Abstract
Background
Doxorubicin (DOXO) chemotherapy increases risk for cardiovascular disease in part by inducing endothelial dysfunction in conduit arteries. However, the mechanisms mediating DOXO-associated endothelial dysfunction in (intact) arteries and treatment strategies are not established.
Objectives
We tested the hypothesis that DOXO impairs endothelial function in conduit arteries via excessive mitochondrial reactive oxygen species (ROS) and that these effects could be prevented by treatment with a mitochondrial-targeted antioxidant (MitoQ).
Methods
Endothelial function (endothelium-dependent dilation [EDD] to acetylcholine) and vascular mitochondrial ROS were assessed 4 weeks following administration (10 mg/kg intraperitoneal injection) of DOXO. A separate cohort of mice received chronic (4 weeks) oral supplementation with MitoQ (drinking water) for 4 weeks following DOXO.
Results
EDD in isolated pressurized carotid arteries was 55% lower 4 weeks following DOXO (peak EDD, DOXO: 42 ± 7% vs. sham: 94 ± 3%; p = 0.006). Vascular mitochondrial ROS was 52% higher and manganese (mitochondrial) superoxide dismutase was 70% lower after DOXO versus sham (p = 0.0008). Endothelial function was rescued by administration of the mitochondrial-targeted antioxidant, MitoQ, to the perfusate. Exposure to plasma from DOXO-treated mice increased mitochondrial ROS in cultured endothelial cells. Analyses of plasma showed differences in oxidative stress-related metabolites and a marked reduction in vascular endothelial growth factor A in DOXO mice, and restoring vascular endothelial growth factor A to sham levels normalized mitochondrial ROS in endothelial cells incubated with plasma from DOXO mice. Oral MitoQ supplementation following DOXO prevented the reduction in EDD (97 ± 1%; p = 0.002 vs. DOXO alone) by ameliorating mitochondrial ROS suppression of EDD.
Conclusions
DOXO-induced endothelial dysfunction in conduit arteries is mediated by excessive mitochondrial ROS and ameliorated by mitochondrial-specific antioxidant treatment. Mitochondrial ROS is a viable therapeutic target for mitigating arterial dysfunction with DOXO.
Key Words: chemotherapy, mitochondrial antioxidant, reactive oxygen species
Abbreviations and Acronyms: ACh, acetylcholine; DOXO, doxorubicin; EDD, endothelium-dependent dilation; L-NAME, N(gamma)-nitro-L-arginine; MitoQ, mitoquinol mesylate, or [10-(2,5-dihydroxy-3,4-dimethoxy-6-methylphenyl) decyl] triphenyl-phosphonium, monomethanesulfonate; NO, nitric oxide; SNP, sodium nitroprusside; SOD, superoxide dismutase; VEGF, vascular endothelial growth factor
Central Illustration
Approximately 650,000 people undergo chemotherapy treatment annually (1). In many cases, these treatments are effective in treating the cancer, but severely damage the cardiovascular system of the surviving patients (2). As a result, cardiovascular disease is a leading cause of later morbidity and mortality among chemotherapy-treated cancer survivors (3).
Anthracyclines are first-line chemotherapy agents for several common cancers, including leukemias, breast cancer, and lymphomas, that exert particularly toxic effects on the cardiovascular system (4). Doxorubicin (DOXO) is the most commonly used anthracycline (5), and its use markedly increases the risk of subsequent cardiovascular disease (6). As such, elucidating the mechanisms underlying the adverse cardiovascular effects of DOXO is an ongoing research priority, as it may help identify therapeutic approaches.
To date, the majority of research on DOXO has focused on its effects on the heart and, specifically, cardiomyocytes. Mechanistic studies in preclinical models implicate excessive bioactivity of reactive oxygen species (ROS) such as superoxide and consequent oxidative stress in DOXO-induced damage to cardiomyocytes (7,8). The excessive ROS bioactivity in cardiomyocytes after DOXO treatment appears to be mediated by some combination of increased ROS production and suppression of endogenous antioxidant enzymes (8,9). Several sources of excessive ROS production have been identified, including the mitochondria (10). Indeed, DOXO directly induces mitochondrial dysfunction and stimulates superoxide production in cardiomyocytes (11,12).
Recent work suggests an important role of the vascular endothelium in DOXO-associated cardiovascular effects (13). DOXO is administered systemically, and vascular endothelial cells are exposed to circulating DOXO before the compound is taken up by cardiomyocytes and other tissues. In vitro studies performed in endothelial cell culture indicate that DOXO induces mitochondrial dysfunction, down-regulates antioxidant enzymes, and increases ROS bioactivity (14,15). Consistent with these observations, endothelial function of intact arteries assessed by endothelium-dependent dilation (EDD) is impaired in DOXO-treated animal models (16,17) and patients compared with untreated control subjects (18). However, the mechanisms by which DOXO induces this endothelial dysfunction in vivo have not been established, nor is there evidence that therapeutic strategies based on these mechanisms can preserve endothelial function after DOXO.
Here, we systematically investigated the underlying mechanisms by which DOXO impairs endothelial function in intact (conduit) arteries and show that a mitochondrial-specific antioxidant can prevent these effects.
Methods
Detailed descriptions of all procedures and statistical analyses are provided in the Supplemental Material. Studies were approved by the University of Colorado Boulder Institutional Care and Use Committee (Protocol# 2618) and conformed to the Guide for the Use of Laboratory Animals.
Male C57BL6/J mice (4 months of age) were randomly assigned to receive DOXO (10 mg/kg intraperitoneal injection; n = 4) or sham (intraperitoneal injection of saline; n = 4). This method of administration causes cardiovascular dysfunction in 4-month-old C57BL6/J mice (19). An additional cohort of mice received DOXO (n = 4) or DOXO + the mitochondrial-targeted antioxidant mitoquinol mesylate (10-[2,5-dihydroxy-3,4-dimethoxy-6-methylphenyl] decyl) (MitoQ) (250 μmol/l in drinking water; n = 5) for 4 weeks (20), initiated on the same day immediately following DOXO. Data are presented separately for each cohort for vascular endothelial function, but DOXO samples were combined for biochemical experiments. Estrogen may be protective against DOXO-induced cardiac dysfunction (21); thus, only male mice were used in the present study to determine the mechanism by which DOXO causes vascular endothelial dysfunction without the confounding effects of female sex hormones.
All mice were euthanized 4 weeks following treatment via cardiac puncture (under inhaled isoflurane anesthesia). Blood was spun and plasma was isolated and saved for cell culture experiments (22) and for assessment of circulating factors, as described in the Supplemental Appendix. Following sacrifice, carotid arteries were excised and cannulated on glass pipettes for assessment of endothelium-dependent and -independent dilation, as described in the Supplemental Appendix. Then, the thoracic aorta was dissected and cleaned, and 1-mm rings were used for assessment of total (22) and mitochondrial-specific ROS (20) or were flash frozen, as described in the Supplemental Appendix. All data are reported as mean ± SEM.
Results
Animal characteristics
Young (4-month-old) male C57BL6/J mice were administered with a single intraperitoneal injection of sterile 1× phosphate-buffered saline (Sham) or DOXO (10 mg/kg in sterile 1× phosphate-buffered saline) and sacrificed 4 weeks later. As observed in previous studies (16,23), body mass at the time of sacrifice was lower (−12%) in the group administered with DOXO (Table 1). Lower body mass with DOXO was associated with lower heart, quadriceps, gastrocnemius, and epididymal white adipose tissue mass, food consumption (−17%), and water intake (−26%) (Table 1). There were no significant differences in the mass of other tissues and organs (Supplemental Table 1).
Table 1.
Body Mass, Tissue Mass, Energy Intake, and Water Intake
| Sham (n = 4) | DOXO (n = 4) | p Value | |
|---|---|---|---|
| Body mass, g | 28.5 ± 0.9 | 25.2 ± 1.0 | 0.017 |
| Heart, mg | 151.5 ± 15.1 | 123.5 ± 4.5 | 0.042 |
| Quadriceps, mg | 389.8 ± 18.8 | 290.0 ± 14.8 | 0.003 |
| Gastrocnemius, mg | 309.0 ± 4.3 | 233.8 ± 20.4 | 0.029 |
| Epididymal white adipose tissue, mg | 468.5 ± 19.9 | 187.1 ± 25.7 | <0.001 |
| Food intake, kcals/day | 12.6 ± 0.4 | 10.5 ± 0.3 | 0.024 |
| Water intake, ml/day | 3.8 ± 0.1 | 2.8 ± 0.1 | 0.003 |
Values are mean ± SEM.
DOXO = doxorubicin.
Effect of DOXO on vascular endothelial function
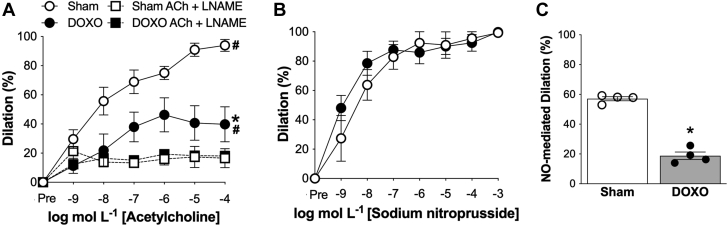
Carotid artery resting (DOXO: 419 ± 10 μm vs. sham: 412 ± 11 μm; p = 0.702) and maximal (DOXO: 449 ± 12 μm vs. sham: 457 ± 11 μm; p = 0.653) diameters did not differ between groups. Carotid artery EDD, measured by the increase in luminal diameter in response to increasing concentrations of acetylcholine, was 57% lower in DOXO versus sham control mice (peak EDD: DOXO, 40 ± 7% vs. sham, 94 ± 3%; p = 0.006), indicating a marked impairment in vascular endothelial function with DOXO treatment (Figure 1A). To determine how DOXO impairs EDD, we first measured endothelium-independent dilation as the dilatory response to sodium nitroprusside, a nitric oxide (NO) donor. We found no difference between groups (peak: DOXO versus sham: p = 0.724) (Figure 1B), indicating that DOXO does not impair EDD by reducing vascular smooth muscle relaxation to NO. Next, we assessed the effects of DOXO on NO-mediated EDD. Administration of the NO synthase inhibitor, N(gamma)-nitro-L-arginine (L-NAME), reduced EDD in both groups, but the decrease was greater in the sham mice, abolishing the group differences in EDD (p = 0.546) (Figure 1A). As a result, NO-mediated dilation (peak response to acetylcholine [ACh] alone [−] peak response to ACh + L-NAME) was 67% lower in the DOXO compared with sham mice (p < 0.001) (Figure 1C). Together, these observations indicate that DOXO impairs NO-mediated EDD rather than decreasing vascular smooth muscle sensitivity to NO.
Figure 1.
Doxorubicin-Induced Endothelial Dysfunction in Conduit Arteries Is Mediated by Reduced Nitric Oxide Signaling
(A) Dose-response endothelium-dependent dilation (EDD) to acetylcholine (ACh) in isolated carotid arteries, with and without the nitric oxide (NO)-synthase inhibitor, L-NAME. (B) Dose-response endothelium independent dilation to the NO donor sodium nitroprusside (SNP). (C) Peak NO-mediated dilation to ACh, assessed as the difference between peak EDD in the absence versus presence of the NO synthase inhibitor N(ω)-nitro-L-arginine methyl ester (L-NAME). n = 4 (sham); n = 4 (doxorubicin [DOXO]). Data are the mean ± SEM. ∗p < 0.05 vs. sham; #p < 0.05 within group response for ACh vs. ACh + L-NAME.
Excess total ROS-associated suppression of endothelial function by DOXO
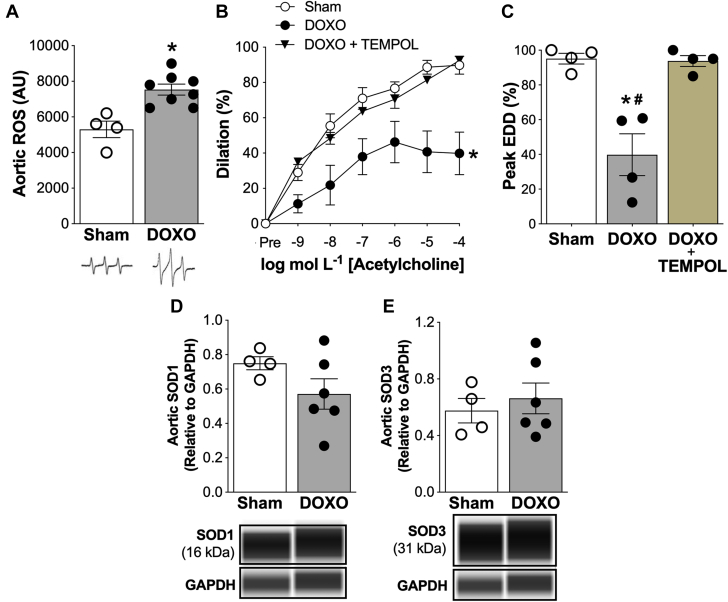
Excess bioactivity of ROS is a key mechanism underlying reductions in NO bioavailability in the endothelium in several physiological (e.g., aging) and pathophysiological states (24). To determine the effect of DOXO on vascular ROS bioactivity, we first assessed ROS in the aorta using electronic paramagnetic resonance spectroscopy and found it was 40% greater (p = 0.002) in the DOXO- than Sham-treated mice (Figure 2A). Next, to determine if this excess ROS had a functional role in the impaired EDD of DOXO-treated mice, we assessed EDD in isolated carotid arteries with and without prior incubation with the superoxide dismutase (SOD) mimetic/ROS scavenger, TEMPOL. We found that TEMPOL administration completely restored peak EDD in the DOXO mice to sham levels (Figures 2B and 2C), indicating that impaired EDD with DOXO is likely due to excessive vascular ROS bioactivity. Moreover, we found no differences in the abundance of the cytosolic (SOD1) and extracellular (SOD3) isoforms of SOD, a major endogenous antioxidant enzyme, in the aorta of the DOXO and sham animals (SOD1: p = 0.157; SOD3: p = 0.854) (Figures 2D and 2E). These observations indicate that DOXO-associated EDD impairment is likely due to excessive bioactivity of ROS without stimulating expected compensatory increases in SOD1 or SOD3.
Figure 2.
Impaired Conduit Artery Endothelial Function With Doxorubicin Is Mediated by Excessive Reactive Oxygen Species
(A) Whole-cell reactive oxygen species (ROS) in aortic segments. Representative electron paramagnetic resonance spectra are shown above. (B) Dose-response and (C) peak endothelium-dependent dilation (EDD) in isolated carotid arteries in response to acetylcholine in the presence or absence of the superoxide dismutase (SOD) mimetic, TEMPOL (1.0 μm, 60 min incubation to scavenge ROS). Aortic protein abundance of (D) SOD1 and (E) SOD3; representative images from the WES capillary electrophoresis automated Western blot system are shown above. Protein abundance of target proteins are normalized to protein abundance of GAPDH. Data are the mean ± SEM. n = 4 (sham); n = 4 (doxorubicin [DOXO]); n = 4 (DOXO + TEMPOL). ∗p < 0.05 vs. sham; #p < 0.05 doxorubicin (DOXO) + TEMPOL vs. DOXO.
Role of mitochondrial-specific ROS in DOXO-induced endothelial dysfunction
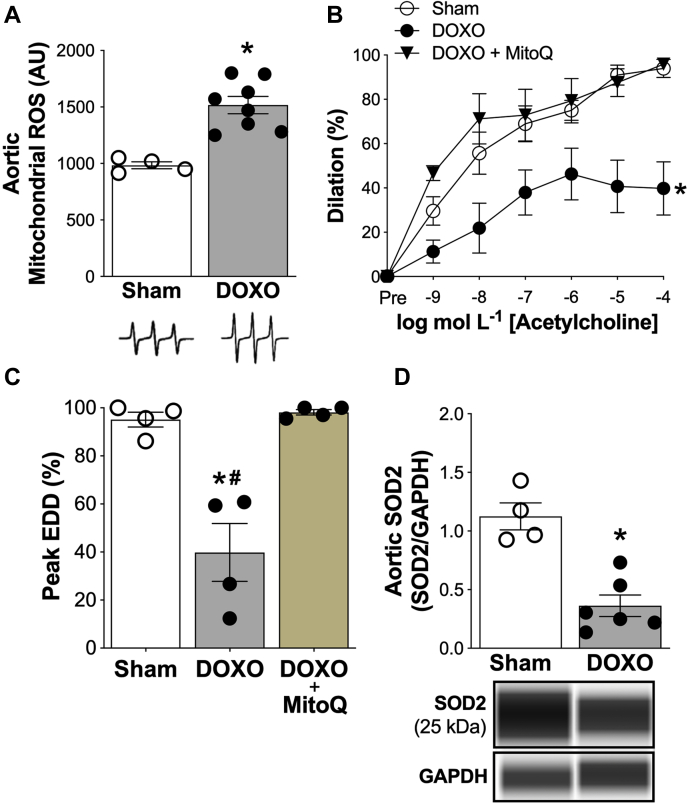
DOXO is reported to induce excess bioactivity of mitochondrial ROS in endothelial cells in vitro (25), but the effects of DOXO on mitochondrial ROS in intact arteries has not been investigated. Accordingly, we next assessed mitochondrial ROS bioactivity in the aorta. We found that aortic mitochondrial ROS was 52% greater (p < 0.001) in DOXO-treated mice compared with sham (Figure 3A). To determine if this excess mitochondrial ROS plays a role in DOXO-induced endothelial dysfunction, we incubated carotid arteries from DOXO-treated mice with the mitochondrial-targeted antioxidant, MitoQ, prior to assessing EDD. Incubation with MitoQ fully restored peak EDD (%) in DOXO treated mice to levels observed in sham control mice (Figures 3B to 3C). To determine if increased vascular mitochondrial ROS after DOXO treatment developed in conjunction with reduced endogenous mitochondrial antioxidant defenses, we also assessed aortic protein abundance of manganese SOD, the mitochondrial isoform of SOD (SOD2). We found that aortic SOD2 abundance was ∼70% lower in DOXO versus sham (p < 0.001) (Figure 3D). Together, these results indicate that the greater total bioactivity of vascular ROS and tonic ROS-related suppression of EDD in DOXO-compared with sham-treated mice is mediated by excessive bioactivity of mitochondrial ROS in the absence of appropriate compensatory up-regulation mitochondrial SOD antioxidant defenses.
Figure 3.
Excessive Reactive Oxygen Species From Mitochondria Mediate Doxorubicin-Induced Endothelial Dysfunction in Conduit Arteries
(A) Mitochondrial reactive oxygen species (ROS) in aortic segments. Representative electron paramagnetic resonance spectra are shown above. (B) Dose-response and (C) peak endothelium-dependent dilation (EDD) to acetylcholine in the presence of the mitochondrial-targeted antioxidant, MitoQ (1.0 μmol/l, 60 min incubation to scavenge mitochondrial-specific reactive oxygen species). (D) Aortic protein abundance of superoxide dismutase-2 (SOD2); representative images from the WES capillary electrophoresis automated Western blot system are shown above. Protein abundance of target proteins are normalized to protein abundance of GAPDH. Data are the mean ± SEM. n = 4 (sham); n = 4 (doxorubicin [DOXO]); n = 5 (DOXO + MitoQ). ∗p < 0.05 vs. sham; #p < 0.05 doxorubicin (DOXO) + MitoQ vs. DOXO.
Stimulation of total and mitochondrial ROS bioactivity by DOXO treatment is induced by systemic circulating factors
We have recently shown that in vivo pharmacological treatments can influence ROS bioactivity in cultured endothelial cells by inducing changes in the circulating milieu that persist after the treatment compound has been cleared from the circulation (22). We hypothesized that this mechanism might be involved in excessive ROS stimulation after DOXO treatment, as DOXO and its primary metabolite, doxorubicinol, are cleared from plasma within 24 to 96 h of administration (26). To test this hypothesis, we used an ex vivo model in which human umbilical vein endothelial cells (HUVECs) are treated for 24 h with ROS with plasma obtained from DOXO and sham-treated mice (upon sacrifice) after which ROS was assessed (22).
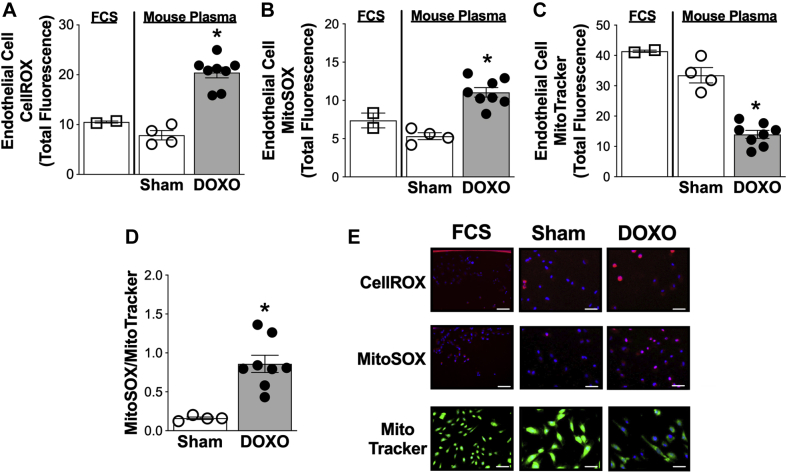
We first sought to establish that total ROS bioactivity was increased in HUVECs exposed to plasma from DOXO-treated mice compared with sham control mice. To do so, we assessed total cellular ROS using the CellROX fluorescent probe (Thermo Fisher [Waltham, Massachusetts]; catalog# C10422) and found that ROS bioactivity was greater in HUVECs incubated with plasma from DOXO-treated mice compared with sham control mice (p < 0.001) (Figures 4A and 4E).
Figure 4.
Plasma From Doxorubicin-Treated Mice Increases Total and Mitochondrial Specific Reactive Oxygen Species in Vascular Endothelial Cells
Quantification of (A) whole cell reactive oxygen species (ROS) (CellROX) and (B) mitochondrial ROS (MitoSOX) in human umbilical vein endothelial cells (HUVECs) following a 24-h incubation with plasma from sham and doxorubicin (DOXO)-treated mice or with fetal cow serum (FCS) (control condition). (C) HUVEC mitochondrial number (MitoTracker Green) following a 24-h incubation with plasma from sham- and DOXO-treated mice. (D) Mitochondrial ROS expressed relative to mitochondrial number in HUVECs following 24-h incubation with plasma from sham- and DOXO-treated mice. (E) Representative images of CellROX, MitoSOX, and MitoTracker in HUVECs following 24-h incubation with plasma from sham- and DOXO-treated mice. Data are the mean ± SEM. ∗p < 0.05 vs. sham.
Next, we aimed to determine if enhanced mitochondrial ROS contributed to the greater total HUVEC ROS induced by DOXO administration by assessing mitochondrial ROS bioactivity using the MitoSOX fluorescent probe (Thermo Fisher, Catalog# M36008). We observed greater mitochondrial ROS in HUVECs exposed to plasma obtained from DOXO compared with sham-treated animals (p < 0.001) (Figures 4B and 4E). To determine if this greater ROS bioactivity was linked to changes in mitochondrial volume, we incubated HUVECs with the fluorescent probe, MitoTracker Green (Thermo Fisher, Catalog# M7514). We found that mitochondrial volume in HUVECs was 40% lower (p < 0.001) following incubation with plasma from DOXO-treated mice compared with sham control mice (Figures 4C and 4E). By accounting for mitochondrial volume, we established that DOXO-induced stimulation of mitochondrial ROS is a result of greater mitochondrial ROS per volume of mitochondria, and not due to an increase in mitochondrial number (DOXO- vs. sham-treated mice; p = 0.002) (Figure 4D). Collectively, these findings demonstrate that plasma from DOXO-treated mice increases total ROS bioactivity in endothelial cells ex vivo, and that greater mitochondrial ROS contributes to this effect, independent of DOXO-induced reductions in endothelial cell mitochondrial volume.
DOXO-induced changes in systemic factors related to mitochondrial ROS bioactivity
Metabolomics
We next sought to identify changes in circulating molecular factors and pathways that may have contributed to the higher mitochondrial ROS bioactivity associated with DOXO treatment. To do so, we first performed a targeted metabolomics analysis on plasma from DOXO- and sham-treated mice, focusing on central carbon and nitrogen metabolites, which are key components of redox homeostasis (27). Partial least squares discriminant analysis showed a discrimination of the plasma metabolite profiles of the 2 groups (Supplemental Figure 1), indicating clear differences in the DOXO- and sham-treated mice. We found that selective metabolites associated with mitochondrial ROS differed in plasma from the DOXO versus sham animals, including: diphosphate (p = 0.048), which typically is reduced during states of mitochondrial oxidative stress (28); lactate (p = 0.031), a byproduct of glycolysis that in some physiological states may be reduced to preserve redox homeostasis in mitochondria (28); guanine (p = 0.046), a nucleotide base that could be reduced by excess mitochondrial ROS (29); ribose phosphate (p = 0.044), a product of the pentose phosphate pathway that is commonly lower during periods of oxidative stress and is associated with mitochondrial dysfunction (30); and 5-hydroxyindoleacetate (p = 0.033), a primary metabolite of serotonin that is implicated in oxidative stress and mitochondrial toxicity (31) (Supplemental Figure 2). To determine associations of these plasma metabolites with mitochondrial-specific aortic ROS, we performed linear regression analyses. We found that plasma diphosphate (p = 0.053) and lactate (p = 0.067) tended to be positively associated with aortic mitochondrial ROS (Supplemental Figure 3). A full report of metabolite abundance is provided in Supplemental Table 2.
Dot blot array
To expand on this initial analysis of potential circulating signals influenced by DOXO treatment, we next targeted inflammatory pathways, given that DOXO administration is associated with higher levels of pro-inflammatory proteins in the circulation (32). To address this aim, we assessed plasma concentrations of 20 different cytokines and chemokines via a dot-blot array. We found that all but 2 of these markers did not differ significantly in plasma from DOXO-treated mice compared with sham control mice (Table 2). The exceptions were the anti-inflammatory cytokine interleukin-4, which was only slightly (<5%), but significantly (p = 0.043) lower in the DOXO mice, and vascular endothelial growth factor-A (VEGF-A), which stood out as being 40% lower in the DOXO treated group (p < 0.001). Moreover, plasma VEGF-A was inversely associated (p = 0.001) with mitochondrial ROS (Supplemental Figure 4). These findings indicated that VEGF-A, a protein signaling molecule in endothelial cells, may be associated with DOXO-induced modulation of ROS in endothelial cells.
Table 2.
Plasma Cytokine, Chemokine, and VEGF-A Levels (pg/ml) Following Sham (n = 4) and DOXO (n = 8) Administration
| Sham (n = 4) | DOXO (n = 8) | p Value | |
|---|---|---|---|
| IL-1α | 197 ± 1 | 196 ± 2 | 0.862 |
| IL-1β | 347 ± 3 | 347 ± 1 | 0.686 |
| IL-2 | 258 ± 16 | 272 ± 6 | 0.342 |
| IL-3 | 715 ± 32 | 699 ± 16 | 0.211 |
| IL-4 | 870 ± 4 | 844 ± 5 | 0.043 |
| IL-5 | 722 ± 1 | 714 ± 2 | 0.091 |
| IL-6 | 95 ± 1 | 89 ± 7 | 0.097 |
| IL-9 | 185 ± 4 | 194 ± 4 | 0.113 |
| IL-10 | 284 ± 1 | 277 ± 5 | 0.062 |
| IL-12-p70 | 162 ± 1 | 156 ± 6 | 0.216 |
| IL-13 | 155 ± 2 | 155 ± 1 | 0.303 |
| IL-17A | 150 ± 2 | 142 ± 8 | 0.053 |
| CCL5 | 251 ± 2 | 250 ± 1 | 0.607 |
| CXCL1 | 227 ± 1 | 228 ± 1 | 0.764 |
| GM-CSF | 808 ± 13 | 788 ± 8 | 0.225 |
| IFN-γ | 1081 ± 2 | 1080 ± 1 | 0.644 |
| MCP-1 | 204 ± 1 | 201 ± 3 | 0.075 |
| M-CSF | 317 ± 1 | 318 ± 2 | 0.905 |
| TNF-α | 47 ± 5 | 34 ± 2 | 0.055 |
| VEGF-A | 228 ± 3 | 138 ± 2 | <0.001 |
Values are mean ± SEM and are expressed relative to an internal positive control. Values in bold indicate a statistical difference. Plasma was analyzed by immunoblot.
CCL = chemokine (C-C motif) ligand (CCL); CXCL = chemokine (C-X-C motif) ligand (CXCL); GM-CSF = granulocyte-macrophage colony stimulating factor; IFN = interferon; IL = interleukin; MCP = monocyte chemoattractant protein; M-CSF = monocyte-colony stimulating factor; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
Restoring VEGF-A in plasma from DOXO-treated mice reduces mitochondrial ROS bioactivity in endothelial cells
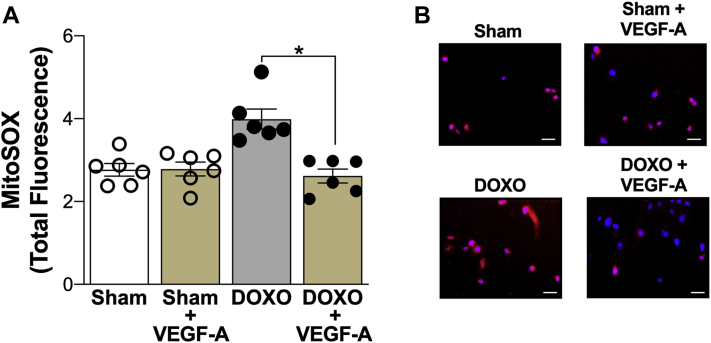
We next sought to determine the possible effects of reduced circulating levels of VEGF-A in regulating DOXO-induced mitochondrial ROS bioactivity in HUVECs. To accomplish this, we supplemented plasma from DOXO-treated mice with 90 pg/ml VEGF-A to normalize VEGF-A to concentrations observed in sham control mice, and then assessed the effect on mitochondrial ROS in HUVECs via MitoSOX fluorescence after a 24-h incubation period. We found that restoring VEGF-A in plasma from DOXO-treated mice normalized mitochondrial ROS in HUVECs compared with sham plasma (Figure 5). To demonstrate that this effect of VEGF-A supplementation of plasma was specific to DOXO-treated mice, we also supplemented sham plasma with the equivalent concentration of VEGF-A and found no change in mitochondrial ROS (sham vs. sham + VEGF-A; p = 0.937). Overall, these observations suggest that reductions in circulating VEGF-A may contribute to greater mitochondrial-derived ROS bioactivity in endothelial cells following DOXO.
Figure 5.
Vascular Endothelial Growth Factor-A Contributes to Doxorubicin-Associated Modulation of Mitochondrial Reactive Oxygen Species in Endothelial Cells
(A) Quantification and (B) representative images of mitochondrial reactive oxygen species (MitoSox fluorescence) in human umbilical vein endothelial cells following 24-h incubation with plasma from Sham-treated mice, Sham-treated plasma + supplementation with vascular endothelial growth factor (VEGF-A) (90 pg/ml), plasma from doxorubicin (DOXO)-treated mice, and DOXO-treated plasma + supplementation with VEGF-A (90 pg/ml). Data are the mean ± SEM. ∗p < 0.05 vs. sham.
In vivo supplementation with MitoQ prevents DOXO-induced vascular endothelial dysfunction by suppressing bioactivity of mitochondrial ROS and preserving NO signaling
Collectively, our findings strongly implicated excess mitochondrial ROS as a key mechanism mediating DOXO-induced endothelial dysfunction. As such, we postulated that targeting excess mitochondrial ROS following DOXO administration may be an effective strategy for preventing endothelial dysfunction with DOXO. To explore this possibility, we next determined if in vivo supplementation of the mitochondria-specific antioxidant MitoQ (250 μmol/l in drinking water for 4 weeks) could prevent DOXO-induced endothelial dysfunction versus administration of DOXO alone. MitoQ supplementation was initiated immediately following DOXO administration, rather than concomitantly, as the National Cancer Institute advises against adjunct antioxidant administration with chemotherapy (33). This guideline is based on concerns that antioxidant therapy may interfere with the effectiveness of these cancer-suppressing drugs and may lead to adverse clinical outcomes (34).
Animal characteristics
In vivo MitoQ supplementation did not influence body weight (DOXO: 26.2 ± 1.0 g vs. DOXO + MitoQ: 26.2 ± 1.4 g; p = 0.927), energy intake (DOXO: 10.7 ± 0.3 kcals/day vs. DOXO + MitoQ: 10.3 ± 0.6 kcals/day; p = 0.794), or water intake (DOXO: 2.6 ± 0.1 ml vs. DOXO + MitoQ: 2.7 ± 0.2 ml; p = 0.945) in DOXO-treated mice. MitoQ supplementation did not influence heart, skeletal muscle, or adipose tissue mass (Supplemental Table 3).
Vascular endothelial function: NO-mediated EDD
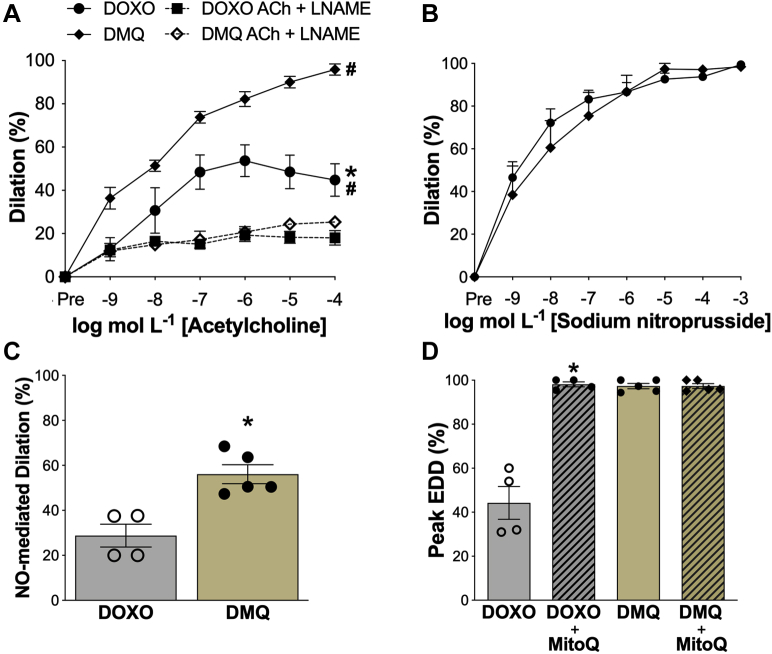
Carotid artery resting (DOXO: 415 ± 9 μm vs. DOXO + MitoQ: 417 ± 32 μm; p = 0.946) and maximal (DOXO: 455 ± 13 μm vs. DOXO + MitoQ: 448 ± 8 μm; p = 0.667) diameters did not differ between the groups (resting, p = 0.90; maximal, p = 0.981). Most importantly, MitoQ supplementation completely prevented DOXO-induced impairments in carotid artery EDD (peak EDD: DOXO + MitoQ vs. DOXO; p = 0.002) (Figure 6A).
Figure 6.
MitoQ Supplementation Prevents Doxorubicin-Induced Endothelial Dysfunction in Conduit Arteries by Preventing Excessive Mitochondrial Reactive Oxygen Species-Associated Suppression of Endothelium-Dependent Dilation and Nitric Oxide Signaling
(A) Dose-response endothelium-dependent dilation (EDD) to acetylcholine (ACh) and ACh in the presence of the nitric oxide (NO)-synthase inhibitor, N(ω)-nitro-L-arginine methyl ester (L-NAME). (B) Dose-response EDD to the NO donor sodium nitroprusside (SNP). (C) Peak NO-mediated dilation to ACh, assessed as the difference between peak EDD in the absence versus presence of the NO synthase inhibitor L-NAME. (D) Peak EDD in the presence of MitoQ (1.0 μmol/l, 60 min incubation to scavenge mitochondrial-specific reactive oxygen species). DMQ: doxorubicin (DOXO) + in vivo MitoQ. Data are the mean ± SEM. n = 4 (doxorubicin [DOXO]); n = 5 (DOXO + MitoQ). ∗p < 0.05, DOXO vs. DOXO + MitoQ; #p < 0.05 vs. LNAME.
Next, we determined if in vivo MitoQ supplementation prevented DOXO-induced impairments in EDD by influencing endothelium-independent dilation (i.e., by altering vascular smooth muscle sensitivity to NO). We found no difference in the dilatory responses to sodium nitroprusside between DOXO and DOXO + MitoQ mice (peak EDD; p = 0.637) or Sham and DOXO + MitoQ mice (peak EDD, p = 0.645), indicating no effects of MitoQ on smooth muscle sensitivity to NO (Figure 6B).
We then sought to determine if MitoQ supplementation prevented DOXO treatment-induced impairments in NO-mediated EDD. Administration of the NO synthase inhibitor L-NAME abolished group differences in peak EDD (DMQ vs. DOXO: p = 0.525; DMQ vs. sham: p = 0.453), indicating that MitoQ supplementation prevented the DOXO-induced impairment in NO-mediated EDD (Figure 6C).
Last, to determine if in vivo MitoQ supplementation prevented DOXO-induced reductions in NO-mediated EDD by inhibiting mitochondrial ROS-related suppression of EDD, we incubated carotid arteries from DOXO + MitoQ mice with MitoQ, prior to assessing EDD. There was no further improvement in EDD following acute MitoQ incubation (Peak EDD: DMQ vs. DMQ + MitoQ; p = 0.997) (Figure 6D). Together, these observations provide experimental evidence that in vivo MitoQ supplementation might be an effective strategy for preventing DOXO treatment-associated stimulation of mitochondrial ROS, vascular endothelial dysfunction, and specifically, the NO-mediated component of EDD.
Discussion
DOXO chemotherapy has been shown to impair endothelial function (i.e., reduce EDD) in conduit arteries of cancer survivors (18). However, the underlying mechanisms and associated therapeutic targets have not been established, because mechanism-focused investigations to date have been largely limited to endothelial cell culture models (35,36). In the present study, we first determined that DOXO treatment impaired endothelial function in conduit arteries as a result of decreased NO-mediated EDD and not due to reduced vascular smooth muscle responsiveness to NO. Next, we identified excessive ROS as a key mechanism underlying DOXO-induced endothelial dysfunction in conduit arteries, and then identified mitochondria as a key source of the excess ROS bioactivity. Subsequently, we established that systemic circulating factors from the DOXO-treated mice stimulated increased endothelial mitochondrial ROS and identified potential molecular transducers that differed in plasma obtained from the DOXO and sham groups. Because lower concentrations of VEGF-A in the DOXO mice appeared to be the strongest signal, we restored VEGF-A to sham control levels and showed that doing so normalized endothelial mitochondrial ROS. Last, to establish excessive mitochondrial ROS as a potential therapeutic target for preventing DOXO treatment-induced endothelial dysfunction in conduit arteries, we supplemented the drinking water of mice with the mitochondrial-targeted antioxidant, MitoQ, for 4 weeks following DOXO administration. Oral MitoQ supplementation prevented the impairment in EDD in DOXO-treated mice by preventing excessive mitochondrial ROS-driven oxidative stress and consequent reductions in NO-mediated EDD. Overall, our findings demonstrate that excessive mitochondrial ROS bioactivity is a key mechanism in DOXO-induced endothelial dysfunction in conduit arteries and provide initial evidence for the potential efficacy of mitochondrial ROS-lowering therapies to preserve vascular health in cancer survivors treated with such drugs.
DOXO accumulates in the mitochondria (37) and damages mitochondrial DNA (38), which can lead to an increased production of ROS from the mitochondria (39). Moreover, and consistent with our findings, in vitro experiments have shown that treatment of cultured cardiomyocytes and endothelial cells with DOXO results in elevated production of mitochondrial ROS (15) and reduced abundance of mitochondrial antioxidant enzymes (11,40). The present results extend these observations by demonstrating that DOXO treatment in vivo stimulates excess mitochondrial ROS in intact conduit arteries while suppressing SOD2 abundance, that is, rather than inducing an appropriate physiological compensatory increase in this important mitochondrial antioxidant. These findings also are in agreement with previous work reporting excess production of mitochondrial ROS and impaired vascular endothelial function in mice with genetic SOD2 insufficiency (41). It should be noted that other ROS generating pathways may also contribute, including oxido-reductases such as endothelial NO synthase or NADPH cytochrome P450 reductase (42).
Another key finding from our experiments was that changes in the composition of the circulating milieu may contribute to DOXO treatment-associated stimulation of excess vascular ROS, consistent with recent observations from our laboratory in the setting of aging (22). The scope of the present investigation precluded an exhaustive interrogation of the identity of the factors involved. However, using a targeted plasma metabolomics analysis, there was a tendency to an overall lower abundance of nucleic acid bases (adenosine, thymidine, cytidine, and guanine), as well as glycolytic and pentose phosphate intermediates, all of which may be associated with states of higher oxidative stress (28,30,43). Particularly, diphosphate and lactate were positively related to vascular mitochondrial ROS. In a second targeted analysis, we assessed the abundance of circulating cytokines, chemokines, and VEGF-A in plasma from DOXO and sham mice, given that DOXO chemotherapy increases circulating abundance of pro-inflammatory cytokines (32) and inflammation can stimulate synthesis of ROS (44). This analysis revealed substantially lower circulating VEGF-A concentrations in the DOXO-treated mice. As a follow-up, we subsequently showed that restoring VEGF-A to sham levels normalized mitochondrial ROS in endothelial cells, demonstrating a role for VEGF-A in regulating endothelial cell mitochondrial ROS with DOXO. These results are in agreement with previous findings in mice in which whole-body overexpression of VEGF-B prevented DOXO-induced impairments in aortic EDD and cardiomyocyte mitochondrial respiration (17).
Chronic oral supplementation with the mitochondrial targeted antioxidant MitoQ reverses age-related impairments in NO-mediated endothelial function by mitigating excessive mitochondrial ROS-related suppression of EDD (20,45). The present study demonstrates that chronic MitoQ supplementation also prevents the increases in mitochondrial ROS and consequent reductions in NO-mediated EDD in conduit arteries induced by DOXO treatment. This is potentially clinically relevant as MitoQ is commercially available and safe for use in humans (46,47), and we have shown that 6 weeks of oral supplementation with this compound improves endothelial function in healthy older adults (45). Patients who have undergone DOXO chemotherapy also have vascular endothelial dysfunction (18), and nonspecific (48) and mitochondrial-targeted (49,50) antioxidant therapies can be effective in settings of DOXO-induced cardiomyopathy. As such, MitoQ may hold promise for anthracycline-treated patients, although the use of antioxidant treatment with chemotherapy should strictly adhere to National Cancer Institute guidelines (33).
Study limitations
Future preclinical studies should consider other experimental approaches to facilitate translation to patient populations, including use of tumor-bearing animals to model human cancer; administering DOXO intravenously in smaller consecutive doses over time; larger sample sizes; and mechanistic analyses to determine if the treatment has direct or off target effects. Additionally, future studies should assess cardiac function in parallel with vascular function.
Endothelial cells produce endothelin (ET)-1, a pro-vasoconstrictor and pro-atherogenic molecule that is elevated in the setting of heart failure (51) and in patients treated with DOXO chemotherapy (52,53). Moreover, ET-1 stimulates mitochondrial ROS production in endothelial cells (54). Thus, strategies aimed at preventing DOXO-induced increases in ET-1 may lower endothelial mitochondrial ROS production and improve cardiovascular function.
Conclusions
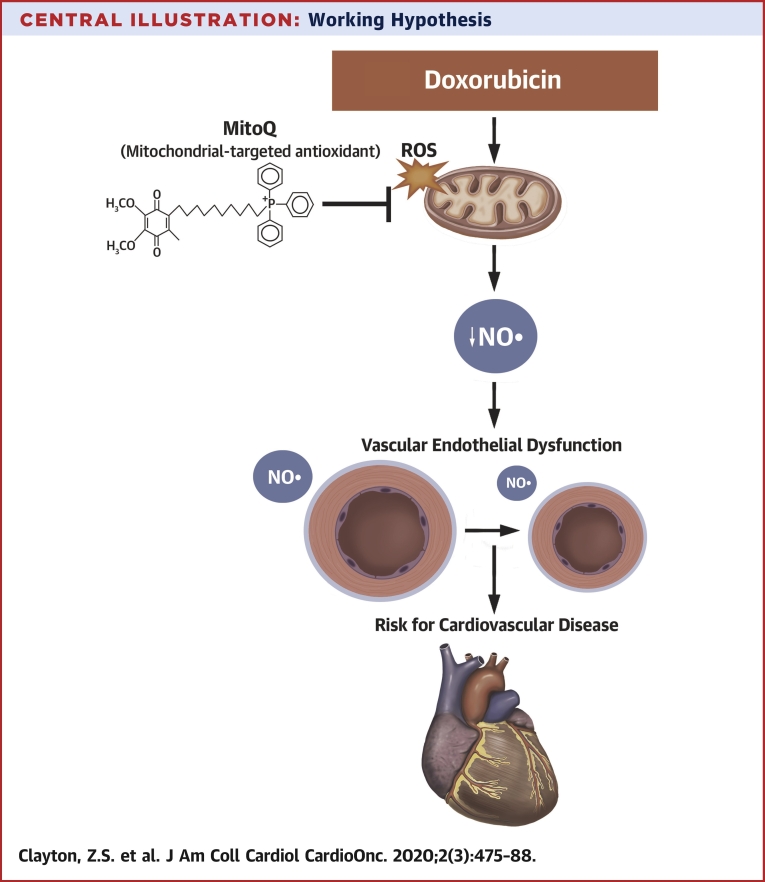
Our results demonstrate that DOXO-induced endothelial dysfunction in intact conduit arteries is mediated by reduced NO signaling secondary to excessive bioactivity of mitochondrial ROS in the absence of an appropriate compensatory upregulation of mitochondrial SOD (Central Illustration). Importantly, we show that chronic oral supplementation with the mitochondrial-targeted antioxidant MitoQ can prevent DOXO-induced endothelial dysfunction in conduit arteries of nontumor-bearing mice by mitigating excessive mitochondrial ROS suppression of EDD. Healthy vascular endothelial function is associated with a marked reduction in future risk of heart failure (55), the major clinical cardiovascular consequence of anthracycline treatment (56). As such, lifestyle and pharmacological strategies that preserve endothelial function hold promise for preventing heart failure and other adverse cardiovascular outcomes associated with anthracycline cancer therapy.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study demonstrates that excessive mitochondrial reactive oxygen species is a key mechanism underlying doxorubicin-associated vascular endothelial dysfunction. Furthermore, this study suggests that oral mitochondrial-targeted antioxidant supplementation is a potential therapeutic strategy for treating excess mitochondrial reactive oxygen species-related suppression of vascular endothelial dysfunction with doxorubicin.
TRANSLATIONAL OUTLOOK: Future studies should determine the long-term efficacy of mitochondrial-targeted antioxidant supplementation in treating doxorubicin-induced vascular endothelial function in tumor-bearing animals, so as to more closely resemble the human condition. Supplementation should occur at a time following chemotherapy that is aligned with the guidelines put forth by the National Cancer Institute. A detailed assessment of cardiac function is also necessary prior to human studies.
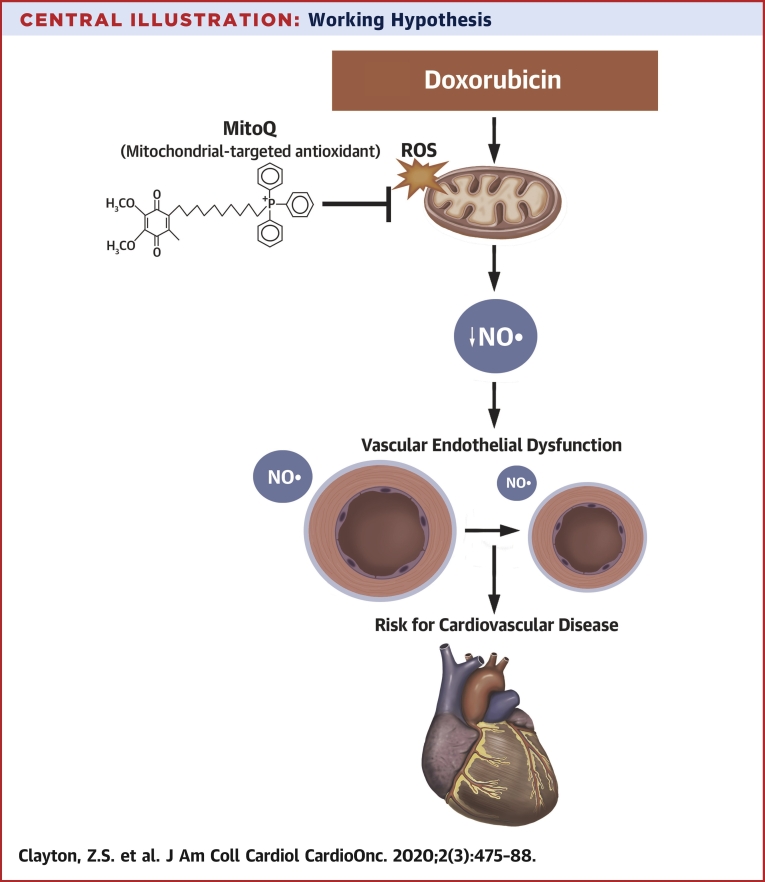
Central Illustration.
Working Hypothesis
An increase in mitochondria-derived reactive oxygen species (ROS) with doxorubicin contributes to a state of oxidative stress and reduction in nitric oxide (NO) signaling that promotes the development of endothelial dysfunction in conduit arteries. Mitochondria-targeted antioxidant treatment with MitoQ may be a therapeutic strategy for reducing oxidative stress and preserving conduit artery endothelial function with doxorubicin to reduce cardiovascular disease risk.
Acknowledgments
We thank Dr. Michael Murphy for the donation of MitoQ and Jill Miyamato-Ditmon and Zachary Cook for assistance with data collection.
Footnotes
Dr. Clayton was supported by T32 DK007135 & F32 HL151022. Dr. Seals was supported by R01 AG055822. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For an expanded Methods section as well as supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Halpern M.T., Yabroff K.R. Prevalence of outpatient cancer treatment in the United States: estimates from the Medical Panel Expenditures Survey (MPES) Cancer Invest. 2008;26:647–651. doi: 10.1080/07357900801905519. [DOI] [PubMed] [Google Scholar]
- 2.Truong J., Yan A.T., Cramarossa G. Chemotherapy-induced cardiotoxicity: detection, prevention, and management. Can J Cardiol. 2014;30:869–878. doi: 10.1016/j.cjca.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Meinardi M.T., Gietema J.A., van Veldhuisen D.J. Long-term chemotherapy-related cardiovascular morbidity. Cancer Treat Rev. 2000;26:429–447. doi: 10.1053/ctrv.2000.0175. [DOI] [PubMed] [Google Scholar]
- 4.Lipshultz S.E., Franco V.I., Miller T.L. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med. 2015;66:161–176. doi: 10.1146/annurev-med-070213-054849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaklamani V.G., Gradishar W.J. Epirubicin versus doxorubicin: which is the anthracycline of choice for the treatment of breast cancer? Clin Breast Cancer. 2003;4(Suppl 1):S26–S33. doi: 10.3816/cbc.2003.s.012. [DOI] [PubMed] [Google Scholar]
- 6.McGowan J.V., Chung R., Maulik A. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng S., Kruger A., Kleschyov A.L. Gp91phox-containing NAD(P)H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic Biol Med. 2007;42:466–473. doi: 10.1016/j.freeradbiomed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Doroshow J.H., Locker G.Y., Myers C.E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980;65:128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies K.J., Doroshow J.H. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261:3060–3067. [PubMed] [Google Scholar]
- 10.Wallace K.B. Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovasc Toxicol. 2007;7:101–107. doi: 10.1007/s12012-007-0008-2. [DOI] [PubMed] [Google Scholar]
- 11.Chaiswing L., Cole M.P., Ittarat W. Manganese superoxide dismutase and inducible nitric oxide synthase modify early oxidative events in acute adriamycin-induced mitochondrial toxicity. Mol Cancer Ther. 2005;4:1056–1064. doi: 10.1158/1535-7163.MCT-04-0322. [DOI] [PubMed] [Google Scholar]
- 12.Zhou S., Starkov A., Froberg M.K. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61:771–777. [PubMed] [Google Scholar]
- 13.Luu A.Z., Chowdhury B., Al-Omran M. Role of endothelium in doxorubicin-induced cardiomyopathy. J Am Coll Cardiol Basic Trans Science. 2018;3:861–870. doi: 10.1016/j.jacbts.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewirtz D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 15.Gorini S., De Angelis A., Berrino L. Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, trastuzumab, and sunitinib. Oxid Med Cell Longev. 2018;2018:7582730. doi: 10.1155/2018/7582730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang K., Liu Y., Tang H. Glabridin prevents doxorubicin-induced cardiotoxicity through gut microbiota modulation and colonic macrophage polarization in mice. Front Pharmacol. 2019;10:107. doi: 10.3389/fphar.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Räsänen M., Degerman J., Nissinen T.A. VEGF-B gene therapy inhibits doxorubicin-induced cardiotoxicity by endothelial protection. Proc Natl Acad Sci U S A. 2016;113:13144–13149. doi: 10.1073/pnas.1616168113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dengel D.R., Ness K.K., Glasser S.P. Endothelial function in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30:20–25. doi: 10.1097/MPH.0b013e318159a593. [DOI] [PubMed] [Google Scholar]
- 19.Demaria M., O'Leary M.N., Chang J. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7:165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gioscia-Ryan R.A., LaRocca T.J., Sindler A.L. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadeddu Dessalvi C., Pepe A., Penna C. Sex differences in anthracycline-induced cardiotoxicity: the benefits of estrogens. Heart Fail Rev. 2019;24:915–925. doi: 10.1007/s10741-019-09820-2. [DOI] [PubMed] [Google Scholar]
- 22.Ballak D.B., Brunt V.E., Sapinsley Z.J. Short-term interleukin-37 treatment improves vascular endothelial function, endurance exercise capacity, and whole-body glucose metabolism in old mice. Aging Cell. 2019 doi: 10.1111/acel.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilliam L.A., Ferreira L.F., Bruton J.D. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol (1985) 2009;107:1935–1942. doi: 10.1152/japplphysiol.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lum H., Roebuck K.A. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 25.Handy D.E., Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal. 2012;16:1323–1367. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Asperen J., van Tellingen O., Tijssen F. Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdr1a P-glycoprotein. Br J Cancer. 1999;79:108–113. doi: 10.1038/sj.bjc.6690019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foyer C.H., Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakao K., Minato N., Uemoto S. Springer Japan KK; Kyoto: 2015. Innovative Medicine: Basic Research and Development. [PubMed] [Google Scholar]
- 29.Ohno M., Oka S., Nakabeppu Y. Quantitative analysis of oxidized guanine, 8-oxoguanine, in mitochondrial DNA by immunofluorescence method. Methods Mol Biol. 2009;554:199–212. doi: 10.1007/978-1-59745-521-3_13. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen T.T., Quan X., Hwang K.H. Mitochondrial oxidative stress mediates high-phosphate-induced secretory defects and apoptosis in insulin-secreting cells. Am J Physiol Endocrinol Metab. 2015;308:E933–E941. doi: 10.1152/ajpendo.00009.2015. [DOI] [PubMed] [Google Scholar]
- 31.Nocito A., Dahm F., Jochum W. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology. 2007;133:608–618. doi: 10.1053/j.gastro.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Yu L.R., Cao Z., Makhoul I. Immune response proteins as predictive biomarkers of doxorubicin-induced cardiotoxicity in breast cancer patients. Exp Biol Med (Maywood) 2018;243:248–255. doi: 10.1177/1535370217746383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Cancer Institute Antioxidants and cancer prevention. 2017. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/antioxidants-fact-sheet Available at:
- 34.Jung A.Y., Cai X., Thoene K. Antioxidant supplementation and breast cancer prognosis in postmenopausal women undergoing chemotherapy and radiation therapy. Am J Clin Nutr. 2019;109:69–78. doi: 10.1093/ajcn/nqy223. [DOI] [PubMed] [Google Scholar]
- 35.Kotamraju S., Konorev E.A., Joseph J. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- 36.Vásquez-Vivar J., Martasek P., Hogg N. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry. 1997;36:11293–11297. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- 37.Anderson A.B., Xiong G., Arriaga E.A. Doxorubicin accumulation in individually electrophoresed organelles. J Am Chem Soc. 2004;126:9168–9169. doi: 10.1021/ja0492539. [DOI] [PubMed] [Google Scholar]
- 38.Yin J., Guo J., Zhang Q. Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the PINK1/parkin pathway. Toxicol In Vitro. 2018;51:1–10. doi: 10.1016/j.tiv.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Yen H.C., Oberley T.D., Gairola C.G. Manganese superoxide dismutase protects mitochondrial complex I against adriamycin-induced cardiomyopathy in transgenic mice. Arch Biochem Biophys. 1999;362:59–66. doi: 10.1006/abbi.1998.1011. [DOI] [PubMed] [Google Scholar]
- 40.He H., Wang L., Qiao Y. Doxorubicin induces endotheliotoxicity and mitochondrial dysfunction. Front Pharmacol. 2019;10:1531. doi: 10.3389/fphar.2019.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wenzel P., Schuhmacher S., Kienhöfer J. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res. 2008;80:280–289. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duquaine D., Hirsch G.A., Chakrabarti A. Rapid-onset endothelial dysfunction with adriamycin: evidence for a dysfunctional nitric oxide synthase. Vasc Med. 2003;8:101–107. doi: 10.1191/1358863x03vm476oa. [DOI] [PubMed] [Google Scholar]
- 43.Kawanishi S., Hiraku Y., Oikawa S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res. 2001;488:65–76. doi: 10.1016/s1383-5742(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 44.Pierce G.L., Lesniewski L.A., Lawson B.R. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossman M.J., Santos-Parker J.R., Steward C.A.C. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension. 2018;71:1056–1063. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gane E.J., Weilert F., Orr D.W. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 47.Snow B.J., Rolfe F.L., Lockhart M.M. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 48.Akolkar G., da Silva Dias D., Ayyappan P. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2017;313:H795–H809. doi: 10.1152/ajpheart.00253.2017. [DOI] [PubMed] [Google Scholar]
- 49.Rocha V.C., França L.S., de Araújo C.F. Protective effects of mito-TEMPO against doxorubicin cardiotoxicity in mice. Cancer Chemother Pharmacol. 2016;77:659–662. doi: 10.1007/s00280-015-2949-7. [DOI] [PubMed] [Google Scholar]
- 50.Chandran K., Aggarwal D., Migrino R.Q. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J. 2009;96:1388–1398. doi: 10.1016/j.bpj.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giannessi D., Del Ry S., Vitale R.L. The role of endothelins and their receptors in heart failure. Pharmacol Res. 2001;43:111–126. doi: 10.1006/phrs.2000.0758. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita J., Ogawa M., Nomura K. Plasma endothelin-1 and doxorubicin cardiotoxicity. N Engl J Med. 1994;331:1528–1529. doi: 10.1056/NEJM199412013312218. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita J., Ogawa M., Shirakusa T. Plasma endothelin-1 as a marker for doxorubicin cardiotoxicity. Int J Cancer. 1995;62:542–547. doi: 10.1002/ijc.2910620509. [DOI] [PubMed] [Google Scholar]
- 54.Sun X., Kumar S., Sharma S. Endothelin-1 induces a glycolytic switch in pulmonary arterial endothelial cells via the mitochondrial translocation of endothelial nitric oxide synthase. Am J Respir Cell Mol Biol. 2014;50:1084–1095. doi: 10.1165/rcmb.2013-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam C.S., Brutsaert D.L. Endothelial dysfunction: a pathophysiologic factor in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2012;60:1787–1789. doi: 10.1016/j.jacc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.