ABSTRACT
Cation channel of Spermatozoa (CatSper) is one of the voltage-gated ion channels consisting of voltage sensor domains (VSDs) and pore-gate domains. CatSper is exclusively expressed in spermatozoa and indispensable for Ca2+ influx into cytosol. Recently, we have reported that the VSD of ascidian CatSper induces Ca2+-permeable pathways in heterologous expression systems. However, it is not known whether ion permeability through the VSD of CatSper is conserved in mammals. In the present study, electrophysiology and fluorometry in Xenopus oocytes revealed that Ca2+-permeable paths are also formed by expressing the VSD of murine CatSper. We also examined the permeability to monovalent cations other than Na+ in the VSD of ascidian CatSper.
KEYWORDS: Cation permeability, catsper, ion channel, spermatozoa, voltage sensor domain
Introduction
Voltage-gated ion channels mediate the passive ion transportation in response to membrane potential changes. In voltage-gated K+ channels, the pore-forming subunits consist of six trans-membrane regions which are divided into two functional domains: the voltage sensor domain (VSD; S1 ~ S4) and the pore-gate domain (S5 ~ S6) [1]. The VSD has been considered to exclusively function as the regulator of the pore of voltage-gated ion channels, but multiple studies have reported that direct ion permeation pathways exist in some mutated VSDs and in the voltage-gated proton channel [2–7]. Thus, the VSD of voltage-gated ion channels plays more roles in physiological and pathological situations than as considered in the past several decades.
Cation channel of Spermatozoa (CatSper) belongs to the voltage-gated ion channel superfamily [8]. It has been suggested that the four distinct α subunits of CatSper (CatSper1, CatSper2, CatSper3 and CatSper4) and several accessary subunits form a CatSper complex [8–13]. Each α subunit consists of six trans-membrane regions with homologous domains to the VSD and the pore-gate domain of voltage-gated ion channels. Gene knockout of the CatSper α subunits causes male infertility due to the lack of Ca2+ supply into spermatozoa which is essential for hyperactivation of the sperm motility in mice [12,14–17]. Electrophysiological analyses by direct whole-cell recordings from mammalian spermatozoa revealed that the CatSper currents are potentiated by intracellular alkalization, and monovalent currents are elicited in divalent-free condition [18,19].
Recently, we have reported that the VSD of the ascidian CatSper3 subunit (CiCS3 VSD) forms Ca2+ permeation pathways in the plasma membrane of Xenopus oocytes and cultured mammalian cells [20]. That was the first case that showed evidence for divalent cation permeation through a VSD as well as Ca2+ current of CatSper in heterologous expression systems. However, several important issues remain to be addressed. First, mechanisms of ion permeability through the VSD of ascidian CatSper3 are elusive. Detailed information of permeability to monovalent cations is essential for understanding mechanisms of ion permeation through CatSper VSD. Second, it remains unclear whether the Ca2+ permeability of VSD is unique to the ascidian CatSper3. In the present study, we studied the permeability of the VSD of the ascidian CatSper3 to monovalent cations. We also tested if the VSD of CatSper derived from mouse has Ca2+ permeability by heterologous expression in Xenopus oocytes. We found that the VSD of ascidian CatSper3 is permeable to various monovalent cations, such as K+, Cs+ and Li+. On the other hand, we did not obtain any evidence for permeation to protons. Overexpression of the VSD of murine CatSper3 (mCS3 VSD) gave rise to Ca2+ permeation pathways which are activated upon membrane hyperpolarization, suggesting the conservation of Ca2+ permeability between the VSDs of murine and ascidian CatSper subunit.
Materials and Methods
Ethics approval
Experiments using Xenopus laevis and mice were performed in accordance with the guidelines of the Animal Care and Use Committee of Osaka University Medical School.
cDNA cloning of the voltage sensor domain of murine CatSper3
Total RNA from a testis of C57BL/6 mouse was extracted with TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA). The cDNA fragment of murine CatSper3 (mCS3; NM_001252187.1) was obtained by RT-PCR and subcloned into a custom modified version of the pcs2+ vector, and sequenced. The primers used for cloning the mCS3 are 5ʹ- ccggatccatgtcccaacattttcaccacaaccc-3ʹ and 5ʹ-ccggatccctaacttagtcccgagaggctggacg-3ʹ. The cDNA of the voltage sensor domain of murine CatSper3 (mCS3 VSD; M1-T176) was subcloned into the pcs2+ vector by PCR using the following primers; 5ʹ-ccggatccatgtcccaacattttcaccacaaccc-3ʹ and 5ʹ-ccggatccctaagtgtagaccgtctcccccac-3ʹ.
Construction of a chimeric protein and mutagenesis
Constructions of chimeric proteins were done by PCR. A cDNA fragment of iR-pHluorin [21] was kindly gifted by Dr. Hiroyuki Katayama and Dr. Atsushi Miyawaki (RIKEN, Japan). The fusion construct of the VSD of ascidian CatSper3 [20] and iR-pHluorin (CiCS3 VSD-iR-pHluorin) was created with the primers: 5ʹ- cccaagcttatggagaagaaaagtcgg −3ʹ, 5ʹ- cgcccttgctcaccatctttttcatcgtgtcta −3ʹ, 5ʹ- tagacacgatgaaaaagatggtgagcaagggcg −3ʹ and 5ʹ- ccggatccttacttgtacagctcgtcca −3ʹ. The fusion construct of CiHv1/VSOP [3] and iR-pHluorin (iR-pHluorin-CiHv1/VSOP) was created with the primers: 5ʹ- ccaagcttatggtgagcaagggcgaggag −3ʹ, 5ʹ- ccggatccatggaaggcgataattgc −3ʹ, 5ʹ- ccggatcccttgtacagctcgtccatgc −3ʹ and 5ʹ- ccctcgagttaaacatcagcagaggctg −3ʹ. M1-K48 of mCS3 was replaced with M1-G103 of ascidian voltage-sensing phosphatase (Ci-VSP) [22,23] and named Membrane-Targeted mCS3 VSD (MT-mCS3 VSD). The primers used for the construction of MT-mCS3 VSD were 5ʹ-cccggatccatggagggattcgacggttc-3ʹ, 5ʹ-atctggaagaaagtgctacctacaccagtagtag-3ʹ, 5ʹ-ctactactggtgtaggtagcactttcttccagat-3ʹ and 5ʹ-ccggatccctaagtgtagaccgtctcccccac-3ʹ. Site-directed mutagenesis was performed using a QuikChange kit (Agilent Technologies, Santa Clara, CA). The primers used for the construction of R151Q and K154Q are 5ʹ-catccagtctctacagatcctcaagcttatctc−3ʹ and 5ʹ-gtctctacgaatcctccagcttatctcctacag −3ʹ, respectively.
Two-electrode voltage clamp recordings and fluorometry in oocytes
Two-electrode voltage clamp (TEVC) recordings and fluorometry were done as described previously [20]. Briefly, Xenopus oocytes were defolliculated by treating with type I collagenase (1.0 mg/mL; Sigma-Aldrich, St. Louis, MO) in ND96 solution; 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 0.1 mg/mL Gentamycin, 5 mM Pyruvate-Na, pH = 7.5 adjusted by NaOH [24]. The defolliculated oocytes were then injected with 50 nL of cRNA solution. The concentration of cRNA synthesized using mMESSAGE mMACHINE transcription kit (Thermo Fisher Scientific) was 1 – 1.5 ng/nL and 0.1 – 0.2 ng/nL for mCS3 VSD and CiCS3 VSD, respectively.
Current recordings were conducted at 2–3 days after cRNA injection by TEVC using an amplifier (OC-725; Warner Instruments, Hamden, CT). Acquired data were digitized by an AD/DA converter (InstruTECH LIH 8 + 8; HEKA Elektronik, Lambrecht/Pfalz, Germany), and analyzed with PatchMaster software (HEKA Elektronik). The interval between traces was 10 seconds. Current-Voltage (I-V) relationships were obtained by plotting the average current amplitudes at the end of the test pulses against the membrane potential. Glass electrodes had resistance of 0.3–0.5 M ohm after filling with 3 M KCl solution. Perfusion of the bath solution was done using a gravity perfusion system. The composition of the bath solutions are described below.
32 K+ solution: 32 mM KOH, 5 mM MgCl2, 140 mM sucrose, 5 mM HEPES, pH 7.5 (pH adjusted by methanesulfonic acid: MeSO3H)
96 K+ solution: 96 mM KOH, 5 mM MgCl2, 40 mM Sucrose, 5 mM HEPES, pH 7.5
32 Li+ solution: 32 mM LiOH, 5 mM MgCl2, 140 mM Sucrose, 5 mM HEPES, pH 7.5
96 Li+ solution: 96 mM LiOH, 5 mM MgCl2, 40 mM Sucrose, 5 mM HEPES, pH 7.5
32 Cs+ solution: 32 mM CsOH, 5 mM MgCl2, 140 mM Sucrose, 5 mM HEPES, pH7.5
96 Cs+ solution: 96 mM CsOH, 5 mM MgCl2, 40 mM Sucrose, 5 mM HEPES, pH 7.5
32 NMDG solution: 32 mM NMDG, 5 mM MgCl2, 140 mM sucrose, 5 mM HEPES, pH 7.5
96 NMDG solution: 96 mM NMDG, 5 mM MgCl2, 40 mM sucrose, 5 mM HEPES, pH 7.5
pH 6 solution: 96 mM NMDG, 5 mM MgCl2, 40 mM sucrose, 5 mM 2-Morpholinoethanesulfonic acid, pH = 6.0 (MeSO3H)
For the fluorometry, macroscopic currents and fluorescence changes were simultaneously recorded using an amplifier (OC-725C) and an inverted microscope (IX70; Olympus, Tokyo, Japan) equipped with a stable 75W xenon lamp (Ushio, Tokyo, Japan). Oocytes were injected with 50 nL of 1 mM Fluo-3 (DOJINDO MOLECULAR TECHNOLOGIES, INC, Kumamoto, Japan) 1–3 hrs before recordings for visualizing changes of intracellular Ca2+ concentration. The GFP-A-BASIC-OMF was used for excitation of Fluo-3 and iR-pHluorin, and an objective lens (× 20, NA 0.70; Olympus) was used to collect fluorescence. A photomultiplier (PMT) module (H5784-02; Hamamatsu Photonics, Shizuoka, Japan) was used for the acquisition of fluorescence. The output signals from the PMT module and the TEVC amplifier were digitized and stored using an A/D converter (1322A; Molecular Devices, Sunnyvale, CA) and pCLAMP8 software (Molecular Devices), respectively. Data were analyzed by Clampfit 10.3 software (Molecular Devices), and the fluorescence signal was digitally filtered at 500 Hz. The interval between traces was 30 seconds. Fluorescence changes (ΔF) were calculated by normalizing with the average signal intensity of 300 ms before the test pulses. Fluorescence-Voltage (F-V) relationships were obtained by plotting average ΔF for 50 ms from the end of the test pulses against membrane potential. The composition of Ca2+ free solution is 96 mM NaCl, 2 mM KCl, 5 mM MgCl2, 5 mM HEPES, pH7.5 (NaOH).
Results
The VSD of ascidian CatSper3 is permeable to K+, Li+ and Cs+
In our previous study, we found that the VSD of ascidian CatSper3 (CiCS3 VSD) is permeable to Na+ as well as Ca2+[2, [20]. However, permeability to other monovalent cations has not been examined. We tried to compare the reversal potentials under different bi-ionic conditions but it was unsuccessful since the kinetics of tail currents was too rapid. Permeability to K+, Li+ and Cs+ was examined by comparing the current amplitude in two different concentrations of extracellular cations, 32 mM and 96 mM, in the same cell. All solutions contained Mg2+ at 5 mM because oocytes are unstable without divalent cation (See Methods). Currents recorded in 96 mM solution were larger than those in 32 mM solution in all cases, indicating that the CiCS3 VSD is permeable to K+, Li+ and Cs+ (Figure 1 A-C). In recordings with perfusion from 32 mM NMDG solution to 96 mM NMDG solution, no obvious increase of the current amplitude was observed (Figure 1D), suggesting that the CiCS3 VSD is not permeable to NMDG. The charge carrier of the current recorded in NMDG solutions could be either Mg2+ or protons which are contained in the bath solutions.
Figure 1.
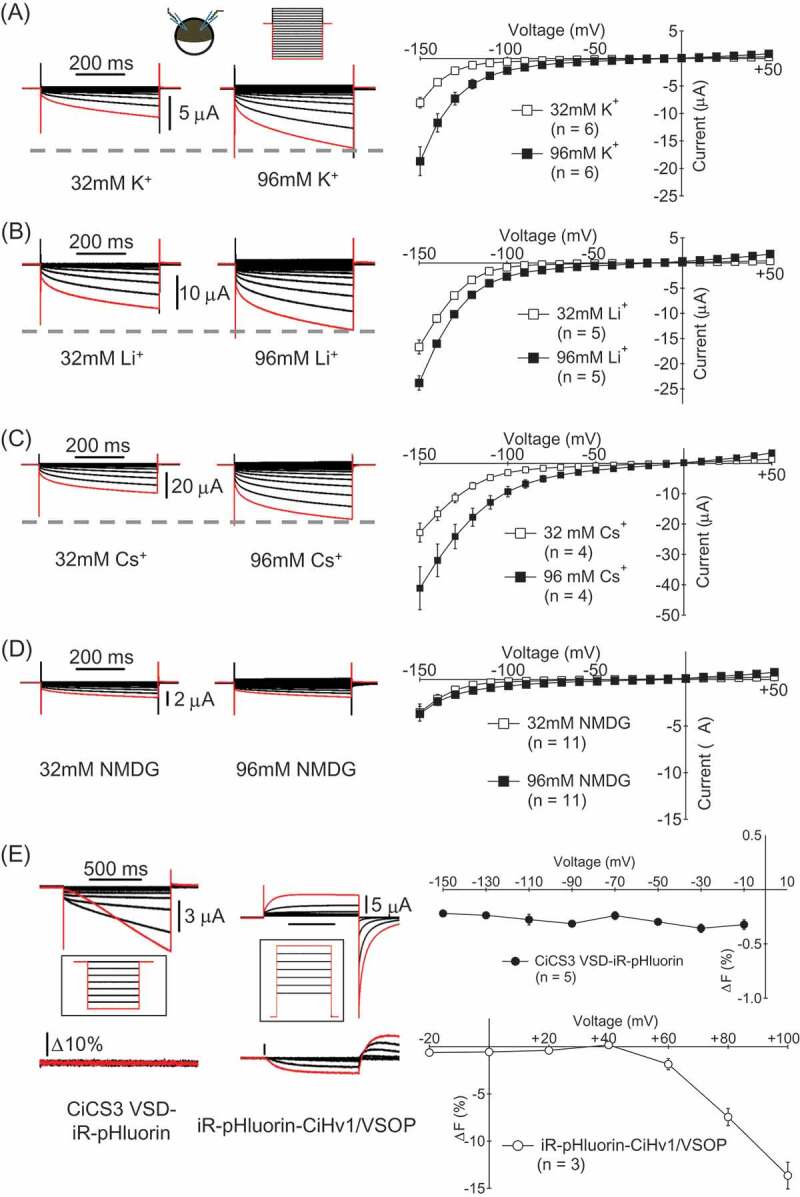
Permeability of CiCS3 VSD to monovalent cations (A-D) Current traces and I-V relationships of the recordings (mean ± SEM) for examining K+ (A), Li+ (B), Cs+ (C) and NMDG (D) permeability. The same cells were recorded in the solutions with two different concentrations of cations. Voltage steps were from + 50 mV to −150 mV by 10 mV decrement. Holding potential (Vh) was at −10 mV. The current evoked by a step pulse to −150 mV is colored by red. (E) Test of proton permeability. Currents and fluorescence changes of iR-pHluorin were recorded from an oocyte expressing CiCS3 VSD-iR-pHluorin (left) or iR-pHluorin-CiHv1/VSOP (right) in the pH6 solution. Voltage steps for CiCS3 VSD-iR-pHluorin were from −150 mV to −10 mV. Vh was −10 mV. The voltage steps for iR-pHluorin-CiHv1/VSOP expressing cells were from −20 mV to + 100 mV. Vh was −80 mV.
To know whether CiCS3 VSD is permeable to protons, pH fluorometry was performed using the pH-sensitive green fluorescence protein, iR-pHluorin [21]. iR-pHluorin fused to the C-terminus of CiCS3 VSD (CiCS3 VSD-iR-pHluorin) was expressed in Xenopus oocytes. A fusion protein of iR-pHluorin and the voltage-gated proton channel cloned from Ciona intestinalis, CiHv1/VSOP [3], which was expressed as a positive control (iR-pHluorin-CiHv1/VSOP), showed clear fluorescence change upon depolarization of the membrane (Figure 1E). However, no fluorescence change was observed upon hyperpolarization in cells expressing the CiCS3 VSD-iR-pHluorin, which showed robust inward current (Figure 1E), suggesting that the proton does not permeate through the CiCS3 VSD. This supports the idea that the currents recorded in NMDG solutions are most likely carried by Mg, but not by protons.
Ca2+ permeable paths are formed by the voltage sensor domain of murine CatSper3
Our previous study on the VSD of ascidian CatSper3 prompted us to test if the VSD of the murine CatSper3 (mCS3 VSD: See Figure 2A) also has ion permeability. 25.3% of amino acids in the trans-membrane regions are identical between the mCS3 VSD and the CiCS3 VSD. When the mCS3 VSD was expressed in Xenopus oocytes, the currents evoked by voltage steps were indistinguishable from those in uninjected cells (Figure 2B). To facilitate the expression of the mCS3 VSD in the plasma membrane, we utilized the N-terminal cytoplasmic region of the ascidian voltage-sensing phosphatase (Ci-VSP) which is known to facilitate the protein translocation to the plasma membrane [22,23]. In oocytes expressing the chimeric protein of mCS3 VSD and N-terminus of Ci-VSP (MT-mCS3 VSD), slowly-activating inward currents were elicited upon membrane hyperpolarization (Figure 2 B and C). Although the amount of injected cRNA for MT-mCS3 VSD was 5–10 times larger than that for the CiCS3 VSD [20], amplitudes of the currents in cells expressing the MT-mCS3 VSD (−0.40 ± 0.060 nA, n = 10, at −120 mV) were approximately 4 times smaller than those of the CiCS3 VSD (−1.69 ± 0.29 nA, n = 7, at −120 mV). As in our former study on ascidian CatSper3 [20], rebound currents were evoked upon depolarization to −10 mV from hyperpolarizing voltage steps. This suggests that the calcium-activated chloride channels endogenously expressed in Xenopus oocytes were activated following the membrane hyperpolarization, implying that Ca2+ influx or release of Ca2+ from intracellular stores occurred upon membrane hyperpolarization as already reported for the CiCS3 VSD [20]. Given that positively charged amino acids on the S4 segment are known to play key roles in the activation of the currents in most of voltage-gated ion channels, we examined the effect of a mutation in the S4 segment of the MT-mCS3 VSD. Substitution of the first arginine to a glutamine (R151Q) did not affect the inward currents in oocytes. Upon mutation of Lys-154 (K154Q), the current magnitude was remarkably smaller than that of WT (Fig. S1).
Figure 2.
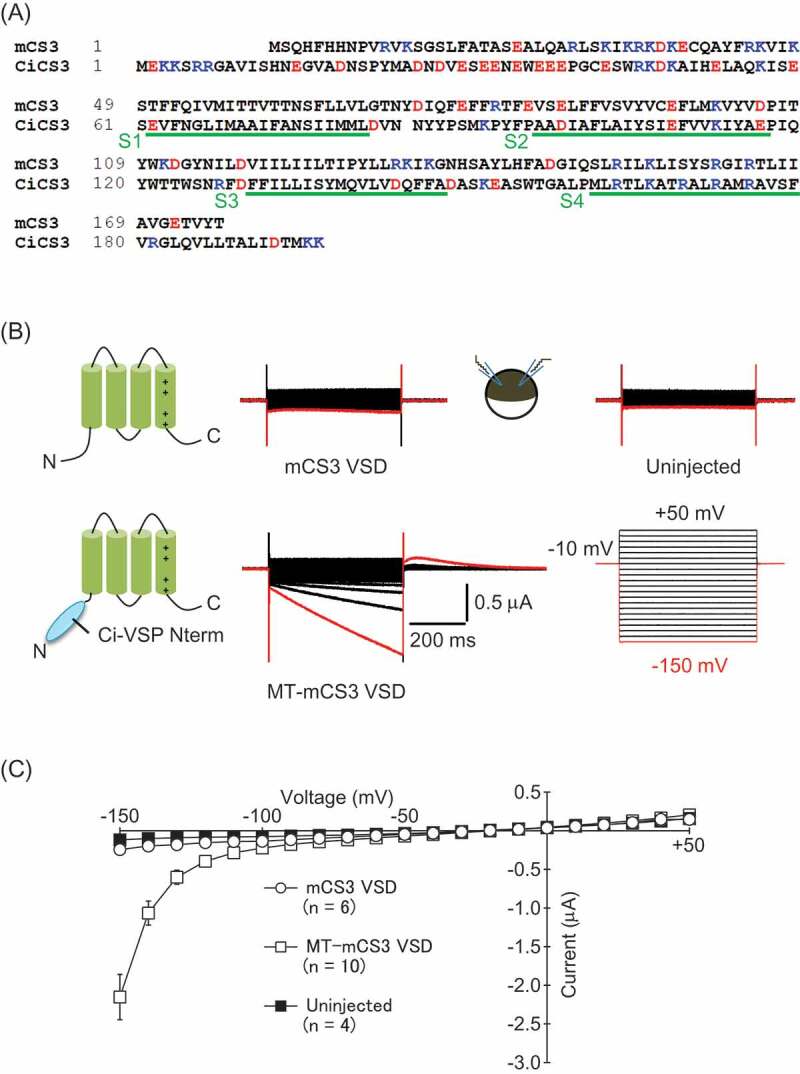
Inward currents are elicited in cells expressing the voltage-sensor domain of murine CatSper3. (A) Alignment of the amino acid sequence of the mCS3 VSD and the CiCS3 VSD (LC329335 in DDBJ). Negatively and positively charged amino acids are colored by red and blue, respectively. The putative trans-membrane regions are indicated by green lines. (B) Representative current traces from Xenopus oocytes injected with the cRNA of the mCS3 VSD (left upper) and the MT-mCS3 VSD (left lower), and an uninjected oocyte (right upper). The bath solution was ND69 solution. Voltage steps were from + 50 mV to −150 mV by 10 mV decrement (right lower). Vh was at −10 mV. The current evoked by a step pulse to −150 mV is colored by red. (C) Current-Voltage relationships of the recordings (mean ± SEM).
To verify that the ion permeation paths induced by expression of the MT-mCS3 VSD are permeable to Ca2+, we performed simultaneous recordings of macroscopic ionic currents and intracellular fluorescence changes of one of the Ca2+ indicators, Fluo-3. In Xenopus oocytes expressing the MT-mCS3 VSD, inward currents and increases of intracellular fluorescence were observed upon membrane hyperpolarization when the cells were bathed in ND96 solution that contains Ca2+ (Figure 3 A left and B). In contrast, such fluorescence changes were not detected from cells bathed in nominally Ca2+ free solution (Figure 3 A right and B) though the ionic currents were elicited. Neither ionic current nor fluorescence change was elicited in intact cells bathed in ND96 solution (Figure 3B). These results indicate that Ca2+ influx occurs upon membrane hyperpolarization in oocytes expressing the MT-mCS3 VSD. Notably, the Ca2+ signal was detected from the step pulse to −50 mV that is within the physiological range of the membrane potential.
Figure 3.
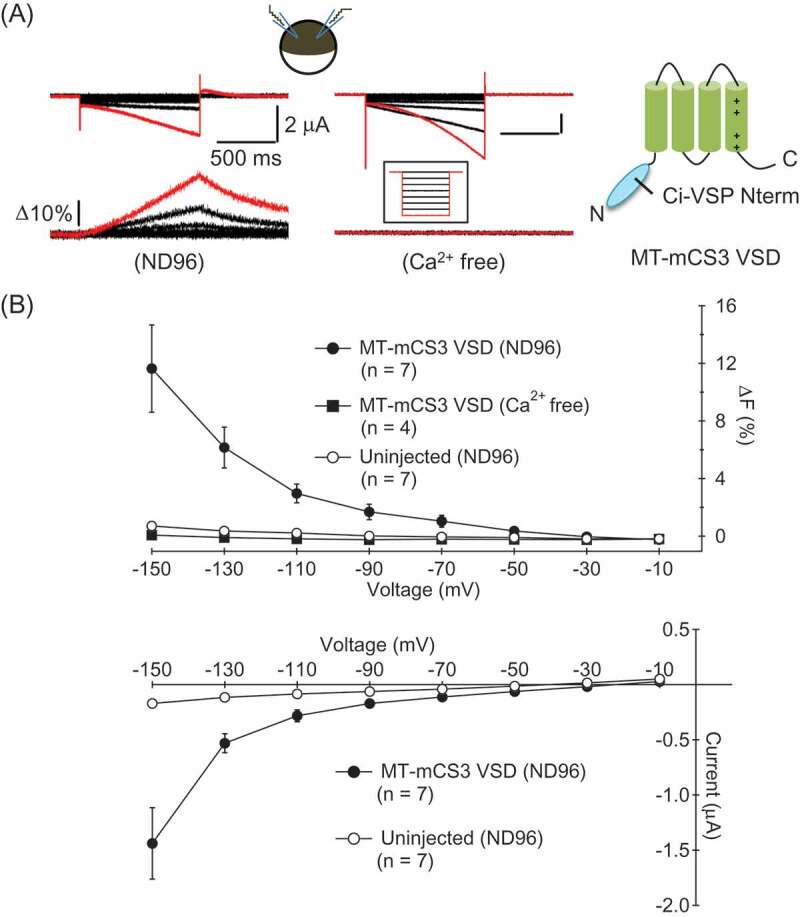
The ion permeation paths induced by the MT-mCS3 VSD are permeable to Ca2+ [2]+ . (A) Representative current traces and fluorescence changes from oocytes expressing the MT-mCS3 VSD bathed in ND96 (Left) or Ca2+ free solution (Right). The voltage steps were from −150 mV to −10 mV by 20 mV increment. Vh was at −10 mV. The traces evoked by a step pulse to −150 mV are colored by red. The recordings in ND96 and Ca2+ free solution were from different cells. (B) Fluorescence-Voltage relationships (upper) and Current-Voltage relationships (lower) of the recordings (mean ± SEM).
Discussion
In this study, we addressed the two issues which have remained unclear in our previous study; (1) Permeability of the VSD of ascidian CatSper3 (CiCS3 VSD) to monovalent cations, (2) Conservation of Ca2+ permeability of CatSper3 among species. We showed that the CiCS3 VSD was permeable to various monovalent cations, and the VSD of murine CatSper3 (mCS3 VSD) induced the Ca2+ -permeable pathways in the plasma membrane of Xenopus oocytes. The mCS3 VSD-induced Ca2+ influx was observed in the physiological range of the membrane potential.
Direct current recordings from murine spermatozoa revealed that the CatSper is permeable to monovalent cations, such as Na+ and Cs+, as well as Ca2+, [18]. As is consistent with this, we demonstrated that the CiCS3 VSD was permeable to monovalent cations including K+, Li+ and Cs+, indicating that the CiCS3 VSD functions as a non-selective cation channel (Figure 1). However, the CiCS3 VSD was not thought to be permeable to protons as far as examined with a pH-sensitive green fluorescence protein (Figure 1E).
In our recordings, no obvious tail current was observed as in the CiCS3 VSD, and therefore, it was impossible to determine the ion selectivity of the CiCS3 VSD from the reversal potential in different bi-ionic conditions. Similarly, omega currents (or gating pore currents) through the VSDs of Shaker K+ channel and Nav1.4 channel show no tail current [6,25]. This suggests that the rapid closing of the ion permeable pathway is a common characteristic among ion-permeable VSDs except Hv1 [2,3]. Further analyses, such as single channel analysis, are required for understanding more detailed biophysical properties of the CiCS3 VSD.
Our previous study revealed that the VSD of ascidian CatSper3 (CiCS3 VSD) forms hyperpolarization-activated Ca -permeable pathways in the plasma membrane of Xenopus oocytes and cultured cells [20]. As is the case with the CiCS3 VSD, inward currents and Ca influx were evoked upon membrane hyperpolarization in the oocytes expressing the mCS3 VSD (Figures. 2 and 3). In addition, the inward currents recorded in the nominally Ca free solution indicated that the paths induced by the mCS3 VSD are permeable to other ions, such as Na+ (Figure 3). These common observations suggest that the ion permeability of the CatSper3 VSD is an intrinsic trait conserved among species despite the low homology between the primary structure of the mCS3 VSD and the CiCS3 VSD. In voltage-gated Ca channels, negatively charged amino acids in the pore-gate domain are important for the Ca permeation [26]. By analogy to this, negatively charged amino acids in trans-membrane regions may play key roles for the Ca permeability in the CiCS3 VSD and the mCS3 VSD. In fact, although the entire homology is low (only 25.3% amino acid identity), three negatively charged amino acids in the S2 segment are conserved between the ascidian and the murine CatSper3 (Figure 2A).
Facilitation of membrane targeting of the mCS3 VSD by making chimeric protein with the N-terminal cytoplasmic region of Ci-VSP was necessary to detect the ion conductance. The expression level of the mCS3 VSD in the plasma membrane of Xenopus oocytes was much lower than that of the CiCS3 VSD which showed robust current without requiring such modification [20]. We also investigated the biophysical properties of VSDs of ascidian CatSper1, CatSper2 and CatSper4, but ionic currents were not recorded reproducibly. That may be because of insufficient expression in Xenopus oocytes.
The ionic currents were detectable at more negative voltages than −100 mV in cells expressing the mCS3 VSD (Figure 2), whereas Ca fluorometry indicated that the Ca influx started to be elicited by a step pulse to −50 mV as in ascidian CatSper3 (Figure 3B)20. Chávez et al. showed that the membrane potential of murine spermatozoa reaches approximately −70 mV during the final maturation of ejaculated spermatozoa called capacitation [27]. Thus, the ion permeation pathways induced by the VSD of mCS3 are capable of conducting Ca in the physiological situation if it is functional in spermatozoa. However, previous studies suggested that the CatSper is activated by depolarization of the membrane [18]. This issue should be addressed by investigating the function of whole CatSper complex in heterologous expression systems. To know whether Ca enters into the cytosol of spermatozoa through the VSD of CatSper α subunits, it may be worth testing if the infertility of male mice in which spermatozoa lack CatSper complex can be rescued by overexpression of the CatSper VSDs in the sperm membrane.
Supplementary Material
Acknowledgments
We thank Drs. Hiroyuki Katayama and Atsushi Miyawaki (RIKEN) for providing iR-pHluorin. We thank the members of the Laboratory of Integrative Physiology in Osaka University for discussion and advice. HA was supported by IPBS, one of the Programs for Leading Graduate Schools, and by a Research Fellowship from the Japan Society for the Promotion of Science for Young Scientists (17J02119 to HA). This work was supported by Grants-in-Aids from Ministry of Education, Culture, Sports, Science and Technology (MEXT) (JP25253016 to HT and YO, 15H05901 to YO, 16H02617 to YO, 26670103 to HT) and CREST (JST) (JPMJCR14M3 to HT and YO).
Biography
HA conducted the experiments and analyzed the data. HT and YO designed and supervised the research. HA, HT and YO wrote the paper.
Disclosure statement
The authors declare no competing interest.
supplementary data
supplementary data can be accessed here
References
- 1.Moreau A, Gosselin-Badaroudine P, Chahine M.. Molecular biology and biophysical properties of ion channel gating pores. Q Rev BiophysInternet.2014. cited2014 December 2;47:364–388. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25382261 [DOI] [PubMed] [Google Scholar]
- 2.Sasaki M, Takagi M, Okamura Y.. A voltage sensor-domain protein is a voltage-gated proton channel. ScienceInternet.2006. cited2014 May 4;312:589–592. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16556803 [DOI] [PubMed] [Google Scholar]
- 3.Ramsey IS, Moran MM, Chong JA, et al. A voltage-gated proton-selective channel lacking the pore domain. Nature. Internet. 2006;440:1213–1216. Available from. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4084761&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokolov S, Scheuer T, Catterall WA. Ion permeation through a voltage-sensitive gating pore in brain sodium channels having voltage sensor mutations. Neuron. 2005;47:183–189. [DOI] [PubMed] [Google Scholar]
- 5.Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. Internet. 2004;427:548–553. Available from. http://www.ncbi.nlm.nih.gov/pubmed/14765197 [DOI] [PubMed] [Google Scholar]
- 6.Tombola F, Pathak MM, Isacoff EY. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. NeuronInternet.2005. cited2014 April 29;45:379–388. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15694325 [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Blunck R. The isolated voltage sensing domain of the Shaker potassium channel forms a voltage-gated cation channel. Elife [Internet] 2016. [cited 2016 Oct 6]; 5:1–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27710769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lishko PV, Kirichok Y, Ren D, et al. The control of male fertility by spermatozoan ion channels. Annu Rev PhysiolInternet2012. [cited 2014 May 1];74:453–475. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3914660&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.J-J C, Miki K, Kim D, et al. CatSperζ regulates the structural continuity of sperm Ca2+ signaling domains and is required for normal fertility. Elife. Internet. 2017;6:1–25. Available from. http://www.ncbi.nlm.nih.gov/pubmed/28226241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung -J-J, Navarro B, Krapivinsky G, et al. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat CommunInternet.2011. cited2014 May 4;2:153 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3999383&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J-L J, Am O, Wang S, et al. Catsper3 and catsper4 encode two cation channel-like proteins exclusively expressed in the testis. Biol ReprodInternet.2005. cited2014 May 4;73:1235–1242. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16107607 [DOI] [PubMed] [Google Scholar]
- 12.Quill TA, Sugden SA, Kl R, et al. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc Natl Acad Sci U S A. 2003;100:14869–14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren D, Navarro B, Perez G, et al. A sperm ion channel required for sperm motility and male fertility. Nature. Internet. 2001;413:603–609. Available from. http://www.ncbi.nlm.nih.gov/pubmed/11595941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson AE, Quill TA, Westenbroek RE, et al. Identical phenotypes of CatSper1 and CatSper2 null sperm. J Biol ChemInternet.2005. cited2014 April 30;280:32238–32244. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16036917 [DOI] [PubMed] [Google Scholar]
- 15.Carlson AE, Westenbroek RE, Quill T, et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A. Internet. 2003;100:14864–14868. Available from. http://www.ncbi.nlm.nih.gov/pubmed/14657352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin J, Jin N, Zheng H, et al. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol ReprodInternet.2007. cited2014 May 4;77:37–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17344468 [DOI] [PubMed] [Google Scholar]
- 17.Qi H, Moran MM, Navarro B, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A. Internet. 2007;104:1219–1223. Available from. http://www.ncbi.nlm.nih.gov/pubmed/17227845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. NatureInternet.2006. cited2014 May 4;439:737–740. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16467839 [DOI] [PubMed] [Google Scholar]
- 19.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. NatureInternet.2011. cited2014 April 29;471:387–391. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21412339 [DOI] [PubMed] [Google Scholar]
- 20.Arima H, Tsutsui H, Sakamoto A, et al. Induction of divalent cation permeability by heterologous expression of a voltage sensor domain. Biochim Biophys Acta - Biomembr. Internet. 2018;1860:981–990. Available from. http://www.ncbi.nlm.nih.gov/pubmed/29317195 [DOI] [PubMed] [Google Scholar]
- 21.Katayama H, Kogure T, Mizushima N, et al. A sensitive and quantitative technique for detecting autophagic events based on lysosomal delivery. Chem Biol. Internet. 2011;18:1042–1052. Available from. http://linkinghub.elsevier.com/retrieve/pii/S1074552111002043 [DOI] [PubMed] [Google Scholar]
- 22.Murata Y, Iwasaki H, Sasaki M, et al. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. NatureInternet.2005. cited2014 May 1;435:1239–1243. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15902207 [DOI] [PubMed] [Google Scholar]
- 23.Tsutsui H, Jinno Y, Tomita A, et al. Improved detection of electrical activity with a voltage probe based on a voltage-sensing phosphatase. J PhysiolInternet.2013. cited2014 May 4;591:4427–4437. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23836686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 1992;207:266–279. [DOI] [PubMed] [Google Scholar]
- 25.Struyk AF, Cannon SC. A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J Gen PhysiolInternet.2007. cited2014 May 4;130:11–20. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2154364&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sather WA, McCleskey EW. Permeation and selectivity in calcium channels. Annu Rev PhysiolInternet.2003. cited2014 May 29;65:133–159. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12471162 [DOI] [PubMed] [Google Scholar]
- 27.Chávez JC, De La Vega-Beltrán JL, Escoffier J, et al. Ion Permeabilities in Mouse Sperm Reveal an External Trigger for SLO3-Dependent Hyperpolarization. PLoS OneInternet.2013. cited2014 May 4;8:e60578 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3618424&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


