Abstract
Mitochondria modulate inflammatory processes in various model organisms, but it is unclear how much mitochondria regulate immune responses in human blood leukocytes. Here, we examine the effect of i) experimental perturbations of mitochondrial respiratory chain function, and ii) baseline inter-individual variation in leukocyte mitochondrial energy production capacity on stimulated cytokine release and glucocorticoid (GC) sensitivity. In a first cohort, whole blood from 20 healthy women and men was stimulated with increasing concentrations of the immune agonist lipopolysaccharide (LPS). Four inhibitors of mitochondrial respiratory chain Complexes I, III, IV, and V were used (LPS + Mito-Inhibitors) to acutely perturb mitochondrial function, GC sensitivity was quantified using the GC-mimetic dexamethasone (DEX) (LPS + DEX), and the resultant cytokine signatures mapped with a 20-cytokine array. Inhibiting mitochondrial respiration caused large inter-individual differences in LPS-stimulated IL-6 reactivity (Cohen’s d = 0.72) and TNF-α (d = 1.55) but only minor alteration in EC50-based LPS sensitivity (d = 0.21). Specifically, inhibiting mitochondrial Complex IV potentiated LPS-induced IL-6 levels by 13%, but inhibited TNF-α induction by 72%, indicating mitochondrial regulation of the IL-6/TNF-α ratio. As expected, DEX treatment suppressed multiple LPS-induced pro-inflammatory cytokines (IFN-γ, IL-6, IL-8, IL-1β, TNF-α) by >85% and increased the anti-inflammatory cytokine IL-10 by 80%. Inhibiting Complex I potentiated DEX suppression of IL-6 by a further 12% (d = 0.73), indicating partial mitochondrial modulation of glucocorticoid sensitivity. Finally, to examine if intrinsic mitochondrial respiratory capacity may explain a portion of immune reactivity differences across individuals, we measured biochemical respiratory chain enzyme activities and mitochondrial DNA copy number in isolated peripheral blood mononuclear cells (PBMCs) from a second cohort of 44 healthy individuals in parallel with LPS-stimulated IL-6 and TNF-α response. Respiratory chain function, particularly Complex IV activity, was positively correlated with LPS-stimulated IL-6 levels (r = 0.45, p = 0.002). Overall, these data provide preliminary evidence that mitochondrial behavior modulates LPS-induced inflammatory cytokine signatures in human blood.
Keywords: Mitochondria, Inflammation, Human blood, LPS, Cytokines, TNF-α, IL-6
Highlights
-
•
Inhibiting mitochondrial respiration alters IL-6 and TNF-α reactivity to LPS in human blood.
-
•
Mitochondrial respiratory chain function only mildly affects sensitivity to LPS.
-
•
Pro- and anti-inflammatory cytokine profiles are under mitochondrial regulation.
-
•
Inhibiting Complex I potentiates DEX suppression of IL-6 response.
-
•
Baseline leukocyte mitochondrial function partly explains inter-individual differences in IL-6 reactivity.
1. Introduction
Chronic inflammation is a hallmark of multiple health disorders that challenge modern medicine (Rea et al., 2018; Ferrucci and Fabbri, 2018). Experimental pre-clinical studies indicate that pro-inflammatory cytokines, especially when their levels are chronically elevated, may directly contribute to disease onset or progression, particularly for heart disease (Hann et al., 1998), sepsis (van der Poll et al., 2017), neurodegeneration (Chitnis and Weiner, 2017), autoimmune disorders like rheumatoid arthritis (Panga and Raghunathan, 2018), and the aging process itself (Rea et al., 2018). Inflammation can also be acutely induced by psychosocial stress (Marsland et al., 2017). But little is known about the basis for inter-individual differences in inflammatory reactivity – why do some individuals produce large amounts of pro-inflammatory cytokines, while in response to the same stimulus others exhibit more modest or qualitatively different immune responses? Although a fraction of the inter-individual variability in human immune response is attributed to genomic variation (Piasecka et al., 2018), evidence suggests that additional mechanisms must influence immune function, including cellular energetics (Breda et al., 2019).
Immune responses require a substantial rise in cellular energy demand supplied in large part by mitochondria (Hotamisligil, 2017), which can contribute to inflammatory responses in many ways. The rise in energy demand fuels a number of intracellular processes such as biosynthesis of macromolecules, gene expression, protein synthesis – including cytokines – and their exocytosis, metabolic reprogramming, and other signaling processes essential for the acquisition of specific immunological phenotypes (Fox et al., 2005). This includes pro- and anti-inflammatory macrophage differentiation (Huang et al., 2014) and lymphocyte activation (Chapman et al., 2020). Mitochondria also serve as a signaling platform for various innate immunological signaling pathways in macrophages and non-immune cells such as fibroblasts (Chandel, 2015; Garaude et al., 2016; Koshiba et al., 2011). Both innate and adaptive immune responses and their associated intracellular signaling pathways (West et al., 2011; Weinberg et al., 2015) are also under regulation of reactive oxygen species (ROS) generated by electron transfer within the respiratory chain Complexes I and III (Breda et al., 2019). On the other hand, Complex II is involved in activating macrophages via succinate (Tannahill et al., 2013) whereas Complex IV as a critical controller of oxygen flux can regulate anti-viral signaling (Zhao et al., 2012; Li et al., 2006). Mitochondria can also release immunogenic components including their mitochondrial DNA (mtDNA), small peptides, and ATP into the cytoplasm and extracellular space, which are sensed as “bacteria-like” damage associated molecular patterns (DAMPs) that engage canonical innate immune cascades (Meyer et al., 2018). The release of immunogenic circulating cell-free mtDNA (ccf-mtDNA) (Strahler et al., 2015; Boyapati et al., 2017) in response to acute psychosocial stress (Trumpff et al., 2019a, 2019b; Lindqvist et al., 2016) and elevated levels in psychopathology (Lindqvist et al., 2018) also implicate mitochondria in the stress-immune axis in humans. Studies have also found correlations between baseline pro-inflammatory cytokine levels and measures of cellular energetics (Boeck et al., 2018), mitochondrial disease and increased risk of sepsis in children (Eom et al., 2017), and between mitochondrial respiration and T-cell activation in a mouse model of mitochondrial disease (Tarasenko et al., 2017). But whether mitochondria in circulating immune cells influence immune reactivity to acute challenge has not been examined in healthy individuals.
To begin examining this question, we first systematic reviewed the literature for studies reporting associations between mitochondrial function and cytokine response in human health and disease. Our analysis showed that few studies provided indirect correlative evidence for some cytokines (e.g., TNF-α, IL-1β) but a lack of evidence for certain cytokines (e.g., IL-6, IFN-γ). The key findings from the systematic review are presented in Supplemental Table 1, highlighting the gap in knowledge around the influence of mitochondrial function on inflammatory cytokines in humans.
Here, we hypothesized that in healthy human blood: i) inhibiting mitochondrial respiration would exaggerate LPS-induced pro-inflammatory cytokines and alter multi-cytokine signatures, ii) mitochondrial function is necessary for glucocorticoid (GC) suppression of pro-inflammatory cytokines, and iii) baseline mitochondrial energy production capacity would in part explain the inter-individual differences in LPS-driven immune responses. Overall, this work provides initial evidence that mitochondria modulate different aspects of immune responses in human leukocytes.
2. Methods
2.1. Participants
For the main study (Cohort1), a total of 20 healthy adults (age 24–70 years, mean age = 33) were recruited from the Columbia University Irving Medical Center area. Recruitment was by flyers and via email/phone communications. Informed consent was obtained in compliance with guidelines of the Institutional Review Board of the New York State Psychiatric Institute. Exclusion criteria included pregnancy, cognitive deficit, flu or seasonal infection 4 weeks prior, involvement in a therapeutic or exercise trial and mitochondrial disease diagnosis. Before the blood draw, participants completed a brief questionnaire to collect information on their sex, age, ethnicity, health condition and medication.
A total of 60 mL of blood was collected by venipuncture in the antecubital fossa. Whole blood was processed within 10-15 min after collection for LPS- stimulation, mitochondrial inhibitors, and glucocorticoid suppression experiments as well as for total blood cell count. Complete blood count (CBC) was performed on 13 participants and included proportions of white blood cells (WBC), red blood cells, platelets, and differential WBC counts using an automated hematologic analyzer (XN-9000 Sysmex systems), yielding the percentage of total WBC that are neutrophils, lymphocytes, monocytes, eosinophils, and basophils.
A second study (Cohort 2) of 44 healthy nonsmoking, no exercise, sedentary, adults (age 20–45 years, mean age = 32) was conducted to test if baseline mitochondrial function measured directly in isolated PBMCs was correlated with LPS-stimulated inflammatory cytokine levels. These subjects were part of the ‘Exercise and Inflammation Study’ recruited from the Columbia University and Medical Center/New York Presbyterian Hospital community. Only the baseline (prior to exercise intervention) time point was used in this study. Recruitment was done by flyers posted throughout the Medical Center and electronic bulletin boards. The study was approved by the institutional review board (IRB) #6956R (Formerly #5948)’ and was registered at ClinicalTrials.gov: NCT01335737.
2.2. LPS-stimulation, mitochondrial inhibitors, and glucocorticoid suppression
For LPS stimulation experiments, 16 mL of whole blood was collected in vacutainers with sodium heparin (BD #67878) and was diluted with 1x RPMI without Phenol red (Thermofisher #11835055). For dose-dependent lipopolysaccharide (LPS) stimulation, blood was incubated for 6 h at 37 °C and 5% CO2 with bacterial endotoxin LPS from Escherichia coli (Sigma-Aldrich, #L2880) at increasing concentrations ranging from 3.2 pg/mL to 10 ng/mL per well, in a 96-well tissue culture plate (Eppendorf, #30730127). In all whole blood experiments, samples were centrifuged twice at 4οC, with a first spin at 1,000g for 5 min followed by a second spin at 2,000g for 10 min to obtain cell-free plasma, which was stored at -80οC for subsequent cytokine quantification. In Cohort 2, 1 ng/mL LPS (Sigma-Aldrich #L4130) was used to stimulate heparinized blood for 4 h at 37 °C. Plasma was collected post centrifugation at 2,040 g for 5 min and stored at -20 °C for subsequent cytokine quantification (Sloan et al., 2018).
For mitochondrial respiration perturbation experiments, inhibitors of Complex I-Rotenone (Sigma-Aldrich #R8875), Complex III-Antimycin A (Sigma-Aldrich #A8674), Complex IV-Potassium Cyanide (KCN) (Sigma-Aldrich #201810) and Complex V-Oligomycin (Sigma-Aldrich #75351) were used at final concentration of 100 nM except KCN which was 100uM. The inhibitors were dissolved in DMSO and co-treated with LPS for 6 h. Inhibitor concentrations were selected based either on our preliminary results (Trumpff et al., 2019b) or previous reports, Rot- (Worth et al., 2014), Anti A- (van Raam et al., 2008), KCN- (Jang et al., 2016), Oligo- (Ehinger et al., 2016) and DEX- (Alm, 2012).
In glucocorticoid suppression experiments, cortisol-mimetic Dexamethasone (DEX, Sigma-Aldrich #D4902) was co-incubated with LPS and whole blood in a 96-well culture plate at a final concentration of 100 nM. Each plate included an untreated control for baseline measures that was incubated at 37οC and 5% CO2 for 6 h. Plasma was collected and stored as described above for subsequent cytokine assessments.
2.3. Cytokine assays
2.3.1. IL-6 ELISA
To assess IL-6 levels in response to increasing dose of LPS, sandwich ELISA method was used following instructions provided by BD OptEIA IL-6 ELISA kit (BD #555220) with minor modifications. Briefly, the capture Ab against IL-6 was coated on to a 96-well plate at a dilution of 1:500 (100ul/well) and was incubated overnight at 4οC. The coated plate was washed with 1x wash buffer and blocked with serum for 1 h at room temperature (RT). The wells with Ab were aspirated followed by 3 washes with the wash buffer. A diluent containing serum (from the manufacturer) was added to the wells marked for standards and samples after which the plate was incubated for 2 h at RT. Plasma samples were diluted twice with the provided diluent in the assay. Detection Ab-Streptavidin HRP conjugate (100 μL/well) was added to each well at 1:500 dilution following 5 washes and the plate was incubated for 1 h at RT. A substrate solution was added after 7 washes to each well and incubated for 30 min in dark. The reaction was interrupted by a stop solution and the plate was immediately read in a micro-plate reader (SpectraMax M3 Molecular Devices) at 450 nm and 570 nm. The background OD at 570 nm was used as internal optical control across wells in a plate. A standard curve was generated from each assay to extrapolate the unknown plasma IL-6 concentration from the linear range of the standards. The final concentration was obtained by correcting for sample dilution factor and batch variation. To control for batch variation, the same plasma sample was run on each plate. The detection sensitivity for IL-6 was 4.7 pg/mL and the intra- and inter-assay CV were <10%. To derive absolute cytokine concentrations in the plasma and to determine EC50 of LPS using increasing concentrations of LPS (ELISA), non-linear curve fits were performed using 5-parameter (5-PL) logistic regression. The EC50 of LPS-induced IL-6 reflects each participant’ sensitivity to LPS exposure tested across treatment groups. EC50 values could not be determined from the dose-response curve for 4 individuals.
2.3.2. Multiplex cytokine array
Cytokine signatures including 20 inflammatory cytokines and chemokines were measured in plasma using the ProcartaPlex bead Immunoassay (ThermoFisher #EPX 200-12185-901) on a Luminex-200 instrument (Luminex technologies) following the manufacturer’s protocol. IL-6 and TNF-α levels in unstimulated and LPS- stimulated plasma were obtained from the Luminex assay for downstream analyses. Briefly, plasma samples collected from LPS, LPS + Inhibitors and LPS + DEX experiments were batched for the 20-plex assay. Each batch of samples were run with the 2 control plasma samples to identify any batch effects between plates. These batch controls were LPS-treated blood to ensure detectable cytokine levels. Samples were diluted 1:5 with the assay diluent prior to the assay and run in duplicates. Standards and samples were prepared. Magnetic beads coated with Abs were added to the 96-well plate and washed with a magnetic plate washer. Samples and standards were added to the respective wells with the beads and incubated for 120 min on an orbital shaker at room temperature. Plates with beads were washed at the end of the incubation and detection Ab was added to the wells and incubated for 30 min followed by washes and incubation with streptavidin for 30 min. Beads were washed and resuspended in a reading buffer. The plates were read, and data was acquired in a Luminex 200 analyzer (Luminex, USA). Data QC and visualization was performed using xPONENT software v 4.2 and files were exported for statistical analyses. The assay sensitivities for all the 20-cytokines can be found on the manufacturer website. A detailed description of inflammation measures performed in Cohort 2 is described elsewhere (Sloan et al., 2018).
2.4. Mitochondrial enzyme activities and mitochondrial DNA copy number (mtDNAcn)
To quantify mitochondrial respiratory capacity and mitochondrial DNA content in circulating leukocytes, peripheral blood mononuclear cells (PBMCs) were isolated from 44 individuals in Cohort 2. Blood (8.5 mL x 5) was collected in acid citrate dextrose (ACD-A) tubes (VWR #VT4606). Blood was centrifuged at 500×g for 15 min at RT and platelet-rich plasma removed. Hank’s Balanced Salt Sodium (HBSS) without phenol red, calcium and magnesium (Life Technologies, #14175103) was added to replace the removed plasma. PBMCs were then isolated by density gradient separation by layering the diluted blood over 4 mL of Ficoll Paque Plus (VWR, #95021-205) in 15 mL conical tubes and centrifuged at 400×g for 30min (no brake) at RT. Total PBMCs at the Ficoll interface were collected in a 50 mL conical tube, diluted 1:1 in HBSS and pelleted by centrifugation at 500×g for 10 min at RT. An additional wash was carried out with HBSS at 200×g for 10min to maximally deplete platelets. Cells were re-suspended in HBSS and counted on the Countess II FL Automated Cell Counter (Thermo Fisher Scientific, AMQAF1000) in a 1:1 ratio of cells to trypan blue and stored at -80 °C until measurements were taken.
The biochemical activity of mitochondrial enzymes were measured and integrated into a composite index of mitochondria energy production capacity, the mitochondrial health index (MHI) (Picard et al., 2018). Briefly, mitochondrial enzymes were selected and results interpreted on the basis of: 1) their known biological function (markers of energy production capacity or mitochondrial content); 2) their robustness in a microplate format designed for high throughput; 3) their ability to respond metabolic and biological stressors; and 4) the knowledge that the subunits that compose them are encoded by either the mitochondrial or nuclear genomes. Thus, enzymatic activities were spectrophotometrically quantified for citrate synthase (CS), cytochrome c oxidase (COX, Complex IV), succinate dehydrogenase (SDH, Complex II), NADH-Ubiquinone Oxidoreductase (Complex I) and expressed per million cells. In parallel, mtDNA and nuclear DNA abundance were quantified by TaqMan-based multiplex quantitative real-time polymerase chain reaction (qPCR) to normalize for cell number and calculate mtDNA copy number (mtDNAcn) as described in (Picard et al., 2018).
2.5. Statistical analysis
All statistical analyses were performed using GraphPad Prism v8.2 and MetaboAnalyst v3.0 (Xia and Wishart, 2016). To identify emergent cytokine patterns after mitochondrial respiration inhibition, we performed i) Spearman rank correlation analysis to test significant inter-cytokine relationships and Pearson analysis for cytokine- MHI relationships, ii) Linear regression analysis to test significant associations between cytokines, and iii) Hierarchical clustering using Ward algorithm and Euclidean distance measure to identify similar responsive cytokine groups. We also performed Partial Least Square Discriminant Analysis (PLS-DA) modeling and ranked cytokines based on variable in projection (VIP) score for the first PLS-DA component, yielded a hierarchy of cytokines most useful in distinguishing treatment effects.
In Cohort 1, Spearman rank correlations were used due to small sample size and non-normally distributed data to assess the strength of the association between the variables, including correlation among cytokines pre- and post-LPS stimulation, LPS + Mito-Inhibitors, and LPS + DEX treatments. In Cohort 2, we performed linear regressions between mitochondrial measures in PBMCs and both unstimulated and LPS-stimulated cytokine levels to quantify the strength and direction of their associations. The association between age and measures of mitochondria content and function in Cohort 2 were also quantified using linear regressions.
To examine intra-individual differences from pre-to post-LPS treatment, with mitochondrial inhibitors, and with DEX treatment, we used pairwise comparisons two-tailed T-test (significance set as P < 0.05). To detect group differences across untreated, LPS + Mito-inhibitors and LPS + DEX, relative to the LPS measures, one- or two-way ANOVAs were used. Missing data were handled using mixed effects models with post-hoc analysis. Adjusted P values were used for multiple comparisons and F values were derived from mixed-effects models. Effect sizes (Cohen’s d) were calculated from t-test and ANOVA results (Cohen, 1988).
3. Results
3.1. Inhibiting mitochondrial respiratory chain function influences LPS-induced IL-6 and TNF-α levels
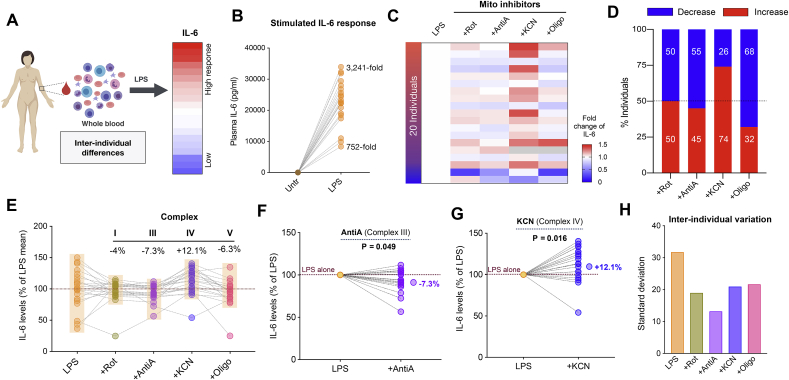
To examine immune responses across participants, we first exposed whole blood to 10 ng/mL LPS and measured IL-6 levels at 6 h (Fig. 1A). LPS exposure significantly (p < 0.0001) elevated IL-6 levels by 752- to 3,241 - fold compared to baseline (Fig. 1B), demonstrating robust immune response to LPS challenge.
Fig. 1.
Inhibition of mitochondrial respiratory capacity causes large inter-individual differences in acute LPS-induced IL-6 levels in human blood. (A) Experimental design illustrating the quantification of cytokine levels from whole human blood (n = 20) before and after LPS stimulation (10 ng/mL). (B) Fold change of LPS-stimulated IL-6 levels relative to the unstimulated levels. (C) Effect of mitochondrial inhibitors on stimulated IL-6 levels relative to LPS alone: +Rotenone (Rot) for Complex I, +Antimycin (Anti) A for Complex III, +KCN for Complex IV and +Oligomycin (Oligo) for Complex V. Missing data are shown in grey. (D) Proportion of individuals showing either elevated (pro-inflammatory) or reduced (anti-inflammatory) IL-6 levels in response to each mitochondrial inhibitor. (E) IL-6 levels plotted relative to the mean LPS response (100%) where semi-transparent boxes illustrate reduced inter-individual variability in mitochondrial inhibition. Each data point is a participant and lines indicate blood from the same individual treated with different inhibitors. Median % change is shown for each inhibitor relative to LPS. (F–G) % change in IL-6 levels for LPS + AntiA (Complex III inhibition) and LPS + KCN (complex IV inhibition) relative to LPS-stimulated levels shown for each participant. Median changes are indicated relative to LPS alone with effect sizes (Cohen’s d) and P values from paired t-test. (H) Inhibition of mitochondrial respiration reduces inter-individual variability, quantified by the group standard deviation.
We then examined how each individual responded to the inhibition of various respiratory chain components, including Complexes I, III, IV, and V (Fig. 1C) and computed the proportion of individuals in whom mitochondrial inhibitors had either a pro- or anti-inflammatory effect relative to their LPS-only levels (Fig. 1D). Complex I inhibition with Rotenone elevated IL-6 levels in half of the participants but reduced IL-6 levels in the rest with a non-significant median reduction of 3.95% (d = -0.29, p = 0.36). Complex III inhibition with Antimycin-A had an anti-inflammatory effect in 55% of individuals, leading to a median reduction of IL-6 by 7.3% (d = -0.66, p = 0.049) (Fig. 1E and F). In contrast, Complex IV inhibition by KCN was mainly pro-inflammatory where 74% of the individuals showed an elevated IL-6 level relative to LPS alone and a median increase of 12.1% (d = 0.88, p = 0.016) (Fig. 1E,G). On the contrary, inhibiting Complex V with oligomycin led to an anti-inflammatory response in 68% of with an overall 6.3% reduction (Fig. 1E) in IL-6 levels compared to LPS alone (d = -0.41, p = 0.22).
Consistent with substantial inter-individual differences in cytokine levels previously reported (Damsgaard et al., 2009; Rohleder, 2014), the between-person standard deviation (SD) was 31.8% after LPS stimulation, reflecting substantial inter-individual differences in IL-6 responses. Interestingly, all respiratory chain inhibitors made participants respond more similarly to one another, evident from significantly reduced inter-individual differences (Fig. 1H). The group standard deviation after inhibition were: SDRot = 19%, SDAntiA = 13.2%, SDKCN = 20.9%, and SDOligo = 21.6%, representing about half of LPS only condition. These results suggest that mitochondrial respiratory capacity may contribute to inter-individual differences in LPS-induced IL-6 responses and that inhibiting mitochondrial respiratory chain function can influence both the magnitude and direction of LPS-induced inflammation in human leukocytes.
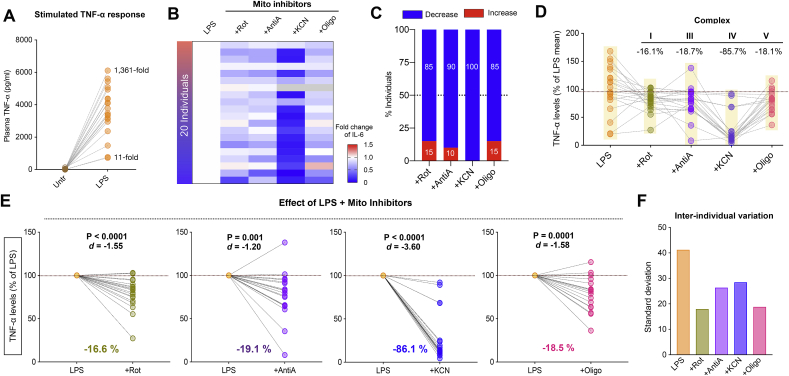
We then extended the same analysis to the cytokine TNF-α (Fig. 2) and found that LPS exposure significantly elevated TNF-α levels by 11- to 1361-fold (p < 0.0001) compared to baseline (Fig. 2A). Inhibition of respiratory chain Complexes I, III, IV, and V at the specific LPS dose of 10 ng/mL had an overall anti-inflammatory effect on LPS-stimulated levels (Fig. 2B), where 85–100% individuals showed decreased TNF-α levels with respiratory chain inhibitors (Fig. 2C), in all cases with large effect sizes. Complex I inhibition decreased TNF-α levels in 85% of individuals by a median 16.1% (d = -1.55, p < 0.0001). Complex III inhibition had an anti-inflammatory effect in 90% of individuals, leading to a median reduction of 18.7% (d = -1.20, p = 0.001). Interestingly, Complex IV inhibition led to a robust anti-inflammatory response in 100% of individuals with a median decrease of 85.7% in the TNF-α (d = -3.60, p < 0.0001). Similarly, inhibiting Complex V led to an anti-inflammatory response of TNF-α levels in 85% of individuals with an overall reduction of 18.1% compared to LPS alone (d = -1.58, p = 0.0001) (Fig. 2D-E). And as for IL-6, respiratory chain inhibitors reduced inter-individual variation in TNF-α (Fig. 2F).
Fig. 2.
Inhibition of mitochondrial respiratory capacity reduces LPS-induced TNF-α levels in human blood. (A) Fold change of LPS-stimulated TNF-α levels relative to unstimulated levels. (B) Effect of mitochondrial respiratory chain inhibitors (+Rot for Complex I, +Anti A for Complex III, +KCN for Complex IV and +Oligo for Complex V) on LPS-stimulated TNF-α levels. Missing data are shown in grey. (C) Proportion of individuals showing either increase or decrease in TNF-α levels upon mitochondrial respiratory chain inhibition. (D) TNF-α abundance relative to LPS, illustrating the inter-individual differences across inhibitors (highlighted semi-transparent boxes) where each datapoint is an individual (n = 19–20). Median % change in TNF-α response is shown for each inhibitor. (E) % change in TNF-α levels upon respiratory chain inhibition relative to LPS treatment alone presented with effect sizes (Cohen’s d) and P values from paired t-test. (F) Inhibition of mitochondrial respiration reduces inter-individual variation in stimulated TNF-α levels.
To examine if cell type composition in whole blood may have contributed to inter-individual differences in IL-6 and TNF-α response to LPS, we correlated stimulated IL-6 and TNF-α levels with the proportion (% of total cells) obtained from complete blood counts available from a subset of participants (Supplemental Figure S1A). As expected, the majority of leukocytes were neutrophils and lymphocytes, together composing 81–93% of all cells. Both IL-6 and TNF-α levels tended to be positively correlated with neutrophil count (r = 0.18–0.43) and negatively associated with lymphocyte count (r = -0.17 to -0.51). Stimulated IL-6 levels were also negatively correlated with eosinophil count (r = -0.68, p = 0.01, n = 13) whereas stimulated TNF-α levels were negatively correlated with basophil count (r = -0.61, p = 0.03, n = 13) (Supplemental Figure S1B). There was no correlation of stimulated cytokine levels with baseline monocyte count. These observations suggest that variable cell type proportions at baseline may in part contribute to stimulated cytokine levels in whole blood and call for future studies in isolated cell populations.
3.2. Minor influence of mitochondrial respiratory chain function on LPS sensitivity
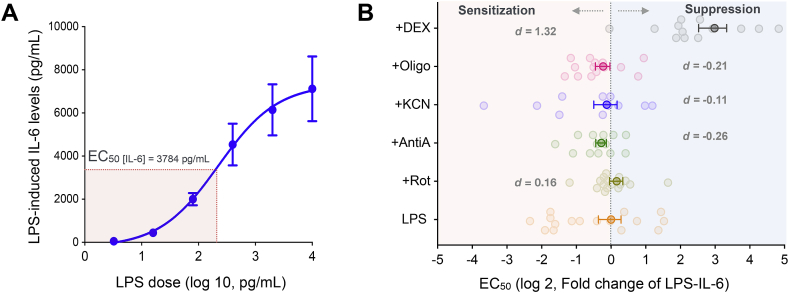
We next sought to determine if mitochondrial modulation influenced immune cells’ sensitivity across a range of LPS concentrations (3.2 pg/mL to 10 ng/mL). Increasing LPS concentrations caused a dose-dependent increase in IL-6 response fitted with a sigmoidal function (Fig. 3A). Again, there were large variations in LPS-sensitivity across individuals (CV = 88.6%) (Fig. 3B). The median LPS EC50 for IL-6 was 90 pg/mL (range 3.2–10 pg/mL) across 20 individuals. Inhibiting mitochondrial respiratory chain complexes led to only small alterations in the LPS-sensitivity. Complex I inhibition sensitized cells to LPS by 20% (d = 0.16) whereas inhibiting Complexes III, IV and V suppressed LPS-induced IL-6 levels by 30%, 60% and 38% respectively (d = -0.26, -0.11, -0.21 respectively, all N.S.) (Fig. 3B). In contrast, as expected from glucocorticoid suppression, DEX treatment decreased sensitivity as illustrated by a 7.9-fold higher LPS EC50 (d = 1.32, p = 0.001). Finally, although our dataset was not powered to examine sex differences, men tended to show about half the sensitivity to LPS (EC50 = 147.5 ± 48.8, mean ± SEM) compared to women (EC50 = 71.2 ± 19.4; d = -1.02, p = 0.32).
Fig. 3.
Sensitivity to LPS exposure is mildly affected by mitochondrial respiratory chain function. (A) Dose-dependent increase in IL-6 levels and LPS EC50 (LPS concentration necessary to reach the half-maximal IL-6 levels) measured in whole blood. (B) Effect of mitochondrial respiratory chain inhibitors on EC50 relative to LPS alone. Negative values represent sensitization (low EC50) and positive values represent suppression (high EC50) of LPS-response. Effect sizes computed as Cohen’s d. Data presented are mean ± SEM, n = 20.
3.3. Inhibition of mitochondrial complex IV alters acute inflammation induced cytokine signatures
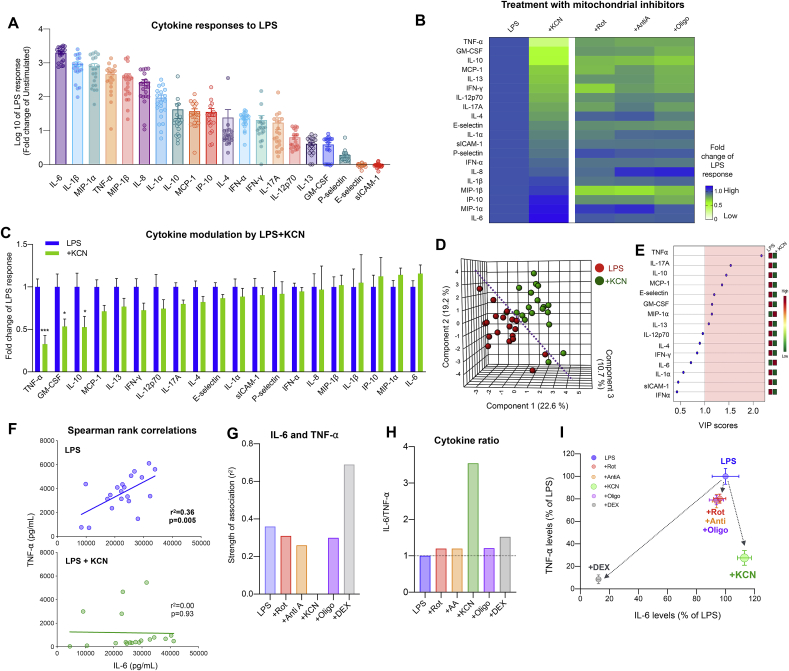
To assess the effect of mitochondrial respiration capacity on pro- and anti-inflammatory cytokine signatures, we simultaneously measured the levels of 20 cytokines in LPS-treated samples (LPS conc. at 10 ng/mL) ± mitochondrial respiratory chain inhibitors. This confirmed the stimulatory effects of LPS on multiple known inflammatory cytokines, chemokines, and interferons (Fig. 4A), including IL-6 which exhibited the strongest induction (~2,000-fold) relative to unstimulated levels (Supplemental Table S2). Again, marked inter-individual differences were noted in stimulated cytokine levels.
Fig. 4.
Inhibition of mitochondrial Complex IV alters pro- and anti-inflammatory cytokine profiles. (A) LPS-stimulated cytokine levels relative to the unstimulated levels. (B) Heatmap illustrating the effect of mitochondrial respiratory chain inhibitors on stimulated cytokine response relative to LPS-only. Cytokines order is based on LPS + KCN-group. (C) KCN-specific effects on stimulated cytokine response relative to LPS. P values from two-way ANOVA with Dunnett multiple comparison. ∗P < 0.05, ∗∗∗P < 0.005. (D) Partial Least square discriminant analysis (PLS-DA) model derived from cytokine response of each individual with or without KCN. (E) Cytokines are ranked by the variable in projection (VIP) reflecting their contribution to group separation in the overall model. By convention, VIP scores >1 are considered significant. (F) Effect of KCN on the association between stimulated IL-6 and TNF-⍺ levels compared to LPS alone. Strength of association (r2) and P values are obtained from linear regression. (G) Effect of all inhibitors on the correlation between IL-6 and TNF-α levels. (H) Modulation of the IL-6/TNF-⍺ ratio by inhibitors, expressed relative to LPS alone (dotted line). (I) Bi-plot illustrating the effect of inhibitors on TNF-⍺ and IL-6 levels, expressed relative to LPS alone. Data shown in (C) and (I) are mean ± SEM, n = 19–20.
We then examined how respiratory chain inhibition influenced levels across this cytokine panel. Overall, all inhibitors altered LPS-stimulated cytokine levels significantly compared to LPS (P < 0.005). Rotenone, Antimycin-A, KCN, and Oligomycin decreased the LPS-induced elevation of most cytokines, but some inhibitors potentiated the release of some cytokines (Fig. 4B, Supplemental Figure S2). In particular, KCN exposure most potently reduced the LPS induction of a large portion of the cytokines (Fig. 4C), including TNF-α whose levels were suppressed by 72% relative to LPS alone (d = -1.59, p = 0.0003).
To explore and visualize the overall effect of mitochondrial inhibition on the inflammatory phenotype, we ran a partial least square discriminant analysis (PLS-DA) with leave-one-out cross-validation (LOOCV). This procedure tests whether all 20 cytokines considered together in the same model contain enough information to distinguish between LPS and LPS + KCN treatments. The model for KCN yielded a prediction accuracy of 83% and produced a reasonable separation of treatments (Fig. 4D, Supplemental Table S3). In comparison, models for other inhibitors did not perform as well (accuracies 55–65%, near chance level), reflecting their smaller effect sizes. In comparison, DEX treatment yielded the most robust of the models tested, consistent with the large immunosuppressive effects of DEX (see Fig. 6B). Among the 8 significant cytokines (VIP score >1 in PLS-DA model) that distinguished the LPS + KCN treatment, TNF-α, IL-17A, IL-10 were the top 3 cytokines, all downregulated by KCN (Fig. 4E).
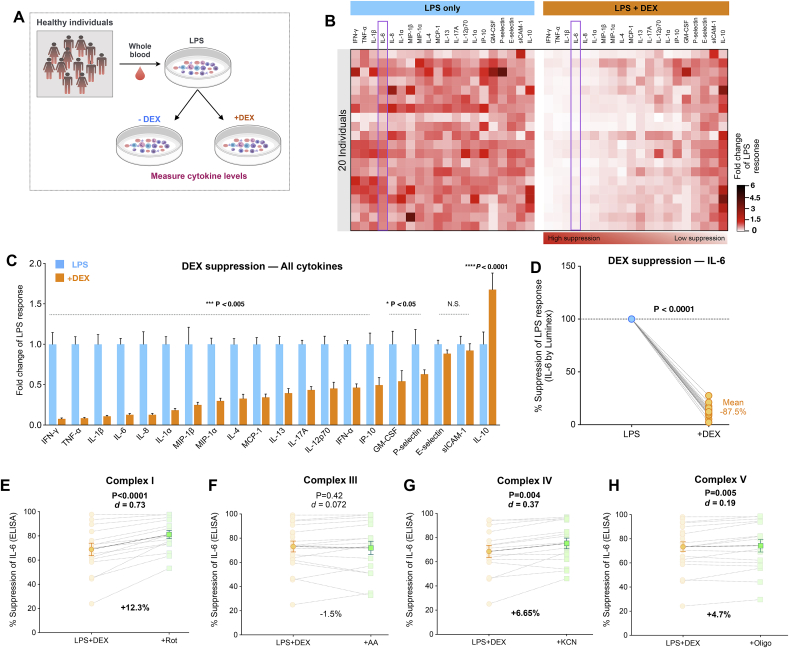
Fig. 6.
Glucocorticoid signaling suppresses most LPS-induced pro-inflammatory cytokines and increases IL-10. (A) Experimental design to probe DEX-mediated effects on cytokine signatures. (B) Effect of DEX on LPS-induced inflammatory cytokine responses relative to LPS alone. The cytokines are ordered from most-to least-suppressed by DEX. (C) Fold change in cytokine response to LPS by DEX exposure relative to LPS alone. P values from one-way ANOVA. (D) % DEX suppression of stimulated IL-6, paired t-test, n = 19–20. (E–H) Effect of respiratory chain inhibitors on %DEX-suppression. Effect sizes (Cohen’s d) and % changes in DEX-suppression are presented, with P values from paired t-test. Data are means ± SEM, n = 20 except in E-H where n = 15–16.
Because TNF-α can regulate the expression of IL-6 (Katz et al., 2004), and both cytokines are often co-released (Arango Duque and Descoteaux, 2014), we next examined the influence of perturbing mitochondrial respiratory chain function on these two cytokines. As predicted, LPS-induced IL-6 and TNF-α levels were significantly correlated upon stimulation, but KCN treatment abolished this coupling (Fig. 4F,G,I). While other inhibitors (Complexes I, III and V) caused a 18–20% reduction in both IL-6 and TNF-α levels, KCN (Complex IV) reduced TNF-α levels by 72% but increased the IL-6 by 13% (Fig. 4C). As a result, KCN treatment increased the IL-6 to TNF-α ratio by 3.5-fold, whereas other mitochondrial inhibitors only modestly increased the ratio by 0.2–0.5-fold (Fig. 4H). KCN also reduced IL-10 levels by 47% (p = 0.17) compared to mean LPS levels whereas Complexes I, III and V inhibitors reduced IL-10 levels by 24%, 26% and 28% respectively (Supplemental Figure S2A-C). These results suggest that KCN and its target respiratory chain complex, Complex IV, has a particularly strong influence on the pro- and anti-inflammatory cytokine levels in whole blood leukocytes.
3.4. Inhibiting mitochondrial respiratory chain alters cytokine signatures
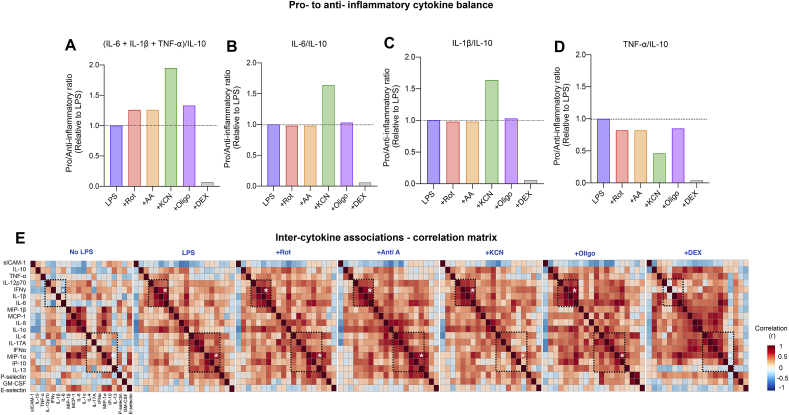
To further examine cytokine signatures, we first explored the ratios of pro- and anti-inflammatory cytokines. To generate a cumulative pro/anti-inflammatory index, we included IL-6, TNF-α, and IL-1β as pro-inflammatory cytokines and used IL-10 as the anti-inflammatory cytokine. As a proof-of-concept, relative to LPS alone, DEX robustly decreased the pro-/anti-inflammatory index, consistent with its potent anti-inflammatory effects. In contrast, inhibiting any of the mitochondrial respiration complexes increased this index by 20-100% (Fig. 5A), tilting the balance towards a pro-inflammatory state. Individual cytokine ratios such as IL-6/IL-10, IL-1 β/IL-10, and TNF-α/IL-10 were also differentially affected by respiratory chain inhibitors (Fig. 5B–D), with KCN consistently showing the most robust immune modulatory effect.
Fig. 5.
Perturbation of mitochondrial respiratory capacity modifies cytokine signatures. (A) Effect of mitochondrial respiration inhibitors and GC-mimetic DEX on group-level pro-to anti-inflammatory cytokine ratio, expressed relative to LPS (dotted line). Respiration inhibitors promote a pro-inflammatory state relative to LPS. (B–D) Complex IV inhibition by KCN increases pro-to anti-inflammatory cytokine ratios in IL-6 and IL-1β but decreases in TNF-⍺. (E) Effect of treatments on inter-cytokine correlations (Spearman rank). Cytokines are rank-ordered based on hierarchical clustering of cytokines from the LPS-group. The dotted regions highlight specific cytokine clusters with altered correlation strength across treatment groups. Each square is a correlation with n = 19–20 individuals.
We next systematically examined the inter-cytokine correlations, visualized as correlation matrices that reveals their co-regulation (Fig. 5E). At baseline before LPS stimulation, only 20 pairs of cytokines were correlated to an appreciable degree (r > 0.5) and after LPS addition, 28 pairs of cytokines were correlated. In contrast, when we pharmacologically inhibited mitochondrial respiration complexes, the inter-correlated cytokine pairs increased to 42 (Rot), 51 (Anti A), 31 (KCN) and 67 (Oligo). Representative cytokine correlations are shown in Supplemental Fig. 3A-C. Together with the simple cytokine ratios, these results highlight the co-regulation of several pro- and anti-inflammatory cytokines, and the respiration chain complex-specific influence on cytokine signatures.
3.5. Effects of glucocorticoid signaling on inflammatory signatures
We then extended this multi-cytokine approach to examine the specific effects of GC-mediated anti-inflammatory signaling (schematic Fig. 6A). DEX significantly suppressed all well-known LPS-stimulated pro-inflammatory cytokines by 70-90%, reported in the order of most (p < 0.0001) to least suppressed (p < 0.05) cytokines IFN-γ, TNF-α, IL-1β, IL-6, IL-8, IL-1α, MIP-1β, MIP-1α in Fig. 6B and C. In contrast, DEX had no effect on cell adhesion proteins like P-selectin, E-selectin and sICAM-1 (N.S.), and rather upregulated the anti-inflammatory cytokine IL-10 by 60% (p < 0.0001) (Fig. 6C). The range of DEX-mediated suppression of LPS-induced IL-6 levels was 72-97% across individuals with an average suppression of 87% (Fig. 6D).
Given that glucocorticoid signaling influences mitochondrial behavior (Psarra et al., 2005; Du et al., 2009) and, that mitochondria modulate inflammatory cytokine production, we reasoned that a portion of the anti-inflammatory action of DEX may involve mitochondria. Therefore, we tested if mitochondrial respiratory capacity modulated DEX-mediated suppression of IL-6 response to LPS. We extracted the % DEX suppression for IL-6 in each participant and compared it to the % suppression after inhibition of mitochondrial respiration by various Complex inhibitors (Fig. 6E–H). In doing so, we found that inhibiting Complex I augmented DEX suppression of IL-6 levels by 12.3% (d = 0.73, p < 0.0001, n = 15) whereas Complex III inhibition had almost no effect on %DEX suppression (d = 0.072, p = 0.42, n = 15). Additionally, Inhibition of Complex IV and V potentiated IL-6 suppression by 6.6% (d = 0.36, p = 0.004, n = 15) and 4.7% (d = 0.19, p = 0.005, n = 15) respectively.
3.6. Associations between intrinsic mitochondrial respiratory capacity and cytokine responses
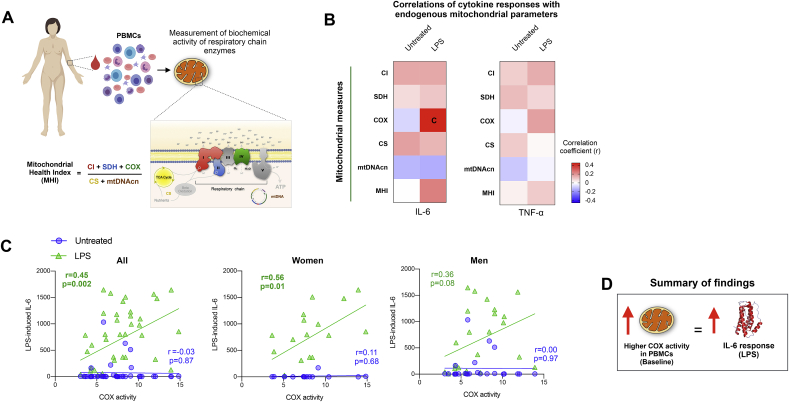
We next hypothesized that intrinsic leukocytes mitochondrial bioenergetic capacity accounts for a portion of inter-individual differences in cytokine responses. We therefore measured respiratory chain enzymatic activities for Complexes I, II, IV, citrate synthase, and mtDNA copy number in an independent cohort (Cohort 2, n = 44) of women and men in whom a sufficient number of peripheral blood mononuclear cells (PBMCs) could be isolated to enable reliable measure of mitochondrial function (Fig. 7A). Individual mitochondrial metrics were also integrated into an index of mitochondrial functional capacity, the MHI (Picard et al., 2018). In the same individuals, whole blood was stimulated with LPS (1 ng/mL) and IL-6 and TNF-α levels quantified after 4 h.
Fig. 7.
Associations between intrinsic bioenergetic capacity and cytokine production. (A) Schematic illustrating the measurement of mitochondrial respiratory chain activity, mtDNA copy number (mtDNAcn, and the mitochondrial health index (MHI) from human peripheral blood mononuclear cells (PBMCs). (B) Correlation between baseline mitochondrial measures and baseline and LPS-stimulated IL-6 and TNF-α responses shown as a heatmap with positive correlations (r) red and negative correlations blue. The correlation shown in the box marked with a “c” is shown in the next panel. (C) Correlation between baseline Complex IV (COX) activity measured in PBMCs and IL-6 levels at baseline (untreated) and after LPS stimulation (1 ng/mL) in all participants, and disaggregated by sex. (D) Schematic summarizing the overall correlation between mitochondrial function and cytokine response. Correlation coefficients (r) are derived from Pearson correlation analysis, n = 39–42 (Women = 15-18, Men = 24). TCA: tricarboxylic acid cycle, also known as “Krebs cycleˮ. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To approach this question from an unbiased perspective, all mitochondrial measures were correlated with cytokine levels in both untreated (no-LPS) and LPS-stimulated conditions, and the magnitude and direction of the associations between mitochondrial content (citrate synthase), respiratory chain activities, mtDNAcn, and the MHI were visualized as a heatmap (Fig. 7B). The majority (72%) of correlations were positive (chance would be 50%), suggesting that individuals with higher mitochondrial content and function produce more cytokines, particularly after LPS stimulation. In particular, baseline Complex IV (COX) activity (marked ‘C’ in the IL-6 heatmap) was positively correlated with LPS-stimulated IL-6 levels (r = 0.45, p = 0.002, n = 44). These associations were generally similar with TNF-α, but of lower magnitude. Both women and men showed pronounced positive correlation between baseline COX activity and stimulated IL-6 (Fig. 7C), but the effect size was larger in women (r = 0.56, p = 0.01, n = 18) than men (r = 0.36, p = 0.08, n = 24). Overall, these findings suggest that inherent mitochondrial respiratory capacity of leukocytes may account for 10–30% of the variance in immune reactivity across individuals. These data cannot rule out possible sex-differences in these associations.
In sensitivity analyses examining the association between the age of participants and various measures of mitochondrial behavior in PBMCs, age was not associated with mitochondrial content or functions (r2 = 0.00–0.03, N.S.) (supplementary Figure S4).
4. Discussion
This study examined how mitochondrial respiratory capacity modulates blood cytokine response upon LPS and DEX exposure. Acute pharmacological inhibition of mitochondrial respiration reduced inter-individual variation in cytokine levels, altered overall cytokine signatures, but only mildly modulated sensitivity to glucocorticoid signaling in Cohort 1. Complex IV activity in isolated leukocytes was positively correlated with LPS-stimulated plasma cytokine levels in Cohort 2. Together, these results suggest that intrinsic mitochondrial respiratory capacity may explain a fraction of inter-individual differences in inflammatory cytokine responses to LPS. Largely, our findings in human blood extent the scientific literature on mitochondria’s role in acute inflammation by providing initial evidence that mitochondrial respiratory capacity influences not only cytokine levels but also the cytokine signatures produced by blood leukocytes in humans.
Experimentally examining immune responses in vitro from human blood has several advantages that allow the isolation of potential mediators of immune processes (Strahler et al., 2015). First, it is possible to vary the strength of the immune challenge, such as exposure to lipopolysaccharide (LPS), a component within the cell wall of Gram-negative bacteria that stimulates various cell types to release IL-6, IL-1β, TNF-α, IL-8, and other pro-inflammatory cytokines (Mosher et al., 2006; Spierenburg et al., 2018). Second, the in vitro approach allows the manipulation of different aspects of mitochondrial function with selective inhibitors, including inhibition of specific respiratory chain components. Finally, this approach also makes it possible to combine known immunomodulators, such as immunosuppressive glucocorticoid (GC) signaling via dexamethasone (DEX), with mitochondrial modulators and thus study their interaction. Additionally, recognizing that immune responses come in different types reflected by their different cytokine signatures (Duffy et al., 2014), it is also possible to examine not only the magnitude but also the type of immune response by simultaneously probing multiple pro- and anti-inflammatory cytokines. Compared to isolated cells where systemic factors are removed, whole blood conditions preserve potential physiologically relevant functional interactions among different circulating leukocytes. However, it should be noted that factors other than the immune cells themselves, such as pre-existing circulating cytokines, levels of metabolites, or extracellular mitochondria (Al Amir Dache et al., 2020; Song, 2020) could also influence immune cell responsiveness to LPS stimulation.
We observed large inter-individual differences in magnitude of LPS-stimulated pro-inflammatory cytokine levels consistent with prior literature, including for IL-6 and TNF-α (Copeland et al., 2005; Wurfel et al., 2005), indicating that cells from different individuals vary widely in their ability to produce cytokines (high and low responders). We also noted modest associations between stimulated IL-6 and TNF-α and baseline leukocyte cell counts, suggesting that 15–21% of the inter-individual variability in LPS responses could in part be attributed to whole blood cell type composition. This leaves >80% of the variance in cytokine release to be explained by other factors within these cells. While inter-individual variability in immune responses have previously been attributed to polymorphisms in immune responsive genes (Li et al., 2016), sex-hormones (Taneja, 2018), age, or experimental factors such as LPS exposure time and dosage, our findings add mitochondrial respiratory capacity to the list of potential immunomodulators in humans.
In LPS sensitivity assays that examined EC50 of LPS-induced IL-6 levels, we observed only a minor effect of mitochondrial function on leukocyte sensitivity to LPS. Mitochondrial inhibitors caused a small effect size shift towards a less sensitive, or more tolerant state (i.e., high EC50). While mitochondria have been implicated as modulators as well as targets of LPS-induced inflammation in isolated macrophages (Van den Bossche et al., 2016), our data illustrates a potential link between mitochondrial respiratory capacity and LPS sensitivity in human blood leukocytes. We speculate that changes in mitochondrial function could contribute to a small fraction glucocorticoid resistance in humans and animals chronically exposed to stress (Niraula et al., 2018; Walsh et al., 2018), but testing this hypothesis requires further work.
Multiple studies have emphasized the critical balance between pro- and anti-inflammatory cytokines and the relevance of inter-cytokine interactions in health and disease (Cicchese et al., 2018). Here we asked if mitochondrial respiration affected this pro/anti-inflammatory balance and found that inhibition of respiratory chain function in blood changed not only the overall inflammatory cytokine levels, but also the ratios of pro- and anti-inflammatory cytokines. Pro/anti-inflammatory cytokine ratios in humans and mice are indicators of susceptibility to infection and disease risk (Chae, 2018; Andres-Rodriguez et al., 2019). Interestingly, inhibiting Complex IV significantly elevated the pro/anti-inflammatory cytokine ratio, specifically the IL-6/IL-10 and IL-1β/IL-10 ratio indicating an overactive inflammatory response. This effect could possibly result from a disrupted balance of Th1(IL-6,IL-1β)/Th2(IL-10) cell types since T cells rely on mitochondria for energy and metabolic support during inflammation (Dumitru et al., 2018). Why this effect was specifically induced by inhibition of Complex IV remains unclear but may relate to the role of Complex IV as the ultimate site of oxygen consumption within the mitochondrion. Moreover, Complex I inhibition elevated both IL-1β and IFN-γ levels, which were also more strongly correlated to each other upon Complex I inhibition, indicating that both cytokines may be under regulation of a common signaling factor from mitochondria. Combined, these results demonstrate that alterations in mitochondrial energetics in general and especially perturbation of Complex IV, modifies LPS-related cytokine signatures in blood leukocytes.
We reported previously that acute stimulation of fibroblasts with DEX is sufficient to extrude mtDNA into the cytoplasm (Trumpff et al., 2019b) along with evidence that a subtype of GR (gamma) resides in mitochondria and regulates ATP production (Morgan et al., 2016). Since circulating levels of GCs in acute and chronic stress can be modulated by the hypothalamic-pituitary-adrenal (HPA) axis via GR activation (Perrin et al., 2019), we explored the possibility that inhibiting mitochondrial respiration would alter GC sensitivity and its ability to suppress the IL-6 response. Notably, GCs significantly suppress LPS-stimulated production of cytokines by upregulating anti-inflammatory mediators like IL-10 (Mann et al., 2019). Accordingly, DEX strongly downregulated multiple pro-inflammatory cytokines. Interestingly, inhibiting mitochondrial respiration in addition to DEX further suppressed cytokine release – in other words, inhibiting mitochondrial respiration potentiated the immunosuppressive properties of DEX. These results suggest that GC signaling in immune cells may involve mitochondria either directly, or indirectly through some aspects of cellular energetics or metabolic signaling.
We also tested whether inherent mitochondrial functional capacity of leukocytes can explain inter-individual differences in LPS-mediated cytokine responses in people. In Cohort 1 experiments with inhibitors, we find that inhibition of respiratory chain function decreased the release of several cytokines, particularly TNF-α and IL-10, whereas IL-6 was modestly increased by Complex IV inhibition. In Cohort 2, we found that higher intrinsic COX activity in isolated PBMCs was correlated with higher stimulated IL-6 levels, and to a lesser extent TNF-α. These results are in part contrary to our first hypothesis that mitochondrial dysfunction would increase cytokine release and suggest a more nuanced view of mitochondrial signaling in specific cytokine pathways. Complementary to our finding in Cohort 2, compared to patients with robust TNF-α response to LPS, immunoparalyzed pediatric sepsis patients with LPS-stimulated TNF-α levels ≤200 pg/mL also had lower mitochondrial respiratory capacity in PBMCs (Weiss et al., 2019). Generally, our combined results indicate that pharmacological perturbations of mitochondria respiratory function influence cytokine responses (Cohort 1), and that baseline measures of PBMCs mitochondrial respiratory chain capacity are associated with cytokine release, support the conclusion that a fraction of inter-individual variation in cytokine response may be influenced by mitochondrial behavior within human leukocytes.
One interpretation of these findings is that higher mitochondrial energy production capacity at baseline, particularly Complex IV activity, may enable more vigorous acute cytokine production in healthy adults. In summary, associations between mitochondrial measures and cytokine responses are pertinent to understand immune responses to acute challenges like LPS. This study calls for both replication and validation in large and diverse cohorts as well as in primary immune cell-subtypes.
4.1. Limitations
This study specifically included healthy individuals to examine the role of mitochondrial respiration in LPS-induced inflammation in an ex vivo whole blood model. Although the within-subject experimental design allowed us to test individual-specific effects of mitochondrial respiratory chain activity despite inter-individual differences in cytokine responses, there are several limitations of the approach. First, whole blood arguably better reflects the physiological cellular mixture in human circulation than isolated and purified cell preparations, but individuals show different proportions of immune cell types that likely differentially produce specific cytokines. Thus, studies in isolated cell types may produce slightly different results and reveal even more profound modulatory effects of mitochondrial respiration on specific cytokines. Second, the sample size in both cohorts is relatively small and precludes definite conclusions about inter-individual and sex-related differences in cytokine behavior. Similarly, while various studies have reported sexual dimorphism in mitochondrial function (Ventura-Clapier et al., 2017), in psychological stress driven endocrine-immune function (Bekhbat and Neigh, 2018; Rainville et al., 2018), as well as in LPS-induced systemic inflammation (Marsland et al., 2017), the sex-stratified correlations between mitochondrial function and cytokine response in Cohort 2 should be interpreted with caution. Studies with larger sample size are needed to establish whether functional differences between the mitochondria of women and men contribute to sex differences in stress-immune signaling.
5. Conclusion
Collectively, our results demonstrate that experimental manipulation of mitochondrial respiratory chain function, particularly Complex IV, mildly exaggerates LPS-induced IL-6 levels, markedly reduces TNF-α levels, and more generally alters multi-cytokine signatures. We also show that mitochondrial bioenergetics moderately influence sensitivity to GC-mediated IL-6 suppression, providing additional evidence that mitochondria modulate different aspects of immune responses, and possibly how immune cells are influenced by endocrine factors. This study in human blood extends in vitro work demonstrating immunomodulation by mitochondrial energetics and provides proof-of-concept data that intrinsic inter-individual variation in mitochondrial phenotypes contribute to differences in immune responses in acute inflammation.
Funding source
This work was supported by the Wharton Fund to RPS and MP, and NIH grants GM119793 to MP, MH119336 to MP, ALM and BK.
Author contributions
KRK and MP designed the study with input from NR. CT and KRK prepared the IRB protocol. MM and JT collected and processed the samples. KRK performed the stimulation experiments, and MM performed the MHI experiments. VL and RPS provided additional samples and participant information. KRK performed analyzes. GS, ALM and BAK provided critical comments on the manuscript. KRK, CT and MP drafted the manuscript. All authors contributed to the final version of the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We thank the participants who contributed to the study, Sloan Krakovsky and Carlos Acosta for their help with sample collection, as well as Carla Basualto and Atif Towheed for useful discussions about this work. We acknowledge the assistance of the Columbia Stem Cell Initiative (CSCI) and Columbia Center for Advanced Lab medicine (CALM).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100080.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Al Amir Dache Z. Blood contains circulating cell-free respiratory competent mitochondria. Faseb. J. 2020;34(3):3616–3630. doi: 10.1096/fj.201901917RR. [DOI] [PubMed] [Google Scholar]
- Alm J.J. Transient 100 nM dexamethasone treatment reduces inter- and intraindividual variations in osteoblastic differentiation of bone marrow-derived human mesenchymal stem cells. Tissue Eng. C Methods. 2012;18(9):658–666. doi: 10.1089/ten.TEC.2011.0675. [DOI] [PubMed] [Google Scholar]
- Andres-Rodriguez L. Immune-inflammatory pathways and clinical changes in fibromyalgia patients treated with Mindfulness-Based Stress Reduction (MBSR): a randomized, controlled clinical trial. Brain Behav. Immun. 2019;80:109–119. doi: 10.1016/j.bbi.2019.02.030. [DOI] [PubMed] [Google Scholar]
- Arango Duque G., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M., Neigh G.N. Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety. Brain Behav. Immun. 2018;67:1–12. doi: 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17(3):684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Boeck C. The association between cortisol, oxytocin, and immune cell mitochondrial oxygen consumption in postpartum women with childhood maltreatment. Psychoneuroendocrinology. 2018;96:69–77. doi: 10.1016/j.psyneuen.2018.05.040. [DOI] [PubMed] [Google Scholar]
- Boyapati R.K. Advances in the understanding of mitochondrial DNA as a pathogenic factor in inflammatory diseases. F1000Research. 2017;vol. 6:169. doi: 10.12688/f1000research.10397.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breda C.N.S. Mitochondria as central hub of the immune system. Redox Biol. 2019;26:101255. doi: 10.1016/j.redox.2019.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae B.S. Pretreatment of low-dose and super-low-dose LPS on the production of in vitro LPS-induced inflammatory mediators. Toxicol. Res. 2018;34(1):65–73. doi: 10.5487/TR.2018.34.1.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel N.S. Evolution of mitochondria as signaling organelles. Cell Metabol. 2015;22(2):204–206. doi: 10.1016/j.cmet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Chapman N.M., Boothby M.R., Chi H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 2020;20:55–70. doi: 10.1038/s41577-019-0203-y. [DOI] [PubMed] [Google Scholar]
- Chitnis T., Weiner H.L. CNS inflammation and neurodegeneration. J. Clin. Invest. 2017;127(10):3577–3587. doi: 10.1172/JCI90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchese J.M. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018;285(1):147–167. doi: 10.1111/imr.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 2nd. Hillsdale, N.J. : L. Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral sciences. [Google Scholar]
- Copeland S. Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 2005;12(1):60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsgaard C.T. Whole-blood culture is a valid low-cost method to measure monocytic cytokines - a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J. Immunol. Methods. 2009;340(2):95–101. doi: 10.1016/j.jim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Du J. Dynamic regulation of mitochondrial function by glucocorticoids. Proc. Natl. Acad. Sci. U. S. A. 2009;106(9):3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy D. Functional analysis via standardized whole-blood stimulation systems defines the boundaries of a healthy immune response to complex stimuli. Immunity. 2014;40(3):436–450. doi: 10.1016/j.immuni.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Dumitru C., Kabat A.M., Maloy K.J. Metabolic adaptations of CD4(+) T cells in inflammatory disease. Front. Immunol. 2018;9:540. doi: 10.3389/fimmu.2018.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehinger J.K. Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat. Commun. 2016;7:12317. doi: 10.1038/ncomms12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom S. Cause of death in children with mitochondrial diseases. Pediatr. Neurol. 2017;66:82–88. doi: 10.1016/j.pediatrneurol.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.J., Hammerman P.S., Thompson C.B. Fuel feeds function: energy metabolism and the T-cell response. Nat. Rev. Immunol. 2005;5(11):844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Garaude J. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 2016;17(9):1037–1045. doi: 10.1038/ni.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann D.M. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual. Life Res. 1998;7(4):301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- Huang S.C.-C. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014;15:846. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang D.H. Impairment of mitochondrial respiration following ex vivo cyanide exposure in peripheral blood mononuclear cells. Clin. Toxicol. 2016;54(4):303–307. doi: 10.3109/15563650.2016.1139712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M.R., Irish J.C., Devins G.M. Development and pilot testing of a psychoeducational intervention for oral cancer patients. Psycho Oncol. 2004;13(9):642–653. doi: 10.1002/pon.767. [DOI] [PubMed] [Google Scholar]
- Koshiba T. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci. Signal. 2011;4(158):ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- Li Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 2006;38(5–6):283–291. doi: 10.1007/s10863-006-9052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. A functional genomics approach to understand variation in cytokine production in humans. Cell. 2016;167(4):1099–1110 e14. doi: 10.1016/j.cell.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Lindqvist D. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Transl. Psychiatry. 2016;6(12):e971. doi: 10.1038/tp.2016.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology. 2018;43(7):1557–1564. doi: 10.1038/s41386-017-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann E.H. High-dose IL-2 skews a glucocorticoid-driven IL-17(+)IL-10(+) memory CD4(+) T cell response towards a single IL-10-producing phenotype. J. Immunol. 2019;202(3):684–693. doi: 10.4049/jimmunol.1800697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland A.L. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav. Immun. 2017;64:208–219. doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. Mitochondria: an organelle of bacterial origin controlling inflammation. Front. Immunol. 2018;9:536. doi: 10.3389/fimmu.2018.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.J. Glucocorticoid receptor isoforms direct distinct mitochondrial programs to regulate ATP production. Sci. Rep. 2016;6:26419. doi: 10.1038/srep26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher C.E., Danoff-Burg S., Brunker B. Post-traumatic growth and psychosocial adjustment of daughters of breast cancer survivors. Oncol. Nurs. Forum. 2006;33(3):543–551. doi: 10.1188/06.ONF.543-551. [DOI] [PubMed] [Google Scholar]
- Niraula A. Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. J. Neurosci. 2018;38(9):2328–2340. doi: 10.1523/JNEUROSCI.2568-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panga V., Raghunathan S. A cytokine protein-protein interaction network for identifying key molecules in rheumatoid arthritis. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0199530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin A.J. Glucocorticoid resistance: is it a requisite for increased cytokine production in depression? A systematic review and meta-analysis. Front. Psychiatr. 2019;10:423. doi: 10.3389/fpsyt.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecka B. Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc. Natl. Acad. Sci. U. S. A. 2018;115(3):E488–E497. doi: 10.1073/pnas.1714765115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M. A mitochondrial health index sensitive to mood and caregiving stress. Biol. Psychiatr. 2018;84(1):9–17. doi: 10.1016/j.biopsych.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poll T. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017;17(7):407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- Psarra A.M. Glucocorticoid receptor isoforms in human hepatocarcinoma HepG2 and SaOS-2 osteosarcoma cells: presence of glucocorticoid receptor alpha in mitochondria and of glucocorticoid receptor beta in nucleoli. Int. J. Biochem. Cell Biol. 2005;37(12):2544–2558. doi: 10.1016/j.biocel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- van Raam B.J. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS One. 2008;3(4) doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville J.R., Tsyglakova M., Hodes G.E. Deciphering sex differences in the immune system and depression. Front. Neuroendocrinol. 2018;50:67–90. doi: 10.1016/j.yfrne.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Rea I.M. Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med. 2014;76(3):181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Sloan R.P., McKinley P.S., Bartels M. Aerobic exercise training and inducible inflammation- results of a randomized controlled trial in healthy, young adults. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.010201. e010201. PMID: 30371169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X. Existence of circulating mitochondria in human and animal peripheral blood. Int. J. Mol. Sci. 2020;21(6):2122. doi: 10.3390/ijms21062122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierenburg E.A.J. Stability of individual LPS-induced ex vivo cytokine release in a whole blood assay over a five-year interval. J. Immunol. Methods. 2018;460:119–124. doi: 10.1016/j.jim.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Strahler J., Rohleder N., Wolf J.M. Acute psychosocial stress induces differential short-term changes in catecholamine sensitivity of stimulated inflammatory cytokine production. Brain Behav. Immun. 2015;43:139–148. doi: 10.1016/j.bbi.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Taneja V. Sex hormones determine immune response. Front. Immunol. 2018;9:1931. doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill G.M. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasenko T.N. Cytochrome c oxidase activity is a metabolic checkpoint that regulates cell fate decisions during T cell activation and differentiation. Cell Metabol. 2017;25(6):1254–1268 e7. doi: 10.1016/j.cmet.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpff C. Predictors of ccf-mtDNA reactivity to acute psychological stress identified using machine learning classifiers: a proof-of-concept. Psychoneuroendocrinology. 2019;107:82–92. doi: 10.1016/j.psyneuen.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpff C. Acute psychological stress increases serum circulating cell-free mitochondrial DNA. Psychoneuroendocrinology. 2019;106:268–276. doi: 10.1016/j.psyneuen.2019.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R. Mitochondria: a central target for sex differences in pathologies. Clin. Sci. (Lond.) 2017;131(9):803–822. doi: 10.1042/CS20160485. [DOI] [PubMed] [Google Scholar]
- Walsh C.P. Development of glucocorticoid resistance over one year among mothers of children newly diagnosed with cancer. Brain Behav. Immun. 2018;69:364–373. doi: 10.1016/j.bbi.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg S.E., Sena L.A., Chandel N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42(3):406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.L., Zhang D., Bush J. Mitochondrial Dysfunction Is Associated with an Immune Paralysis Phenotype in Pediatric Sepsis. Shock. 2019 Nov 20 doi: 10.1097/SHK.0000000000001486. published online ahead of print. PMID: 31764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A.P., Shadel G.S., Ghosh S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth A.J. Inhibition of neuronal cell mitochondrial complex I with rotenone increases lipid beta-oxidation, supporting acetyl-coenzyme A levels. J. Biol. Chem. 2014;289(39):26895–26903. doi: 10.1074/jbc.M114.591354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurfel M.M. Identification of high and low responders to lipopolysaccharide in normal subjects: an unbiased approach to identify modulators of innate immunity. J. Immunol. 2005;175(4):2570–2578. doi: 10.4049/jimmunol.175.4.2570. [DOI] [PubMed] [Google Scholar]
- Xia J., Wishart D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinf. 2016;55:14 10 1–14 10 91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- Zhao Y. COX5B regulates MAVS-mediated antiviral signaling through interaction with ATG5 and repressing ROS production. PLoS Pathog. 2012;8(12) doi: 10.1371/journal.ppat.1003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.