Since the first reported cases in December 2019 in Wuhan, China, coronavirus disease (COVID-19) outbreak has rapidly spread around the world (1). This infection often requires ICU admissions and invasive mechanical ventilation (2). To prevent diaphragmatic atrophy and to enhance weaning, the early use of ventilatory modes allowing spontaneous breathing is usually recommended as soon as possible but should be balanced with potential harmful effects. Indeed, a high respiratory drive is sometimes observed in patients with acute respiratory distress syndrome (ARDS), and thus, spontaneous breathing could lead to uncontrolled transpulmonary pressures and possibly to patient self-inflicted lung injuries (P-SILI) (3, 4). Strong efforts could also simply reflect the nonresolution of the underlying disease and thus invite to delay the weaning process of mechanical ventilation. Lacking specific respiratory monitoring, surrogate measures of respiratory drive should be assessed. Airway occlusion pressure (P0.1) is a simple, noninvasive measurement method for estimating respiratory drive during mechanical ventilation (3, 5). It is automatically available in almost all ventilators. P0.1 is the negative airway pressure generated during the first 100 ms of an occluded inspiration. Because of the very short duration and zero flow, it is independent from respiratory muscle weakness as well as respiratory system compliance and resistance. However, it provides little information about the magnitude of dynamic lung stress (5, 6). It has been proposed to target a range between 1.5 and 3.5 cm H2O of P0.1 (3). The airway pressure deflection generated by the patient’s respiratory effort during an end-expiratory airway occlusion (ΔPocc) is a recently validated noninvasive technique for detecting excessive respiratory effort and dynamic lung stress during assisted mechanical ventilation (6). Bertoni and colleagues showed that measurements of ΔPocc allow a reliable and bedside estimation of respiratory muscle pressure (Pmus) by using a conversion factor (predicted Pmus = −0.75 × ΔPocc) (6). Besides, in a recent editorial, Gattinoni and colleagues suggested that P0.1 and ΔPocc should be determined in patients with COVID-19 to assess excessive inspiratory efforts (7). The validity of P0.1 or ΔPocc measurements in intubated and mechanically ventilated patients with COVID-19 has not been evaluated.
We hypothesized that mechanically ventilated patients with COVID-19 with ARDS often present high respiratory drive and excessive inspiratory efforts (as suggested by elevated P0.1 and ΔPocc measurements) and that this could rapidly lead to a relapse of respiratory failure during the weaning process of mechanical ventilation.
Therefore, the aim of this study was to assess the threshold values of P0.1 and ΔPocc predicting the occurrence of relapse in the following 24-hour period after measurements in intubated and mechanically ventilated patients with COVID-19 pneumonia.
Methods
We conducted a retrospective, bicenter study at the Sainte Anne Military Hospital and the Marseille University North Hospital. This study enrolled critically ill patients with mild to severe ARDS due to COVID-19 (positive result of a real-time RT-PCR assay in nasal or pulmonary samples), intubated and mechanically ventilated, in supine position, and with spontaneous breathing (pressure support ventilation [PSV] or airway pressure release ventilation [APRV]).
The study was approved by the institutional review board of the Sainte Anne Military Hospital (no. 0011873-2020-05), which waived the requirement for informed consent from patients and their relatives, given the retrospective and observational nature of the study.
P0.1 and ΔPocc measurements were performed by the clinician in charge in each patient on the first day on APRV mode or PSV mode. P0.1 was measured at least three times (1 min between each measurement), and the mean P0.1 was notified. ΔPocc was defined as the maximal deflection in airway pressure from positive end-expiratory pressure (PEEP) during an end-expiratory airway occlusion (6). Measurements were repeated at least three times, and the highest value was recorded.
Automated measurements were performed with four commercialized ventilators: Evita XL (Dräger), Evita Infinity V500 (Dräger), Avea (CareFusion), and Carescape R860 (GE Healthcare). The accuracy and precision of values of P0.1 displayed by these ventilators have been validated (8, 9). The same ventilator was used for a given patient.
The main endpoint of the study was a relapse of respiratory failure during the weaning process of invasive mechanical ventilation in the 24-hour period following measurements defined by the presence of at least one of the following criteria: decrease of PaO2/FiO2 ratio ≥20%, or severe hypoxemia (oxygen saturation as measured by pulse oximetry [SpO2] <88% under FiO2 ≥60% for >15 min), new onset of respiratory acidosis (pH <7.35), or increase of PaCO2 ≥10 mm Hg in patients with preceding respiratory acidosis. Ventilator settings were optimized in case of respiratory worsening as follows: 2 cm H2O stepwise increase of pressure support (PS) until 14 cm H2O when respiratory rate was >35/min or Vt was <6 ml/kg of predicted body weight (PBW), decrease of PS until 0 cm H2O or increase of sedation (without loss of spontaneous breathing) in case of Vt >8 ml/kg of PBW, and 2 cm H2O stepwise increase of PEEP until 16 cm H2O when SpO2/FiO2 was <150 (10). If temporary deoxygenations were observed (e.g., following an accidental ventilator disconnection, airway suctioning, transport to computed tomographic scan) and were not followed by any medical intervention (i.e., change of ventilator settings, increase in sedations), they were not considered a relapse of respiratory failure.
The method of weaning was similar in the two ICUs. Briefly, all patients were initially ventilated in volume-controlled mode. When the PaO2/FiO2 ratio was greater than 150 mm Hg during at least 6 hours without neuromuscular blockers, and/or use of prone positioning or inhaled nitric oxide in the last 12 hours, volume-controlled mode was switched to APRV mode (minimal timehigh:timelow was 1 s:1.5–2 s, Phigh was set to achieve a Vt of 6–8 ml/kg of PBW with a maximal driving pressure of 15 cm H2O, Plow was the corresponding PEEP during volume-controlled mode). When spontaneous minute ventilation was above 50% in APRV mode, ventilator settings were switched to PSV. The PS was then decreased every 4 hours if Vt remained ≥6 ml/kg of PBW and respiratory rate remained <35/min. PEEP was gently (2 cm H2O stepwise) decreased every 8–12 hours if PaO2/FiO2 ratio remained ≥200 mm Hg. Extubation was considered when PS was ≤4 cm H2O with Vt >6 ml/kg of PBW and respiratory rate <35/min, PEEP was ≤6 cm H2O, and FiO2 was ≤40% with PaO2/FiO2 ratio ≥200 mm Hg. A spontaneous breathing trial using a T-tube was not systematically performed before extubation.
Statistical analysis was performed using R software, version 3.5.1 (The R Foundation for Statistical Computing). Nonparametric variables were compared using a Mann-Whitney test. Abilities of P0.1 or ΔPocc to predict a relapse of respiratory failure were represented by a receiver operating characteristic (ROC) curve analysis. Areas under the curves (AUCs) were presented with their 95% confidence interval (95% CI). The diagnostic cutoff was determined by the highest Youden index value. Because some patients underwent several P0.1 and ΔPocc measurements, we analyzed only the first values of P0.1 and ΔPocc measurements.
Results
Twenty-eight patients with COVID-19 admitted in the two ICUs from March 10 through April 14, 2020, were included. Population characteristics are displayed in Table 1.
Table 1.
Baseline Characteristics, Treatments, and Main Outcomes of Included Patients
| Demographic data | |
| Age, yr | 66 (57–73) |
| Sex, M | 22 (78.6) |
| Comorbidities | |
| Any | 25 (89) |
| ≥3 | 11 (39) |
| Arterial hypertension | 20 (71) |
| Diabetes | 5 (18) |
| BMI >25 kg/m2 | 9 (32) |
| Obstructive sleep apnea | 7 (25) |
| Chronic obstructive pulmonary disease | 4 (14) |
| Coronary heart disease | 3 (11) |
| Chronic kidney disease | 2 (7) |
| Malignancy | 3 (11) |
| Time from onset of symptoms to | |
| ICU admission, d | 8 (5–11) |
| Invasive mechanical ventilation, d | 9 (5–11) |
| SAPS II score at admission | 59 (39–65) |
| SOFA score at admission | 7 (4–9) |
| Minimal PaO2/FiO2 ratio, mm Hg | 110 (98–128) |
| Mild ARDS | 1 (4) |
| Moderate ARDS | 19 (68) |
| Severe ARDS | 8 (29) |
| Treatments for ARDS | |
| Continuous infusion of neuromuscular blockers | 24 (86) |
| Prone position | 19 (68) |
| Inhaled nitric oxide | 6 (21) |
| Almitrine infusion | 2 (7) |
| Extracorporeal membrane oxygenation | 1 (4) |
| Outcomes | |
| VFD at Day 30 | 0 (0–5) |
| Weaning before Day 30 | 11 (39) |
| Tracheostomy | 17 (61) |
| Renal replacement therapy | 2 (7) |
| Discharge from ICU before Day 30 | 11 (39) |
| 30-d mortality | 1 (4) |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; BMI = body mass index; SAPS II = Simplified Acute Physiology Score; SOFA = Sequential Organ Failure Assessment; VFD = ventilator-free days.
n = 28. Data are expressed as n (%) or median (interquartile range).
A total of 28 paired measurements of P0.1 (3 measures, mean value of the 3) and ΔPocc (highest value of 3 measures) were performed (4 on APRV mode and 24 on PSV mode). Time from the onset of invasive mechanical ventilation to first measurements was 8.5 (interquartile range [IQR], 4–12) days. Before measurements, median Richmond Agitation-Sedation Scale was −4 (IQR, −4 to −4). Ventilator settings before measurements were as follows: PS at 6 (IQR, 4–11) cm H2O, PEEP at 12 (IQR, 10–14) cm H2O, and Vt of 6.6 (IQR, 6.3–7.3) ml/kg of PBW. Median rapid shallow breathing index was 49 (IQR, 40–62) breaths/min/L. Median minute ventilation was 11.1 (IQR, 8.9–12.6) L/min. Results of last blood gas analysis before measurements were as follows: PaO2 83 (IQR, 77–97) mm Hg, PaCO2 46.2 (IQR, 39.7–49.3) mm Hg, pH 7.43 (IQR, 7.42–7.46), and PaO2/FiO2 ratio 203 (IQR, 187–238) mm Hg.
Mean P0.1 value was 4.4 ± 3.0 cm H2O. Notably, 14 measurements (50%) were >3.5 cm H2O, and 7 (25%) were ≥6.0 cm H2O. Twelve ΔPocc measurements (43%) were <−15 cm H2O.
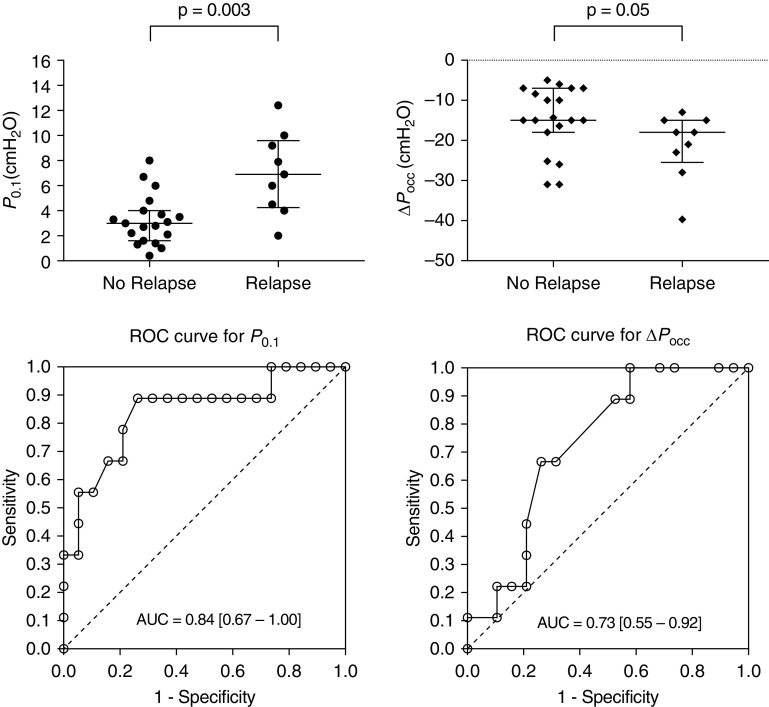
Of the 28 measurements, 9 (32%) were followed by a relapse of respiratory failure. As illustrated in the Figure 1, median P0.1 were significantly higher in those cases (6.9 [IQR, 4.3 to 9.6] cm H2O vs. 3 [IQR, 1.6 to 4] cm H2O), and median ΔPocc were lower (−18 [IQR, −26 to −15] cm H2O vs. −15 [IQR, −18 to −7] cm H2O).
Figure 1.
Abilities of airway occlusion pressure (P0.1) and end-expiratory airway occlusion (ΔPocc) to predict relapse of respiratory failure during the weaning process of invasive mechanical ventilation. (Top) Scatter dot plots describing P0.1 (left) and ΔPocc (right) values (median with interquartile range) followed or not by a relapse. (Bottom) ROC curves of P0.1 (left) and ΔPocc (right) for prediction of relapse of respiratory failure. AUC = area under the curve; ROC = receiver operating characteristic.
One measurement was followed by a successful extubation with a value of P0.1 at 3.0 cm H2O.
ROC curve showed that P0.1 had a satisfactory accuracy to predict a relapse with an AUC of 0.84 (95% CI, 0.67–1.00), P = 0.004 (Figure 1). The maximum value of the Youden index was obtained for a P0.1 ≥4 cm H2O. The prognostic performance of this threshold showed a sensitivity of 89% (95% CI, 52–100), a specificity of 74% (95% CI, 49–91), a positive predictive value of 62% (95% CI, 32–86), a negative predictive value of 93% (95% CI, 68–100), a positive likelihood ratio (LR) of 3.38 (95% CI, 1.54–7.42), a negative LR of 0.15 (95% CI, 0.02–0.98), and a diagnostic accuracy of 79% (95% CI, 59–92).
ROC curve demonstrated that ΔPocc had an acceptable accuracy to predict a relapse with an AUC of 0.73 (95% CI, 0.55–0.92), P = 0.05 (Figure 1). The maximum value of the Youden index was obtained for a ΔPocc < −10 cm H2O. The prognostic performance of this threshold showed a sensitivity of 100% (95% CI, 66–100), a specificity of 42% (95% CI, 20–67), a positive predictive value of 45% (95% CI, 23–68), a negative predictive value of 100% (95% CI, 63–100), a positive LR of 1.73 (95% CI, 1.18–2.53), a negative LR of 0.00 (95% CI, 0.00–0.00), and a diagnostic accuracy of 61% (95% CI, 41–78).
Prognostic performances of the other thresholds are presented in Table 2.
Table 2.
Prognostic Performance of Different Threshold of P0.1 and ΔPocc to Predict Relapse of Respiratory Failure during the Weaning Process of Invasive Mechanical Ventilation
| Thresholds (cm H2O) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR− | Diagnostic Accuracy (%) | Youden Index |
|---|---|---|---|---|---|---|---|---|
| P0.1 | ||||||||
| ≥3 | 89 (52–100) | 47 (24–71) | 44 (22–69) | 90 (55–100) | 1.69 (1.04–2.74) | 0.23 (0.03–1.58) | 61 (41–78) | 0.36 |
| ≥4 | 89 (52–100) | 74 (49–91) | 62 (32–86) | 93 (68–100) | 3.38 (1.54–7.42) | 0.15 (0.02–0.98) | 79 (59–92) | 0.63 |
| ≥5 | 67 (30–93) | 84 (60–97) | 67 (30–93) | 84 (60–97) | 4.22 (1.36–13.16) | 0.40 (0.15–1.02) | 79 (59–92) | 0.51 |
| ≥6 | 67 (30–93) | 84 (60–97) | 67 (30–93) | 84 (60–97) | 4.22 (1.36–13.16) | 0.40 (0.15–1.02) | 79 (59–92) | 0.51 |
| ≥7 | 44 (14–79) | 95 (74–100) | 80 (28–99) | 78 (56–93) | 8.44 (1.10–65.12) | 0.59 (0.32–1.06) | 79 (59–92) | 0.39 |
| ΔPocc | ||||||||
| <−10 | 100 (66–100) | 42 (20–67) | 45 (23–68) | 100 (63–100) | 1.73 (1.18–2.53) | 0.00 (0.00–0.00) | 61 (41–78) | 0.42 |
| <−15 | 67 (30–93) | 68 (43–87) | 50 (21–79) | 81 (54–96) | 2.11 (0.94–4.73) | 0.49 (0.18–1.29) | 68 (48–84) | 0.35 |
| <−20 | 44 (14–79) | 79 (54–94) | 50 (16–84) | 75 (51–91) | 2.11 (0.68–6.58) | 0.70 (0.38–1.32) | 68 (48–84) | 0.23 |
| <−25 | 22 (03–60) | 79 (54–94) | 33 (04–78) | 68 (45–86) | 1.06 (0.24–4.73) | 0.99 (0.65–1.50) | 61 (41–78) | 0.01 |
| <−30 | 11 (00–48) | 89 (67–99) | 33 (01–91) | 68 (46–85) | 1.06 (0.11–10.17) | 0.99 (0.75–1.31) | 64 (44–81) | 0.00 |
Definition of abbreviations: ΔPocc = airway pressure deflection generated by respiratory effort during an end-expiratory airway occlusion; LR+ = positive likelihood ratio; LR− = negative likelihood ratio; NPV = negative predictive value; P0.1 = airway occlusion pressure; PPV = positive predictive value.
Data in parentheses are 95% confidence intervals.
Finally, AUC for P0.1 was not significantly different than AUC for ΔPocc (DeLong’s test, P = 0.32).
We then split measurements into four categories: low ΔPocc (≥−15 cm H2O)/low P0.1 (<4 cm H2O), high ΔPocc (<−15 cm H2O)/low P0.1, low ΔPocc/high P0.1 (≥4 cm H2O), and high ΔPocc/high P0.1. Proportions of relapse of respiratory failure were, respectively, 0/11 (0%), 1/4 (25%), 3/5 (60%), and 5/8 (62.5%) (P = 0.015).
Discussion
In this cohort of patients with COVID-19, we found that P0.1 was frequently above 3.5 cm H2O, suggesting high neural respiratory drive (3, 11). Even if ranges of P0.1 up to 6.0 cm H2O have been reported in patients with ARDS, a quarter of our measurements were above this value (5). P0.1 is an easy and reliable tool to measure the respiratory drive, available worldwide. Recently, Telias and colleagues demonstrated that P0.1 directly displayed by the ventilator correlates with invasive measures of respiratory drive (electrical activity of the diaphragm and muscular pressure measured with esophageal pressure). They also showed that P0.1 was well correlated with pressure–time product per minute, a surrogate of inspiratory effort (9).
We found that P0.1 had a reliable accuracy to predict relapse of respiratory failure in the first 24 hours after measurement of P0.1 and ΔPocc in our population. A P0.1 ≥4.0 cm H2O had an excellent specificity and negative predictive value. Relapse might be a consequence of P-SILI and myotrauma, or also due to the nonresolution of the COVID-19 pneumonia. High drive and excessive respiratory efforts could possibly lead to P-SILI through different mechanisms such as an excessive global and regional lung stress, a pulmonary edema, or patient–ventilator asynchronies (3). The diaphragm is also sensitive to an excessive respiratory load, ensuing load-induced diaphragm injury (myotrauma).
ΔPocc measurements were also frequently less than −15 cm H2O, which can correspond, after application of conversion factor, to Pmus greater than 10 cm H2O, indicating excessive respiratory efforts (6). Indeed, Bertoni and colleagues propose to target a range of Pmus between 5 and 10 cm H2O during spontaneous breathing (3). Even if ΔPocc was less discriminating than P0.1, its regular measurement is also interesting to predict a relapse of respiratory failure during mechanical ventilation weaning. Moreover, we found that high ΔPocc and high P0.1 association is at higher risk of relapse of respiratory failure.
This study has several limitations including its retrospective design and the limited number of patients included. A comparative measure of respiratory drive and inspiratory efforts such as electrical activity of the diaphragm or muscular pressure measured with esophageal catheter might have helped to confirm our results.
In conclusion, in this COVID-19 pandemic context, with limited time and material resources, serial measurements of P0.1 and ΔPocc could be a valuable bedside clinical tool to predict relapse of respiratory failure in the next 24 hours and therefore to potentially delay the weaning process of mechanical ventilation, especially when P0.1 is ≥4 cm H2O and ΔPocc is <−15 cm H2O.
Supplementary Material
Footnotes
Author Contributions: P.E., M.C., and C.G. contributed to the study concept and design. P.E., M.C., P.G., E.P’h., and C.G. contributed to the acquisition of data. P.E., S.H., P.G., K.B., and C.G. contributed to the analysis and interpretation of data. P.E., J.B., J.M.-F., E.M., L.P., and C.G. contributed to drafting the manuscript and critically revising the manuscript for important intellectual content. All authors read and approved the final manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202005-1582LE on August 5, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertoni M, Spadaro S, Goligher EC. Monitoring patient respiratory effort during mechanical ventilation: lung and diaphragm-protective ventilation. Crit Care. 2020;24:106–108. doi: 10.1186/s13054-020-2777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 5.Telias I, Damiani F, Brochard L. The airway occlusion pressure (P0.1) to monitor respiratory drive during mechanical ventilation: increasing awareness of a not-so-new problem. Intensive Care Med. 2018;44:1532–1535. doi: 10.1007/s00134-018-5045-8. [DOI] [PubMed] [Google Scholar]
- 6.Bertoni M, Telias I, Urner M, Long M, Del Sorbo L, Fan E, et al. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. 2019;23:346. doi: 10.1186/s13054-019-2617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beloncle F, Piquilloud L, Olivier P-Y, Vuillermoz A, Yvin E, Mercat A, et al. Accuracy of P0.1 measurements performed by ICU ventilators: a bench study. Ann Intensive Care. 2019;9:104–109. doi: 10.1186/s13613-019-0576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Telias I, Junhasavasdikul D, Rittayamai N, Piquilloud L, Chen L, Ferguson ND, et al. Airway occlusion pressure as an estimate of respiratory drive and inspiratory effort during assisted ventilation. Am J Respir Crit Care Med. 2020;201:1086–1098. doi: 10.1164/rccm.201907-1425OC. [DOI] [PubMed] [Google Scholar]
- 10.Umbrello M, Fumagalli J, Pesenti A, Chiumello D. Pathophysiology and management of acute respiratory distress syndrome in obese patients. Semin Respir Crit Care Med. 2019;40:40–56. doi: 10.1055/s-0039-1685179. [DOI] [PubMed] [Google Scholar]
- 11.Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46:606–618. doi: 10.1007/s00134-020-05942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.