Abstract
Purpose:
Low-dose radiation has become increasingly used in the management of indolent non-Hodgkin lymphoma (NHL), but has not been studied specifically for cases of ocular adnexal involvement. The objective of this study is to investigate the effectiveness of low-dose radiation in the treatment of NHL of the ocular adnexa.
Methods and Materials:
We reviewed the records of 20 NHL patients with 27 sites of ocular adnexal involvement treated with low-dose radiation consisting of 2 successive fractions of 2 Gy at our institution between 2005 and 2011. The primary endpoint of this study is freedom from local relapse (FFLR).
Results:
At a median follow-up time of 26 months (range 7–92), the overall response rate for the 27 treated sites was 96%, with a complete response (CR) rate of 85% (n = 23) and a partial response rate of 11% (n = 3). Among all treated sites with CR, the 2-year FFLR was 100%, with no in-treatment field relapses. The 2-year freedom from regional relapse rate was 96% with 1 case of relapse within the ipsilateral orbit (outside of the treatment field). This patient underwent additional treatment with low-dose radiation of 4 Gy to the area of relapse achieving a CR and no evidence of disease at an additional 42 months of follow-up. Orbital radiation was well tolerated with only mild acute side effects (dry eye, conjunctivitis, transient periorbital edema) in 30% of treated sites without any reports of long-term toxicity.
Conclusions:
Low-dose radiation with 2 Gy × 2 is effective and well tolerated in the treatment of indolent NHL of the ocular adnexa with high response rates and durable local control with the option of reirradiation in the case of locoregional relapse.
Summary
Low-dose radiation is an effective treatment for indolent non-Hodgkin lymphoma (NHL), but has not been studied in NHL of the ocular adnexa. We report our experience of 20 patients with 27 sites of orbital NHL involvement treated with 2 Gy × 2. Our results show that low-dose radiation in the treatment of orbital lymphoma is effective and well tolerated, with high response rates, durable local control, and minimal side effects.
Introduction
Approximately 50% of patients with non-Hodgkin lymphoma (NHL) have extranodal disease (1). Lymphomas of the ocular adnexa are uncommon, but comprise at least 10% of extranodal NHL (1–4). The majority of lymphomas of the ocular adnexa are indolent and radiosensitive (5, 6). Radiation therapy is currently the standard management for patients with stages I and II primary lymphoma of the ocular adnexa (7). Historically, patients have received treatment with conventional doses of 24–36 Gy with local control rates >95% (1, 3, 5, 8–10).
Taking advantage of the radiosensitivity of indolent lymphoma, there has been a recent trend towards reducing radiation doses to as low as 2 successive fractions of 2 Gy (6). Low-dose radiation in the palliative treatment of indolent NHL has shown promising results with effective control rates and minimal toxicity (6, 11–15). Ganem et al first reported a series of patients with indolent NHL treated with palliative radiation to 4 Gy in 2 fractions (11). Subsequently, additional reports were published of patients with NHL effectively treated with low-dose radiation (5, 11–15).
Although low-dose radiation therapy is increasingly used in patients with advanced stage indolent lymphoma, it has not yet been explored in the management of patients with limited involvement of the ocular adnexa. The use of radiation to target lesions in the ocular adnexa can be challenging because of the proximity of radiosensitive tissues such as the lens, lacrimal gland, or the retina (9). Retinal toxicity may result in visual impairment, which can significantly impact quality of life (2, 14). At our institution, we offer low-dose radiation consisting of 2 Gy × 2 fractions as an alternative to conventional radiation doses in cases requiring symptom palliation, reirradiation of recurrent disease, or when length of treatment is a barrier to completing treatment. The objective of this study is to investigate the effectiveness of low-dose radiation in the treatment of NHL of the ocular adnexa. We report our experience of 20 patients with NHL involving 27 orbital sites treated with low-dose radiation therapy with 2 Gy × 2.
Methods and Materials
Patient characteristics
We reviewed the records of 20 patients with NHL of the ocular adnexa treated with radiation therapy to a dose of 4 Gy delivered in 2 successive fractions of 2 Gy between 2005 and 2011 at our institution. All patients had histologically proven NHL and were staged clinically according to the Ann Arbor staging system. Patients with synchronous bilateral orbital disease involvement at diagnosis were staged as IE. Of the 20 patients, 2 presented with recurrent orbital disease in the setting of prior orbital radiation therapy with conventional doses and were offered the low-dose regimen to treat the area of relapse. Seven patients presented with stage IE disease and were deemed suitable candidates for definitive radiation therapy. After discussion of treatment options, these 7 patients chose to pursue low-dose radiation therapy. The remaining 11 patients had evidence of nonlocalized disease and were offered the low-dose radiation regimen as palliative treatment.
We identified a total of 27 sites of orbital disease involvement, which were treated among these 20 patients. There were 14 patients with single orbital involvement and 6 with bilateral orbital involvement treated with low-dose radiation therapy to both orbits, one of whom experienced an out-of-field relapse following low-dose radiation therapy and received retreatment to the ipsilateral orbit with low-dose radiation therapy, thus undergoing 3 courses of the low-dose regimen. Each orbit was documented as an independent site, bringing the total number of sites to 27.
Treatment details
Radiation therapy was individualized based on site and extent of orbital disease involvement. Patients underwent 2-dimensional treatment planning often for clinically visible tumors (n = 10 sites) or 3-dimensional treatment planning with CT scan-based simulation (n = 17 sites). Immobilization was frequently accomplished with the use of a thermoplastic mask. The gross tumor volume (GTV) encompassed the extent of disease as detected by clinical exam and by review of imaging, primarily using the planning computed tomography (CT) scan and magnetic resonance imaging (MRI) or positron emission tomography (PET) scans when available. The clinical target volume (CTV) included the GTV plus adequate margin to fully encompass the involved orbital site at risk (ie, the entire conjunctiva). In 1 patient, the CTV encompassed the entire orbit because of multiple sites of disease within the orbit. For patients with superficial disease limited to the eyelids or conjunctiva, treatment was generally delivered using electron beams (6–12 MeV) with or without bolus as deemed necessary to provide optimal surface dose. Our institutional experience treating lymphomas of the ocular adnexa with electron beams has been previously published (16). Deeper lesions were generally treated with photon beams (4–6 MV) or higher energy electron beams (9–16 MeV). Lens protection was a high priority in treatment planning with lens shielding used as appropriate. Patients were treated to a total dose of 4 Gy in 2 fractions over 2 consecutive days referred to as low-dose radiation therapy.
Response rate
Following treatment, patients were reevaluated during regularly scheduled clinical visits. Response rates were classified as complete response (CR), partial response (PR), or stable disease (SD). Evaluation of treatment response was assessed by clinical exam primarily. Radiographic studies were referenced when available. CR was defined as resolution of disease by physical examination with or without radiographic studies including CT, MRI, or PET scans. PR was defined as a reduction in size or activity of the tumor burden by physical exam with or without radiographic studies, in the absence of CR. SD was defined as no reduction in size or activity of the tumor burden by physical exam and/or radiographic studies. Patients were asked about side effects on follow-up visits; however, documentation of side effects was not standardized.
Statistical analysis
The primary endpoint of this study was freedom from local relapse (FFLR). Local relapse was defined by events occurring within the radiation treatment field. Regional relapse was defined by events occurring outside of the radiation treatment field, but within the ipsilateral orbit and recorded as freedom from regional relapse (FFRR). Time to event was measured from the completion date of radiation therapy. Relapse rates were estimated using the Kaplan-Meier method. All analyses were performed using SAS, version 9.3 software (SAS Institute, Cary, NC).
Results
The median follow-up time was 26 months (range 7–92) among all patients and 33 months among patients who achieved a CR to treatment. Patient characteristics are detailed in Table 1 and further site characteristics are listed in Table 2. Of the total 27 sites treated with low-dose radiation therapy, 26 (96%) sites received partial orbital radiation, whereas only 1 site (4%) received entire orbital radiation.
Table 1.
Patient characteristics
| Characteristic | Patients (N = 20) n (%) |
|---|---|
| Age at treatment (y) | |
| Median | 70 |
| Range | 38–88 |
| Sex | |
| Male | 10 (50) |
| Female | 10 (50) |
| Clinical stage | |
| IE | 7 (35) |
| IIE | 3 (15) |
| IIIE | 1 (5) |
| IV | 9 (45) |
| Histology | |
| Follicular | 11 (55) |
| Grade 1 | 8 (40) |
| Grade 2 | 3 (15) |
| Grade 3 | 0 (0) |
| Extranodal marginal zone | 8 (40) |
| lymphoma/mucosa-associated | |
| lymphoid tissue | |
| Mantle cell lymphoma | 1 (5) |
| Prior treatment | |
| Chemotherapy | 6 (30) |
| Radiation | 8 (40) |
Table 2.
Orbital characteristics
| Characteristic | Sites (N = 27) n (%) |
|---|---|
| Laterality | |
| Left | 13 (48) |
| Right | 14 (52) |
| Site of involvement | |
| Conjunctiva | 9 (33) |
| Lacrimal gland | 11 (41) |
| Retrobulbar | 2 (7) |
| Eyelid | 5 (19) |
The overall response rate (CR+PR) for the 27 treated sites was 96%. CR was observed in 23 (85%) sites, PR was observed in 3 (11%) sites, and SD in 1 (4%) site. Among the 3 cases with PR, 1 patient had reduction in size of the tumor on physical exam and reduction in the hypermetabolic activity on PET/CT. In the other 2 cases, PR was defined by physical exam alone. One patient with synchronous bilateral orbital disease involvement achieved a PR to treatment in the right orbit and a CR to treatment in the left orbit. The right orbit had previously received conventional dose radiation with local relapse. This patient has since been followed clinically without evidence of disease progression at 26 months of follow-up. Imaging findings did not always correlate with clinical exam with respect to defining treatment response. Often, there was no evidence of disease on physical exam, but there were minimal changes interpreted as indicative of disease activity on CT/MRI. These patients were regarded as CR based on physical exam and have remained free of disease at last follow-up. There was no significant difference in CR rate based on site of ocular involvement, histology, or clinical stage.
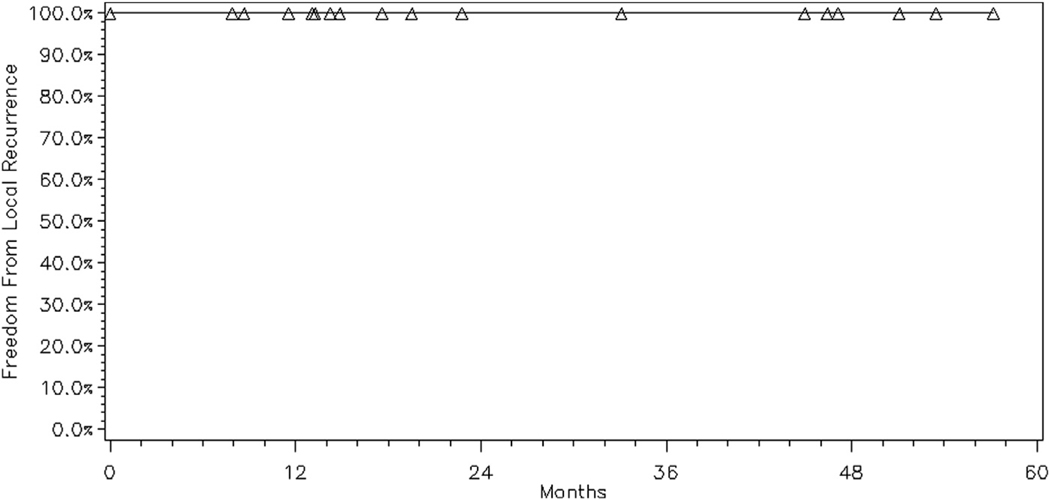
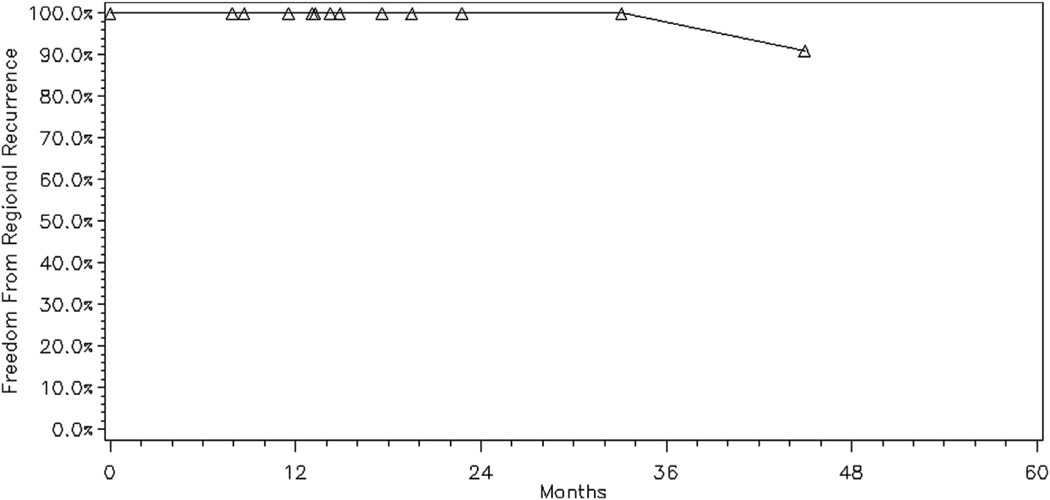
Among all 23 treated sites with CR, there were no in-treatment field relapses. The 2-year FFLR rate was 100% (Fig. 1) and the 2-year FFRR rate was 96% (Fig. 2). There was 1 regional relapse observed in this cohort, outside of the treatment field, but within the ipsilateral orbit. This patient had presented with synchronous bilateral orbital involvement with grade 2 follicular lymphoma of the retrobulbar tissues treated with 4-MV photon beams who developed disease relapse (confirmed on imaging) at 45 months in the ipsilateral orbit within the lower eyelid soft tissue, outside of the previous radiation treatment field. The patient underwent additional treatment with low-dose radiation therapy to the area of relapse with CR and no evidence of disease at an additional 46 months of follow-up. During our follow-up period, 5 patients experienced distant relapses treated with systemic therapy. Even when censoring these patients at the time of their salvage therapy, the remaining 15 patients had the same rates of FFLR and FFRR.
Fig. 1.
Freedom from local relapse for all sites with complete response treated with low-dose radiation therapy (N = 23).
Fig. 2.
Freedom from regional relapse for all sites with complete response treated with low-dose radiation therapy (N = 23).
Three patients (15%) experienced new sites of disease within the contralateral orbit. One patient received low-dose radiation to 4 Gy to the contralateral orbit with CR and is included in this cohort. The second patient received conventional dose radiation to 36 Gy (according to standard of care in 2008) to the contralateral orbit with CR. The third patient was only recently diagnosed with contralateral disease involvement and has chosen to undergo clinical surveillance without further intervention at this time. It is worth noting that at our institution during the same period, 8 patients with 9 sites of ocular adnexal NHL received conventional dose radiation therapy to the orbit ranging between 24 and 36 Gy.
Radiation was well tolerated with mild acute side effects including dry eye, conjunctivitis, and transient periorbital edema (Table 3). The one patient with acute conjunctivitis received treatment with corticosteroid eye drops with resolution of symptoms. Among the 4 cases of periorbital edema, symptoms resolved without intervention. Of note, all of the patients who experienced periorbital edema following treatment had received treatment with electron beam therapy with energies of 6 to 9 MeV. There were no cases of long-term toxicity such as retinopathy or keratitis. One patient aged 71 did develop bilateral cataracts 7 years after low-dose radiation to both orbits; however, the cataract formation was considered to be unlikely related to the history of radiation as this patient had other risk factors for development of cataracts. Otherwise, no patients were found to have visual impairment during the follow-up of this review.
Table 3.
Acute side effects follwing low-dose radiation therapy
| Toxicity | Site (n = 27) n (%)* |
|---|---|
| Dry eye | 1 (4) |
| Acute conjunctivitis | 1 (4) |
| Transient periorbital edema | 4 (15) |
| None reported | 22 (81) |
Total number of reported complications exceeds number of sites because of the possibility of more than 1 side effect per treated site.
Discussion
Radiation therapy is the standard treatment for patients with stage I and II primary orbital lymphoma. Doses of 24 to 36 Gy are associated with excellent local control. Indolent forms of NHL are highly radiosensitive and may respond to low-dose radiation. This is of particular importance in lymphoma of the ocular adnexa where the lesions may be in close proximity to radiosensitive ocular structures such as the retina, lens, and lacrimal gland. This study describes our experience of 20 patients with 27 sites of ocular adnexal NHL involvement treated with low-dose radiation therapy. We observed an overall response rate of 96% with a CR rate of 85% and no cases of in-field relapse at a median follow-up of 33 months.
Our results compare favorably with previously published data using low-dose radiation for indolent NHL (11–15). In the largest series reported to date, Haas et al reported 109 patients with indolent NHL involving 304 symptomatic disease sites treated with 4 Gy with an overall response rate of 92%, CR rate of 61% and a median time to local progression of 42 months among patients with an initial CR (13). The CR rates reported in other series using low-dose radiation in the treatment of NHL ranged from 37% to 84% (11, 12, 14, 15). Some of these reports included patients with indolent or aggressive lymphomas, which may explain the variation in response rates. Most of our patients had indolent NHL, mostly follicular lymphoma and marginal B cell lymphoma, which is consistent with reports in the literature regarding ocular adnexal lymphoma (9). We did observe a CR in 1 patient with mantle cell lymphoma that has been sustained at a follow-up of 52 months.
Local control rates in the treatment of lymphoma of the ocular adnexa using conventional dose radiation typically range from 95% to 100% (3, 4, 9, 10), which are similar to our experience of FFLR using a low-dose radiation regimen. Letschert et al suggested treating low-grade orbital lymphoma lesions with 30 Gy to achieve a local control rate >90% and using 40 Gy for patients with intermediate- and high-grade lesions (10). The lower dose limit of radiation has been a source of controversy in the literature with regard to orbital NHL management. Minehan et al suggested the use of at least 24 Gy for treatment of primary orbital lymphoma based on previous studies reporting local recurrence rates of 15% to 33% with <20 Gy (8). Hoskin et al recently presented preliminary data from the UK phase 3 randomized trial Follicular Radiation Therapy Trial investigating low-dose radiation therapy of 4 Gy compared with 24 Gy for 548 patients with follicular and marginal zone lymphoma (17). At a median follow-up of 22 months, the local control rate in the group that received 4 Gy was inferior to that of the group that received 24 Gy (80.4% vs 93.7%) (17). The disease location within the orbit may have influenced our excellent response rates as compared with other studies investigating 2 Gy × 2 in the general treatment of NHL. Because of the anatomical boundaries of the orbit, the tumor burden in these patients is likely much less than in patients with more generalized lymphoma in other sites. In addition, patients who present with ocular adnexal NHL often have visible disease or present with symptoms such as mass effect or decreased visual acuity that may prompt earlier medical attention. This may have influenced the high response rates we observed in our patients.
Although our cohort has not experienced any in-field relapses, there was 1 case of relapse within the ipsilateral orbit outside of the radiation treatment field. This patient had received partial orbit radiation and suffered an out-of-field relapse 45 months later. The area of relapse was successfully treated with low-dose radiation to 4 Gy with a CR, highlighting an important point that treatment with low-dose radiation does not prevent further treatment with radiation therapy if necessary. Pfeffer et al observed a 33% out-of-field recurrence rate in a group of patients with orbital lymphoma treated with partial orbit radiation compared with 0% recurrence rate among patients treated with radiation that encompassed the entire orbit (2). Similarly, Uno et al reported a patient who had received orbital radiation with an electron field that did not include the entire conjunctiva and later experienced an out-of-field relapse within the outer lateral conjunctiva (18). There is no consensus regarding the optimal planning target volume that is recommended in the irradiation of orbital lymphoma (18). In our cohort, all sites except 1 received partial orbit radiation with only 1 case of orbital relapse outside of the irradiated field. Although this could be interpreted as evidence in favor of whole orbit radiation, this case was successfully retreated with an additional course of low-dose radiation without toxicity. Patients should be followed closely after treatment with attention placed on examining both eyes as 3 of our patients developed contralateral ocular adnexal involvement within a median follow-up period of 26 months.
There are a few limitations worth noting in this study. First, this is a retrospective review, which could introduce bias into our analysis. Our data were not collected systematically or at similar time intervals. There was a lack of consistency in the documentation of response. There was also heterogeneity in radiation treatment techniques. Second, our overall sample size was small, although orbital extranodal NHL is uncommon. Third, our follow-up period is short with a median follow-up of 33 months among patients achieving a CR, which may not be long enough to capture local relapse. Fourth, our measurements of treatment response were defined by clinical exam, which did not always include imaging. Treatment response is difficult to evaluate accurately and FFLR may be of more importance in these patients. Fifth, the disease location within the orbit may have influenced our excellent response rates as discussed previously.
Our results confirm that low-dose radiation is well tolerated with only 30% of patients experiencing minimal acute side effects and none exhibiting moderate or severe sequelae. In contrast, Stafford et al reported a 52% rate of acute complications with doses ranging from 19 to 48 Gy in the treatment of lymphoma of the ocular adnexa (9). Kaushik et al investigated the risk of retinopathy in 67 patients with orbital NHL treated with conventional dose radiation to the entire orbit and found 12% of their patients developed radiation retinopathy (19). Only patients who received at least 20 Gy developed radiation retinopathy and the mean dose in patients with retinopathy was 33 Gy (19). Both of these studies highlight cases of orbital toxicity that occurred with the use of doses as low as 19 to 20 Gy.
In addition to the favorable side effect profile, low-dose radiation is extremely convenient for patients because it is delivered over the course of 2 successive days. Many patients traveling from long distances or who have a poor performance status can easily undergo this form of radiation therapy. This low-dose approach might also be considered in patients with contraindications to high-dose radiation such as the presence of Sjögren syndrome. Our study describes the first experience using low-dose radiation therapy to treat lymphoma of the ocular adnexa. Further study of the low-dose radiation approach with longer follow-up period is warranted.
Conclusions
In summary, we report that low-dose radiation is effective and well tolerated in the treatment of indolent NHL of the ocular adnexa with high response rates and durable local control. We recommend 2 Gy × 2 as excellent treatment of NHL of the ocular adnexa for the purpose of disease control in patients with advanced stage indolent disease with the option of reirradiation in the case of locoregional relapse. The ease and brevity of treatment and low risk of treatment toxicity make this an attractive option for patients. We believe this effective fractionation holds promise as initial definitive treatment for those with localized disease and warrants further study among patients who present with early-stage indolent NHL of the orbital adnexa.
Footnotes
Conflict of interest: none.
References
- 1.Le QT, Eulau SM, George TI, et al. Primary radiotherapy for localized orbital malt lymphoma. Int J Radiat Oncol Biol Phys 2002;52:657–663. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MR, Rabin T, Tsvang L, et al. Orbital lymphoma: Is it necessary to treat the entire orbit? Int J Radiat Oncol Biol Phys 2004; 60:527–530. [DOI] [PubMed] [Google Scholar]
- 3.Bolek TW, Moyses HM, Marcus RB Jr., et al. Radiotherapy in the management of orbital lymphoma. Int J Radiat Oncol Biol Phys 1999; 44:31–36. [DOI] [PubMed] [Google Scholar]
- 4.Smitt MC, Donaldson SS. Radiotherapy is successful treatment for orbital lymphoma. Int J Radiat Oncol Biol Phys 1993;26:59–66. [DOI] [PubMed] [Google Scholar]
- 5.Ganem G, Cartron G, Girinsky T, et al. Localized low-dose radiotherapy for follicular lymphoma: History, clinical results, mechanisms of action, and future outlooks. Int J Radiat Oncol Biol Phys 2010;78: 975–982. [DOI] [PubMed] [Google Scholar]
- 6.Chan EK, Fung S, Gospodarowicz M, et al. Palliation by low-dose local radiation therapy for indolent non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2011;81:e781–e786. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Non-Hodgkin’s lymphomas v.1.2013. Accessed January 13, 2013, www.nccn.org. [DOI] [PubMed]
- 8.Minehan KJ, Martenson JA Jr., Garrity JA, et al. Local control and complications after radiation therapy for primary orbital lymphoma: A case for low-dose treatment. Int J Radiat Oncol Biol Phys 1991;20: 791–796. [DOI] [PubMed] [Google Scholar]
- 9.Stafford SL, Kozelsky TF, Garrity JA, et al. Orbital lymphoma: Radiotherapy outcome and complications. Radiother Oncol 2001;59: 139–144. [DOI] [PubMed] [Google Scholar]
- 10.Letschert JG, Gonzalez Gonzalez D, Oskam J, et al. Results of radiotherapy in patients with stage I orbital non-Hodgkin’s lymphoma. Radiother Oncol 1991;22:36–44. [DOI] [PubMed] [Google Scholar]
- 11.Ganem G, Lambin P, Socie G, et al. Potential role for low dose limited-field radiation therapy (2 × 2 grays) in advanced low-grade non-Hodgkin’s lymphomas. Hematol Oncol 1994;12:1–8. [DOI] [PubMed] [Google Scholar]
- 12.Sawyer EJ, Timothy AR. Low dose palliative radiotherapy in low grade non-Hodgkin’s lymphoma. Radiother Oncol 1997;42:49–51. [DOI] [PubMed] [Google Scholar]
- 13.Haas RL, Poortmans P, de Jong D, et al. High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J Clin Oncol 2003;21:2474–2480. [DOI] [PubMed] [Google Scholar]
- 14.Luthy SK, Ng AK, Silver B, et al. Response to low-dose involved-field radiotherapy in patients with non-Hodgkin’s lymphoma. Ann Oncol 2008;19:2043–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas RL, Poortmans P, de Jong D, et al. Effective palliation by low dose local radiotherapy for recurrent and/or chemotherapy refractory non-follicular lymphoma patients. Eur J Cancer 2005;41:1724–1730. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson SS, Findley DO. Treatment of orbital lymphoid tumors with electron beams. Front Radiat Ther Oncol 1991;25: 187–200. [DOI] [PubMed] [Google Scholar]
- 17.Hoskin P, Kirkwood A, Popova B, et al. Fort RT: A phase 3 multi-center prospective randomized trial of low dose radiation therapy for follicular and marginal zone lymphoma. Int J Radiat Oncol Biol Phys 2013;85:22. [Google Scholar]
- 18.Uno T, Isobe K, Shikama N, et al. Radiotherapy for extranodal, marginal zone, b-cell lymphoma of mucosa-associated lymphoid tissue originating in the ocular adnexa: A multiinstitutional, retrospective review of 50 patients. Cancer 2003;98:865–871. [DOI] [PubMed] [Google Scholar]
- 19.Kaushik M, Pulido JS, Schild SE, et al. Risk of radiation retinopathy in patients with orbital and ocular lymphoma. Int J Radiat Oncol Biol Phys 2012;84:1145–1150. [DOI] [PubMed] [Google Scholar]