Abstract
N-Iminopyridinium ylides are competent monodentate directing groups for cobalt-catalysed annulation of sp2 C–H bonds with internal alkynes. The pyridine moiety in the ylide serves as an internal oxidant and is cleaved during the reaction. The annulation reactions possess excellent compatibility with heterocyclic substrates, tolerating furan, thiophene, pyridine, pyrrole, pyrazole, and indole functionalities.
First-row transition-metal, and more specifically, cobalt-catalysed carbon–hydrogen bond functionalization has received substantial attention in recent years.1 Compared to their 4d and 5d analogues, non-noble transition metals are abundant and cheap. Additionally, the reaction manifolds accessible by 3d transition metals sometimes differ from those of their 4d and 5d counterparts.
We have recently reported palladium-catalysed, N-iminopyridinium ylide-directed sp3 C–H arylation and alkylation reactions.2a Furthermore, copper-promoted sp2 C–H/N–H couplings were also disclosed.2b Monodentate N-iminopyridinium ylide directing groups appear to be nearly as efficient as the widely used bidentate auxiliaries in palladium-catalysed C–H functionalization.2,3 Their performance in copper-promoted sp2 C–H amination is superior to those of other monodentate directing groups.2b Thus, we were interested in exploring the reactivity of N-iminopyridinium ylides in cobalt-catalysed C–H functionalization.
A pioneering report by Matsunaga and Kanai in 2014 described Cp*Co(III)-catalysed coupling of alkynes with aromatic C–H bonds.4a,b Our group reported a method for cobalt-catalysed, aminoquinoline- and picolinamide-directed coupling of alkynes with C(sp2)–H bonds. The reaction employs a simple Co(OAc)2 hydrate catalyst, and oxygen from the air as a terminal oxidant.4c Subsequently, many similar high-valent cobalt catalysed transformations have been reported.5,6 Simple cobalt salt promoted transformations typically possess high regioselectivity for reactions with unsymmetrical alkynes and require a bidentate directing group.5 Only on rare cases, monodentate directing groups can be used.5i Reactions that are catalysed by various cyclopentadienylcobalt(III) complexes can be performed by employing simpler, monodentate, directing groups, but the regioselectivity observed for unsymmetric alkynes may be lower.6 Other metals and low-valent cobalt catalysis have been employed as well.7 Among cobalt-catalysed transformations that result in the formation of isoquinolones, few papers report the use of monodentate directing groups.6e,m The compatibility of these methodologies with heterocyclic substrates is underexplored. We report here N-iminopyridinium ylide-directed, cobalt-catalysed coupling of sp2 C–H bonds with alkynes that uses the N–N bond as an internal oxidant forming isoquinolones. These transformations are highly compatible with heterocyclic substrates.
The reaction optimization studies are presented in Table 1. The use of simple cobalt salts such as Co(OAc)2 and Co(acac)2 did not afford any product, and thus cyclopentadienylcobalt(III) complexes were employed. After screening a number of solvents, cobalt complexes, and additives, we found that the best results were obtained by using a [Cp*Co(MeCN)3][SbF6]2 catalyst in HFIP solvent with added pivalic acid. Subsequently, modification of the ylide moiety was undertaken (entries 1–3). Relatively low yield was obtained by employing a ylide derived from commercially available 1-aminopyridinium iodide and ptoluoyl chloride (entry 1). Switching to 4-methoxypyridinium derivative 2 and 4-tert-butylpyridinium ylide 3, previously utilized for copper promoted C–H amination,2b allowed the yields to be increased to 81–82% (entries 2 and 3). Pivalic acid additive was found to be optimal for the reaction (entries 4 and 5).
Table 1.
Optimization of the reaction conditionsa
 | ||
|---|---|---|
| Entry | Deviation from standard conditions | Yieldb (%) |
| 1 | R1 = H, 1 | 24 |
| 2 | R1 = OMe, 2 | 82 |
| 3 | R1 = tBu, 3 | 81 |
| 4 | 1-AdCO2H instead of PivOH | 77 |
| 5 | Without PivOH | 55 |
| 6 | Cp*CoI2(CO) (20 mol%) AgSbF6 (40 mol%) 1-AdCO2H (50 mol%) | 60 |
| 7 | [CpCo(MeCN)3][SbF6]2 | 7 |
Reaction conditions: ylide (0.2 mmol), HFIP (1 mL).
Isolated yields. Abbreviations: HFIP = 1,1,1,3,3,3-hexafluoroisopropanol, 1-AdCO2H = 1-adamantanecarboxylic acid, PivOH = pivalic acid.
Best catalytic performance was observed for [Cp*Co(MeCN)3] [SbF6]2, while in situ generated active species (entry 6) and [CpCo(MeCN)3][SbF6]2 (entry 7) gave lower yields.
After identification of the optimal reaction conditions, we evaluated the generality of the directed C–H annulation of aryl ylides with symmetric alkynes such as diphenylacethylene and 3-hexyne (Scheme 1). Various functional groups such as alkoxy (5), halides (6, 7, 13, 14, and 17), esters (10 and 15), nitrile (12), trifluoromethyl (9), and sulfone (11) on the aryl rings are well tolerated furnishing good to excellent yields of isoquinolones regardless of the substituent electronic properties and positions on the arene. The annulation of substrates with substituents at the meta position of an aryl group occurred regioselectively at the less hindered site (13–15). Compared to meta and para substituted compounds, ortho substituted substrates gave products in somewhat lower yields (16 and 17). The annulation is not limited to aromatic substrates. Acrylic acid derivatives are reactive as well (18 and 19). In addition to diphenylacetylene, 3-hexyne can be employed as a substrate for the annulation to generate the corresponding products (20–22). Comparison of t-butyl pyridinium ylides with the corresponding methoxy pyridinium derivatives is instructive. In some cases, performance is nearly identical (4). For most other substrates, the methoxy derivative performs substantially better (14, 16, and 17) even with shorter reaction times.
Scheme 1.

Reaction scope with respect to ylides.a a Reaction scale: 0.2 mmol, HFIP 1 mL. b Time: 20 h. c Time: 48 h. d Catalyst: [Cp*Co(MeCN)3][SbF6]2 (20 mol%), PivOH (30 mol%), 48 h. e Time: 72 h. f Catalyst: [Cp*Co(MeCN)3] [SbF6]2 (20 mol%), PivOH (30 mol%), 72 h. Isolated yields reported. Please see the ESI† for details.
The annulation protocol was investigated with respect to heteroaryl substrates (Scheme 2). Thiophene derivatives gave the expected products in good yields, irrespective of the substitution pattern (23–25). The best results were obtained with 3-substituted thiophene which afforded the cyclized product 25 regioselectively and reacted faster than other substrates. Furan derivatives are competent substrates (26–28) but if a ylide derived from furan-2-carboxylic acid was employed, the product was isolated in a low yield (26). Notably, a brominated furan derivative is reactive (28). Furthermore, nitrogen-containing heteroarene substrates participate in the transformation. Pyridine (29 and 30), pyrrole (31), pyrazole (32), and indole moieties (33) are all compatible with the reaction conditions, irrespective of the potentially strong coordinating ability of the heteroatom which may interfere with the C–H functionalization step of the annulation reaction.8 In all cases except that for the 6-methoxypyridinium substrate (30), formation of only one product isomer was observed. For 30, two isomers (C2 : C4 = 5.9 : 1) were obtained. Next, the annulation of heterocycles with 3-hexyne was investigated. Thiophenes and benzo[b]thiophene gave products in good yields (34–37). However, the furan-containing substrate afforded 38 in a modest yield. In most cases the methoxy-substituted pyridine ylide derivative performs substantially better compared with the ylide derived from t-butylpyridine.
Scheme 2.

Reaction scope with respect to heteroaryl ylides.a a Reaction scale: 0.2 mmol, HFIP 1 mL. b Time: 48 h. c Time: 20 h. d Catalyst: [Cp*Co (MeCN)3][SbF6]2 (20 mol%), PivOH additive (30 mol%), 48 h. e Catalyst: [Cp*Co(MeCN)3][SbF6]2 (20 mol%), PivOH additive (30 mol%), 72 h. f Product major isomer is shown (5.9 : 1). Isolated yields are reported. Please see the ESI† for details.
Regioselectivity of the reactions with unsymmetrical alkynes was investigated next (Scheme 3). The annulation with ethyl hept-2-ynoate exhibited good selectivity (39 and 40). The use of ethyl 3-phenylpropiolate resulted in about 2 : 1 ratio of isomeric products 41 and 42. Poor selectivity was observed in the reaction with 4-phenylbut-3-yn-2-one (43 and 44). The selectivities are consistent with those observed for cyclopentadienylcobalt(III)- catalysed annulation reactions with alkynes.6
Scheme 3.

Reactions with unsymmetrical alkynes.a aReaction scale: 0.2 mmol, HFIP 1 mL. b [Cp*Co(MeCN)3][SbF6]2 (20 mol%), PivOH (30 mol%), 48 h. c [Cp*Co(MeCN)3][SbF6]2 (15 mol%), PivOH (20 mol%), 48 h.
We carried out preliminary mechanistic experiments to delineate the annulation mechanism. The C–H bond cleavage step is reversible as determined by D/H scrambling (Scheme 4). Two deuterium atoms at the ortho positions of the ylide were completely replaced with hydrogens in the absence of alkyne (45 to 45-d3). The reaction of deuterated ylide with 3-hexyne gave the isoquinoline product with 52% hydrogen incorporation (45 to 46-h). When the reaction was stopped before completion, H/D scrambling in both the recovered ylide starting material and product was observed (45 to 45-h and 46-h, eqn (3)). These results suggest that the C–H bond cleavage step is not the rate determining step.
Scheme 4.

Scrambling experiments.
In a competitive annulation using a 1 : 1 molar mixture of tert-butyl pyridinium ylide 3 and methoxy pyridinium ylide 47, a 1 : 1.35 mixture of 6-methyl-isoquinoline 4 and 6-ethylisoquinoline 48 was obtained (Scheme 5). Somewhat higher reaction rate for the methoxy pyridinium ylide may reflect the higher coordinating ability of a more electron-rich ylide.
Scheme 5.

Competition reaction.
We attempted to prepare the cyclometalated complex via C–H activation of the pyridinium ylide with a stoichiometric amount of [Cp*Co(MeCN)3][SbF6]2, but the desired cobaltacycle could not be isolated. Reversible C–H cobaltation may have contributed to the failure to isolate the complex. However, the Cp*Co(III)-pyridinium ylide complex 50 was prepared by chelation-assisted oxidative addition of the pyridinium ylide Ar–I bond to Cp*Co(CO)2, generated in situ from cobalt carbonyl (Scheme 6, eqn (1)).9 Complex 50 was characterized by single crystal X-ray diffraction and NMR spectroscopy. Iodide abstraction by treatment of AgSbF6 in acetonitrile afforded cationic cobalt complex 51, which is a competent annulation catalyst, affording isoquinolone 4 in 68% yield (Scheme 6, eqn (2)).
Scheme 6.
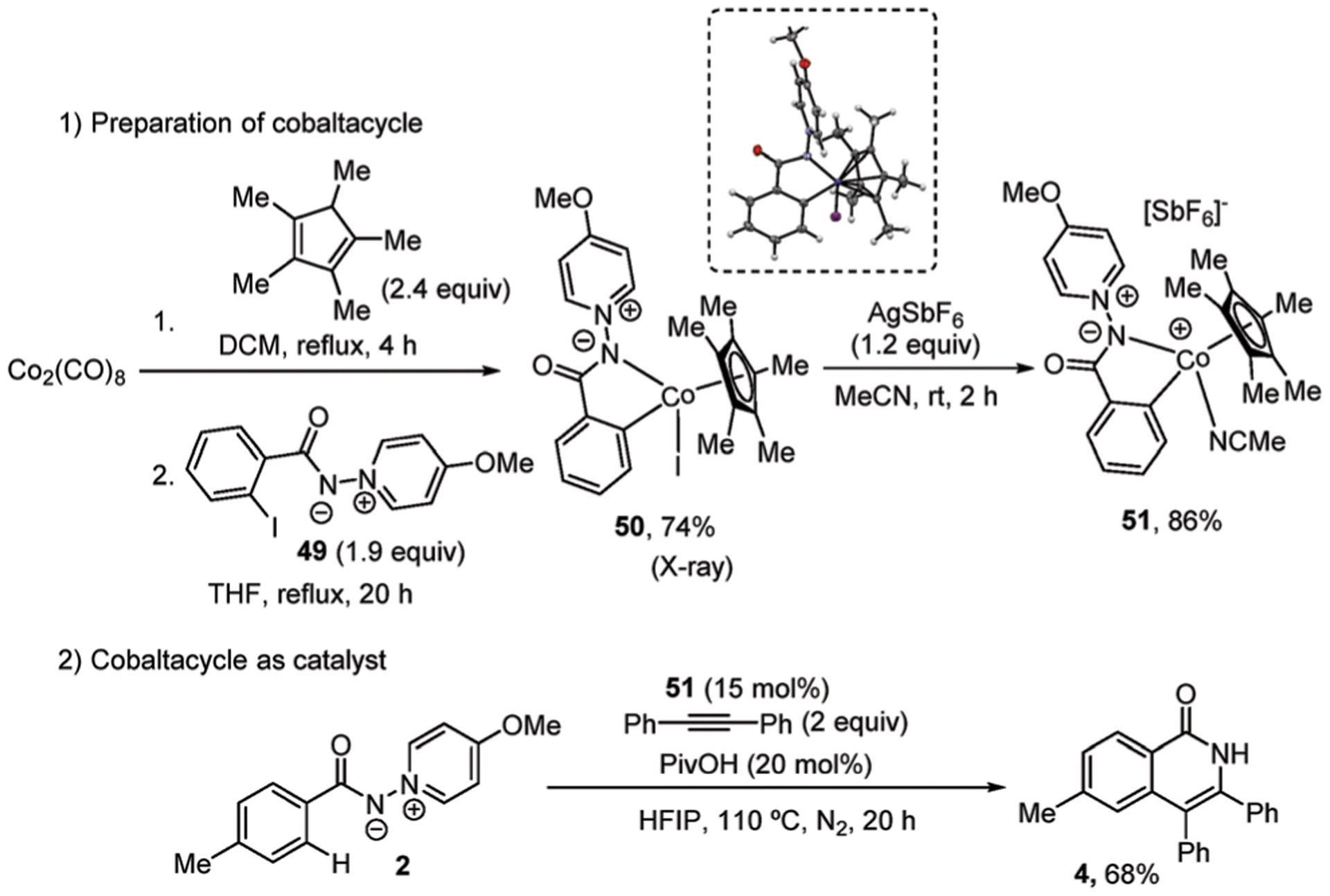
Cp*Co(III)-pyridinium ylide complex.
Based on the mechanistic studies and the reported mechanism for high valent cobalt catalysed annulations with alkynes in the literature, we propose the catalytic cycle in Scheme 7.6e,i,k,m,10 The reaction starts with ligand exchange followed by subsequent chelation assisted C–H activation to give the cobaltacycle A. Ligand exchange between the alkyne and nitrile, followed by migratory insertion forms the seven-membered cobaltacycle B. Next, intramolecular C–N bond formation furnishes the isoquinoline and releases the pyridine and Co(III) catalyst closing the catalytic cycle.
Scheme 7.

Proposed catalytic cycle.
In conclusion, we have shown that N-iminopyridinium ylides act as competent monodentate directing groups for cobalt-catalysed annulation of sp2 C–H bonds with internal alkynes. Coupling reactions proceed in hexafluoroisopropanol solvent at increased temperatures and are catalysed by the [Cp*Co(MeCN)3][SbF6]2 complex. The pyridine moiety in the ylide is cleaved during the reaction, serving as an internal oxidant. The annulation reactions possess excellent compatibility with heterocyclic substrates, tolerating furan, thiophene, pyridine, pyrrole, pyrazole, and indole functionalities.
Supplementary Material
Acknowledgments
We are grateful to the Welch Foundation (Chair E-0044) and NIGMS (Grant No. R01GM077635) for supporting this work. We thank Dr Xiqu Wang for collecting diffraction data and solving the X-ray structures of 41 and 50.
Footnotes
Electronic supplementary information (ESI) available. CCDC 1991068 and 2014034. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc05294a
Conflicts of interest
The authors declare no conflict of interest.
Notes and references
- 1.(a) Gandeepan P, Müller T, Zell D, Cera G, Warratz S and Ackermann L, Chem. Rev, 2019, 119, 2192–2452; [DOI] [PubMed] [Google Scholar]; (b) Kulkarni AA and Daugulis O, Synthesis, 2009, 4087–4109; [Google Scholar]; (c) Moselage M, Li J and Ackermann L, ACS Catal, 2016, 6, 498–525; [Google Scholar]; (d) Baccalini A, Vergura S, Dolui P, Zanoni G and Maiti D, Org. Biomol. Chem, 2019, 17, 10119–10141. [DOI] [PubMed] [Google Scholar]
- 2.(a) Le KKA, Nguyen H and Daugulis O, J. Am. Chem. Soc, 2019, 141, 14728–14735; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kwak SH and Daugulis O, J. Org. Chem, 2019, 84, 13022–13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Zaitsev VG, Shabashov D and Daugulis O, J. Am. Chem. Soc, 2005, 127, 13154–13155; [DOI] [PubMed] [Google Scholar]; (b) Daugulis O, Roane J and Tran LD, Acc. Chem. Res, 2015, 48, 1053–1064; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rej S, Ano Y and Chatani N, Chem. Rev, 2020, 120, 1788–1887. [DOI] [PubMed] [Google Scholar]
- 4.(a) Ikemoto H, Yoshino T, Sakata K, Matsunaga S and Kanai M, J. Am. Chem. Soc, 2014, 136, 5424–5431; [DOI] [PubMed] [Google Scholar]; (b) Yoshino T and Matsunaga S, Adv. Synth. Catal, 2017, 359, 1245–1262; [Google Scholar]; (c) Grigorjeva L and Daugulis O, Angew. Chem., Int. Ed, 2014, 53, 10209–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Kalsi D and Sundararaju B, Org. Lett, 2015, 17, 6118–6121; [DOI] [PubMed] [Google Scholar]; (b) Landge VG, Jaiswal G and Balarman E, Org. Lett, 2016, 18, 812–815; [DOI] [PubMed] [Google Scholar]; (c) Kuai C, Wang L, Li B, Yang Z and Cui X, Org. Lett, 2017, 19, 2102–2105; [DOI] [PubMed] [Google Scholar]; (d) Mei R, Ma W, Zhang Y, Guo X and Ackermann L, Org. Lett, 2019, 21, 6534–6538; [DOI] [PubMed] [Google Scholar]; (e) Nguyen TT, Grigorjeva L and Daugulis O, ACS Catal, 2016, 6, 551–554; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhai S, Qiu S, Chen X, Wu J, Zhao H, Tao C, Li Y, Cheng B, Wang H and Zhai H, Chem. Commun, 2018, 54, 98–101; [DOI] [PubMed] [Google Scholar]; (g) Mei R, Sauermann N, Oliveira JCA and Ackermann L, J. Am. Chem. Soc, 2018, 140, 7913–7921; [DOI] [PubMed] [Google Scholar]; (h) Martínez ÁM, Rodríguez N, Gómez-Arrayás R and Carretero JC, Chem. – Eur. J, 2017, 23, 11669–11676; [DOI] [PubMed] [Google Scholar]; (i) Nguyen TT, Grigorjeva L and Daugulis O, Angew. Chem., Int. Ed, 2018, 57, 1688–1691; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Zhang LB, Hao X-Q, Liu Z-J, Zheng X-X, Zhang S-K, Niu J-L and Song M-P, Angew. Chem., Int. Ed, 2015, 54, 10012–10015. [DOI] [PubMed] [Google Scholar]
- 6.(a) Bera SS, Debbarma S, Ghosh AK, Chand S and Maji MS, J. Org. Chem, 2017, 82, 420–430; [DOI] [PubMed] [Google Scholar]; (b) Kong L, Yu S, Zhou X and Li X, Org. Lett, 2016, 18, 588–591; [DOI] [PubMed] [Google Scholar]; (c) Zhang Z-Z, Liu B, Xu J-W, Yan S-Y and Shi B-F, Org. Lett, 2016, 18, 1776–1779; [DOI] [PubMed] [Google Scholar]; (d) Tanaka R, Ikemoto H, Kanai M, Yoshino T and Matsunaga S, Org. Lett, 2016, 18, 5732–5735; [DOI] [PubMed] [Google Scholar]; (e) Yu X, Chen K, Guo S, Shi P, Song C and Zhu J, Org. Lett, 2017, 19, 5348–5351; [DOI] [PubMed] [Google Scholar]; (f) Xu X, Yang Y, Zhang X and Yi W, Org. Lett, 2018, 20, 566–569; [DOI] [PubMed] [Google Scholar]; (g) Wang H, Moselage M, González MJ and Ackermann L, ACS Catal, 2016, 6, 2705–2709; [Google Scholar]; (h) Barsu N, Sen M, Premkumar JR and Sundararaju B, Chem. Commun, 2016, 52, 1338–1341; [DOI] [PubMed] [Google Scholar]; (i) Kong L, Yang X, Zhou X, Yu S and Li X, Org. Chem. Front, 2016, 3, 813–816; [Google Scholar]; (j) Dutta PK and Sen S, Eur. J. Org. Chem, 2018, 5512–5519; [Google Scholar]; (k) Liang Y and Jiao N, Angew. Chem., Int. Ed, 2016, 55, 4035–4039; [DOI] [PubMed] [Google Scholar]; (l) Prakash S, Muralirajan K and Cheng C-H, Angew. Chem., Int. Ed, 2016, 55, 1844–1848; [DOI] [PubMed] [Google Scholar]; (m) Sivakumar G, Vijeta A and Jeganmohan M, Chem. – Eur. J, 2016, 22, 5899–5903; [DOI] [PubMed] [Google Scholar]; (n) Mandal R and Sundararaju B, Org. Lett, 2017, 19, 2544–2547. [DOI] [PubMed] [Google Scholar]
- 7.(a) Zheng X-X, Du C, Zhao X-M, Zhu X, Suo J-F, Hao X-Q, Niu J-L and Song M-P, J. Org. Chem, 2016, 81, 4002–4011; [DOI] [PubMed] [Google Scholar]; (b) Cera G, Haven T and Ackermann L, Chem. Commun, 2017, 53, 6460–6463; [DOI] [PubMed] [Google Scholar]; (c) Misal Castro LC, Obata A, Aihara Y and Chatani N, Chem. – Eur. J, 2016, 22, 1362–1367; [DOI] [PubMed] [Google Scholar]; (d) Matsubara T, Ilies L and Nakamura E, Chem. – Asian J, 2016, 11, 380–384; [DOI] [PubMed] [Google Scholar]; (e) Yang J and Yoshikai N, Angew. Chem., Int. Ed, 2016, 55, 2870–2874; [DOI] [PubMed] [Google Scholar]; (f) Fallon BJ, Derat E, Amatore M, Aubert C, Chemla F, Ferreira F, Perez-Luna A and Petit M, J. Am. Chem. Soc, 2015, 137, 2448–2451. A metal-free approach: [DOI] [PubMed] [Google Scholar]; (g) Zhao Y, Shi C, Su X and Xia W, Chem. Commun, 2020, 56, 5259–5262. [DOI] [PubMed] [Google Scholar]
- 8.(a) Desai LV, Stowers KJ and Sanford MS, J. Am. Chem. Soc, 2008, 130, 13285–13293; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cernak T, Dykstra KD, Tyagarajan S, Vachal P and Krska SW, Chem. Soc. Rev, 2016, 45, 546–576; [DOI] [PubMed] [Google Scholar]; (c) Shang M, Sun S-Z, Dai H-X and Yu J-Q, J. Am. Chem. Soc, 2014, 136, 3354–3357; [DOI] [PubMed] [Google Scholar]; (d) Shang M, Wang M-M, Saint-Denis TG, Li M-H, Dai H-X and Yu J-Q, Angew. Chem., Int. Ed, 2017, 56, 5317–5321; [DOI] [PubMed] [Google Scholar]; (e) Liu Y-J, Xu H, Kong W-J, Shang M, Dai H-X and Yu J-Q, Nature, 2014, 515, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Sanjosé-Orduna J, Gallego D, Garcia-Roca A, Martin E, Benet-Buchholz J and Pérez-Temprano MH, Angew. Chem., Int. Ed, 2017, 56, 12137–12141; [DOI] [PubMed] [Google Scholar]; (b) Martínez de Salinas S, Sanjosé-Orduna J, Odena C, Barranco S, Benet-Buchholz J and Pérez-Temprano MH, Angew. Chem., Int. Ed, 2020, 59, 6239–6243. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Ji X, Wu W and Jiang H, Chem. Soc. Rev, 2015, 44, 1155–1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


