Abstract
Rapid climate warming in the Arctic results in multifaceted disruption of biodiversity, faunal structure, and ecosystem health. Hypotheses have linked range expansion and emergence of parasites and diseases to accelerating warming globally but empirical studies demonstrating causality are rare. Using historical data and recent surveys as baselines, we explored climatological drivers for Arctic warming as determinants of range expansion for two temperature-dependent lungworms, Umingmakstrongylus pallikuukensis and Varestrongylus eleguneniensis, of muskoxen (Ovibos moschatus) and caribou (Rangifer tarandus), in the Canadian Arctic Archipelago from 1980 through 2017. Our field data shows a substantial northward shift of the northern edge of the range for both parasites and increased abundance across the expanded ranges during the last decade. Mechanistic models parameterized with parasites’ thermal requirements demonstrated that geographical colonization tracked spatial expansion of permissive environments, with a temporal lag. Subtle differences in life histories, thermal requirements of closely related parasites, climate oscillations and shifting thermal balances across environments influence faunal assembly and biodiversity. Our findings support that persistence of host-parasite assemblages reflects capacities of parasites to utilize host and environmental resources in an ecological arena of fluctuating opportunity (alternating trends in exploration and exploitation) driving shifting boundaries for distribution across spatial and temporal scales.
Subject terms: Climate-change ecology, Ecosystem ecology, Biogeography, Ecology, Ecology
Introduction
The unprecedented rate of warming in the Arctic is having repercussions across terrestrial and marine systems, leading to changes in ecosystem structure and function1,2, including shifting diversity and dynamics of wildlife disease/pathogens3,4. Increased temperatures, resulting in the northward shift of isotherms, are altering the zones of climatic suitability, or permissive environments, for pathogens and parasites, and this is being increasingly linked to invasions, range shifts and disease outbreaks5–9. Empirical observations and mechanistic models from the Arctic were the first to establish an unequivocal and direct link between climate warming and changing host–pathogen dynamics for nematode parasites, resulting in alterations in pathogen ecology and distribution7,8.
In the Arctic, understanding the outcomes of climate-driven perturbations on host–pathogen interactions is essential to a synoptic view of a biosphere in accelerating transition. Pathogens are fundamental components of ecosystems and understanding host–pathogen dynamics is critical for conserving healthy wildlife populations which are the foundations for food security and well-being of northern communities and Indigenous peoples10. The Arctic offers a unique landscape for testing hypotheses and models on host-parasite dynamics because of its relative simplicity, minimal confounding variables, and rapid rate of change11. Studying responses of Arctic host-parasite systems to climate warming provides broader insights on how climate oscillations on evolutionary to ecological time scales have historically determined and continue to influence the dynamics, evolution, and biogeography of parasites and the biosphere12,13.
Umingmakstrongylus pallikuukensis (hereafter referred to as UP) and Varestrongylus eleguneniensis (hereafter referred to as VE) are protostrongylid nematodes of muskoxen (Ovibos moschatus) and caribou (Rangifer tarandus), two important species which have experienced recent local to global population declines14,15. Protostrongylid nematodes have indirect temperature-dependent lifecycles (Fig. 1): larval development, and thus geographic ranges of this group of parasites, are limited by species specific climatic envelopes and availability of suitable thermal habitats and intermediate hosts that facilitate or limit the persistence of host-parasite assemblages. As model systems, these nematodes provide a direct pathway to understanding the impacts of climate change on host-parasite systems16.
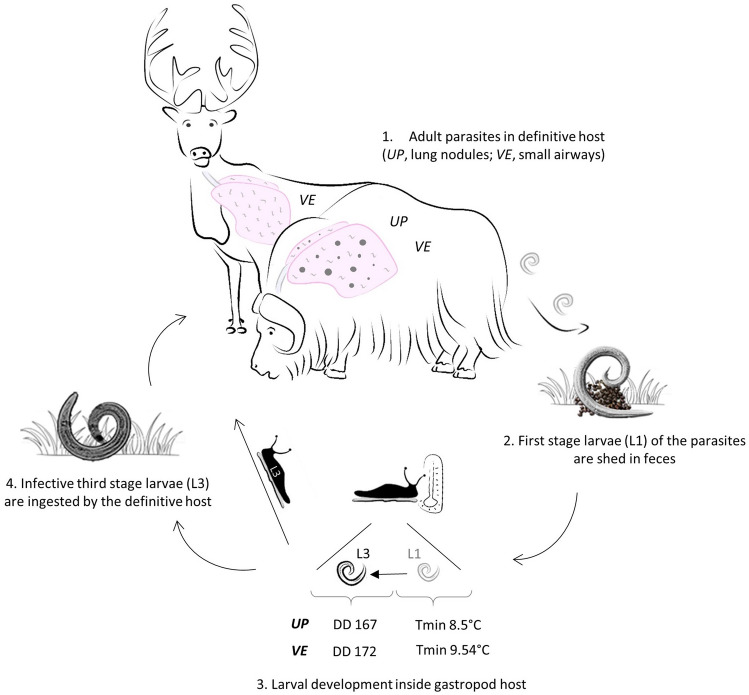
Figure 1.
The lifecycle of the protostrongylid nematodes Umingmakstrongylus pallikuukensis and Varestrongylus eleguneniensis. The lifecycle is indirect, involving gastropods as intermediate hosts (IH) where first stage larvae (L1) develop into infective third stage larvae (L3), the rate of which is determined by gastropod temperature. The L3 are ingested by the definitive hosts either as larvae that have emerged from the IH into the environment, or while still inside the gastropods. Umingmakstrongylus pallikuukensis (UP) and Varestrongylus eleguneniensis (VE) follow this life cycle pattern but differ in key thermal parameters (lower developmental threshold—Tmin, development degree-days—DD) and other life history features (e.g., host specificity, fecundity, life span)17–19. Umingmakstrongylus pallikuukensis is specific to muskoxen, is long-lived, and highly fecund, whereas VE can infect both caribou and muskoxen, has a shorter lifespan and much lower fecundity. (Artwork: M. Tomaselli).
The muskox lungworm, UP, has served as the ‘poster child’ for the effect of climate warming on parasites in the Arctic, and was the first helminth parasite globally in which changes in development and geographic distribution were linked to patterns of accelerating warming7,8. High temperature anomalies in the late 1980s, followed by sustained above-average temperatures, resulted in a tipping point for this parasite leading to a shift from a multi-year to a single year lifecycle in the core of its range on the central Arctic mainland7. More recently, continued and accelerating warming in Arctic environments has been associated with northward geographic expansion with invasion and establishment of UP and of VE, a related lungworm that infects both muskoxen and caribou, onto Victoria Island, in the Canadian Arctic Archipelago8. The recent invasion of these two parasites, which have similar life histories but different ecological characteristics and lifecycle dynamics (Fig. 1), provides a unique opportunity to assess, in real time, the effects of incremental climate warming, short-term thermal oscillations and the implications for tipping points7, and thresholds on parasite invasion (e.g.,9,13 ). In this study, we describe spatial and temporal patterns of abundance of UP and VE in the Arctic from 1980 to 2017, including historical distribution and northward spread. We then compare our observational data to the predicted thermal niches of UP and VE for 1980–2017, determined using a mechanistic model based on development degree-days inside the gastropod intermediate host. We contribute to broader insights about climate warming and oscillation across temporal and spatial scales and processes in the biosphere that determine the distribution of pathogens and disease.
Materials and methods
Study area
The study area mainly covers the Arctic and Subarctic regions of the Inuit Nunangat in Canada, with the Inuit communities of Ulukhaktok, Northwest Territories, and Kugluktuk and Cambridge Bay, Nunavut in the core study area (Fig. 2). This region has experienced rapid environmental changes in the last few decades: e.g., the annual mean temperature north of 60º north latitude increased by 2.3 °C (likely range 1.7–3.0 °C) from 1948 to 2016. This is roughly three times the global mean warming rate of 0.8 °C20,21. Climate models predict an increasing rate of future Arctic warming, with a projected rise in annual temperature by the end of century (2081–2100 avg) between 2.1 (RCP2.6) and 7.8 °C (RCP8.5)21.
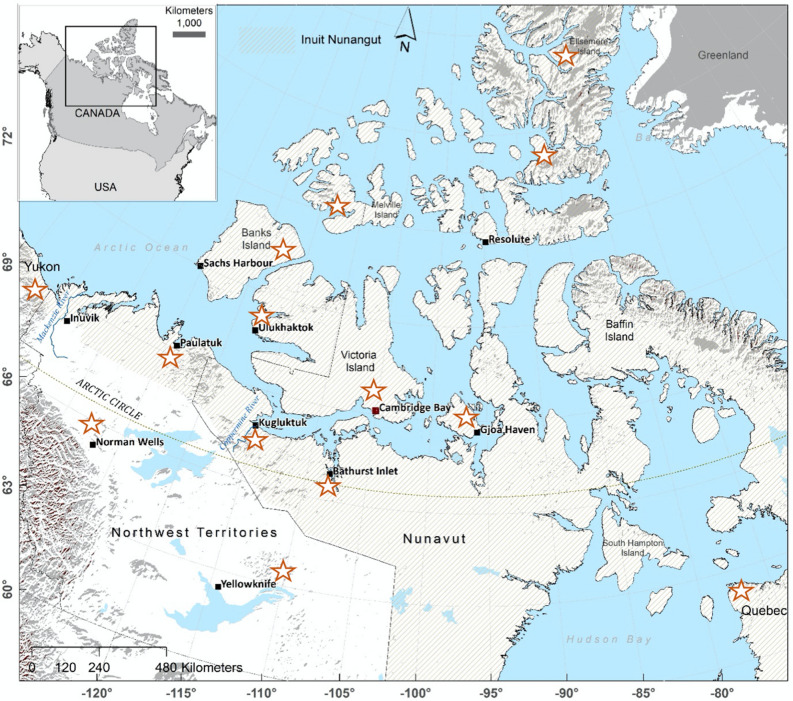
Figure 2.
Map showing the study area and general localities (☆) where the muskox fecal samples were collected between 2013 and 2017. Fecal surveys were carried out mainly within Inuit Nunagut region, as well as in the Sahtu (Norman Wells) and North Slave (east of Yellowknife) areas of Northwest Territories, and northern Yukon (west of Inuvik). The map was generated in ArcGIS software version 10.6 (ESRI 2011, ArcGIS Desktop: Release 10. Redlands, CA, Environmental Systems Research Institute, https://www.esri.com/en-us/home).
Muskoxen and caribou are distributed throughout much of northern Canada, although their abundance varies14,15. Barren-ground caribou herds occur on the mainland, and the Dolphin and Union caribou herd, a unique ecotype, winters on the mainland and seasonally migrates to calve and summer on Victoria Island14. The Committee on the Status of Endangered Wildlife in Canada has recommended the Dolphin and Union caribou as Endangered14. The muskox populations of Victoria and Banks Islands have declined by greater than 50–70% since the early 2000s while the populations on the adjacent mainland have remained relatively stable15.
Field and laboratory methods
We drew on the literature, archival data (since the 1980s), along with field-survey and monitoring based on new collections and fecal sampling to determine the historical and recent distribution of UP and VE. Survey data for 1988–2012 (historical data) were derived from previously published studies8,22,23 and data for the recent period, 2013–2017, originated from new sampling and collections following standard protocols.
Between April 2013 and December 2017, 1380 muskox fecal samples were collected from various regions of the Canadian Arctic and Subarctic. Samples were collected opportunistically by regional biologists and field scientists during fieldwork, and by hunters, guides and outfitters during sport, commercial and subsistence hunts. Samples were also collected strategically at targeted sites, with an attempt to detect the northern and eastern edges of the parasites’ ranges. Samples were frozen upon collection and sent to the University of Calgary for further analyses. All the samples were stored at -20 °C and analyzed within 1 to 6 months of collection.
Fecal samples were analyzed using the modified beaker Baermann technique24. Briefly, 5 g (on average) of fecal pellets were placed in between the two layers of mesh and a cheesecloth and submerged in the beaker full of tap water for 24 h under light. First stage larvae (L1) were extracted and counted using total and aliquot counts (in heavy infections). Samples were run in pairs in areas of low larval abundance (northern and eastern edges of ranges) and the larval counts were averaged. Extracted L1 were identified to species using morphological keys25–27, counted, and quantified on a per gram basis (LPG). Voucher specimens of larvae determined as UP and VE have been held in ethanol and cryo-archived in museum collections of the Museum of Southwestern Biology, University of New Mexico, and databased in the Arctos platform (https://arctos.database.museum) [MSB:Para:31452 to MSB:Para:31458].
Modelling
We parameterized a spatially explicit process-based mechanistic model (Degree-day model28) to determine annual accumulations of development degree-days (ADD) from 1980 through to 2017 and mapped the geographical extent of the thermal niches in which UP and VE could develop from an L1 to an infective L3 within a single summer (Fig. 3). For each cell in the gridded map, the temperature values were converted to ADD units following Eq. (1):
| 1 |
where k is the upper summation limit for the year. Th is the temperature of the h-th hour of the day (we used 3 hourly temperature readings—0:00, 3:00, 6:00, …, 21:00, so 8 time slices/day) and Tmin is the minimum threshold temperature for development (8.5 °C for UP29 and 9.54 °C for VE18). Th > 21 °C were defaulted to 21 °C to account for behavioral thermoregulation by the slug intermediate host; slugs tend to seek microclimates at 21 °C when the outside temperature exceeds 21 °C30 and this cut-off value was previously validated in experimental field studies by Kutz et al7.
Figure 3.
Modelling framework to generate the maps showing permissive thermal conditions for development. First, for each grid cells, degree-day (DD) units accumulated in a day (dDD) was determined by calculating the degree-units (D) for each time slice of the day (8 time slices per day). The value D for each time slice is calculated as the difference between the temperature at that time, or the upper temperature cut-off (21 °C), whichever is smaller, and the lower development threshold (Tmin). To calculate dDD, total D units in a day are summed and divided by 8 (see31,32). Finally, annual cumulative degree-days (ADD) for each year was derived by summing the dDD values for the entire year. The final step involved the conversion of the ADD units into potential transmission index (PTI) units by dividing the ADD units by the threshold DD units for each species. The maps were generated in ArcGIS software version 10.6 (ESRI 2011, ArcGIS Desktop: Release 10. Redlands, CA, Environmental Systems Research Institute, https://www.esri.com/en-us/home).
The potential thermal niches were delimited by the geographic areas where the minimum thermal conditions for complete development of L1 to infective L3 could be obtained during a single summer: 167 degree-days (DD) above the developmental threshold (Tmin) of 8.5 °C for UP29 and 171.4 DD above the threshold of 9.54 °C for VE18 (Fig. 3).
The temperature data were derived from the North American Regional Reanalysis (NARR)33. The NARR is a state-of-the-art climate model reanalysis that provides fine spatial (grid resolution of approximately 0.3-degree (32 km) at the lowest latitude) and temporal (3 hourly) resolution across North America. Thermal parameters; lower development threshold (Tmin) and development degree-days (DD) for the two species were derived from our earlier work18,29. Calculation of degree-day units was based on horizontal cut-off method34 which assumes that development continues at a constant rate at temperatures above the upper threshold. The upper-temperature limit of 21 °C was used for both species, which is based on the behavioural thermoregulation by the main gastropod intermediate host, Deroceras laeve.
We used a Parasite Transmission Index (PTI), derived by dividing the DD values by the threshold number of DD required by the parasites for complete development into infective stage larvae to standardize comparisons between the two species, which have different heating requirements. A PTI below 1 indicates insufficient summer heating or DD for development from L1 to L3 in gastropod intermediate hosts, a PTI of 1 indicates enough DD for development and a PTI of 2 means double the number of required degree days have been accumulated. The total area of suitable thermal conditions for each year was estimated by calculating the entire area of the grid cells where PTI ≥ 1. To determine the trend in ADD accumulation around Cambridge Bay (Victoria Island), the mean of ADD estimates for the grid cells that lie within the 50 km radius of Cambridge Bay location (69 06′29" N, 105 08′18" W) (land only) were extracted from 1980 through to 2017. The analysis was performed in ArcGIS software version 10.6 (ESRI 2011, ArcGIS Desktop: Release 10. Redlands, CA, Environmental Systems Research Institute), using the Spatial analyst toolbox.
We acknowledge that UP can overwinter in gastropod hosts and resume development the following year35. It is unknown if VE has a similar life history. For simplicity, we modelled for single year development but recognized that for UP at least, and probably VE, this is a conservative estimate of the thermal niche.
Statistical analyses
Statistical analyses were performed using R statistical software, version 3.4.236. Prevalence for each parasite species was quantified as the percent of fecal samples with larvae of the specific parasite, the intensity of infection as the median of the larval counts (LPG) of infected hosts only, and abundance as the mean larval counts from all individuals sampled37.
A generalized linear model (negative binomial model, log link) was fitted to determine if the intensity of infection for both parasite species in muskoxen around Cambridge Bay changed between 2009 and 2017. The response variable was the number of larvae counted in 5 gm of feces, and the explanatory variables were year and species. The nonparametric Mann–Kendall (MK) trend test (significance level, 5%) was applied to identify the trends in ADD accumulated around Cambridge Bay from 1980 through to 2017.
Results
Range expansion and changes in abundance
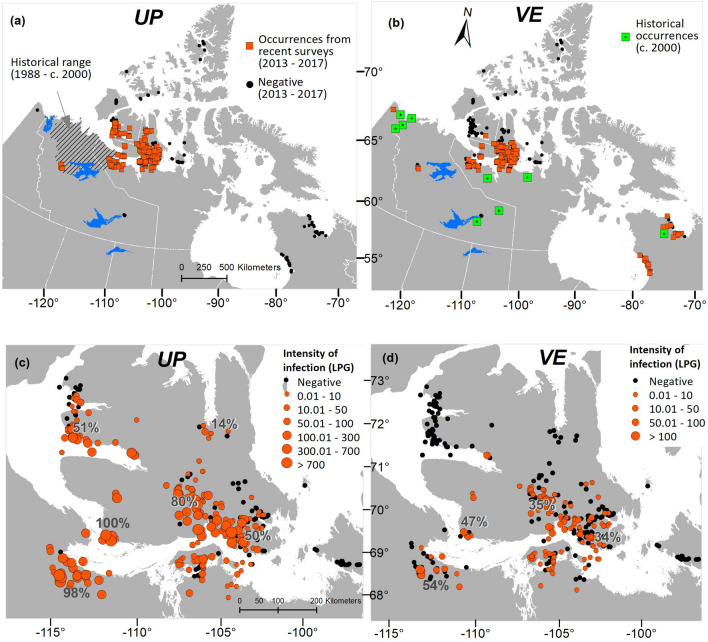
Populations of both UP and VE have undergone substantial geographic expansion, especially in the last decade (2009–2017) (Fig. 4a,b; Supplementary table 1) based on geographically extensive and site intensive surveys of muskoxen (larvae in fecal samples) across the Canadian Arctic, with a main focus on Victoria Island and the adjacent mainland (Fig. 2). Geographic colonization is most notable onto and across Victoria Island, (Northwest Territories and Nunavut), where UP is now found at least 400 km and VE at least 200 km north of the ranges previously reported by Kutz et al.8. Additionally, UP has expanded the eastern limit of its mainland range by approximately 500 km (Fig. 4). In contrast, VE was already broadly distributed across the temperate, subarctic and arctic mainland of North America22,25,38,39. Based on surveys and monitoring during the past decade, we did not detect either parasite on any islands of the Arctic Archipelago other than Victoria Island; UP was also absent from Quebec and the eastern Nunavut mainland (Fig. 4).
Figure 4.
Northward expansion of the geographic ranges of Umingmakstrongylus pallikuukensis (UP) and Varestrongylus eleguneniensis (VE). (a,b) Maps show the historical and current ranges of UP and VE in muskoxen. The historical range for UP was restricted to the mainland and bounded by the Mackenzie River in the west and the Coppermine River in the east (see methods, Fig. 2), prevalence in muskoxen approached 100% in this area16,23. The historical occurrences for VE were derived from Kutz, et al.22 and Verocai et al.38. Point occurrences are provided for VE as there were fewer surveys, however, the parasites were likely distributed continuously across the mainland in their migratory caribou hosts38. (c,d) The 2013–2017 range and intensity of infection (LPG, larval counts per gram of feces) for UP and VE on Victoria Island and the adjacent mainland. The text overlaid on the maps represent the prevalence of the parasites in muskoxen during this time. These figures show a clear latitudinal gradient in prevalence and infection intensities, indicating the northern invasion trajectory, as well as species specific differences in those parameters. The maps were generated in ArcGIS software version 10.6 (ESRI 2011, ArcGIS Desktop: Release 10. Redlands, CA, Environmental Systems Research Institute, https://www.esri.com/en-us/home).
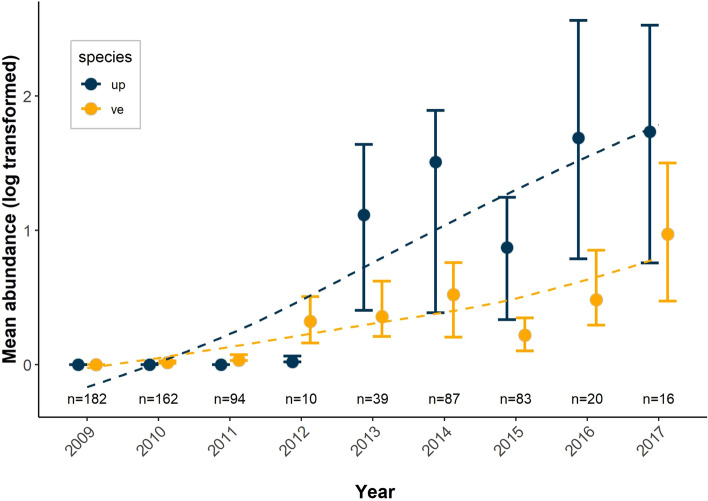
Near Cambridge Bay, Victoria Island, where muskoxen were monitored annually, we observed a rapid increase in abundance of both parasites following the initial detection of VE in 2010 and UP in 2012. Although UP was detected in the region later, the rate of increase in abundance of this parasite was significantly higher than that of VE. (Fig. 5, Supplementary Tables 2, 3).
Figure 5.
The increase in the larval abundance for UP and VE where muskoxen were continually sampled near Cambridge Bay from 2009 to 2017. The error bars represent the 95% confidence interval around the mean larvae per gram count (log transformed) of all sampled muskoxen. The lines represent the fit of loess regression. Abundance data from 2009 to 2012 were derived from Kutz et al.8.
Northward expansion of the parasites’ thermally suitable areas.
We found that the geographic area thermally suitable for the development and persistence of UP and VE, from landscape to regional scales, has expanded substantially on northward and eastward trajectories since the 1980s. This is represented in geographical space by the areas with the potential transmission index (PTI) values ≥ 1(Fig. 6). The 2012–2017 isolines of threshold PTI were considerably further north and east for both parasites compared to early 1980s (Fig. 6). The current northern limit of the observed distribution of UP based on field sampling is largely concordant with that of the northern extent of the modelled thermal niche for the same period. In contrast, the northern range limit of VE lags behind the northwestern extent of its thermal niche (Fig. 6f). The eastern range of UP on the mainland has also expanded consistent with the expansion of the thermally suitable areas; however, the extent of this expansion could not be determined because of insufficient sampling at the potential eastern range limits. VE is presumed to have been present historically in all mainland barren-ground caribou herds22,38, but sampling of muskoxen on the mainland east of the core study area was not adequate to determine distribution in this host.
Figure 6.
Northward expansion of the zones of suitable climate, based on parasite species specific accumulated degree days, from 1980 through to 2017. (a–f) Maps are showing the geographic extent of the thermal niches (represented by red shaded areas, from above 1 to darker red for values above 3 units of PTI) of the northern range of UP and VE during three six-year periods 1980–1985, 1996–2001, and 2012–2017. Darker shades of red indicate a greater number of accumulated degree days. The maps are produced by modelling degree-day accumulation for the two parasites using the 2 m air temperature (3 hourly) North American Regional Reanalysis (NARR) dataset by the National Centers for Environmental Prediction (NCEP)33. The average DD accumulated in six-year intervals, ten years apart, are displayed. Squares represent the known presence or absence of each parasite during the respective time frames. In maps a and b, no occurrence points are available as no surveys were done during that period. However, neither UP nor VE were detected on Victoria Island until 2008 and 2010, respectively, and neither have been found on any other arctic island despite targeted sampling since the late 1990s, thus they are both presumed to have been absent from the arctic archipelago in the 1980s. Occurrence points on maps c and d were obtained from published literature and our data. The maps were generated in ArcGIS software version 10.6 (ESRI 2011, ArcGIS Desktop: Release 10. Redlands, CA, Environmental Systems Research Institute, https://www.esri.com/en-us/home).
Compared to the early 1980s, during the period 2012–2017, approximately 310,000 sq. km more of the Canadian Arctic became thermally suitable for development of UP and 420,000 sq. km for development of VE (Fig. 6). However, there was considerable inter-annual variability in suitability. For example, in 1996–2001, the modelled thermally suitable range for both parasites was greater than that in 2012–2017. The summers of 1996, 1998, and 2000 were the warmest summers during our study period, and the high temperatures in those years are reflected in thermal suitability across a much greater geographic area during the period 1996–2001, including most of Victoria Island, the nearby islands and much of the eastern Canadian Arctic mainland (Fig. 6c, d). The apparent discrepancy between our results—greater predicted geographic range in the late 1990s compared to recent years—and the annual trends for increasing Arctic temperatures since the 1990s, are due to different warming patterns over time: while high temperatures in the late 1990s were driven by warmer summers, in the last decade they have been primarily driven by warmer winters40,41 (Supplementary Figs. 4, 5). This highlights the importance of seasonal and spatial patterns of warming and cooling on relatively short time scales of years to decades. The dynamics of ocean–atmosphere regimes, shifting between warm and cold cycles, such as El Niño Southern Oscillation (ENSO) or the Pacific Decadal Oscillation (PDO), may have pervasive effects in terrestrial systems and in predicting the outcomes of climate-drivers on the dynamics of host-parasite systems and the abundance, persistence and distribution of pathogens and disease (e.g.,9,13).
Annual variation in thermal suitability for the two parasites around Cambridge Bay, NU
For both parasites, significant positive trends in the annual PTI around Cambridge Bay (UP: Kendall’s Tau (τ) = 0.22, p = 0.04, Mann–Kendall’s statistic (S) = 157; VE: τ = 0.21, p = 0.05, S = 152) with high interannual variability (Coefficient of variation (CV) for UP: 25.7%, CV for VE: 28.4%) were observed from 1980 to 2017 (Fig. 7). Previous research by Kutz, et al.7 described 1988–89 as a temperature and developmental tipping point in the core of the UP range; also coinciding with a recognized major tipping point in the acceleration of warming at high latitudes42. This is also reflected in our data for Cambridge Bay where, prior to 1988, development of UP within a single year was unlikely, but most years following 1988 have been suitable. Near Cambridge Bay from 2000 to present, except for 2004 and 2005, all years have been suitable for the development of UP within a single year. The annual thermal pattern is similar for VE, however, because of the higher threshold for development and higher heating requirements of this parasite, there are fewer suitable years, and those have a lower PTI compared to UP. Both parasites consistently had a PTI > 1 from 2010–2017 (Fig. 7).
Figure 7.
Annual variation in DD accumulation around Cambridge Bay from 1980 to 2017. (a) Heatmap and (b) graph showing the trend and variability in the potential transmission index for UP and VE within 50 km of Cambridge Bay from 1980 through to 2017. The PTI < 1 (blue colour in the heatmap) indicates unsuitable thermal conditions for L1–L3 development in a single summer. In the graph, the lines represent the fit of loess regression (span 0.4). The vertical dotted red line represents the year 2009 after which the PTI for both parasites was consistently > 1.
Discussion
It is well accepted that climate warming can result in distributional shifts of species, including pathogens, in space and through time, directly by dissolving the climatic barriers, indirectly by impacting other related biological systems (e.g., vector dynamics, host movement), or by influencing both processes concurrently9,43–46. In polar areas, where historically a cold and unfavourable climate has arguably been the major limiting factor for most species occurrences, high rates of climate warming in the last few decades have resulted in faster and more widespread poleward movement of species and restriction of refugial zones43,45,47–49. Despite the rapid warming and predictions of range expansion and pathogen emergence, there are few empirical examples globally that unequivocally demonstrate temperature as a driver3,7,50.
Kovats et al.51 recommended certain minimum requirements be met before linking pathogen emergence to climate change. These included (i) the monitoring of the pathogen should be done with adequate spatial and temporal coverage, (ii) controlled lab and field-based studies should investigate the pathogen’s physiological and epidemiological response (including the direction of response) to the environmental variable, and (iii) a long-term assessment of climatic conditions (including variability) in the region of interest and its correlation with changing patterns of distribution and abundance are necessary to conclude that the climate change is driving the emergence of the pathogen. Our work meets these requirements and demonstrates a direct association between parasite range expansion in the Arctic and climate change, coherent with expectations from empirical data from the lab18,29, monitoring data from the field, and degree-day modelling. Hence, this work provides strong empirical evidence of climate mediated changes in host parasite dynamics and distribution in the Arctic.
UP and VE were detected on Victoria Island for the first time in 2008 (southwest corner) and 2010 (Cambridge Bay), respectively. Based on the results of fecal examinations and characteristics of muskox lung nodules (i.e., small dimension and absence of calcification), it was concluded these were recent invasion and establishment events8. Our data demonstrate that the prevalence and intensity of infection of both parasites on Victoria Island follow a latitudinal gradient, with both being more abundant at southern latitudes on the island compared to the northern edge of their ranges (Fig. 4a,d, Supplementary Table 1), establishing clear evidence of a trajectory of range expansion. Our data showing increasing trends in abundance of the two lungworms near Cambridge Bay on southeast Victoria Island, (Fig. 5, Supplementary Tables 2, 3), further support interpretations about recent geographic colonization and continuing range expansion of these parasites on the island. Although logistical constraints prevented ongoing systematic sampling across the area of expansion, the data available also suggest an increase in abundance over time across the new range. Both patterns, movement on a northward trajectory and increasing abundance of parasites, are consistent with a dynamic and changing thermal environment emergent from a regional tipping point documented in 1970s42.
Despite periodic suitable thermal conditions for establishment, neither parasite was detected on Victoria Island until the 2000s8. For UP, which has only muskoxen as a definitive host, establishment on the island may have been prevented by the limited frequency of movement of muskoxen between the mainland and Victoria Island. This parasite is long lived in muskoxen and has high fecundity, with the average infected muskox shedding literally millions of freeze and desiccation tolerant larvae/day19. Consequently, once established, it is likely to persist in a region for several years during periodic episodes of unsuitable thermal conditions19,52. It is possible that the warmer summers of the late 1990s would have supported establishment if the parasite had been moved to Victoria Island by muskoxen, or less likely, anthropogenically as fomites on contaminated equipment (skidoos, sleds, etc.) when people travel between the mainland and island8, or through predators (passed through gastrointestinal tract)53. While there is recent genetic evidence and local knowledge of muskox movement between Victoria Island and mainland54 this appears to have been relatively rare historically55.
In contrast, the establishment of VE on the island may have been more constrained by temperature and other life history characteristics of the parasite. Introduction of the parasite to the island could have occurred through the annual migration of the Dolphin and Union caribou herd, or by infrequent movement of mainland muskoxen, or barren-ground caribou, to the island. Presuming that Dolphin and Union caribou could be infected on the mainland during winter through third stage larvae on the vegetation25,56, VE may have been transported to Victoria Island regularly with the annual seasonal migration of these caribou between the island and the mainland8,9,14. Our models suggest that thermal conditions on the island were periodically suitable for parasite establishment over the last 37 years, and increasingly so since the late 1990s. Nevertheless, the higher thermal requirements for the development, the substantially shorter lifespan in caribou, and the low fecundity of VE17, may have prevented its establishment on the island until there were multiple consecutive years with suitable conditions to facilitate establishment and persistence (i.e., 2010–2017). Other mechanisms for VE to be introduced to the island exist. Traditional knowledge indicates that some Dolphin and Union caribou periodically over-summer on the mainland (A. Hanke, pers. comm) so summer infection with subsequent migration to the island the following year may be a source of introduction. Conversely, introduction to Victoria Island may have been through muskox movement, similar to that for UP, with subsequent spill-over to Dolphin and Union caribou on their summer range on the island8. Finally, sporadic movement onto the island of barren-ground caribou (as reported through traditional knowledge [Hanke, pers. Comm]), in which VE is endemic38, could also have introduced the parasite to the island. Establishing the introduction route for VE to the island and determining whether maintenance on the island is dependent on caribou or muskoxen alone, or both species together, will require elucidation through parasite genetic analyses and more complex transmission dynamics models.
Unique to our analyses, we were able to simultaneously study two phylogenetically related parasites with similar life histories but different thermal requirements. Initially, given that VE has two definitive hosts, one of which is a migratory caribou, we expected that this parasite would have expanded its range more quickly than UP, both because it had a higher potential host density (both muskoxen and caribou) and it could be moved more quickly across the landscape within its migratory caribou host. Our data showed the contrary, with UP leading the expansion front on Victoria Island. This is further supported by our degree-day models which showed that PTI of VE is consistently lower than that of UP for any given time and location, and the empirical data demonstrated that the observed distribution limit of VE lagged behind the spatial limit of its potential thermal niche. Whereas the higher thermal requirements of VE explain the lower PTI, the apparent lag of VE to fill its potential thermal niche is more likely related to its lower fecundity and a shorter lifespan.
These findings suggest that, at least in this system, the life history, as well as the physiological and thermal requirements of the parasites, may be stronger determinants of distribution and range expansion than host factors such as density and migration behaviour. A key component of the life cycle of these parasites that was not investigated was the ecology and distribution of the gastropod intermediate hosts. Deroceras laeve, the meadow slug, is considered the primary intermediate host species for both parasites23,29. This slug is present on the island at low densities57. Features of parasite species-specific development, survival, and emergence rates within D. laeve, as well as gastropod diversity, density and distribution relative to muskox and caribou habitat use, likely play a role in differential rates and patterns of range expansion. It is the thermal arena, however, that serves to determine parasite development and persistence in the environment and ultimately is the limiting factor that controls distribution.
We used a process-based mechanistic model using a thermal constant required for the development of the larval stages of the parasites (development degree-days: DD) to reveal the change in the geographical extents of the thermal niche for two invasive parasites over space and time, and to determine if the observed parasite range expansion could be explained by the ongoing Arctic warming. These models quickly demonstrated spatial and temporal differences in the parasites’ thermal niches, and different expansion rates between two parasite species that have generated new hypotheses. The results supported a tipping point in the late 1980s as proposed by Kutz et al.7, and identified a possible second ‘tipping point’ where intermittent suitability in annual temperatures have flipped to a phase of continuous suitability. This observation is consistent with the proposal for a shifting thermal balance driven by climatological conditions that serve to either limit or facilitate expansion, invasion, establishment and persistence for parasites (e.g.,9,13). Degree-day models have previously been widely used to understand the broader implications of climate warming on the distributional changes of species58–62, however, they are based on fairly simple ecological assumptions and do not incorporate key transmission variables such as transmission rates, population densities, spatial distributions of hosts etc. Increasingly robust models for dynamic transmission populated with parasite life-history traits and host population and movement data will help to further disentangle the complexities associated with the differential patterns of range expansion of these lungworms and in other parasite-host systems (e.g.,63).
Empirical data and baselines in conjunction with mechanistic models, provide strong evidence that Arctic warming has led to progressive changes in the distribution of permissive environments and has relaxed thermal constraints, facilitating range expansion, establishment, and persistence of two northern nematode parasites. While annual degree-day (ADD) accumulation across our study area generally increased over time, our detailed examination of conditions on Victoria Island demonstrated that this increase was characterized by considerable interannual variability in occurrence of conditions conducive for parasite development. We described a shifting thermal balance over time from years that were historically and predominantly non-permissive for parasite development to years that were predominantly permissive, facilitating invasion, persistence and emergence of the parasites. Thus, the interaction between hosts, parasites, thermal tolerances and thresholds, and fluctuating climatic conditions, is critical in limiting or facilitating the spatial and temporal potential for emerging disease. Habitats and host-parasite assemblages we have explored on Victoria Island are not idiosyncratic. General mechanisms are at play at high latitudes, as evidenced by similar trends in distribution, invasion, host association and explosive emergence of disease for phylogenetically distant filarioid nematodes, with arthropod and vector-borne transmission, among reindeer and other cervids across northern Finland64,65.
A wobbling climate over time drives episodes of environmental sloshing (recurrent environmental-faunal expansion and contraction) that occurs under alternating regimes of warming and cooling. Sloshing events unfold at varying temporal and spatial scales, from glacial-interglacial cycling of the Quaternary to shallow ecological time such as thermal shifts emerging from the ENSO or PDO and are central to faunal assembly relative to latitude and altitude that shapes the biosphere12,13. Changing patterns and amplitudes of warming and cooling influence the continuity of host-parasite systems across evolutionary to ecological time in the Arctic and globally12,13. Our observations provide direct insights about the central role of climate drivers in creating recurring opportunities for exploitation of geographical space by parasites66. Exemplified are the processes of intercontinental expansion and geographic colonization that characterized the assembly of mosaic parasite faunas linking Eurasia and North America and at intracontinental scales globally during the Pleistocene (e.g.,12,13). Contemporary communities, including a protostrongylid fauna with species of Varestrongylus and Umingmakstrongylus, and a diverse assemblage of other mammalian parasites reflect this intricate history67.
Anthropogenic warming now dominates climate conditions in northern systems, with accelerating warming evident since the 1970s. Trajectories for incremental warming in ecological time can be further influenced by decadal scale ocean–atmosphere dynamics and oscillations of the PDO and ENSO which directly influence temperature and environments in terrestrial systems13,68,69. Empirical observations from the Central Canadian Arctic in our study, as well as studies from Finland, demonstrate that these short-term shifts in warming and cooling may have important effects on opportunities for invasion that ultimately determine the distribution of pathogens and diseases. Our observations further confirm a general relationship linking capacities for parasites in utilizing host and environmental resources while exploring and exploiting opportunities emerging from ecological disruption which lead to shifting boundaries for distribution70,71. These dynamics emerge from ecological fitting and interactions of physical environment (Darwin’s nature of the conditions) and host-parasite biology (nature of the organism) under regimes of changing environmental settings66.
Warming is exerting pervasive and cascading environmental effects in the Arctic, and will continue to do so, with numerous and compounding ecological, social, and political perturbations2,72. Wildlife remains a critical resource for arctic peoples, foundational to their cultural well-being, and simultaneously an important source of food and income in a landscape where food-insecurity is widespread10,73. Recent disease outbreaks74,75, local and severe muskox declines15,76, widespread declines in caribou14,77, and enigmatic mortalities of seals78 and a massive shift in the structure of the Bering Sea ecosystem79,80, highlight the critical urgency of understanding biotic outcomes of unprecedented climate change and the downstream reverberations in host–pathogen interactions across the Arctic ecosystem.
Supplementary information
Acknowledgements
We thank Tracy Davison, Marsha Branigan, Morgan Anderson, Myles Lamont, Shane Black, Donald McLennan, Fabien Mavrot, Juliette Di Francesco and Steeve Côté for their help during the sample collection in the field, Angie Schneider, Kamala Sapkota, Auguste Condemine, Mickael Combe and Cassandra Bruce for assistance during sample processing and analysis. We thank Sylvia Checkley, Manigandan Lejeune, Guilhmere Verocai, and Andy Dobson for their technical assistance and for providing valuable suggestions and input throughout the study. We thank James Wang for technical support in the laboratory. We are grateful to Ekaluktutiak Hunters and Trappers Organization, Department of the Environment of the Government of Nunavut and the Government of Northwest Territories for the support throughout the project. The work was supported by funding to SK from NSERC Discovery, Research Tools and Instruments ( #RGPIN/04171-2014), Northern supplement Grants; ArcticNet Network Centre of Excellence (#03650-GF099007), Polar Knowledge Canada (#1516-166), and Canada North Outfitting. PK was partially supported by a scholarship from the NSERC-CREATE Host-Parasite Interactions (HPI) graduate training program.
Author contributions
P.K. and S.K. conceptualized the study. P.K. performed the research and analyzed the data. P.K. and S.K. drafted the manuscript, which was revised multiple times by all the coauthors. L.-M.R. and M.T. helped during field sampling and analysis. P.P. provided technical help in constructing and running the models. A.M. helped in statistical analyses, provided critical feedback from the start and helped to refine the final draft of the manuscript. E.H. revised and edited the manuscript and provided critical feedback to strengthen the final draft. All the co-authors approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-74358-5.
References
- 1.Arneth, A. et al. Summary for Policymakers. (2019).
- 2.Post E, et al. Ecological consequences of sea-ice decline. Science. 2013;341:519–524. doi: 10.1126/science.1235225. [DOI] [PubMed] [Google Scholar]
- 3.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- 4.Dobson A, Molnár PK, Kutz S. Climate change and Arctic parasites. Trends Parasitol. 2015;31:181–188. doi: 10.1016/j.pt.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Chan FT, et al. Climate change opens new frontiers for marine species in the Arctic: current trends and future invasion risks. Glob. Chang. Biol. 2019;25:25–38. doi: 10.1111/gcb.14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Descamps S, et al. Climate change impacts on wildlife in a High Arctic archipelago—Svalbard, Norway. Glob. Chang. Biol. 2017;23:490–502. doi: 10.1111/gcb.13381. [DOI] [PubMed] [Google Scholar]
- 7.Kutz SJ, Hoberg EP, Polley L, Jenkins EJ. Global warming is changing the dynamics of Arctic host–parasite systems. Proc. R. Soc. Lond. B Biol. Sci. 2005;272:2571–2576. doi: 10.1098/rspb.2005.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kutz SJ, et al. Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Glob. Chang. Biol. 2013;19:3254–3262. doi: 10.1111/gcb.12315. [DOI] [PubMed] [Google Scholar]
- 9.Hoberg EP, Brooks DR. Evolution in action: climate change, biodiversity dynamics and emerging infectious disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370:20130553. doi: 10.1098/rstb.2013.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomaselli M, Gerlach SC, Kutz SJ, Checkley SL. Iqaluktutiaq voices: local perspectives about the importance of muskoxen, contemporary and traditional use and practices. Arctic. 2018;71:1–14. doi: 10.14430/arctic4697. [DOI] [Google Scholar]
- 11.Kutz SJ, et al. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host–parasite interactions. Vet. Parasitol. 2009;163:217–228. doi: 10.1016/j.vetpar.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Hoberg, E. P., Galbreath, K. E., Cook, J. A., Kutz, S. J. & Polley, L. Northern host–parasite assemblages: history and biogeography on the borderlands of episodic climate and environmental transition. In Advances in Parasitology vol. 79. 1–97 (Elsevier, Amsterdam, 2012). [DOI] [PubMed]
- 13.Hoberg EP, et al. Arctic systems in the Quaternary: ecological collision, faunal mosaics and the consequences of a wobbling climate. J. Helminthol. 2017;91:409–421. doi: 10.1017/S0022149X17000347. [DOI] [PubMed] [Google Scholar]
- 14.COSEWIC. COSEWIC Assessment and Status Report on the Caribou (Rangifer tarandus) Dolphin and Union population in Canada 2017 (2017)
- 15.Cuyler C, et al. Muskox status, recent variation, and uncertain future. Ambio. 2019;49:1–15. doi: 10.1007/s13280-019-01205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutz S, Hoberg E, Polley L. A new lungworm in muskoxen: an exploration in Arctic parasitology. Trends Parasitol. 2001;17:276–280. doi: 10.1016/S1471-4922(01)01882-7. [DOI] [PubMed] [Google Scholar]
- 17.Kafle P, Sullivan J, Verocai GG, Kutz SJ. Experimental life-cycle of Varestrongylus eleguneniensis(Nematoda: Protostrongylidae) in a captive Reindeer (Rangifer tarandus tarandus) and a Muskox (Ovibos moschatus moschatus) J. Parasitol. 2017;103:584–587. doi: 10.1645/17-19. [DOI] [PubMed] [Google Scholar]
- 18.Kafle P, Peacock SJ, Grond S, Orsel K, Kutz S. Temperature-dependent development and freezing survival of protostrongylid nematodes of Arctic ungulates: implications for transmission. Parasit. Vectors. 2018;11:400. doi: 10.1186/s13071-018-2946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutz SJ, Hoberg EP, Polley L. Experimental infections of muskoxen (Ovibos moschatus) and domestic sheep with Umingmakstrongylus pallikuukensis (Nematoda: Protostrongylidae): parasite development, population structure, and pathology. Can. J. Zool. 1999;77:1562–1572. doi: 10.1139/z99-137. [DOI] [Google Scholar]
- 20.Osborn TJ, Jones P. The CRUTEM4 land-surface air temperature data set:Construction, previous versions and dissemination via Google earth. Earth Syst. Sci. Data. 2014;6:61–68. doi: 10.5194/essd-6-61-2014. [DOI] [Google Scholar]
- 21.Zhang X, et al. Changes in temperature and precipitation across Canada; Chapter 4. In: Bush E, Lemmen DS, et al., editors. Canada’s Changing Climate Report. Government of Canada: Ottawa, Ontario; 2019. pp. 112–193. [Google Scholar]
- 22.Kutz SJ, et al. Serendipitous discovery of a novel protostrongylid (Nematoda : Metastrongyloidea) in caribou, muskoxen, and moose from high latitudes of North America based on DNA sequence comparisons. Can. J. Zool. 2007;85:1143–1156. doi: 10.1139/Z07-091. [DOI] [Google Scholar]
- 23.Hoberg EP, Polley L, Gunn A, Nishi JS. Umingmakstrongylus pallikuukensis gen. nov. et sp. nov. (Nematoda: Protostrongylidae) from muskoxen, Ovibos moschatus, in the central Canadian Arctic, with comments on biology and biogeography. Can. J. Zool. Rev. Can. Zool. 1995;73:2266–2282. doi: 10.1139/z95-269. [DOI] [Google Scholar]
- 24.Forrester SG, Lankester MW. Extracting protostrongylid nematode larvae from ungulate feces. J. Wildl. Dis. 1997;33:511–516. doi: 10.7589/0090-3558-33.3.511. [DOI] [PubMed] [Google Scholar]
- 25.Kafle P, et al. Morphological keys to advance the understanding of protostrongylid biodiversity in caribou (Rangifer spp.) at high latitudes. Int. J. Parasitol. Parasites Wildl. 2017;6:331–339. doi: 10.1016/j.ijppaw.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kafle, P., Lejeune, M., Verocai, G. G., Hoberg, E. P. & Kutz, S. J. Morphological and morphometric differentiation of dorsal-spined first-stage larvae of lungworms (Nematoda: Protostrongylidae) infecting muskoxen (Ovibos moschatus) in the central Canadian Arctic. Int. J. Parasitol. Parasites Wildl.4 (2015). [DOI] [PMC free article] [PubMed]
- 27.Hoberg E, et al. Caudal polymorphism and cephalic morphology among first-stage larvae of Parelaphostrongylus odocoilei (Protostrongylidae: Elaphostrongylinae) in Dall’s sheep from the Mackenzie mountains, Canada. J. Parasitol. 2005;91:1318–1325. doi: 10.1645/GE-3503.1. [DOI] [PubMed] [Google Scholar]
- 28.Snyder RL, Spano D, Cesaraccio C, Duce P. Determining degree-day thresholds from field observations. Int. J. Biometeorol. 1999;42:177–182. doi: 10.1007/s004840050102. [DOI] [Google Scholar]
- 29.Kutz SJ, Hoberg EP, Polley L. Umingmakstrongylus pallikuukensis (Nematoda : Protostrongylidae) in gastropods: larval morphology, morphometrics, and development rates. J. Parasitol. 2001;87:527–535. doi: 10.1645/0022-3395(2001)087[0527:UPNPIG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Dainton, B. H. Field and laboratory observations on slug and snail behaviour. Monograph-British Crop Protection Council (1989).
- 31.Felber R, Stoeckli S, Calanca P. Generic calibration of a simple model of diurnal temperature variations for spatial analysis of accumulated degree-days. Int. J. Biometeorol. 2018;62:621–630. doi: 10.1007/s00484-017-1471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purcell LC. Comparison of thermal units derived from daily and hourly temperatures. Crop Sci. 2003;43:1874–1879. doi: 10.2135/cropsci2003.1874. [DOI] [Google Scholar]
- 33.Mesinger F, et al. North American regional reanalysis. Bull. Am. Meteorol. Soc. 2006;87:343–360. doi: 10.1175/BAMS-87-3-343. [DOI] [Google Scholar]
- 34.Roltsch WJ, Zalom FG, Strawn AJ, Strand JF, Pitcairn MJ. Evaluation of several degree-day estimation methods in California climates. Int. J. Biometeorol. 1999;42:169–176. doi: 10.1007/s004840050101. [DOI] [Google Scholar]
- 35.Kutz SJ, Hoberg EP, Nishi J, Polley L. Development of the muskox lungworm, Umingmakstrongylus pallikuukensis (Protostrongylidae), in gastropods in the Arctic. Can. J. Zool. 2002;80:1977–1985. doi: 10.1139/z02-197. [DOI] [Google Scholar]
- 36.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 37.Rózsa L, Reiczigel J, Majoros G. Quantifying parasites in samples of hosts. J. Parasitol. 2000;86:228–232. doi: 10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Verocai GG, et al. The biogeography of the caribou lungworm, Varestrongylus eleguneniensis (Nematoda: Protostrongylidae) across northern North America. Int. J. Parasitol. Parasites Wildl. 2020;11:93–102. doi: 10.1016/j.ijppaw.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verocai GG, Kutz SJ, Simard M, Hoberg EP. Varestrongylus eleguneniensis sp n. (Nematoda: Protostrongylidae): a widespread, multi-host lungworm of wild North American ungulates, with an emended diagnosis for the genus and explorations of biogeography. Parasit. Vectors. 2014;7:22. doi: 10.1186/1756-3305-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bintanja R, van der Linden EC. The changing seasonal climate in the Arctic. Sci. Rep. 2013;3:1556. doi: 10.1038/srep01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bush, E. & Lemmen, D. S. Canada’s Changing Climate Report; Government of Canada, Ottawa, ON. 444 (2019).
- 42.Trenberth, K. E. Observation: surface and atmospheric climate change. Climate Change 2007: The Physical Science Basis. Contribution of working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (2007).
- 43.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 44.Hoar BM, Ruckstuhl K, Kutz S. Development and availability of the free-living stages of Ostertagia gruehneri, an abomasal parasite of barrenground caribou (Rangifer tarandus groenlandicus), on the Canadian tundra. Parasitology. 2012;139:1093–1100. doi: 10.1017/S003118201200042X. [DOI] [PubMed] [Google Scholar]
- 45.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 46.Pecl GT, et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science. 2017;355:eaai9214. doi: 10.1126/science.aai9214. [DOI] [PubMed] [Google Scholar]
- 47.Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006;12:450–455. doi: 10.1111/j.1365-2486.2006.01116.x. [DOI] [Google Scholar]
- 48.Hope AG, Waltari E, Payer DC, Cook JA, Talbot SL. Future distribution of tundra refugia in northern Alaska. Nat Clim Change. 2013;3:931–938. doi: 10.1038/nclimate1926. [DOI] [Google Scholar]
- 49.Hope AG, et al. Arctic biodiversity: increasing richness accompanies shrinking refugia for a cold-associated tundra fauna. Ecosphere. 2015;6:1–67. doi: 10.1890/ES15-00104.1. [DOI] [Google Scholar]
- 50.Laaksonen, S. et al. Climate change promotes the emergence of serious disease outbreaks of filarioid nematodes. Ecohealth (2010). [DOI] [PMC free article] [PubMed]
- 51.Kovats RS, Campbell-Lendrum DH, McMichel AJ, Woodward A, Cox JSH. Early effects of climate change: do they include changes in vector-borne disease? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:1057–1068. doi: 10.1098/rstb.2001.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoberg EP. Invasive processes, mosaics and the structure of helminth parasite faunas. Rev. Sci. Tech. 2010;29:255. doi: 10.20506/rst.29.2.1972. [DOI] [PubMed] [Google Scholar]
- 53.Bryan HM, et al. Identification of Parelaphostrongylus odocoilei (Nematoda: Protostrongylidae) first-stage larvae in the feces of gray wolves (Canis lupus) by molecular methods. J. Wildl. Dis. 2010;46:297–302. doi: 10.7589/0090-3558-46.1.297. [DOI] [PubMed] [Google Scholar]
- 54.Bird S, et al. Geography, seasonality, and host-associated population structure influence the fecal microbiome of a genetically depauparate Arctic mammal. Ecol. Evol. 2019;23:13202–13217. doi: 10.1002/ece3.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prewer E, Kutz S, Leclerc LM, Kyle CJ. Already at the bottom? Demographic declines are unlikely further to undermine genetic diversity of a large Arctic ungulate: muskox, Ovibos moschatus (Artiodactyla: Bovidae) Biol. J. Linn. Soc. 2020;129:459–469. doi: 10.1093/biolinnean/blz175. [DOI] [Google Scholar]
- 56.Kutz SJ, Hoberg EP, Polley L. Emergence of third-stage larvae of Umingmakstrongylus pallikuukensis from three gastropod intermediate host species. J. Parasitol. 2000;86:743–749. doi: 10.1645/0022-3395(2000)086[0743:EOTSLO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan J. Developing a Systematic Sampling Framework for Terrestrial Gastropods in the Canadian Arcitc Calgary, Alberta. Calgary: University of Calgary; 2016. [Google Scholar]
- 58.Kearney M, Porter W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 59.Kearney M, Porter WP. Mapping the fundamental niche: physiology, climate, and the distribution of a nocturnal lizard. Ecology. 2004;85:3119–3131. doi: 10.1890/03-0820. [DOI] [Google Scholar]
- 60.Simon JA, et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol. Appl. 2014;7:750–764. doi: 10.1111/eva.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanhanen H, Veteli TO, Paivinen S, Kellomaki S, Niemela P. Climate change and range shifts in two insect defoliators: gypsy moth and nun moth-a model study. Silva Fenn. 2007;41:621. doi: 10.14214/sf.469. [DOI] [Google Scholar]
- 62.Yang G-J, et al. A growing degree-days based time-series analysis for prediction of Schistosoma japonicum transmission in Jiangsu province, China. Am. J. Trop. Med. Hyg. 2006;75:549–555. doi: 10.4269/ajtmh.2006.75.549. [DOI] [PubMed] [Google Scholar]
- 63.Molnár, P. K., Sckrabulis, J. P., Altman, K. A. & Raffel, T. R. Thermal performance curves and the metabolic theory of ecology-a practical guide to models and experiments for parasitologists. J. Parasitol. (2017). [DOI] [PubMed]
- 64.Laaksonen S, Oksanen A, Hoberg E. A lymphatic dwelling filarioid nematode, Rumenfilaria andersoni (Filarioidea; Splendidofilariinae), is an emerging parasite in Finnish cervids. Parasites Vectors. 2015;8:228. doi: 10.1186/s13071-015-0835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laaksonen, S. et al. Filarioid nematodes, threat to arctic food safety and security. In Game Meat Hygiene: Food Safety and Security, 213–223 (Wageningen Academic Publishers, Wageningen, 2017).
- 66.Brooks DR, Hoberg EP, Boeger WA. The Stockholm Paradigm: Climate Change and Emerging Disease. Chicago: University of Chicago Press; 2019. [Google Scholar]
- 67.Cook JA, et al. The Beringian Coevolution Project: holistic collections of mammals and associated parasites reveal novel perspectives on evolutionary and environmental change in the North. Arctic Science. 2016;3:585–617. doi: 10.1139/as-2016-0042. [DOI] [Google Scholar]
- 68.Stocker, T. F. et al. Climate Change 2013: The Physical Science Basis, Working Group 1 (WG1) Contribution to the Intergovernmental Panel on Climate Change (IPCC) 5th Assessment Report (AR5). Cambridge, UK and New York, New York, USA (2013)
- 69.Berkelhammer, M. Synchronous modes of terrestrial and marine productivity in the North Pacific. Front. Earth Sci.7 (2019).
- 70.Agosta SJ, Janz N, Brooks DR. How specialists can be generalists: resolving the" parasite paradox" and implications for emerging infectious disease. Zoologia (Curitiba) 2010;27:151–162. doi: 10.1590/S1984-46702010000200001. [DOI] [Google Scholar]
- 71.Araujo, S. B. et al. Understanding host-switching by ecological fitting. PLoS ONE10 (2015). [DOI] [PMC free article] [PubMed]
- 72.IPCC . Climate Change: Synthesis Report. In: Core Writing TeamPachauri RK, Meyer LA, editors. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC. Geneva: IPCC; 2014. p. 151. [Google Scholar]
- 73.Ford JD, Beaumier M. Feeding the family during times of stress: experience and determinants of food insecurity in an Inuit community. Geograph J. 2011;177:44–61. doi: 10.1111/j.1475-4959.2010.00374.x. [DOI] [PubMed] [Google Scholar]
- 74.Kutz S, et al. Erysipelothrix rhusiopathiae associated with recent widespread muskox mortalities in the Canadian Arctic. Can Vet J Rev Vet Can. 2015;56:560–563. [PMC free article] [PubMed] [Google Scholar]
- 75.Tomaselli M, et al. Contagious ecthyma, rangiferine brucellosis, and lungworm infection in a muskox (Ovibos moschatus) from the Canadian Arctic, 2014. J. Wildl. Dis. 2016;52:719–724. doi: 10.7589/2015-12-327. [DOI] [PubMed] [Google Scholar]
- 76.Kutz S, et al. Muskox health ecology symposium 2016: gathering to share knowledge on Umingmak in a time of rapid change. Arctic. 2017;70:225–236. doi: 10.14430/arctic4656. [DOI] [Google Scholar]
- 77.Vors LS, Boyce MS. Global declines of caribou and reindeer. Glob. Chang. Biol. 2009;15:2626–2633. doi: 10.1111/j.1365-2486.2009.01974.x. [DOI] [Google Scholar]
- 78.Fisheries, N. Diseased ice seals|NOAA fisheries. NOAAhttps://www.fisheries.noaa.gov/alaska/marine-life-distress/diseased-ice-seals (2020).
- 79.Jones, T. et al. Unusual mortality of Tufted puffins (Fratercula cirrhata) in the eastern Bering Sea. PloS ONE14 (2019). [DOI] [PMC free article] [PubMed]
- 80.Pörtner, H. O. Intergovernmental panel on climate change: summary for policymakers. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request.