Abstract
Background:
Chronic Hepatitis D virus (HDV) infection results in the most severe form of viral hepatitis with a rapid progression to cirrhosis. However, non-invasive fibrosis tests that can accurately predict cirrhosis have not been adequately validated. We aimed to develop a clinically useful non-invasive score that can accurately detect cirrhosis.
Material and methods:
Patients with chronic HDV diagnosed by liver histology or serum PCR were evaluated. Data regarding demographics, laboratory, imaging, vibration-controlled transient elastography (VCTE), and liver biopsy were collected. The total cohort was randomized into a training and validation cohort. The training cohort was used to develop a novel score, the Delta-4 fibrosis score (D4FS) which was then compared to other non-invasive tests in the validation cohort by area under receiver operating characteristics (AUROC).
Results:
77 patients with chronic HDV were evaluated: mean age 42.6 (SD:11.1) years, 59.7% male, and 57.1% Asian. The total cohort was then separated into a training (n = 45) and validation (n = 32) cohort with no significant differences in terms of clinical characteristics between the two. From the training cohort, the D4FS was derived from variables of statistical and clinical interest (gamma-glutamyl transpeptidase (GGT), platelet count, alanine aminotransferase (ALT), and liver stiffness measurement (LSM)). The D4FS demonstrated the best AUROC in the validation cohort (0.94) followed by VCTE (0.90), FIB-4 (0.86), APRI (0.81), and AAR (0.71).
Discussion:
The D4FS is a clinically useful non-invasive fibrosis score that can accurately detect cirrhosis in patients with chronic HDV infection. Further studies should be performed to further validate clinical utility.
Keywords: Hepatitis D, Hepatitis B, Liver cirrhosis, Biomarkers, Delta hepatitis
1. Introduction
Chronic Hepatitis D virus (HDV) is the most severe form of viral hepatitis estimated to affect 62–72 million worldwide (Chen et al., 2018). Results from multiple studies have reported that HDV and hepatitis B virus (HBV) co-infection is associated with more aggressive disease compared to HBV infection alone (Fattovich et al., 2000; Beguelin et al., 2017). Approximately 10–15% of HBV/HDV co-infected patients will progress to cirrhosis within 2 years and ~50% will suffer a liver-related complication within 5 years (Heidrich et al., 2012; Bonino et al., 1987). Thus, identification of HDV infected patients with rapid progression of disease, advance fibrosis or cirrhosis, is desperately needed to recognize which patients need variceal and hepatocellular carcinoma screening, initiate treatment with interferon or an investigative therapeutic, and decide who needs to be referred to a liver transplant center.
However, determination of disease stage (i.e. fibrosis) in HDV infected patients is not well studied. While liver biopsy can be performed to stage disease, it is invasive, costly, subject to complications, and may be contraindicated in the setting of severe coagulopathy (Takyar et al., 2017a). These issues also limit its routine use especially if repeated investigations are needed. Non-invasive fibrosis tests in the form of “indirect” and “direct” fibrosis markers, algorithms that integrate these markers, and imaging modalities (i.e. vibration-controlled transient elastography (VCTE)) offer a solution to this problem. These tests have revolutionized the management of an assortment of different chronic liver diseases including HBV, hepatitis C virus (HCV), and non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) such that liver biopsy can be avoided in most cases (Soresi et al., 2014).
Nevertheless, these tests have not been substantially validated in chronic HDV and initial reports have suggested that non-invasive serum-based fibrosis tests may not be as accurate as they are in HBV and HCV infection (Takyar et al., 2017b; Lutterkort et al., 2017). This may be due to the fact that these tests were specifically created and validated in HBV and HCV cohorts or perhaps due to the significant levels of hepatic inflammation that exists in HDV infection which can cause artificial elevations in “indirect” marker-based scores (Kim et al., 2016). VCTE may become the non-invasive test of choice in chronic HDV infection due to its excellent performance in HBV and HCV however this modality has not yet been adequately studied in HDV. In addition, VCTE may also perform better in certain liver diseases and can also be limited by artificial elevations is liver stiffness measurements (LSMs) in the setting of hepatic inflammation (Castera, 2009; Degos et al., 2010). Possible solutions to this problem includes stepwise combination algorithms of non-invasive markers or combining different non-invasive modalities such as serologic markers with imaging-based tests (Castera, 2009; Sebastiani et al., 2006). These combinations have been previously explored with promising results in improving diagnostic performance in HBV and HCV (Boursier et al., 2011; Crespo et al., 2012).
In this current study, we aimed to develop a novel non-invasive fibrosis score for chronic HDV infection that can accurately detect cirrhosis utilizing routine lab markers with or without VCTE.
2. Materials and methods
2.1. Patients
We performed a retrospective cohort study of consecutive patients with chronic HDV infection who underwent VCTE at the NIH Clinical Center between January 2006 and April 2019. All patients were enrolled in a natural history of liver diseases protocol [NCT00001971] that has been approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board. Each patient gave written informed consent for participation.
Demographic, laboratory, imaging, VCTE, and liver biopsy data was collected. In those patients who did not undergo liver biopsy, imaging (Ultrasound, CT, MRI), was required to be included in this study. Laboratory, imaging, and liver biopsy data was only used if it was within 3, 6, and 12 months (respectively) of the VCTE date. All patients were diagnosed with HDV either serologically by detectable HDV-RNA on PCR (ARUP Laboratories, Salt Lake City, UT) or by histology with positive staining for hepatitis delta antigen (HDAg) in hepatocytes. Chronicity of at least 6 months was established based on clinical and laboratory findings.
Patients were excluded if there were no available or reliable LSM, lack of imaging within 6 months of a VCTE in those patients without liver biopsy, had undetectable HDV RNA by the time of VCTE, moderate to large volume ascites, alcoholic liver disease, non-alcoholic steatohepatitis, acute viral hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis. LSM was measured using FibroScan® (Echosens, Paris, France). A reliable LSM was defined as having at least 10 valid measurements, a success rate ≥ 60%, and an interquartile range (IQR) under 30% of the median value. LSM results were as reported as median values in kilopascals (kPa). A separate cohort of chronic HBV mono-infected patients was also utilized to test the performance of our non-invasive HDV fibrosis score.
2.2. Liver histology
Liver biopsy was performed either via the percutaneous or transjugular approach as clinically indicated. All liver biopsy specimens were read and scored by an expert hepatopathologist (DK). Hepatic fibrosis was assessed using the Ishak fibrosis score (0–6) (Ishak et al., 1995). Hepatic inflammation was assessed using the histology activity index (HAI) (0–18). (Knodell et al., 1981).
2.3. Diagnosis of cirrhosis
Patients were determined to have cirrhosis either by histology (Ishak fibrosis score ≥ 5)16 or clinical criteria. Clinical criteria was defined as having imaging consistent with cirrhosis (i.e. nodular liver) with two or more signs of portal hypertension (i.e. thrombocytopenia (PLT < 150 × 109/L), collaterals, splenomegaly, varices, or ascites) (Castera, 2009; Lu et al., 2006). Splenomegaly was defined based on cut-offs established based on age and height (Chow et al., 2016).
2.4. APRI, FIB-4, and AAR calculation
The APRI, FIB-4, and AAR scores were selected as comparative tests in this study since they are the most widely utilized and validated non-invasive serum fibrosis tests for HBV and HCV. The APRI was calculated as (AST (IU/L)/ULN of AST (IU/L))/PLT (109/L) × 100 (Wai et al., 2003). The FIB-4 was calculated as (age (years) × AST (IU/L))/[PLT (109/L) × ALT1/2 (IU/L) (Sterling et al., 2006). The AAR was calculated as AST (IU/L)/ALT (IU/L). (Williams and Hoofnagle, 1988).
2.5. Statistical analysis
Baseline patient characteristics were described using frequencies for categorical variables and means versus medians (depending on distribution) for continuous variables. Variables that were evaluated include demographics (age, sex, race), laboratory results (platelet count (PLT), prothrombin time, total bilirubin, albumin, ALT, AST, ALP, and GGT), and LSM. All variables used were logarithmically transformed to adjust for normality. Wilcoxon rank-sum tests, chi-squared tests and Fisher's exact tests were used to compare baseline variables across competing groups. A 2-sided P value of less than 0.05 was considered statistically significant.
For the development of the Delta-4 fibrosis score (D4FS), the entire cohort was randomly separated into a training and validation cohort stratified by the presence of cirrhosis and availability of histology. Significant predictors of cirrhosis in the training cohort were considered variables of interest. The clinical and laboratory characteristics of the training and validation cohort was compared after randomization to rule out any significant differences.
Based on the variables of interest and clinical judgement, the D4FS was developed. The diagnostic value of the D4FS was assessed by calculating areas under the receiver operating characteristic (ROC) curves. An area under the ROC (AUROC) of 1.0 represents an ideal test while an AUROC of 0.5 indicates no discriminatory ability. The diagnostic performance of other non-invasive testing modalities including VCTE by itself, APRI (Wai et al., 2003), FIB-4 (Sterling et al., 2006), and AST-to-ALT ratio (AAR) (Williams and Hoofnagle, 1988) were also assessed using ROC curves. Cirrhosis was defined as a: VCTE ≥12.5 kPa (Degos et al., 2010); APRI > 2 (Wai et al., 2003); FIB-4 > 3.6 (Kim et al., 2010); and AAR ≥ 1 (Williams and Hoofnagle, 1988).
The ideal cut-off for the D4FS to identify cirrhosis was identified using the distance criterion. This criterion chooses the point closest to the point on the ROC curve where 1-Specificity (Sp) = 0 and Sensitivity (Se) = 1. The Se, Sp, and positive and negative predictive value (PPV and NPV) using the ideal cut-off were also calculated. All statistical analysis was performed using SAS 9.4 (Cary, NC).
3. Results
3.1. Patient characteristics of the entire cohort
From January 2006 and April 2019, 93 patients with chronic HDV underwent liver biopsy and/or imaging at the NIH (Fig. 1). A total of 16 patients were excluded from the study: 13 patients had no available or valid VCTE result, 2 patients cleared HDV-RNA by the time of VCTE, and 1 patient was decompensated with large volume ascites. A total of 77 patients with chronic HDV were therefore included in the analysis. Infection with HDV was diagnosed by HDAg detection in liver tissue in 8 patients (10.4%) and by detectable serum HDV-RNA in 69 patients (89.6%). 18 patients (23.4%) were classified as cirrhotic: 10 by histologic criteria and 8 by clinical criteria.
Fig. 1.
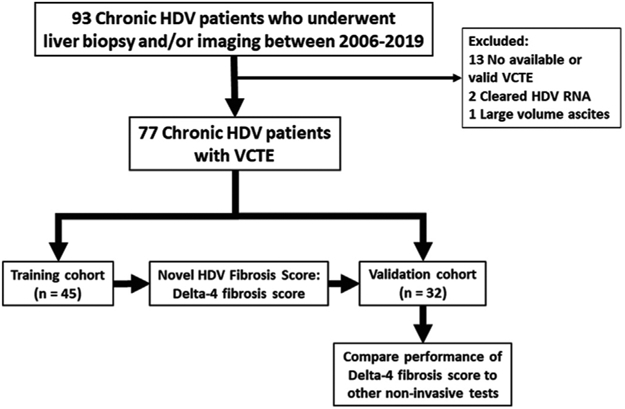
Study flow diagram.
Abbreviations: HDV, hepatitis d virus; VCTE, vibration-controlled transient elastography; RNA, ribonucleic acid.
3.2. Patient characteristics of the training cohort
Baseline characteristics of the training cohort is shown in Table 1. The mean age of the 45 patients in the training cohort was 43.3 (SD 12.3) years and 23 (51%) were male. 23 (51%) in the training cohort were Asian, and 17 (38%) were White. HBeAg and HBeAb was positive in 7 (16%) and 31 (69%) patients, respectively. 25 (56%) were on anti-nucleo(s)tide therapy. 10 (22.2%) were classified as cirrhotic: 6 by histologic criteria and 4 by clinical criteria. Mean HAI was 9.7 (SD 2.7).
Table 1.
“Training” vs “Validation cohort” characteristics.
| Training cohort (n = 45) |
Validation cohort (n = 32) |
P | |
|---|---|---|---|
| Age at VCTE | 43.3 (12.3) | 41.8 (9.5) | 0.79 |
| Gender | 0.07 | ||
| Male | 23 (51.1%) | 23 (71.9%) | |
| Female | 22 (48.9%) | 9 (28.1%) | |
| Race | 0.33 | ||
| White | 17 (37.8%) | 10 (31.3%) | |
| Black | 5 (11.1%) | 1 (3.1%) | |
| Asian | 23 (51.1%) | 21 (65.6%) | |
| Laboratory | |||
| ALP (IU/L) | 85.5 (27.0) | 81.8 (34.4) | 0.81 |
| AST (IU/L) | 68.5 (64.8) | 76.2 (63.6) | 0.31 |
| ALT (IU/L) | 95.7 (101.5) | 118.7 (149.2) | 0.26 |
| Total bilirubin (mg/dL) | 0.7 (0.3) | 0.9 (1.6) | 0.90 |
| GGT (IU/L) | 58.0 (50.1) | 57.2 (47.0) | 0.80 |
| Albumin (g/dL) | 4.0 (0.5) | 4.1 (0.4) | 0.59 |
| PT (seconds) | 14.1 (1.1) | 14.4 (1.7) | 0.58 |
| PLT (K/μL) | 170.0 (75.5) | 154.1 (77.1) | 0.59 |
| HBeAg + | 7 (15.6%) | 4 (12.5%) | 0.75 |
| HBeAb+ | 31 (68.9%) | 24 (75.0%) | 0.62 |
| Anti-nucleo(s)tide therapy | 25 (55.8%) | 21 (65.6%) | 0.39 |
| Histology | 20 (44.4%) | 14 (43.8%) | |
| HAI (0–18) | 9.7 (2.7) | 8.9 (1.7) | 0.47 |
| Ishak fibrosis score (0–6) | 3.7 (1.4) | 2.9 (1.8) | 0.16 |
| Ishak fibrosis score ≥ 5 | 6 (30.0%) | 4 (28.6%) | 1.0 |
| Clinical diagnosis of cirrhosis | 4 (8.9%) | 4 (12.5%) | 1.0 |
| LSM (kPa) | 13.5 (16.5) | 12.5 (10.9) | 0.29 |
Values expressed as mean (standard deviation) or n (%).
Abbreviations: VCTE, vibration-controlled transient elastography; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; PT, prothrombin time; PLT, platelet count; HBeAg, hepatitis B-e antigen; HBeAb, hepatitis B-e antibody; HAI, histology activity index; LSM, liver stiffness measurement.
3.3. Predictors of cirrhosis in the training cohort and the development of the Delta-4 fibrosis score (D4FS)
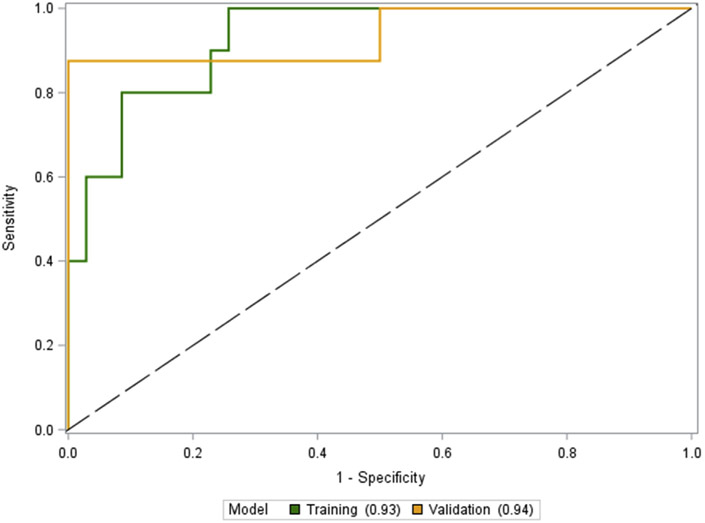
After the entire HDV cohort (n=77) was randomly divided into the training cohort (n = 45) and validation cohort (n = 32), variables associated with the presence of cirrhosis were first assessed by univariate analysis. (Table 2). Variables that were of interest due to a P < 0.05 included AST (P = 0.008), GGT (P = 0.01), albumin (P = 0.01), PLT (P = 0.0006), and LSM (P < 0.0001). Of those, GGT, PLT, and LSM were chosen as components of the D4FS. In addition, ALT was also incorporated into the score due to the severity of hepatic necroinflammation that occurs in chronic HDV infection (El-Shabrawi et al., 2010). The formula for the D4FS is depicted below. The AUROC for the D4FS for predicting cirrhosis in the training cohort is shown in Fig. 2. The D4FS performed with an AUROC of 0.93 (95% CI 0.85–1.0). The optimal cut-off for the D4FS for the detection of cirrhosis was calculated to be 7.8.
Table 2.
Training cohort (n = 45) – “Cirrhosis” vs “No cirrhosis”.
| Cirrhosis (n = 10) |
No cirrhosis (n = 35) |
P | |
|---|---|---|---|
| Age at VCTE | 43.0 (9.8) | 43.3 (13.0) | 0.87 |
| Gender | 0.10 | ||
| Male | 6 (60.0%) | 16 (48.5%) | |
| Female | 4 (40.0%) | 17 (51.5%) | |
| Race | 0.92 | ||
| White | 4 (40.0%) | 13 (39.4%) | |
| Black | 1 (10.0%) | 4 (12.1%) | |
| Asian | 5 (50.0%) | 16 (48.5%) | |
| Laboratory | |||
| ALP (IU/L) | 99.4 (33.1) | 81.5 (24.1) | 0.13 |
| AST (IU/L) | 82.7 (30.1) | 64.4 (71.5) | 0.008 |
| ALT (IU/L) | 91.4 (51.7) | 96.9 (112.3) | 0.54 |
| Total bilirubin (mg/dL) | 0.9 (0.5) | 0.6 (0.2) | 0.14 |
| GGT (IU/L) | 83.2 (41.3) | 50.7 (50.6) | 0.01 |
| Albumin (g/dL) | 3.6 (0.6) | 4.2 (0.3) | 0.01 |
| PT (seconds) | 14.7 (1.0) | 14.0 (1.0) | 0.04 |
| PLT (K/μL) | 105.3 (48.1) | 188.5 (72.0) | 0.0006 |
| LSM (kPa) | 33.5 (26.5) | 7.8 (4.0) | < 0.0001 |
Values expressed as mean (standard deviation) or n (%).
Abbreviations: VCTE, vibration-controlled transient elastography; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; PT, prothrombin time; PLT, platelet count; LSM, liver stiffness measurement.
Fig. 2.
Performance of the D4FS in the “Training” and “Validation” cohort.
3.4. Patient characteristics of the validation cohort
32 patients comprised of the validation cohort. In general, the characteristics of the training and validation cohorts were similar (Table 1). There was no difference in the mean HAI, Ishak fibrosis score, LSM, or the number of patients classified as cirrhotic. All of the mean baseline laboratory markers in addition to HBeAg and HBeAb status were comparable as well. However, the validation cohort had more males: 23 (72%) and Asians: 21 (66%).
3.5. Performance of the D4FS in the validation cohort and comparison to pre-existing non-invasive fibrosis tests
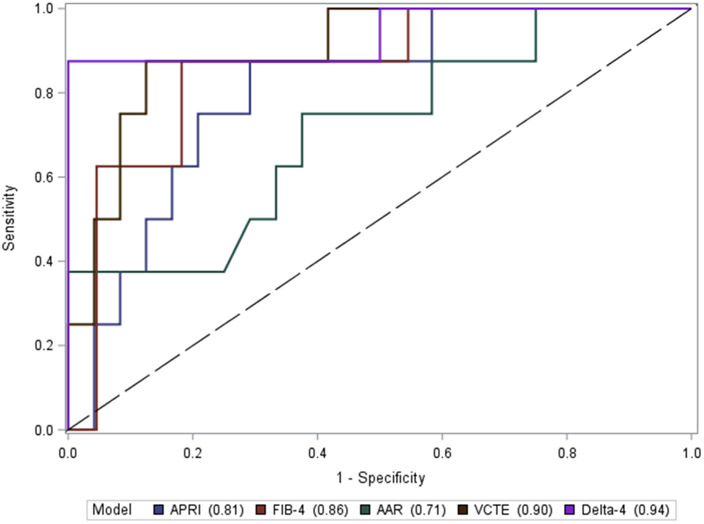
The AUROC for the D4FS in the validation cohort is demonstrated in Fig. 2. The D4FS demonstrated an AUROC of 0.94 (95% CI 0.81–1.0). At the calculated optimal cut-off of 7.8 from the training cohort, the D4FS performed with a Se 87.5%, Sp 83.3%, PPV 63.6%, and NPV 95.2%. 27 of 32 (84.3%) were correctly classified as cirrhotic at this cut-off (Table 3). When compared to other non-invasive tests, the D4FS performed the best followed by VCTE (AUROC = 0.90, 95% CI 0.78–1.0), FIB-4 (AUROC = 0.86, 95% CI 0.70–1.0), APRI (AUROC = 0.81, 95% CI 0.64–0.97), and AAR (AUROC = 0.71, 95% CI 0.49–0.93). The corresponding AUROC comparing all of the tests are shown in Fig. 3.
Table 3.
Diagnostic accuracy of the D4FS in the “Validation cohort” (n = 32).
| Non-invasive test | Cut-offs for cirrhosis | Patients with cirrhosis (n = 8) | Patients without cirrhosis (n = 24) | Se (%) | Sp (%) | PPV (%) | NPV (%) | Correctly classified |
|---|---|---|---|---|---|---|---|---|
| D4FS | ≥ 7.8 | 7 | 4 | 87.5% | 83.3% | 63.6% | 95.2% | 27 (84.3%) |
| < 7.8 | 1 | 20 |
Abbreviations: D4FS, Delta-4 fibrosis score; Se, Sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
Fig. 3.
Performance of the D4FS compared to other non-invasive tests in the “Validation” cohort. Abbreviations: APRI, AST to platelet ratio index; AST, aspartate aminotransferase; FIB-4, fibrosis-4 index; AAR, aspartate aminotransferase to ALT ratio; ALT, alanine aminotransferase; VCTE, vibration-controlled transient elastography.
3.6. Baseline characteristics of the HBV cohort and the performance of the D4FS compared to other pre-existing non-invasive fibrosis tests
Baseline characteristics of the HBV cohort are shown in Supplemental Table 1. 112 HBV mono-infected patients were included with a mean age of 46.3 (SD 14.0) years with 67.9% males. The mean LSM was 7.2 (SD 4.7) kPa and 12 patients (30.0%) were classified as having cirrhosis. The performance of the D4FS compared to other non-invasive tests is shown in Supplemental Fig. 1. VCTE by itself outperforms the D4FS with a AUROC of 0.93 compared to 0.90.
4. Discussion
Chronic HDV infection is the chronic viral hepatitis with the most aggressive hepatic inflammation resulting in advanced liver disease and an associated increased risk of HCC and mortality (Takyar et al., 2017b; Niro et al., 2010). In this study, we developed a novel non-invasive fibrosis score, the D4FS, that can accurately detect cirrhosis in this patient population despite the severity of inflammation as evidenced by the high AST/ALT levels and HAI.
The D4FS is an easy to calculate, non-invasive score that combines three commonly used serologic markers (PLT, GGT, and ALT) with VCTE. PLT and GGT are both biomarkers of liver disease severity that have been incorporated in numerous non-invasive serum tests while VCTE is perhaps the most well validated non-invasive imaging technique for estimating fibrosis (Wai et al., 2003; Lu et al., 2018). ALT was also included in our score despite it not being statistically significant in the training cohort because it is the most liver-specific biomarker evaluated in this study and thus reflective of ongoing chronic liver inflammation (Kim et al., 2008). ALT has been added to numerous other non-invasive tests to attempt to account for ongoing hepatic injury such as the FIB-421, AAR (Williams and Hoofnagle, 1988), and FibroMeter® (Echosens, Paris, France) (Cales et al., 2008). ALT has also been added to pre-existing non-invasive tests such as FibroTest® (Biopredictive, Paris, France) resulting in the ActiTest® (Biopredictive, Paris, France) also to deal with the issue of hepatic inflammation skewing test results (Yakoob et al., 2015).
The D4FS performed the best in HDV infection for detecting cirrhosis with an AUROC of 0.94 compared to VCTE alone which performed with an AUROC of 0.90. We suspect that the improved performance of the D4FS may be due to the significant hepatic inflammation in HDV affecting LSMs (Chan et al., 2009). The combination of serologic markers and LSM in our score exploits a synergism between LSM and serologic tests that has been previously reported in hepatitis C virus (HCV) and HBV infected patients to resolve discordances between tests and improve diagnostic accuracy and thus may alleviate this weakness of LSM (Boursier et al., 2011; Crespo et al., 2012). In addition, the D4FS outperformed commonly used non-invasive serologic tests including FIB-4 (AUROC = 0.86), APRI (AUROC = 0.81), and AAR (AUROC = 0.71). Each of these tests are widely accepted and routinely used to quickly assess for the presence of fibrosis and cirrhosis. Finally, at an ideal cut-off of 7.8, the D4FS demonstrated an excellent NPV of 95.2% and ability to rule-out cirrhosis with a Se of 87.5%.
The D4FS is the second non-invasive fibrosis score that has been developed strictly for chronic HDV. The first score was introduced as the Delta Fibrosis Score (DFS) by Lutterkort et al., in 2017, but this was aimed at detecting advanced fibrosis (Ishak fibrosis ≥3) (Lutterkort et al., 2017). However, this score includes serum cholinesterase as part of the score which is not readily available in many countries limiting the applicability of this score. The D4FS differs from the DFS in that all the serologic markers incorporated are routinely measured and readily available.
The strength of this study is that every patient was confirmed to have active HDV infection either by HDAg staining on histology or by detectable HDV-RNA in the serum and chronicity by clinical history. Confirmation of active HDV infection is rare among available HDV studies due to availability of HDV-RNA PCR assays. Additionally, every HDV patient was well characterized with imaging and VCTE data. The performance of non-invasive serologic and imaging fibrosis tests have not been well studied in HDV. Finally, an exploration of the D4FS in HBV mono-infected patients demonstrated worse performance than VCTE. This suggests that the D4FS is truly a test excels in identifying cirrhosis in HDV infection whereas it has limited utility in mono-infected HBV patients.
Our study has several limitations. First, this study was a singlecenter cohort study and lacks an external validation cohort for the Delta-4 score. This limitation is difficult to correct due several reasons. The rarity of HDV infected patients in general results in small cohorts at individual center. This is likely related to undertesting and lack of overall disease awareness (Kushner et al., 2015). Furthermore, confirmation of active HDV infection in these cohorts are often lacking due to the availability of HDV-RNA PCR assays. Moreover, liver biopsy is infrequently pursued in clinical practice and VCTE, which is a necessary component of the score, has limited availability in many of the countries in which HDV is considered endemic. Another limitation of this study is the small number of patients classified as “cirrhosis” in both the training and validation cohorts. This is again related to the relative rarity of HDV and the total size of our HDV cohort. A third limitation of this study is that approximately half of the patients were clinically diagnosed with cirrhosis since these patients never underwent liver biopsy. This likely underestimated the number of patients with cirrhosis since the prevalence of cirrhosis among patients with histologic results was much higher than those without due to our strict criteria of requiring imaging consistent with cirrhosis plus two signs of portal hypertension.
In summary, the D4FS is a clinically useful non-invasive fibrosis score that can accurately detect cirrhosis in patients infected with chronic HDV. This score combines the use of several commonly ordered serologic markers (PLT, GGT, and ALT) with LSM in a novel algorithm that improves on the diagnostic accuracy of LSM alone. Further exploration of the D4FS in additional cohorts should be performed to further validate its clinical utility.
Supplementary Material
Supplemental Fig. 1. Performance of the D4FS compared to other non-invasive tests in HBV mono-infected patients
Acknowledgments
Funding
This work was funded by the intramural research programs of the National Institute of Diabetes & Digestive & Kidney Diseases and National Cancer Institute, National Institutes of Health.
Abbreviations
- HDV
hepatitis D virus
- VCTE
vibration controlled transient elastography
- D4FS
Delta-4 fibrosis score
- AUROC
area under receiver operator characteristics
- ALT
alanine aminotransferase
- LSM
liver stiffness measurement
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NIH
National Institutes of Health
- CT
computer tomography
- MRI
magnetic resonance imaging
- PCR
polymerase chain reaction
- HDAg
hepatitis delta antigen
- RNA
ribonucleic acid
- IQR
interquartile range
- KPa
kilopascals
- HAI
histology activity index
- PLT
platelet
- APRI
ast-to-platelet-ratio index
- FIB-4
fibrosis-4 index
- AAR
AST-to-ALT-ratio
- GGT
gammaglutamyl transferase
- ALP
alkaline phosphatase
- ROC
receiver operating characteristics
- Sp
specificity
- Se
sensitivity
- PPV
positive predictive value
- NPV
negative predictive value
- HBeAg
hepatitis B e antigen
- HBeAb
hepatitis B e antibody
Footnotes
Declaration of competing interest
The authors have no relevant conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2019.104691.
Writing assistance
None.
Informed consent in studies with human subjects
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- Beguelin C, Moradpour D, Sahli R, et al. , 2017. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J. Hepatol 66, 297–303. [DOI] [PubMed] [Google Scholar]
- Bonino F, Negro F, Baldi M, et al. , 1987. The natural history of chronic delta hepatitis. Prog. Clin. Biol. Res 234, 145–152. [PubMed] [Google Scholar]
- Boursier J, de Ledinghen V, Zarski JP, et al. , 2011. A new combination of blood test and fibroscan for accurate non-invasive diagnosis of liver fibrosis stages in chronic hepatitis C. Am. J. Gastroenterol 106, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Cales P, Boursier J, Oberti F, et al. , 2008. FibroMeters: a family of blood tests for liver fibrosis. Gastroenterol. Clin. Biol 32, 40–51. [DOI] [PubMed] [Google Scholar]
- Castera L, 2009. Transient elastography and other non-invasive tests to assess hepatic fibrosis in patients with viral hepatitis. J. Viral Hepat 16, 300–314. [DOI] [PubMed] [Google Scholar]
- Chan HL, Wong GL, Choi PC, et al. , 2009. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J. Viral Hepat 16, 36–44. [DOI] [PubMed] [Google Scholar]
- Chen HY, Shen DT, Ji DZ, et al. , 2018. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut 68, 512–521. [DOI] [PubMed] [Google Scholar]
- Chow KU, Luxembourg B, Seifried E, et al. , 2016. Spleen size is significantly influenced by body height and sex: establishment of normal values for spleen size at US with a cohort of 1200 healthy individuals. Radiology 279, 306–313. [DOI] [PubMed] [Google Scholar]
- Crespo G, Fernandez-Varo G, Marino Z, et al. , 2012. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J. Hepatol 57, 281–287. [DOI] [PubMed] [Google Scholar]
- Degos F, Perez P, Roche B, et al. , 2010. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J. Hepatol 53, 1013–1021. [DOI] [PubMed] [Google Scholar]
- El-Shabrawi MH, Mohsen NA, Sherif MM, et al. , 2010. Noninvasive assessment of hepatic fibrosis and necroinflammatory activity in Egyptian children with chronic hepatitis C virus infection using FibroTest and ActiTest. Eur. J. Gastroenterol. Hepatol 22, 946–951. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Giustina G, Christensen E, et al. , 2000. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut 46, 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrich B, Serrano BC, Idilman R, et al. , 2012. HBeAg-positive hepatitis delta: virological patterns and clinical long-term outcome. Liver Int. 32, 1415–1425. [DOI] [PubMed] [Google Scholar]
- Ishak K, Baptista A, Bianchi L, et al. , 1995. Histological grading and staging of chronic hepatitis. J. Hepatol 22, 696–699. [DOI] [PubMed] [Google Scholar]
- Kim WR, Flamm SL, Di Bisceglie AM, et al. , 2008. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 47, 1363–1370. [DOI] [PubMed] [Google Scholar]
- Kim BK, Kim DY, Park JY, et al. , 2010. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 30, 546–553. [DOI] [PubMed] [Google Scholar]
- Kim WR, Berg T, Asselah T, et al. , 2016. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J. Hepatol 64, 773–780. [DOI] [PubMed] [Google Scholar]
- Knodell RG, Ishak KG, Black WC, et al. , 1981. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1, 431–435. [DOI] [PubMed] [Google Scholar]
- Kushner T, Serper M, Kaplan DE, 2015. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J. Hepatol 63, 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SN, Wang JH, Liu SL, et al. , 2006. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer 107, 2212–2222. [DOI] [PubMed] [Google Scholar]
- Lu XJ, Li XH, Yuan ZX, et al. , 2018. Assessment of liver fibrosis with the gamma-glutamyl transpeptidase to platelet ratio: a multicentre validation in patients with HBV infection. Gut 67, 1903–1904. [DOI] [PubMed] [Google Scholar]
- Lutterkort GL, Wranke A, Yurdaydin C, et al. , 2017. Non-invasive fibrosis score for hepatitis delta. Liver Int. 37, 196–204. [DOI] [PubMed] [Google Scholar]
- Niro GA, Smedile A, Ippolito AM, et al. , 2010. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J. Hepatol 53, 834–840. [DOI] [PubMed] [Google Scholar]
- Sebastiani G, Vario A, Guido M, et al. , 2006. Stepwise combination algorithms of non-invasive markers to diagnose significant fibrosis in chronic hepatitis C. J. Hepatol 44, 686–693. [DOI] [PubMed] [Google Scholar]
- Soresi M, Giannitrapani L, Cervello M, et al. , 2014. Non invasive tools for the diagnosis of liver cirrhosis. World J. Gastroenterol 20, 18131–18150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling RK, Lissen E, Clumeck N, et al. , 2006. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43, 1317–1325. [DOI] [PubMed] [Google Scholar]
- Takyar V, Etzion O, Heller T, et al. , 2017a. Complications of percutaneous liver biopsy with Klatskin needles: a 36-year single-centre experience. Aliment. Pharmacol. Ther 45, 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takyar V, Surana P, Kleiner DE, et al. , 2017b. Noninvasive markers for staging fibrosis in chronic delta hepatitis. Aliment. Pharmacol. Ther 45, 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai CT, Greenson JK, Fontana RJ, et al. , 2003. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38, 518–526. [DOI] [PubMed] [Google Scholar]
- Williams AL, Hoofnagle JH, 1988. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 95, 734–739. [DOI] [PubMed] [Google Scholar]
- Yakoob R, Bozom IA, Thandassery RB, et al. , 2015. Noninvasive biomarkers FibroTest and ActiTest versus liver biopsy in chronic hepatitis C patients: the Middle East experience. Ann. Gastroenterol 28, 265–270. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Performance of the D4FS compared to other non-invasive tests in HBV mono-infected patients