Airway type 2 inflammation in asthma is associated with enhanced steroid responsiveness, though a large proportion of nonresponders have eosinophilic asthma at baseline, which persists despite treatment. In addition to poor medication compliance, type 2 cytokines may further contribute biologically to this phenomenon; IL-5 delays or inhibits eosinophil apoptosis (1), whereas IL-13 may suppress steroid-mediated downregulation of LPS-induced IL-6 production by monocytes (2). In this issue of the Journal, Machida and colleagues (pp. 1105–1114) shed light on the role of the TL1A/DR3 (death receptor 3) axis in group 2 innate lymphoid cell (ILC2) activation in asthma and thus pinpoint the potential of this pathway as a therapeutic target for modulation of eosinophilia in those with severe asthma (3).
ILCs consist of a highly heterogeneous and functionally diverse group of cells, which at barrier surfaces are capable of rapidly responding to microbial and other antigenic stimuli. Humans and mice may differ significantly, with circulating ILC progenitors constitutively present in human peripheral blood and differentiating into mature ILCs within tissues (4). ILC2s express GATA-3 and produce the cytokines IL-4, IL-5, IL-9, IL-13, and amphiregulin in response to pathogens or other stimuli (5, 6) while they are responsive to IL-25, TSLP, and IL-33, among other mediators. Impaired regulation of such responses may drive allergic disease such as asthma, allergic rhinitis, and atopic dermatitis (6, 7). ILC2-derived IL-4, for example, plays a role in the inhibition of Treg cell responses (7), and ILC2s produce IL-2 after allergen challenge. Furthermore, human ILC2s express MHC class II molecules and present allergen-derived peptides to CD4+ T cells, leading to differentiation and propagation of Th2 cell subsets (8). In relation to asthma, initial studies were performed in murine models (5, 6), whereas later increased numbers of ILC2s have been identified in the sputum and BAL of patients with severe eosinophilic asthma compared with control subjects (9). Activated ILC2s also rapidly increase in the airways after allergen challenge (10). ILC2s may have an even more substantial role in the persistence of airway eosinophilia among patients with severe asthma through uncontrolled localized production of type 2 cytokines despite high-dose oral corticosteroid therapy (9). Yet, there is an accentuated need for further research regarding the anatomical location of ILC2s, their interaction with immune and nonhematopoetic cells, and their activation signals.
Machida and colleagues performed whole-lung allergen challenges in patients with mild atopic asthma (n = 10) to investigate the luminal recruitment of DR3+ ILC2s. DR3+ ILC2s increased significantly, from 205 ± 60 to 943 ± 316 cells/ml at 24 hours after challenge, which was accompanied by an increase of TL1A in sputum. Ex vivo analysis revealed that DR3 expression was inducible by physiological concentrations of IL-2, IL-33, and TSLP in a biphasic manner. TL1A in combination with IL-2 induced significantly intracellular IL-5 expression in these cells, which was reduced if dexamethasone (at a physiologically relevant concentration) was present. By costimulation with TSLP (IL-2 + TL1A), no corticosteroid treatment effect on IL-5 expression was observed. Furthermore, DR3 expression was insensitive to dexamethasone treatment. Patients with severe eosinophilic asthma (n = 11) produced significantly greater sputum TL1A levels than those with mild asthma (9.69 ± 2.69 vs. 1.06 ± 0.93 ng/ml).
Does this imply that patients with corticosteroid-resistant eosinophilic asthma truly have ILC2-dominated asthma? Yes and no! Taking a careful look at the data and also pointed out by the authors, there seems to be a group of patients with severe asthma (∼50%) with high TL1A levels and those without. Still, both groups are considered to have oral corticosteroid–dependent severe eosinophilic asthma. The sputum concentrations of TL1A are close to what Machida and colleagues used to stimulate DR3+ ILC2s ex vivo, so it is reasonable to assume that DR3+ ILC2s produce a significant amount of IL-5, driving the eosinophilic inflammation in the lung. Ideally, sputum levels of TSLP and IL-2 would have helped to really corner this particular asthma phenotype and gauge its clinical relevance. The overall small group sizes of the study pose a limit on the general extrapolation of this data set, yet Machida and colleagues pass on an exact recipe for how to identify those ILC2-dominated 50% of patients with severe eosinophilic asthma for further studies: detection of high eosinophils/high TL1A and anti-EPX (eosinophil peroxidase) antibodies in sputum. Anti-EPX antibodies? In a peculiar observation, the authors traced back to a potential mechanism how elevated levels of TL1A may be produced. Monocytes activated by EPX antibody complexes produced significantly more TL1A, which may lead to ILC2 activation and aggravation of a type 2 inflammation. This is certainly food for thought and worth further investigations.
This work clearly highlights the need for a continuous search for mechanisms underlying asthma heterogeneity (see cross-sectional analysis in Figure 1). Canonical types of asthma such as T2 high may result as a consequence of a complex interplay of immunological cells and pathways. As a matter of fact, various (sub)endotypes may occur simultaneously in one individual (11, 12) and may also change over time (see Figure 1). Considerable efforts are necessary to approach a longitudinal deep phenotyping, and studies have been established to address pediatric/transitional aspects (All-Age-Asthma-Cohort [13] and Children’s Respiratory and Environmental Workgroup Birth Cohort [14]) as well as molecular endotype persistence/evolution in adults (Cohort for Reality and Evolution of Adult Asthma in Korea [15] and Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes [16]), to name only a few. It is thus evident that one-size-fits-all treatment approaches are inherently flawed, and deeper understanding of the heterogeneous (targetable) molecular mechanisms in asthma is imperative.
Figure 1.
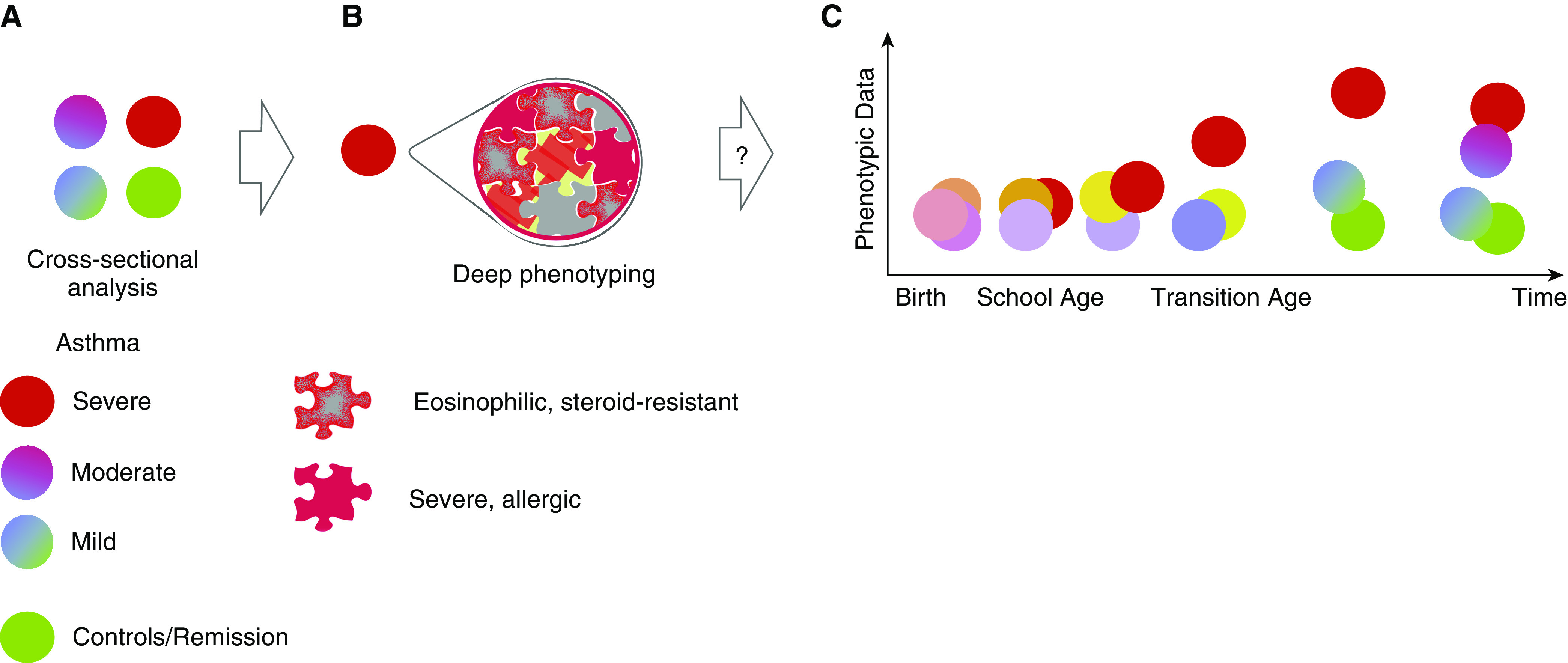
Innate lymphoid group 2 cell (ILC2) endotypes contribute to severe asthma phenotypes across all ages. (A) Severe asthma phenotypes (red circle) are clinically identified in cross-sectional analysis as a result of comparison to symptomatically milder phenotypes (blue/red or green/blue circles) or control subjects (green circle). These differences may then drive a deep phenotyping approach. (B) Severe phenotypes are analyzed by, for example, multiomics or hypothesis-specific tools (e.g., flow cytometry of DR3 [death receptor 3]-positive ILC2s as presented by Machida and colleagues in this issue of the Journal [3]), and a palette of several, possibly coexisting, endotypes emerges. Further characterization of the phenotypic characteristics of each endotype help to establish “ground truth” for asthma heterogeneity. Importantly, this is not limited to severe phenotypes alone but extends also to milder asthma and even asthma remission. (C) It is conceivable that these coexisting endotypes may have developed over time by endogenous and exogenous drivers, but little is known about the prerequisites for many of these endotypes and how they coexist. At young age, risk factors are associated with asthma pathogenesis; however, phenotypic expression is low (light purple vs., e.g., orange circle). During preschool age, first symptomatic episodes distinguish between children with an ever-increasing risk to be diagnosed with asthma at school age (dark orange circle) and those with, for example, intermittent wheeze (purple circle). At which point an ILC2-driven immune response becomes established or even dominates the asthmatic phenotype is not well understood. Moreover, features such as auto-antibodies against eosinophil peroxidase are not routinely tested, so early emergence of such a subendotype goes undetected. Longitudinal deep-phenotyping cohorts heavily rely on cross-sectional data (such as Machida and colleagues’) to trace the origins of these complex endotypes and help to provide other biomarkers, preventive measures, and early disease-modifying strategies.
Supplementary Material
Footnotes
C.S. is supported by the Universities Giessen and Marburg Lung Center; the German Center for Lung Research (DZL); University Hospital Giessen and Marburg research funding according to article 2, section 3 cooperation agreement; and the Deutsche Forschungsgemeinschaft-funded SFB 1021 (C04), KFO 309 (P10), and SK 317/1-1 (project number 428518790). M.W. is supported by the DZL and the University of Lübeck (E42-2012 and JC01-2016).
Originally Published in Press as DOI: 10.1164/rccm.202007-2658ED on July 28, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Pazdrak K, Moon Y, Straub C, Stafford S, Kurosky A. Eosinophil resistance to glucocorticoid-induced apoptosis is mediated by the transcription factor NFIL3. Apoptosis. 2016;21:421–431. doi: 10.1007/s10495-016-1226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spahn JD, Szefler SJ, Surs W, Doherty DE, Nimmagadda SR, Leung DY. A novel action of IL-13: induction of diminished monocyte glucocorticoid receptor-binding affinity. J Immunol. 1996;157:2654–2659. [PubMed] [Google Scholar]
- 3.Machida K, Aw M, Salter BMA, Ju X, Mukherjee M, Gauvreau GM, et al. The role of the TL1A/DR3 axis in the activation of group 2 innate lymphoid cells in subjects with eosinophilic asthma. Am J Respir Crit Care Med. 2020;202:1105–1114. doi: 10.1164/rccm.201909-1722OC. [DOI] [PubMed] [Google Scholar]
- 4.Lim AI, Li Y, Lopez-Lastra S, Stadhouders R, Paul F, Casrouge A, et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell. 2017;168:1086–1100, e10. doi: 10.1016/j.cell.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Neill DR, McKenzie ANJ. Nuocytes and beyond: new insights into helminth expulsion. Trends Parasitol. 2011;27:214–221. doi: 10.1016/j.pt.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138:801–811, e9. doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria J-P, O’Byrne PM, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86, e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Smith SG, Salter B, El-Gammal A, Oliveria J-P, Obminski C, et al. Allergen-induced increases in sputum levels of group 2 innate lymphoid cells in subjects with asthma. Am J Respir Crit Care Med. 2017;196:700–712. doi: 10.1164/rccm.201612-2427OC. [DOI] [PubMed] [Google Scholar]
- 11.Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers. 2015;1:15025. doi: 10.1038/nrdp.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agache I, Akdis CA. Endotypes of allergic diseases and asthma: an important step in building blocks for the future of precision medicine. Allergol Int. 2016;65:243–252. doi: 10.1016/j.alit.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs O, Bahmer T, Weckmann M, Dittrich A-M, Schaub B, Rösler B, et al. The all age asthma cohort (ALLIANCE): from early beginnings to chronic disease. A longitudinal cohort study. BMC Pulm Med. 2018;18:140. doi: 10.1186/s12890-018-0705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gern JE, Jackson DJ, Lemanske RF, Jr, Seroogy CM, Tachinardi U, Craven M, et al. The Children’s Respiratory and Environmental Workgroup (CREW) birth cohort consortium: design, methods, and study population. Respir Res. 2019;20:115–14. doi: 10.1186/s12931-019-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loza MJ, Adcock I, Auffray C, Chung KF, Djukanovic R, Sterk PJ, et al. ADEPT and U-BIOPRED Investigators. Longitudinally stable, clinically defined clusters of patients with asthma independently identified in the ADEPT and U-BIOPRED asthma studies. Ann Am Thorac Soc. 2016;13:S102–S103. doi: 10.1513/AnnalsATS.201508-519MG. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Jung HW, Lee JM, Shin B, Kim HJ, Kim M-H, et al. COREA investigators. Novel Trajectories for identifying asthma phenotypes: a longitudinal study in Korean asthma cohort, COREA. J Allergy Clin Immunol Pract. 2019;7:1850–1857, e4. doi: 10.1016/j.jaip.2019.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


