Abstract
Purpose of Review
Compared to the current standard of implanting bone anabolics for fracture repair, bone fracture targeted anabolics would be more effective, less invasive, less toxic and would allow for control over what phase of fracture healing is being affected. We therefore sought to identify the optimal bone targeting molecule to allow for systemic administration of therapeutics to bone fractures.
Recent Findings
We found that many bone targeting molecules exist, but most have been developed for the treatment of bone cancers, osteomyelitis, or osteoporosis. There are a few examples of bone targeting ligands that have been developed for bone fractures that are selective for the bone fracture over the body and skeleton.
Summary
Acidic oligopeptides have the ideal half-life, toxicity profile, and selectivity for a bone fracture targeting ligand and are the most developed and promising of these bone fracture targeting ligands. However, many other promising ligands have been developed that could be used for bone fractures.
Keywords: Bone fracture targeting, Systemic administration, Bone fracture drugs, Acidic oligopeptides, Bisphosphonates, Drug targeting
Introduction
The 18.3 million bone fractures treated annually in the US represent one of the greatest cost to the US healthcare system (176.1 billion dollars annually)(1) and result in over 216.5 million days of work lost. Patients with comorbidities (e.g., diabetes or osteoporosis) are up to six times as likely to experience fractures and are also likely to experience twice as slow healing rates.(2–5) Patients over 65 who break hips have a 33% chance of dying within a year from complications associated with fracture-induced immobility.(6) For patients over 85, these rates can reach as high as 65%.(1) Those who survive often never walk unaided. Common morbidities associated with months of such immobility result in heart attacks, strokes, embolisms, pressure ulcers, muscle and bone loss, increased risk of infection, depression, and wage loss.(7)
Despite these ramifications and the dramatic need to accelerate bone fracture repair to minimize associated morbidities and mortality, most fracture treatments still consist solely of stabilization and opioid pain treatment. (7,8) Little has been done to improve fracture healing from a pharmacological standpoint.
Systemically administered anabolics show promise for accelerating bone healing but would benefit from targeting systemic administration. Such targeting can be accomplished using compounds that home to the hydroxyapatite in bone that is exposed to the bloodstream when bone is damaged. To aid other researchers in identifying which of these targeting compounds are best suited to treat various bone conditions, this review analyzes which bone-targeting molecules have been applied to treat various forms of bone damage in recent years.
Current Bone Fracture Therapeutics
Efforts have been made to improve fracture repair by augmenting the differentiation and activity of osteoblasts. Currently, the only clinically approved (US) pharmaceutical interventions are BMP2 and BMP7(9), which have been approved for open tibia trauma. BMPs activate BMP receptors, which signal through the SMAD pathway to elicit their osteogenic effects on mesenchymal stem cells (MSCs) and osteoblasts.(10) Their use is relatively limited because of ectopic bone growth side effects caused by leakage from the administration site (11), cancer risks, and invasiveness of drug administration.(12)
Many researchers have ramified the clinical success of BMP fracture repair, elucidating interesting biological and chemical factors that promote MSC differentiation, angiogenesis, collagen production, and mineralization needed to reach the injury site to elicit their effects there.(13) Most investigative strategies have looked to deliver therapeutics to the injury site in the form of a drug-eluting hydrogel,(14) drug-impregnated cement,(15) or as part of biocompatible scaffolds.(16) Many such strategies have potential to improve on some of the negative side effects produced with BMP administration. However, each relies on an invasive application at the fracture site during surgery. Surgery is highly invasive and can have poor outcomes in geriatric patients.(17) Furthermore, it is difficult to maintain a pharmacological effect from a single drug dose, and doing so often requires using a huge bolus of drug during its single administration. Additionally, there is no temporal control over which fracture healing phase is affected. These therapeutics are therefore too invasive and insufficiently effective as fracture repair therapies.
Systemic Anabolics Currently in Use or Being Tested
Systemically administrable anabolic agents represent a more promising step in the right direction toward accelerated bone healing, but they still present challenges when it comes to treating non-systemic conditions, such as fractures. On the one hand, such anabolics allow physicians to control how long a drug is delivered, as well as which phase of fracture repair it affects. They also improve upon the single bolus by allowing for multiple smaller injections. Several systemically administered anabolics such as teriparatide, abaloparatide, and antisclerostin antibodies (e.g., romosozumab) are quite successful in treating osteoporosis.(18) However, several attempts have been made to bring teriparatide to the clinic for fracture repair, resulting in conflicting studies(19–22). Although systemically administrable anabolics safely improve bone density in osteoporosis, achieving a local fracture concentration capable of improving repair would be blunted by dose-limiting toxicities (such as hypercalcemia(12)). Other anabolics, if not locally implanted like BMP2, lead to possible ectopic mineralization.(23) Many of the biochemical pathways involved in tissue repair are shared between tissues, and anabolics involved in bone repair will affect other tissues as well. Therefore, a targeting platform is needed to allow these potent anabolics to be selectively delivered directly—and exclusively—to the fracture.
Targeting Systemic Administration
Such a systemically administered drug that achieves anabolic concentrations at the fracture site without reaching side-effect-inducing blood concentrations can be achieved by the means of targeting. Targeting a bone anabolic agent concentrates it at the fracture site, thus resulting in the following benefits: fewer side effects, noninvasive administration, reduced ectopic bone formation from anabolic leakage, an expanded range of fractures that can benefit from its use. Attempts to achieve such bone targeting has revolved primarily around targeting the crystallin component of bone hydroxyapatite (HA), which makes up more than 70% of the bone.(24) Though HA remains unexposed in unfractured bone, a fracture reveals the HA, exposing it to the bloodstream. With this exposure, a targeting system can be designed to localize to increased-capillary-supply regions where bone is forming, resorbing, or damaged. This targeting allows the treatment to localize according to the needs of different orthopedic indications. In addition, different HA-binding ligands have preferences for different exposed HA surfaces relative to the others to allow skeletal selectivity. A variety of such compounds have been investigated for their ability to localize therapeutics to damaged HA to treat orthopedic diseases. The remainder of this review compares these compounds in order to elucidate instances in which each might prove useful for a pharmaceutical scientist.
Bisphosphonates
Due to their high affinity for HA, bisphosphonates are likely the most utilized drug in the toolbox for homing molecules to bone to treat bone disease. They have been around since the 1960s, and a number of bisphosphonate products are currently available. These molecules were originally developed as water softeners that could pull the calcium out of hard water. Their high affinity for HA inhibits farnesyl pyrophosphate (FFPS) synthase in OC to shut down bone turnover. Their structure is derived from a stabilized pyrophosphate. Pyrophosphate naturally regulates bone mineralization with an unstable, rapidly-cleaved anhydride bond. The carbon-phosphate part of bone is very stable, giving bisphosphonates up to 20 years’ residence time in the bone.(54) The deprotonated hydroxyls in the two phosphates that are in a bisphosphonate molecule are separated by approximately 2.9 to 3.1 Å.(55) This is similar to the native spacing of oxygen atoms in HA. The hydroxyl group that extends from the geminal bisphosphonate carbon also interacts with HA and further provides affinity to the bone.(55) The other R group (see table 1), usually a nitrogen-containing carbon chain or aromatic ring, influences the molecule’s ability to inhibit FFPS and thus determines its potency and contribute to its calcium binding. Bisphosphonates have been used extensively to target treatments for cancer, osteomyelitis, osteoporosis, dental diseases, and other mineral-based diseases. Recently, one of the first bisphosphonate-based therapeutics completed a successful phase I clinical trial for delivering cytarabine to cancer-induced bone lesions. The safety profile has improved upon targeting, and over half the bone lesions treated had reduced activity.(29) Other therapeutics have been developed to deliver a bortezomib bisphosphonate conjugate with a special boron linker that will not cleave in the bloodstream to treat multiple myeloma.(28) The bisphosphonate targeted bortezomib was twice as effective as the untargeted bortezomib at reducing the tumor burden in multiple myeloma, and the targeted form prevented most of the side effects from bortezomib including paralysis and loss of platelets. A special effort was made to use a bisphosphonate that would not prevent resorption via FFPS inhibition once the bortezomib was released. However, the pyrophosphate-like structure of bisphosphonates has proven to inhibit mineralization without any interaction with osteoclasts.(56) It remains unknown whether these non-active bisphosphonates would be effective for targeting bone fractures as they might inhibit mineralization, thus disrupting the basic bone unit in the long run.
Table 1:
Overview of common bone targeting ligands
| Name | Structure | Cons | Pros | Select Citations |
|---|---|---|---|---|
| Bisphosphonate | 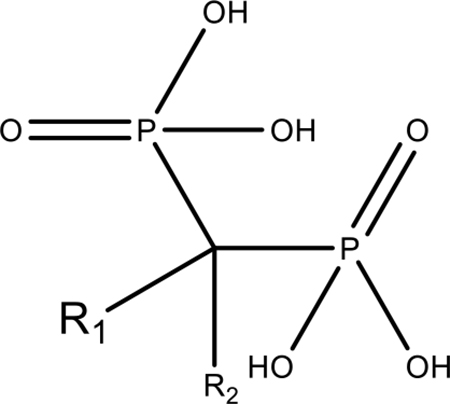 |
Selectivity Half life Skeletal Side effects |
Affinity Dual action Targeting for osteoporosis |
(25–32) |
| Tetracycline |  |
Toxicity Poor Selectivity |
Binds growing bone | (33,34) |
| Acidic Oligopeptide |  |
Peptide | Half life Selectivity Tunable Nontoxic |
(35–42) |
| TRAP binding Peptide | 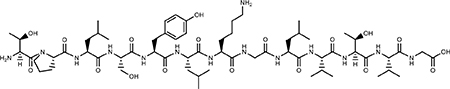 |
Peptide Poor Specificity |
Nontoxic | (43) |
| Pyrophosphoric Acid | 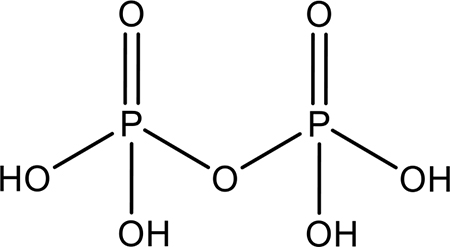 |
Liposomal formulation and leakage 20-day half-life |
High affinity Nontoxic |
(44) |
| (DSS)n | 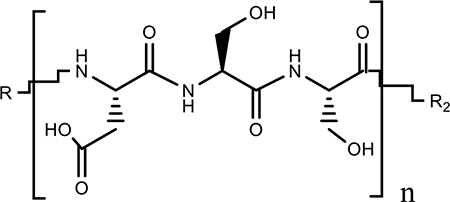 |
Peptide Poor Affinity Poor Specificity |
Specific for bone forming surfaces Nontoxic |
(11,45,46) |
| VTK | 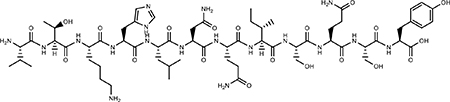 |
Peptide Requires Phosphoserines can inhibit osteoblast mineralization | nontoxic | (47–50) |
| EHBP7 | 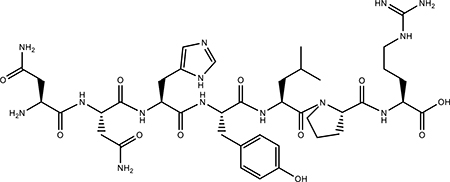 |
Peptide Enamel specific |
Nontoxic | (51) |
| SDSSD | 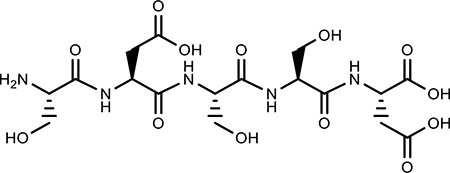 |
Peptide Poor Specificity |
Osteoblast specific | (52) |
| CH6 | 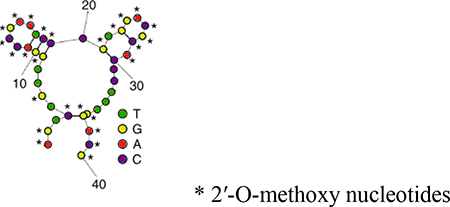 |
Nucleotide Expensive |
Nontoxic Cell penetrating Good for gene delivery |
(53) |
Bisphosphonates have also been used to deliver radionuclides such as 177Lu for a targeted radiotherapy of bone metastasis. The bisphosphonates have allowed for highly-selective delivery of radionucleotides to the metastasis, thus minimizing the soft tissue damage during radiotherapy.(30,57) Other work has focused on delivering nanoparticles and micelles with chemotherapeutics to bone cancers.(58–62) Bisphosphonates have proven effective at improving the pharmacokinetics of chemotherapeutics and a number of antibiotics in treating osteomyelitis.(63–65)
More relevant to fracture treatment is using bisphosphonates to target anabolics for osteoporosis.(66) Young et al. have worked on using bisphosphonates to deliver prostaglandin E2 agonists via a releasable ester linkage for the treatment of osteoporosis. Upon cleavage and hydrolysis, the bone conjugate works to deliver both an antiresorptive bisphosphonate and an anabolic prostaglandin EP2 agonist.(31,67,68) This combination completely restored the bone lost due to ovariectomy and improved the overall mechanical properties of the bone. The targeted combination therapy was better than its constituents delivered simultaneously but not tethered together, giving credence to the power of bone targeting.(69) The group found that identifying the right linkage is critical to the success of these conjugates. Other osteoporosis treatments have focused on localizing estrogens(70) or other anabolics to the bone.(27,71)
Bisphosphonates have also been used to deliver anabolics for the treatment of periodontal disease.(72,73) With limited success, some studies have even attempted to use bisphosphonates to localize proteins and cells to bone surfaces.(74,75) Though bisphosphonates are effective tools for localizing compounds of various sizes to HA, they have also been shown to lead to osteonecrosis of the jaw (BRONJ), atypical sub-trochanteric fractures in the femur, and esophageal cancer in fairly high frequencies.(25,76) Though arguments can be made that concentrations of BP for targeting are far lower than concentrations that induce side effects, the association with BRONJ has prevented most bisphosphonate-based drugs from fully developing(77). Most research today in this field focuses on non-active bisphosphonates as targeting ligands or dual-action targeting compounds where the skeletal activity of the bisphosphonate is desired.
Tetracycline
Tetracycline is a broad-spectrum antibiotic that blocks protein synthesis in bacteria. It is originally derived from the bacterial genus streptomyces and has been used as a therapeutic agent for decades.(78,79) Its use has drastically declined due to its unexpected skeletal side effects of discoloring bones and teeth and preventing normal tooth development in children, because of its affinity for calcium. Tetracycline should therefore be taken without milk in order to prevent it from having reduced bioavailability resulting from its binding to calcium ions.(80) These same properties that result in skeletal side effects enable its use as a biomarker for dynamic histomorphometry measurements in bone, as it emits fluorescence at 390 nM. The β-diketone system at positions 1 and 2, the enol system at positions 4 and 6, and the carboxamide group at position 5 are responsible for the chelating behavior.(55) Additionally, the chelation of tetracycline is permanent, meaning the unwanted side effects are also permanent.(81)
The ability to bind and label primarily growing bone have led people to localize anabolics to the bone surface by conjugating them to tetracycline and tetracycline-derived analogs. Tetracyclines have been used to create bone-targeted PLGA nanoparticle systems to deliver the drug simvastatin to osteoporotic bone with the localization only minimal success.(33) The tetracycline nanoparticles primarily localized to the liver and spleen with less than 3% accumulating in the bone, which moderately improved the effects of simvastatin on ovariectomized rats. There are concerns with the toxicities associated with tetracycline in that its presence in the body leads not only to undesirable skeletal effects, but also several organ toxicities. Tetracyclines have been shown to decrease collagenase levels and osteoblast activity levels,(80) which has led some groups to make attempts at modifying the structure of tetracycline to reduce some of its organ toxicities. Neale et al. found that they could retain 50% of the full tetracycline bone-binding ability with just one of the rings and use it to localize estrogen for the treatment of osteoporosis which was able to separate the skeletal effects of estrogen from its uterine effects thus improving its safety profile.(34) Tetracycline preferentially binds to areas of low crystallinity where bone is forming, rather than to resorption surfaces.(82) This and its oral bioavailability make it attractive as a targeting ligand. However, its toxicity and skeletal side effects have prevented it from being fully developed. The osteoblastic toxicities associated with tetracyclines and the strong affinity towards growing bone make it a far better targeting ligand for osteosarcoma and bone metastases over the previous work in osteoporosis. Similarly, tetracyclines could target the growing bone on of bone fractures but it would be important to use a modified version such as described in Neale et al.
Acidic Oligopeptides
Acidic oligopeptides are the means by which nature homes protein to bone. Proteins such as bone sialoprotein and osteopontin have highly acidic stretches of their sequences in which up to 22% of their sequences are glutamic acid.(83) These proteins serve a role in localizing to bone, nucleating HA, and mineralizing collagen.(84) These amino acids contain side chains with carboxylic acid that are believed to chelate the calcium component of the bone.(55,85) Affinity for the hydroxyapatite in the bone increases as the number of repeating units of aspartic acid or glutamic acid increase. Typically, affinity towards bone plateaus around 6–8 repeated units.(38) However, longer chains improve the amount of compound delivered and retained in vivo.(40,41) The interaction between acidic oligopeptides and HA is a non-chiral interaction as L and D enantiomers of amino acids have similar affinities to HA. However, in vivo, the D enantiomer degrades more slowly, leading to greater accumulation.(38,41,86,87) Acidic oligopeptides are very attractive as targeting ligands for bone due to their lack of obvious toxicities, especially compared to bisphosphonates and tetracyclines.(60) Acidic oligopeptides also have more desirable half-lives for treating acute bone disorders than tetracyclines and bisphosphonates, which can have half-lives in the bone for years rather than hours or days for acidic oligopeptides.(76,81,87) Acidic oligopeptides are also tunable and can localize a nanoparticle using as few as three aspartic acid molecules all the way out to much longer polymers to generate the desired half-life.(50)
Acidic oligopeptides have also been used to target therapy for a number of bone-related diseases. The clinically-approved asfotase alfa relies on a chain of 10 aspartic acids to localize tissue-nonspecific alkaline phosphatase to bone for treating hypophosphatasia. Acidic oligopeptides have also been used preclinically to localize antibiotics to treat osteomyelitis,(88) as well as to deliver liposomes and micelles carrying chemotherapy agents for osteosarcomas and bone metastasis.(42,89) They have also been shown to effectively deliver radionucleotides for imaging(41,90) and nanoparticles for photothermally treating bone tumors.(91)
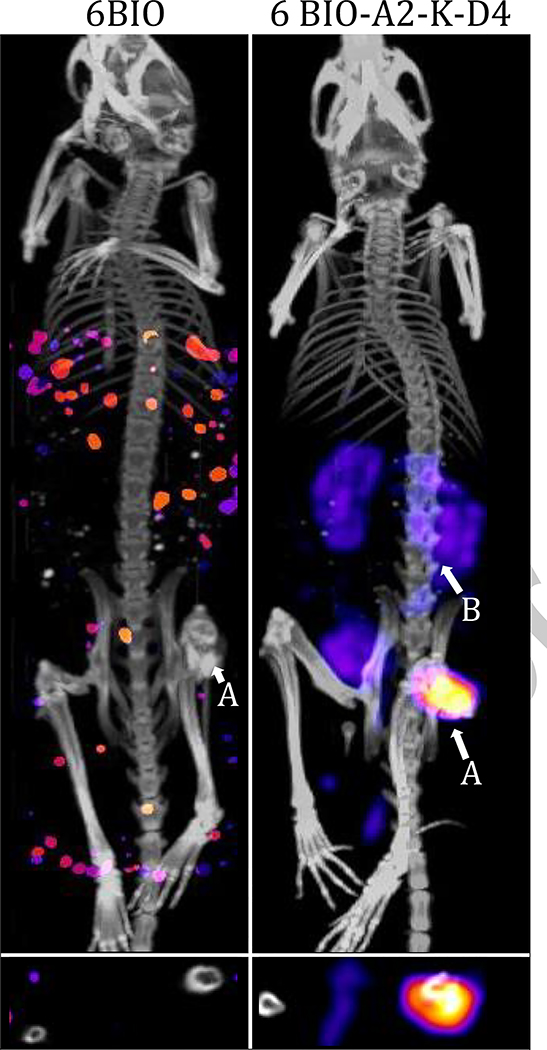
Furthermore, there is evidence that acidic oligopeptides can be used for more than targeting bone infections and the large osteolytic lesions that form in bone cancer. They have been reported to improve treatment in osteoporosis when localizing anabolics such as EP1 agonists to bone via acidic oligopeptides.(82) Our group, Low et al., have reported that acidic oligopeptides can be used to localize compounds to bone fractures specifically (Fig. 1).(35) This was a remarkable result because acidic oligopeptides tend to target longer, mature, HA crystals rather than the short growing HA crystals in a fracture. We further reported that these compounds can be used to improve the efficacy and safety of bone anabolics such as 6-BIO.(36) Our group has demonstrated that this platform can localize small molecules in the absence of a micelle, liposome, or nanoparticle through direct conjugation to the small molecule as with the conjugation to dasatinib. This promising subcutaneously-injected drug was well tolerated and reduced the time for fractured femurs to return to full strength by greater than 50%.(37)
Figure 1.
Femoral fracture targeting of 125I labeled 6-BIO vs targeted 6- BIO. Uptake in the kidneys (B) is likely due to amino acid reuptake receptors. Adapted from Low SA, Galliford C V, Yang J, Low PS, City SL, Chemistry P, et al. Biodistribution of fracture-targeted GSK3β inhibitor-loaded micelles for improved fracture healing. Vol. 16, Biomacromolecules. 2015. 3145–3153 p. Copyright (2015) American Chemical Society. Used with permission.
Acidic oligopeptides are a promising potential targeting platform for bone fractures. They have exceptional safety profiles and half-lives for treating acute skeletal damage such as bone fracture, their propensity to adsorb to mature HA does not inhibit their affinity towards fractures, and they themselves are osteogenically inert, allowing for natural healing. Still, they are peptides and can be degraded; therefore, work will need to be done to identify the appropriate enantiomeric configurations and lengths to provide the proper pharmacokinetics for bone fracture repair.
Tartrate-Resistant Acid Phosphate (TRAP)
A newer method that has been used to target bone fractures specially uses tartrate-resistant acid phosphate (TRAP), a protein deposited on bone resorptive surfaces. Benoit et al. have demonstrated that nanoparticles targeted using a peptide that binds to TRAP have increased localization in bone fractures.(92) Though the particles do have a fairly low target-to-background ratio for the spleen and liver, there is about a threefold selectivity for fractured femurs relative to unfractured femurs.(93) Benoit et al. were able to use TRAP to deliver GSK-3β inhibitors to elicit moderate improvements in density and strength of the bone fractures. TRAP has only moderate bone delivery capacities and will preferentially deliver payloads to resorption pits, not bone formation surfaces such as those that are present in the early stages of bone fractures. Localizing TRAP to the fractures is only slightly better than using untargeted nanoparticles, which preferential localization has been attributed to ELVIS (Extravasation through Leaky Vasculature and Inflammatory cell-mediated Sequestration) mechanism.(94,95)
Pyrophosphate
A promising new targeting technique for bones is that of pyrophosphorylated cholesterol or bone-targeting liposomes, which, in their bone-targeting capacity, are comparable to bisphosphonates. Wang et al. demonstrated that these bone-targeted liposomes could selectively localize to the fracture more than would be predicted by the ELVIS effect. They used them to selectively deliver salvianolic acid A, a potent bone anabolic, to mice in a glucocorticoid-induced delayed fracture healing model.(44) The targeted agent saw significant improvement relative to the untargeted free therapeutic. Though it retained the targeting capacity of a bisphosphonate, the pyrophosphorylated cholesterol had the advantage of a shorter half-life than a bisphosphonate. Pyrophosphate is metabolized into one of the main components of HA and is a safe additive. It is widely used in the food and oral care industry. This helps it keep the best of both worlds: a shorter-lived (like acidic oligopeptides) but higher-affinity (like bisphosphonates) targeting ligand. More work needs to be done to validate this new targeting technology and tune its pharmacokinetics, but it could be useful in delivering therapeutics to fractures.
(DSS)6
The tripeptide repeat of aspartate-serine-serine (DSS)n was identified as an important region for HA binding in dentin phosphoprotein (DPP). DPP is one of the main non-collagen proteins found in the dentin extracellular matrix and plays an important role in the nucleation of HA during dentin mineralization. DPP’s HA binding region contains a large number of repeats of the sequence Asp-Ser-Ser, which are heavily phosphorylated. It has been shown that unphosphorylated repeats of DSS can be used to localize to HA. The binding does not require the serines to be phosphorylated. Furthermore, replacing the serines with alanines did not diminish the binding affinity, but only affected the maximum monolayer DSS repeat concentration deemed to be optimal. It was shown that increasing the number of repeats proportionally increased the targeting capacity until about 6 repeats.(45) DSS peptide has been shown to preferentially bind to bone-forming surfaces over resorption pits where there is low crystallinity HA.(96) DSS6 was found to favorably bind to mantle dentin, which consists of small and randomly-oriented crystals, rather than the enamel surface, which consists of elongated and well-oriented HA crystal. These properties make it attractive as a targeting ligand in terms of its abilities to localize to newly growing bone rather than to existing bone.
DSS has been shown to be a successful delivery platform for the treatment of osteoporosis. Saidak et al showed that (DSS)6 could be used to deliver an osteogenic cyclic peptide ligand for α5β1 integrin. This α5β1 integrin priming stimulates the differentiation of MSC into osteoblasts. The targeting of this compound allows its effect to be constrained to the desired regions and improves long bone mass and microarchitecture.(46) (DSS)6 has also proven to be effective at localizing liposomes with siRNAs to bone-forming surfaces. Dioleoyl trimethylammonium propane (DOTAP)-based cationic liposomes encapsulate siRNAs and achieve 10x greater delivery than untargeted liposomes. This liposomal system has been able to achieve almost a two-fold greater knockdown in PLEK 01 RNA relative to untargeted liposomes.(97) The system has potential to make siRNA more commonly used in the treatment of bone diseases. However, it does have relatively high liver and kidney uptake. (DSS)6 does not have the highest affinity relative to bisphosphonates but is biocompatible, nontoxic, and prefers bone-forming surfaces.
VTK and Other Phage-Display Derived Peptides
Phage-display techniques have led to the development of new targeting ligands that are specific to HA or to osteoblasts. One of the most studied of these is VTKHLNQISQSY (VTK) a 12mer that was identified as having strong and specific affinity toward HA and bone-like material.(47) Surprisingly, it does not contain any aspartic acids or glutamic acids and is positively charged. However, follow-up studies have shown that VTK has dramatically improved the phosphorylation of its serines.(48) VTK-modified biomaterial has been shown to improve osteogenic differentiation of MSCs and biomineral deposition.(49) However, there are reports that VTK can inhibit osteoblast mineralization, which could generate undesired skeletal side effects as a targeting ligand. (50) Between this and its poorly understood adsorption mechanism, VTK requires additional preliminary work before being fully developed as a targeting platform for bone fractures.
Phage-display has been used to identify other HA-specific peptides, such as EHBP7(NNHYLPR), based on its ability to bind enamel.(51) It has been successfully used to localize KSLW (a broad-spectrum antimicrobial) to tooth surfaces to reduce biofilm formation.(98) Not only has phage-display allowed for the development of HA-specific ligands, but it has also been used to develop a number of ligands specific to different components of the bone.(79) Targeting ligands that specifically interact with osteoblasts are of particular interest, because they allow for a more specific stimulation of the desired cells in the bone. Two ligands that have allowed osteoblast-specific interaction are SDSSD and CH6. SDSSD was discovered via phage-display and binds to osteoblasts via periostin.(52) SDSSD has been used to localize nanomicelles encapsulating siRNA and microRNA to osteoblasts. The biodistribution of the compound suggests a broad uptake of the SDSSD targeting ligand in the brain, heart, liver, and lung in addition to the bone. This undesirable localization in other tissues makes SDSSD a poor targeting ligand for treating bone fractures because it could lead to off-target side effects in many tissues. The CH6 aptamer is a single-stranded oligonucleotide modified with 2′-O-methyl-nucleotide that can directly target osteoblasts at the cellular level. It was discovered via aptamer exponential enrichment (cell-SELEX) and has been shown to selectively localize lipid nanoparticles loaded with siRNA to osteoblasts.(53) These aptamer biodistributions are much more specific to the bone than SDSSD, and after 12 hours has most of its accumulation in the bone. It appears to achieve greater localization and therapeutic effects than (DSS)6 does with the same payload. CH6 seems to stimulate micropinocytosis and helps to facilitate lysosomal escape for its payload. However, nucleotide-based aptamers such as CH6 do suffer from nuclease degradation and are expensive to make. Nevertheless, CH6 is a promising osteoblast-specific aptamer that could be used to deliver osteoblast-specific gene therapies.
Conclusions
The above targeting systems have allowed for the development of systemic administration for bone disease treatments, many of which can be or have already been applied to localize compounds specifically to bone fractures. Their cargo has been delivered in the form of targeting ligands linked to nanoparticles, liposomes, and micelles loaded with therapeutic small molecules or siRNA. Additionally, these targeting ligands have been used to directly localize peptides, proteins, or small molecules through releasable or non-releasable conjugation. Overall, the safety of drugs is dramatically improved when the localization of a drug is confined to the desired location. The efficacy of the compound is increased because the concentration at the desired location is amplified relative to the administered systemic dose due to accumulation. Each targeting ligand has its pros and cons but their use has helped to further develop systemic noninvasive medicines for the treatment of bone diseases. This development allows for a noninvasive course of drug administration rather than surgically implanting therapeutics. It also allows for repeated therapeutic administration such that the therapeutic index can be maintained with much less drug and for longer periods of time. This temporal control over when in the bone fracture treatment the compound is delivered allows compounds that affect the different phases of fracture healing to differentially be administered during the ideal time of fracture repair.
Bone fracture targeting is a step in the direction of making more precise medicines and helping to bring pharmacological solutions into the treatment regime of fractures. Further developments need to be made to identify the ideal targeting ligand for bone fractures. There is most likely not a one-size-fits-all solution, because the therapeutic payload significantly influences the biodistribution. In some cases, an aptamer like CH6 might make the most sense for gene therapy, whereas acidic oligopeptides might be appropriate for peptide-based therapeutics due to their ease of synthesis, half-life, and biocompatibility. Future work can also focus on targeting components of bone fractures other than just the mineral aspect. Most targeting platforms have revolved entirely around targeting HA. However, collagen, fibronectin, or even osteoblast targeting could also be developed to localize compounds to bone fractures.(79,99,100). Targeting therapeutics to bone fractures is only half the battle. Once the ideal targeting platforms are identified, choosing the compounds that best exert their anabolic effects when locally delivered from a systemic injection is the next half of the battle. Developing these targeting strategies has great potential to help get people moving faster and to reduce the enormous global burden bone fractures place on humanity.
Abbreviations
- 6BIO
6-Bromoindirubin-3′-oxime
- EP1
Prostaglandin E2 receptor 1
- HA
Hydroxy apatite
- BMP
Bone morphogenetic proteins
- MSC
Mesenchymal stem cells
- LIPUS
low-intensity pulsed ultrasound
- BRONJ
osteonecrosis of the jaw
- FFPS
farnesyl pyrophosphate
- TRAP
Tartrate-resistant acid phosphate
- ELVIS
Extravasation through Leaky Vasculature and Inflammatory cell-mediated Sequestration
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Jeffery Nielsen reports having patents pending.
Dr. Stewart Low reports that he is an employee of Novosteo, which works on targeting bone anabolics to fractures. This is primarily why he understands the field in such depth, qualifying him to discuss the topic.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
* of importance
** of high importance
- 1.The Bone and Joint Initative. The Burden of Musculoskeletal Diseases in the United States The Burden of Musculoskeletal Diseases in the United States, Fourth Edition. 2019. [Google Scholar]
- 2.Nyman JS. Effect of diabetes on the fracture resistance of bone. Clin Rev Bone Miner Metab. 2013;11(1):38–48. [Google Scholar]
- 3.Nyman JS, Even JL, Jo CH, Herbert EG, Murry MR, Cockrell GE, et al. Increasing duration of type 1 diabetes perturbs the strength-structure relationship and increases brittleness of bone. Bone. 2011;48(4):733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyman JS, Kalaitzoglou E, Clay Bunn R, Uppuganti S, Thrailkill KM, Fowlkes JL. Preserving and restoring bone with continuous insulin infusion therapy in a mouse model of type 1 diabetes. Bone Reports. 2017;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson S, Ibe I, Cahill S, Chung YH, Lee FY. Bone Quality and Fracture-Healing in Type-1 and Type-2 Diabetes Mellitus. J Bone Jt Surg - Am Vol. 2019;101(15):1399–410. [DOI] [PubMed] [Google Scholar]
- 6.Schnell S, Friedman SM, Mendelson DA, Bingham KW, Kates SL. The 1-Year Mortality of Patients Treated in a Hip Fracture Program for Elders. Geriatr Orthop Surg Rehabil. 2010;1(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpintero P, Caeiro JR, Carpintero R, Morales A, Silva S, Mesa M, et al. Complications of hip fractures : A review. World J Orthop. 2014;5(4):402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Einhorn TA, Gerstenfeld L. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31(6):735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebara S, Nakayama K. Mechanism for the action of bone morphogenetic proteins and regulation of their activity. Spine (Phila Pa 1976). 2002;27(16 SUPPL.):10–5. [DOI] [PubMed] [Google Scholar]
- 11.Bergeron E, Leblanc E, Drevelle O, Giguère R, Beauvais S, Grenier G, et al. The evaluation of ectopic bone formation induced by delivery systems for bone morphogenetic protein-9 or its derived peptide. Tissue Eng - Part A. 2012;18(3–4):342–52. [DOI] [PubMed] [Google Scholar]
- 12.Haas AV, LeBoff MS. Osteoanabolic Agents for Osteoporosis. J Endocr Soc. 2018;2(8):922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyllönen L, D’Este M, Alini M, Eglin D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Vol. 11, Acta Biomaterialia. 2015. 412–434 p. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Malcolm DW, Benoit DSW. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials. 2017;139:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhongade ML, Tiwari IR. A comparative evaluation of the effectiveness of an anorganic bone matrix/cell binding peptide with an open flap debridement in human infrabony defects: A clinical and radiographic study. J Contemp Dent Pract. 2007;8(6):25–34. [PubMed] [Google Scholar]
- 16.Shih YRV, Hwang Y, Phadke A, Kang H, Hwang NS, Caro EJ, et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Vol. 111, Proceedings of the National Academy of Sciences of the United States of America. 2014. 990–995 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaupre LA, Jones CA, Saunders LD, Johnston DWC, Frcs C, Buckingham J, et al. Best Practices for Elderly Hip Fracture Patients A Systematic Overview of the Evidence. J GEN INTERN MED [Internet]. 2005;20(C):1019–25. Available from: http://www.labolsa.com/finanzas/precio+del+aluminio+por+kilo+en+colombia [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClung MR. Romosozumab for the treatment of osteoporosis. Osteoporos Sarcopenia [Internet]. 2018;4(1):11–5. Available from: 10.1016/j.afos.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.JOHANSSON T PTH 1–34 ( teriparatide ) may not improve healing in proximal humerus fractures. Acta Orthop. 2016;87(1):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhandari M, Jin L, See K, Burge R, Mbchb NG, Witvrouw R, et al. Does Teriparatide Improve Femoral Neck Fracture Healing : Results From A Randomized Placebo-controlled Trial. Clin Orthop Relat Res. 2016;474(5):1234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prospective HA, Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, et al. Teriparatide for Acceleration of Fracture Repair in Humans: A Prospective, Randomized, Double-Blind Study of 102 Postmenopausal Women With Distal Radial Fractures*. JBMR. 2010;25(2):404–14. [DOI] [PubMed] [Google Scholar]
- 22.Aspenberg P, Johansson T. Teriparatide improves early callus formation in distal radial fractures Teriparatide improves early callus formation in distal radial fractures Analysis of a subgroup of patients within a randomized trial. Acta Orthop ISSN. 2010;81(2):234–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, et al. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng - Part B Rev. 2016;22(4):284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shea JE, Miller SC. Skeletal function and structure: Implications for tissue-targeted therapeutics. Adv Drug Deliv Rev. 2005;57(7):945–57. [DOI] [PubMed] [Google Scholar]
- 25.Cole LE, Vargo-gogola T, Roeder RK. Targeted delivery to bone and mineral deposits using bisphosphonate ligands [Internet]. Vol. 99, Advanced Drug Delivery Reviews. Elsevier B.V.; 2016. 12–27 p. Available from: 10.1016/j.addr.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 26.Farrell KB, Karpeisky A, Thamm DH, Zinnen S. Bisphosphonate conjugation for bone specific drug targeting. Bone Reports [Internet]. 2018;9(June):47–60. Available from: 10.1016/j.bonr.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yewle JN, Puleo DA, Bachas LG. Bifunctional bisphosphonates for delivering PTH (1–34) to bone mineral with enhanced bioactivity. Biomaterials [Internet]. 2013;34(12):3141–9. Available from: 10.1016/j.biomaterials.2013.01.059 [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Xiao L, Tao J, Srinivasan V, Boyce BF, Ebetino FH, et al. Synthesis of a bone-targeted bortezomib with in vivo anti-myeloma effects in mice. Pharmaceutics. 2018;10(3):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zinnen SP, Karpeisky A, Von Hoff DD, Plekhova L, Alexandrov A. First-in-Human Phase I Study of MBC-11, a Novel Bone-Targeted Cytarabine-Etidronate Conjugate in Patients with Cancer-Induced Bone Disease. Oncologist. 2019;24(3):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergmann R, Meckel M, Kubíček V, Pietzsch J, Steinbach J, Hermann P, et al. 177Lu-labelled macrocyclic bisphosphonates for targeting bone metastasis in cancer treatment. EJNMMI Res. 2016;6(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arns S, Gibe R, Moreau A, Monzur Morshed M, Young RN. Design and synthesis of novel bone-targeting dual-action pro-drugs for the treatment and reversal of osteoporosis. Bioorganic Med Chem. 2012;20(6):2131–40. [DOI] [PubMed] [Google Scholar]
- 32.Young RN, Grynpas MD. Targeting therapeutics to bone by conjugation with bisphosphonates. Curr Opin Pharmacol [Internet]. 2018;40:87–94. Available from: 10.1016/j.coph.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 33.Chai G, Hu F. Tetracycline-grafted PLGA nanoparticles as bone-targeting drug delivery system. Int J Nanomedicine. 2015;10:5671–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neale JR, Richter NB, Merten KE, Taylor KG, Singh S, Waite LC, et al. Bioorganic & Medicinal Chemistry Letters Bone selective effect of an estradiol conjugate with a novel tetracycline-derived bone-targeting agent. Bioorg Med Chem Lett [Internet]. 2009;19(3):680–3. Available from: 10.1016/j.bmcl.2008.12.051 [DOI] [PubMed] [Google Scholar]
- 35.Low SA, Galliford CV, Yang J, Low PS, City SL, Chemistry P, et al. Biodistribution of fracture-targeted GSK3β inhibitor-loaded micelles for improved fracture healing. Vol. 16, Biomacromolecules. 2015. 3145–3153 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Low SA, Galliford CV., Jones-Hall YL, Roy J, Yang J, Low PS, et al. Healing efficacy of fracture-targeted GSK3β inhibitor-loaded micelles for improved fracture repair. Nanomedicine [Internet]. 2017;12(3):185–93. Available from: pmid: 28093944 [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Park S, Nam Y, Nielsen J, Low SA, Srinivasarao M, et al. Bone-Fracture-Targeted Dasatinib-Oligoaspartic Acid Conjugate Potently Accelerates Fracture Repair [Internet]. Vol. 29, Bioconjugate Chemistry. 2018. 3800–3809 p. Available from: pmid: 30380292 [DOI] [PubMed] [Google Scholar]
- 38.Nakato T, Yoshitake M, Matsubara K, Tomida M, Kakuchi T. Relationships between structure and properties of poly(aspartic acid)s. Vol. 31, Macromolecules. 1998. 2107–2113 p. [Google Scholar]
- 39.Ishizaki J, Waki Y, Takahashi-Nishioka T, Yokogawa K, Miyamoto KI. Selective drug delivery to bone using acidic oligopeptides. Vol. 27, Journal of Bone and Mineral Metabolism. 2009. 1–8 p. [DOI] [PubMed] [Google Scholar]
- 40.Sekido T, Sakura N, Higashi Y, Miya K, Nitta Y, Nomura M, et al. Novel drug delivery system to bone using acidic oligopeptide: Pharmacokinetic characteristics and pharmacological potential. Vol. 9, Journal of Drug Targeting. 2001. 111–121 p. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa K, Ishizaki A, Takai K, Kitamura Y, Makino A, Kozaka T, et al. Evaluation of Ga-DOTA-(D-Asp)n as bone imaging agents: D-aspartic acid peptides as carriers to bone. Sci Rep. 2017;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita S, Katsumi H, Hibino N, Isobe Y, Yagi Y, Tanaka Y, et al. Development of PEGylated aspartic acid-modified liposome as a bone-targeting carrier for the delivery of paclitaxel and treatment of bone metastasis [Internet]. Vol. 154, Biomaterials. Elsevier Ltd; 2018. 74–85 p. Available from: 10.1016/j.biomaterials.2017.10.053 [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Newman MR, Ackun-farmmer M, Michael P, Sheu T, Puzas JE, et al. Fracture-Targeted Delivery of β-Catenin Agonists via Peptide-Functionalized Nanoparticles Augments Fracture Healing. Vol. 11, ACS Nano. 2017. 9445–9458 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Jia Z, Akhter MP, Gao X, Wang X, Wang X, et al. Bone-targeting liposome formulation of Salvianic acid A accelerates the healing of delayed fracture Union in Mice. Nanomedicine Nanotechnology, Biol Med. 2018;14(7):2271–82. [DOI] [PubMed] [Google Scholar]
- 45.Yarbrough DK, Hagerman E, Eckert R, He J, Choi H, Cao N, et al. Specific binding and mineralization of calcified surfaces by small peptides. Calcif Tissue Int. 2010;86(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saidak Z, Henaff C Le, Azzi S, Marty C, Nascimento S Da, Sonnet P, et al. Wnt/beta-Catenin Signaling Mediates Osteoblast Differentiation Triggered by Peptide-induced alpha 5 beta1 Integrin Priming in Mesenchymal Skeletal Cells. J Biol Chem. 2015;290(11):6903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segvich SJ, Smith HC, Kohn DH. The adsorption of preferential binding peptides to apatite-based materials. Biomaterials [Internet]. 2009;30(7):1287–98. Available from: 10.1016/j.biomaterials.2008.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Addison WN, Miller SJ, Ramaswamy J, Mansouri A, Kohn DH, McKee MD. Phosphorylation-dependent mineral-type specificity for apatite-binding peptide sequences. Biomaterials [Internet]. 2010;31(36):9422–30. Available from: 10.1016/j.biomaterials.2010.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dinjaski N, Plowright R, Zhou S, Belton DJ, Perry CC, Kaplan DL. Osteoinductive recombinant silk fusion proteins for bone regeneration. Acta Biomater. 2017;49:127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramaswamy J, Nam HK, Ramaraju H, Hatch NE, Kohn DH. Inhibition of osteoblast mineralization by phosphorylated phage-derived apatite-specific peptide. Biomaterials [Internet]. 2015;73:120–30. Available from: 10.1016/j.biomaterials.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao J, Shi X, Wu YB, Gong SQ. Identification of specific hydroxyapatite {001} binding heptapeptide by phage display and its nucleation effect. Materials (Basel). 2016;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Ye X, Cai M, Liu X, Xiao J, Zhang C, et al. Osteoblast-Targeting-Peptide Modified Nanoparticle for siRNA/microRNA Delivery. ACS Nano. 2016;10(6):5759–68. [DOI] [PubMed] [Google Scholar]
- 53.Liang C, Guo B, Wu H, Shao N, Li D, Liu J, et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat Med. 2015;21(3):288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roussignol X, Currey C, Duparc F, Dujardin F. Indications and results for the Exogen™ ultrasound system in the management of non-union: A 59-case pilot study. Orthop Traumatol Surg Res [Internet]. 2012;98(2):206–13. Available from: 10.1016/j.otsr.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 55.Rotman SG, Grijpma DW, Richards RG, Moriarty TF, Eglin D, Guillaume O. Drug delivery systems functionalized with bone mineral seeking agents for bone targeted therapeutics [Internet]. Vol. 269, Journal of Controlled Release. Elsevier; 2018. 88–99 p. Available from: 10.1016/j.jconrel.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 56.Cole LE, Vargo-Gogola T, Roeder RK. Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Adv Drug Deliv Rev. 2016;99:12–27. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa K, Kawashima H, Shiba K, Washiyama K, Yoshimoto M, Kiyono Y, et al. Development of [90Y]DOTA-conjugated bisphosphonate for treatment of painful bone metastases. Nucl Med Biol [Internet]. 2009;36(2):129–35. Available from: 10.1016/j.nucmedbio.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 58.Miller K, Erez R, Segal E, Shabat D, Satchi-Fainaro R. Targeting bone metastases with a bispecific anticancer and antiangiogenic polymer-alendronate-taxane conjugate. Angew Chemie - Int Ed. 2009;48(16):2949–54. [DOI] [PubMed] [Google Scholar]
- 59.Wang G, Mostafa NZ, Incani V, Kucharski C, Uludaĝ H. Bisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseases. J Biomed Mater Res - Part A. 2012;100 A(3):684–93. [DOI] [PubMed] [Google Scholar]
- 60.Chaudhari KR, Kumar A, Khandelwal VKM, Mishra AK, Monkkonen J, Murthy RSR. Targeting efficiency and biodistribution of zoledronate conjugated docetaxel loaded pegylated pbca nanoparticles for bone metastasis. Adv Funct Mater. 2012;22(19):4101–14. [Google Scholar]
- 61.Miller K, Clementi C, Polyak D, Eldar-Boock A, Benayoun L, Barshack I, et al. Poly(ethylene glycol)-paclitaxel-alendronate self-assembled micelles for the targeted treatment of breast cancer bone metastases. Biomaterials [Internet]. 2013;34(15):3795–806. Available from: 10.1016/j.biomaterials.2013.01.052 [DOI] [PubMed] [Google Scholar]
- 62.Yu L, Cai L, Hu H, Zhang Y. Experiments and synthesis of bone-targeting epirubicin with the water-soluble macromolecular drug delivery systems of oxidized-dextran. J Drug Target. 2014;22(4):343–51. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka KSE, Dietrich E, Ciblat S, Métayer C, Arhin FF, Sarmiento I, et al. Synthesis and in vitro evaluation of bisphosphonated glycopeptide prodrugs for the treatment of osteomyelitis. Bioorganic Med Chem Lett. 2010;20(4):1355–9. [DOI] [PubMed] [Google Scholar]
- 64.Houghton TJ, Tanaka KSE, Kang T, Dietrich E, Lafontaine Y, Delorme D, et al. Linking bisphosphonates to the free amino groups in fluoroquinolones: Preparation of osteotropic prodrugs for the prevention of osteomyelitis. J Med Chem. 2008;51(21):6955–69. [DOI] [PubMed] [Google Scholar]
- 65.Sedghizadeh PP, Sun S, Junka AF, Richard E, Sadrerafi K, Mahabady S, et al. Design, Synthesis, and Antimicrobial Evaluation of a Novel Bone-Targeting Bisphosphonate-Ciprofloxacin Conjugate for the Treatment of Osteomyelitis Biofilms. Vol. 60, Journal of Medicinal Chemistry. 2017. 2326–2343 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu XM, Wiswall AT, Rutledge JE, Akhter MP, Cullen DM, Reinhardt RA, et al. Osteotropic β-cyclodextrin for local bone regeneration. Biomaterials. 2008;29(11):1686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen G, Arns S, Young RN. Determination of the Rat in Vivo Pharmacokinetic Profile of a Bone-Targeting Dual-Action Pro-Drug for Treatment of Osteoporosis. Bioconjug Chem. 2015;26(6):1095–103. [DOI] [PubMed] [Google Scholar]
- 68.Gil L, Han Y, Opas EE, Rodan GA, Ruel R, Seedor JG, et al. Prostaglandin E2-bisphosphonate conjugates: Potential agents for treatment of osteoporosis. Bioorganic Med Chem. 1999;7(5):901–19. [DOI] [PubMed] [Google Scholar]
- 69.Liu CC, Hu S, Chen G, Georgiou J, Arns S, Kumar NS, et al. Novel EP4 receptor agonist-bisphosphonate conjugate drug (C1) promotes bone formation and improves vertebral mechanical properties in the ovariectomized rat model of postmenopausal bone loss. J Bone Miner Res. 2015;30(4):670–80. [DOI] [PubMed] [Google Scholar]
- 70.Morioka M, Kamizono A, Takikawa H, Mori A, Ueno H, Kadowaki S ichiro, et al. Design, synthesis, and biological evaluation of novel estradiol-bisphosphonate conjugates as bone-specific estrogens. Vol. 18, Bioorganic and Medicinal Chemistry. 2010. p. 1143–8. [DOI] [PubMed] [Google Scholar]
- 71.Yang Y, Aghazadeh-Habashi A, Panahifar A, Wu Y, Bhandari KH, Doschak MR. Bone-targeting parathyroid hormone conjugates outperform unmodified PTH in the anabolic treatment of osteoporosis in rats. Drug Deliv Transl Res. 2017;7(4):482–96. [DOI] [PubMed] [Google Scholar]
- 72.Chen F, Liu XM, Rice KC, Li X, Yu F, Reinhardt RA, et al. Tooth-binding micelles for dental caries prevention. Antimicrob Agents Chemother. 2009;53(11):4898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Killeen AC, Rakes PA, Schmid MJ, Zhang Y, Narayana N, Marx DB, et al. Impact of Local and Systemic Alendronate on Simvastatin-Induced New Bone Around Periodontal Defects. J Periodontol. 2012;83(12):1463–71. [DOI] [PubMed] [Google Scholar]
- 74.Yao W, Lane NE. Targeted delivery of mesenchymal stem cells to the bone. Bone [Internet]. 2015;70:62–5. Available from: 10.1016/j.bone.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katsumi H, Sano JI, Nishikawa M, Hanzawa K, Sakane T, Yamamoto A. Molecular design of bisphosphonate-modified proteins for efficient bone targeting in vivo. PLoS One. 2015;10(8):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown JP, Morin S, Leslie MW, Papaioannou A, Cheung AM, Davison KS, et al. Bisphosphonates for treatment of osteoporosis: Expected benefits, potential harms, and drug holidays. Can Fam Physician. 2014;60(4):324–33. [PMC free article] [PubMed] [Google Scholar]
- 77.Rotman SG, Grijpma DW, Richards RG, Moriarty TF, Eglin D, Guillaume O. Drug delivery systems functionalized with bone mineral seeking agents for bone targeted therapeutics. J Control Release. 2018;269(November 2017):88–99. [DOI] [PubMed] [Google Scholar]
- 78.Low SA, Kopeček J. Targeting polymer therapeutics to bone. Adv Drug Deliv Rev [Internet]. 2012;64(12):1189–204. Available from: pmid: 22316530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Newman MR, Benoit DSW. Local and targeted drug delivery for bone regeneration. Curr Opin Biotechnol. 2016;40:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stapleton M, Sawamoto K, Alm CJ, Mackenzie WG, Mason RW, Orii T, et al. Development of Bone Targeting Drugs. Int J Mol Sci. 2017;18(1345):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cutbirth ST. A Restorative Challenge: Tetracycline-Stained Teeth. Denistry Today. 2015;(July):3–6. [PubMed] [Google Scholar]
- 82.Miller SC, Pan H, Wang D, Bowman BM, Kopečková P, Kopeček J. Feasibility of using a bone-targeted, macromolecular delivery system coupled with prostaglandin E1 to promote bone formation in aged, estrogen-deficient rats. Pharm Res. 2008;25(12):2889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vincent K, Durrant MC. Journal of Molecular Graphics and Modelling A structural and functional model for human bone sialoprotein [Internet]. Vol. 39, Journal of Molecular Graphics and Modelling. Elsevier Inc.; 2013. 108–117 p. Available from: 10.1016/j.jmgm.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 84.Tavafoghi M, Cerruti M. The role of amino acids in hydroxyapatite mineralization. J R Soc Interface. 2016;13(123). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamashita S, Katsumi H, Hibino N, Isobe Y, Yagi Y, Kusamori K, et al. Development of PEGylated carboxylic acid-modified polyamidoamine dendrimers as bone-targeting carriers for the treatment of bone diseases [Internet]. Vol. 262, Journal of Controlled Release. Elsevier; 2017. 10–17 p. Available from: 10.1016/j.jconrel.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto M, Hosoda H, Kitajima Y, Morozumi N, Minamitake Y, Tanaka S, et al. Structure-activity relationship of ghrelin: Pharmacological study of ghrelin peptides. Biochem Biophys Res Commun. 2001;287(1):142–6. [DOI] [PubMed] [Google Scholar]
- 87.Wang D, Sima M, Mosley RL, Davda JP, Tietze N, Miller SC, et al. Pharmacokinetic and biodistribution studies of a bone-targeting drug delivery system based on N-(2hydroxypropyl)methacrylamide copolymers. Mol Pharm. 2006;3(6):717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takahashi T, Yokogawa K, Sakura N, Nomura M, Kobayashi S, Miyamoto KI. Bone-targeting of quinolones conjugated with an acidic oligopeptide. Pharm Res. 2008;25(12):2881–8. [DOI] [PubMed] [Google Scholar]
- 89.Low SA, Yang J, Kopeček J. Bone-targeted acid-sensitive doxorubicin conjugate micelles as potential osteosarcoma therapeutics. Bioconjug Chem. 2014;25(11):2012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yanagi M, Uehara T, Uchida Y, Kiyota S, Kinoshita M, Higaki Y, et al. Chemical design of 99mTc-labeled probes for targeting osteogenic bone region. Bioconjug Chem. 2013;24(7):1248–55. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Yang J, Liu H, Wang X, Zhou Z, Huang Q, et al. Osteotropic peptide-mediated bone targeting for photothermal treatment of bone tumors [Internet]. Vol. 114, Biomaterials. Elsevier Ltd; 2017. 97–105 p. Available from: 10.1016/j.biomaterials.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Newman MR, Ackun-Farmmer M, Baranello MP, Sheu TJ, Puzas JE, et al. Fracture-Targeted Delivery of β-Catenin Agonists via Peptide-Functionalized Nanoparticles Augments Fracture Healing. ACS Nano. 2017;11(9):9445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y, Newman MR, Benoit DSW. Development of controlled drug delivery systems for bone fracture-targeted therapeutic delivery: A review. Eur J Pharm Biopharm. 2018;127(February):223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jia Z, Zhang Y, Chen YH, Dusad A, Yuan H, Ren K, et al. Simvastatin prodrug micelles target fracture and improve healing. J Control Release [Internet]. 2015;200:23–34. Available from: 10.1016/j.jconrel.2014.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Jia Z, Yuan H, Dusad A, Ren K, Wei X, et al. The Evaluation of Therapeutic Efficacy and Safety Profile of Simvastatin Prodrug Micelles in a Closed Fracture Mouse Model. Pharm Res [Internet]. 2016;33(8):1959–71. Available from: 10.1007/s11095-016-1932-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dang L, Liu J, Li F, Wang L, Li D, Guo B, et al. Targeted delivery systems for molecular therapy in skeletal disorders. Int J Mol Sci. 2016;17(3):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat Med. 2012;18(2):307–14. [DOI] [PubMed] [Google Scholar]
- 98.Huang Z Bin, Shi X, Mao J, Gong SQ. Design of a hydroxyapatite-binding antimicrobial peptide with improved retention and antibacterial efficacy for oral pathogen control. Vol. 6, Scientific Reports. Nature Publishing Group; 2016. 1–11 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wahyudi H, Reynolds AA, Li Y, Owen SC, Yu SM. Targeting collagen for diagnostic imaging and therapeutic delivery. J Control Release. 2016;240:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ponnapakkam T, Katikaneni R, Sakon J, Stratford R, Gensure RC. Treating osteoporosis by targeting parathyroid hormone to bone. Vol. 19, Drug Discovery Today. 2014. p. 204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]