PREFACE
The American College of Cardiology (ACC) develops a wide range of policy documents to provide members with guidance on clinical topics. Although Clinical Practice Guidelines remain the primary mechanism for offering evidence-based recommendations, such guidelines may contain gaps in their guidance regarding clinical decision making, particularly when equipoise is present in a topic. Expert Consensus Documents are intended to provide guidance for clinicians in areas in which evidence may be limited, new and evolving, or lack sufficient data to fully inform clinical decision making.
To increase the impact of ACC clinical policy on patient care, an ACC Presidential Task Force was formed in 2014 to examine the processes and format of ACC’s clinical documents. The main recommendation of the Task Force was a new focus on concise decision pathways and/or key points of care, instead of the traditional longer documents. The Task Force also established criteria for identifying high-value clinical topics to be addressed, as well as an innovative approach to collecting stakeholder input through roundtable or think tank meetings. To complement the new focus on brief decision pathways and key points, Expert Consensus Documents were rebranded “Expert Consensus Decision Pathways.”
Although Decision Pathways have a new format, they maintain the same goal of Expert Consensus Documents: to develop clinical policy based on expert opinion in areas in which important clinical decisions are not adequately addressed by existing trials. Expert Consensus Decision Pathways are designed to complement the guidelines and bridge remaining gaps in clinical guidance. In some cases, topics covered by Expert Consensus Decision Pathways will be addressed subsequently by ACC/American Heart Association (AHA) guidelines as the evidence base evolves. The writing groups are charged with developing algorithms that are more actionable and can be implemented in the form of tools or applications to accelerate the use of these documents at the point of care. ECDPs are intended not to provide a single correct answer, but to encourage clinicians to ask certain questions and consider important factors as they reach a decision on a treatment plan together with patients. There may be multiple pathways that can be taken for treatment decisions, and the goal is to help clinicians and patients make a more informed decision together.
James L. Januzzi, MD, FACC
Chair, ACC Task Force on Expert Consensus Decision Pathways
1. INTRODUCTION
Despite major therapeutic advances leading to improved outcomes over the past 2 decades, cardiovascular (CV) disease remains the leading cause of morbidity and mortality in patients with type 2 diabetes (T2D) (1). Over that time, the prevalence of T2D has increased, while the excess risk of adverse CV events in patients with T2D (compared with patients without diabetes) has remained largely unchanged (2). Accordingly, the development of treatment strategies to improve CV outcomes in this vulnerable patient population remains a major priority.
Diabetes is typically thought of as a disease of elevated blood glucose (3). Although large clinical trials have consistently demonstrated an improvement in microvascular outcomes with intensive versus conservative glucose control, similar results have not been demonstrated for CV outcomes, despite the clinically important differences in hemoglobin A1C (A1C) achieved between treatment groups in glucose-lowering trials (4-7). On the basis of its extensive record of safety and efficacy with regard to glucose lowering, current Standards of Medical Care documents from the American Diabetes Association (ADA) recommend metformin together with lifestyle management as the first-line approach for patients with T2D (8).
The opportunity for CV disease prevention in patients with T2D has recently expanded. Certain sodium-glucose cotransporter 2 (SGLT) inhibitors and glucagon-like peptide 1 receptor agonists (GLP-1RAs) have shown significant reductions in the risk of major adverse cardiovascular events (MACE) (9-12). Although the exact mechanisms of CV benefit remain uncertain, they appear to be unrelated to the direct glucose-lowering effects of these agents.
The arrival of these new agents proven to reduce adverse CV outcomes in patients with T2D has triggered a major paradigm shift beyond glucose control, to a broader strategy of comprehensive CV risk reduction (8). The potential of these new compounds has also stimulated reexamination of the traditional roles of various medical specialties in the management of T2D, compelling CV disease specialists to adopt a more active role in prescribing drugs that may previously have been seen primarily as glucose-modifying therapies and creating a need for a collaborative, interprofessional, and multidisciplinary approach to managing this high-risk patient group. The purpose of this document is to summarize key elements from emerging studies, and to provide succinct, practical guidance on the use of specific glucose-lowering agents for reducing CV risk in patients with T2D and clinical atherosclerotic cardiovascular disease (ASCVD).
1.1. A Focus on Comprehensive CV Risk Reduction in T2D
Although the primary focus of patients, clinicians, and the healthcare system should be the prevention of T2D (13), a significant proportion of patients cared for by CV clinicians have known T2D, undiagnosed diabetes, or pre-diabetes (14). Because most morbidity and mortality in T2D comes from macrovascular events (15), the CV specialist has a key role in optimizing these patients’ care.
The CV specialist is well-positioned to address 3 key areas in the management of patients with T2D: screening for T2D in their patients with or at high risk of CVD, aggressively treating CV risk factors, and incorporating the data for newer antihyperglycemic agents into routine practice. Data from the National Cardiovascular Disease Registry PINNACLE program show that only 13% of outpatients in the United States with coronary artery disease cared for by cardiologists are screened for T2D (16). There is also a need for improvement in comprehensive CV risk factor control among patients with T2D (17), as current care delivery is often fragmented, episodic, and focused on treating acute events. Comprehensive risk factor control reduces events and improves survival in patients with T2D (18,19). This includes encouraging a healthy diet, regular physical activity, and weight loss, and assiduous control of blood pressure, (20) lowering of blood lipids, (21,22), and use of antiplatelet agents in accordance with current treatment guidelines (8,22,23).
Beyond these core recommendations, specialists in CV medicine should be aware of the strong clinical evidence regarding new glucose-lowering therapies that lower CV risk. A firm understanding of the net clinical benefit of these agents is important given that patients with T2D and CV disease frequently follow-up with their CV specialists. Such encounters are an ideal time to review the patient’s overall management and to consider the institution of these novel agents to favorably impact patient care and outcomes.
2. METHODS
The ACC created the Heart House Roundtable, a structured format of interactive discussion among a broad group of stakeholders from different professions and disciplines within medicine to address high-value topics and issues that clinicians and patients face daily, such as the treatment of CV disease in patients with T2D. The planning committee for the “Managing CV Disease Risk in Diabetes” Roundtable was led by Mikhail Kosiborod, MD, FACC, FAHA, and Larry Sperling, MD, FACC. To accommodate the multiple perspectives concerning new therapeutic options for patients with T2D, the Roundtable included several experts in diverse medical specialty areas, such as cardiology, family medicine, internal medicine, and endocrinology, and included physicians, nurses, advanced practice providers, and pharmacists. Recognizing the significant impact of recently available cardiovascular outcomes trial data, the planning committee carefully crafted an agenda that allowed participants to review and discuss the real-world challenges faced in working toward comanaging T2D and CV disease for improved patient outcomes.
Each of the 3 sessions consisted of 3 presentations, followed by individual table discussions. The interactive discussions were facilitated by questions developed by the planning committee with the goal of obtaining deep and broad insights into specific issues in each of the topic areas, along with recommendations for solutions, to inform the development of practical guidance, support, and tools for clinicians.
During the interactive table discussions, ACC consistently heard the following from participants: 1) there is a need for a paradigm shift from focusing on glycemic control alone to focusing more comprehensively on reducing CV risk and preventing CV death; and 2) there is a need to acknowledge that some of these emerging medical therapies have been proven to reduce CV death in patients with established or who are at high risk for CV disease, and that CV clinicians therefore have a role in prescribing them. Thus, the ACC saw an opportunity to provide guidance to fill the current gap between CV clinicians and diabetes care providers who jointly manage patients with T2D and ASCVD. To support this effort, a writing committee of multidisciplinary experts was convened and tasked with developing an ECDP providing guidance on the use of novel antidiabetic agents proven to reduce CV risk in patients with T2D and established ASCVD. The first writing committee meeting convened via teleconference on October 25, 2017, and recurred approximately every 2 weeks. An in-person writing committee meeting was held on December 18, 2017. At the end of the meeting, individual writing assignments were distributed and the work on assembling a formal document began. Drafted in sections, the documents were reviewed by the cochairs as they were written, and were edited to reconcile with each other and thereby convey the group consensus. Biweekly teleconferences were held to incorporate feedback of the entire panel into the document being assembled. Conference calls of the writing committee were confidential and were attended only by committee members and ACC staff. Differences were resolved by consensus among the group, and no portions of the document required administrative decision overrides.
The work of the writing committee was supported only by the ACC and did not have any commercial support. Writing committee members were all unpaid volunteers. All members of the writing committee and peer reviewers were required to fully disclose any relationships with industry (RWI) or other real or perceived conflicts of interest. The full panel and the cochairs take responsibility for the content of this document. In keeping with ACC policy, most members of the writing committee had no relevant relationships with industry. The formal peer review process was completed consistent with ACC policy and included a public comment period to obtain further feedback. Following reconciliation of all comments, this document was approved for publication.
3. ASSUMPTIONS AND DEFINITIONS
To facilitate interpretation of the recommendations provided in this Expert Consensus Decision Pathway, specific assumptions were made by the Writing Committee as follows:
3.1. General Clinical Assumptions
The focus of this effort, including Expert Consensus Decision Pathway recommendations, only applies to patients with both T2D and clinically evident atherosclerotic cardiovascular disease (ASCVD), which is defined below. Extending inferences beyond this specific population should be done with caution.
The Writing Committee endorses the evidence-based approaches to CV disease risk reduction recommended in the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (24).
The Writing Committee endorses the evidence-based approaches to diabetes management in the ADA Standards of Care (3).
The Writing Committee endorses the evidence-based approaches to heart failure (HF) therapy and management enumerated in the 2013 ACCF/AHA Guideline for the Management of Heart Failure and the 2016 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure and 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction (25-27).
Optimal patient care decisions should properly reflect the patient’s preferences and priorities as well as those of the managing clinician.
This Expert Consensus Decision Pathway is not intended to supersede good clinical judgement. The treating clinician should seek input as needed from relevant experts (e.g., pharmacists, cardiologists, endocrinologists).
This Expert Consensus Decision Pathway is based on the best data currently available. New information is being generated rapidly (e.g., cardiovascular outcomes trials of additional agents), and as these data become available, they will impact the recommendations made here. Clinicians should be careful to incorporate relevant information published after this document.
A background effort aimed at comprehensive risk reduction is essential, using the full complement of diet, exercise, and lifestyle recommendations, as well as risk factor modification and other preventive medical therapies described in the ADA Standards of Care and/or the applicable AHA/ACC guidelines or clinical consensus recommendations.
Although implementing relevant portions of these recommendations in the acute inpatient setting may be reasonable, this document is primarily focused on management in the outpatient ambulatory setting.
3.2. Definitions
ASCVD:
atherosclerotic cardiovascular disease. ASCVD is defined by the inclusion criteria for the randomized trials referenced in this document but is consistent with the definition used in other recent guidelines, such as those for lipid therapy (28). Those criteria generally include a history of an acute coronary syndrome or myocardial infarction, stable or unstable angina, coronary heart disease with or without revascularization, other arterial revascularization, stroke, or peripheral artery disease assumed to be atherosclerotic in origin. A number of trials included a minority of patients without clinical ASCVD but required a high burden of risk factors in those patients.
MACE:
In the context of this document, this is either a “3-point MACE” composite of nonfatal myocardial infarction (MI), nonfatal stroke, or CV mortality, or a “4-point MACE,” which also includes unstable angina.
4. PATHWAY SUMMARY GRAPHIC
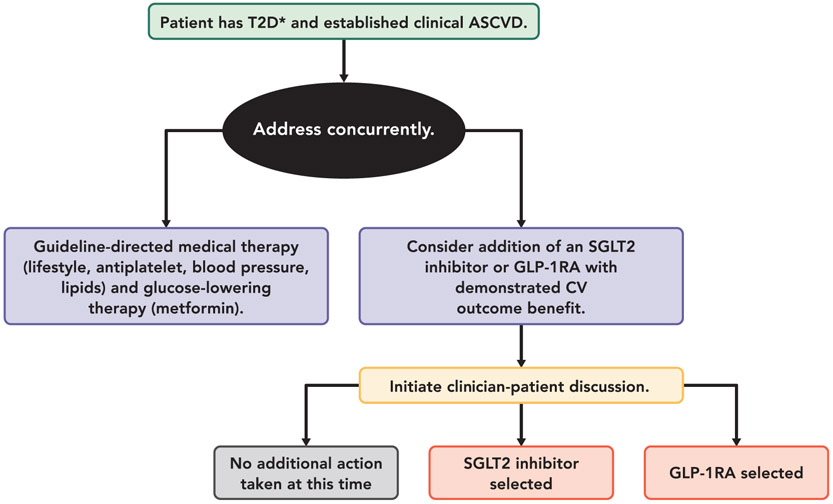
Figure 1 provides an overview of what is covered in the Expert Consensus Decision Pathway. See each section for more detailed considerations and guidance.
FIGURE 1. Summary Graphic.
*Most trials of SGLT2i and GLP-1RA required baseline A1C ≥7% (Example: EXSCEL Trial required HbA1c ≥ 6.5%), and most patients were already on metformin as first-line therapy if tolerated and not contraindicated
Abbreviations: ASCVD = atherosclerotic cardiovascular disease; CV = cardiovascular; GLP-1RA = glucagon-like peptide-1 receptor agonist; SGLT2 = sodium-glucose cotransporter-2; T2D = type 2 diabetes.
5. DESCRIPTION AND RATIONALE
CV specialists should be aware of the evidence supporting the use of SGLT2 inhibitors and GLP-1RAs to reduce risk in patients with T2D and established ASCVD.
5.1. SGLT2 Inhibitors
SGLT2 inhibitors have emerged as important new oral therapies for patients with T2D (1). Large, randomized controlled clinical trials in patients with T2D, most of whom had established ASCVD (1), have demonstrated that 2 drugs in this class, empagliflozin and canagliflozin (see Tables 1 and 2), reduce MACE and HF hospitalization (2-4). Empagliflozin also significantly reduced the risk of CV and all-cause mortality.
TABLE 1.
FDA Indications and Doses for SGLT2 Inhibitors With Cardiovascular Outcomes Trial Data
| Empagliflozin | Canagliflozin | |
|---|---|---|
| Doses | ■ 10 mg PO daily | ■ 100 mg PO daily |
| ■ 25 mg PO daily | ■ May increase to 300 mg daily if needed in those who have an eGFR ≥60 mL/min/1.73 m2 | |
| FDA-approved Indications | ■ Improve glycemic control in adults with T2D | ■ Improve glycemic control in adults with T2D |
| ■ Reduce risk of CV death in adults with T2D and CV disease | ||
| Dose modifications* | ■ eGFR ≥45 mL/min/1.73 m2: No dose adjustment required. | ■ eGFR ≥60 mL/min/1.73 m2: No dose adjustment required. |
| ■ eGFR <45 mL/min/1.73 m2: Do not initiate; discontinue if eGFR persistently below 45 mL/min/1.73 m2* | ■ eGFR 45 to 59 mL/min/1.73 m2: Do not exceed 100 mg/day. | |
| ■ eGFR <45 mL/min/1.73 m2: Do not initiate; discontinue if eGFR persistently below 45 mL/min/1.73 m2 |
SGLT2 inhibitor doses are modified in patients with impaired renal function because the medications are less effective in lowering glucose concentrations when renal function is impaired, rather than because of specific safety concerns. The CV benefit of these medications appears to be present down to eGFR OF 30 mL/min/1.73 m2. Trials of SGLT2 inhibitors are underway in patients with CKD using progression of kidney disease as their key clincial outcomes.
CKD = chronic kidney disease; CV = cardiovascular; eGFR = estimated glomerular filtration rate; FDA = Food and Drug Administration; PO = orally; SGLT2 = sodium-glucose cotransporter-2; T2D = type 2 diabetes.
TABLE 2.
Summary of the Published SGLT2 Inhibitor Cardiovascular Outcomes Trials
| EMPA-REG OUTCOME (29) |
CANVAS/ CANVAS-R (12) |
|
|---|---|---|
| Patients enrolled | n = 7,020 | n = 10,142 |
| Drug | Empagliflozin | Canagliflozin |
| Dose | 10 or 25 mg PO daily | 100 or 300 mg PO daily |
| Median duration of follow-up (years) | 3.1 | 2.4 |
| Mean baseline A1C (%) | 8.1 | 8.2 |
| Mean duration of diabetes (years) | N/A* | 13.5 |
| Baseline metformin use (%) | 74 | 77 |
| Baseline statin use (%) | 77 | 75 |
| Baseline prevalence of CV disease/HF (%) | 100/11 | 72/14 |
| Primary outcome (HR [95% CI])† | 0.86 (0.74-0.99) | 0.86 (0.75-0.97) |
| CV death (HR [95% CI]) | 0.62 (0.49-0.77) | 0.87 (0.72-1.06) |
| Fatal or non-fatal MI (HR [95% CI]) | 0.87 (0.70-1.09) | 0.89 (0.73-1.09) |
| Fatal or non-fatal stroke (HR [95% CI]) | 1.18 (0.89-1.56) | 0.87 (0.69-1.09) |
| All-cause mortality (HR [95% CI]) | 0.68 (0.57-0.82) | 0.87 (0.74-1.01) |
| HF hospitalization (HR [95% CI]) | 0.65 (0.50-0.85) | 0.67 (0.52-0.87) |
Mean duration of diabetes was not provided for EMPA-REG OUTCOME, but 57% of patients enrolled had diabetes for more than 10 years.
The primary outcome was three point MACE, a composite of nonfatal myocardial infarction, nonfatal stroke, and CV death. The p value for superiority for the primary endpoint for empagliflozin (all doses) vs. placebo was 0.04, and the p value for superiority for the primary endpoint for canagliflozin (all doses) vs. placebo was 0.02. The p values for the other comparisons are available in the primary EMPA-REG OUTCOME report but were not published in the CANVAS/CANVAS-R report. The incidence of the primary endpoint in EMPA-REG was 43.9 and 37.4 events per 1,000 patient-years in the placebo and active therapy groups, respectively. In the CANVAS/CANVAS-R trials the incidence of the primary endpoint was 31.5 and 26.9 events per 1,000 patient-years observation, respectively.
Hazard ratios and (95% confidence intervals) are presented.
A1C = hemoglobin A1C; CANVAS/CANVAS-R = Canagliflozin Cardiovascular Assessment Study; CI = confidence interval; CV = cardiovascular; EMPA-REG OUTCOME = Empagliflozin Cardiovascular Outcome Event Trial in T2D Patients-Remove Excess Glucose Outcomes; HF = heart failure; HR = hazard ratio; PO = orally; SGLT2 = sodium-glucose cotransporter-2.
5.1.1. SGLT2 Inhibitors: Mechanism of Action
SGLT2 is a sodium-glucose cotransporter in the proximal tubule of the nephron that is responsible for approximately 90% of urinary glucose reabsorption. Inhibition of SGLT2 results in glucose lowering through induction of glucosuria. This effect is more pronounced in the setting of hyperglycemia, where significant amounts of glucose are filtered into the urine. Glucosuria diminishes significantly as blood glucose normalizes (5). As such, the risk of hypoglycemia for patients taking SGLT2 inhibitors is quite low unless they are used concomitantly with insulin or insulin secretagogues (such as sulfonylureas and glinides). Beyond their effect on blood glucose, SGLT2 inhibitors also cause diuretic and natriuretic effects, weight loss, and lowering of systolic blood pressure (29).
5.1.2. SGLT2 Inhibitors: CV Benefits
To date, 2 large cardiovascular outcomes trials, the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in T2D Patients-Remove Excess Glucose) (30) and the CANVAS (Canagliflozin Cardiovascular Assessment Study) Program (comprising the CANVAS and CANVAS-R trials) (12) have demonstrated significant reductions in MACE in patients randomized to receive SGLT2 inhibitor therapy compared with placebo (Table 2). Large observational analyses based on administrative claims data, national registries data, and electronic medical records of patients treated in current clinical practice have reported similar clinical benefits (see Table 3) (31,32).
TABLE 3.
Selected Observational Studies of CV Benefits of SGLT2 Inhibitors
| CVD-REAL (31) | Patorno et al. (32) | EASEL (33) | CVD-REAL 2 (34) | |
|---|---|---|---|---|
| Size | n = 309,056 | n = 224,999 | n = 25,258 | n >400,000 |
| Agent | Canagliflozin (53%), Dapagliflozin (42%), Empagliflozin (5%) | Canagliflozin | Canagliflozin (58%), Empagliflozin (26%), Dapagliflozin (16%) | Dapagliflozin (75%), Empagliflozin (9%), Ipragliflozin (8%), Canagliflozin (4%), Tofogliflozin (3%), Luseogliflozin (1%) |
| Mean duration of follow-up | <1 year | <1 year | 1.6 years | >1 year |
| Baseline A1C | N/R | 8.8-8.9 | N/R | N/R |
| Proportion with established cardiovascular disease* at baseline | 13% | 16% to 18% | 100% | 27% |
| All-cause death, MI, stroke HR (95% CI) | N/R | N/R | 0.67 (0.60-0.75) | N/R |
| Hospital admission for MI or stroke HR (95% CI) | N/R | 0.89 (0.68-1.17) | N/R | N/R |
| CV death | N/R | N/R | N/R | N/R |
| MI | N/R | 0.91 (0.64-1.29) | 0.81 (0.64-1.03) | 0.81 (0.74-0.88) |
| Stroke | N/R | 0.81 (0.54-1.22) | 0.85 (0.66-1.10) | 0.68 (0.55-0.84) |
| All-cause death | 0.49 (0.41-0.57) | 0.66 (0.25-1.74) | 0.57 (0.49-0.66) | 0.51 (0.37-0.70) |
| HF hospitalization | 0.61 (0.51-0.73) | 0.70 (0.54-0.92) | 0.57 (0.45-0.73) | 0.64 (0.50-0.82) |
The specific definitions of established cardiovascular disease vary by study but generally include a history of myocardial infarction, unstable angina, stroke, transient ischemic attack, coronary revascularization, heart failure, or peripheral artery disease.
A1C = hemoglobin A1C; CI = confidence interval; CV = cardiovascular; CVD-REAL = Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors; EASEL = Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World; HF = heart failure; HR = hazard ratio; MACE = major adverse cardiovascular event; MI = myocardial infarction; N/R = not reported; SGLT2 = sodium-glucose cotransporter-2.
Patients randomized to empagliflozin experienced a 14% relative risk reduction in the primary composite endpoint of CV death, MI, or stroke (hazard ratio [HR]: 0.86; 95% CI: 0.74 to 0.99) compared with placebo. This reduction in the primary outcome, as well as the observed 32% reduction in all-cause mortality (HR: 0.68; 95% CI: 0.57 to 0.82) were driven predominantly by a 38% reduction in CV death (HR: 0.62; 95% CI: 0.49 to 0.77) (35). The effects of empagliflozin on fatal or nonfatal MI were more modest (HR: 0.87; 95% CI: 0.70 to 1.09), and there was the suggestion of an increased risk of fatal or nonfatal stroke, with confidence limits that broadly overlapped 1.00 (HR: 1.18; 95% CI: 0.89 to 1.56). Importantly, a secondary endpoint of HF hospitalization was reduced by 35% (HR: 0.65; 95% CI: 0.50 to 0.85). Separation in the cumulative event curves suggested an early benefit of the compound (36) and was consistent across patient subgroups with or without prevalent HF at study entry (37). To date, empagliflozin is the only SGLT2 inhibitor specifically approved by the U.S. Food and Drug Administration (FDA) to reduce the risk of CV death in adults with T2D and established CV disease (38).
The CANVAS program enrolled 4,330 and 5,812 patients, 72% of whom had established ASCVD, to CANVAS and CANVAS-R, respectively. Study participants were randomized to placebo or canagliflozin (100 or 300 mg in CANVAS, and 100 mg with an optional increase to 300 mg in CANVAS-R). Results from CANVAS and CANVAS-R are mostly consistent with those of EMPA-REG OUTCOME. Analyses of the effects of canagliflozin versus placebo on CV and all-cause death, while underpowered, were directionally consistent with the primary endpoint (12,39). As with EMPA-REG OUTCOME, no difference in outcomes was seen between SGLT2 inhibitor doses. The combined analysis of the 2 CANVAS trials demonstrated a 14% relative reduction in the primary endpoint of triple MACE (a composite of nonfatal MI, nonfatal stroke, and CV death; HR: 0.86; 95% CI: 0.75 to 0.97 from 31.5 to 26.9 events per 1000 person years) compared with placebo (12,39). Point estimates for each of the individual components of the primary outcome were consistently in favor of SGLT2 inhibitor therapy: CV death (HR: 0.87; 95% CI: 0.72 to 1.06); fatal or nonfatal MI (HR: 0.89; 95% CI: 0.73 to 1.09), and fatal or nonfatal stroke (HR: 0.87; 95% CI: 0.69 to 1.09), as was the point estimate for reduction in all-cause mortality (HR: 0.87; 95% CI: 0.74 to 1.01). In exploratory results from the CANVAS program, a significant 33% reduction in the secondary endpoint of hospitalization for HF (HR: 0.67; 95% CI: 0.52 to 0.87) was observed, similar to the results of EMPA-REG OUTCOME. Prospective cardiovsacular outcomes trials of the SGLT2 inhibitors dapagliflozin (40) and ertugliflozin (41) as well as the SGLT2 and SGLT1 inhibitor sotagliflozin (42) are currently underway.
Observational data from large international studies of insurance claims, registries, and electronic medical records from a broad population of T2D patients seen in clinical practice, referenced in Table 3, are largely consistent with the results observed in the randomized trials in the previous text. For example, in CVD-REAL (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors), compared with a propensity-matched cohort of patients receiving other oral medications for T2D, those receiving SGLT2 inhibitors had a 51% lower associated risk of all-cause mortality (HR: 0.49; 95% CI: 0.41 to 0.57) and a 39% lower associated risk of hospitalization for HF (HR: 0.61; 95% CI: 0.51 to 0.73) (31). Comparable results were found in the even larger CVD-REAL 2 study, with a 49% lower risk of all-cause mortality, 36% lower risk of hospitalization for HF, and lower risks of MI and stroke (34). These observational data have important limitations and may overestimate the effectiveness of these medications, despite the use of sophisticated statistical techniques (43). Nonetheless, these observations from outside of the tightly controlled clinical trial setting provide support for the CV effects of this class of medications.
5.1.3. SGLT2 Inhibitors: Non-CV Benefits
Both empagliflozin and canagliflozin have favorable effects on kidney function (12,44,45). In the EMPA-REG OUTCOME study, empagliflozin slowed the progression of kidney disease, reducing incident or worsening nephropathy, which was defined as a progression to macroalbuminuria (HR: 0.61, 95% CI: 0.53 to 0.70). Mechanisms to explain these observations may include tubuloglomerular feedback, reduction in glomerular hypertension, containment of hyperfiltration injury, and effects on sodium-hydrogen exchange. In the CANVAS and CANVAS-R trials, progression of albuminuria occurred less frequently (HR: 0.73; 95% CI: 0.67 to 0.79) and regression of albuminuria occurred more frequently (HR: 1.70; 95% CI: 1.51 to 1.91) among those assigned to canagliflozin than among those assigned to placebo. A renal composite outcome in CANVAS of 40% reduction in estimated glomerular filtration rate (eGFR), renal replacement therapy, or renal death was improved by 40% in those assigned to active therapy (HR: 0.60; 95% CI: 0.47 to 0.77), although whether this effect was due to a benefit seen in 1, 2, or all 3 of the components of the composite endpoint has not yet been reported (12).
5.1.4. SGLT2 Inhibitors: Safety Concerns
The contraindications and safety concerns of SGLT2 inhibitors are included in Table 4.
TABLE 4.
Contraindications and Cautions for SGLT2 Inhibitors
| Contraindications | Cautions* |
|---|---|
| ■ History of serious hyper-sensitivity reaction to drug ■ Severe renal impairment, ESRD, or dialysis† |
■ May cause intravascular volume contraction, particularly in patients with renal impairment or low systolic blood pressure, those on diuretics, or the elderly |
| ■ Increased incidence of bone fractures reported with canagliflozin | |
| ■ Hypoglycemia risk increased with insulin and insulin secretagogues (e.g., sulfonylureas); a lower dose of insulin or the insulin secretagogue may be required | |
| ■ Increased risk of mycotic genital infections. | |
| ■ Euglycemic ketoacidosis in vulnerable patients | |
| ■ History of prior amputation, severe peripheral vascular disease, neuropathy, or diabetic foot ulcers. This caution is for canagliflozin and ertugliflozin. No increased risk of amputation has been seen for empagliflozin or dapagliflozin to date. | |
| ■ History of osteoporosis. This caution is for canagliflozin. |
SGLT2 inhibitors are not for the treatment of type 1 diabetes.
SGLT2 inhibitors have shown benefit for CV event reduction down to eGFR of 30 mL/min/m2
ESRD = end-stage renal disease; SGLT2 = sodium-glucose cotransporter 2.
An increased risk for genital mycotic infections (mostly candida vaginitis in women, balanitis in men) has been seen with SGLT2 inhibitors (12,29,46,47). These infections are not usually serious, tend to resolve with a brief course of antifungal agents, and rarely recur (12,29). Although there have been spontaneous postmarketing reports of pyelonephritis and urosepsis requiring hospitalization in patients receiving SGLT2 inhibitors, large clinical trials have shown no difference in the rates of any urinary tract infections or serious urinary tract infections between SGLT2 inhibitors and placebo. Rare postmarketing reports of necrotizing fasciitis of the perineum (12 cases over 5 years, with more than 1.7 million patients being prescribed SGLT2 inhibitors in 2017) have led the FDA to request a warning to SGLT2 inhibitor prescribing instructions; whether these infections are causally related to SGLT2 inhibitor use is unclear.
Case reports have pointed to an increased risk of diabetic ketoacidosis with SGLT2 inhibitors in the absence of significant hyperglycemia, often called “euglycemic diabetic ketoacidosis,” although moderate hyperglycemia is common. This risk has been shown to be very low in the large randomized controlled trials of patients with T2D, particularly in those not requiring insulin therapy (48). Patients with signs or symptoms of ketoacidosis, such as dyspnea, nausea, vomiting, and abdominal pain, should be instructed to discontinue SGLT2 inhibitors and seek immediate medical attention (29). Providers should be aware of precipitating factors and treatment strategies, which have been reviewed recently (49).
Canagliflozin has been associated with increased risk for lower limb amputation (6.3 vs. 3.4 amputations per 1,000 patient-years of observation after a median follow-up of 126 weeks; p < 0.001) (12,22) prompting the FDA to add a black box warning to the canagliflozin prescribing information in May 2017 (50). A numerical excess of amputations in the phase III trials with ertugliflozin (0.1% [n = 1] with placebo vs. 0.5% [n = 8] with the 15 mg dose) is reported in the prescribing information. In post-hoc analyses from the EMPA-REG OUTCOME study, this risk has not been observed with empagliflozin or with dapagliflozin to date (51-53). Whether amputation risk represents a class effect remains unclear, but vigilance is suggested in those with a history of amputation, peripheral arterial disease, neuropathy, or diabetic foot ulcers.
Bone fractures (including from low-trauma events) were observed to be more common among those treated with canagliflozin than with placebo in CANVAS but not in the CANVAS-R trial (3). Last, given a diuretic and antihypertensive effect, SGLT2 inhibitors may increase the risk of volume depletion and hypotension; in large randomized control trials, this risk was slightly higher with canagliflozin than with placebo but was not increased with empagliflozin. Although there were early potential concerns about acute kidney injury with SGLT2 inhibitors, these risks have not been observed in large randomized control trials to date. The FDA labels for all SGLT2 inhibitors currently suggest discontinuing therapy in the context of acute kidney injury or renal impairment; we await the results of dedicated prospective trials testing the safety and efficacy of these medications in patients with renal insufficiency.
5.1.5. Hypothetical Mechanisms Underlying CV Benefits of SGLT2 Inhibitors
The CV benefits of SGLT2 inhibitors may derive from their diuretic and natriuretic effect, weight loss, and lowering of blood pressure (30). They may also derive from effects on the sympathetic nervous system, and inhibition of the sodium-hydrogen exchanger, possibly reducing cardiac injury, hypertrophy, fibrosis, remodeling, and systolic dysfunction (54,55). SGLT2 inhibitors may shift myocardial metabolism away from free fatty acids and glucose oxidation toward more energy-favorable ketone bodies (which may improve myocardial work efficiency and function) (56). In a randomized, placebo-controlled trial of more than 600 patients, those receiving canagliflozin had lower age-related increases of both amino-terminal pro-B-type natriuretic peptide and highly sensitive troponin I (57) compared with placebo–results that support a myocardial protective effect of SGLT2 inhibitors. A summary of possible mechanisms is detailed in Table 5.
TABLE 5.
Potential Mechanisms by Which SGLT2 Inhibition Decreases CV Events
| Effect | Consequence |
|---|---|
| ■ Diuresis | ■ Reduced filling pressures, pre-/afterload reduction |
| ■ Natriuresis | ■ Reduced filling pressures, pre-/afterload reduction |
| ■ Blood pressure lowering | ■ Reduced myocardial work, reduced filling pressures, pre-/afterload reduction |
| ■ Weight loss | ■ Improved CV risk profile, lower blood pressure |
| ■ Reduction in/prevention of albuminuria, slowing of kidney function decline | ■ Reduction in kidney risk profile, possibly fewer incident CV events, including less HF |
| ■ Effects on myocardial and kidney metabolism: shift to more efficient ketone-based metabolism | ■ Improved metabolic efficiency, less myocardial workload |
| ■ Blockade of sodium-hydrogen cotransporter | ■ Tissue protection: reduction in kidney and myocardial injury |
| ■ Reduction in sympathetic tone | ■ Reduce blood pressure and arrhythmia |
CV = cardiovascular; HF = heart failure; SGLT2 = sodium-glucose cotransporter 2.
5.2. GLP-IRAs
GLP-1RA has demonstrated benefits for CV risk in patients with T2D. Of the 6 FDA-approved GLP-1RAs, to date only liraglutide has been definitively demonstrated to significantly reduce CV events. A similar benefit was observed in a moderately sized trial of semaglutide, which was designed and powered as a noninferiority trial (see Tables 6 and 7). A third, exenatide once weekly, showed numerically favorable results for 3-point MACE when compared with placebo; however, these results did not reach statistical significance (58). A fourth GLP-1RA, lixisenatide, does not appear to lower risk for ASCVD events in those randomized after an acute coronary syndrome (59). Taken together, these results suggest the potential for clinically relevant heterogeneity within the class, although some of the differences between compounds may also be due to the doses tested or specific drug characteristics, or due to variations between patient populations and trial designs.
TABLE 6.
FDA Indications and Doses for GLP-1RAs With Cardiovascular Outcomes Trial Data
| Liraglutide | Semaglutide | Lixisenatide | Exenatide QW | |
|---|---|---|---|---|
| Doses | ■ Initiate 0.6 mg SC daily. | ■ Initiate 0.25 mg SC per week. | ■ 10 mcg SC daily | ■ 2 mg SC per week |
| ■ Titrate slowly to 1.8 mg or maximally tolerated dose based on prescribing information. | ■ Titrate slowly to maximally tolerated dose based on prescribing information. | ■ Titrate as tolerated to 20 mcg daily based on prescribing information. | ||
| FDA-approved indications | ■ Improve glycemic control in adults with T2D | ■ Improve glycemic control in adults with T2D | ■ Improve glycemic control in adults with T2D | ■ Improve glycemic control in adults with T2D |
| Reduce risk of MI, CVA, or CV death in adults with T2D and CV disease | ||||
| Dose modifications | ■ Up-titrate slowly to reduce nausea and vomiting. | ■ Up-titrate slowly to reduce nausea and vomiting. | ■ Up-titrate slowly to reduce nausea and vomiting. | ■ Discontinue if pancreatitis is suspected and do not restart if pancreatitis is confirmed. |
| ■ Discontinue if pancreatitis is suspected and do not restart if pancreatitis is confirmed. | ■ Discontinue if pancreatitis is suspected and do not restart if pancreatitis is confirmed. | ■ Discontinue if pancreatitis is suspected and do not restart if pancreatitis is confirmed. | ■ CrCl ≥60 mL/min: no dosage adjustment required | |
| ■ No dose adjustment necessary with renal or hepatic impairment; data in end-stage renal disease are limited. | ■ No dose adjustment necessary with renal or hepatic impairment; data in end-stage renal disease are limited. | ■ CrCl ≥30 mL/min: no dosage adjustment required ■ CrCl = 15 to 29 mL/min: use caution and monitor renal function | ■ CrCl = 30 to 59 mL/min: use caution ■ CrCl <30 mL/min: use not recommended | |
| ■ <15 mL/min: use not recommended |
CrCl = creatinine clearance; CV = cardiovascular; CVA = cerebrovascular accident; FDA = U.S. Food and Drug Administration; GLP-RA = glucagon-like peptide-1 receptor agonists; T2D = type 2 diabetes; SC = subcutaneous; QW = once weekly.
TABLE 7.
Summary of the GLP-1RA Cardiovascular Outcomes Trials
| LEADER (10) | SUSTAIN-6* (11) | EXSCEL (58) | ELIXA (59) | |
|---|---|---|---|---|
| Patients enrolled | 9,340 | 3,297 | 14,752 | 6,068 |
| Drug | Liraglutide | Semaglutide | Exenatide QW | Lixisenatide |
| Dose | 1.8 mg or max tolerated dose per day | 0.5 mg or 1 mg per week | 2 mg per week | 10 mcg or 20 mcg per day |
| Duration of follow up (years) | 3.8 | 2.1 | 3.2 | 2.1 |
| Baseline A1C | 8.7 | 8.7 | 8.0 | 7.7 |
| Mean duration of diabetes (years) | 12.8 | 13.9 | 12 | 9.3 |
| Baseline metformin use (%) | 76 | 73 | 77 | 66 |
| Baseline statin use (%) | 72 | 73 | 74 | 93 |
| Baseline prevalence of CV disease†/HF (%) | 81/18 | 72/24 | 73.1/16.2 | 100/22 |
| Primary outcome, HR (95% CI)‡ | 3-point MACE 0.87 (0.78-0.97) | 3-point MACE 0.74 (0.58-0.95) | 3-point MACE 0.91 (0.83-1.00) | 4-point MACE 1.02 (0.89-1.17) |
| CV death, HR (95% CI) | 0.78 (0.66-0.93) | 0.98 (0.65-1.48) | 0.88 (0.76-1.02) | 0.98 (0.78-1.22) |
| Fatal or nonfatal MI, HR (95% CI)§ | 0.86 (0.73-1.00) | 0.74 (0.51-1.08) | 0.97 (0.85-1.10) | 1.03 (0.87-1.22) |
| Fatal or nonfatal stroke, HR (95% CI)§ | 0.86 (0.71-1.06) | 0.61 (0.38-0.99) | 0.85 (0.70-1.03) | 1.12 (0.79-1.58) |
| All-cause mortality, HR (95% CI) | 0.85 (0.74-0.97) | 1.05 (0.74-1.50) | 0.86 (0.77-0.97) | 0.94 (0.78-1.13) |
| HF hospitalization, HR (95% CI) | 0.87 (0.73-1.05) | 1.11 (0.77-1.61) | 0.94 (0.78-1.13) | 0.96 (0.75-1.23) |
As noted in the text, SUSTAIN-6 was designed and powered as a noninferiority trial. Testing for superiority for the primary CV outcome was not prespecified.
SUSTAIN-6 reported that 72.2% of patient had established CV disease with or without chronic kidney disease, and 10.7% had chronic kidney disease without cardiovascular disease. In total, 83% had established CV disease including chronic kidney disease of stage 3 or higher.
Three-point MACE is a composite of CV death, myocardial infarction, or stroke. The 4-point MACE used in the ELIXA trial the composite of CV death, myocardial infarction, stroke, or hospitalization for unstable angina.
The risk estimates and 95% CI for SUSTAIN-6 and ELIXA are for nonfatal MI (excluding fatal MI) or nonfatal stroke (excluding fatal stroke). The effect estimates for the composite endpoints of fatal or nonfatal MI and fatal or nonfatal stroke were not available in the primary manuscripts.
A1C = hemoglobin A1C; CV = cardiovascular; ELIXA = Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome; EXSCEL =Exenatide Study of Cardiovascular Event Lowering; GLP-RA = glucagon-like peptide-1 receptor agonists; HF = heart failure; MACE = major adverse cardiovascular event; MI = myocardial infarction; QW = once weekly; LEADER =The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; SUSTAIN-6 = Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes.
5.2.1. GLP-1RAs: Mechanisms of Action
GLP-1 is a peptide hormone released from the distal ileum and colon after oral nutrient intake (60). Following administration of GLP-1RA, supraphysiologic concentrations of GLP-1 reduce glucose by increasing glucose-dependent insulin secretion and decreasing glucagon secretion, and by delaying gastric emptying, which leads to satiety (60).
5.2.2. GLP-1RAs: CV Benefits
Most GLP-1RA cardiovascular outcomes trials (see Table 7) used a 3-point MACE outcome of CV death, nonfatal myocardial infarction (MI), or nonfatal stroke. Inclusion criteria varied across trials. The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial randomized 9,340 patients with established ASCVD (81% of the total) or older patients with ASCVD risk factors (19% of the total) to either liraglutide or placebo (10). The 3-point MACE composite was reduced by 13% (HR: 0.87; 95% CI: 0.78 to 0.97; p = 0.01 for superiority) with liraglutide versus placebo. All components of the composite contributed to a reduction in 3-point MACE, and all-cause mortality was reduced by 15% (HR: 0.85; 95% CI: 0.74 to 0.97; p = 0.02). The reduction in all-cause mortality was driven by reduction in CV death. No reduction in HF events was noted in the LEADER trial (HR: 0.87; 95% CI: 0.73 to 1.05; p = 0.14). To date, liraglutide is the only GLP-1RA approved by the FDA to reduce the risk of MACE in adults with T2D and established CV disease (38).
The preapproval SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes) enrolled 3,297 patients using the same trial inclusion criteria as LEADER and had the same primary composite endpoint (11). Although the study was not powered for superiority, semaglutide reduced 3-point MACE by 26% (HR: 0.74; 95% CI: 0.58 to 0.95), with a consistent magnitude and direction of effect for the key components of nonfatal stroke (HR: 0.61; 95% CI: 0.38 to 0.99) and nonfatal MI (HR: 0.74; 95% CI: 0.51 to 1.08). No reduction in all-cause mortality (HR: 1.05; 95% CI: 0.74 to 1.50) or CV mortality (HR: 0.98; 95% CI: 0.65 to 1.48) was observed. No reduction in HF events was seen. A planned cardiovascular outcomes trials will assess whether an oral version of semaglutide is superior to placebo for CV event reduction (NCT02692716).
The EXSCEL (Exenatide Study of Cardiovascular Event Lowering) trial enrolled 14,752 subjects, approximately 70% of whom had established ASCVD, randomizing them to once-weekly exenatide versus usual care (58). Three-point MACE was directionally lower for exenatide compared with placebo, but this difference did not reach statistical significance (HR: 0.91, 95% CI: 0.83 to 1.00). All-cause mortality was lower in the once-weekly exenatide group (HR: 0.86, 95% CI: 0.77 to 0.97), and the direction and magnitude of effect of CV mortality was similar (HR: 0.88, 95% CI: 0.76 to 1.02). There was no difference in hospitalization for HF between exenatide and placebo. Lixisenatide, which was tested in 6,068 patients with acute coronary syndrome in the ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome) trial, did not show evidence for a reduction in a 4-point MACE composite outcome (CV death, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina), or in any components of that MACE composite, or hospitalization for HF. Two phase 2 trials have tested whether liraglutide can improve outcomes in heart failure. The FIGHT trial (Functional Impact of GLP-1 for Heart Failure Treatment, n = 300) and the LIVE trial (A Randomised, Double-blind, Placebo-controlled Study of the Effect of LIraglutide on Left VEntricular Function in Chronic Heart Failure Patients With and Without Type 2 Diabetes, n = 243) examined the use of liraglutide in patients with reduced ejection fraction (61,62). In FIGHT, there was no difference in the primary endpoint of a rank score of a composite of death, time to heart failure hospitalization, or time-averaged proportional change in NT-proBNP concentration by randomized treatment group (61). In LIFE, there was no difference in the primary endpoint (left ventricular ejection fraction) by randomized treatment group, but serious cardiac events were more common in the active (12 events) than in the placebo (3 events) arm (p = 0.04) (62). Ongoing cardiovascular outcome trials of other GLP-1RA may shed further light on whether this class of medications is safe and effective in patients with heart failure. Prospective cardiovascular outcomes trials of the GLP-1RAs dulaglutide and albiglutide are currently underway (63,64).
5.2.3. GLP-1RAs: Non-CV Benefits
Although it has yet to be confirmed in an independent randomized trial, analyses of existing trials suggest that the GLP-1RAs may provide renal benefits. In LEADER, liraglutide was associated with an approximately 20% reduction in the risk of a composite outcome of new-onset persistent macroalbuminuria, persistent doubling of the serum creatinine level, end-stage renal disease, or death due to renal disease regardless of baseline eGFR. This result was primarily due to a 26% reduction in persistent macroalbuminuria. In SUSTAIN-6, semaglutide was associated with a 36% reduction in the risk of persistent macroalbuminuria, persistent doubling of the serum creatinine accompanied by an eGFR ≤45 mL/min/1.73 m2, or the need for continuous renal replacement therapy (although this benefit was driven mainly by reduction in albuminuria).
Weight loss, ranging from 2% to 4% of total body weight for liraglutide and exenatide and up to 10% for semaglutide, may occur with use of GLP-1RA therapy, although generally at higher doses than those targeted for CV risk reduction (65,66). Whether associated with weight loss or another mechanism, GLP-1RAs may also modestly lower blood pressure, but can also lead to elevations in heart rate. Compared with placebo (plus usual care), use of liraglutide produced a 20% reduction in the occurrence of confirmed hypoglycemia and a 31% reduction in severe hypoglycemia (10).
5.2.4. GLP-1RAs: Safety Concerns
The contraindications and safety concerns of GLP-1RAs are included in Table 8. The most frequently reported side effects of GLP-1RAs are nausea and vomiting (60). These gastrointestinal symptoms are usually transient for longer-acting GLP-1RAs and can be mitigated by gradual dose escalation (67) and educating patients to reduce meal size. GLP-1RAs may also increase the risk of gallbladder disease, including acute cholecystitis (10,68). As short-acting GLP-1RAs delay gastric emptying, the absorption of concomitantly administered oral medications may be impacted, although the clinical relevance of this theoretical concern remains unclear. Caution should be used in patients with prior gastric surgery. GLP-1RAs can lead to elevations in heart rate. GLP-1RAs are unlikely to cause hypoglycemia on their own, but they may do so when they are used in combination with insulin or insulin secretagogues–most commonly sulfonylureas (29).
TABLE 8.
Contraindications and Cautions for GLP-1RAs
| Contraindications | Cautions |
|---|---|
| ■ History of serious hyper-sensitivity reaction to drug | ■ Use liraglutide with caution in patients with history of pancreatitis. |
| ■ Severe renal impairment or ESRD (exenatide, lixisenatide) ■ Personal or family history of medullary thyroid cancer |
■ Use liraglutide and semaglutide with caution in patients with severe renal impairment or ESRD |
| ■ MEN2 | ■Hypoglycemia risk increased with insulin and insulin secretagogues (e.g., sulfonylureas); a Lower dose of insulin or the insulin secretagogue may be required. |
| ■ Shorter-acting agents may delay gastric emptying, so may slow absorption of concomitantly administered oral medications and are not recommended in patients with clinically meaningful gastroparesis. This effect is usually transient with longer-acting GLP-1RA | |
| ■ Care should be taken in patients with prior gastric surgery | |
| ■ Semaglutide has been associated with diabetic retinopathy complications which may be related to its associated rapid and marked glucose and A1C reductions |
A1C = hemoglobin A1C; ESRD = end-stage renal disease; GLP-1RA = glucagon-like peptide-1 receptor agonists; MEN2 = multiple endocrine neoplasia syndrome type 2.
Although post-marketing case reports have suggested possible associations between GLP-1RAs and acute pancreatitis, LEADER did not demonstrate any increase in the risk of pancreatitis (10). Moreover, the FDA and the European Medicines Agency have not identified a causal link between this class of drugs and either pancreatitis or pancreatic cancer (67). Liraglutide and semaglutide should be used with caution in patients with severe renal impairment or end-stage renal disease. In the SUSTAIN-6 trial, semaglutide was associated with an increase in diabetic retinopathy complications versus placebo–an effect hypothesized to be related to its efficacy in rapidly reducing blood glucose and A1C. Therefore, patients should be advised to undergo appropriate, guideline-recommended eye examinations before starting therapy if an examination has not been completed within the last 12 months (3).
5.2.5. Hypothetical Mechanisms Underlying CV Benefits of GLP-1RAs
The mechanisms by which GLP-1RAs achieve positive CV effects have not been fully elucidated (see Table 9). GLP-1RAs have been shown to lower systolic blood pressure by 1 to 6 mm Hg (65) and reduce low-density lipoprotein cholesterol by up to 16% (69). The significant weight loss that sometimes accompanies GLP-1RA therapy may also help to explain the benefit; however, taken together, these effects are insufficient to fully account for the observed CV benefit. GLP-1 receptors are also present in the myocardium and vasculature, but the role of these receptors in the observed cardiovascular benefits is unknown (70,71).
TABLE 9.
Hypothesized Mechanisms of GLP-1RA to Lower CV Events
| Effect | Consequence |
|---|---|
| ■ Blood pressure reduction | ■ Reduced myocardial work, reduced filling pressures, pre-/afterload reduction |
| ■ Weight loss | ■ Improved CV disease risk profile, lower blood pressure |
| ■ Low-density lipoprotein cholesterol reduction | ■ Reduced atherogenesis |
| ■ Anti-inflammatory action | ■ Upregulated nitric oxide and suppressed NF-κB activation |
CV = cardiovascular; GLP-RA = glucagon-like peptide-1 receptor agonists; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells.
5.3. Considerations for Optimal Therapy Initiation and Treatment Individualization: Recommendations
The CV benefits of some SGLT2 inhibitors and GLP-1RAs appear robust, creating new options to improve the CV outcomes of their patients with T2D and established ASCVD. There are a number of circumstances in which clinicians might consider starting one of these agents with demonstrated CV benefit (see Table 10). Consider initiating a clinician-patient discussion about the use of either a GLP-1RA or SGLT2 inhibitor at the time of a clinical follow-up visit for patients with T2D and clinical ASCVD. Alternatively, or in conjunction with a patient-clinician discussion, consider discussing these medications with the person caring for the patient’s diabetes. Similarly, a new diagnosis of T2D in a patient with clinical ASCVD or a new diagnosis of clinical ASCVD in patient with T2D offers the opportunity to begin a clinician-patient discussion about starting therapy with an SGLT2 inhibitor or GLP-1RA demonstrated to improve CV outcomes.
TABLE 10.
Opportunities* to Initiate a GLP-1RA or SGLT2 Inhibitor With Demonstrated CV Benefit in Patients With Clinical ASCVD† and T2D in the Context of Background Metformin† Therapy
| ■ In a patient with T2D and ASCVD‡ |
| ■ At the time of diagnosis of clinical ASCVD,‡ in a patient with T2D on a drug regimen that does not include a GLP1-RA or SGLT2 inhibitor with CV benefit |
| ■ At the time of diagnosis of T2D in a patient with clinical ASCVD‡ |
| ■ At hospital discharge after admission for an ASCVD- or diabetes-related clinical event§ |
At the time of hospital discharge or in the outpatient setting. Clinical judgment may lead some practitioners to prescribe an SGLT2 inhibitor or GLP-1 receptor agonist with the intent of reducing CV risk in patients with an A1C <7%; however, the data supporting CV benefits of these agents in this patient population are currently limited. Increased vigilance regarding hypoglycemia surveillance is warranted, especially if on background insulin, sulfonylurea or glinide therapy.
Patients who are being treated with metformin, who cannot tolerate metformin, or for whom metformin is contraindicated.
A minority of patients included in CANVAS, LEADER, SUSTAIN-6, and EXSCEL trials could be characterized as high-risk primary prevention patients. These patients did not have established ASCVD but did have prespecified ASCVD risk factors.
Hospitalized patients were not included in most of the CV outcome trials discussed here. There is a lack of practical and safety data regarding in-hospital addition of SGLT2 inhibitors or GLP-1RA to a patient’s regimen.
ASCVD = atherosclerotic cardiovascular disease; CV = cardiovascular; GLP1RA = glucagon-like peptide-1 receptor agonist; SGLT2 = sodium-glucose cotransporter-2; T2D = type 2 diabetes.
Patients with T2D may become eligible for initiation of these new T2D therapies if they are subsequently hospitalized or diagnosed with ASCVD or HF (38), although it is important to note that hospitalized patients were not included in most of the cardiovascular outcome trials discussed here, and hospital inpatient formularies may not include these agents. However, outpatient adherence to therapy after acute MI can be favorably influenced by initiation of medications at discharge. These factors must be weighed if contemplating in-hospital addition of SGLT2 inhibitors or GLP-1RAs.
Because T2D is common among patients with ASCVD or HF, CV specialists should consider periodic screening for T2D in these patients by measuring A1C at intervals based on most recently measured A1C (e.g., annually in patients close to an A1C of 6.5%). Patients newly diagnosed with T2D as per ADA SOC guidelines should begin guideline-based therapy with lifestyle changes and metformin, and discuss addition of an SGLT2 inhibitor or GLP-1RA with demonstrated CV benefit. Among the SGLT2 inhibitors, empagliflozin is currently the preferred agent based on the available evidence and overall benefit-risk balance. Because there is no evidence of a graded dose response regarding CV disease outcomes, SGLT2 inhibitors with demonstrated CV benefit should be initiated at the lowest available dose (e.g., 10 mg for empagliflozin, 100 mg for canagliflozin, and so on). No further up-titration is needed for CV risk reduction, although dose may be increased by the doctor managing the patient’s glucose, and cardiologists should make patients aware that this may happen for non-CVD risk reduction reasons. Among the GLP-1RAs with demonstrated CV benefit, the most convincing data for CV benefit are for liraglutide, which should currently be the preferred member of this class for CV event reduction until additional information becomes available. In accordance with randomized controlled trials, a GLP-1RA with demonstrated CV benefit should be initiated at the lowest dose and up-titrated slowly to the maximal tolerated dose, noting that the goal dose for liraglutide is 1.8 mg daily for CVD reduction.
Prior to initiating T2D therapies aimed at CV disease risk reduction, a detailed clinician-patient risk discussion is recommended (72). This discussion should review risks, potential benefits, and different treatment options. Specifically, potential side effects, drug-drug interactions, and safety issues should be explained clearly, patient preference and other concerns elicited, and cost discussed, because SGLT2 inhibitors and GLP1-RA are expensive, and out-of-pocket cost could be considerable for many patients (73).
5.3.1. Should I Recommend an SGLT2 Inhibitor or a GLP-1RA for My Patient?
Since selected SGLT2 inhibitors and GLP-1RA have been demonstrated to have CV benefit in patients with T2D and ASCVD, clinician-patient discussions regarding use of these agents must include discussion of which specific agent is most appropriate (Table 11). As noted, patient preferences and medical history can help guide that decision.
TABLE 11.
Patient and Clinician Preferences and Priorities for Considering SGLT2 Inhibitors with Demonstrated CV Benefit Versus GLP-1RAs With Demonstrated CV Benefit
| Consider Using an SGLT2 Inhibitor First When Patient and Clinician Priorities Include: |
Consider Using a GLP-1RA First When Patient and Clinician Priorities Include: |
|---|---|
| Reducing MACE and CV death | Reducing MACE and CV death |
| Preventing heart failure hospitalization | Substantial weight loss |
| Reducing blood pressure | Once weekly (subcutaneous) dosing |
| Orally administered therapies | Therapy when eGFR consistently <45 ml/min/1.73 m2* |
| Consider alternative agents if: ■ Significant CKD* ■ History of prior amputation, severe peripheral arterial disease, neuropathy, or diabetic foot ulcers (avoid canagliflozin) ■ History of recurrent genital candidiasis ■ History of diabetic ketoacidosis ■ History of osteoporosis (avoid canagliflozin) |
Consider alternative agents if: ■ Persistent nausea, even at low ■ History of pancreatitis ■ History of gastroparesis ■ History of MEN2 or medullary thyroid cancer ■ History of proliferative retinopathy (semaglutide) |
eGFR <45 ml/min/1.73 m2 is currently a caution due to a decrease in glycemic efficacy (not due to safety), but SGLT2 inhibitors are currently being investigated for nephroprotection in these patients.
CKD = chronic kidney disease; CV = cardiovascular; DPP4 = dipeptidyl-peptidase 4; eGFR = estimated glomerular filtration rate; GLP-RAs = glucagon-like peptide-1 receptor agonists; MACE = major adverse cardiovascular event; MEN2 = multiple endocrine neoplasia type 2; SGLT2 = sodium-glucose cotransporter-2.
The SGLT2 inhibitors with demonstrated CV benefit appear to reduce both MACE and HF risk but increase the risk of genital mycotic infections (with possible additional risks of rare events as previously outlined). GLP-1RAs with demonstrated CV benefit offer reductions in MACE but are associated with transient nausea and vomiting, especially when initiating therapy or up-titrating doses. Both classes of agents have nonglycemic benefits in systolic blood pressure and weight and have a low risk of hypoglycemia on their own or when used with metformin and other oral glucose-lowering medications (except for insulin secretagogues). Differences in the route of administration (oral for SGLT2 inhibitors, subcutaneous for GLP-1RA) may influence patient and physician decision making; however, the GLP-1RAs are given with a small needle and pen device to ease administration and patient acceptance. Cost should also be considered, as insurance coverage for these agents can vary significantly. Until further data from ongoing clinical trials become available, patients at high risk for HF (and possibly those with established HF) may derive more benefit from an SGLT2 inhibitor with demonstrated CV benefit, whereas those with osteoporosis, prior amputations, severe peripheral artery disease, peripheral neuropathy, or active lower extremity soft tissue ulcers or infections may have a more favorable benefit/risk balance if initially treated with a GLP-1RA with demonstrated CV benefit rather than canagliflozin.
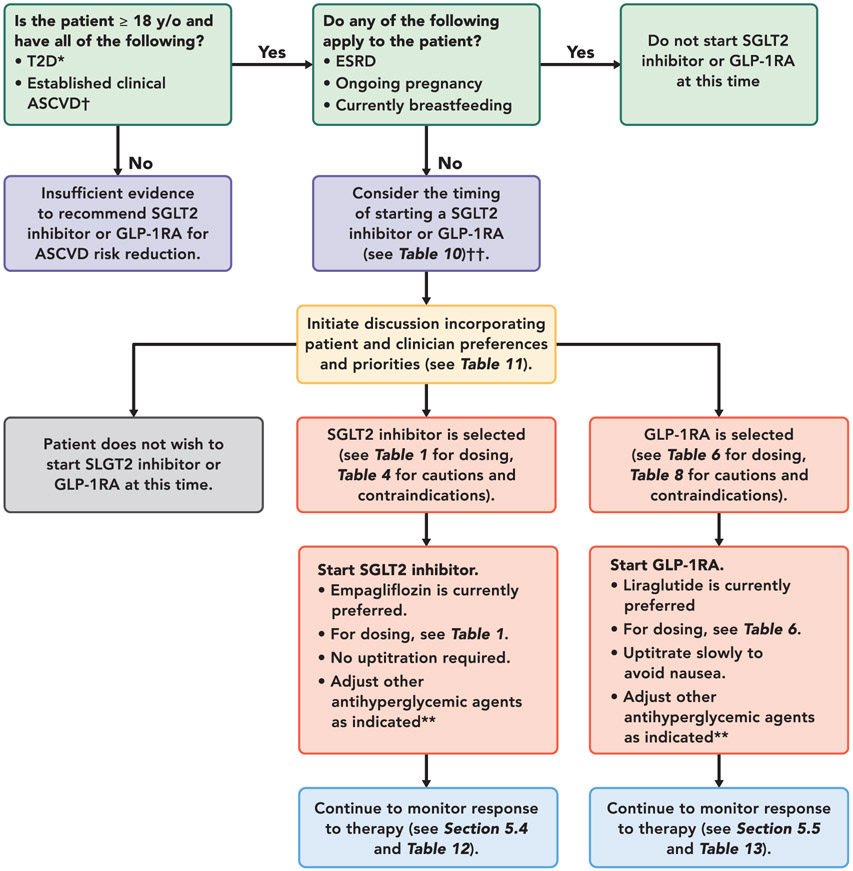
Figure 2 offers 1 approach to deciding which drug to use in which patient, Table 11 outlines patient and clinician preferences to consider when selecting an SGLT2 inhibitor or GLP-1RA. Table 12 provides an overview of considerations for initiating and monitoring an SGLT2 inhibitor. Table 13 provides an overview of considerations for initiating and monitoring a GLP-1RA.
FIGURE 2. Approach to Managing Patients With Established ASCVD and T2D.
*SGLT2 inhibitors and GLP-1RA have not been studied in and do not have an FDA -approved indication for patients with type 1 diabetes.
†Some cardiovascular outcome trials included primary prevention patients with multiple risk factors for ASCVD.
‡ The available evidence for cardiovascular event reduction in patients with T2D and clinical ASCVD is derived from trials in which most participants were treated with metformin at baseline. Please see “Do Patients Need to Be on Metformin Before Initiating an SGLT2 Inhibitor or a GLP-1RA?” section in text.
**Please see Table 12 and Table 13. If A1C well-controlled at baseline, or known history of frequent hypoglycemic events, reduce dose of sulfonylurea by 50% or basal insulin dose by 20% when starting therapy.
Abbreviations:
ASCVD = atherosclerotic cardiovascular disease; ESRD = end-stage renal disease; FDA = Food and Drug Administration; GLP-1RA = glucagon-like peptide-1 receptor agonist; SGLT2 = sodium-glucose cotransporter-2; T2D = type 2 diabetes; y/o = years old
TABLE 12.
Considerations for Drug Initiation and Monitoring in Patients Starting an SGLT2 Inhibitor With Demonstrated CV Benefit
| ■ If A1C well-controlled at baseline, or known history of frequent hypoglycemic events, reduce dose of sulfonylurea by 50% or basal insulin dose by 20% when starting therapy. |
| ■ Avoid hypovolemia. May need to reduce thiazide or loop diuretic dose. |
| ■ Educate patients regarding symptoms of low blood pressure (light headedness, orthostasis, weakness) |
| ■ Instruct patients to more closely monitor glucose at home for the first 4 weeks of therapy |
| ■ Educate patients regarding symptoms of diabetic ketoacidosis (nausea, vomiting, weakness) and that diabetic ketoacidosis can occur even if blood glucose readings are in the 150-250 mg/dL range. If patient experiences diabetic ketoacidosis-like symptoms, he or she should be instructed to seek medical attention. |
| ■ Educate patients regarding foot care and follow-up foot pulse examination (particularly canagliflozin) |
| ■ Monitor kidney function |
| ■ Educate patients regarding potential for genital mycotic infections |
A1C = hemoglobin A1C; CV = cardiovascular; SGLT2 = sodium-glucose cotransporter-2.
TABLE 13.
Considerations for Drug Initiation and Monitoring in Patients Starting a GLP-1RA With Demonstrated CV Benefit
| ■ If A1C well-controlled at baseline, or known history of frequent hypoglycemic events, reduce dose of sulfonylurea by 50% or basal insulin dose by 20% when starting therapy. |
| ■ Discontinue DPP-4 inhibitor before starting (if applicable) |
| ■ Start at lowest dose and up-titrate slowly to mitigate nausea to the doses used in CV outcome trials* |
| ■ Instruct patients to more closely monitor glucose at home for the first 4 weeks of therapy |
| ■ Advise patients to undergo appropriate, guideline-recommended eye examinations before starting therapy if not done within the last 12 months |
| ■ Increase in diabetic retinopathy complications (for semaglutide) |
Higher doses of GLP1-RA can sometimes be used for weight loss, but have not been shown to offer additional CV risk reduction.
A1C = hemoglobin A1C; CV = cardiovascular; DPP4 = dipeptidyl peptidase-4; GLP-1RA = glucagon-like peptide-1 receptor agonist.
5.3.2. Do Patients Need to Be on Metformin Before Initiating an SGLT2 Inhibitor or a GLP-1RA?
The available evidence for CV event reduction in patients with T2D and clinical ASCVD is derived from trials in which most participants were treated with metformin at baseline. Thus, we recommend that patients with T2D and clinical ASCVD treated with metformin (or in whom metformin is contraindicated or not tolerated) should have an SGLT2 inhibitor or GLP-1 RA with proven CV benefit added to their treatment regimen. Clinical judgement may lead some practitioners to prescribe an SGLT2 inhibitor or GLP-1RA with the intent of reducing cardiovascular risk in patients that are not on background metformin therapy. To be explicitly clear, there are no definitive clinical trial data that provide evidence of benefit to the approach of using an SGLT2 inhibitor or GLP-1RA for the reduction of cardiovascular risk in such patients. However, limited data suggest there is no heterogeneity in the cardiovascular benefits of SGLT2 inhibitor or GLP-1RA as a function of background antihyperglycemic therapy, with those patients not receiving metformin demonstrating comparable reduction in risk (29,74). Thus, background antihyperglycemic therapy in these patients may arguably not be pertinent.
The majority of patients with T2D and ASCVD in completed cardiovascular outcomes trials of SGLT2 inhibitors and GLP-1 RA had A1C ≥7%. Thus, the evidence for cardiovascular benefit of these agents in patients with well-controlled A1C remains limited. However, secondary analyses from several studies demonstrate that baseline A1C does not modify the cardiovascular benefits of these agents. Nevertheless, if added to patients with well controlled T2D, dose adjustment of background medications may be required to avoid hypoglycemia when adding a new agent in the context of insulin, sulfonylurea, or glinide therapy, particularly in patients at or near glycemic goals. (Please see Sections 5.4 and 5.5 and Table 4.) Full efforts to achieve glycemic and blood pressure targets and to adhere to lipid, antiplatelet, antithrombotic, and tobacco cessation guidelines should continue after an SGLT2 inhibitor or GLP-1RA is added.
5.3.3. Should SGLT2 Inhibitors and GLP-1RA Be Used Concomitantly?
No trials to date have studied the CV outcome effects of concomitant use of both an SGLT2 inhibitor with demonstrated CV benefit and a GLP-1RA with demonstrated CV benefit in patients with ASCVD. Mechanistically, these drug classes have opposite effects on glucagon, suggesting the possibility of an interaction, and the out of pocket cost of using drugs from both classes together would likely be very high. However, DURATION-8 (Xenatide Once Weekly Plus Dapagliflozin Once Daily Versus Exenatide or Dapagliflozin Alone in Patients With T2D Inadequately Controlled With Metformin Monotherapy)–a 28-week, multicenter, double-blind, phase 3, randomized controlled trial, demonstrated greater reductions in blood pressure and body weight in patients randomly allocated to the combination of exenatide and dapagliflozin (75). These limited data suggest that the nonglycemic effects of the medication classes may be additive. Combination therapy with both an SGLT2 inhibitor and a GLP-1RA for glycemic management also accords with current T2D management guidelines (3). Therefore, it appears reasonable to use both an SGLT2 inhibitor and a GLP-1RA with demonstrated CV benefit concomitantly if clinically indicated, even though such combination therapy has not been studied for CVD risk reduction.
5.4. What to Monitor When Prescribing an SGLT2 Inhibitor
Patients starting an SGLT2 inhibitor should be informed about the higher risk of genital mycotic infections, and that this risk could be lowered with meticulous attention to personal hygiene. Topical antifungal agents can be used for initial treatment. Oral antifungals can be used but require close attention to QTc duration in patients who are also taking certain antiarrhythmic agents or other QTc-prolonging drugs.
Patients should be informed about the unlikely risk of euglycemic diabetic ketoacidosis and advised to seek immediate care if they develop symptoms potentially associated with diabetic ketoacidosis (e.g., nausea, vomiting, abdominal pain, generalized weakness). Substantial initial reductions in insulin dose (i.e., >20%) should be avoided after initiation of SGLT2 inhibitors. Patients on a complex insulin regimen or with history of labile blood glucose should have an SGLT2 inhibitor initiated in collaboration with the diabetes care provider. Conversely, patients only requiring oral glucose-lowering medications are at lower risk of euglycemic diabetic ketoacidosis. Approximately 5% to 10% of adult-onset diabetes is late-onset type 1; these patients have an increased risk of diabetic ketoacidosis.
Patients taking insulin or an insulin secretagogue (i.e., a sulfonylurea or glinide) should be advised of the risk of hypoglycemic events when adding newer antihyperglycemic therapies for cardiovascular benefit. In these patients, reducing sulfonylurea or glinide dose by 50% and to at most 50% of the maximum recommended dose, discontinuing these agents if already on a minimal dose, and/or reducing total daily insulin dose by 20% could reduce the risk of hypoglycemia. Dose adjustment of insulin or sulfonylureas requires individualization to each patient, and this is suggested as a reasonable starting point for such adjustment. Complex insulin regimens or “brittle” diabetes should be carefully managed in coordination with the patient’s diabetes care provider. These patients should be advised to self-monitor blood glucose levels closely during the first 3 to 4 weeks after initiating SGLT2 inhibitors. In contrast, the risk of hypoglycemia is not significantly increased with the addition of SGLT2 inhibitors in patients who are not taking either insulin or an insulin secretagogue, although it is possible that dose adjustments of other agents may occasionally be needed to minimize the risk of hypoglycemia for patients who are at or near glycemic targets.
Patients should be advised that there is a diuretic effect that may be observed with SGLT2 inhibitors and potentially additive natriuretic effects when SGLT2 inhibitors are administered with loop diuretics (76). Patients, especially the elderly or those on diuretic therapy, should be advised to monitor for signs of volume depletion such as orthostatic lightheadedness and to contact their clinician if these occur.
Therapy with SGLT2 inhibitors may cause a modest (and likely hemodynamically mediated and reversible) decrease in eGFR. However, longer-term nephroprotective effects have been consistently observed in large clinical trials. Because some patients may be “hyperresponders,” monitoring of renal function in the first several weeks of therapy is reasonable, particularly in patients with impaired renal function at baseline.
Increased risk of lower limb amputation has been noted with canagliflozin, as described in the previous text. This increased amputation risk has not been consistently observed with other SGLT2 inhibitors to date, although previous trials did not systematically monitor for amputation. Caution is advised when prescribing canagliflozin to patients with a history of prior amputations, significant peripheral artery disease, or active lower extremity soft tissue ulcers or infections.
5.5. What to Monitor When Prescribing a GLP-1RA
The strategy to reduce hypoglycemic events with GLP-1RA is the same as that for SGLT2 inhibitors, as outlined in the previous text. Patients initiated on a GLP-1RA should be informed that transient nausea and vomiting are a relatively common side effect. Nausea and vomiting can be minimized by starting with the lowest dose, up-titrating gradually once every few weeks, and eating smaller portions. This nausea and vomiting does not imply gastrointestinal pathology and is usually self-limited in patients treated with longer-acting GLP-1RAs. However, GLP-1RA should be used with caution in patients who have had problems with clinically significant gastroparesis. If treatment is suspended, reinitiation should again be at the lowest dose, with gradual up-titration to avoid recurrent nausea and vomiting. GLP-1RA should not be coadministered with DPP4 inhibitors given that they both work through GLP-1 signaling and have not been approved for use together.
An increased risk of diabetic retinopathy complications has been noted with semaglutide, predominantly in patients with a prior history of proliferative retinopathy. Therefore, the risks and benefits of semaglutide therapy should be considered carefully in these patients. Patients should have a recent eye examination prior to semaglutide initiation, as recommended by the current guidelines (77). This increased risk has not been consistently observed with other GLP-1RAs to date and is hypothesized to be due to the rapid and sustained reductions in blood glucose observed with semaglutide (68).
5.5.1. Systems Factors in Caring for Patients With T2D and CV Disease
Challenges to utilization of and adherence to evidence-based and guideline-recommended therapies remain (78). CV specialists have recognized preventing morbid CV outcomes as central to their clinical mission and have typically taken ownership of therapies that are effective in preventing those events. Because of their effects on major CV events, SGLT2 inhibitors and GLP-1RA classes are 2 of the newest examples of therapies that support this goal. However, some CV specialists may be reluctant to use them, perhaps because these agents were originally approved for glucose reduction, or because of incomplete knowledge of their benefits and/or risks, lack of familiarity with their use and monitoring, or because of systems factors that discourage CV specialists from using them.
One potential approach to optimize their use would be employing what might be called the “consultative” approach, in which the discussion of these agents is encouraged in conversations or communication with the person caring for the patient’s diabetes and/or with the patient. This approach requires clear, open communication and does not require the CV medicine specialist to or preclude them from initiating and monitoring these medications. An alternative might be a more comprehensive “team” approach, such as that which has been implemented for patients with other chronic diseases, such as human immunodeficiency virus (HIV) or organ transplantation. Members of the care team for patients with diabetes include primary care physicians, endocrinologists, cardiologists, podiatrists, ophthalmologists, pharmacists, nurses, advanced practice providers, and dietitians. With both approaches, the key elements are patient-centered care, shared decision making, and integration across disciplines and patient care roles.
Given the data supporting comprehensive CV risk reduction in patients with T2D, CV clinicians should be both champions and change agents as strong advocates for our patients, recognizing unmet needs in healthcare delivery, and extending our comfort zone in implementing the use of new evidence-based therapies that reduce CV event rates.
5.6. Unresolved Questions
Several important clinical questions regarding the use of SGLT2 inhibitors and GLP-1RA remain unanswered. First, the pivotal trials that showed evidence of CV benefit for SGLT2 inhibitors and GLP-1RAs enrolled a high proportion of patients who were treated with metformin at baseline (10,12,30). Whether initiating an SGLT2 inhibitor and GLP-1RA with CV benefit prior to starting metformin would be expected to lead to cardiovascular risk reduction is unknown. However, in the EMPA-REG OUTCOME and LEADER trials, no evidence was found to suggest that the effects of either empagliflozin or liraglutide were modified by baseline medication use, including metformin. Second, the published trials of SGLT2 inhibitors and the GLP-1RA enrolled patients a majority of whom had A1C $7% at baseline (average A1C above 8%). Thus, the cardiovascular benefits of these drugs in patients with T2D and A1C <7% have not been proven (12,29,74), although secondary analyses from EMPA-REG OUTCOME trial suggest that the cardiovascular benefits of empagliflozin may be independent of either baseline A1C or change in A1C during the trial (79). The Writing Committee emphasizes the importance of these drugs to CV specialists based on their effects on CV risk reduction rather than glucose lowering, despite limitations in our current understanding regarding their role in the absence of metformin background and/or A1C <7%. However, increased vigilance to avoid hypoglycemia in patients with A1C near or below target levels at SGLT2 inhibitor or GLP-1RA initiation is warranted, especially if the patient’s existing T2D therapies include sulfonylureas, glinides, or insulin. (Please see Sections 5.4, and 5.5 and Table 4.) Ongoing trials will seek to address the role of a SGLT2 inhibitor and GLP-1RA for CV event reduction in a wide array of populations, including those with chronic kidney disease and HF as outlined in Table 14.
TABLE 14.
Ongoing or Recently Completed but Unpublished Cardiovascular Outcomes Trials for SGLT2 Inhibitors and GLP-1RA (80)
| Drug | Trial Acronym | Class | Trial Primary Endpoint* | Clinicatrials.gov Reference |
|---|---|---|---|---|
| Dapagliflozin | DECLARE-TIMI-58† | SGLT2 inhibitor | 1) CV death or HHF; 2) 3-point MACE | NCT01730534 |
| Dapagliflozin | Dapa-HF | SGLT2 inhibitor | CV death, HHF or urgent HF visit | NCT03036124 |
| Empagliflozin | EMPEROR-PRESERVED | SGLT2 inhibitor | CV death, HHF | NCT03057951 |
| Empagliflozin | EMPEROR-REDUCED | SGLT2 inhibitor | CV death, HHF | NCT03057977 |
| Ertugliflozin | VERTIS CV | SGLT2 inhibitor | 3-point MACE | NCT01986881 |
| Sotagliflozin | SCORED | SGLT2 inhibitor | 3-point MACE | NCT03315143 |
| Sotagliflozin | SOLOIST-WHF | SGLT2 inhibitor | CV death, HHF | NCT03521934 |
| Albiglutide | HARMONY OUTCOMES | GLP-1RA | 3-point MACE | NCT02465515 |
| Dulaglutide | REWIND | GLP-1RA | 3-point MACE | NCT01394952 |
| Semaglutide (oral) | PIONEER 6 | GLP-1RA | 3-point MACE | NCT02692716 |
Three-point MACE is the composite of nonfatal myocardial infarction, nonfatal stroke, and CV death.
Two primary endpoints.
CV = cardiovascular; GLP-1RA = glucagon-like peptide-1 receptor agonists; HF = heart failure; HHF = hospitalization for heart failure; MACE = major adverse cardiovascular event; SGLT2 = sodium-glucose cotransporter-2.
6. DISCUSSION AND IMPLICATION OF PATHWAY
This Expert Consensus Decision Pathway represents a change in the paradigm of how the CV specialist should approach the care of patients with T2D. Previously, CV care in patients with diabetes was centered around risk factor optimization, and the medications used for glycemic control were not expected to demonstrate direct CV benefit. The recent development of 2 novel classes of therapies–SGLT2 inhibitors and GLP-1RAs–has, for the first time, demonstrated that treatments developed for glucose lowering can directly improve CV outcomes. In large, well-conducted, randomized clinical trials, specific medications in these 2 classes have been proven to reduce rates of acute MI, stroke, and CV death, and, in the case of SGLT2 inhibitors, to reduce HF hospitalizations, in patients with T2D (majority with established ASCVD). These benefits appear to be independent of their effects on A1C; thus, CV specialists are now faced with the need to incorporate the use of these agents in patients with T2D and clinical ASCVD, purely to optimize outcomes. This Expert Consensus Decision Pathway provides a practical guide to CV specialists for the initiation and monitoring of SGLT2 inhibitors and GLP-1RA with this express goal of reducing CV risk.
The Expert Consensus Decision Pathway and treatment algorithms described here should be used in concert with established risk factor modification guidelines for the prevention of MACE in patients with T2D, including guidelines on lipids (21,22), blood pressure (20), and antiplatelet therapy (23). This Expert Consensus Decision Pathway should also be applied in the context of guideline-directed diabetes care (3). Although intended to facilitate clinical decision making, the information provided in this pathway should complement rather than supersede good clinical judgement.
The treatment of patients with T2D and clinical ASCVD is increasingly complex. It involves physicians and advanced practice providers across a wide array of specialties, including primary care, endocrinology, cardiology, nephrology, podiatry, and ophthalmology, among others. It also involves associated providers such as nurses, pharmacists, and dieticians. Ultimately, the main goals of care for these high-risk patients should be improving survival and quality of life. Achieving these important goals requires a team-based approach to achieve optimal outcomes. If used appropriately, the SGLT2 inhibitors and GLP-1RAs discussed in this document should significantly reduce CV morbidity and mortality in these patients. We have highlighted the potential benefits and risks associated with these novel therapies and have sought to provide a context for the rational utilization of these medications. The evidence for specific agents in these classes is still emerging, and other cardiovascular outcomes trials in T2D are currently underway (see Table 14). As such, this area of care for affected patients is likely to continue evolving rapidly. We anticipate that the algorithms proposed here will change as new evidence emerges, but that the overarching goal of improving CV outcomes in patients with T2D and clinical ASCVD will remain consistent.
Appendix
APPENDIX 1. AUTHOR RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES (RELEVANT)–2018 ACC EXPERT CONSENSUS DECISION PATHWAY ON NOVEL THERAPIES FOR CARDIOVASCULAR RISK REDUCTION IN PATIENTS WITH TYPE 2 DIABETES AND ATHEROSCLEROTIC CARDIOVASCULAR DISEASE
To avoid actual, potential, or perceived conflicts of interest that may arise as a result of industry relationships or personal interests among the writing committee, all members of the writing committee, as well as peer reviewers of the document, are asked to disclose all current healthcare-related relationships, including those existing 12 months before initiation of the writing effort. The ACC Task Force on Clinical Expert Consensus Documents reviews these disclosures to determine which companies make products (on market or in development) that pertain to the document under development. Based on this information, a writing committee is formed to include a majority of members with no relevant relationships with industry (RWI), led by a chair with no relevant RWI. RWI is reviewed on all conference calls and updated as changes occur. Author RWI pertinent to this document is disclosed in the table below and peer reviewer RWI is disclosed in Appendix 2. Additionally, to ensure complete transparency, authors’ comprehensive disclosure information–including RWI not pertinent to this document–is available online (see Online Appendix). Disclosure information for the ACC Task Force on Clinical Expert Consensus Documents is also available online, as is the ACC disclosure policy for document development.
| Committee Member |
Employment | Consultant | Speakers Bureau |
Ownership/ Partnership/ Principal |
Personal Research | Institutional, Organizational, or Other Financial Benefit |
Expert Witness |
|---|---|---|---|---|---|---|---|
| Sandeep R. Das (Co-Chair) | UT Southwestern Medical Center–Associate Professor of Medicine | None | None | None | None | None | None |
| Brendan M. Everett (Co-Chair) | Brigham and Women’s Hospital–Assistant Professor of Medicine | None | None | None | None | None | None |
| Kim K. Birtcher | University of Houston College of Pharmacy–Clinical Professor | None | None | None | None | None | None |
| Jenifer M. Brown | Brigham and Women’s Hospital–Fellow in Cardiology | None | None | None | None | None | None |
| William T. Cefalu | American Diabetes Association–Chief Scientific, Medical and Mission Officer | None | None | None | None | None | None |
| James L. Januzzi Jr. | Massachusetts General Hospital–Professor of Medicine, Harvard | None | None | None | ■ Boehringer Ingelheim (DSMB)* | None | None |
| Medical School | ■ Janssen Pharmaceuticals (DSMB)* | ||||||
| Rita Rastogi Kalyani | Johns Hopkins University School of Medicine–Associate Professor of Medicine | None | None | None | None | None | None |
| Mikhail Kosiborod | Saint Luke’s Mid America Heart Institute and University of Missouri-Kansas City–Professor of Medicine | ■ AstraZeneca* ■ Boehringer Ingelheim* ■ Janssen* ■ Merck (Diabetes)* ■ Novo Nordisk ■ Sanofi* | None | None | ■ AstraZeneca* ■ Boehringer Ingelheim* | None | None |
| Melissa L. Magwire | Shawnee Mission Health–Endocrine Clinic Coordinator | None | ■ Janssen ■ Novo Nordisk*■ Sanofi-Aventis | None | none | None | None |
| Pamela B. Morris | Medical University of South Carolina–Director, Seinsheimer Cardiovascular Health Program; Co-Director, Women’s Heart Care | None | None | None | Amgen | None | None |
| Laurence S. Sperling | Emory Heart Disease Prevention Center–Professor of Medicine (Cardiology); Emory University–Director, Professor of Global Health, Hubert Department of Global Health, Rollins School of Public Health | None | None | None | None | None | None |
This table represents all relationships of committee members with industry and other entities that were reported by authors, including those not deemed to be relevant to this document, at the time this document was under development. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5,000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Please refer to http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/relationships-with-industry-policy for definitions of disclosure categories or additional information about the ACC/AHA Disclosure Policy for Writing Committees.
Significant relationship.
ACC = American College of Cardiology; AHA = American Heart Association; DSMB = data and safety monitoring board.
APPENDIX 2. PEER REVIEWER INFORMATION–2018 ACC EXPERT CONSENSUS DECISION PATHWAY ON NOVEL THERAPIES FOR CARDIOVASCULAR RISK REDUCTION IN PATIENTS WITH TYPE 2 DIABETES
This table represents the individuals, organizations, and groups that peer reviewed this document. A list of corresponding comprehensive healthcare-related disclosures for each reviewer is available online.
| Reviewer | Representation | Employment |
|---|---|---|
| John B. Buse | Official Reviewer–ADA | University of North Carolina School of Medicine–Director, Diabetes Care |
| Christopher P. Cannon | Official Reviewer–ADA | Brigham and Women’s Hospital, Harvard Medical School–Professor of Medicine |
| Jill Crandall | Official Reviewer–ADA | Albert Einstein College of Medicine–Professor, Department of Medicine (Endocrinology) |
| David D’Alessio | Official Reviewer–ADA | Duke Molecular Physiology Institute–Associate Director |
| Ian de Boer | Official Reviewer–ADA | University of Washington (Seattle)–Associate Professor of Medicine, Division of Nephrology |
| Judith Fradkin | Official Reviewer–ADA | National Institute of Diabetes and Digestive and Kidney Diseases–Director of the Division of Diabetes, Endocrinology, and Metabolic Diseases |
| Ty J. GLuckman | Official Reviewer–ACC BOG | Providence Heart and Vascular Institute–Medical Director |
| Sherita Hill Golden | Official Reviewer–ADA | Johns Hopkins University School of Medicine, Welch Center for Prevention, Epidemiology, and Clinical Research–Hugh P. McCormick Family Professor of Endocrinology and Metabolism |
| Joshua J. Neumiller | Official Reviewer–ADA | Washington State University, College of Pharmacy–Vice-Chair & Associate Professor, Department of Pharmacotherapy; Editor-in-Chief, Diabetes Spectrum |
| Barbara S. Wiggins | Official Lead Reviewer-Task Force on Expert Consensus Decision Pathways | Medical University of South Carolina–Clinical Pharmacy Specialist Cardiology, Department of Pharmacy Services |
| Seth J. Baum | Organizational Reviewer–ASPC | American Society for Preventive Cardiology–President |
| James M. Falko | Organizational Reviewer–NLA | University of Colorado–Clinical Professor of Medicine |
| Stuart T. Haines | Organizational Reviewer–APA | University of Mississippi School of Pharmacy–Professor, Department of Pharmacy Practice; Director, Pharmacy Professional Development |
| Barbara A. Hutchinson | Organizational Reviewer–ABC | Chesapeake Cardiac Care–President; Association of Black Cardiologists–Immediate Past President |
| Daniel L. Hurley | Organizational Reviewer–President, AACE | Mayo Clinic–Consultant, Division of Endocrinology, Diabetes, Metabolism and Nutrition; Mayo Clinic College of Medicine & Science–Associate Professor of Medicine |
| Paul Jellinger | Organizational Reviewer–AACE | University of Miami Miller School of Medicine–Professor of Clinical Medicine; The Center for Diabetes & Endocrine Care–Cardiologist |
| Joshua Joseph | Organizational Reviewer–AHA | The Ohio State University Wexner Medical Center–Assistant Professor of Medicine, Division of Endocrinology, Diabetes and Metabolism |
| Cynthia A. Lamendola | Organizational Reviewer–PCNA | Stanford Medicine and Stanford Health Care–Nurse Practitioner; Clinical Research Study Coordinator |
| Anthony L. McCall | Organizational Reviewer–Endocrine Society | University of Virginia School of Medicine–Professor of Medicine & Diabetes/Endocrinology (Emeritus); Cornell University–Professor of Nutritional Sciences, Cornell University Cornell |
| Joanna Mitri | Organizational Reviewer–Joslin Diabetes Center | Joslin Diabetes Center–Research Associate, Section on Clinical, Behavioral, and Outcomes Research Lipid Clinic, Adult Diabetes Section; Harvard Medical School–Clinical Instructor |
| Mary H. Parker | Organizational Reviewer–ASHP | Durham VA Medical Center–Acting Associate Chief of Staff for Ambulatory Care |
| David Sperling | Organizational Reviewer–AAFP | Northeast Ohio Medical University–Associate Professor, Department of Family and Community Medicine |
| Veronica Wilbur | Organizational Reviewer–AANP | Westchester University–Assistant Professor Graduate Nursing |
| Suzanne V. Arnold | Content Reviewer-Roundtable Participant | Saint Luke’s Mid America Heart Institute/University of Missouri-Kansas City–Associate Professor |
| Michael Blaha | Content Reviewer-ACC Expert | Johns Hopkins Ciccarone Center for the Prevention of Heart Disease–Director of Clinical Research |
| Roger S. Blumenthal | Content Reviewer-Co-Chair, 2019 Prevention Guideline Writing Committee | Johns Hopkins Hospital Ciccarone Preventive Cardiology Center–Pollin Professor of Cardiology |
| Matthew Aaron Cavender Deborah Croy |
Content Reviewer-Roundtable Participant Content Reviewer-Roundtable Participant |
University of North Carolina at Chapel Hill–Assistant Professor of Medicine, Interventional Cardiology Bland County Medical Clinic–Adult Cardiovascular Nurse Practitioner |
| Prakash Deedwania | Content Reviewer-Diabetes & Cardiometabolic Syndrome Working Group | University of California San Francisco Fresno–Chief of Cardiology |
| Reviewer | Representation | Employment |
| Robert N. Piana | Content Reviewer-Task Force on Expert Consensus Decision Pathways | Vanderbilt University Medical Center–Professor of Medicine (Cardiology) |
| Jorge Plutzky | Content Reviewer-Roundtable Participant | Brigham and Women’s Hospital, Harvard Medical School–Director, The Vascular Disease Prevention Program |
| Carla Weidner | Content Reviewer-Cardiovascular Team Council | St. Luke’s University Health Network–Cardiac Nurse Practitioner |
| Deborah J. Wexler | Content Reviewer-ACC Expert | Harvard Medical School–Associate Professor of Medicine; Massachusetts General Hospital–Associate Clinical Chief, Diabetes Unit |
| Nathan D. Wong | Content Reviewer-Prevention Council and Roundtable Participant | University of California, Irvine–Professor and Director, UCI Heart Disease Prevention Program |
AACE = American Association of Clinical Endocrinology; AAFP = American Academy of Family Physicians; AANP = American Association of Nurse Practitioners; ABC = Association of Black Cardiologists; ACC = American College of Cardiology; ADA = American Diabetes Association; AHA = American Heart Association; APA = American Pharmacists Association; ASHP = American Society of Health-System Pharmacists; ASPC = American Society for Preventive Cardiology; BOG = Board of Governors; CVD = cardiovascular disease; NLA = National Lipid Association; PCNA = Preventive Cardiovascular Nurses Association; VA = Veterans Administration.
APPENDIX 3. ABBREVIATIONS
| A1C = hemoglobin A1C | GLP-1RA = glucagon-like peptide-1 receptor agonist |
| ACC = American College of Cardiology | HFSA = Heart Failure Society of America |
| ADA = American Diabetes Association | MACE = major adverse cardiovascular event |
| AHA = American Heart Association | MI = myocardial infarction |
| ASCVD = atherosclerotic cardiovascular disease | SGLT2 = sodium-glucose cotransporter-2 |
| CV = cardiovascular | T2D = type 2 diabetes mellitus |
| eGFR = estimated glomerular filtration rate |
Footnotes
Endorsed by the American Diabetes Assocation
This document was approved by the American College of Cardiology Clinical Policy Approval Committee in October 2018.
The American College of Cardiology requests that this document be cited as follows: Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL Jr, Kalyani RR, Kosiborod M, Magwire ML, Morris PB, Sperling LS. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;72:3200-23.
REFERENCES
- 1.American Diabetes Association. Statistics About Diabetes: American Diabetes Association; Available at: http://www.diabetes.org/diabetes-basics/statistics/. Accessed January 29, 2018. [Google Scholar]
- 2.Rawshani A, Rawshani A, Gudbjornsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;377:300–1. [DOI] [PubMed] [Google Scholar]
- 3.Professional Practice Committee. Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S3. [DOI] [PubMed] [Google Scholar]
- 4.King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122:844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. [DOI] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S86–104. [DOI] [PubMed] [Google Scholar]
- 9.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. [DOI] [PubMed] [Google Scholar]
- 10.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 12.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperling LS, Mechanick JI, Neeland IJ, et al. The CardioMetabolic Health Alliance: Working Toward a New Care Model for the Metabolic Syndrome. J Am Coll Cardiol. 2015;66:1050–67. [DOI] [PubMed] [Google Scholar]
- 15.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 16.Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong ND, Patao C, Wong K, et al. Trends in control of cardiovascular risk factors among US adults with type 2 diabetes from 1999 to 2010: comparison by prevalent cardiovascular disease status. Diab Vasc Dis Res. 2013;10:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. [DOI] [PubMed] [Google Scholar]
- 19.Bittner V, Bertolet M, Barraza Felix R, et al. Comprehensive cardiovascular risk factor control improves survival: the BARI 2D Trial. J Am Coll Cardiol. 2015;66:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–248. [DOI] [PubMed] [Google Scholar]
- 21.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 focused update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:1785–822. [DOI] [PubMed] [Google Scholar]
- 23.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–164. [DOI] [PubMed] [Google Scholar]
- 24.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 26.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–88. [DOI] [PubMed] [Google Scholar]
- 27.Yancy CW, Januzzi JL Jr., Allen LA, et al. 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71:201–30. [DOI] [PubMed] [Google Scholar]
- 28.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- 29.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 31.Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patorno E, Goldfine AB, Schneeweiss S, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ. 2018;360:k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udell JA, Yuan Z, Rush T, et al. Cardiovascular outcomes and risks after initiation of a sodium glucose cotransporter 2 inhibitor: results from the EASEL population-based cohort study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World). Circulation. 2018;137:1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosiborod M, Lam CSP, Kohsaka S, et al. Lower cardiovascular risk associated with SGLT-2i in >400, 000 patients: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71:2628–39. [DOI] [PubMed] [Google Scholar]
- 35.Fitchett D, Inzucchi SE, Lachin JM, et al. Cardiovascular mortality reduction with empagliflozin in patients with type 2 diabetes and cardiovascular disease. J Am Coll Cardiol. 2018;71:364–7. [DOI] [PubMed] [Google Scholar]
- 36.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;37:1526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2018;39:363–70. [DOI] [PubMed] [Google Scholar]
- 38.American Diabetes Association. Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clin Diabetes. 2018;36:14–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137:323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raz I, Mosenzon O, Bonaca MP, et al. DECLARE-TIMI 58: Participants’ baseline characteristics. Diabetes Obes Metab. 2018;20:1102–10. [DOI] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov. CardiovascularOutcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease, The VERTIS CV Study (MK-8835-004). Available at: https://clinicaltrials.gov/ct2/show/results/NCT01986881. Accessed August 15, 2018.
- 42.ClinicalTrials.gov. Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED). Available at: https://clinicaltrials.gov/ct2/show/NCT03315143. Accessed August 15, 2018.
- 43.Suissa S Lower risk of death with SGLT2 inhibitors in observational studies: real or bias? Diabetes Care. 2018;41:6–10. [DOI] [PubMed] [Google Scholar]
- 44.Wanner C, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:1801–2. [DOI] [PubMed] [Google Scholar]
- 45.Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–21. [DOI] [PubMed] [Google Scholar]
- 46.Li D, Wang T, Shen S, et al. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19:348–55. [DOI] [PubMed] [Google Scholar]
- 47.Jabbour S, Seufert J, Scheen A, et al. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20:620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erondu N, Desai M, Ways K, et al. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38:1680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–42. [DOI] [PubMed] [Google Scholar]
- 50.Kaptoge S, Di Angelantonio E, White IR, et al. , Emerging Risk Factors Collaboration. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inzucchi SE, Iliev H, Pfarr E, et al. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41:e4–5. [DOI] [PubMed] [Google Scholar]
- 52.Verma S, Mazer CD, Al-Omran M, et al. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–7. [DOI] [PubMed] [Google Scholar]
- 53.Fadini GP, Avogaro A. SGLT2 inhibitors and amputations in the US FDA Adverse Event Reporting System. Lancet Diabetes Endocrinol. 2017;5:680–1. [DOI] [PubMed] [Google Scholar]
- 54.Baartscheer A, Schumacher CA, Wust RC, et al. Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia. 2017;60:568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uthman L, Baartscheer A, Bleijlevens B, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, Lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61:722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME Study? A unifying hypothesis. Diabetes Care. 2016;39:1115–22. [DOI] [PubMed] [Google Scholar]
- 57.Januzzi JL Jr., Butler J, Jarolim P, et al. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol. 2017;70:704–12. [DOI] [PubMed] [Google Scholar]
- 58.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. [DOI] [PubMed] [Google Scholar]
- 60.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77. [DOI] [PubMed] [Google Scholar]
- 63.Gerstein HC, Colhoun HM, Dagenais GR, et al. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2018;20:42–9. [DOI] [PubMed] [Google Scholar]
- 64.ClinicalTrials.gov. Effect of Albiglutide, When Added to Standard Blood Glucose Lowering Therapies, on Major Cardiovascular Events in Subjects With Type 2 Diabetes Mellitus. Available at: https://clinicaltrials.gov/ct2/show/NCT02465515. Accessed August 15, 2018.
- 65.Davidson JA. Incretin-based therapies: focus on effects beyond glycemic control alone. Diabetes Ther. 2013;4:221–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nauck MA, Meier JJ, Cavender MA, et al. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–70. [DOI] [PubMed] [Google Scholar]
- 67.Nauck M Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 69.Avogaro A, Vigili de Kreutzenberg S, Fadini GP. Cardiovascular actions of GLP-1 and incretin-based pharmacotherapy. Curr Diab Rep. 2014;14:483. [DOI] [PubMed] [Google Scholar]
- 70.Fye WB. Introduction: the origins and implications of a growing shortage of cardiologists. J Am Coll Cardiol. 2004;44:221–32. [PubMed] [Google Scholar]
- 71.Richards P, Parker HE, Adriaenssens AE, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol. 2015;65:1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Avorn J The Psychology of clinical decision making–implications for medication use. N Engl J Med. 2018;378:689–91. [DOI] [PubMed] [Google Scholar]
- 74.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frias JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–16. [DOI] [PubMed] [Google Scholar]
- 76.Wilcox CS, Shen W, Boulton DW, et al. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc. 2018;7:e007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marathe PH, Gao HX, Close KL. American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes. 2017;9:320–4. [DOI] [PubMed] [Google Scholar]
- 78.Hirsh BJ, Smilowitz NR, Rosenson RS, et al. Utilization of and adherence to guideline-recommended lipid-lowering therapy after acute coronary syndrome: opportunities for improvement. J Am Coll Cardiol. 2015;66:184–92. [DOI] [PubMed] [Google Scholar]
- 79.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41:356–63. [DOI] [PubMed] [Google Scholar]
- 80.Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care. 2018;41:14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]