Abstract
Rationale: The World Health Organization recommends the use of isoniazid (INH) alone or in combination with rifapentine to treat latent tuberculosis infections. The recent rise of drug-resistant tuberculosis has complicated the choice of treatment regimen for latent tuberculosis infection.
Objectives: To evaluate the effects of INH preventive therapy on the contacts of patients with multidrug-resistant tuberculosis.
Methods: In a prospective cohort study conducted between September 2009 and August 2012, we identified 4,500 index patients with tuberculosis and 14,044 tuberculosis-exposed household contacts who we followed for 1 year for the occurrence of incident tuberculosis disease. Although Peruvian national guidelines specify that INH preventive therapy should be provided to contacts aged 19 years old or younger, only half this group received INH preventive therapy.
Measurements and Main Results: Among 4,216 contacts under 19 years of age, 2,106 contacts (50%) initiated INH preventive therapy at enrollment. The protective effect of INH was more extreme in contacts exposed to drug-sensitive tuberculosis (adjusted hazard ratio, 0.30; 95% confidence interval, 0.18–0.48) and to multidrug-resistant tuberculosis (adjusted hazard ratio, 0.19; 95% confidence interval, 0.05–0.66) compared with those exposed to mono–INH-resistant tuberculosis (adjusted hazard ratio, 0.80; 95% confidence interval, 0.23–2.80). In the second independent study, tuberculosis occurred in none of the 76 household contacts who received INH preventive therapy compared with 3% (8 of 273) of those who did not.
Conclusions: Household contacts who received INH preventive therapy had a lower incidence of tuberculosis disease even when they had been exposed to an index patient with multidrug-resistant tuberculosis. INH may have a role in the management of latent multidrug-resistant tuberculosis infection.
Keywords: tuberculosis, multidrug-resistant tuberculosis, INH, INH preventive therapy
At a Glance Commentary
Scientific Knowledge on the Subject
Few data exist on the efficacy of isoniazid (INH) in preventing tuberculosis progression among people exposed to multidrug-resistant tuberculosis.
What This Study Adds to the Field
We found that INH preventive therapy protected contacts of multidrug-resistant tuberculosis patients from developing tuberculosis disease. Our findings suggest that INH may have a role in the management of multidrug-resistant latent tuberculosis infection.
The World Health Organization (WHO) estimates that there were 10 million new cases of tuberculosis in 2017 and that between one-quarter and one-third of the world’s population has latent tuberculosis infection (LTBI) (1, 2). Although the treatment of LTBI has been shown to prevent tuberculosis disease progression, only a minority of those at risk receive preventive therapy (2). WHO’s recently revised guidelines now recommend testing and treatment of LTBI for an expanded group at risk of tuberculosis disease, including the household contacts of patients with pulmonary tuberculosis (2). Recommended regimens for LTBI include 6–9 months of isoniazid (INH), a 3-month regimen of rifapentine plus INH, 3–4 months of INH and rifampicin, and 3–4 months of rifampicin alone (2).
The recent rise of drug-resistant tuberculosis has complicated the choice of an LTBI regimen. Although several small studies have suggested that regimens tailored to specific drug susceptibility profiles can be effective, most either lacked control arms or compared these regimens to no treatment rather than an alternative regimen (3). WHO concludes that the current lack of evidence on optimal regimens prevents the formulation of definitive recommendations for contacts exposed to drug-resistant tuberculosis (2).
In countries that implement preventive therapy for those at high risk, household contacts of patients with multidrug-resistant (MDR) tuberculosis often receive standard LTBI regimens before the time that the index patient’s drug susceptibility tests are available to the treating clinician. In areas where rapid diagnostic tests for MDR are not yet available, contacts may receive INH for months before the eventual diagnosis of MDR (4, 5). Here, we examined the risk of disease progression of individuals who received INH preventive therapy as part of routine tuberculosis management stratified by the drug resistance profile of the index patient.
Some of the results of these studies have been previously reported in the form of a preprint (https://doi.org/10.1101/479865) (6).
Methods
Setting and Recruitment
This study was conducted in Lima, Peru, in a catchment area of approximately three million residents. We identified and enrolled all patients newly diagnosed with pulmonary tuberculosis and over 15 years of age who presented at 106 district health centers. We confirmed the microbiological status of their pulmonary tuberculosis disease with either a positive sputum smear or mycobacterial culture. We then recruited their household contacts into a prospective cohort study.
Baseline and Follow-up Assessments of Index Patients and Household Contacts
We collected data from index patients on the duration of symptoms before diagnosis, presence of cavitary disease on chest radiography, sputum smear status, and mycobacterial culture results. We performed drug susceptibility testing on isolates from culture-positive patients. We collected the following data from both index patients and household contacts at the time of enrollment: age, height, weight, sex, occupation, history of tuberculosis disease, alcohol use, education, type of housing, frequency of public transportation use, tobacco history, symptoms of tuberculosis, bacillus Calmette-Guérin (BCG) vaccination, recreational drug use, and comorbidities, including HIV and diabetes mellitus. All enrolled household contacts were assessed for the presence of tuberculosis disease and received a tuberculin skin test to determine infection status at baseline, 6-month follow-up, and 12-month follow-up.
INH Preventive Therapy for Household Contacts
The 2006 Peruvian National Tuberculosis Program guidelines recommended that household contacts aged 19 years or younger and adults with specified comorbidities should receive a course of INH preventive therapy (7). Healthcare providers sometimes chose to discontinue INH preventive therapy in household contacts if the index patient was subsequently found to be infected with a strain that was resistant to INH, but many such household contacts received a full course of INH preventive therapy. We used medical records from participating hospitals and health clinics to determine whether household contacts received INH preventive therapy and the duration of their regimen.
Incident Tuberculosis Disease
We identified incident tuberculosis among household contacts during scheduled household visits at 2, 6, and 12 months after enrollment and through a review of tuberculosis registries at the participating health clinics to ensure we obtained all the incident tuberculosis cases among household contacts during the 1-year follow-up. We considered household contacts to have coprevalent tuberculosis it was diagnosed in the contact within 2 weeks of the diagnosis of the index patient and to have secondary tuberculosis otherwise. We defined tuberculosis disease among contacts younger than 18 years of age according to the consensus guidelines for classifying tuberculosis disease in children (8). Paired-end whole genome sequencing using the Illumina HiSeq 4000 platform was performed on isolates from all culture-positive incident tuberculosis cases and their index cases if the index cases were also culture positive.
A detailed description of the study setting, design, study design, outcome definition, and data collection process has been previously reported in the supplementary document of Becerra and colleagues (9). We also provided a brief version of data collection and variable assessments in the online supplement of this article.
Analyses
We restricted the analysis to household contacts under 19 years of age because older contacts received INH preventive therapy only if they had comorbidities that substantially increased their risk of tuberculosis disease. We used a Cox frailty proportional hazards model to evaluate risk factors for incident tuberculosis disease, accounting for clustering within households (10). We first performed a univariate analysis to examine the effect of INH preventive therapy on tuberculosis incidence, followed by a multivariate model that adjusted for the following potential confounders: age, sex, alcohol use, tobacco use, recreational drug use, and employment status of the index patient; age, sex, alcohol use, tobacco use, employment status, use of public transportation, BCG vaccination history, and tuberculosis history of the household contact; and household socioeconomic status, incarceration history, residential district, and education level. We used backward stepwise regression criteria with α level = 0.2 to the multivariate models. To evaluate whether the effect of INH preventive therapy varied by the index patient’s resistance profile, we included the resistance profile and an interaction term for resistance and INH preventive therapy use. Because the spectrum of INH resistance–causing mutations that lead to INH monoresistance may differ from those that lead to MDR tuberculosis, we classified strains as sensitive, mono–INH-resistant, or MDR tuberculosis (resistant to both INH and rifampin). Previous studies have shown that the effectiveness of INH preventive therapy treatment is reduced if the treatment duration is less than 3 months (11). We therefore repeated these analyses by stratifying on the duration of treatment. We conducted two sensitivity analyses. We first restricted the analyses to household contacts less than 6 years old as we considered this age group the most likely to have acquired tuberculosis from the index patient rather than from a community exposure. Second, we restricted the analyses to household contacts who were infected at baseline. We also repeated these analyses in the subset of household contacts exposed to index patients for whom quantitative INH resistance (mean inhibitory concentrations) was available. All the analyses were performed using the R program (12). The institutional review board number of the study cohort is 19332. The institutional review board approved the use of a small proportion of data without additional patient consent.
Analyses of Publicly Available Data
We analyzed publicly available data from a second independent prospective cohort study conducted in Lima, Peru, between 2010 and 2013 by Grandjean and colleagues (13). This study measured incident tuberculosis over 2 years of follow-up in 1,055 household contacts of 213 index patients with MDR tuberculosis and 2,362 household contacts of 487 index patients with drug-susceptible tuberculosis. Drug susceptibility testing for INH and rifampin was performed on isolates from all index patients and secondary cases whose isolates were available using microscopic observation drug susceptibility assays in regional laboratories. Results were confirmed by proportion methods in the Peru National Reference Laboratory (14). INH preventive therapy was reportedly discontinued in this group after MDR tuberculosis index cases were confirmed, but data on the duration of INH preventive therapy were not available. Among the incident cases with drug susceptibility tests results available, 86% of those exposed to MDR tuberculosis also had MDR tuberculosis, and 98% of those exposed to drug-sensitive tuberculosis also had drug-sensitive tuberculosis. We analyzed the data using the approach described above.
Results
We identified 4,500 patients with tuberculosis and 14,839 household contacts. We received consent forms from 14,044 household contacts (94.6%). The retention rates for enrolled household contacts at 12 months of follow-up was 92.0%. Among the enrolled household contacts, 12,767 had been exposed to index patients with microbiologically confirmed tuberculosis. Of these, 4,216 were aged 19 years old or less (Figure E1 in the online supplement); 2,096 (50%) of these received a course of INH preventive therapy. Table 1 shows that the distribution of baseline characteristics did not vary by the index case drug-resistant profiles. Tables 2 and E1–E3 shows the baseline characteristics stratified by INH preventive therapy status. The mean duration of the INH preventive therapy was 115 days among household contacts of patients with MDR tuberculosis compared with 142 days for household contacts of patients with tuberculosis that was resistant to INH alone and 148 days for household contacts exposed to MDR tuberculosis (Figure E2). At 12 months, tuberculosis disease was diagnosed in 146 contacts who were less than 19 years old. Based on the distribution of the number of SNPs identified by whole genome sequencing that differed between the household pairs (Figure E3), we chose a cutoff of 10 SNPs or fewer to identify strains that we assumed had been transmitted from the index patient to the secondary case. Among the 52 secondary cases who were culture positive and for whom whole genome sequencing was therefore available, the isolates of 38 (73%) matched those of the index patients.
Table 1.
Baseline Characteristics of Household Contacts ≤19 Years Old, Stratified by DST Profile of Index Case
| Characteristic | DS Index Cases [n (%)] | INH-R Index Cases [n (%)] | MDR Index Cases [n(%)] | P Value* | |
|---|---|---|---|---|---|
| Age (N = 4,216) | |||||
| 0–5 yr | 1,143 (36) | 134 (35) | 242 (36) | 0.55 | |
| 6–10 yr | 741 (23) | 80 (21) | 150 (23) | 0.55 | |
| 11–15 yr | 703 (22) | 85 (22) | 152 (23) | 0.55 | |
| 16–19 yr | 577 (18) | 87 (23) | 122 (18) | 0.55 | |
| Sex (N = 4,216) | |||||
| F | 1,592 (50) | 191 (49) | 337 (51) | 0.94 | |
| M | 1,572 (50) | 195 (51) | 329 (49) | 0.94 | |
| HIV seropositive (n = 4,164) | |||||
| No | 3,124 (100) | 378 (100) | 658 (100) | 0.52 | |
| Yes | 4 (0) | 0 (0) | 0 (0) | 0.52 | |
| Diabetes mellitus (n = 4,202) | |||||
| No | 3,156 (100) | 381 (100) | 661 (100) | 0.07 | |
| Yes | 1 (0) | 1 (0) | 2 (0) | 0.07 | |
| BCG scars (N = 4,216) | |||||
| 0 | 593 (19) | 90 (23) | 141 (21) | 0.05 | |
| >1 | 2,571 (81) | 296 (77) | 525 (79) | 0.05 | |
| Smoking status (n = 4,209) | |||||
| None or light smoking | 3,139 (99) | 382 (99) | 663 (100) | 0.84 | |
| Heavy smoking | 20 (1) | 2 (1) | 3 (0) | 0.84 | |
| Alcohol use (n = 4,195) | |||||
| None or light drinker | 3,112 (99) | 375 (98) | 653 (99) | 0.37 | |
| Heavy drinker | 39 (1) | 8 (2) | 8 (1) | 0.37 | |
| Nutritional status† (n = 4,173) | |||||
| Normal weight | 2,568 (82) | 316 (83) | 545 (83) | 0.98 | |
| Underweight | 77 (2) | 10 (3) | 16 (2) | 0.98 | |
| Overweight | 487 (16) | 57 (15) | 97 (15) | 0.98 | |
| Use of public transportation (n = 4,120) | |||||
| Nonuser | 1,159 (37) | 135 (36) | 237 (37) | 0.34 | |
| 1–3 d/wk | 994 (32) | 137 (37) | 218 (34) | 0.34 | |
| 4–7 d/wk | 952 (31) | 100 (27) | 188 (29) | 0.34 | |
| Socioeconomic status‡ (n = 4,128) | |||||
| Low | 1,210 (39) | 144 (39) | 268 (40) | 0.85 | |
| Middle | 1,369 (44) | 166 (45) | 283 (43) | 0.85 | |
| High | 520 (17) | 55 (15) | 113 (17) | 0.85 | |
| TB infected at baseline (n = 4,068) | |||||
| No | 2,214 (72) | 256 (68) | 441 (69) | 0.09 | |
| Yes | 842 (28) | 118 (32) | 197 (31) | 0.09 | |
| TB history (N = 4,216) | |||||
| No | 3,102 (98) | 375 (97) | 651 (98) | 0.49 | |
| Yes | 62 (2) | 11 (3) | 15 (2) | 0.49 | |
| Employment (n = 4,214) | |||||
| No | 2,917 (92) | 351 (91) | 606 (91) | 0.42 | |
| Yes | 245 (8) | 35 (9) | 60 (9) | 0.42 | |
| Being a student (n = 4,214) | |||||
| No | 1,137 (36) | 141 (37) | 258 (39) | 0.4 | |
| Yes | 2,025 (64) | 245 (63) | 408 (61) | 0.4 | |
| Index case age (N = 4,216) | |||||
| 16–30 yr | 1,857 (59) | 204 (53) | 400 (60) | 0.02 | |
| 31–45 yr | 746 (24) | 118 (31) | 154 (23) | 0.02 | |
| 46–60 yr | 297 (9) | 40 (10) | 70 (11) | 0.02 | |
| >60 yr | 264 (8) | 24 (6) | 42 (6) | 0.02 | |
| Index case sex (n = 4,126) | |||||
| F | 1,437 (45) | 135 (35) | 288 (43) | <0.01 | |
| M | 1,727 (55) | 251 (65) | 378 (57) | <0.01 | |
| Index case smoking status (n = 4,125) | |||||
| None or light smoker | 3,074 (99) | 363 (96) | 621 (97) | <0.01 | |
| Heavy smoker | 36 (1) | 14 (4) | 17 (3) | <0.01 | |
| Index case drinking status (n = 4,053) | |||||
| None or light drinker | 2,720 (90) | 330 (87) | 581 (91) | 0.21 | |
| Heavy drinker | 315 (10) | 48 (13) | 59 (9) | 0.21 | |
| Index case employment (n = 4,200) | |||||
| No | 2,104 (67) | 233 (61) | 459 (69) | 0.02 | |
| Yes | 1,046 (33) | 152 (39) | 206 (31) | 0.02 | |
| Index case marijuana use (n = 4,206) | |||||
| No | 2,760 (87) | 327 (85) | 573 (87) | 0.33 | |
| Yes | 399 (13) | 59 (15) | 88 (13) | 0.33 | |
| Index case cocaine use (n = 4,206) | |||||
| No | 2,643 (84) | 321 (83) | 562 (85) | 0.71 | |
| Yes | 516 (16) | 64 (17) | 100 (15) | 0.71 | |
| Household incarceration history (N = 4,216) | |||||
| No | 2,854 (90) | 359 (93) | 584 (88) | 0.02 | |
| Yes | 310 (10) | 27 (7) | 82 (12) | 0.02 | |
| Household education (N = 4,216) | |||||
| Low | 663 (21) | 77 (20) | 133 (20) | <0.01 | |
| Medium | 1,814 (57) | 191 (49) | 402 (60) | <0.01 | |
| High | 687 (22) | 118 (31) | 131 (20) | <0.01 | |
| Household district (N = 4,216) | |||||
| Cercado de Lima | 276 (9) | 46 (12) | 51 (8) | <0.01 | |
| Comas | 214 (7) | 20 (5) | 13 (2) | <0.01 | |
| El Agustino | 229 (7) | 18 (5) | 99 (15) | <0.01 | |
| La Victoria | 346 (11) | 24 (6) | 81 (12) | <0.01 | |
| Los Olivos | 332 (10) | 39 (10) | 79 (12) | <0.01 | |
| Rimac | 310 (10) | 60 (16) | 37 (6) | <0.01 | |
| San Martin de Porres | 713 (23) | 97 (25) | 168 (25) | <0.01 | |
| Santa Anita | 186 (6) | 7 (2) | 28 (4) | <0.01 | |
| Others | 558 (18) | 75 (19) | 110 (17) | <0.01 |
Definition of abbreviations: BCG = bacillus Calmette-Guérin; DS = drug-sensitive; DST = drug sensitivity test; INH-R = isoniazid-resistant; MDR = multidrug-resistant; TB = tuberculosis.
Compared the two groups using a χ2 test.
Nutritional status was defined by the World Health Organization body mass index z-score tables.
Socioeconomic status was defined using a principal component analysis based on housing quality, water supply, and sanitation.
Table 2.
Baseline Characteristics of Household Contacts ≤19 Years Old, Stratified by Isoniazid Preventive Therapy
| Characteristic | No INH Preventive Therapy [n(%)] | INH Preventive Therapy [n(%)] | P Value* | ||
|---|---|---|---|---|---|
| Age (N = 4,216) | |||||
| 0–5 yr | 664 (31) |
855 (41) |
<0.01 | ||
| 6–10 yr | 439 (21) |
532 (25) |
<0.01 | ||
| 11–15 yr | 489 (23) |
451 (22) |
<0.01 | ||
| 16–19 yr | 528 (25) |
258 (12) |
<0.01 | ||
| Sex (N = 4,216) | |||||
| F | 1,087 (51) |
1,033 (49) |
0.21 | ||
| M | 1,033 (49) |
1,063 (51) |
0.21 | ||
| HIV seropositive (n = 4,164) | |||||
| No | 2,086 (100) |
2,074 (100) |
0.14 | ||
| Yes | 4 (0) |
0 (0) |
— | ||
| Diabetes mellitus (n = 4,202) | |||||
| No | 2,111 (100) |
2,087 (100) |
0.99 | ||
| Yes | 2 (0) |
2 (0) |
— | ||
| BCG scars (N = 4,216) | |||||
| 0 | 423 | 20% | 401 | 19% | 0.39 |
| >1 | 1,697 | 80% | 1,695 | 81% | — |
| Smoking status (n = 4,209) | |||||
| ≤1 cigarette/d | 2,093 (99) |
2,091 (100) |
<0.01 | ||
| >1 cigarette/d | 22 (1) |
3 (0) |
— | ||
| Alcohol use (n = 4,195) | |||||
| <3 drinks/d | 2,061 (98) |
2,079 (99) |
<0.01 | ||
| ≥3 drinks/d | 44 (2) |
11 (1) |
— | ||
| Nutritional status† (n = 4,173) | |||||
| Normal weight | 1,748 (83) |
1,681 (81) |
0.12 | ||
| Underweight | 44 (2) |
59 (3) |
— | ||
| Overweight | 308 (15) |
333 (16) |
— | ||
| Use of public transportation (n = 4,120) | |||||
| Nonuser | 736 (35) |
795 (39) |
0.02 | ||
| 1–3 d/wk | 709 (34) |
640 (32) |
0.02 | ||
| 4–7 d/wk | 652 (31) |
588 (29) |
0.02 | ||
| Socioeconomic status‡ (n = 4,128) | |||||
| Low | 821 (40) |
801 (39) |
0.20 | ||
| Middle | 931 (45) |
887 (43) |
0.20 | ||
| High | 325 (16) |
363 (18) |
0.20 | ||
| TB infected at baseline (n = 4,068) | |||||
| No | 1,417 (70) |
1,494 (73) |
0.01 | ||
| Yes | 613 (30) |
544 (27) |
0.01 | ||
| TB history (N = 4,216) | |||||
| No | 2,042 (96) |
2,086 (100) |
<0.01 | ||
| Yes | 78 (4) |
10 (0) |
<0.01 | ||
| Employment (n = 4,214) | |||||
| No | 1,893 (89) |
1,981 (95) |
<0.01 | ||
| Yes | 226 (11) |
114 (5) |
<0.01 | ||
| Being a student (n = 4,214) | |||||
| No | 809 (38) |
727 (35) |
0.02 | ||
| Yes | 1,311 (62) |
1,367 (65) |
0.02 | ||
| Index case age (N = 4,216) | |||||
| 16–30 yr | 1,264 (60) |
1,197 (57) |
<0.01 | ||
| 31–45 yr | 438 (21) |
580 (28) |
<0.01 | ||
| 46–60 yr | 252 (12) |
155 (7) |
<0.01 | ||
| >60 yr | 166 (8) |
164 (8) |
<0.01 | ||
| Index case sex (n = 4,126) | |||||
| F | 836 (39) |
1,024 (49) |
<0.01 | ||
| M | 1,284 (61) |
1,072 (51) |
<0.01 | ||
| Index case smoking status (n = 4,125) | |||||
| None or light smoker | 2,037 (99) |
2,021 (98) |
0.45 | ||
| Heavy smoker | 30 (1) |
37 (2) |
0.45 | ||
| Index case drinking status (n = 4,053) | |||||
| None or light drinker | 1,798 (89) |
1,833 (90) |
0.25 | ||
| Heavy drinker | 222 (11) |
200 (10) |
0.25 | ||
| Index case employment (n = 4,200) | |||||
| No | 1,412 (67) |
1,384 (66) |
0.62 | ||
| Yes | 697 (33) |
707 (34) |
0.62 | ||
| Index case INH profile (N = 4,216) | |||||
| Sensitive | 1,534 (72) |
1,630 (78) |
<0.01 | ||
| Monoresistant | 185 (9) |
201 (10) |
<0.01 | ||
| MDR | 401 (19) |
265 (13) |
<0.01 | ||
| Index case marijuana use (n = 4,206) | |||||
| No | 1,774 (84) |
1,811 (90) |
<0.01 | ||
| Yes | 336 (16) |
284 (10) |
<0.01 | ||
| Index case cocaine use (n = 4,206) | |||||
| No | 1,715 (81) |
1,811 (86) |
<0.01 | ||
| Yes | 396 (19) |
284 (14) |
<0.01 | ||
| Household incarceration history (N = 4,216) | |||||
| No | 1,863 (88) |
1,943 (92) |
<0.01 | ||
| Yes | 257 (12) |
162 (8) |
<0.01 | ||
| Household education (N = 4,216) | |||||
| Low | 900 (42) |
1,384 (34) |
<0.01 | ||
| Medium | 801 (38) |
707 (41) |
<0.01 | ||
| High | 419 (20) |
1,384 (25) |
<0.01 | ||
| Household district (N = 4,216) | |||||
| Cercado de Lima | 238 (11) |
135 (6) |
0.62 | ||
| Comas | 112 (5) |
135 (6) |
0.62 | ||
| El Agustino | 294 (14) |
52 (2) |
0.62 | ||
| La Victoria | 273 (13) |
178 (8) |
0.62 | ||
| Los Olivos | 212 (10) |
238 (11) |
0.62 | ||
| Rimac | 84 (4) |
323 (15) |
0.62 | ||
| San Martin de Porres | 373 (18) |
605 (29) |
0.62 | ||
| Santa Anita | 138 (7) |
83 (4) |
0.62 | ||
| Others | 396 (19) | 347 (17) | 0.62 | ||
Definition of abbreviations: BCG = bacillus Calmette-Guérin; INH = isoniazid; MDR = multidrug resistant; TB = tuberculosis.
Compared the two groups using a χ2 test.
Nutritional status was defined by the World Health Organization body mass index z-score tables.
Socioeconomic status was defined using a principal component analysis based on housing quality, water supply, and sanitation.
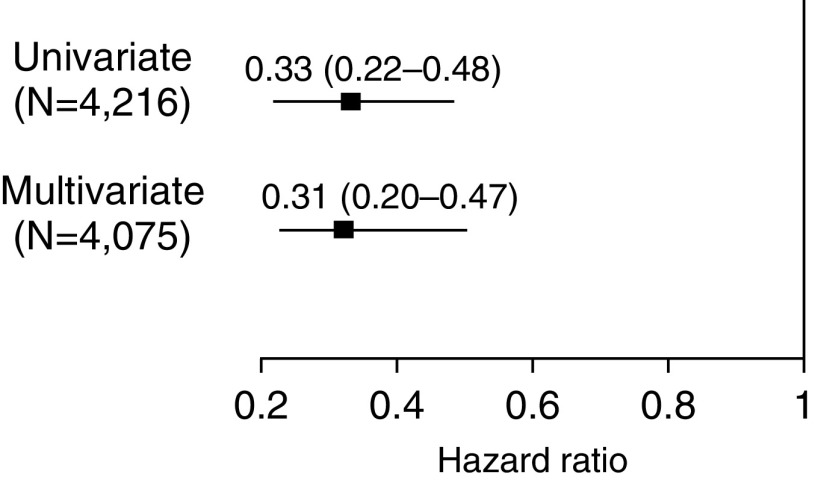
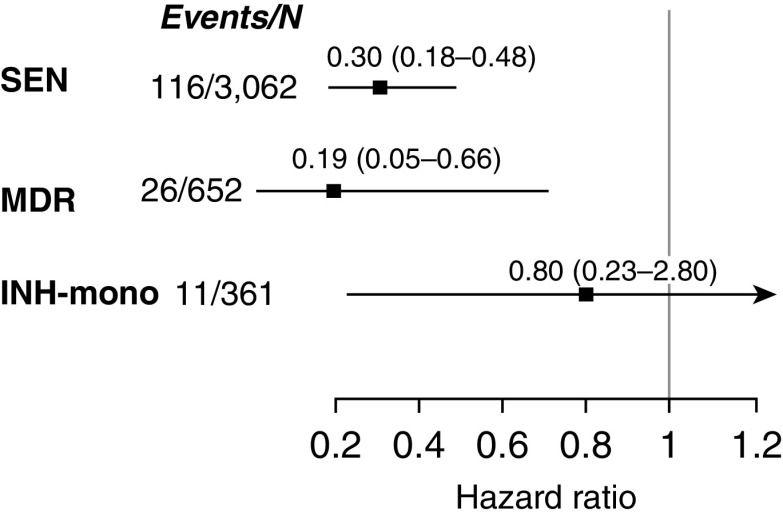
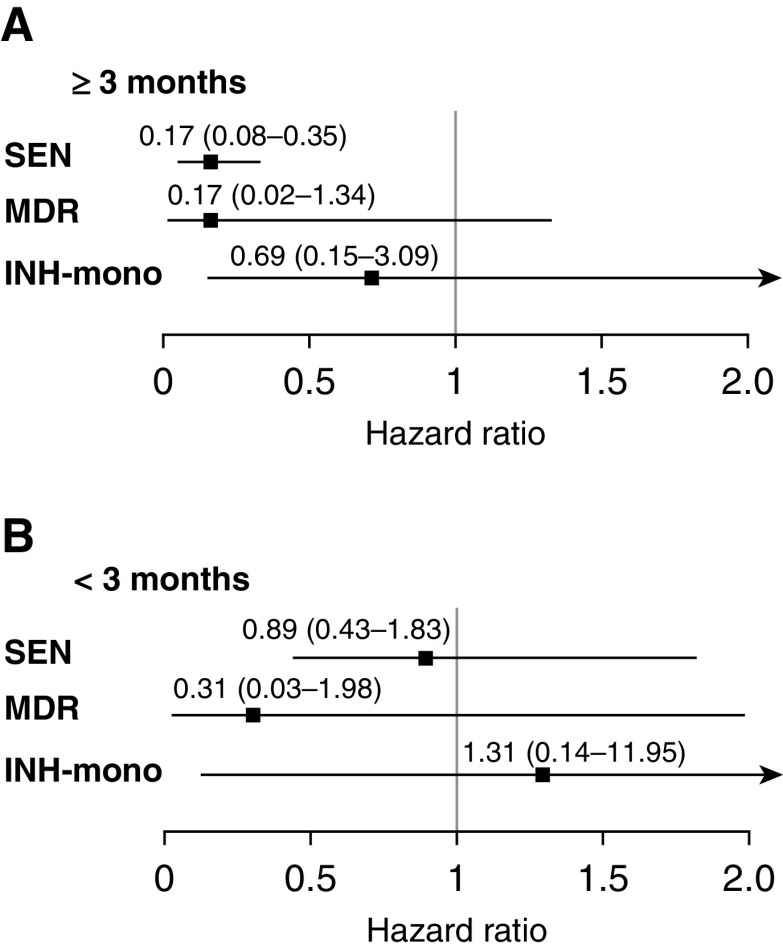
Compared with those who did not receive treatment, household contacts who received INH preventive therapy were one-third as likely to have tuberculosis disease diagnosed in both the univariate and multivariate models (Figure 1) (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.22–0.48; adjusted HR, 0.31; 95% CI, 0.20–0.47) (Table E4). Figure 2 and Table E5 show that INH was more effective in household contacts exposed to drug-sensitive or MDR tuberculosis than in those exposed to strains resistant to INH alone (INH preventive therapy vs. no INH preventive therapy adjusted HR, 0.30; 95% CI, 0.18–0.48 in subgroup with INH-sensitive tuberculosis; adjusted HR, 0.19; 95% CI, 0.05–0.66 in subgroup with MDR tuberculosis; adjusted HR, 0.80; 95% CI, 0.23–2.80 in subgroup with mono–INH-resistant tuberculosis). INH efficacy increased with the duration of therapy across all three resistance categories (Figure 3 and Table E5). None of the participants who were 5 years old or younger who received more than 3 months treatment developed tuberculosis disease during follow-up (Tables 3–5 ). When we restricted the analyses to a subcohort who were infected at baseline, the protective effect of INH preventive therapy on the contacts of patients with MDR tuberculosis remained strong (adjusted HR, 0.14; 95% CI, 0.02–1.07) (Table E6). Among 1,276 household contacts for whom index patient minimal inhibitory concentrations (MICs) were available, the effectiveness of INH preventive therapy did not vary by INH MIC; among 92 household contacts who received INH preventive therapy after being exposed to an index patient with an MIC of greater than 5 μg/ml, none developed (0 of 92) active tuberculosis, whereas 4% (14 of 368) of those who did not receive INH preventive therapy developed the disease.
Figure 1.
Effect of isoniazid preventive therapy on disease incidence of household contacts ≤19 years of age. Multivariate model adjusted for index case age, recreational drug use, household contact age, sex, bacillus Calmette-Guérin vaccination scar, nutritional status, being a student or not, tuberculosis history, household socioeconomic status, and household residential district.
Figure 2.
The effect of isoniazid preventive therapy on tuberculosis incidence in household contacts 19 years old or younger by the isoniazid resistance status of index patient adjusted for index case age, recreational drug use, household contact age, sex, bacillus Calmette-Guérin vaccination scar, nutritional status, being a student or not, tuberculosis history, household socioeconomic status, and household residential district. INH-mono = mono–isoniazid resistant; MDR = multidrug resistant; SEN = sensitive.
Figure 3.
(A and B) The effect of ≥3 months (A) or <3 months (B) of isoniazid preventive therapy on tuberculosis incidence in household contacts 19 years old or younger by the isoniazid resistance status of the index patient adjusted for index case age, recreational drug use, household contact age, sex, bacillus Calmette-Guérin vaccination scar, nutritional status, being a student or not, tuberculosis history, household socioeconomic status, and household residential district. For definition of abbreviations, see Figure 2.
Table 3.
The Effect of Isoniazid Preventive Therapy on Disease Incidence of Children 5 Years of Age or Younger (Complete Data Set)*
| Isoniazid Preventive Therapy | Isoniazid Sensitive |
MDR |
Mono–Isoniazid Resistant |
|||
|---|---|---|---|---|---|---|
| Cases/Person-Year | HR (95% CI) | Cases/Person-Year | HR (95% CI) | Cases/Person-Year | HR (95% CI) | |
| No | 19/566 | Ref | 10/145 | Ref | 3/58 | Ref |
| Yes | 9/785 | 0.28 (0.12–0.58) | 2/144 | 0.19 (0.04–0.98) | 1/90 | 0.25 (0.02–2.76) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; INH = isoniazid; MDR = multidrug resistant; Ref = reference.
Stratified by INH profiles of index cases and adjusted for index case age, recreational drug use, household contact age, sex, bacillus Calmette-Guérin vaccination scar, nutritional status, being a student or not, tuberculosis history, household socioeconomic status, and household residential district
Likelihood ratio test for interaction term = 0.413.
Table 5.
The Effect of Isoniazid Preventive Therapy on Disease Incidence of Children 5 Years of Age or Younger (Household Contacts Who Received Isoniazid Preventive Therapy <3 Months)*
| Isoniazid Preventive Therapy | Isoniazid Sensitive |
MDR |
Mono–Isoniazid Resistant |
|||
|---|---|---|---|---|---|---|
| Cases/Person-Year | HR (95% CI) | Cases/Person-Year | HR (95% CI) | Cases/Person-Year | HR (95% CI) | |
| No | 19/566 | Ref | 10/145 | Ref | 3/58 | Ref |
| Yes | 10/273 | 1.49 (0.5–4.44) | 1/77 | 0.38 (0.04–3.46) | 1/42 | 2.04 (0.14–29.64) |
For definition of abbreviations, see Table 3.
Stratified by INH profiles of index cases and adjusted for index case age, recreational drug use, household contact age, sex, bacillus Calmette-Guérin vaccination scar, nutritional status, being a student or not, tuberculosis history, household socioeconomic status, and household residential district
Likelihood ratio test for interaction term = 0.158.
Table 4.
The Effect of Isoniazid Preventive Therapy on Disease Incidence of Children 5 Years of Age or Younger (Household Contacts Who Received Isoniazid Preventive Therapy ≥3 Months)*
| Isoniazid Preventive Therapy | Isoniazid Sensitive |
MDR |
Mono–Isoniazid Resistant |
|||
|---|---|---|---|---|---|---|
| Cases/Person-Year | HR (95% CI) | Cases/Person-Year | HR (95% CI) | Cases/Person-Year | HR (95% CI) | |
| No | 19/566 | Ref | 10/145 | Ref | 3/58 | Ref |
| Yes | 1/470 | 0.06 (0.01 to 0.43) | 0/54 | 0 (0 to infinity) | 0/64 | 0 (0 to infinity) |
For definition of abbreviations, see Table 3.
Stratified by INH profiles of index cases and adjusted for index case age, recreational drug use, household contact age, sex, bacillus Calmette-Guérin vaccination scar, nutritional status, being a student or not, tuberculosis history, household socioeconomic status, and household residential district
Likelihood ratio test for interaction term = 0.768.
Second Independent Data Set
The previously reported cohort described above included 1,121 household contacts ≤19 years age whose INH preventive therapy status was known. INH preventive therapy use was associated with reduced rates of incident tuberculosis in both univariate and analyses that adjusted for age, socioeconomic status, and tuberculosis history (HR, 0.1; 95% CI, 0.03–0.30; adjusted HR, 0.11; 95% CI, 0.02–0.49). INH preventive therapy not only protected household contacts of index patients with drug-sensitive tuberculosis (adjusted HR, 0.13; 95% CI, 0.03–0.57), but none of 76 household contacts of index patients with MDR tuberculosis who received INH preventive therapy developed tuberculosis compared with eight of 273 (3%) household contacts without INH preventive therapy.
Discussion
Here, we found that INH preventive therapy use is associated with reduced rates of tuberculosis disease among household contacts of patients with tuberculosis even when the index patients were infected with INH-resistant and MDR strains of tuberculosis. Notably, INH effectiveness was higher among household contacts of MDR tuberculosis than among people exposed to strains resistant to INH alone. INH effectiveness increased with the duration of therapy regardless of the tuberculosis resistance profile of the index patient. Among those less than 5 years of age (the group most likely to have been infected by the index patient), none of the children who received at least 3 months of INH preventive therapy developed tuberculosis disease. We found that the effectiveness of INH preventive therapy was not associated with the INH MIC of the index patient’s tuberculosis strain; no household contact who was exposed to an index patient with an INH MIC greater than 5 μg/ml developed disease.
Few data exist on the effectiveness of INH in preventing tuberculosis progression among people exposed to drug-resistant tuberculosis (Table E7). In a study from Brazil, investigators reported that among 190 MDR tuberculosis–exposed contacts, disease occurred in two of 45 (4%) who received INH preventive therapy and in 13 of 145 (9%) who did not (15). A similar study from Israel reported no cases over 6 years of follow-up among 71 MDR tuberculosis–exposed contacts who received INH preventive therapy (16). A study in South African children found that those who received no preventive therapy were four times more likely to develop tuberculosis disease than those who received an individualized regimen that included high-dose INH, but the study could draw no conclusions about the efficacy of INH alone because the regimens were tailored to the drug susceptibility profile of the index strain (17). Another study in South African children found no cases over 1 year of follow-up among 21 MDR tuberculosis–exposed children who received ofloxacin, ethambutol, and high-dose INH (18). An Australian study compared tailored preventive regimens to either INH preventive therapy or no treatment among MDR tuberculosis–exposed contacts (19). Two contacts in the INH preventive therapy/no treatment arm developed tuberculosis disease within 54 months, but the study did not specify whether the two incident patients received INH preventive therapy or not. Finally, a study conducted in Beijing followed students during an MDR TB outbreak and found two cases among five INH preventive therapy recipients and four cases among 16 INH preventive therapy nonrecipients over 6 months of follow-up (20). Other studies that reported on regimens that included INH among contacts of patients with MDR/drug-resistant tuberculosis lacked control arms (21, 22).
We considered possible explanations for the observed effectiveness of INH preventive therapy among contacts of patients with INH-resistant tuberculosis. It is possible that household contacts were not infected by the INH-resistant disease of their index patient but instead acquired a drug-sensitive infection from an unknown contact in the community. The finding that the majority of the household contacts who developed tuberculosis in both studies either harbored strains that were almost genetically identical or shared the same drug susceptibility tests profiles with their index case argues against this explanation, as does our finding that the protective effect of INH preventive therapy was more marked in those younger than 5 years old, whom we considered much less likely than older contacts to have been infected by someone other than the index case. We also considered the possibility that INH preventive therapy use might be confounded by socioeconomic status in these observational studies. Although we have tried to adjust for possible confounding, we still cannot rule out the possibility of residual confounding. We note, however, that because the distributions of these variables were very similar between household contacts exposed to drug-sensitive tuberculosis and MDR tuberculosis, any residual confounding would be expected to have a similar impact in the drug-sensitive and MDR tuberculosis–exposed household contacts. Therefore, our findings should be robust even if there was some residual confounding by socioeconomic status. Furthermore, the reduced efficacy of INH preventive therapy among people who received less than 1 month of treatment is within the range reported in a seminal randomized trial, again suggesting that residual confounding is unlikely to explain our findings (13).
Finally, we considered the possibility that INH might be effective against LTBI even when the relevant strains are found to be resistant to INH in media-based growth assays. This raises the possibility that the mechanism by which INH reduces tuberculosis risk among those with LTBI may differ from its mechanism in tuberculosis disease. INH is known to be a prodrug that is converted to its active metabolite, an INH–nicotinamide adenine dinucleotide (NAD) adduct, by a Mycobacterium tuberculosis (MTB) catalase peroxidase encoded by the KatG gene (23). The INH–NAD adduct then binds to InhA (an enoyl-acyl carrier protein reductase) and inhibits the synthesis of essential mycolic acids in MTB cell walls. Mutations in KatG that reduce the activity of the catalase peroxidase, block the conversion of INH to its active form, and result in INH resistance. Several studies have raised the possibility that the conversion of INH to its active form may occur independently of the mycobacterial catalyst peroxidase. One group found that the presence of copper increased the INH sensitivity of an otherwise INH-resistant strain, suggesting that the interaction of INH and copper ions may facilitate the conversion of INH to its active form (23, 24). Two recent studies showed that eosinophil- or neutrophil-derived myeloperoxidase was able to produce the INH–NAD adduct (25, 26). Another research study identified the metabolites of oxidized INH–NAD adducts in the urine of people who were not infected with MTB, thereby raising the possibility that INH can be activated by host enzymes (27). Other studies have suggested that INH may employ nonspecific antibacterial mechanisms against MTB in addition to impacting mycolic acid synthesis. For example, INH is a strong ligand for iron, copper, and zinc, and it might be involved in metal ion uptake by MTB, which could disrupt metal homeostasis and inhibit MTB growth (27–31). Other investigators have posited a role for a host immunomodulation of INH (32–34). In one study, investigators examined the impact of INH on cultured human promyelocytic leukemia (HL-60) cells as a model for human phagocytes and found that it protected them from MTB-induced oxidative stress–mediated necrosis (33). In another study, INH was found to induce the differentiation of proinflammatory monocytes in HL-60 cells. The investigators speculate that INH works by bolstering the proinflammatory response in monocytes in granulomas rather than through a direct bactericidal effect (34). None of these hypotheses directly address the question of why INH fails to cure active tuberculosis disease in patients with INH-resistant strains. It is possible that these mechanisms clear MTB in the early stage of infection when the MTB is restricted to the granuloma and bacterial load is low but are less effective when the MTB is released outside the granuloma and the bacterial load is much higher.
We also found that the protective effect of INH differs in contacts exposed to MDR tuberculosis strains compared with mono–INH-resistant strains. Given the small number of patients with INH resistance alone, it is possible that this difference is the result of statistical imprecision. However, previous studies have shown that the distribution of the INH resistance–causing mutation differs between MDR and mono–INH-resistant strains, with mono–INH-resistant strains being more likely than MDR strains to harbor InhA promoter mutations and less likely to have KatG mutations (35). Because InhA is the downstream target of the INH–NAD adduct, it is possible that mono–INH-resistant strains remain resistant to INH regardless of whether INH conversion goes through an MTB-dependent or independent pathway.
Our study has some limitations. Like any observational study, it is possible that unmeasured factors associated with both tuberculosis susceptibility and INH preventive therapy use have created the appearance of an association that is not causal. The contacts of MDR tuberculosis cases also received INH for a shorter period of time than contacts of pan-sensitive or mono–INH-resistant cases, presumably because clinicians halted INH preventive therapy once the index patients’ MDR tuberculosis status was confirmed. Given the dose effect we observed, we would expect to see an even more extreme effect of INH preventive therapy if contacts of patients with MDR tuberculosis had received the same duration of INH preventive therapy as those exposed to drug-sensitive strains. Furthermore, we were unable to assess the effect of INH preventive therapy on adult contacts of patients with MDR tuberculosis given that INH preventive therapy is not indicated for adult contacts without comorbidities in Peru. Finally, almost all household contacts in our cohort were HIV-negative, so we were not able to evaluate the synergistic effect between INH preventive therapy and highly active antiretroviral therapy in HIV-positive household contacts exposed to MDR tuberculosis.
In conclusion, we found that INH preventive therapy protected against tuberculosis among contacts of patients with MDR tuberculosis. Given the safety profile of INH and its wide use across the globe, INH may have a role in the management of MDR LTBI.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the patients and their families who gave their time and energy to contribute to this study, the National Tuberculosis Program at the Peruvian Ministry of Health, and the healthcare personnel at the 106 participating health centers in Lima, Peru.
Footnotes
Supported by the NIH and the National Institute of Allergy and Infectious Diseases grants U01AI057786, U19AI076217, U19AI109755 (Center for Excellence in Translational Research), and U19AI111224 (Tuberculosis Research Unit).
Author Contributions: M.C.B. and M.M. led the study design. L.L. oversaw data collection and management with R.C., C.C., J.G., R.Y., and Z.Z. R.C. managed laboratory efforts. L.G. conducted the second independent cohort study. M.M. supervised data analysis and interpretation in conjunction with C.-C.H. C.-C.H. and M.M. wrote the first draft of the manuscript, and all authors contributed to manuscript revision.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201908-1576OC on June 17, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Geneva: 2019. Global tuberculosis report 2019. [accessed 2019 Apr 1]. Available from: http://www.who.int/tb/publications/factsheet_global.pdf?ua=1. [Google Scholar]
- 2.World Health Organization. Geneva: 2018. Latent tuberculosis infection, updated and consolidated guidelines for programmatic management. [accessed 2018 Dec 1]. Available from: http://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/ [PubMed] [Google Scholar]
- 3.Fox GJ, Dobler CC, Marais BJ, Denholm JT. Preventive therapy for latent tuberculosis infection-the promise and the challenges. Int J Infect Dis. 2017;56:68–76. doi: 10.1016/j.ijid.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson KR, Theron D, Kendall EA, Franke MF, Barnard M, van Helden PD, et al. Implementation of genotype MTBDRplus reduces time to multidrug-resistant tuberculosis therapy initiation in South Africa. Clin Infect Dis. 2013;56:503–508. doi: 10.1093/cid/cis920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C-C, Becerra MC, Calderon R, Contreras C, Jerome G, Grandjean L, et al. Isoniazid preventive therapy protects against tuberculosis among household contacts of isoniazid-resistant patients [preprint]; bioRxiv; 2018. 2019. [accessed 2020 Sept 1]. Available from: https://www.biorxiv.org/content/10.1101/479865v1. [Google Scholar]
- 7.Ministerio de Salud. Lima: 2006. Norma técnica de salud para el control de la tuberculosis. [accessed 2018 Oct 10]. Available from: ftp://ftp2.minsa.gob.pe/descargas/dgsp/ESN-tuberculosis/normaspublicaciones/NTSTBC.pdf. [Google Scholar]
- 8.Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease: consensus from an expert panel. J Infect Dis. 2012;205:S199–S208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becerra CM, Huang C-C, Lecca L, Bayona J, Contreras C, Calderon R, et al. Transmissibility and potential for disease progression of drug resistant Mycobacterium tuberculosis: prospective cohort study. BMJ. 2019;24:l5894. doi: 10.1136/bmj.l5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Quigley J, Stare J. Proportional hazards models with frailties and random effects. Stat Med. 2002;21:3219–3233. doi: 10.1002/sim.1259. [DOI] [PubMed] [Google Scholar]
- 11.International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the UAT trial. Bull World Health Organ. 1982;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- 12.R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017. [accessed 2018 Mar 4] Available from: https://www.R-project.org/ [Google Scholar]
- 13.Grandjean L, Gilman RH, Martin L, Soto E, Castro B, Lopez S, et al. Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: a prospective cohort study. PLoS Med. 2015;12:e1001843. doi: 10.1371/journal.pmed.1001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caviedes L, Lee TS, Gilman RH, Sheen P, Spellman E, Lee EH, et al. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures: the Tuberculosis Working Group in Peru. J Clin Microbiol. 2000;38:1203–1208. doi: 10.1128/jcm.38.3.1203-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kritski AL, Marques MJ, Rabahi MF, Vieira MA, Werneck-Barroso E, Carvalho CE, et al. Transmission of tuberculosis to close contacts of patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 1996;153:331–335. doi: 10.1164/ajrccm.153.1.8542139. [DOI] [PubMed] [Google Scholar]
- 16.Attamna A, Chemtob D, Attamna S, Fraser A, Rorman E, Paul M, et al. Risk of tuberculosis in close contacts of patients with multidrug resistant tuberculosis: a nationwide cohort. Thorax. 2009;64:271. doi: 10.1136/thx.2008.100974. [DOI] [PubMed] [Google Scholar]
- 17.Schaaf HS, Gie RP, Kennedy M, Beyers N, Hesseling PB, Donald PR. Evaluation of young children in contact with adult multidrug-resistant pulmonary tuberculosis: a 30-month follow-up. Pediatrics. 2002;109:765–771. doi: 10.1542/peds.109.5.765. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Prats AJ, Zimri K, Mramba Z, Schaaf HS, Hesseling AC. Children exposed to multidrug-resistant tuberculosis at a home-based day care centre: a contact investigation. Int J Tuberc Lung Dis. 2014;18:1292–1298. doi: 10.5588/ijtld.13.0872. [DOI] [PubMed] [Google Scholar]
- 19.Denholm JT, Leslie DE, Jenkin GA, Darby J, Johnson PD, Graham SM, et al. Long-term follow-up of contacts exposed to multidrug-resistant tuberculosis in Victoria, Australia, 1995 -2010. Int J Tuberc Lung Dis. 2012;16:1320–1325. doi: 10.5588/ijtld.12.0092. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Pang Y, Song Y, Dong W, Zhang T, Wen S, et al. Implications of a school outbreak of multidrug-resistant tuberculosis in Northern China. Epidemiol Infect. 2018;145:584–588. doi: 10.1017/S0950268817003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seddon JA, Hesseling AC, Finlayson H, Fielding K, Cox H, Hughes J, et al. Preventive therapy for child contacts of multidrug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis. 2013;57:1676–1684. doi: 10.1093/cid/cit655. [DOI] [PubMed] [Google Scholar]
- 22.Tochon M, Bosdure E, Salles M, Beloncle C, Chadelat K, Dagorne M, et al. Management of young children in contact with an adult with drug-resistant tuberculosis, France, 2004-2008. Int J Tuberc Lung Dis. 2011;15:326–330. [PubMed] [Google Scholar]
- 23.Bernardes-Génisson V, Deraeve C, Chollet A, Bernadou J, Pratviel G. isoniazid: an update on the multiple mechanisms for a singular action. Curr Med Chem. 2013;20:4370–4385. doi: 10.2174/15672050113109990203. [DOI] [PubMed] [Google Scholar]
- 24.Youatt J. The influence of copper on the uptake of hydrazides. Aust J Exp Biol. 1962;40:201–206. doi: 10.1038/icb.1962.23. [DOI] [PubMed] [Google Scholar]
- 25.Khan SR, Morgan AG, Michail K, Srivastava N, Whittal RM, Aljuhani N, et al. Metabolism of isoniazid by neutrophil myeloperoxidase leads to INH‐NAD+ adduct formation: a comparison of the reactivity of isoniazid with its known human metabolites. Biochem Pharmacol. 2016;106:46–55. doi: 10.1016/j.bcp.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Babu D, Morgan AG, Reiz B, Whittal RM, Almas S, Lacy P, et al. Eosinophil peroxidase oxidizes isoniazid to form the active metabolite against M. tuberculosis, isoniazid‐NAD+ Chem Biol Interact. 2019;305:48–53. doi: 10.1016/j.cbi.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Mahapatra S, Woolhiser LK, Lenaerts AJ, Johnson JL, Eisenach KD, Joloba ML, et al. A novel metabolite of antituberculosis therapy demonstrates host activation of isoniazid and formation of the isoniazid-NAD+ adduct. Antimicrob Agents Chemother. 2012;56:28–35. doi: 10.1128/AAC.05486-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez GM. Control of iron metabolism in Mycobacterium tuberculosis. Trends Microbiol. 2006;14:320–327. doi: 10.1016/j.tim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Ratledge C. Iron, mycobacteria and tuberculosis. Tuberculosis (Edinb) 2004;84:110–130. doi: 10.1016/j.tube.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, et al. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zumla A, Atun R, Maeurer M, Mwaba P, Ma Z, O’Grady J, et al. Viewpoint: scientific dogmas, paradoxes and mysteries of latent Mycobacterium tuberculosis infection. Trop Med Int Health. 2011;16:79–83. doi: 10.1111/j.1365-3156.2010.02665.x. [DOI] [PubMed] [Google Scholar]
- 32.Khan SR, Manialawy Y, Siraki AG. Isoniazid and host immune system interactions: a proposal for a novel comprehensive mode of action. Br J Pharmacol. 2019;176:4599–4608. doi: 10.1111/bph.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan SR, Aljuhani N, Morgan AGM, Baghdasarian A, Fahlman RP, Siraki AG. Cytoprotective effect of isoniazid against H2O2 derived injury in HL-60 cells. Chem Biol Interact. 2016;244:37–48. doi: 10.1016/j.cbi.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Babu D, Khan SR, Srivastava N, Kyoung Suh LY, Morgan AG, Aljuhani N, et al. Isoniazid induces a monocytic-like phenotype in HL-60 cells. Arch Biochem Biophys. 2019;664:15–23. doi: 10.1016/j.abb.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Hazbón MH, Brimacombe M, Bobadilla del Valle M, Cavatore M, Guerrero MI, Varma-Basil M, et al. Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2006;50:2640–2649. doi: 10.1128/AAC.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.