Bronchopulmonary dysplasia (BPD) is a common pulmonary complication of prematurity, resulting in long-term pulmonary morbidity or death. BPD is conceptually understood as an irreversible growth arrest of the premature lung caused by multiple pre- and postnatal injurious events leading to two main histomorphological characteristics: alveolar simplification and dysmorphic pulmonary vasculature. The incidence of BPD is approximately 40% and still increasing, leading to a substantial burden for patients and healthcare systems (1). Yet, there is no curative therapy available. Thus, concepts to promote pulmonary regeneration are urgently needed. In this context, a study in this issue of the Journal demonstrates the capacity of the lung to resume alveolar development after significant hyperoxia injury. This study also suggests that a key mesenchymal cell type is critical for this regenerative process.
In this issue of the Journal, Zysman and colleagues (pp. 1088–1104) evaluate the dynamics of the cell-cycle kinase inhibitor p16 (also known as p16INK4a or cyclin-dependent kinase inhibitor 2A) in a murine hyperoxia model (2). The authors observed an increase in p16-positive nuclei in the lung tissue from mice exposed to high oxygen conditions. Next, they found that deficiency of p16INK4a enhances lung regeneration following hyperoxia-induced lung injury as well as after pneumonectomy, a model of compensatory growth in adult lung. They show that p16INK4a deficiency restores normal lung architecture into adulthood but does not protect against early hyperoxia-induced lung injury. A similar trend is observed when p16INK4a-expressing cells are depleted after hyperoxia conditions. Interestingly, the authors show that the number of Pdgfra (platelet-derived growth factor α)-positive fibroblasts and an associated lipid profile (lipid-content staining and ADRP expression) are significantly increased in p16INK4a-deficient animals (see interaction 1 in Figure 1). In line with the restored alveolar tissue, the authors also find an increase in the number of alveolar type 2 (AT2) cells. Together, these data underscore the capacity for tissue repair in the lung alveolus and highlight cell dynamics acting in parallel between the alveolar mesenchyme and epithelium.
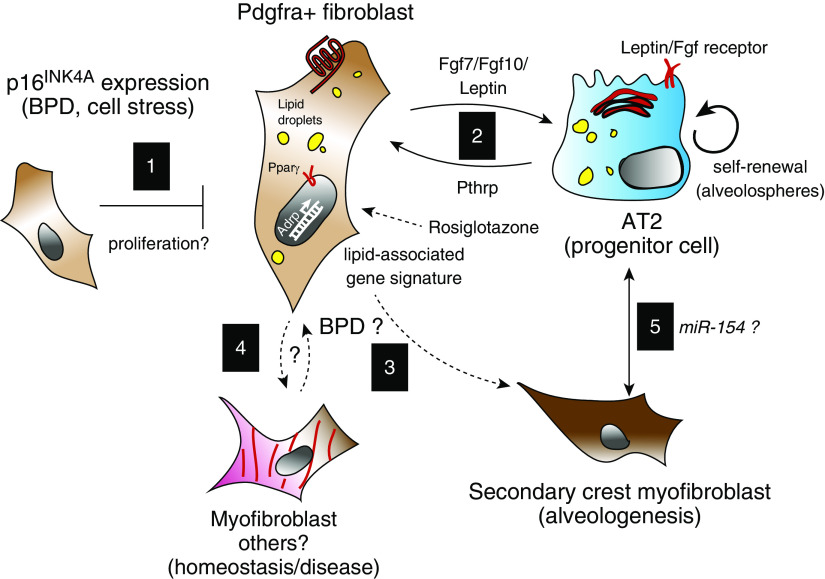
Figure 1.
Modeling p16INK4A expression in the context of the developing alveolar niche. (Interaction 1) Zysman and colleagues show that under hyperoxic conditions fibroblasts express p16INK4A. Fibroblasts that are Pdgfra positive and express p16INK4A display an increased lipid profile. It has been shown that Pdgfra-positive fibroblasts support AT2 cell self-renewal via an exchange of secreted factors (interaction 2). Under normal conditions, in the developing lung, Pdgfra-positive fibroblasts also include a transient secondary crest myofibroblast. In certain pathological conditions, myofibroblasts contribute to impaired lung function. Future work could explore how acquisition of the p16INK4A signature results in differentiation of these fibroblast subsets and impact on the alveolar niche (interactions 3–5). AT2 = alveolar type 2; BPD = bronchopulmonary dysplasia.
The cellular and functional heterogeneity within the mesenchyme, including fibroblasts and smooth muscle, has been under intense investigation in multiple organs. Recent studies have relied on new tools for genetic lineage tracing as well as single-cell transcriptomics to dissect fibroblast subsets and their associated function. In the lung alveolar compartment, electron microscopy and histology were used to characterize fibroblasts with lipid droplets, termed lipofibroblasts (LIFs), in rodents. Furthermore, these lipid droplet–containing mesenchymal cells were mapped in close proximity to AT2 cells (3). Zysman and colleagues found an increase in LIFs and enriched set of genes implicated in lipid content and storage from whole-lung homogenates as well as an enhanced neutral lipid profile in the p16INK4a knockouts. Transgenic and knock-in mouse lines have further shown that the resident alveolar fibroblasts are mostly composed of Pdgfra- or Pdgfrb-expressing cells. Interestingly, in three-dimensional alveolar epithelial organoid models, Pdgfra+ fibroblasts, which includes LIFs, but not Pdgfrb+ fibroblasts, have the unique ability to support alveolar epithelial organoid growth and differentiation (4, 5). These data indicate that alveolar fibroblasts may be functionally classified by their abilities to support epithelial cell growth. Thus, they have been referred to as mesenchymal alveolar niche or (alveolar) type 2–associated stromal cells (see interaction 2 in Figure 1) (5, 6). Taken together, future work will be needed to determine the relevance of a lipid-associated profile and alveolar cell growth and differentiation.
Extracellular ligands such as leptin, retinoic acid, Pthrp (parathyroid hormone-related protein), Fgfs (Fgf7/Fgf10), cytokines, Bmp (bone morphogenic protein) antagonists, and Wnt ligands have been implicated in the fibroblast/AT2 cell niche (5, 7–9). Furthermore, a Wnt/Fgf responsive subset of AT2 cells has been shown to exhibit enhanced progenitor capabilities (8, 9). The data presented by Zysman and colleagues suggests that the increased numbers of lipofibroblast/AT2 may restore alveolar repair after hyperoxia. However, whether p16INK4a actively inhibits the proliferation of LIFs and/or impairs their capacity to sustain the AT2 progenitor cells via the content of secreted ligands is unclear.
In injury models, including the hyperoxia model used by Zysman and colleagues, it is not unexpected that the expression levels of p16INK4a, a potent cell cycle inhibitor, increases, possibly because of underlying cell stress in response to reactive oxygen species. The key findings from Zysman and colleagues indicate that clearance of the cells expressing p16INK4a results in a significant improvement across a variety parameters of lung function and that alveolar fibroblasts are sensitive to this cell cycle regulation. The latter finding raises many additional questions or clarifications, including what mechanisms, transcriptional signatures, and pathways are active in distinct subsets of lung fibroblasts, and are these critical for cell identity? Addressing these questions would also require a thorough characterization of the proliferation, expansion, and contraction of specific mesenchymal cell subsets (see interaction 1 in Figure 1). In particular, it will be important to better characterize the p16INK4a-positive fibroblast population in terms of heterogeneity, identification of specific surface markers, and signaling pathway activity.
Insights into the lineage relationship between alveolar fibroblasts and myofibroblasts might offer clues for targeted therapies to promote alveologenesis after lung injury. The concept of LIF transdifferentiating to myogenic cell types such as the myofibroblast (MYF) was pioneered almost 20 years ago. Using molecular and metabolic studies, it was suggested that a transdifferentiation of LIFs to MYFs occurs upon hyperoxia-induced lung injury (10). Whether the MYFs in question in this study were alveologenesis-specific secondary crest myofibroblasts (SCMF) or activated (pathogenic) MYFs was not shown (see interactions 3 and 4 in Figure 1). Recently, a reversible switch of LIF to activated MYF in idiopathic pulmonary fibrosis was reported (11). In a follow-up study, the metabolic drug Metformin was shown to induce lipogenic differentiation in activated MYFs to reverse lung fibrosis (see interaction 4 in Figure 1) (12). Interestingly, it was proposed that Pdgfra-positive/LIF cells could give rise to MYFs during realveolarization after pneumonectomy (see interaction 4 in Figure 1) (13).
How AT2s interact with other mesenchymal cell types such as the SCMF is currently unknown (see interaction 5 in Figure 1). It was recently demonstrated using a cell-autonomous approach that overexpression of miR-154 in AT2 cells leads to defective alveologenesis, suggesting that AT2 cells could be instrumental in this developmental process (14). In this context, it will be interesting to define the link between the LIF-AT2 interaction upon deletion of p16INK4a-positive fibroblasts and the process of alveologenesis, particularly whether AT2s impact the formation and/or the functionality of SCMFs. BPD results from the disruption of intricate processes underlying the development of the alveolar tissue. The current study and new questions may help to address the paucity of information regarding the cell turnover and differentiation of the mesenchyme and the impact on the alveolar niche.
Supplementary Material
Footnotes
Supported by NIH (grant R01-HL143059 to S.B.) as well as the Deutsche Forschungsgemeinschaft (BE4443/1-1, BE4443/4-1, BE4443/6-1, KFO309 P7, and SFB1213 projects A02 and A04).
Originally Published in Press as DOI: 10.1164/rccm.202006-2164ED on July 15, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zysman M, Baptista RB, Essari L-A, Taghizadeh S, Thibault de Menonville C, Giffard C, et al. Targeting p16INK4a promotes lipofibroblasts and alveolar regeneration after early-life injury. Am J Respir Crit Care Med. 2020;202:1088–1104. doi: 10.1164/rccm.201908-1573OC. [DOI] [PubMed] [Google Scholar]
- 3.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell. 2017;170:1134–1148, e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung M-I, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development. 2018;145:dev163014. doi: 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rehan VK, Torday JS. The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury. Antioxid Redox Signal. 2014;21:1893–1904. doi: 10.1089/ars.2013.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359:1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boros LG, Torday JS, Paul Lee WN, Rehan VK. Oxygen-induced metabolic changes and transdifferentiation in immature fetal rat lung lipofibroblasts. Mol Genet Metab. 2002;77:230–236. doi: 10.1016/s1096-7192(02)00140-3. [DOI] [PubMed] [Google Scholar]
- 11.El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell. 2017;20:261–273, e3. doi: 10.1016/j.stem.2016.10.004. [Published erratum appears in Cell Stem Cell 20:571.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kheirollahi V, Wasnick RM, Biasin V, Vazquez-Armendariz AI, Chu X, Moiseenko A, et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun. 2019;10:2987. doi: 10.1038/s41467-019-10839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK. Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol. 2012;47:517–527. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao CM, Carraro G, Rako ZA, Kolck J, Sedighi J, Zimmermann V, et al. Failure to down-regulate miR-154 expression in early postnatal mouse lung epithelium suppresses alveologenesis, with changes in Tgf-β signaling similar to those induced by exposure to hyperoxia. Cells. 2020;9:859. doi: 10.3390/cells9040859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.