Abstract
The previous meta-analysis of clinical trials revealed a beneficial effect of vitamin E supplementation on serum C-reactive protein (CRP) concentrations; however, it is unknown whether this vitamin has the same influence on other inflammatory biomarkers. Also, several clinical trials have been published since the release of earlier meta-analysis. Therefore, we aimed to conduct a comprehensive meta-analysis to summarize current evidence on the effects of vitamin E supplementation on inflammatory biomarkers in adults. We searched the online databases using relevant keywords up to November 2019. Randomized clinical trials (RCTs) investigating the effect of vitamin E, compared with the placebo, on serum concentrations of inflammatory cytokines were included. Overall, we included 33 trials with a total sample size of 2102 individuals, aged from 20 to 70 years. Based on 36 effect sizes from 26 RCTs on serum concentrations of CRP, we found a significant reduction following supplementation with vitamin E (− 0.52, 95% CI − 0.80, − 0.23 mg/L, P < 0.001). Although the overall effect of vitamin E supplementation on serum concentrations of interleukin-6 (IL-6) was not significant, a significant reduction in this cytokine was seen in studies that used α-tocopherol and those trials that included patients with disorders related to insulin resistance. Moreover, we found a significant reducing effect of vitamin E supplementation on tumor necrosis factor-α (TNF-α) concentrations at high dosages of vitamin E; such that based on dose–response analysis, serum TNF-α concentrations were reduced significantly at the dosages of ≥ 700 mg/day vitamin E (Pnon-linearity = 0.001). Considering different chemical forms of vitamin E, α-tocopherol, unlike other forms, had a reducing effect on serum levels of CRP and IL-6. In conclusion, our findings revealed a beneficial effect of vitamin E supplementation, particularly in the form of α-tocopherol, on subclinical inflammation in adults. Future high-quality RCTs should be conducted to translate this anti-inflammatory effect of vitamin E to the clinical setting.
Subject terms: Physiology, Biomarkers, Diseases, Medical research
Introduction
Vitamin E is the most abundant lipid-soluble antioxidant present in body tissues1. Intake of the antioxidant has a beneficial effect on the prevention and management of chronic diseases including stroke, hypertension, diabetes mellitus, and fatty liver disease2,3. Subclinical inflammation is a low-grade chronic inflammation with only minor elevation in C-reactive protein (CRP) levels occurring in the absence of classic clinical signs of inflammation. The major function of such inflammation is to restore tissue homeostasis4–6. Whether the beneficial effect of vitamin E on these chronic conditions is mediated through the inflammatory processes is not clear. It has been shown that vitamin E has an inhibitory effect on pro-inflammatory cytokine expression through inhibition of activation of the key nuclear transcription factor NF-κB7,8. Also, there is evidence from observational studies indicating an overall favorable link between vitamin E intake and serum levels of pro-inflammatory cytokines9,10. Despite the aforesaid points, findings from clinical trials investigating the effect of vitamin E supplementation on subclinical inflammation are conflicting11–43. Some clinical trials have shown a significant reducing effect of vitamin E supplementation on serum concentrations of C-reactive protein (CRP) and interleukin-6 (IL-6)26,37,40, while others did not find any significant effect17,19,27. Surprisingly, in a randomized clinical trial (RCT), vitamin E supplementation resulted in a significant increase in serum concentrations of CRP and IL-633. An earlier meta-analysis of vitamin E supplementation on serum CRP levels suggested a beneficial effect of vitamin E in the form of either α-tocopherol or γ-tocopherol44. Also, a meta-analysis in 2014 revealed a beneficial effect of vitamin E coated dialyzer on subclinical inflammation; however, authors in that meta-analysis included only clinical trials that performed on hemodialysis patients45.
Overall, there is a need for a comprehensive meta-analysis summarizing all available findings in this area. Therefore, the current meta-analysis was conducted to summarize current evidence on the effects of vitamin E supplementation on inflammatory biomarkers in adults.
Methods
This study was performed based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol for reporting systematic reviews and meta-analyses.
Search strategy
We performed a comprehensive literature search in the online databases of PubMed, Scopus, Web of Science, and Google Scholar up to November 2019. In the search, we purposed to identify clinical trials investigating the effects of vitamin E supplementation on inflammatory cytokines in adults. The following keywords were used in the search strategy: ("vitamin E" OR tocopherol OR tocotrienol OR "VIT E") AND ("Inflammation" OR "inflammatory " OR "Interleukin-10" OR IL-10 OR "Interleukin-8" OR IL-8 OR "Interleukin-6" OR IL-6 OR "Tumor necrosis factor" OR TNF OR "C-reactive protein" OR " high-sensitivity c-reactive protein" OR CRP OR hs-CRP OR "Transforming growth factor beta" OR "cytokines" OR "cytokine" OR "Acute phase reactant" OR "Matrix metalloproteinase" OR "e-selectin" OR "p-selectin" OR "Intercellular adhesion molecule-1" OR "Monocyte chemotactic protein 1" OR MCP-1 OR "Inflammation Mediator" OR "Neurogenic Inflammation" OR "Myokine" OR "Adipokine" OR "Interleukin-1B" OR IL-1B OR "interleukins" OR "interleukin" OR "Systemic inflammation" OR "Biological marker" OR "Fibrinogen”). No language or time restriction was applied. Reference lists of the relevant studies were manually screened to avoid missing any eligible publication. Unpublished studies were not considered. The literature search was conducted by two independent investigators.
Inclusion criteria
We included eligible studies that met the following criteria: (1) randomized controlled clinical trials, (2) studies that conducted on adult subjects (≥ 18 years), (3) studies that administered vitamin E in different chemical forms including alpha-, beta-, gamma-, and delta-tocopherol and alpha-, beta-, gamma-, and delta-tocotrienol, (4) RCTs with at least one week’s duration of intervention, and (5) controlled trials that reported mean changes and their standard deviations (SDs) of inflammatory cytokines throughout the trial for both intervention and control groups or presented required information for calculation of those effect sizes. If > 1 article were published for one dataset, the more complete one was included. Clinical trials with an additional intervention group were considered as 2 separate studies.
Exclusion criteria
In the current meta-analysis, we excluded experimental studies, those with a cohort, cross-sectional, and case–control design, review articles, and ecological studies. We also excluded trials without a placebo or control group and those which were performed on children or adolescents.
Data extraction
Two independent investigators performed data extraction from each eligible RCT. The following information was extracted: name of the first author, publication year, individuals’ characteristics (mean age and sex), study design, sample size (control and intervention groups), type of vitamin E prescribed, the dosage of vitamin E, duration of intervention, mean changes and their SDs of inflammatory biomarkers throughout the trial for the intervention and control groups, and the confounding variables adjusted in the analyses. If data on inflammatory biomarkers were reported in different units, we converted them to the most frequently used unit.
Risk of bias assessment
We applied the Cochrane quality assessment tool for assessing the risk of bias for each study included in the current meta-analysis46,47. This tool contained seven domains including random sequence generation, allocation concealment, reporting bias, performance bias, detection bias, attrition bias, and other sources of bias. Each domain was given a “high risk” score if the study comprised methodological defects that may have affected its findings, a “low risk” score if there was no defect for that domain, and an “unclear risk” score if the information was not sufficient to determine the impact. The overall risk of bias for an RCT was considered: (1) Low; if all domains had “low risk”, (2) Moderate; if one or more domains had “unclear risk”, and (3) High; if one or more domains had “high risk”48. The risk of bias assessment was done independently by two reviewers.
Statistical analysis
Mean changes and their SDs of inflammatory cytokines in the vitamin E and control groups were used to obtain the overall effect sizes. When mean changes were not reported, we calculated them by considering changes in cytokine concentrations during the intervention. We also converted standard errors (SEs), 95% confidence intervals (CIs), and interquartile ranges (IQRs) to SDs using the method of Hozo et al.49. To obtain the overall effect sizes, we applied a random-effects model that takes between-study variations into account. Heterogeneity was determined by the I2 statistic and Cochrane’s Q test. I2 value > 50% or P < 0.05 for the Q-test was considered as significant between-study heterogeneity50–52. To find probable sources of heterogeneity, subgroup analyses were performed according to the predefined variables including duration of the intervention (≥ 8 vs. < 8 weeks), type (alpha-tocopherol vs. gamma-tocopherol vs. mixed type) and dosage of vitamin E (≥ 500 vs. < 500 mg/day), participants’ health condition (apparently healthy vs. unhealthy individuals), baseline serum levels of inflammatory cytokines (elevated vs. normal levels), and adjustment for baseline levels of the outcome variable (adjusted vs. non-adjusted). To determine the non-linear effects of vitamin E dosage (mg/d) on cytokine concentrations, fractional polynomial modeling was applied. Sensitivity analysis was used to detect the dependency of the overall effect size on a particular study. The possibility of publication bias was examined by the formal test of Begg. The meta-analysis was carried out by the use of the Stata, version 11.2 (StataCorp). P-value < 0.05 was considered as significant level.
Results
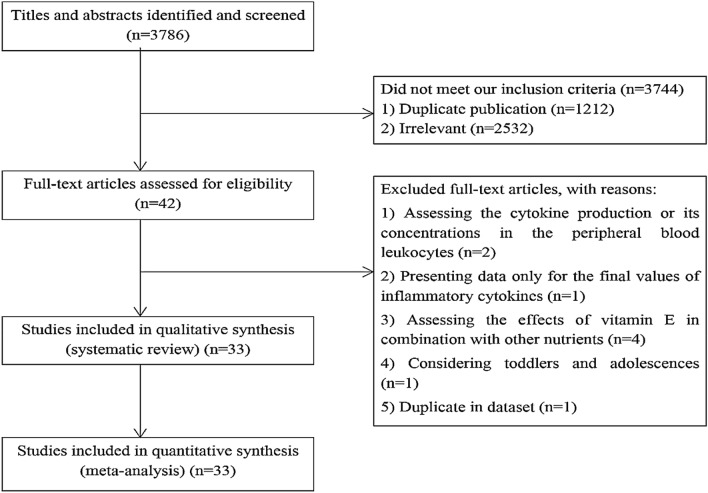
Out of 3786 publications that were identified in our initial search, 1212 duplicate articles were excluded. After screening the remaining 2574 records, 2532 unrelated articles were also removed based on title and abstract assessment. Then, 42 publications remained for further evaluation of the full text. Out of those 42 studies, two RCTs were excluded due to assessing the cytokine production or its concentrations in the peripheral blood leukocytes53,54. The study of Silva et al. was also excluded because they did not report baseline values of cytokine concentrations making us unable to calculate mean changes of cytokines throughout the trial55. We also excluded four RCTs in which the effects of vitamin E in combination with other nutrients such as vitamin C, α-lipoic acid, and omega-3 fatty acids were investigated56–59. The study of Tang et al. performed on iron-deficient infants and toddlers was excluded as well60. Moreover, two eligible articles were published on the same dataset35,61, of which the more complete one was included35 and the other one was excluded61. After these exclusions, 33 eligible RCTs remained for inclusion in the current systematic review and meta-analysis11–43; out of them, 26 studies assessed serum concentrations of CRP11,13–20,22,25–31,33–35,37,38,40–43, 14 studies assessed serum concentrations of IL-617,20–23,26–31,33,37,38, and 12 studies evaluated serum concentrations of tumor necrosis factor-α (TNF-α) following vitamin E supplementation12,17,20,21,24,29–32,36,38,39. Data on the other inflammatory cytokines including IL-1 (n = 1), IL-2 (n = 2), IL-4 (n = 1), and IL1β (n = 1) were not sufficient to perform a meta-analysis. Flow diagram of study selection is outlined in Fig. 1.
Figure 1.
Flow diagram of the study selection.
Characteristics of the included studies
The characteristics of 33 RCTs included in the current systematic review and meta-analysis are illustrated in Table 1. These RCTs were published between 2000 and 2018 and were from the USA14,20,27,29,42,43, Europe13,15,19,21,22, Asia11,18,23–26,28,30–37,39–41, Canada12, and Australia16,17,38. Four studies were exclusively performed on male subjects23,24,28,35 and others on both genders. The sample size of included RCTs varied from 16 to 110 participants, resulting in a total sample size of 2102 individuals. The mean age of participants was between 20 and 70 years. The dosage of vitamin E supplements varied from 15 to 1080 mg/day and duration of intervention ranged from 1 to 104 weeks across selected RCTs. Most studies employed a parallel design, while only one study was cross-over22. Concerning the type of vitamin E, a total of 27 studies administered α-tocopherol11–15,17–26,28,30–37,39,42,43, 3 studies administered γ-tocopherol16,20,29, and 3 other studies performed the intervention with a combination of different types of tocopherols. In six trials, participants in the vitamin E and control groups received concentrated red grape juice19 or supplements of α-lipoic acid26,30 or n-3 fatty acids14,18,32 in addition to the main intervention. RCTs were performed on healthy individuals14,16,23,24,29, patients with type 2 diabetes11,17,25,31,33,36,38, metabolic syndrome20,30, non-alcoholic fatty liver disease21,39,40, cardiovascular diseases (CVDs)12,13,22,35,41,42, rheumatoid arthritis18,32, erectile dysfunction28, and renal calculi34. Furthermore, five trials included hemodialysis patients15,19,26,37,43. Of 33 RCTs, 11 studies controlled the baseline values of inflammatory cytokines in their analyses11,16–19,25,36,37,39,40,43. Only one study30 could be considered as a high-quality study with a totally low risk of bias for all domains of the Cochrane Risk of Bias Assessment Tool. Two RCTs29, 38 were of moderate-quality in which one or more domains had an unclear risk of bias. Others had low-quality since they had a high risk of bias for one or more domains (Supplemental Table 1).
Table 1.
Summary of clinical trials on the effects of vitamin E supplementation on inflammatory biomarkers in adults aged ≥ 20 years.
| Author, year | Design | Participants, n | Health condition | Age, yeara | Intervention | Duration (week) | Outcomes (changes)b | Adjust/matchingc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Control group | Treatment group | Control group | |||||||
| Upritchard et al. 2000 | RA/parallel | M/F: 25 Int: 12, Con: 13 | DM | Int: 56 ± 14, Con: 60 ± 6 | 800 IU/day α-tocopherol | 4 | CRP: − 2.70 ± 3.70 | CRP: 0.20 ± 1.75 | 4 | |
| Keith et al. 2001 | RA/DB/parallel | M/F: 56 Int: 30, Con: 26 | CVD | Int: 70, Con: 64 | 1000 IU/day α-tocopherol | 12 | TNF-α: 0.40 ± 2.22 | TNF-α: 0.99 ± 2.65 | ||
| Murphy et al. 2004 | RA/DB/parallel | M/F: 110 Int: 55, Con: 55 | CVD | Int: 65 ± 9, Con: 66 ± 13 | 400 IU/day α-tocopherol acetate | 26 | CRP: − 10.47 ± 10.48 | CRP: − 14.19 ± 33.09 | ||
| Lopez et al. 2004 | RA/DB/parallel | M/F: 40 Int: 20, Con: 20 | Healthy | Int: 33 ± 7, Con: 33 ± 10 | 800 IU/day all-rec α-tocopherol | 12 | CRP: 0.10 ± 4.75 | CRP: 0. ± 1.61 | ||
| M/F: 40 Int: 20, Con: 20 | Int: 29 ± 6, Con: 30 ± 9 | 800 IU/day all-rec α-tocopherol + 1.5 g n-3 PUFA | CRP: 0.40 ± 4.79 | CRP: 1.10 ± 6.28 | ||||||
| Hodkova et al. 2006 | RA/parallel | M/F: 29 Int: 15, Con: 14 | HD | Int: 63 ± 6, Con: 60 ± 8 | 400 mg/day α-tocopherol | 5 | CRP: − 0.72 ± 2.56 | CRP: 1.13 ± 3.28 | 1 | |
| Singh et al. 2007 | RA/DB/parallel | M/F: 26 Int: 14, Con: 12 | Healthy | Int: 20–40, Con: 20–40 | 100 mg/day γ-tocopherol | 5 | CRP: 0.50 ± 2.76 | CRP: − 0.30 ± 2.10 | 2,4 | |
| M/F: 25 Int: 13, Con: 12 | 200 mg/day γ-tocopherol | CRP: − 0.40 ± 1.45 | CRP: − 0.30 ± 2.10 | |||||||
| Wu et al. 2007 | RA/DB/parallel | M/F: 36 Int: 18, Con: 18 | DM | Int: 64 ± 30, Con: 62 ± 30 | 500 mg/day α-tocopherol | 6 |
CRP: 0.02 ± 1.34 TNF-α: 0.03 ± 0.15 IL-6: − 0.33 ± 0.65 |
CRP: − 0.20 ± 1.46 TNF-α: 0.03 ± 0.19 IL-6: 0.15 ± 0.71 |
4 | |
| M/F: 37 Int: 19, Con: 18 | Int: 58 ± 17, Con: 62 ± 30 | 500 mg/day mixed tocopherols |
CRP: − 0.21 ± 0.79 TNF-α: 0.02 ± 0.13 IL-6: 0.05 ± 0.45 |
CRP: − 0.20 ± 1.46 TNF-α: 0.03 ± 0.19 IL-6: 0.15 ± 0.71 |
||||||
| Aryaeian et al. 2008 | RA/DB/parallel | M/F: 43 Int: 21, Con: 22 | rheumatoid arthritis | Int: 49 ± 12, Con: 48 ± 11 | 400 mg/day α-tocopherol | 12 | CRP: − 4.99 ± 11.00 | CRP: − 0.96 ± 4.77 | 4,9 | |
| M/F: 44 Int: 22, Con: 22 | Int: 44 ± 13, Con: 46 ± 3 | 400 mg/day α-tocopherol + CLA | CLA | CRP: − 2.06 ± 4.04 | CRP: − 1.72 ± 6.60 | |||||
| Castilla et al. 2008 | RA/parallel | M/F: 16 Int: 8, Con: 8 | HD | 33–79 | 800 IU/day α-tocopherol | Nothing | 2 | CRP: 3.50 ± 8.44 | CRP: 0.69 ± 6.36 | 4 |
| M/F: 16 Int: 8, Con: 8 | 800 IU/day α-tocopherol plus red grape juice | Red grape juice | CRP: − 1.10 ± 6.46 | CRP: 2.40 ± 10.78 | ||||||
| Devaraj et al. 2008 | RA/DB/parallel | M/F: 40 Int: 20, Con: 20 | MetS | Int: 51 ± 11, Con: 56 ± 11 | 800 mg/day α-tocopherol | 6 |
CRP: − 0.86 ± 3.15 TNF-α: − 0.04 ± 0.21 IL-6: 0.20 ± 1.46 |
CRP: − 0.38 ± 3.29 TNF-α: 0.08 ± 0.29 IL-6: 0.50 ± 1.64 |
1,3 | |
| M/F: 40 Int: 20, Con: 20 | Int: 50 ± 9, Con: 56 ± 11 | 800 mg/day γ-tocopherol |
CRP: − 1.49 ± 2.80 TNF-α: − 0.02 ± 0.27 IL-6: − 0.30 ± 1.02 |
CRP: − 0.38 ± 3.29 TNF-α: 0.08 ± 0.29 IL-6: 0.50 ± 1.64 |
||||||
| M/F: 40 Int: 20, Con: 20 | Int: 57 ± 14, Con: 56 ± 11 | 800 mg/day mixed tocopherols |
CRP: − 2.20 ± 3.05 TNF-α: − 0.14 ± 0.30 IL-6: 0.30 ± 1.26 |
CRP: − 0.38 ± 3.29 TNF-α: 0.08 ± 0.29 IL-6: 0.50 ± 1.64 |
||||||
| Balmer et al. 2009 | RA/DB/parallel | M/F: 28 Int: 14, Con: 14 | NASH | Int: 47 ± 14, Con: 47 ± 12 | 800 IU/day α-tocopherol | 104 |
TNFα: − 0.75 ± 0.66 IL-6: − 0.47 ± 2.44 |
TNFα: − 1.65 ± 0.85 IL-6: 0.69 ± 1.88 |
1 | |
| Dalgard et al. 2009 | RA/DB/crossover | M/F: 48 Int: 24, Con: 24 | CVD | Int: 63 ± 7, Con: 57 ± 6 | 15 mg/day α-tocopherol + fruits juice | Fruits juice | 4 |
CRP: − 0.20 ± 0.74 IL-6: 0.10 ± 0.81 |
CRP: − 0.10 ± 2.00 IL-6: − 0.10 ± 0.96 |
|
| Ghiasvand et al. 2009 | RA/DB/parallel | M: 17 Int: 9, Con: 8 | Healthy | Int: 24 ± 2, Con: 21 ± 2 | 400 IU/day α-tocopherol | 6 | IL-6: − 0.10 ± 1.27 | IL-6: 0.16 ± 1.43 | 1,2 | |
| M: 17 Int: 9, Con: 8 | Int: 27 ± 5, Con: 24 ± 3 | 400 IU/day α-tocopherol + EPA | EPA | IL-6: − 2.83 ± 1.49 | IL-6: − 3.81 ± 0.94 | |||||
| Ghiasvand et al. 2010 | RA/DB/parallel | M: 17 Int: 9, Con: 8 | Healthy | Int: 23 ± 2, Con: 21 ± 2 | 400 IU/day α-tocopherol | 6 | TNF-α: 1.44 ± 3.60 | TNF-α: 0.37 ± 3.62 | 1,2 | |
| M: 17 Int: 9, Con: 8 | Int: 27 ± 5, Con: 24 ± 3 | 400 IU/day α-tocopherol + EPA | EPA | TNF-α: 3.50 ± 2.40 | TNF-α: 0.88 ± 4.45 | |||||
| Rafraf et al. 2012 | RA/DB/parallel | M/F: 83 Int: 42, Con: 41 | DM | Int: 35 ± 7, Con: 35 ± 8 | 400 mg/day α-tocopheryl acetate | 8 | CRP: − 0.03 ± 0.59 | CRP: 0.07 ± 0.79 | 1,2,3,4,6,8 | |
| Ahmadi et al. 2013 | RA/parallel | M/F: 41 Int: 17, Con: 24 | HD | Int: 45 ± 13, Con: 49 ± 12 | 400 IU/day α-tocopherol | 8 |
CRP: − 2.00 ± 5.17 IL-6: − 10.00 ± 20.26 |
CRP: 0.19 ± 3.92 IL-6: 10.9 ± 25.50 |
- | |
| M/F: 44 Int: 24, Con: 20 | Int: 53 ± 10, Con: 49 ± 11 | 400 IU/day α-tocopherol + lipoic acid | lipoic acid |
CRP: − 1.60 ± 4.08 IL-6: − 11.00 ± 20.13 |
CRP: − 2.50 ± 4.71 IL-6: − 7.50 ± 17.15 |
|||||
| Daud et al. 2013 | RA/DB/parallel | M/F: 81 Int: 41, Con: 40 | HD | Int: 59 ± 12, Con: 58 ± 13 | 220 mg/day tocotrienol-rich fraction, all types | 16 |
CRP: 1.30 ± 16.90 IL-6: 1.00 ± 2.12 |
CRP: 1.30 ± 23.86 IL-6: 0.60 ± 4.40 |
||
| El-sisi et al. 2013 | RA/DB/parallel | M: 40 Int: 20, Con: 20 | Erectile dysfunction | 40–60 | 400 IU/day α-tocopherol | 6 |
CRP: 0.66 ± 2.55 IL-6: − 3.17 ± 2.32 |
CRP: 0.17 ± 1.74 IL-6: 0.79 ± 2.67 |
||
| Mah et al. 2013 | RA/DB/parallel | M/F: 30 Int: 16, Con: 14 | Healthy smokers | Int: 21 ± 4, Con: 22 ± 4 | 500 mg/day γ-tocopherol | 1 |
CRP: − 1.73 ± 5.28 TNF-α: − 0.29 ± 0.40 IL-6: − 0.02 ± 0.48 |
CRP: − 0.96 ± 2.31 TNF-α: − 0.05 ± 0.2 IL-6: − 0.38 ± 0.80 |
||
| Manning et al. 2013 | RA/DB/parallel | M/F: 76 Int: 36, Con: 40 | MetS | Int: 57 ± 10, Con: 57 ± 9 | 100 IU/day α-tocopherol | 52 |
CRP: 0.50 ± 2.42 TNFα: − 0.09 ± 0.44 IL-6: − 0.30 ± 0.44 |
CRP: 0.00 ± 2.01 TNFα: 0.00 ± 0.32 IL-6: 0.10 ± 0.79 |
2 | |
| M/F: 75 Int: 41, Con: 34 | Int: 54 ± 13, Con: 55 ± 10 | 100 IU/day α-tocopherol + lipoic acid | Lipoic acid |
CRP: − 0.20 ± 2.75 TNFα: 0.30 ± 0.40 IL-6: 0.40 ± 1.07 |
CRP: 0.30 ± 1.68 TNFα: 0.00 ± 0.44 IL-6: 0.99 ± 0.93 |
|||||
| Shadman et al. 2013 | RA/DB/parallel | M/F: 36 Int: 17, Con: 19 | Overweight DM | Int: 48 ± 4, Con: 45 ± 6 | 100 IU/day α-tocopherol | 8 |
CRP: − 0.98 ± 2.71 TNFα: − 2.80 ± 1.83 IL-6: − 0.60 ± 1.83 |
CRP: − 0.55 ± 1.58 TNFα: − 3:00 ± 1.50 IL-6: − 0.89 ± 0.98 |
1,2,3 | |
| Aryaeian et al. 2014 | RA/DB/parallel | M/F: 43 Int: 21, Con: 22 | rheumatoid arthritis | Int: 49 ± 12, Con: 48 ± 11 | 400 mg/day α-tocopherol | 12 | TNFα: − 1.17 ± 2.88 | TNFα: − 1.13 ± 2.74 | 1,2,10 | |
| M/F: 44 Int: 22, Con: 22 | Int: 44 ± 13, Con: 46 ± 13 | 400 mg/day α-tocopherol + CLA | TNFα: − 2.41 ± 2.59 | TNFα: − 2.36 ± 3.63 | ||||||
| Gopalan et al. 2014 | RA/DB/parallel | M/F: 88 Int: 46, Con: 42 | CVD | Int: 52 ± 9, Con: 52 ± 8 | 400 mg/day mixed tocotrienols | 104 | CRP: − 0.57 ± 4.27 | CRP: 2.12 ± 10.76 | ||
| Hejazi et al. 2015 | RA/SB/parallel | M/F: 27 Int: 14, Con: 13 | DM | Int: 48 ± 62, Con: 47 ± 8 | 400 IU/day α-tocopherol | 6 |
CRP: 1.30 ± 8.17 IL-6: 15.40 ± 8.96 |
CRP: − 0.70 ± 4.69 IL-6: 5.60 ± 3.83 |
||
| Modi et al. 2015 | RA/parallel | M/F: 72 Int: 36, Con: 36 | Renal calculi | Int: 39 ± 5, Con: 40 ± 4 | 800 mg/day α-tocopherol | 1 | CRP: 0.09 ± 1.40 | CRP: 2.99 ± 4.28 | ||
| Ramezani et al. 2015 | RA/DB/parallel | M/F: 42 Int: 20, Con: 22 | CVD | Int: 56 ± 2, Con: 55 ± 1 | 400 IU/day α-tocopherol | 8 | CRP: − 1.57 ± 2.41 | CRP: − 1.29 ± 1.99 | ||
| Khatami et al. 2016 | RA/DB/parallel | M/F: 60 Int: 30, Con: 30 | DM | Int: 61 ± 10, Con: 62 ± 14 | 1200 IU/day α-tocopherol | 12 | TNF-α: − 32.8 ± 24.90 | TNF-α: 3.0 ± 24.09 | 1,2,3,4,6,7 | |
| Sohrabi et al. 2016 | RA/parallel | M/F: 46 Int: 23, Con: 23 | HD | Int: 56 ± 9, Con: 57 ± 10 | 1800 IU/week all-rec α-tocopherol + whey protein | 8 |
CRP: − 0.98 ± 0.23 IL-6: − 1.18 ± 2.70 |
CRP: − 0.34 ± 0.87 IL-6: − 3.96 ± 14.25 |
4 | |
| M/F: 46 Int: 23, Con: 23 | Int: 58 ± 8, Con: 55 ± 6 | 1800 IU/week all-rec α-tocopherol | Nothing |
CRP: 0.002 ± 0.90 IL-6: − 5.10 ± 17.90 |
CRP: 0.06 ± 0.34 IL-6: 2.77 ± 4.80 |
|||||
| Stonehouse et al. 2016 | RA/DB/parallel | M/F: 57 Int: 28, Con: 29 | DM | Int: 60 ± 7, Con: 61 ± 6 | 552 mg/day tocotrienol, all types | 8 |
CRP: 0.38 ± 1.68 TNF-α: − 0.09 ± 1.32 IL-6: 0.37 ± 5.77 |
CRP: − 0.07 ± 1.52 TNF-α: − 0.4 ± 1.29 IL-6: − 2.13 ± 5.67 |
1,2,3,5,6 | |
| Ekhlasi et al. 2017 | RA/DB/parallel | M/F: 30 Int: 15, Con: 15 | NAFLD | 25–64 | 400 IU/day α-tocopherol | 8 | TNF-α: − 11.66 ± 10.4 | TNF-α: 2.57 ± 10.1 | 1,3,4 | |
| 400 IU/day α-tocopherol + probiotic strain | Probiotic strain | TNF-α: − 15.01 ± 10.0 | TNF-α: − 9.1 ± 10.1 | |||||||
| Pervez et al. 2018 | RA/DB/parallel | M/F: 64 Int: 31, Con: 33 | NAFLD | Int: 45 ± 9, Con: 44 ± 10 | 600 mg/day δ-tocotrienol (90%) and γ-tocotrienol (10%) | 12 | CRP: − 0.74 ± 0.29 | CRP: − 0.26 ± 0.30 | 4 | |
| Devaraj et al. 2007 | RA/DB/parallel | M/F: 90 Int: 44, Con: 46 | CVD | Int: 59 ± 7, Con: 62 ± 6 | 1200 IU/day α-tocopherol | 104 | CRP: − 1.69 ± 1.69 | CRP: 0.61 ± 1.76 | ||
| Rachelle et al. 2011 | RA/DB/parallel | M/F: 50 Int: 25, Con: 25 | HD | Int: , Con: | 400 IU/day α-tocopherol | 8 | CRP: − 5.80 ± 13.29 | CRP: 6.40 ± 11.48 | 4 | |
CRP C-reactive protein, IL-6 interleukin 6, TNF-α tumour necrosis factor-α, DM diabetes mellitus, CVD cardiovascular disease, NASH nonalcoholic steatohepatitis, NAFLD non-alcoholic fatty liver disease, HD hemodialysis, MetS metabolic syndrome, RA randomized, DB double-blinded, M male, F female, Int intervention, Con control.
aValues are mean ± SD or range (for age).
bChanges in cytokine concentrations are presented by common units for CRP (mg/L), IL-6 (pg/mL) and TNF-α (pg/mL).
cAdjustment or matching: age (1), sex (2), BMI (3), baseline values of cytokines (4), DM (5), duration of DM (6), use of medication or supplements (7), dietary intake of vitamin E (8), changes in other variables (9), disease duration (10).
Findings from the systematic review
Among 26 studies assessing the serum concentrations of CRP, 6 studies revealed a significant reducing effect of vitamin E supplementation on serum CRP concentrations11,34,37,40,42,43, whereas others found no significant effect. Four trials showed a significant reduction26,28,30,37 and one study indicated a significant increase in serum IL-6 concentrations33 following vitamin E supplementation, while others revealed no significant change. Of 12 RCTs that examined the effects of vitamin E supplementation on serum TNF-α concentrations, two studies reported a beneficial effect36,39, whereas two trials showed an increasing effect of vitamin E supplementation on serum concentrations of TNF-α21,30. The remaining studies on TNF-α reported no significant effect.
Findings from the meta-analysis
Overall, 33 RCTs in the systematic review were included in the meta-analysis. These trials had a total sample size of 2102 individuals with the age of 20 years and over.
The effect of vitamin E on serum CRP concentrations
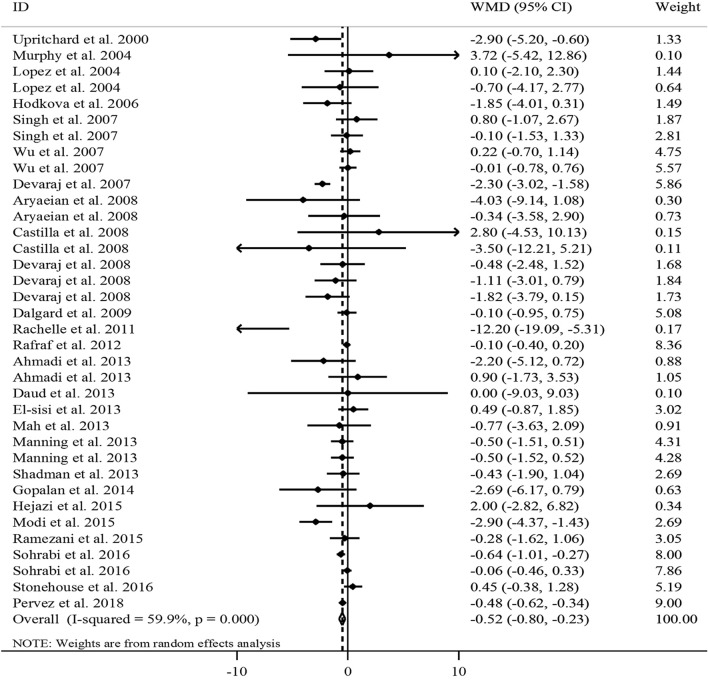
In total, 26 RCTs with a total sample size of 1743 subjects were included in the analysis11,13–20,22,25–31,33–35,37,38,40–43. Combining 36 effect sizes from these studies indicated that vitamin E supplementation, compared with controls, resulted in a significant reduction in serum CRP concentrations [weighted mean difference (WMD) − 0.52, 95% CI − 0.80, − 0.23 mg/L, P < 0.001] (Fig. 2).
Figure 2.
Forest plot for the effect of vitamin E supplementation on serum CRP concentrations, expressed as mean differences between intervention and control groups. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from the random-effects analysis. CRP C-reactive protein, WMD weighted mean difference, CI confidence interval.
However, there was evidence of a moderate between-study heterogeneity (I2 = 59.9, P < 0.001).
To detect potential sources of heterogeneity, subgroup analyses were performed (Table 2). We found that the between-study heterogeneity was explained by the type and dosage of tocopherol, participants’ health condition, and baseline serum concentrations of CRP. From these analyses, we found a significant reducing effect of vitamin E supplementation on serum CRP concentrations in RCTs with intervention duration of ≥ 8 weeks, trials that administered α-tocopherol or a mixed type of tocopherols, and RCTs that prescribed ≥ 500 mg/day vitamin E. Besides, a significant reduction was observed in studies that were conducted on unhealthy participants including hemodialysis patients or subjects with CVDs and disorders related to insulin resistance, trials on individuals with normal or elevated levels of CRP, and studies that provided effect size whether adjusted or did not adjust for baseline serum concentrations of CRP. In the sensitivity analysis, exclusion of any single study did not affect the overall estimate for the effect of vitamin E supplementation on serum CRP concentrations (range of summary estimates: − 0.84, − 0.12). In addition, based on the Begg’s test, no evidence of substantial publication bias was seen (P = 0.20).
Table 2.
Subgroup analyses for the effects of vitamin E supplementation on inflammatory biomarkers in adults aged ≥ 20 years.
| Effect size, n | WMD (95% CI)a | P-withinb | I2 (%)c | P-heterogeneityd | |
|---|---|---|---|---|---|
| Vitamin E supplementation on serum CRP concentrations | |||||
| Overall | 36 | − 0.43 (− 0.54, − 0.33) | < 0.001 | 59.9 | < 0.001 |
| Intervention duration (week) | |||||
| < 8 | 16 | − 0.35 (− 0.72, 0.01) | 0.059 | 47.6 | 0.018 |
| ≥ 8 | 20 | − 0.44 (− 0.55, − 0.33) | < 0.001 | 67.5 | < 0.001 |
| Type of vitamin E | |||||
| α-Tocopherol | 26 | − 0.42 (− 0.59, − 0.25) | < 0.001 | 66.9 | < 0.001 |
| γ-Tocopherol | 4 | − 0.19 (− 1.11, 0.73) | 0.685 | 0.0 | 0.542 |
| Mixed-tocopherols | 6 | − 0.45 (− 0.59, − 0.31) | < 0.001 | 46.7 | 0.095 |
| Dosage of vitamin E (mg/day) | |||||
| < 500 | 19 | − 0.18 (− 0.42, 0.06) | 0.135 | 29.0 | 0.115 |
| ≥ 500 | 17 | − 0.50 (− 0.61, − 0.38) | < 0.001 | 71.8 | < 0.001 |
| Health condition | |||||
| Healthy | 5 | 0.04 (− 0.88, 0.96) | 0.933 | 0.0 | 0.885 |
| Unhealthy | 31 | − 0.44 (− 0.55, − 0.33) | < 0.001 | 64.8 | < 0.001 |
| Insulin resistance-related disorders | 13 | − 0.39 (− 0.51, − 0.26) | < 0.001 | 39.4 | 0.071 |
| CVDs | 5 | − 1.25 (− 1.75, − 0.75) | < 0.001 | 79.0 | 0.001 |
| Hemodialysis | 9 | − 0.41 (− 0.67, − 0.15) | 0.002 | 62.1 | 0.007 |
| Rheumatoid arthritis | 2 | − 1.40 (− 4.13, 1.34) | 0.317 | 30.0 | 0.232 |
| Other disease | 2 | − 1.06 (− 2.06, − 0.07) | 0.037 | 90.9 | 0.001 |
| Baseline concentrations of CRP (mg/L) | |||||
| Normal (< 3 mg/L) | 18 | − 0.31 (− 0.51, − 0.10) | 0.004 | 69.0 | < 0.001 |
| Elevated (≥ 3 mg/L) | 18 | − 0.48 (− 0.60, − 0.35) | < 0.001 | 44.4 | 0.022 |
| Adjustment for baseline values | |||||
| Adjusted | 14 | − 0.39 (− 0.50, − 0.27) | < 0.001 | 60.5 | 0.002 |
| Non-adjusted | 22 | − 0.76 (− 1.07, − 0.45) | < 0.001 | 57.6 | < 0.001 |
| Vitamin E supplementation on serum IL-6 concentrations | |||||
| Overall | 21 | − 0.14 (− 0.29, 0.01) | 0.06 | 74.3 | < 0.001 |
| Intervention duration (week) | |||||
| < 8 | 11 | − 0.12 (− 0.32, 0.07) | 0.220 | 80.6 | < 0.001 |
| ≥ 8 | 10 | − 0.17 (− 0.40, 0.06) | 0.145 | 65.7 | 0.002 |
| Type of vitamin E | |||||
| α-Tocopherol | 15 | − 0.21 (− 0.39, − 0.03) | 0.023 | 79.2 | < 0.001 |
| γ-Tocopherol | 2 | 0.08 (− 0.34, 0.50) | 0.717 | 81.6 | 0.020 |
| Mixed-tocopherols | 4 | − 0.05 (− 0.40, 0.29) | 0.760 | 10.2 | 0.342 |
| Dosage of vitamin E (mg/day) | |||||
| < 500 | 12 | − 0.14 (− 0.34, 0.06) | 0.177 | 82.0 | < 0.001 |
| ≥ 500 | 9 | − 0.15 (− 0.37, 0.07) | 0.190 | 52.5 | 0.032 |
| Health condition | |||||
| Healthy | 3 | 0.37 (− 0.05, 0.79) | 0.086 | 0.0 | 0.387 |
| Unhealthy | 18 | − 0.22 (− 0.38, − 0.06) | 0.008 | 75.5 | < 0.001 |
| Insulin resistance-related disorders | 11 | − 0.22 (− 0.40, − 0.05) | 0.010 | 67.0 | 0.001 |
| CVDs | 1 | 0.20 (− 0.30, 0.70) | 0.435 | – | – |
| Hemodialysis | 5 | − 0.04 (− 1.45, 1.38) | 0.960 | 71.8 | 0.007 |
| Other disease | 1 | − 3.96 (− 5.51, − 2.41) | < 0.001 | – | – |
| Baseline concentrations of IL-6 (pg/mL) | |||||
| Normal (< 4.4 pg/mL) | 15 | − 0.13 (− 0.28, 0.03) | 0.109 | 66.7 | < 0.001 |
| Elevated (≥ 4.4 pg/mL) | 6 | − 0.50 (− 1.18, 0.18) | 0.150 | 85.6 | < 0.001 |
| Adjustment for baseline values | |||||
| Adjusted | 4 | − 0.27 (− 0.56, 0.02) | 0.073 | 53.7 | 0.090 |
| Non-adjusted | 17 | − 0.10 (− 0.27, 0.08) | 0.264 | 77.3 | < 0.001 |
| Vitamin E supplementation on serum TNF-α concentrations | |||||
| Overall | 19 | − 0.03 (− 0.09, 0.02) | 0.25 | 78.9 | < 0.001 |
| Intervention duration (week) | |||||
| < 8 | 8 | − 0.07 (− 0.13, − 0.01) | 0.026 | 32.0 | 0.173 |
| ≥ 8 | 11 | 0.12 (− 0.00, 0.25) | 0.052 | 85.2 | < 0.001 |
| Type of vitamin E | |||||
| α-Tocopherol | 14 | 0.02 (− 0.06, 0.09) | 0.655 | 82.7 | < 0.001 |
| γ-Tocopherol | 2 | − 0.15 (− 0.29, − 0.01) | 0.040 | 0.0 | 0.358 |
| Mixed-tocopherols | 3 | − 0.06 (− 0.15, 0.04) | 0.238 | 57.8 | 0.093 |
| Dosage of vitamin E (mg/day) | |||||
| < 500 | 11 | 0.12 (− 0.00, 0.25) | 0.052 | 72.4 | < 0.001 |
| ≥ 500 | 8 | − 0.07 (− 0.13, − 0.01) | 0.027 | 83.2 | < 0.001 |
| Health condition | |||||
| Healthy | 3 | − 0.22 (− 0.46, 0.02) | 0.072 | 39.9 | 0.189 |
| Unhealthy | 16 | − 0.02 (− 0.08, 0.03) | 0.449 | 81.1 | < 0.001 |
| Insulin resistance-related disorders | 13 | − 0.02 (− 0.08, 0.03) | 0.439 | 84.8 | < 0.001 |
| CVD | 1 | 0.30 (− 0.99, 1.59) | 0.650 | – | – |
| Rheumatoid arthritis | 2 | − 0.04 (− 1.29, 1.21) | 0.944 | 0.0 | 0.994 |
| Baseline concentrations of TNF-α (pg/mL) | |||||
| Normal (< 2.3 pg/mL) | 8 | − 0.04 (− 0.10, 0.01) | 0.125 | 66.1 | 0.004 |
| Elevated (≥ 2.3 pg/mL) | 11 | 0.46 (0.10, 0.83) | 0.013 | 82.5 | < 0.001 |
| Adjustment for baseline values | |||||
| Adjusted | 5 | − 0.01 (− 0.09, 0.07) | 0.820 | 92.0 | < 0.001 |
| Non-adjusted | 14 | − 0.05 (− 0.12, 0.02) | 0.177 | 62.5 | 0.001 |
WMD weighted mean difference, CI confidence interval, CRP C-reactive protein, IL-6 interleukin-6, TNF-α tumor necrosis factor-α.
aObtained from the fixed-effects model.
bRefers to the mean (95% CI).
cInconsistency, percentage of variation across studies due to heterogeneity.
dObtained from the Q-test.
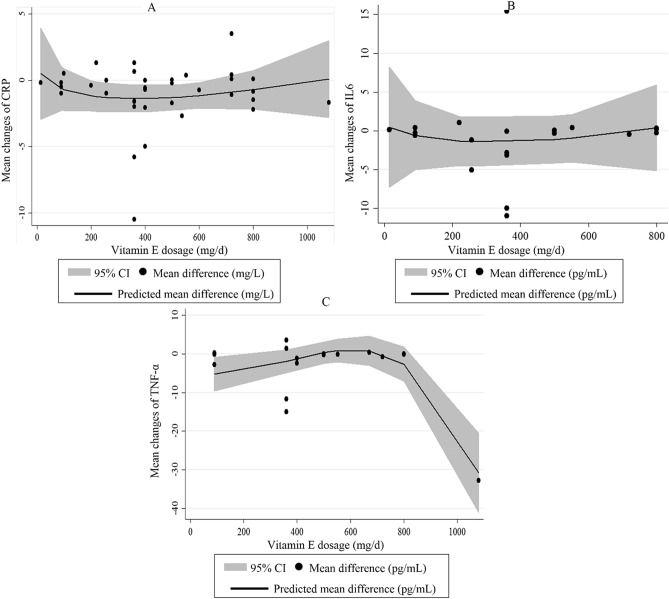
The 26 eligible RCTs on serum CRP concentrations were included in the non-linear dose–response meta-analysis. Although not significant, there was a nearly U-shaped curve of the effect of vitamin E dosage on circulating CRP in which the reducing effect of vitamin E gradually increased, and then, the effect gradually decreased and reached to zero value at the dosages of ≥ 1000 mg/day (Pnon-linearity = 0.39). It seems that the highest reducing effect occurs at the dosages of 300–600 mg/day vitamin E (Fig. 3A). After excluding studies on γ-tocopherol and retaining only those studies that administered α-tocopherol, no change was seen on the non-linear association between vitamin E dosage and changes in CRP levels (Pnon-linearity = 0.32) (Supplemental Figure 1A).
Figure 3.
Non-linear dose–response effects of vitamin E dosage (mg/day) on serum concentrations of (A) CRP, (B) IL-6 and (C) TNF-α. The 95% CI is demonstrated in the shaded regions. CRP C-reactive protein, IL-6 interleukin-6, TNF-α tumor necrosis factor-α.
The effect of vitamin E on serum IL-6 concentrations
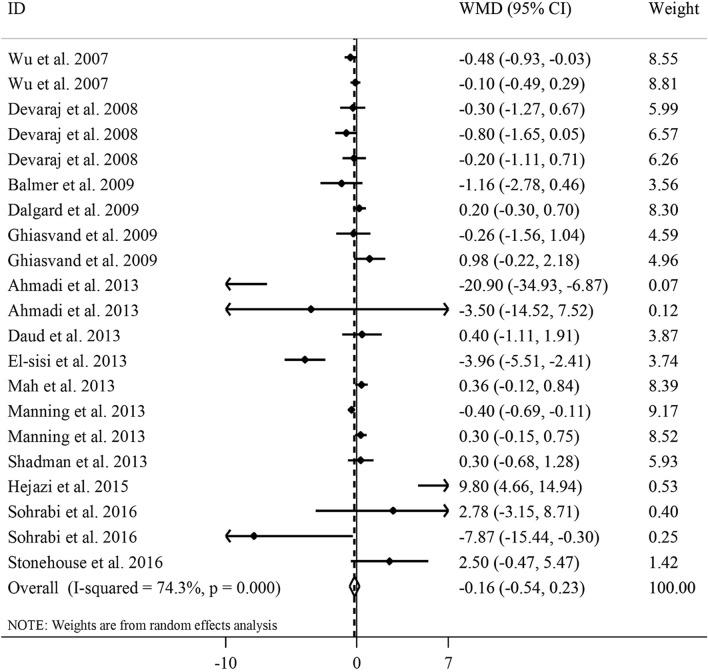
In total, 21 effect sizes from 14 RCTs17,20–23,26–31,33,37,38, including 902 people, were included in the meta-analysis. Combining the effect sizes, we found no significant effect of vitamin E supplementation on serum IL-6 concentrations (WMD − 0.16, 95% CI − 0.54, 0.23 pg/mL, P = 0.42) (Fig. 4).
Figure 4.
Forest plot for the effect of vitamin E supplementation on serum IL-6 concentrations, expressed as mean differences between intervention and control groups. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from the random-effects analysis. IL-6 interleukin-6, WMD weighted mean difference, CI confidence interval.
Heterogeneity between studies was significant in this regard (I2 = 74.3, P < 0.001). In the subgroup analyses, the type of vitamin E prescribed and participants’ health condition could explain the between-study heterogeneity. Also, vitamin E supplementation resulted in a significant reduction in serum concentrations of IL-6 in RCTs that used α-tocopherol for their intervention and those trials that were performed on unhealthy participants including those with disorders related to insulin resistance. Based on findings from the sensitivity analysis, no single study influenced the overall effect of vitamin E supplementation on serum IL-6 concentrations (range of summary estimates − 0.61, 0.30). Moreover, Begg’s test rejected our hypothesis about the presence of substantial publication bias (P = 0.62). In the non-linear dose–response analysis, we failed to find a significant effect of vitamin E dosage on serum IL-6 concentrations (Pnon-linearity = 0.57) (Fig. 3B). Such finding was also observed after excluding studies on γ-tocopherol and retaining only those that did supplementation with α-tocopherol (Pnon-linearity = 0.60) (Supplemental Figure 1B).
The effect of vitamin E on serum TNF-α concentrations
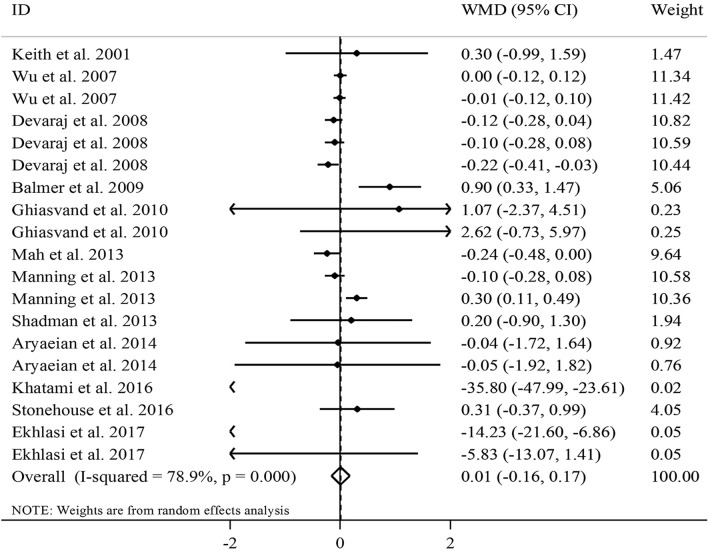
Combining 19 effect sizes from 12 RCTs12,17,20,21,24,29–32,36,38,39, including a total sample size of 792 participants, we found no significant effect of vitamin E supplementation on serum TNF-α concentrations (WMD − 0.01, 95% CI − 0.16, 0.17 pg/mL, P = 0.93) (Fig. 5).
Figure 5.
Forest plot for the effect of vitamin E supplementation on serum TNF-α concentrations, expressed as mean differences between intervention and control groups. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from the random-effects analysis. TNF-α tumor necrosis factor-α, WMD weighted mean difference, CI confidence interval.
Significant between-study heterogeneity was seen (I2 = 78.9, P < 0.001). Subgroup analyses according to the duration of intervention, type of vitamin E, and participants’ health condition explained the between-study heterogeneity. From these analyses, we observed that vitamin E supplementation significantly reduced serum TNF-α concentrations at the dosage of ≥ 500 mg/day, in RCTs with a duration of < 8 weeks, and those studies that administered γ-tocopherol rather than other types of tocopherol. Surprisingly, vitamin E supplementation resulted in a significant increase in serum TNF-α concentrations in individuals with elevated levels of TNF-α. Based on the sensitivity analysis, we found that the overall effect size of vitamin E supplementation on serum TNF-α concentrations did not depend on a particular study (range of summary estimates − 0.20, 0.21). In addition, no evidence of substantial publication bias was found based on the Begg test (P = 0.34). Based on the dose–response analysis, we observed a significant non-linear effect of vitamin E dosage on serum concentrations of TNF-α; such that serum TNF-α concentrations were reduced significantly at the dosage of ≥ 700 mg/day vitamin E (Pnon-linearity = 0.001) (Fig. 3C). When we did dose–response analysis on studies that conducted supplementation with α-tocopherol, the significant effect of vitamin E remained significant (Pnon-linearity = 0.004) (Supplemental Figure 1C).
Discussion
In the current meta-analysis, we found that vitamin E supplementation can exert a significant reducing effect, around 0.52 mg/L, on serum levels of CRP in adults. Although this meta-analysis failed to find a significant effect of vitamin E on serum levels of IL-6 overall, there was a significant reducing effect in studies that used α-tocopherol and those that conducted on individuals with disorders associated with insulin resistance. The overall effect size for the effect of vitamin E on serum levels of TNF-α was not significant; however, we observed a significant beneficial effect in studies that used vitamin E at the dosage of ≥ 500 mg/day, those with < 8 weeks’ duration of intervention, and studies that administered vitamin E in the form of γ-tocopherol.
Vitamin E is the most widely studied antioxidant in humans. In vitro studies have revealed a protective role for α-tocopherol in the development of atherosclerotic plaque as on cultured endothelial cells62. Indeed, vitamin E inhibits the expression of adhesion molecules stimulated by oxidized low-density lipoprotein (LDL)63. Likewise, in vivo animal studies have shown that α-tocopherol can significantly reduce circulating CRP and enhance the scavenging activity of reactive oxygen species (ROSs)64. In contrast, results from clinical trials on major cardiovascular properties of vitamin E are not conclusive65. In the current meta-analysis, we observed an overall significant reducing effect of vitamin E supplementation in the form of α-tocopherol or mixed isoforms on serum levels of CRP. Similarly, in an earlier meta-analysis of clinical trials, a significant reduction in circulating CRP was observed in patients with hemodialysis following supplementation with vitamin E coated dialyzer45. Also, a meta-analysis in 2015 revealed a significant beneficial effect of vitamin E supplements, in the isoforms of α and γ-tocopherols, on serum levels of CRP44. That meta-analysis44; however, had some limitations which might have distorted the findings. For example, a considerable number of publications19,20,25,27,30,31,42 were missed in that study despite meeting the inclusion criteria. Also, the authors excluded studies that used the isoforms of vitamin E other than α- and γ-tocopherols. Despite the conclusions on inflammation, they had only focused on serum levels of CRP.
The reduction of CRP concentrations for an average of 0.52 mg/L following vitamin E in our meta-analysis is an important finding in the clinical setting when compared to lifestyle intervention and bariatric surgery that reduce CRP levels by 0.13 and 0.16 mg/L, respectively66. Almost all participants in the studies included in our analysis had a low-grade inflammation (CRP < 10 mg/L); and therefore, 0.5 mg/L reduction in serum CRP is clinically significant in these ranges. Earlier reports found a direct association between CRP concentrations and the risk of CVDs in different populations around the world. In a population-based study, two-fold higher mortality from CVDs was reported in serum CRP of > 3 mg/L, while the optimal level confirmed by the AHA and the CDC is < 1 mg/L67. In the Women's Health Study, by increasing quintiles of CRP (≤ 0.49, > 0.49 to 1.08, > 1.08 to 2.09, > 2.09 to 4.19, and > 4.19 mg/L), the corresponding relative risks of a first cardiovascular event were 1.0, 1.4, 1.6, 2.0, and 2.3 (P for trend < 0.001), in the adjusted model68. Most interventions with reducing CVD risk have been linked to lower CRP levels69; however, definitive evidence that lowering CRP levels will necessarily result in reduced cardiovascular events is lacking. Besides, despite well-documented beneficial effects of vitamin E on inflammation, as well as the inverse relationship between vitamin E consumption and the risk of chronic diseases, many large intervention studies have failed to support consistent benefits of vitamin E for the prevention of chronic diseases such as cancer and CVDs70,71.
The measurement of CRP is a powerful predictor for cardiovascular mortality72. However, given the high fluctuations upon inflammation or even health status, CRP measurement alone is not enough to represent the immunomodulatory changes73,74. Therefore, there is a need for evaluating other biomarkers of subclinical inflammation. In our analysis, unlike serum CRP concentrations, the overall effect of vitamin E on circulating IL-6 and TNF-α was not significant. Similarly, in a population-based cohort study, the intake of α-tocopherol was negatively associated with serum levels of CRP but not IL-6 after adjustment for confounding variables (65). However, this finding is not consistent with that of the previous meta-analysis on hemodialysis patients that suggested a significant reduction in circulating IL-6 following supplementation with vitamin E coated dialyzer45.
When we did subgroup analyses, we found a significant reducing effect of vitamin E on serum levels of IL-6 and CRP in studies performed on subjects with insulin resistance-related disorders including type 2 diabetes, metabolic syndrome, and non-alcoholic fatty liver disease. These metabolic conditions are associated with several pathophysiological problems resulting in elevated baseline levels of inflammatory biomarkers75. Since elevated levels of inflammatory biomarkers are more sensitive to the supplementation of antioxidants76, it may explain our findings in this subgroup.
We also found a non-significant U-shape dose–response effect of vitamin E (or α-tocopherol) dosage on the reduction of serum CRP in which the effect had a gradually increasing trend from 0 to 400 mg/day and then it had a decreasing trend from 400 to 1000 mg/day; such that at the dosages of ≥ 1000 mg/day, vitamin E had no significant effect on serum CRP concentrations. It seems that the highest reducing effect occurs at the dosages of 300–600 mg/day. This is consistent with the recommended dietary allowance (RDA) and tolerable upper intake level (UL) of vitamin E for adults that was suggested to be 15 mg (22.4 IU) and 1000 mg α-tocopherol (1500 IU), respectively77. Antioxidants at high doses, not only act as prooxidants but also disrupt redox balance through interaction with physiological concentrations of ROS required for optimal cellular functioning resulting in cellular dysfunction78. In the subgroup meta-analysis, we observed a significant reduction in serum levels of CRP and TNF-α in studies that used vitamin E at the dosage of ≥ 500 mg/day. Likewise, our dose–response meta-analysis revealed a significant reduction in serum TNF-α at the dosage of ≥ 700 mg/day vitamin E. This finding; however, contradicts the earlier meta-analysis in which the dosages lower than 400 IU/day significantly reduced circulating CRP44. Findings from a dose–response meta-analysis revealed that a minimum dosage of 400 IU/day (266 mg/day) α-tocopherol is required for the reduction of LDL79. Therefore, smaller dosages of vitamin E may not sufficient to exert anti-inflammatory effect; however, higher dosages of vitamin E should be used cautiously due to the probability of increased risk of all-cause mortality80.
We also found a significant decline in CRP concentrations in studies with a long duration of the intervention (≥ 8 weeks). This would seem to oppose our findings of TNF-α, in which the beneficial effect was seen for studies with a short duration of the intervention (< 8 weeks). The contradictory findings of CRP and TNF-α might be explained by the fact that most studies that assessed TNF-α levels did not control the analyses for baseline values of this biomarker12,20,21,24,29–32,38, while almost all the included RCTs on circulating CRP performed adjustment for baseline concentrations of this biomarker. Furthermore, the lack of a significant effect on TNF-α in studies with longer duration of follow-up might be due to selecting patients with well-controlled disease conditions resulting in a normal level of TNF-α.
Considering different isoforms of vitamin E, we found that only α-tocopherol significantly reduced serum levels of CRP and IL-6, whereas the effect of γ-tocopherol was not significant.
Both isoforms are the most prevalent forms of vitamin E in diets, supplements, and tissues; however, the levels of α-tocopherol are approximately tenfold higher than γ-tocopherol in tissues due to the preferential transfer of α-tocopherol to lipid particles81. Therefore, α-tocopherol is more likely to potentiate ROS scavenging than γ-tocopherol. Besides, in our analysis, combined administration of both α- and γ-tocopherol reduced serum levels of CRP. Unlike earlier reviews with reports of opposing regulatory function of different isoforms of vitamin E for inflammation82, recent preclinical and clinical studies have shown that different forms of vitamin E have unique anti-inflammatory properties83. Moreover, some clinical trials revealed that supplementation with the α-tocopherol isoform of vitamin E can reduce plasma γ-tocopherol84. This may happen as a result of the function of hepatic α-tocopherol transfer protein (α-TTP)85. α-TTP, together with ATP-binding cassette transporter A1 (ABCA1), potentially incorporates α-tocopherol into the plasma as well as increasing γ-tocopherol metabolism86,87. However, recent studies have rejected the decreased levels of γ-tocopherol induced by α-tocopherol supplementation when they were supplemented in combination20. Thus, there may be benefits in avoidance of potential adverse effects following supplementation with both isoforms.
Considering the above-mentioned points, it seems that the different responses of CRP, IL6, and TNF-α to vitamin E depend on the isoforms of this vitamin, dosage, duration of consumption, and health condition of consumers. For instance, CRP and IL6 levels were reduced by supplementation with α-tocopherol, particularly in subjects with an unhealthy condition such as those with insulin resistance-related disorders, while TNF-α levels were reduced by γ-tocopherol supplementation, or both CRP and TNF-α responded to high dosages of vitamin E (≥ 500 mg/day). Further studies are needed to examine the effect of vitamin E supplementation on other inflammatory biomarkers in adults.
Mechanistic evidence for the anti-inflammatory properties of α-tocopherol is not completely discovered. This tocopherol reduces the production of ROS from monocytes through activating the activation protein 1 (AP-1), which can dephosphorylate protein kinase C (PKC) and inhibit the proliferation of smooth muscle cells88. ROSs are important free radical species that cause endothelial dysfunction by altering cell membrane integrity and subsequent cell death89. Also, ROSs play an important role in increasing the concentrations of inflammatory cytokines90. Moreover, in different cell types, vitamin E may induce an anti-inflammatory effect through inhibiting the COX-2 and 5-LUX mediated eicosanoids and suppressing the NF-κB and JAK-STAT6 or JAK-STAT3 signaling pathways83.
In the current meta-analysis, we gathered all available evidence about the effect of all types of supplemental vitamin E on serum concentrations of inflammatory biomarkers. However, some potential limitations should be addressed when interpreting our findings. There was considerable heterogeneity between the included studies. In the subgroup analysis, type and dosage of tocopherol, participants’ health condition, and baseline serum concentrations of inflammatory cytokines could explain the variation between studies. Moreover, we could not determine a safety margin of supplemental vitamin E due to the lack of serious adverse events in the selected studies. High-dose vitamin E has been debated for the safety such that the dosages of > 400 IU/day have been reported to be associated with increased all-cause mortality by increasing the risk of prolonged bleeding time80. However, other meta-analyses did not report this increase in total mortality91,92. Moreover, some included studies were conducted on patients with different metabolic diseases; and therefore, caution should be taken when extrapolating our findings to the general population. Last but not least, since most studies did not report serum levels of α-tocopherol, it is not clear whether the effect is dependent on the status of serum vitamin E.
Altogether, the results of this meta-analysis are in favor of a CRP-reducing effect of supplementation with α-tocopherol, especially at the dosage of ≥ 500 mg/day, alone or in combination with γ-tocopherol. Higher dosages of vitamin E (> 1000 mg/day) are not effective in the reduction of subclinical inflammation; and therefore, are not recommended. Furthermore, a significant reduction in serum levels of IL-6 was also seen in studies that used α-tocopherol. Despite the confirmed health effects of vitamin E, literature supporting actual clinical benefits on hard endpoint including morbidity or mortality caused by just reduction in serum concentration of CRP is disappointing. Thus, the accumulating evidence on the anti-inflammatory properties of tocotrienol warrants future research on the clinical setting and larger population. Moreover, since there may be benefits in avoidance of potential adverse effects following supplementation with both isoforms, future studies are needed to further examine this issue.
Supplementary information
Acknowledgements
The study is supported financially by Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran. The thesis proposal was approved by Medical Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1398.437).
Abbreviations
- CRP
C-reactive protein
- IL-6
Interleukin 6
- TNF-α
Tumor necrosis factor-α
- DM
Diabetes mellitus
- CVD
Cardiovascular disease
- NASH
Nonalcoholic steatohepatitis
- NAFLD
Non-alcoholic fatty liver disease
- HD
Hemodialysis
- MetS
Metabolic syndrome
- RCT
Randomized clinical trial
Author contributions
O.A., M.S., A.S. and M.S.A. contributed to the systematic search and data extraction. M.A., V.M., B.N. and H.M.-K. contributed to statistical analyses and data interpretation. O.S. and M.S. contributed to manuscript drafting and data interpretation. All authors approved the final manuscript for submission.
Funding
The research protocol was approved & supported by Student Research Committee; Tabriz University of Medical Sciences, Tabriz, Iran (grant number: 64478).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Alizadeh, Email: mdalizadeh@tbzmed.ac.ir.
Omid Sadeghi, Email: omidsadeghi69@yahoo.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-73741-6.
References
- 1.Zingg JM. Vitamin E: a role in signal transduction. Annu. Rev. Nutr. 2015;35:135–173. doi: 10.1146/annurev-nutr-071714-034347. [DOI] [PubMed] [Google Scholar]
- 2.Cheng P, et al. Vitamin E intake and risk of stroke: a meta-analysis. Br. J. Nutr. 2018;120:1181–1188. doi: 10.1017/s0007114518002647. [DOI] [PubMed] [Google Scholar]
- 3.Amanullah I, et al. Effect of vitamin E in non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomised controlled trials. Postgrad. Med. J. 2019;95:601–611. doi: 10.1136/postgradmedj-2018-136364. [DOI] [PubMed] [Google Scholar]
- 4.Kushner I, Samols D, Magrey M. A unifying biologic explanation for "high-sensitivity" C-reactive protein and "low-grade" inflammation. Arthritis Care Res. 2010;62:442–446. doi: 10.1002/acr.20052. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.Asbaghi O, et al. The effect of vitamin d-calcium co-supplementation on inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Cytokine. 2020;129:155050. doi: 10.1016/j.cyto.2020.155050. [DOI] [PubMed] [Google Scholar]
- 7.Kuhad A, Chopra K. Attenuation of diabetic nephropathy by tocotrienol: involvement of NFkB signaling pathway. Life Sci. 2009;84:296–301. doi: 10.1016/j.lfs.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Mishra P, et al. Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFkB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 2019;9:7408. doi: 10.1038/s41598-019-43320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arablou T, Aryaeian N, Djalali M, Shahram F, Rasouli L. Association between dietary intake of some antioxidant micronutrients with some inflammatory and antioxidant markers in active rheumatoid arthritis patients. Int. J. Vitam. Nutr. Res. 2019 doi: 10.1024/0300-9831/a000255. [DOI] [PubMed] [Google Scholar]
- 10.Kooshki A, Samadipour E, Akbarzadeh R. The association between serum C-reactive protein and macronutrients and antioxidants intake in hemodialysis patients. J. Med. Life. 2015;8:43–46. [PMC free article] [PubMed] [Google Scholar]
- 11.Upritchard JE, Sutherland WH, Mann JI. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care. 2000;23:733–738. doi: 10.2337/diacare.23.6.733. [DOI] [PubMed] [Google Scholar]
- 12.Keith ME, et al. A controlled clinical trial of vitamin E supplementation in patients with congestive heart failure. Am. J. Clin. Nutr. 2001;73:219–224. doi: 10.1093/ajcn/73.2.219. [DOI] [PubMed] [Google Scholar]
- 13.Murphy RT, et al. Vitamin E modulation of C-reactive protein in smokers with acute coronary syndromes. Free Radic. Biol. Med. 2004;36:959–965. doi: 10.1016/j.freeradbiomed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Vega-Lopez S, et al. Supplementation with omega3 polyunsaturated fatty acids and all-rac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism. 2004;53:236–240. doi: 10.1016/j.metabol.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Hodkova M, et al. Influence of oral vitamin E therapy on micro-inflammation and cardiovascular disease markers in chronic hemodialysis patients. Ren. Fail. 2006;28:395–399. doi: 10.1080/08860220600683698. [DOI] [PubMed] [Google Scholar]
- 16.Singh I, Turner AH, Sinclair AJ, Li D, Hawley JA. Effects of gamma-tocopherol supplementation on thrombotic risk factors. Asia Pac. J. Clin. Nutr. 2007;16:422–428. [PubMed] [Google Scholar]
- 17.Wu JH, et al. Effects of alpha-tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetes. Clin. Chem. 2007;53:511–519. doi: 10.1373/clinchem.2006.076992. [DOI] [PubMed] [Google Scholar]
- 18.Aryaeian N, et al. Effect of conjugated linoleic acid, vitamin E and their combination on lipid profiles and blood pressure of Iranian adults with active rheumatoid arthritis. Vasc. Health Risk Manag. 2008;4:1423. doi: 10.2147/VHRM.S3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castilla P, et al. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am. J. Clin. Nutr. 2008;87:1053–1061. doi: 10.1093/ajcn/87.4.1053. [DOI] [PubMed] [Google Scholar]
- 20.Devaraj S, Leonard S, Traber MG, Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008;44:1203–1208. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balmer ML, Siegrist K, Zimmermann A, Dufour JF. Effects of ursodeoxycholic acid in combination with vitamin E on adipokines and apoptosis in patients with nonalcoholic steatohepatitis. Liver Int. 2009;29:1184–1188. doi: 10.1111/j.1478-3231.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 22.Dalgard C, et al. Supplementation with orange and blackcurrant juice, but not vitamin E, improves inflammatory markers in patients with peripheral arterial disease. Br. J. Nutr. 2009;101:263–269. doi: 10.1017/s0007114508995660. [DOI] [PubMed] [Google Scholar]
- 23.Ghiasvand R, Djalali M, Djazayery SA, Keshavarz SA, Hosseini M. Effects of eicosapentaenoic acid and vitamin e on the plasma levels of antioxidant vitamins and inflammatory markers, and on erythrocyte antioxidant enzyme activities, in male basketball players. Acta Med. Iran. 2009;47:269–274. [Google Scholar]
- 24.Ghiasvand R, et al. Effect of eicosapentaenoic acid (EPA) and vitamin e on the blood levels of inflammatory markers, antioxidant enzymes, and lipid peroxidation in Iranian basketball players. Iran. J. Public Health. 2010;39:15–21. [PMC free article] [PubMed] [Google Scholar]
- 25.Rafraf M, Bazyun B, Sarabchian MA, Safaeiyan A, Ghaemmaghami Hezaveh SJ. Impact of vitamin E supplementation on blood pressure and Hs-CRP in type 2 diabetic patients. Health Promot. Perspect. 2012;2:72–79. doi: 10.5681/hpp.2012.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadi A, Mazooji N, Roozbeh J, Mazloom Z, Hasanzade J. Effect of alpha-lipoic acid and vitamin E supplementation on oxidative stress, inflammation, and malnutrition in hemodialysis patients. Iran. J. Kidney Dis. 2013;7:461–467. [PubMed] [Google Scholar]
- 27.Daud ZA, et al. Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients. Vasc. Health Risk Manag. 2013;9:747–761. doi: 10.2147/vhrm.s51710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Sisi AA, Hegazy SK, Salem KA, AbdElkawy KS. Atorvastatin improves erectile dysfunction in patients initially irresponsive to Sildenafil by the activation of endothelial nitric oxide synthase. Int. J. Impot. Res. 2013;25:143–148. doi: 10.1038/ijir.2012.46. [DOI] [PubMed] [Google Scholar]
- 29.Mah E, et al. gamma-Tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic. Biol. Med. 2013;65:1291–1299. doi: 10.1016/j.freeradbiomed.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Manning PJ, et al. The effect of lipoic acid and vitamin E therapies in individuals with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2013;23:543–549. doi: 10.1016/j.numecd.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Shadman Z, Taleban FA, Saadat N, Hedayati M. Effect of conjugated linoleic acid and vitamin E on glycemic control, body composition, and inflammatory markers in overweight type2 diabetics. J. Diabetes Metab. Disord. 2013;12:42. doi: 10.1186/2251-6581-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aryaeian N, Djalali M, Shahram F, Djazayery A, Eshragian MR. Effect of conjugated linoleic acid, vitamin e, alone or combined on immunity and inflammatory parameters in adults with active rheumatoid arthritis: a randomized controlled trial. Int. J. Prev. Med. 2014;5:1567–1577. [PMC free article] [PubMed] [Google Scholar]
- 33.Hejazi N, Dabbaghmanesh MH, Mazloom Z, Dashtabi A. Effects of vitamin E on fasting and postprandial oxidative stress, inflammatory markers, glucose status, insulin resistance, blood pressure and pulse rate in type-2 diabetic patients: a randomized clinical trial. Galen Med. J. 2015;4:67–74. [Google Scholar]
- 34.Modi J, et al. Role of vitamin C and E supplementation in reduction of serum level of renal injury marker following shock wave lithotripsy: prospective single centre experience. Urol. Ann. 2015;7:350–354. doi: 10.4103/0974-7796.156143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramezani A, et al. Omega-3 fatty acids/vitamin E behave synergistically on adiponectin receptor-1 and adiponectin receptor-2 gene expressions in peripheral blood mononuclear cell of coronary artery disease patients. Curr. Top. Nutraceut. Res. 2015;13:23. [Google Scholar]
- 36.Khatami PG, Soleimani A, Sharifi N, Aghadavod E, Asemi Z. The effects of high-dose vitamin E supplementation on biomarkers of kidney injury, inflammation, and oxidative stress in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. J. Clin. Lipidol. 2016;10:922–929. doi: 10.1016/j.jacl.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Sohrabi Z, Eftekhari MH, Eskandari MH, Rezaianzadeh A, Sagheb MM. Intradialytic oral protein supplementation and nutritional and inflammation outcomes in hemodialysis: a randomized controlled trial. Am. J. Kidney Dis. 2016;68:122–130. doi: 10.1053/j.ajkd.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 38.Stonehouse W, Brinkworth GD, Thompson CH, Abeywardena MY. Short term effects of palm-tocotrienol and palm-carotenes on vascular function and cardiovascular disease risk: a randomised controlled trial. Atherosclerosis. 2016;254:205–214. doi: 10.1016/j.atherosclerosis.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Ekhlasi G, et al. Effects of symbiotic and vitamin E supplementation on blood pressure, nitric oxide and inflammatory factors in non-alcoholic fatty liver disease. EXCLI J. 2017;16:278–290. doi: 10.17179/excli2016-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pervez MA, Khan DA, Ijaz A, Khan S. Effects of delta-tocotrienol supplementation on liver enzymes, inflammation, oxidative stress and hepatic steatosis in patients with nonalcoholic fatty liver disease. Turk. J. Gastroenterol. 2018;29:170–176. doi: 10.5152/tjg.2018.17297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gopalan Y, et al. Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke. 2014;45:1422–1428. doi: 10.1161/strokeaha.113.004449. [DOI] [PubMed] [Google Scholar]
- 42.Devaraj S, et al. Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am. J. Clin. Nutr. 2007;86:1392–1398. doi: 10.1093/ajcn/86.5.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coloma R, Jocson V. Effects of vitamin E on a biomarker of inflammation and precursors of atherogenesis in chronic hemodialysis patients. Phillippine J. Intern. Med. 2011;49:206–215. [Google Scholar]
- 44.Saboori S, Shab-Bidar S, Speakman JR, Yousefi Rad E, Djafarian K. Effect of vitamin E supplementation on serum C-reactive protein level: a meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2015;69:867–873. doi: 10.1038/ejcn.2014.296. [DOI] [PubMed] [Google Scholar]
- 45.Yang SK, et al. Effects of vitamin E-coated dialyzer on oxidative stress and inflammation status in hemodialysis patients: a systematic review and meta-analysis. Ren. Fail. 2014;36:722–731. doi: 10.3109/0886022x.2014.890858. [DOI] [PubMed] [Google Scholar]
- 46.Higgins JP, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadeghi O, et al. Whole-grain consumption does not affect obesity measures: an updated systematic review and meta-analysis of randomized clinical trials. Adv. Nutr. (Bethesda, MD) 2020;11:280–292. doi: 10.1093/advances/nmz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epstein R, F. C., Williamson E, et al. Psychosocial and Pharmacologic Interventions for Disruptive Behavior in Children and Adolescents [Internet]. Rockville: Agency for Healthcare Research and Quality (US); 2015 Oct. (Comparative Effectiveness Reviews, No. 154.) Appendix C, Risk of Bias Assessment Forms and Summaries. https://www.ncbi.nlm.nih.gov/books/NBK327223/. [PubMed]
- 49.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zahedi H, et al. Dietary inflammatory potential score and risk of breast cancer: systematic review and meta-analysis. Clin. Breast Cancer. 2018;18:e561–e570. doi: 10.1016/j.clbc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Sadeghi O, et al. Dietary intake of fish, n-3 polyunsaturated fatty acids and risk of hip fracture: a systematic review and meta-analysis on observational studies. Crit. Rev. Food Sci. Nutr. 2019;59:1320–1333. doi: 10.1080/10408398.2017.1405908. [DOI] [PubMed] [Google Scholar]
- 52.Nachvak SM, et al. Soy, soy isoflavones, and protein intake in relation to mortality from all causes, cancers, and cardiovascular diseases: a systematic review and dose-response meta-analysis of prospective cohort studies. J. Acad. Nutr. Diet. 2019;119:1483–1500.e1417. doi: 10.1016/j.jand.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Jeng KC, et al. Supplementation with vitamins C and E enhances cytokine production by peripheral blood mononuclear cells in healthy adults. Am. J. Clin. Nutr. 1996;64:960–965. doi: 10.1093/ajcn/64.6.960. [DOI] [PubMed] [Google Scholar]
- 54.Mahalingam D, Radhakrishnan AK, Amom Z, Ibrahim N, Nesaretnam K. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur. J. Clin. Nutr. 2011;65:63–69. doi: 10.1038/ejcn.2010.184. [DOI] [PubMed] [Google Scholar]
- 55.Silva LA, et al. Vitamin E supplementation decreases muscular and oxidative damage but not inflammatory response induced by eccentric contraction. J. Physiol. Sci. 2010;60:51–57. doi: 10.1007/s12576-009-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hosseinpour-Arjmand S, Amirkhizi F, Ebrahimi-Mameghani M. The effect of alpha-lipoic acid on inflammatory markers and body composition in obese patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled trial. J. Clin. Pharm. Ther. 2019;44:258–267. doi: 10.1111/jcpt.12784. [DOI] [PubMed] [Google Scholar]
- 57.Chou C-C, Sung Y-C, Davison G, Chen C-Y, Liao Y-H. Short-term high-dose vitamin C and E supplementation attenuates muscle damage and inflammatory responses to repeated taekwondo competitions: a randomized placebo-controlled trial. Int. J. Med. Sci. 2018;15:1217. doi: 10.7150/ijms.26340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jamilian M, et al. The effects of omega-3 and vitamin E co-supplementation on parameters of mental health and gene expression related to insulin and inflammation in subjects with polycystic ovary syndrome. J. Affect. Disord. 2018;229:41–47. doi: 10.1016/j.jad.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 59.Dewell A, Tsao P, Rigdon J, Gardner CD. Antioxidants from diet or supplements do not alter inflammatory markers in adults with cardiovascular disease risk. A pilot randomized controlled trial. Nutr. Res. (New York, N.Y.) 2018;50:63–72. doi: 10.1016/j.nutres.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang M, et al. Effect of vitamin E with therapeutic iron supplementation on iron repletion and gut microbiome in US iron deficient infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2016;63:379–385. doi: 10.1097/mpg.0000000000001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saboori S, et al. Beneficial effects of omega-3 and vitamin E coadministration on gene expression of SIRT1 and PGC1α and serum antioxidant enzymes in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2016;26:489–494. doi: 10.1016/j.numecd.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Sozen E, Demirel T, Ozer NK. Vitamin E: regulatory role in the cardiovascular system. IUBMB Life. 2019;71:507–515. doi: 10.1002/iub.2020. [DOI] [PubMed] [Google Scholar]
- 63.Cominacini L, et al. Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic. Biol. Med. 1997;22:117–127. doi: 10.1016/s0891-5849(96)00271-7. [DOI] [PubMed] [Google Scholar]
- 64.Sagach VF, et al. The water-soluble vitamin E analogue Trolox protects against ischaemia/reperfusion damage in vitro and ex vivo. A comparison with vitamin E. Pharmacol. Res. 2002;45:435–439. doi: 10.1006/phrs.2002.0993. [DOI] [PubMed] [Google Scholar]
- 65.Lonn E, et al. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care. 2002;25:1919–1927. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]
- 66.Calabro P, Golia E, Yeh TH. Role of C-reactive protein in acute myocardial infarction and stroke: possible therapeutic approaches. Curr. Pharm. Biotechnol. 2012;13:4–16. doi: 10.2174/138920112798868764. [DOI] [PubMed] [Google Scholar]
- 67.Ko A, et al. Association between high sensitivity C-reactive protein and dietary intake in Vietnamese young women. Nutr. Res. Pract. 2014;8:445–452. doi: 10.4162/nrp.2014.8.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 69.Ridker PMJC. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.CIR.0000053730.47739.3C. [DOI] [PubMed] [Google Scholar]
- 70.Ingles DP, Cruz Rodriguez JB, Garcia H. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Curr. Cardiol. Rep. 2020;22:22. doi: 10.1007/s11886-020-1270-1. [DOI] [PubMed] [Google Scholar]
- 71.Abraham A, Kattoor AJ, Saldeen T, Mehta JL. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019;59:2831–2838. doi: 10.1080/10408398.2018.1474169. [DOI] [PubMed] [Google Scholar]
- 72.Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am. J. Kidney Dis. 2000;35:469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 73.Blum A, et al. Variability of C-reactive protein levels among patients with stable coronary artery disease and on statin therapy. Isr. Med. Assoc. J. 2009;11:602–605. [PubMed] [Google Scholar]
- 74.Rahmani S, et al. The effect of whole-grain intake on biomarkers of subclinical inflammation: a comprehensive meta-analysis of randomized controlled trials. Adv. Nutr. (Bethesda, MD) 2020;11:52–65. doi: 10.1093/advances/nmz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wing RR, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the look AHEAD trial. J. Sex. Med. 2010;7:156–165. doi: 10.1111/j.1743-6109.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Block G, et al. Vitamin C treatment reduces elevated C-reactive protein. Free Radic. Biol. Med. 2009;46:70–77. doi: 10.1016/j.freeradbiomed.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Food and Nutrition Board . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 78.Block G, et al. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic. Biol. Med. 2008;45:377–384. doi: 10.1016/j.freeradbiomed.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jialal I, Fuller CJ, Huet BA. The effect of alpha-tocopherol supplementation on LDL oxidation. A dose–response study. Arterioscler. Thromb. Vasc. Biol. 1995;15:190–198. doi: 10.1161/01.atv.15.2.190. [DOI] [PubMed] [Google Scholar]
- 80.Miller ER, 3rd, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 81.Wolf G. How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr. Rev. 2006;64:295–299. doi: 10.1111/j.1753-4887.2006.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 82.Berdnikovs S, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J. Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang QJ. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Handelman GJ, Machlin LJ, Fitch K, Weiter JJ, Dratz EA. Oral α-tocopherol supplements decrease plasma γ-tocopherol levels in humans. J. Nutr. 1985;115:807–813. doi: 10.1093/jn/115.6.807%JTheJournalofNutrition. [DOI] [PubMed] [Google Scholar]
- 85.Manor D, Morley S. The α-tocopherol transfer protein. Vitam. Horm. 2007;76:45–65. doi: 10.1016/S0083-6729(07)76003-X. [DOI] [PubMed] [Google Scholar]
- 86.Traber MG. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 87.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. doi: 10.1096/fasebj.13.10.1145. [DOI] [PubMed] [Google Scholar]
- 88.Ricciarelli R, et al. alpha-Tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem. J. 1998;334(Pt 1):243–249. doi: 10.1042/bj3340243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iannitti T, Palmieri B. Antioxidant therapy effectiveness: an up to date. Eur. Rev. Med. Pharmacol. Sci. 2009;13:245–278. [PubMed] [Google Scholar]
- 90.Papaconstantinou J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells. 2019 doi: 10.3390/cells8111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr. Aging Sci. 2011;4:158–170. doi: 10.2174/1874609811104020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berry D, Wathen JK, Newell M. Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clin. Trials (London, England) 2009;6:28–41. doi: 10.1177/1740774508101279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.