Abstract
The tumor microenvironment is distinct from normal tissue as a result of abnormal vascular network characterized by hypoxia, low pH, high interstitial fluid pressure and elevated glycolytic activity. This poses a barrier to treatments including radiation therapy and chemotherapy. Imaging methods which can characterize such features non-invasively and repeatedly will be of significant value in planning treatment as well as monitoring response to treatment. The three techniques based on magnetic resonance imaging (MRI) are reviewed here. Tumor pO2 can be measured by two MRI methods requiring an exogenous contrast agent: electron paramagnetic resonance imaging (EPRI) and Overhauser magnetic resonance imaging (OMRI). Tumor metabolic profile can be assessed by a third method, hyperpolarized metabolic MR, based on injection of hyperpolarized biological molecules labeled with 13C or 15N and MR spectroscopic imaging. Imaging pO2 in tumors is now a robust pre-clinical imaging modality with potential for implementation clinically. Pre-clinical studies and an initial clinical study with hyperpolarized metabolic MR have been successful and suggest that the method may be part of image-guided radiotherapy to select patients for tailored individual treatment regimens.
Solid tumors have regions of hypoxia as a result of inadequate supply of oxygen and nutrients [1]. Hypoxia can be diffusion limited (chronic) or perfusion limited (acute) [2]. Oxygen is necessary to fix radiation-induced damage to targets of ionizing radiation. Hypoxia has been shown to exist in human tumors and was associated to resistance to radiotherapy [3]. Tumors displaying a hypoxic phenotype may also be resistant to chemotherapy since poor perfusion to these regions can result in sub-optimal doses of the infused drug being delivered to the tumor [4]. A priori knowledge of the tumor physiology will enable implementing appropriate therapies such as dose-painting in radiotherapy, combination with radiation sensitizers or even the use of hypoxia-specific cytotoxins [5]. Several randomized clinical studies testing therapies specific to hypoxic tumors pointed to a need of identifying specific patients whose tumor contain hypoxic populations preferably by non-invasive imaging [6–8]. In addition to the abnormal oxygen status, tumors also display a different metabolic profile with energy derived by aerobic glycolysis compared to normal cells, which derive energy by oxidative phosphorylation. Cancer cells realize energy by glycolysis to lactate even when they are in normoxic environments (Warburg effect) suggesting that profiling tumors on a metabolic basis can also provide imaging biomarkers, which can be integrated in treatment regimens [9].
Imaging techniques which can provide maps of tumor pO2 and metabolic profile can be valuable in guiding therapies, especially radiotherapy where images with information pertinent to physiology and/or metabolism can be integrated to radiation treatment planning [10] or combination therapies with a radio sensitizer, a hypoxia-specific cytotoxin or a hypoxia activated pro-drug [11]. In a recent review, the importance of integrating biological images to guide radiotherapy has been discussed [12,13]. Imaging modalities such as PET and MRI probing metabolism, perfusion and other local features have been discussed for potential use. In this article, we review two imaging techniques, which can provide non-invasive and repeated assessment of tumor pO2, and a novel method, hyperpolarized metabolic MR, based on hyperpolarization of 13C or 15N in biological molecules and magnetic resonance spectroscopic imaging (MRSI) that can profile the tumor metabolically.
Electron paramagnetic resonance imaging (EPRI)
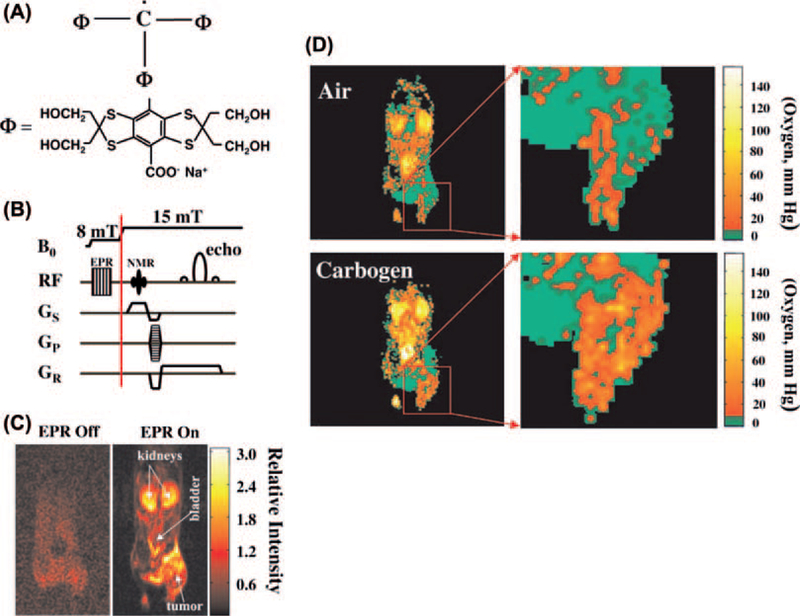
There are several techniques in use in preclinical and clinical studies to obtain pO2 status of tissue [10]. Oxygen electrodes can provide quantitative assessment of tissue pO2, but are invasive point measurements and are not amenable to deep-seated tumors. PET hypoxia imaging is non-invasive yet provides images which are qualitative in nature. These two techniques are in use clinically [14,15]. Several other methods are in development of which Overhauser magnetic resonance imaging (OMRI), electron paramagnetic resonance imaging (EPRI) and 19F MRI represent non-invasive and quantitative imaging approaches for mapping pO2. The OMRI and EPRI require injection of an exogenous paramagnetic agent. OX063, an organic molecule (trityl) with a single unpaired electron and having long in vivo half-life and single narrow EPR spectrum, was designed for this purpose [16]. The structure of OX063 is shown in Figure 1A. Experimental details of a pre-clinical EPR imaging system for pO2 imaging can be found in previous reports [17]. EPR spectroscopy is widely used in analytical chemistry for the detection and characterization of paramagnetic species. The pO2 imaging capability in EPR stems from the fact that molecular oxygen is paramagnetic and affects the relaxation rates of the exogenous paramagnetic agent. Dissolved molecular oxygen cannot be detected directly by EPR. Since molecular oxygen is paramagnetic and imposes a T2 contrast through collisional interactions, the spectral broadening of OX063 is linearly proportional to the oxygen concentration. An EPR imaging spectrometer operating at a frequency of 300 MHz (similar frequency as a 7 T MRI scanner) and a magnetic field of 10 mT was developed and the image formation and reconstruction approached were integrated [17]. Suitable agents for EPR imaging should have simple EPR spectra, and should be administered at levels which are well tolerated and should have pharmacological half-lives longer than the imaging time. OX063 is well suited as a paramagnetic agent in EPRI in vivo, to study tumor pO2, dynamics of pO2 to distinguish chronic versus acute hypoxia, and also monitor changes serially in response to therapy.
Figure 1.
(A) Structural formula of the electron paramagnetic agent OX063, a trityl radical. (B) Overhauser MRI pulse-sequence diagram showing B0 filed cycling and RF and field-gradient waveforms. (C) Interleaved (“EPR off” and “EPR on”) OMRI images (coronal) of a female C3H mouse, bearing SCC tumor on the right hind leg, demonstrating the Overhauser enhancement. Both images were acquired in the presence of the contrast agent. (D) pO2 images of a mouse with SCC tumor during air breathing (upper) and carbogen breathing (lower). The expanded tumor region is given at the right (see [18]).
Overhauser magnetic resonance imaging (OMRI)
The methodology for generating pO2 maps in vivo using the paramagnetic agent OX063 and OMRI was described previously [18]. Briefly, the object being imaged is placed in a resonator assembly tuned to resonant frequencies of both OX063 and 1H when placed in a magnetic field of ~10 mT. The MR images based on tissue water proton density are enhanced by a combination of the presence of OX063 in the region being imaged and saturation of the EPR frequency prior to MRI. The pulse sequence typically used in OMRI scans is shown in Figure 1B. As shown in the figure, a standard gradient recalled echo sequence routinely used in MRI experiments is preceded by an EPR irradiation pulse for Overhauser enhancement (to the left of the red vertical line) of the water 1H signal. Molecular oxygen (O2/pO2) affects the EPR saturation and OMRI maps of the OX063 concentration and pO2 maps can be calculated. The enhancement of the MR image intensity depends on three factors: 1) concentration of the paramagnetic agent OX063; 2) strength of the EPR irradiation; and 3) inversely on the oxygen concentration. OMRI images collected at two different levels of EPR irradiation allow the determination of OX063 and oxygen concentration.
In vivo oxygen imaging
OMRI has been applied to image tumor pO2 in mice [18]. Figure 1C shows images from an SCCVII tumor bearing mouse infused with OX063 (3.8 mmol/kg) through a tail vein catheter. The image on the left (Figure 1C) was obtained without EPR irradiation and the image on the right was collected after EPR irradiation. The result is a significant enhancement of the MR images even at an operating field of 15 mT. Figure 1D shows a pO2 map of a tumor bearing mouse while breathing air, obtained from OMRI experiments with a tumor region shown on the right. Pixels shown in green represent pO2 values < 10 mmHg. The images show that a significant region of the tumor is hypoxic. When the inhalation gas was changed to carbogen (95% O2 + 5% CO2) tumor hypoxia decreased substantially. The dynamic imaging capabilities of OMRI in providing pO2 maps based on physical (collisional) interactions between the reporter molecule (OX063) and molecular oxygen make it advantageous over other pO2 assessment techniques.
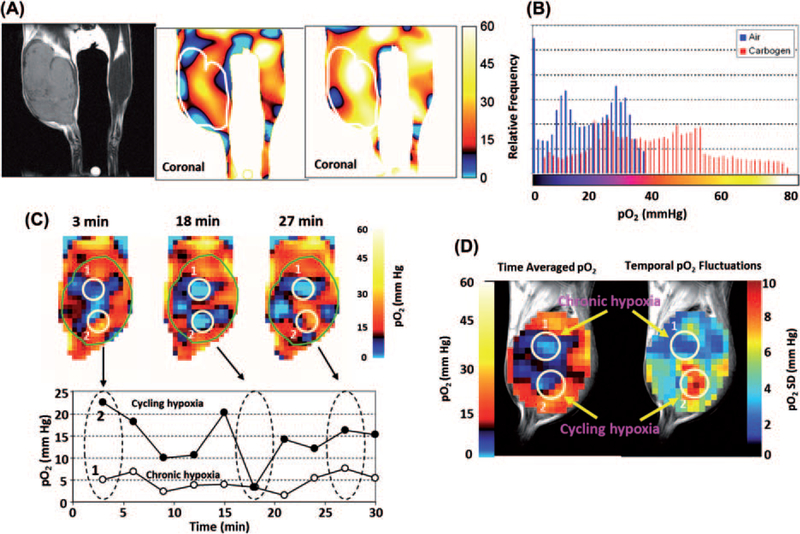
While OMRI as a technique is capable of providing anatomically co-registered pO2 maps, direct detection of the resonance signals of OX063 by EPRI is more sensitive requiring lower doses of the administered agent. The quantitative pO2 imaging in defined phantom objects and in in vivo models was validated in several studies [17]. Figure 2A shows pO2 images from a mouse with the tumor bearing leg and normal leg in the field of view. Figure 2A (left) shows the anatomic image with the tumor bearing leg on the left and the normal leg on the right. The middle image in Figure 2A is a pO2 image from the same mouse obtained from EPRI after infusion of OX063 (1.1 mmol/kg) when the mouse was breathing air. This tumor displayed well-oxygenated regions and hypoxic regions. The pO2 histogram from this image is shown in Figure 2B (blue bars).The histogram shows that the median pO2 was ~16 mmHg when the mouse was breathing air. The pO2 image in Figure 2A (right) was obtained when the breathing gas was carbogen and the corresponding pO2 histogram (Figure 2B, red bars) shows a significant right shift towards higher pO2 values with a median pO2 value of 35.5 mmHg. Additionally, the fractional region of the tumor with pO2 < 10 mmHg decreased from 35% when breathing air to approximately 7% when breathing carbogen. This experiment suggests the dynamic and quantitative pO2 imaging capability. Further developments in EPRI capabilities made it possible to obtain pO2 maps in three dimensions in approximately 3 minutes making it possible to apply this technique to study dynamics of pO2 in tumors. Tumors exhibit cycling hypoxia as well as chronic hypoxia, EPRI studies were conducted to examine whether this technique can distinguish the two types [19]. Figure 2C shows representative pO2 maps at 3, 18 and 27 min (top row) from a set of images of the tumor bearing animal collected every 3 minutes and the scatter graph below shows the pO2 values in a time interval of 0–30 minutes from the two regions of interest (ROIs) marked in the top row. The image data show that while ROI 1 had a pO2 < 10 mmHg and with minimal fluctuations, ROI 2 displayed significant fluctuations with values ranging between 25 and 5 mmHg. The image data suggest that ROI 1 is consistent with a chronically hypoxic tumor region and ROI 2 representing a cyclic hypoxic region. Figure 2D shows a time averaged pO2 map (left) and a map of the magnitude of fluctuations (right) generated from the snapshot images collected every 3 minutes. The data analyzed in this manner show that the map of the magnitude of pO2 fluctuations from EPRI can distinguish both chronically hypoxic and cycling hypoxic regions. In summary, EPRI is a promising non-invasive imaging modality that directly measures oxygen concentration in tumors in a 3D volume with spatial resolution of ~ mm and temporal resolution of minutes. The radiofrequency (300 MHz) power used provides the capability of imaging deep-seated tumors. With scale-up development to humans, EPRI has the capability to provide images of the tumor pO2 and its dynamics, which can be used to deliver appropriate cancer treatment.
Figure 2.
(A) In vivo EPR oxygen mapping of SCC tumor-bearing mouse leg, and visualization of the effect of carbogen (95% O2 plus 5% CO2) breathing on tumor pO2. (B) Histograms of pO2 in the tumor region of the same mouse breathing medical air (blue) and carbogen (red). A net increase in the median pO2 was noted upon carbogen breathing. (see [24]). (C) Non-invasive imaging of chronic and cycling tumor hypoxia in a mouse implanted with a SCCVII tumor. 3D-EPR oxygen images were obtained every 3 min during a 30 min time window. Three representative images acquired at 3, 18, and 27 min are shown. Two ROIs were selected in the tumor (1 and 2), and pO2 was assessed in the ROIs over 30 min. (D) ROI 1 (open circles) indicates a chronically hypoxic region; ROI 2 (closed circles) represents a cycling hypoxic region showing temporal fluctuations in pO2. C, Time-averaged pO2 map (left) and standard deviation map of pO2 (right) calculated from the 10 images taken in the 30 min time window (see [19]).
Hyperpolarized metabolic MR
Conventional MRI has evolved from the 1980s to become a routine diagnostic modality providing images of soft tissue with exquisite resolution using resonance signals from the abundant water protons. However, MRI of molecules probing other nuclei such as 13C, 14N, 19F, 31P etc., has been impractical primarily because of the low sensitivity and significantly lower concentrations compared to tissue water (a few mM versus 80 M). The signal to noise ratio in MRI depends on the polarization of the spin states, of nuclei such as 1H, 13C in addition to their nuclear magnetic moments, and the magnetic field strength of the MRI scanner. The polarization of 13C at a magnetic field strength of 3 T is 2.5 parts per million. Thus, biological molecules enriched with 13C suffer from poor sensitivity because of a lower magnetic moment, lower polarization and lower concentrations. The polarization of nuclei can be increased by different methods of which dynamic nuclear polarization (DNP) is the most successful [20]. In DNP, the higher polarization of a molecule with an unpaired electron spin is transferred to nuclei such as 1H, 13C, 15N, 19F etc. OX063 was also used to polarize 13C labeled agents such as [1-13C]pyruvate ex vivo, which when injected in vivo breaks down to various metabolites such as bicarbonate, lactate, alanine depending on the metabolic profile. The conversion of pyruvate to each of these metabolites can be imaged using chemical shift MRI [21].
The hyperpolarization is achieved by the dissolution DNP method. The idea and schematics of the apparatus, which increases the polarization of 13C nuclei to more than 20% translating into a gain in sensitivity of four orders in magnitude was first published in 2003 [20]. The gain in SNR by DNP was demonstrated by measuring the hyperpolarized 13C spectrum of urea (natural abundance 13C, 59.6 mM) in a 9.4 T NMR spectrometer. The 13C NMR spectrum from hyperpolarized urea was compared to the 13C NMR spectrum from the same sample at thermal polarization (in the 9.4 T magnet) after signal averaging for 65 hours. The polarization of 13C when hyperpolarized was determined to be approximately 20% and the SNR of 4592 was considerably higher than that from urea at normal polarization. This enhanced sensitivity by hyperpolarization provides the needed SNR for in vivo 13C MR imaging of organic molecules. Since the chemical shifts of various organic molecules display significant differences, it is possible to inject a hyperpolarized 13C labeled agent and follow its breakdown by chemical shift imaging or MRSI.
For a molecule to be sufficiently polarized with long signal decay the following characteristics are desired: 1) the molecule should have a carbon site with long intrinsic T1 that can be enriched in 13C (e.g. carbonyl or carboxylic acid). Deuteration can make aliphatic carbons amenable as well; 2) The molecule should be formulated with the paramagnetic agent as a glassy (homogenous) solid when frozen to ensure polarization transfer from the electrons to nuclei; 3) Upon dissolution the loss of polarization should not be significant; 4) the chemical shifts of the injected molecule and products should be distinct; and finally 5) the molecule should have minimal side effects when injected as a bolus at the required doses (typically approx. 0.1 mmol/kg body weight).
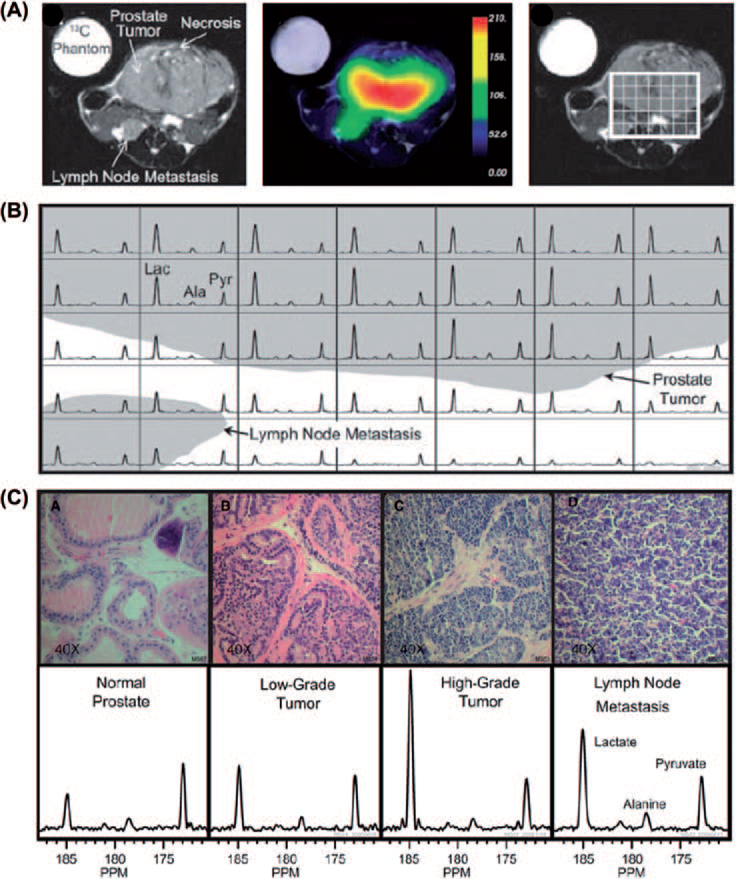
Pyruvate labeled with 13C in the C-1 position is a molecule which satisfies all the conditions listed above and also is a key intermediate involved in major energy generating pathways in cells. Pyruvate is utilized to generate ATP through oxidative phosphorylation generating CO2 as a byproduct, which is in equilibrium with HCO3 in a pH dependent manner with its unique chemical shifts. It is also converted to lactate through aerobic glycolysis or alanine through transamination. Albers et al. have used a transgenic mouse model of prostate cancer to determine the capability of the method to grade malignancy and correlate with histology [22]. Pyruvate is utilized intracellularly producing the above mentioned metabolites, each having its distinct spectrum characterized by the chemical shift. In this study they show spectra from hyperpolarized [1-13C]pyruvate injected in a mouse bearing a high grade tumor and the time course of the various products. These experiments show that the lactate signal reaches a maximum about 20 seconds after the pyruvate bolus arrival. The time course suggests that a time window between 30 and 60 seconds from start of injection is suitable for MRSI when the lactate levels were relatively constant. This study shows the feasibility of performing in vivo metabolic MRI with hyperpolarized 13C labeled agents and that regions can be distinguished based on their metabolic profile and identifies a time window for imaging. They further implemented the imaging experiments in transgenic adenocarcinoma of mouse prostate (TRAMP) mice bearing a large primary tumor and a lymph node metastasis. Figure 3A shows anatomic image indicating the primary tumor and the lymph node metastasis and the corresponding image of pyruvate distribution. A grid was placed on the tumor region from which the spectra in each voxel are displayed in Figure 3B. The spectra show high levels of lactate in the tumor and lymph node metastasis. Further studies in this report examined the relationship of the glycolytic activity monitored by MRI and histological exam of malignancy. Results from this study further support the relationship between the MRI data and histological experiments (Figure 3C). The normal prostate region displayed the lowest lactate/pyruvate ratio whereas this ratio increased in histologically determined tumor grade. From this study, the correlation of the image data (lactate/pyruvate ratio) and the histologic assessment of the tumor grade validate this as an imaging biomarker for tumor grade.
Figure 3.
Hyperpolarized 13C metabolic images of a TRAMP mouse. Upper: Hyperpolarized 13C lactate image following the injection of hyperpolarized [1-13C]pyruvate, overlaid on T2-weighted 1H image. Middle: Hyperpolarized 13C spectra of primary and metastatic tumor regions. Lower: Representative H&E-stained sections and hyperpolarized 13C spectra for one case from each of the histologically defined groups. The 3D MRSI shows substantially elevated lactate in the high-grade primary tumor compared with the low-grade tumor. Ala, alanine; Lac, lactate; Pyr, pyruvate (see [22]).
There is a significant interest in developing imaging biomarkers which not only distinguish malignancies but also provide responses to treatment early in the course of treatment. Most commonly used imaging assessment of treatment response in tumors is by monitoring tumor volume changes by anatomical imaging techniques. While these changes take several weeks, adding a physiologic/biochemical dimension to anatomic images had been shown to be useful using MRS, DWI-MRI, DCE-MRI or FDG-PET. Metabolic MRI with DNP has similar capabilities with additional advantages of rapid imaging, high SNR and no background. Monitoring the metabolic conversion of hyperpolarized pyruvate to lactate and using lactate/pyruvate as a biomarker can be a useful imaging biomarker for treatment response especially with support from the study of Albers et al. where a strong correlation between the malignancy and the lactate/pyruvate ratio was found. The study of Day et al. from Brindle’s group in Cambridge tested this hypothesis of lactate/pyruvate as a biomarker for treatment response using a lymphoma model in mice treated with etoposide [23]. They studied mice implanted with EL-4 tumors studied with metabolic MRI using hyperpolarized [1-13C]pyruvate before and after treatment with etoposide and the lactate/pyruvate monitored with MRSI. The images showed a significant conversion of the injected pyruvate to lactate prior to treatment, and a diminished conversion 24 hours after treatment with etoposide. Day et al. quantified the pyruvate-to-lactate exchange rate before and after treatment and found a 39% decrease as a result of treatment. The images and the quantified data show that lactate/pyruvate ratio can serve as a useful biomarker for early treatment response in experimental animals. Hyperpolarized fumarate has also been used by Gallagher et al. as an agent to report on tumor cell death by monitoring its conversion to malate by exposure to fumarate hydratase entering into the extracellular space from necrotic cells. This study from Brindle’s group showed that 24 hours after treatment, high levels of malate indicate necrotic cells supporting this agent as a biomarker for in vivo necrotic response [24]. Thus, hyperpolarized metabolic MR can utilize multiple probes which can be polarized to follow specific metabolic processes in key biochemical pathways to obtain imaging biomarkers for treatment response.
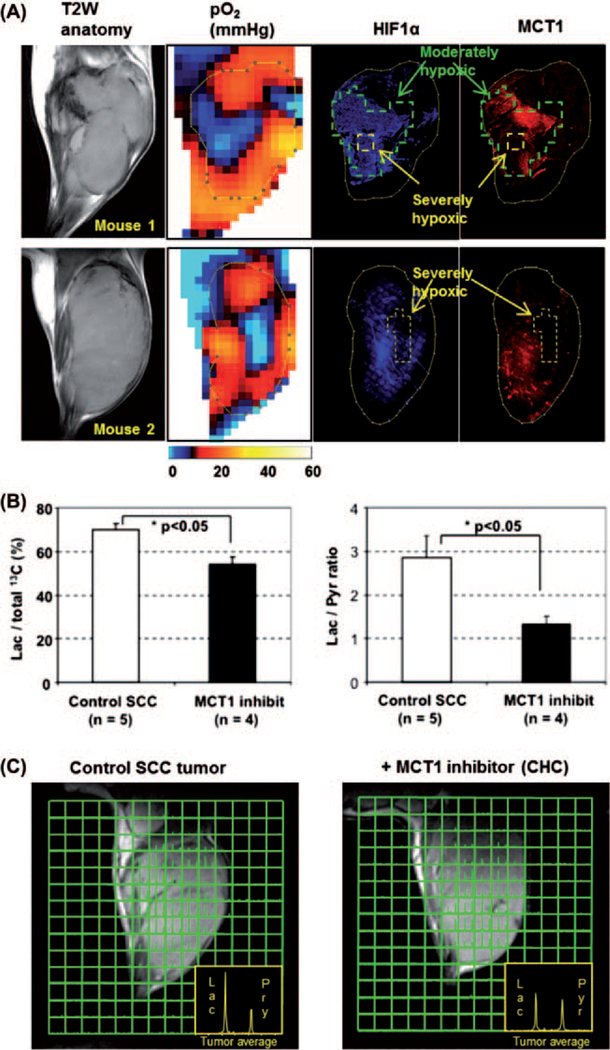
Our group has shown that the flux of pyruvate to lactate can be used to monitor import of carboxylates such as pyruvate through monocarboxylate transporter family. The flux of pyruvate to lactate was monitored by MRSI in a control group of mice and a group of mice treated with the monocarboxylate transporter-1 inhibitor a-cyano-4-hydroxy cinnamate. The results show that a greater than 60% inhibition of lactate to pyruvate ratio was observed in mice treated with the MCT-1 inhibitor (Figure 4C) [25]. In the same study we showed that from the pO2 image-guided histological experiments, a biphasic oxygen dependency of MCT1 and HIF-1a expression was observed (Figure 4A). In the tumor regions with moderate hypoxia (pO2 5–15 mmHg, marked by green line) surrounding severely hypoxic core (yellow line), increased expression of MCT1 was observed as well as overexpression of HIF-1a in the moderately hypoxic regions. However, the expression level of MCT1 was clearly down regulated in the severely hypoxic tumor regions, especially where pO2 was found to be below 2–3 mmHg. On the other hand, HIF-1 expression was high even in severely hypoxic tumor regions when the severely hypoxic area is relatively small (mouse 1). Suppressed expression of HIF-1 was observed only if the size of severely hypoxic regions were beyond 2–3 mm (mouse 2) that is similar to the biphasic expression pattern of HIF-1 near necrotic tumor regions reported in both preclinical models and human tumors.
Figure 4.
Monocarboxylate transporter 1 (MCT1) dependent uptake of [1-13C]pyruvate. (A) Comparison of tumor pO2 and MCT1 expression in vivo. After EPR oxygen imaging and following anatomic MRI scans, tumor tissue slice corresponding to the particular slice of pO2 map was exsected and immunostaining of HIF-1a (blue) and MCT1 (red) was conducted(B) Pretreatment with CHC significantly suppressed lactate/total 13C and lactate/pyruvate ratios calculated from whole tumor regions in 13C MRI images. (C) Metabolic 13C MRI of hyperpolarized 13C-labeled pyruvate in SCC tumors with or without pretreatment of MCT1 inhibitor CHC obtained 30 s after [1-13C] pyruvate injection (see [25]).
The first clinical trial of hyperpolarized [1-13C] pyruvate metabolic imaging of prostate cancer patients was conducted at the University of California in San Francisco. This study was a proof-of-concept study entitled: A phase 1/2a ascending-dose study to assess the safety and tolerability and imaging potential of hyperpolarized pyruvate (13C) injection in subjects with prostate cancer. The secondary objective of this study was to determine the kinetics of hyperpolarized pyruvate delivery and metabolism throughout the prostate, and to determine the signal to noise ratio for pyruvate metabolites in regions of cancer and in surrounding benign prostate as a function of the dose of the hyperpolarized [1-13C]pyruvate. All doses were well tolerated without exception, and excellent contrast-to-noise for [1-13C]lactate was observed even at the lowest dose.
Conclusion
Emerging pre-clinical and clinical imaging modalities are adding important physiological and metabolic dimensions to anatomic images. Such capabilities will provide valuable biological characterization of tumors in terms of non-invasive imaging biomarkers to design appropriate treatments based on tumor microenvironment. These imaging biomarkers will also be useful to monitor treatment response. Imaging pO2 in tumors is now a robust pre-clinical imaging modality with potential for implementation clinically. Pre-clinical studies and the initial clinical study with hyperpolarized metabolic MR have been successful and may be part of image-guided radiotherapy to select patients for tailored individual treatment regimens.
Footnotes
Declaration of interest: Jan Henrik Ardenkjaer-Larsen is an employee of GE Healthcare.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- [1].Brown JM. The hypoxic cell: A target for selective cancer therapy – eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res 1999;59:5863–70. [PubMed] [Google Scholar]
- [2].Chaplin DJ, Olive PL, Durand RE. Intermittent blood flow in a murine tumor: Radiobiological effects. Cancer Res 1987;47:597–601. [PubMed] [Google Scholar]
- [3].Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1955;9:539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006;6:583–92. [DOI] [PubMed] [Google Scholar]
- [5].Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012;9:674–87. [DOI] [PubMed] [Google Scholar]
- [6].Overgaard J Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol 2011;100:22–32. [DOI] [PubMed] [Google Scholar]
- [7].Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiother Oncol 1998; 46:135–46. [DOI] [PubMed] [Google Scholar]
- [8].Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol 1996;6:10–21. [DOI] [PubMed] [Google Scholar]
- [9].Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: Why do cancers have high glycolysis? J Bioenerg Biomembr 2007;39:251–7. [DOI] [PubMed] [Google Scholar]
- [10].Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 2006;82:699–757. [DOI] [PubMed] [Google Scholar]
- [11].Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004;4:437–47. [DOI] [PubMed] [Google Scholar]
- [12].Grau C, Muren LP, Hoyer M, Lindegaard J, Overgaard J. Image-guided adaptive radiotherapy – integration of biology and technology to improve clinical outcome. Acta Oncol 2008;47:1182–5. [DOI] [PubMed] [Google Scholar]
- [13].Grau C, Olsen DR, Overgaard J, Hoyer M, Lindegaard JC, Muren LP. Biology-guided adaptive radiation therapy – presence or future? Acta Oncol 2010;49:884–7. [DOI] [PubMed] [Google Scholar]
- [14].Lewis JS, Welch MJ. PET imaging of hypoxia. Q J Nucl Med 2001;45:183–8. [PubMed] [Google Scholar]
- [15].Vaupel P, Mayer A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev 2007; 26:225–39. [DOI] [PubMed] [Google Scholar]
- [16].Ardenkjaer-Larsen JH, Laursen I, Leunbach I, Ehnholm G, Wistrand LG, Petersson JS, et al. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J Magn Reson 1998;133:1–12. [DOI] [PubMed] [Google Scholar]
- [17].Matsumoto S, Hyodo F, Subramanian S, Devasahayam N, Munasinghe J, Hyodo E, et al. Low-field paramagnetic resonance imaging of tumor oxygenation and glycolytic activity in mice. J Clin Invest 2008;118:1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krishna MC, English S, Yamada K, Yoo J, Murugesan R, Devasahayam N, et al. Overhauser enhanced magnetic resonance imaging for tumor oximetry: Coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci U S A 2002;99:2216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Matsumoto S, Yasui H, Mitchell JB, Krishna MC. Imaging cycling tumor hypoxia. Cancer Res 2010;70:10019–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A 2003;100:10158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I. Molecular imaging with endogenous substances. Proc Natl Acad Sci U S A 2003;100:10435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Albers MJ, Bok R, Chen AP, Cunningham CH, Zierhut ML, Zhang VY, et al. Hyperpolarized 13C lactate, pyruvate, and alanine: Noninvasive biomarkers for prostate cancer detection and grading. Cancer Res 2008;68:8607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med 2007;13:1382–7. [DOI] [PubMed] [Google Scholar]
- [24].Gallagher FA, Kettunen MI, Hu DE, Jensen PR, Zandt RI, Karlsson M, et al. Production of hyperpolarized [1,4–13C2] malate from [1,4–13C2] fumarate is a marker of cell necrosis and treatment response in tumors. Proc Natl Acad Sci USA 2009;106:19801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matsumoto S, Saito K, Yasui H, Morris HD, Munasinghe JP, Lizak M, et al. EPR oxygen imaging and hyperpolarized (13)C MRI of pyruvate metabolism as noninvasive biomarkers of tumor treatment response to a glycolysis inhibitor 3-bromopyruvate. Magn Reson Med 2013;69:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]