produce coloured pertitanic acid, which was measured on spectrophotometric plate reader (BioTek Epoch 2C, Mason technology Ltd., Dublin, Ireland) at 390 nm. The levels of nitrite in PAW were measured using Griess reagent (N-(1-Naphthyl) ethylenediamine dihydrochloride) (Sigma-Aldrich, Ireland), where 50 µl of the reagent was added to 50 µL PAW and incubated in dark for 30 min. After incubation, absorbance of samples was measured at 548 nm. When detecting nitrate concentration, PAW was pre-treated with sulfamic acid to eliminate nitrite interference. Nitrate concentration was then assessed by 2,6-dimethyl phenol (DMP) using the Spectroquant nitrate assay kit (Merck Chemicals, Darmstadt, Germany) with manufactures instructions. During each reactive species assessment, a known standard was included in each plate to convert absorbance values into known concentrations.
Statistical analysis
All experiments were performed in three independent experiments with three technical replicates each. Analysis of variance (ANOVA) and Tukey’s test was carried out using SAS (Version 8.0) and considered statistically significant at p < 0.05. The parametric assumptions were verified before performing ANOVA: normal distribution (Shapiro–Wilk test), homogeneity of variances (Levene test) and data independence (by experimental design).
Acknowledgements
This research was carried out with the financial support of the Irish Department of Agriculture, Food and the Marine (project no 14F845) and Science Foundation Ireland (SFI) under grant number 17/CDA/4653.
Author contributions
C.C, A.P and L.H performed experiments. J.S performed and helped with confocal imaging. C. C and A. P. wrote the manuscript. L.H, J.S, C.O and B.T edited the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whitehead KA, Verran J. Formation, architecture and functionality of microbial biofilms in the food industry. Curr. Opin. Food Sci. 2015;2:84–91. doi: 10.1016/j.cofs.2015.02.003. [DOI] [Google Scholar]
- 2.Alvarez-Ordóñez A, Coughlan LM, Briandet R, Cotter PD. Biofilms in food processing environments: challenges and opportunities. Annu. Rev. Food Sci. Technol. 2019;10:173–195. doi: 10.1146/annurev-food-032818. [DOI] [PubMed] [Google Scholar]
- 3.Galié S, García-Gutiérrez C, Miguélez EM, Villar CJ, Lombó F. Biofilms in the food industry: health aspects and control methods. Front. Microbiol. 2018;9:898. doi: 10.3389/fmicb.2018.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magana M, et al. Options and limitations in clinical investigation of bacterial biofilms. Clin. Microbiol. Rev. 2018;31:e00084–e116. doi: 10.1128/CMR.00084-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papaioannou E, Giaouris ED, Berillis P, Boziaris IS. Dynamics of biofilm formation by Listeria monocytogenes on stainless steel under mono-species and mixed-culture simulated fish processing conditions and chemical disinfection challenges. Int. J. Food Microbiol. 2018;267:9–19. doi: 10.1016/j.ijfoodmicro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Amrutha B, Sundar K, Shetty PH. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb. Pathog. 2017;111:156–162. doi: 10.1016/j.micpath.2017.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Chmielewski RAN, Frank JF. Biofilm formation and control in food processing facilities. Compr. Rev. Food Sci. Food Saf. 2003;2:22–32. doi: 10.1111/j.1541-4337.2003.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 8.Møretrø T, et al. Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int. J. Food Microbiol. 2017;241:251–224. doi: 10.1016/j.ijfoodmicro.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Srey, S., Jahid, I. K. & Ha, S. Do. Biofilm formation in food industries: a food safety concern. Food Control31, 572–585. https://doi.org/10.1016/j.foodcont.2012.12.001 (2013).
- 10.Gallego-Juarez JA, Rodriguez-Corral G, Gaete-Garreton L. An ultrasonic transducer for high power applications in gases. Ultrasonics. 1978;16:267–271. doi: 10.1007/978-1-4419-7472-3_25. [DOI] [Google Scholar]
- 11.Turner, M. Chapter 2 - Physics of Cold Plasma in Cold Plasma in Food and Agriculture (eds. Misra, N. N., Schlüter, O. & Cullen, P. J.) 17–51 (Academic Press, 2016). https://doi.org/10.1016/B978-0-12-801365-6.00002-0
- 12.Pankaj SK, Keener KM. Cold plasma: background, applications and current trends. Curr. Opin. Food Sci. 2017;16:49–52. doi: 10.1016/j.cofs.2017.07.008. [DOI] [Google Scholar]
- 13.Misra, N. N., Schlüter, O. & Cullen, P. J. Plasma in Food and Agriculture in Cold Plasma in Food and Agriculture: Fundamentals and Applications 1–16 (Academic Press., 2016). https://doi.org/10.1016/B978-0-12-801365-6.00001-9
- 14.Sun D, Stylios GK. Effect of low temperature plasma treatment on the scouring and dyeing of natural fabrics. Text. Res. J. 2004;74:751–756. doi: 10.1177/004051750407400901. [DOI] [Google Scholar]
- 15.Kim K, Kim G, Hong YC, Yang SS. A cold micro plasma jet device suitable for bio-medical applications. Microelectron. Eng. 2010;87:1177–1180. doi: 10.1016/j.mee.2009.12.045. [DOI] [Google Scholar]
- 16.Fanelli F, Fracassi F. Atmospheric pressure non-equilibrium plasma jet technology: general features, specificities and applications in surface processing of materials. Surf. Coatings Technol. 2017;322:174–201. doi: 10.1016/j.surfcoat.2017.05.027. [DOI] [Google Scholar]
- 17.Cullen PJ, et al. Translation of plasma technology from the lab to the food industry. Plasma Process. Polym. 2018;15:1700085. doi: 10.1002/ppap.201700085. [DOI] [Google Scholar]
- 18.Perinban S, Orsat V, Raghavan V. Nonthermal plasma-liquid interactions in food processing: a review. Compr. Rev. Food Sci. Food Saf. 2019;18:1985–2008. doi: 10.1111/1541-4337.12503. [DOI] [PubMed] [Google Scholar]
- 19.Laroussi M. Low temperature plasma jets: characterization and biomedical applications. Plasma. 2020;3:54–58. doi: 10.3390/plasma3020006. [DOI] [Google Scholar]
- 20.Nishime TMC, et al. Non-thermal atmospheric pressure plasma jet applied to inactivation of different microorganisms. Surf. Coatings Technol. 2017;312:19–24. doi: 10.1016/j.surfcoat.2016.07.076. [DOI] [Google Scholar]
- 21.Charoux CMG, et al. Effect of non-thermal plasma technology on microbial inactivation and total phenolic content of a model liquid food system and black pepper grains. LWT- Food Sci. Technol. 2020;118:108716. doi: 10.1016/j.lwt.2019.108716. [DOI] [Google Scholar]
- 22.Xu Z, et al. Inactivation effects of non-thermal atmospheric-pressure helium plasma jet on Staphylococcus aureus biofilms. Plasma Process. Polym. 2015;12:827–835. doi: 10.1002/ppap.201500006. [DOI] [Google Scholar]
- 23.Sarangapani C, Patange A, Bourke P, Keener K, Cullen PJ. Recent advances in the application of cold plasma technology in foods. Annu. Rev. Food Sci. Technol. 2018;9:609–629. doi: 10.1146/annurev-food-030117-012517. [DOI] [PubMed] [Google Scholar]
- 24.Patange A, et al. High voltage atmospheric cold air plasma control of bacterial biofilms on fresh produce. Int. J. Food Microbiol. 2019;293:137–145. doi: 10.1016/j.ijfoodmicro.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Thirumdas R, et al. Plasma activated water (PAW): chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018;77:21–31. doi: 10.1016/j.tifs.2018.05.007. [DOI] [Google Scholar]
- 26.Charoux CMG, O’Donnell CP, Tiwari BK. Effect of airborne ultrasonic technology on microbial inactivation and quality of dried food ingredients. Ultrason. Sonochem. 2019;56:313–317. doi: 10.1016/j.ultsonch.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Dolan HL, Bastarrachea LJ, Tikekar RV. Inactivation of Listeria innocua by a combined treatment of low-frequency ultrasound and zinc oxide. LWT - Food Sci. Technol. 2018;88:146–151. doi: 10.1016/j.lwt.2017.10.008. [DOI] [Google Scholar]
- 28.Charoux CMG, Ojha KS, O’Donnell CP, Cardoni A, Tiwari BK. Applications of airborne ultrasonic technology in the food industry. J. Food Eng. 2017;208:28–36. doi: 10.1016/j.jfoodeng.2017.03.030. [DOI] [Google Scholar]
- 29.Gilmore, B. F. et al. Cold plasmas for biofilm control: opportunities and challenges. Trends Biotechnol.36, 627–638. https://10.1016/j.tibtech.2018.03.007 (2018). [DOI] [PubMed]
- 30.Chen TP, Su TL, Liang J. Plasma-activated solutions for bacteria and biofilm inactivation. Curr. Bioact. Compd. 2017;13:59–65. doi: 10.2174/1573407212666160609082945. [DOI] [Google Scholar]
- 31.Xu Z, et al. In vitro antimicrobial effects and mechanism of air plasma-activated water on Staphylococcus aureus biofilm. Plasma Process. Polym. 2020;17:1900270. doi: 10.1002/ppap.201900270. [DOI] [Google Scholar]
- 32.Handorf O, et al. Characterization of antimicrobial effects of plasma-treated water (PTW) produced by microwave-induced plasma (MidiPLexc) on pseudomonas fluorescens biofilms. Appl. Sci. 2020;10:3118. doi: 10.3390/app10093118. [DOI] [Google Scholar]
- 33.Smet C, et al. Inactivation of single strains of Listeria monocytogenes and Salmonella typhimurium planktonic cells biofilms with plasma activated liquids. Front. Microbiol. 2019;10:1539. doi: 10.3389/fmicb.2019.01539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang, M. et al. The synergistic fungicidal effect of low-frequency and low-intensity ultrasound with amphotericin B-loaded nanoparticles on C. albicans in vitro. Int. J. Pharm.542, 232–241. https://doi.org/10.1016/j.ijpharm.2018.03.033 (2018). [DOI] [PubMed]
- 35.Wang J, et al. Multiple low frequency ultrasound enhances bactericidal activity of vancomycin against methicillin-resistant staphylococcus aureus biofilms. Biomed Res. Int. 2018;1:1–7. doi: 10.1155/2018/6023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou R, et al. Plasma-activated water: generation, origin of reactive species and biological applications. J. Phys. D. Appl. Phys. 2020;53:303001. doi: 10.1088/1361-6463/ab81cf. [DOI] [Google Scholar]
- 37.Soler-Arango J, Figoli C, Muraca G, Bosch A, Brelles-Mariño G. The Pseudomonas aeruginosa biofilm matrix and cells are drastically impacted by gas discharge plasma treatment: a comprehensive model explaining plasma-mediated biofilm eradication. PLoS ONE. 2019;14:e0216817. doi: 10.1371/journal.pone.0216817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang Q, et al. Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT- Food Sci. Technol. 2018;96:395–401. doi: 10.1016/j.lwt.2018.05.059. [DOI] [Google Scholar]
- 39.Lukes, P., Brisset, J. L. & Locke, B. R. Biological effects of electrical discharge plasma in water and in gas-liquid environments in Plasma Chem. Catal. Gases Liquids 309–352. (2012) https://doi.org/10.1002/9783527649525.ch8
- 40.Ikawa S, Tani A, Nakashima Y, Kitano K. Physicochemical properties of bactericidal plasma-treated water. J. Phys. D. Appl. Phys. 2016;49:425401. doi: 10.1088/0022-3727/49/42/425401. [DOI] [Google Scholar]
- 41.Patange A, Lu P, Boehm D, Cullen PJ, Bourke P. Efficacy of cold plasma functionalised water for improving microbiological safety of fresh produce and wash water recycling. Food Microbiol. 2019;84:103226. doi: 10.1016/j.fm.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Liao LB, Chen WM, Xiao XM. The generation and inactivation mechanism of oxidation-reduction potential of electrolyzed oxidizing water. J. Food Eng. 2007;78:1326–1332. doi: 10.1016/j.jfoodeng.2006.01.004. [DOI] [Google Scholar]
- 43.Fischer, A. H., Jacobson, K. A., Rose, J. & Zeller, R. Preparation of slides and coverslips for microscopy. Cold Spring Harb. Protoc. pdb.prot4988. https://doi.org/10.1101/pdb.prot4988 (2008) [DOI] [PubMed]
- 44.Gallego-Juárez, J. A. & Riera, E. Technologies and applications of airborne power ultrasound in food processing in Ultrasound Technologies for Food and Bioprocessing (eds. Feng, H., Barbosa-Canovas, G. & Weiss, J.) 617–641 (Springer New York, 2011). https://doi.org/10.1007/978-1-4419-7472-3_25
- 45.Vatanyoopaisarn, S. Comparison of Detachment Methods for Biofilm Removal on Glass and Stainless Steel Surfaces. J. KMITNB (2001).