Abstract
Ex vivo generation of red blood cells (RBCs) from hematopoietic stem cells (HSCs) used for blood transfusion represents one of the focuses in current regenerative medicine. However, massive production of HSCs-based RBCs requires a significant quantity of erythropoietic growth factors, making manufacturing at large scale cost prohibitive. Plant cell culture is proposed to be a promising bioproduction platform for functional human proteins in a safe and cost-efficient manner. This study exploited a proprietary technology, named HypGP engineering technology, for high-yield production of one of the key erythropoietic growth factors--stem cell factor (SCF)--in plant cell culture. Specifically, a designer hydroxyproline (Hyp)-O-glycosylated peptide (HypGP) comprised of 20 tandem repeats of the “Ser-Pro” motif, or (SP)20, was engineered at either the N-terminus or C-terminus of SCF in tobacco BY-2 cells. The (SP)20 tag dramatically increased the secreted yields of SCF up to 2.5 μg/ml. The (SP)20-tagged SCF showed bioactivity in promoting the proliferation of the TF-1 cell line, although the SCF-(SP)20 was 8.4-fold more potent than the (SP)20-SCF. Both the (SP)20-SCF and SCF-(SP)20 exhibited desired function in stimulating the expansion and differentiation of human umbilical cord blood CD34+ cells towards RBCs.
Keywords: Plant cell culture, stem cell factor, hydroxyproline-O-glycosylation, hematopoietic stem cells, proliferation
Graphical Abstract
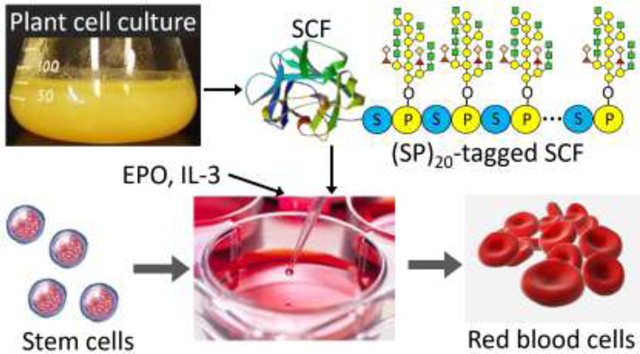
Introduction
A timely and sufficient supply of human blood is critical in both military and civilian healthcare systems [1–3]. Ex vivo generation of clinically available red blood cells (RBCs), or erythrocytes from pluripotent human hematopoietic stem cells (HSCs), is a promising approach for overcoming the limitations associated with the current use of a donor’s blood [1]. HSCs-derived RBCs have the potential to be pathogen free, universally matched to all recipients, and be in abundant supply [2]. However, generating RBCs from HSCs in culture requires massive quantities of erythropoietic growth factors (eryGFs) such as stem cell factor, erythropoietin (EPO), interleukin-3 (IL-3), and insulin-like growth factor-1 (IGF-1), etc., with their activity and purity being critical to successful HSCs expansion and differentiation [4–6]. It has been recognized that the majority of the cost to produce RBCs ex vivo derives from the culture media that requires substantial fortification with eryGFs (referred to as “liquid gold” due to their high value). Although bacteria, yeast, and mammalian cell cultures have been successfully utilized for the production of many human growth factors and cytokines [1, 7], each of these systems has limitations that are associated with safety, cost, scalability, and posttranslational modifications, thus indicating the need for alternative production systems.
We aim to develop an efficient plant cell-based bioproduction platform for high-quality (i.e. animal-free and endotoxin-free) eryGFs that facilitates massive production of stem cell-derived RBCs for clinical applications. Plant cells have emerged as a powerful bioproduction platform for fully functional human proteins in a cost-efficient manner at a large scale [8–10]. From a safety standpoint, plant cell-derived recombinant proteins are endotoxin-deficient and totally animal-free. Thus, the risk of endotoxin and pathogen contamination is minimal [11, 12]. Therefore, human growth factors and cytokines produced from plant cells are particularly well suited for stem cell applications, as both endotoxins and pathogens are known to affect different cellular processes such as cell proliferation and differentiation leading to biased results [1]. However, low protein productivity is still a bottleneck towards the commercialization of the plant cell-based production platform. Furthermore, protein yields are also diminished when the protein of interest is intracellular, which dramatically increases the cost of protein purification.
This technical challenge could be addressed by expressing a recombinant protein fused with a hydroxyproline (Hyp)-O-glycosylated peptide (HypGP) tag, such as one with repeats of a “Ser-Pro” motif. This designer HypGP tag has been demonstrated to function as a molecular carrier in boosting the secretion of fused proteins from cultured plant cells and stabilizing the proteins, which dramatically increases the secreted protein yields and simplifies the protein purification process. High secreted yields of a green fluorescence protein and some therapeutic proteins were earlier achieved with the HypGP engineering in BY-2 cell and hairy root cultures [13–17]. In addition, the highly glycosylated HypGP tag could increase the solubility of fused proteins, which usually improves protein stability and bioavailability [15, 16].
In this research, one of the key eryGFs essential for the ex vivo expansion and differentiation of HSCs toward RBCs, human stem cell factor (SCF), was tested to express in tobacco BY-2 cells with the HypGP engineering technology. SCF is produced by stromal cells in fetal livers and adult bone marrow [18]. It binds to the receptor tyrosine kinase c-KIT (CD117), which exerts its activity in the early stages of hematopoiesis. It also works synergistically with other growth factors, such as EPO and IL-3, to promote the expansion and differentiation of primitive HSCs and multi-potent tag comprised of 20 tandem repeats of the “Ser-Pro” motif or (SP)20 for short. It was found the (SP)20-tagged SCF was dramatically secreted into the BY-2 cell culture media while the recombinant (SP)20-tagged SCF exerted desired bioactivity in promoting the proliferation of human erythroleukemic TF-1 cell and stimulating the expansion and differentiation of HSCs toward RBCs.
2. Materials and Methods
2.1. Construction of expression vectors and BY-2 cell transformation
The genes encoding SCF and (SP)20-tagged SCF, each appended with a C-terminal 6×His tag and with its DNA sequence being optimized for tobacco expression (Fig. 1), were synthesized by GeneArt® Gene Synthesis service (ThermoFisher Scientific, Waltham, MA). A native sequence SCFnat gene was also synthesized. The synthetic gene constructs were subcloned into the plant expression vector pBI121-SStob-EGFP [20, 21] at the XmaI and BsrGI sites to generate pBI121-SStob-SCF, pBI121-SStob-SCFnat, pBI121-SStob-SCF-(SP)20 and pBI121-SStob-(SP)20-SCF, respectively, and then transferred into Agrobacterium tumefaciens LBA4404 by the freeze-thaw method. Here, SStob refers to the tobacco extensin signal sequence [22]. These gene constructs were stably transformed into tobacco Bright Yellow-2 (BY-2) cells by using the Agrobacterium-mediated method as previously described [15, 21]. Transformed BY-2 cell colonies were selected by kanamycin resistance.
Figure 1. Schematic of the gene constructions in pBI121 expression vector.
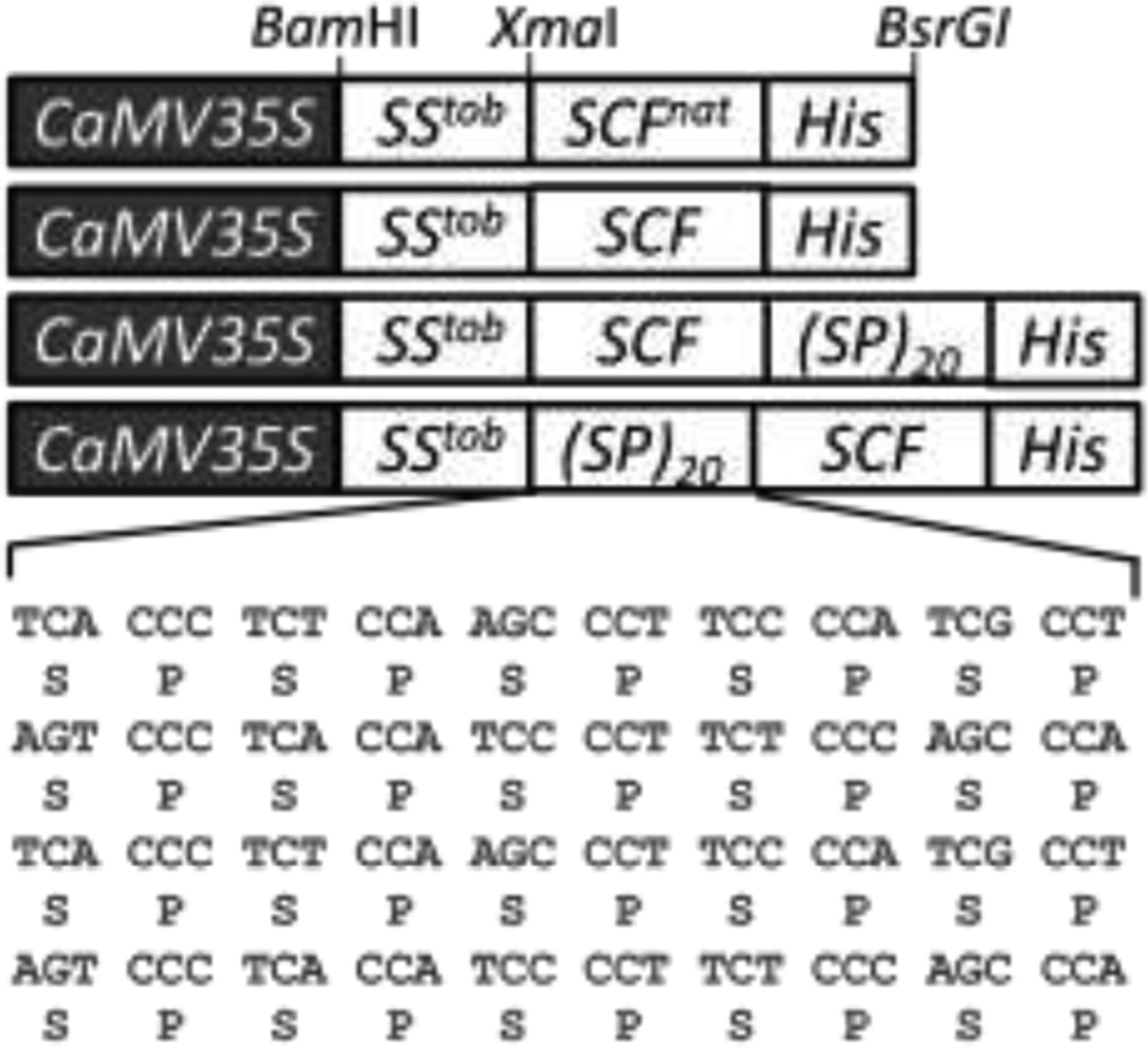
(SP)20: twenty tandem repeats of a “Ser-Pro” motif; CaMV35S: 35S cauliflower mosaic virus promoter; SStob: tobacco extensin signal sequence; SCFnat: native gene sequence of SCF; His: 6×His tag.
2.2. BY-2 cell culture and determination of cell biomass
Transgenic BY-2 cells were cultured in Schenk & Hildebrandt (SH) medium [23] containing 0.4 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 0.1 mg/L kinetin, and 34 g/L sucrose. Flasks (250 mL) containing 80 mL medium were placed on a gyratory shaker rotating at 95 rpm under continuous illumination at 25°C. Subcultures were carried out every 7 to 8 days with a 5% (v/v) inoculum density. Cultured cells were harvested by vacuum filtration before determining the fresh weight (FW). The cells were then dried in an oven at 75 °C for 48 hrs to measure the dry weight (DW).
2.3. Transient protein expression in Nicotiana benthamiana by agroinfiltration
Transient expression of SCF and SCFnat in Nicotiana benthamiana was conducted as previously described. Briefly, selected transformed A. tumefaciens LBA4404 colonies were grown at 28 °C in YEP medium (10 g/L yeast extract, 10g/L bacto-peptone, and 5g/L NaCl) containing 50 μg/mL kanamycin and 40 μg/mL streptomycin until the OD600 reached 0.8 to 1.0. Then, the Agrobacterium culture was diluted to OD600 = 0.4 with the induction media consisting of 10 mM MES (pH 5.6). The final infiltration medium was made by adding 600 μl H2O, 30 μl Silwet L-77, 30 μl Triton X-100, and 30 μl Tween 20 into every 500 mL of the diluted Agrobacterium culture. The infiltration was conducted by inverting 5-week-old N. benthamiana plants into the infiltration medium in a vacuum chamber. The vacuum pressure was brought to 25 in. Hg and then quickly released to zero. Infiltrated plants were watered and maintained in a designated plant growth chamber at 25 °C with light and 90% humidity until harvest.
2.4. Protein purification from culture media
BY-2 cell culture media were harvested after 8–10 days of cell growth and supplemented with ammonium sulfate to a final concentration of 2.0 M. Recombinant proteins were first enriched by hydrophobic interaction chromatography (HIC) on an ÄKTA Start system (GE Healthcare, Chicago, IL). The HIC column packed with Phenyl Sepharose™ High Performance beads (GE Healthcare, Chicago, IL) was equilibrated with 2M ammonium sulfate before the medium samples were loaded. The column was then washed with 1M ammonium sulfate, and target proteins (SCF or (SP)20-tagged SCF) were finally eluted with 1xPBS. The HIC enriched protein samples were then purified with nickel affinity chromatography. Briefly, the protein samples calibrated to the equilibration buffer containing 20 mM sodium phosphate, 0.5 M NaCl, and 20 mM imidazole (pH7.4) were incubated with Ni-NTA agarose resin (Qiagen, Germantown, MD) at 4°C overnight with gentle agitation. The incubated resin was washed three times with 2 column volumes of the equilibration buffer each time before the SCF proteins were eluted with elution buffer containing 20 mM sodium phosphate, 0.5 M NaCl, and 500 mM imidazole (pH 7.4). The eluted protein samples were further concentrated using the10,000 MWCO PES centrifugal concentrator (Sartorius, Germany). The purified samples were sterilized using a 0.22 μm Ultrafree®-MC filter (Merck Millipore, Burlington, MA) prior to cell-based functional assays.
2.5. Dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot assay
The harvested culture media was used directly for the assay of secreted proteins. For intracellular protein assays, frozen BY-2 cells or infiltrated plant leaves (~1.0 g) were ground by mortar and pestle in liquid N2 and then supplemented with SDS extraction buffer (150 mM Tris-HCl, pH 6.8, 30% glycerol, 6% SDS, 5 mM EDTA) at a ratio of 1:2 (w/v). Samples were then centrifuged at 13,000 ×g for 15 min, and the supernatant was assayed.
Protein samples and SCF standard (R&D Systems, Minneapolis, MN)) were resolved on 10% Tris-HCl gels (Bio-Rad, Hercules, CA). Gels were either stained with Coomassie Brilliant Blue R250 or for Western blot assay. For the latter, protein bands were electro-transferred onto a 0.2 μm nitrocellulose membrane. Immunoblot detection of SCF products was carried out using a rabbit-anti-SCF polyclonal antibody (ThermoFisher Scientific Inc., Waltham, MA) as the primary antibody and a goat–anti rabbit IgG (H + L)-peroxidase conjugated (Jackson Immuno Research labs, West Grove, PA) as the secondary antibody. Protein blots were then detected using the SuperSignal® West Pico Chemiluminescent Substrate (ThermoFisher Scientific Inc., Waltham, MA) according to manufacturers’ procedures. For the dot blot assay, 1.5 μL of samples, after being heated at 95 °C for 10 min, were blotted onto nitrocellulose membranes, and the SCF proteins were detected using the same procedure as the Western blot.
2.6. Quantification of recombinant SCF proteins
Recombinant SCF proteins in culture media were quantified by densitometry based on Western blot [14, 24]. Briefly, medium samples and SCF standard were electrophoresed on the same SDS-PAGE gel. The blot images were captured on the Li-Cor Odyssey Fc imaging system (Li-Cor Biosciences, Lincoln, NE), and the target protein products were quantified with the Li-Cor’s Image Studio™ Software. This imaging system provides a broad, linear, and dynamic range to accurately detect both strong and weak bands on the same Western blot. The purified proteins were quantified by the bicinchoninic acid (BCA) assay using the Micro BCA™ Protein Assay kit (ThermoFisher Scientific Inc., Rockford, IL).
2.7. TF-1 cell proliferation assay
A human erythroleukemia cell line TF-1 obtained from the American Type Culture Collection (ATCC CRL-2003, Manassas, VA) was grown in RPMI 1640 media containing 10% fetal bovine serum (FBS) and 2 ng/mL granulocyte-macrophage colony stimulating factor (GMCSF). The biological activity of the purified (SP)20-SCF and SCF-(SP)20 was determined by measuring their dose-dependent effects on TF-1 cell proliferation. Prior to the proliferation assay, actively growing TF-1 cells were washed twice with PBS to remove all traces of the growth factor. Then, in 96-well plates, TF-1 cells (1×104 in 100 μl medium) in triplicate were incubated at 37 °C and 5% CO2 with increasing concentrations of (SP)20-tagged SCF or SCF standard (10−3 to 102 ng/mL) in the assay media (culture media without GM-CSF). After 48 hrs of incubation, the cell viability in the 96-well microplate was measured by the Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Rockville, MD) following the manufacturer’s instructions. The absorbance of each sample was measured at a wavelength of 450 nm with the Synergy HT microplate reader (Biotek, Winooski, VT). The cells cultured in medium with 2 ng/mL GM-CSF and in the absence of SCF were regarded as a positive control and negative control (blank), respectively. The cell viabilities were evaluated by the formula: (Asample-Ablank)/(Acontrol-Ablank)×100%. The potency of the (SP)20-tagged SCF was measured in terms of EC50, the effective concentration of an individual growth factor to cause a half-maximal increase in cell proliferation.
2.8. CD34+ cell expansion and differentiation
Primary human umbilical cord blood cells with the hematopoietic progenitor cell antigen CD34 (CD34+ cells) were purchased from STEMCELL™ Technologies (Vancouver, Canada). The stem cells were expanded once in StemSpan™ Serum-Free Expansion Medium II (SFEM II) supplemented with StemSpan™ CC100 solution (STEMCELL™ Technologies) containing both early- and late-acting recombinant human cytokines. The expanded CD34+ cells were cryopreserved in CryoStor®CS10 (BioLife Solutions, Bothell, WA). For testing the expansion and differentiation of CD34+ cells stimulated by the plant cell-derived SCF, the protocol developed by Kim et al. (2014) [25] was followed. Briefly, CD34+ cells (2×104 cells/ml) were incubated in the SFEM II medium supplemented with 6 IU/ml EPO (STEMCELL™ Technologies), 10 ng/mL IL-3 (STEMCELL™ Technologies), and 100 ng/ml SCF equivalent of SCF-(SP)20 or (SP)20-SCF at 37 °C with 5% CO2 for 7 days. The cells were then incubated in SFEM II supplemented with 3 IU/ml EPO, 10 ng/mL IL-3, and 50 ng/ml SCF equivalent of SCF-(SP)20 or (SP)20-SCF for another 7 days. As a comparison, the positive group was treated with all three commercial eryGFs (SCF, EPO and IL-3), and the negative group was treated with the commercial EPO and IL-3 lacking the SCF. The differentiation of the cultured CD34+ cells was analyzed by cell staining, flow cytometry, and hemoglobin development.
2.9. Cell staining and microscopy
The modified Giemsa stain solution (Fluka® Sigma, St Louis, MO) was used for the staining of the differentiated CD34+ cells. The bright-field microscope, Nikon TE2000u Inverted Microscope (Tokyo, Japan), was used to examine the cell morphology under a 60× objective. For counting cell numbers, the cultured cells were stained with Trypan Blue Solution (CORNING cellgro, Manassas, VA), and loaded onto Countess™ cell counting chamber slides (Invitrogen, Eugene, OR) and read using the Countess™ automated cell counter (Invitrogen, Eugene, OR).
2.10. Hemoglobin content assay
The Drabkin’s reagent supplemented with the 30% Brij® L23 solution (Sigma, St Louis, MO) was used to examine hemoglobin development during the differentiation of the CD34+ cells. To prepare the working solution, one vial of the Drabkin’s reagent was reconstituted with 1 L of water, followed by the addition of the 30% Brij® L23 solution (0.5 ml). For the hemoglobin content assay, CD34+ cells incubated in the expansion and differentiation were collected every other day starting from day 5. Each of the samples was added with 100 μl of the working solution in a 96-well plate. The mixture was incubated at room temperature for 15 min. Then, the absorbance was measured at a wavelength of 540 nm with the Synergy HT microplate reader (BioTek, Winooski, VT).
2.11. Flow cytometry assay
The flow cytometer BD FACSAria™ with the BD FACSDiva software was used to examine the differentiation of the CD34+ cells. About 10,000 events were acquired for each sample. The two antibodies used for immunostaining were fluorescein isothiocyanate (FITC) labeled antibody against CD34 and allophycocyanin (APC)- labeled antibody against the transferrin receptor-1 (CD71) (BioLegend, San Diego, CA). Briefly, harvested cells were first washed with 1X PBS with 3% FBS and then incubated with the two antibodies in 1X PBS with 3% FBS for 30 min on ice in the dark. After incubation, the cells were washed 3 times with 1X PBS and finally resuspended in 300 μL 1X PBS for flow cytometric analysis.
3. Results
3.1. Expression of recombinant SCF in E. coli and N. benthamiana
Before the stable expression of the SCF and (SP)20-tagged SCF in tobacco BY-2 cells, transient expression of SCF in N. benthamiana was conducted to determine the glycosylation status of the recombinant protein and the suitable gene sequence (native vs. codon optimized) to be used in this research. The native SCF gene sequence was expressed in both E. coli and tobacco (N. benthamiana) (Fig. 2a). The non-glycosylated SCF produced by E. coli migrated as a band of 18.5 kDa, which is consistent with the molecular size of SCF calculated based on its peptide sequence. Plant expressed SCF protein showed a band of ~2 kDa larger in molecular size than the E. coli-produced counterpart. This indicated that the SCF protein produced in tobacco plants is likely a glycosylated form of SCF (~21 kDa). This was confirmed by the treatment of the SCF extract with PNGase F, which showed the shift of the SCF band from 21 kDa to 18.5 kDa after the enzyme treatment (Fig. 2b). The SCF expression in N. benthamiana between using the codon optimized gene sequence and the native sequence was then compared. The Western blot analysis showed that the codon optimized SCF gene produced 25 to 30-fold more protein than the native SCF gene (Fig. 2c). The codon optimized SCF gene sequence was used in the following studies to produce the SCF and SCF fusion proteins in BY-2 cells.
Figure 2. Detection of recombinant SCF expression by an anti-SCF Western blot.
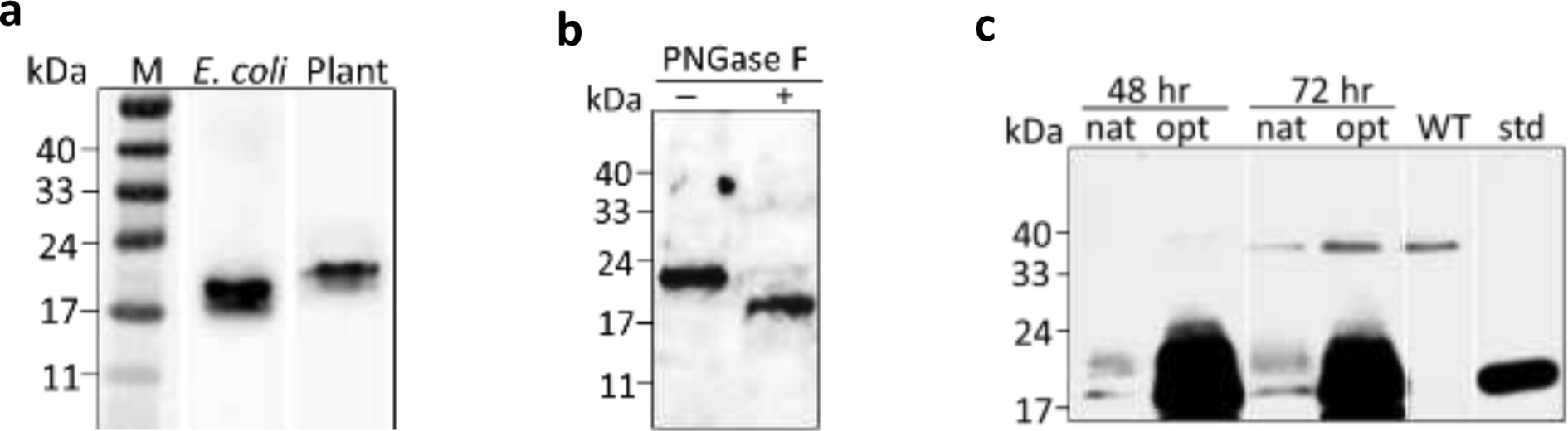
(a) SCF (native gene sequence) expressed in E. coli and tobacco plants (transient expression in N. benthamiana). pET28b(+) expression vector with a T7 promoter was used for the E. coli expression as previously described [14]; (b) Detection of the glycosylation of the SCF expressed in N. benthamiana. SCF crude extract was incubated with (+) and without (−) PNGase F. (New England Biolab, MA) at 37°C for 1 hour before the assay by Western blot; (c) Comparison of the SCF expression in N. benthamiana between using the native gene sequence (nat) and the codon optimized SCF gene sequence (opt). The tobacco leaves collected 48 hr and 72 hr post-infiltration were analyzed. WT: wild-type tobacco; std: SCF standard (30 ng).
3.2. Expression of recombinant SCF proteins in BY-2 cells
Following co-culture of the BY-2 cells with Agrobacterium, numerous positive colonies were obtained on the selection medium containing 100 mg/L kanamycin. It is crucial to screen for elite cell lines that produce high-yield target proteins. More than 50 individual calli for each gene construction were transferred into liquid medium to generate cell suspension cultures. Anti-SCF dot blot was then conducted to pre-screen for the high-secretion cell lines. Four lines of SCF-(SP)20 and (SP)20-SCF were selected for further analysis by Western blot. Hardly any positive SCF signal was detected in the culture media of the SCF calli by dot blot.
As shown in Fig.3a, all four selected cell lines produced secreted and Hyp-O-glycosylated fusion proteins that migrated as a broad band at ~85 kDa, which was consistent with the (SP)20-tagged SCF polypeptide backbone of ~35 kDa (apparent molecular size with one N-glycan attached) and the presence of 20 Hyp-glycans (~2.5 kDa/glycan) [26]. The highest secreted yield of (SP)20-SCF and SCF-(SP)20 was estimated (by densitometry) to be 2.5 μg/mL and 1.4 μg/mL respectively. This corresponded to the cell biomass yields of 10.2 to 10.8 gDW/L for both types of BY-2 cells. In contrast, the expression of the SCF control without a (SP)20 tag did not produce detectable secreted protein.
Figure 3. Anti-SCF Western blot detection of the recombinant SCF, (SP)20-SCF and SCF-(SP)20 expressed in BY-2 cells.
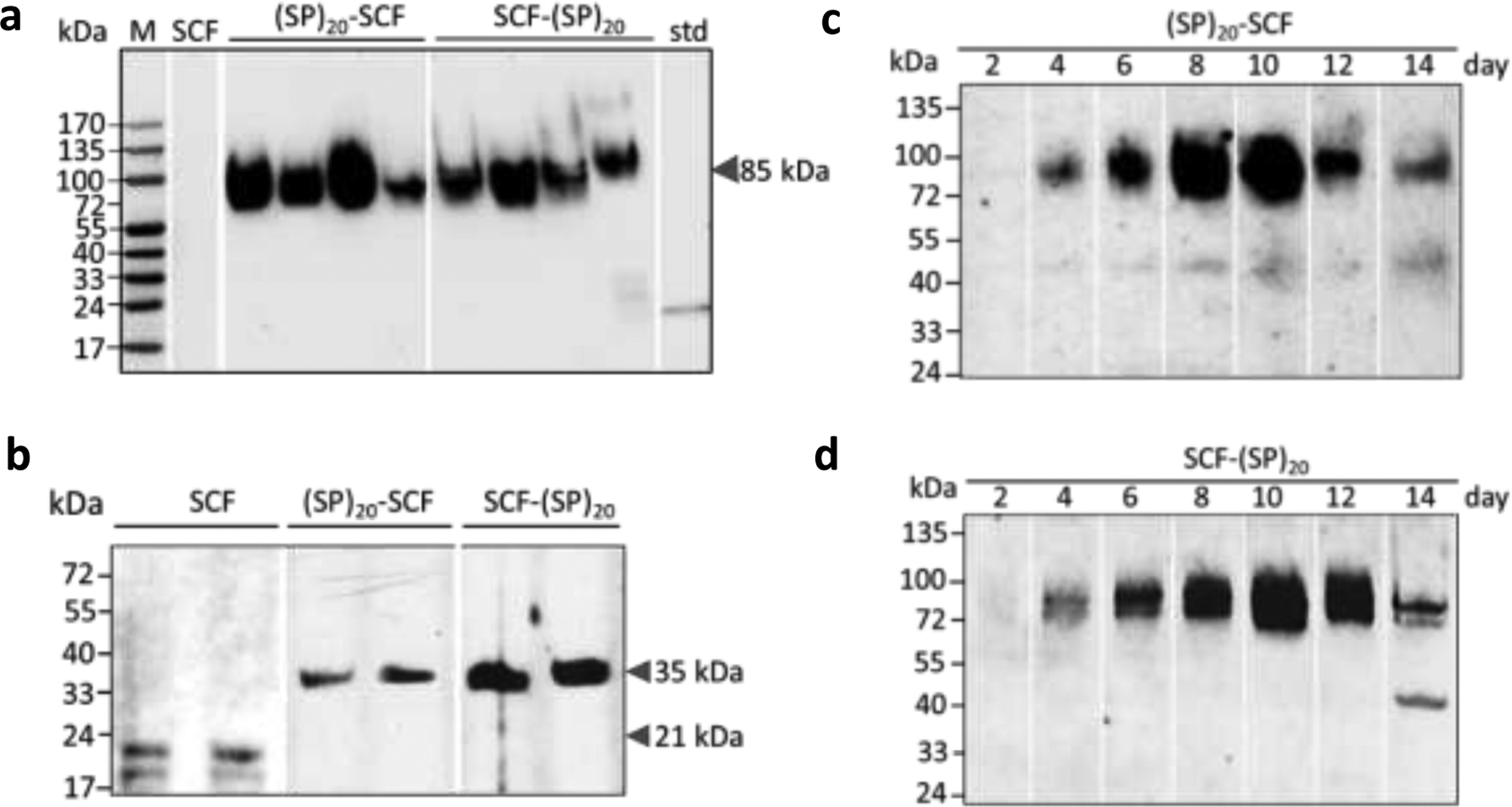
(a) Secreted (SP)20-tagged SCF in BY-2 cell cultures for 10 days. Secreted SCF was undetectable. M: molecular marker; std: SCF standard (30 ng); (b) Intracellular SCF and (SP)20-tagged SCF in cultured BY-2 cells. Both the glycosylated (~21 kDa) and non-glycosylated form (18.5 kDa) of SCF was detected. The (SP)20 tag substantially increases the apparent molecular size of SCF to 35 kDa; (c) and (d) Time course of the (SP)20-SCF and SCF-(SP)20 secretion in BY-2 cell cultures, respectively
When the intracellular accumulation of SCF products was examined, all three types of transgene products could be detected (Fig.3b). Both the N-glycosylated (~21 kDa) and non-glycosylated form (18.5 kDa) of the SCF could be observed. However, the (SP)20-SCF and SCF-(SP)20 showed much smaller molecular size (~35 kDa) than their secreted counterpart (~85 kDa). They were regarded as the non-Hyp-O-glycosylated form of the fusion proteins. Interestingly, the glycosylated form of the (SP)20-SCF or SCF-(SP)20 fusion proteins (~85 kDa) did not accumulate within the BY-2 cells at all. All were secreted into the culture media.
The time course of (SP)20-SCF and (SP)20 secretion was further investigated with the high-expression BY-2 cell line selected in Fig. 3c, d. The anti-SCF Western blotting assay confirmed that only the fully Hyp-O-glycosylated (SP)20-SCF and SCF-(SP)20 fusion proteins (~85 kDa) accumulated in the culture media. The dramatic secretion of the fusion protein started after 4 days of culture, and the maximum accumulation occurred at 8 to 10 days, which correlated with the rapid increase in cell biomass. Beyond day 10, the secreted SCF-(SP)20 fusion protein began to degrade due to the secretion of large amounts of proteases by the cultured BY-2 cells, similar to those reported earlier [13, 27]. This result allows us to determine the suitable time (8–10 days) for harvesting the culture media to purify the target proteins.
3.3. Purification of the recombinant SCF-derived proteins
A two-step purification method, HIC and nickel affinity chromatography, was developed to purify the (SP)20-tagged SCF protein from BY-2 cell culture media. The HIC not only removed large amounts of polysaccharides compounds, such as pectins and arabinogalactan glycoproteins, from culture media, but also substantially enriched the target proteins by 50–80 times.
The HIC enriched protein solutions were subsequently purified by nickel affinity chromatography. As seen in Fig. 4a, the SCF-(SP)20 protein bound to the Ni-NTA resins very well with no target protein detected in the flow though (FT) and the washing buffer, even though the C-terminal 6×His tag was directly connected to the highly glycosylated (SP)20 module. Meanwhile, the SCF-(SP)20 protein bound to the Ni-NTA resins could be efficiently eluted, and most of the target protein (more than 95%) was recovered in the first two elution buffers (E1 and E2). Similar results were obtained with the purification of the (SP)20-SCF fusion protein. The purity of the purified proteins was examined by the SDS-PAGE with Coomassie Blue staining and silver staining (Fig. 4b), which indicated a high purity of the (SP)20-SCF and SCF-(SP)20 proteins obtained in this study. About 1.0 mL of each purified fusion protein was obtained from the 300 ml of BY-2 cell culture medium collected on day 8. Quantification of the protein contents with the BCA assay indicated 300 μg/mL for the (SP)20-SCF, and 270 μg/mL for the SCF-(SP)20, which reflected a recovery rate of 49.4% and 43.2.6%, respectively.
Figure 4. Purification of the (SP)20-tagged SCF from BY-2 cell culture media.
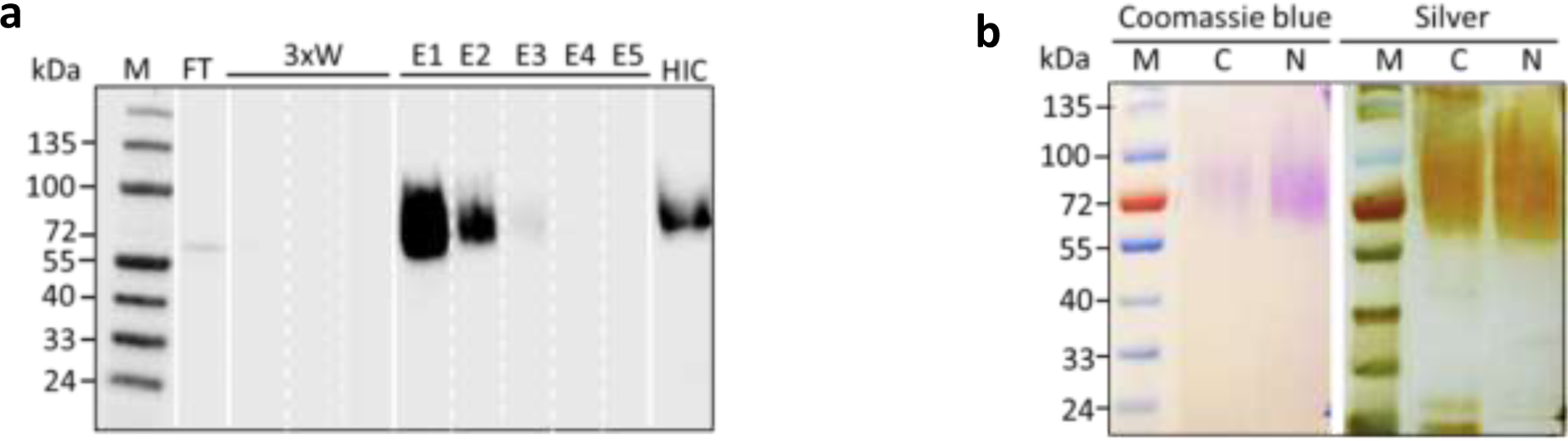
(a) Anti-SCF Western blot assay of the SCF-(SP)20 fractions collected during the nickel affinity chromatography. FT: flow through; 3xW: three times wash with a 2-column volume of equilibration buffer each time; E1-E5: five times elution with a 2-column volume of elution buffer each time; HIC: protein solution eluted from HIC column; (b) SDS-PAGE separation of the purified (SP)20-tagged SCF. The protein bands were detected by the Coomassie brilliant blue staining and silver staining, respectively. M: molecular maker; C: SCF with a C-terminal tag or SCF-(SP)20; N: SCF with a N-terminus tag or (SP)20-SCF
3.4. Bioactivity of (SP)20-tagged SCF in promoting cell proliferation
The bioactivity of the (SP)20-tagged SCF in stimulating the proliferation of a human bone marrow erythroleukemic cell line, TF-1 cell, was first assayed. Both the (SP)20-SCF and SCF-(SP)20 fusion proteins could promote the proliferation of the TF-1 cells (Fig. 5). However, their potency differed depending on the orientation of the (SP)20 tag. While the estimated EC50 of SCF-(SP)20 (9.5±0.3 ng/ml) was close to that of the SCF standard (6.0±0.2 ng/mL), the (SP)20-SCF has a significantly high EC50 of 80±0.8 ng/ml. This indicated that the N-terminal (SP)20 tag with heavy Hyp-O-glycosylation largely blocked the key amino acids at the N-terminus essential for receptor binding of the growth factor.
Figure 5. Dose responsive curve of the plant cell-produced SCF products on the human TF-1 cells using a CCK-8 assay.
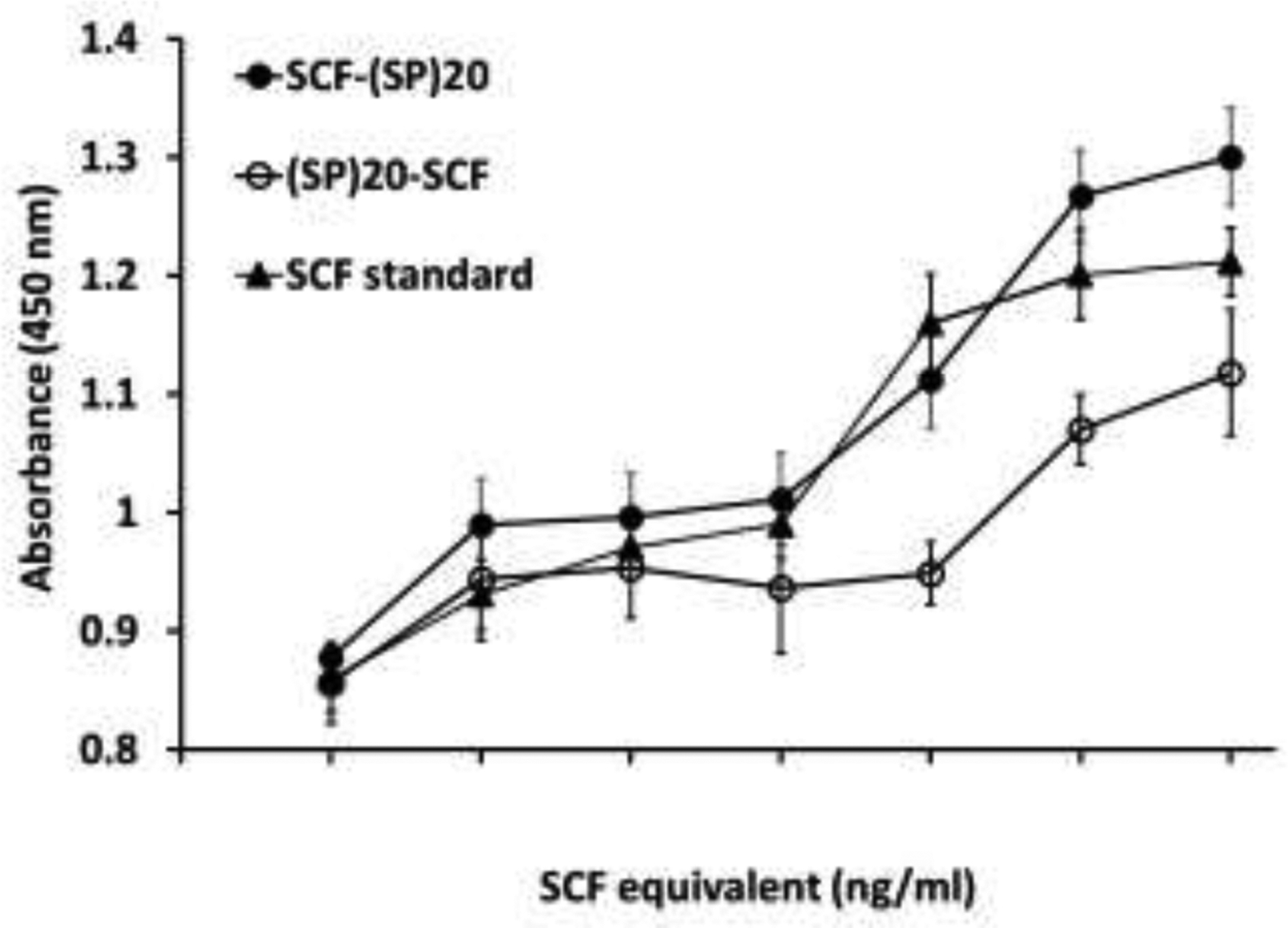
The data represent mean ± standard deviation of quadruplet treatments.
3.5. Function of (SP)20-tagged SCF in stimulating the expansion and differentiation of HSCs
The expansion and differentiation of CD34+ cells stimulated by the (SP)20-tagged SCF in combination with two other essential growth factors (EPO and IL-3) were investigated. There were four groups of treatments used in the assay: a positive group treated with all three commercial growth factors (SCF, EPO and IL-3), a negative group treated with the commercial EPO and IL-3 but lacking the SCF, and two sample groups treated with either the SCF-(SP)20 or (SP)20-SCF in combination with the commercial EPO and IL-3.
Both the SCF-(SP)20 and (SP)20-SCF exhibited the same activity as the commercial SCF in promoting the expansion of CD34+ cells, increasing the cell number by up to 300-fold during the 14-day culture period (Fig. 6a). In contrast, the CD34+ cells in the negative group (without adding SCF) did not show proliferation and could not survive beyond day 9. This indicated that the SCF played a critical role in the early stage of proliferation and survival of the CD34+ cells.
Figure 6. Expansion and differentiation of the CD34+ cells towards RBCs.
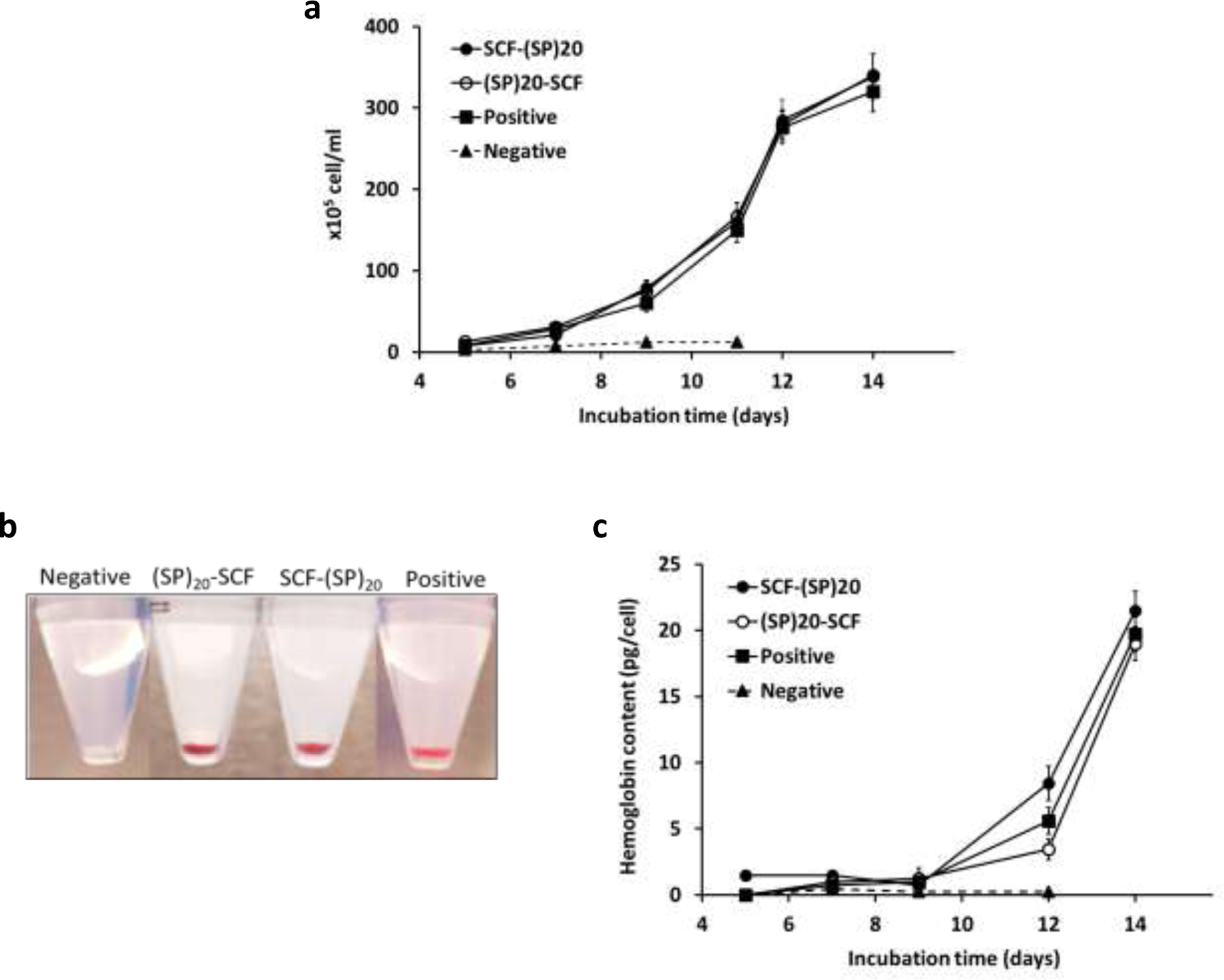
(a) Expansion of the CD34+ cells over 14 days of culture; (b) Cell pellets of the cultured cells on day 14 showing the hemoglobin formation. The cells in the negative control group were collected at day 7; (c) Hemoglobin content of the differentiating CD34+ over 14 days. The data represent mean ± standard deviation, n = 3.
The differentiation of the cultured CD34+ cells towards RBCs were visually observed at first. As shown in Fig. 6b, except for the negative group, the cells harvested from all the other three groups showed a bright red color due to formation of the iron-containing red pigment after 14-days of incubation. This was confirmed by the detection of a dramatic increase of hemoglobin content in the cultured cells from day 12 to day 14 (Fig. 6c).
The differentiation of the cultured CD34+ cells were then analyzed by flow cytometry at day 7 and day 14. As shown in Fig. 7, the BY-2 cell-derived SCF-(SP)20 and (SP)20-SCF have similar functions to the SCF standard in stimulating the CD34+ cell surface marker developments, including the loss of the CD34 and the emergence of the CD71 (transferrin receptor), the representative marker for differentiating erythroblasts [28, 29]. Before the differentiation process, the one-time expanded CD34+ cells were found to partially lose the CD34 surface marker. During the differentiation of the HSCs over 14 days, the CD34 marker disappeared from the CD34+ cells quickly while the CD71 maker developed in the presence of the SCF or its fusion proteins. Between the two sample groups, the SCF-(SP)20 showed a higher potency than the (SP)20-SCF in stimulating the differentiation of the CD34+ cells, as reflected by a higher percentage of the CD71+ cell population developed in the cells treated by the SCF-(SP)20 than the (SP)20-SCF, particularly at day 7. The potency of the SCF-(SP)20 was similar to the SCF standard.
Figure 7. Flow cytometric analysis of the CD34+ cell differentiation after 7 and 14 days of incubation.
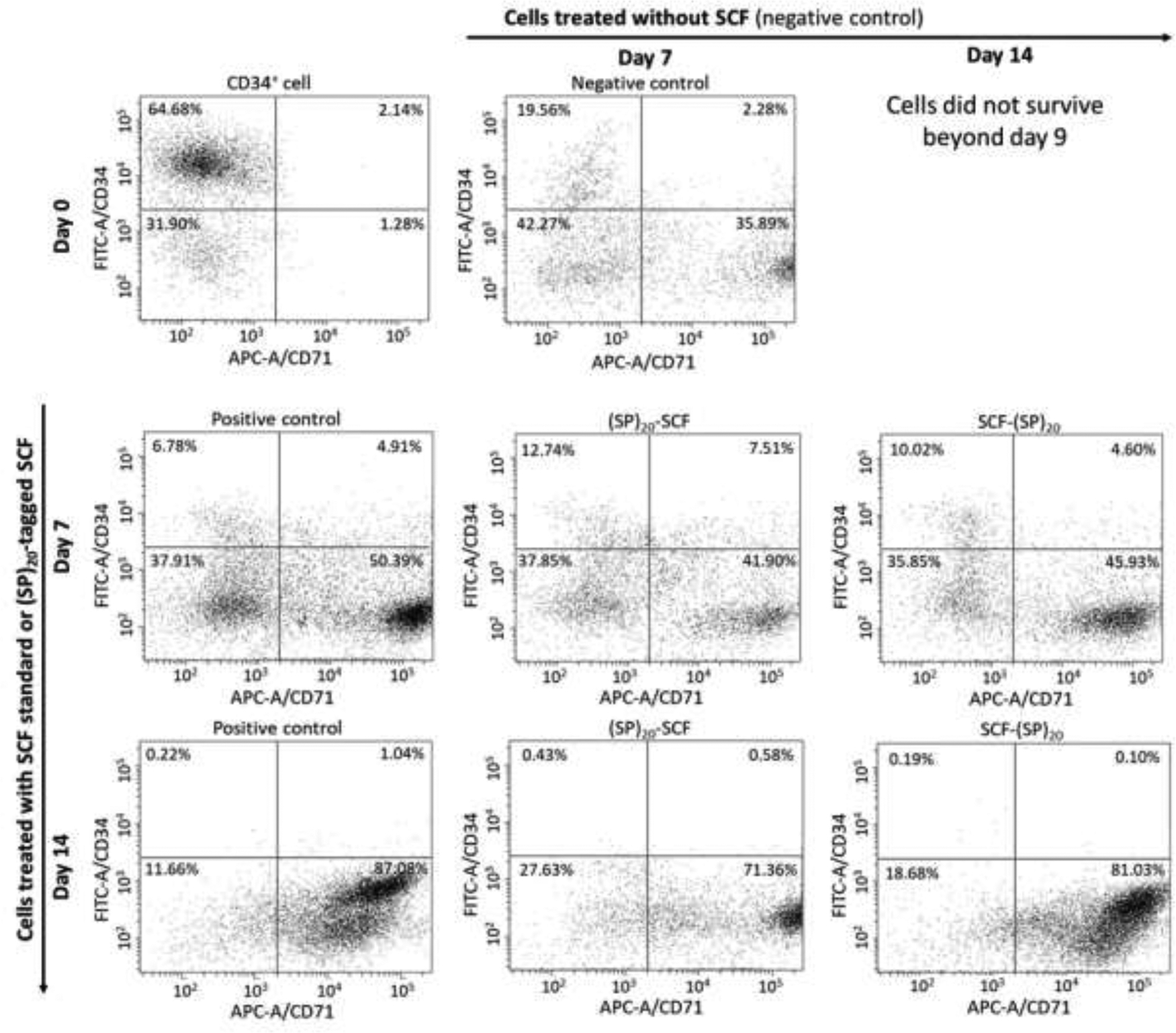
The differentiated cells were labelled with a FITC for the CD34 marker and an APC for the CD71 marker. Positive control: CD34+ cells treated with the commercial SCF in combination with the other growth factors; negative control: CD34+ cells treated with the growth factors lacking the SCF; Two sample groups: CD34+ cells treated with the (SP)20-SCF or SCF-(SP)20 in combination with the other growth factors.
Interestingly, the CD34+ cells in the negative group also showed some levels of differentiation, presumably due to the occurrence of other key growth factors, especially EPO, which efficiently stimulates erythropoiesis [30]. However, the cell did not proliferate in the absence of the SCF (Fig. 6a).
To examine the cell morphology during the differentiation process, the cultured CD34+cells were viewed under a bright-field microscope after Giemsa staining. As seen in Fig. 8, different cell types could be identified based on the cell size, nucleus, and cell cytoplasm composition after 7 and 14 days of incubation. The cells appeared much larger on day 7 than those observed on day 14, which was consistent with the early report that differentiating blood stem cells were large in the early and maturation stages, but became much smaller and enucleated to be RBCs at the end [25]. This characteristic change of cell morphology could be seen in Fig. 8, particularly, matured enucleated RBCs were identified after 14 days of expansion and differentiation. These results indicated the success of ex vivo differentiating CD34+ to RBCs with the plant cell-produced (SP)20 tagged-SCF.
Figure 8. Microscopic images of the differentiating CD34+ cells with a Giemsa staining.
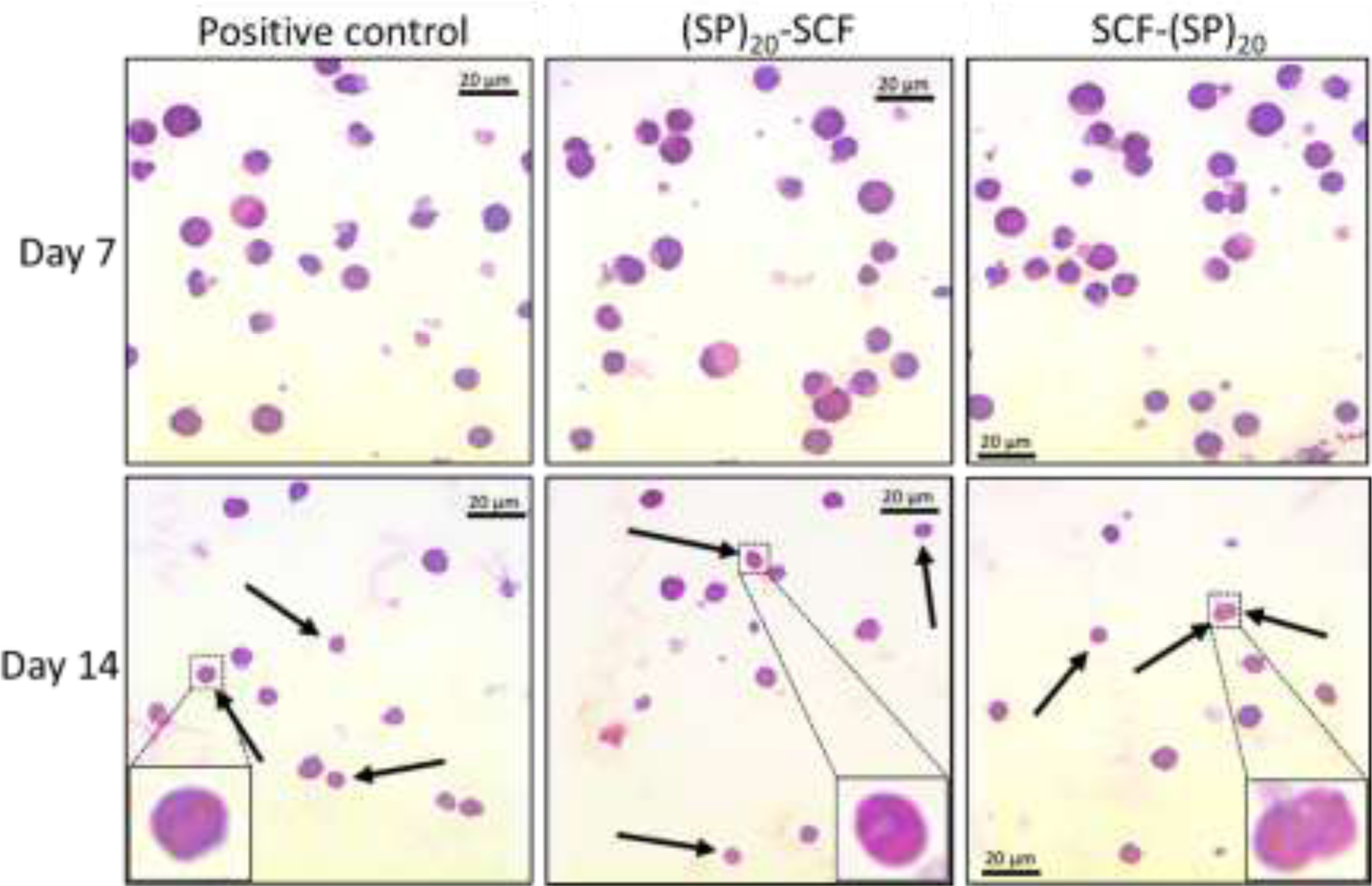
Enucleated RBCs are indicated by the arrows. Some matured RBCs with a donut-like shape are enlarged in the boxes. bar = 20 μm
4. Discussion
Ex vivo production of RBCs from HSCs requires high-quality eryGFs in significant quantities. Plants are proposed to be a promising bioproduction platform for these growth factors and other therapeutic proteins based on the advantages of low cost, high scalability, safety, and eukaryotic protein modification [31]. The expression of functional eryGFs, including SCF, EPO and IL-3, was achieved early on with the plant transient expression in N. benthamiana [1]. However, time-consuming and high-cost purification procedures must be applied to purify the recombinant growth factor from plant leaves. Plant cell suspension culture, in combination with the HypGP engineering, may overcome this problem as the expressed heterologous proteins are expected to dramatically secret into culture media, which will greatly simplify the protein purification process. This study aims to leverage the HypGP engineering technology to produce one of the key eryGFs -- SCF, which is used in the largest amount among all the eryGFs essential for the ex vivo expansion and differentiation of stem cells.
The Hyp-O-glycosylated (SP)20 tag, presenting either at the N-terminus or C-terminus, was able to boost the secreted yields of SCF up to 2.5 μg/mL in BY-2 cell cultures (Fig. 3a). In contrast, the expression of the SCF control without a HypGP tag did not produce detectable proteins, although the target protein could be found to accumulate intracellularly (Fig. 3b). This indicated that the SCF either did not secrete into culture media or the secreted SCF was rapidly degraded due to proteolysis. In both cases, the problem was overcome by the (SP)20 tag. This was also consistent with our previous research, in which the designer (SP)10 and (SP)20 tag dramatically increased the secreted yields of two therapeutic proteins, human growth hormone and interferon α2b, by up to 1500-fold. Meanwhile, the heavily Hyp-O-glycosylated (SP)n tag was found to stabilize the fused proteins against proteolytic degradation [15, 16].
It is worth mentioning that the (SP)20-tagged SCF transgene products appeared as different sizes in the culture media (~85 kDa) and inside the cells (~35 kDa), and they were completely segregated (Fig. 3a, b). Based on the molecule sizes detected and our previous research with the characterization of the (SP)32-tagged EGFP (enhanced green fluoresces protein) [14, 32] and the (AP)20-tagged α1 -antitrypsin expressed in BY-2 cells, we could conclude that the secreted one was the Hyp-O-glycosylated form that migrated as a broad band while the intracellular one was the non-glycosylated form. For the latter, the linear and rigid structure of the (SP)20 tag substantially increased the apparent molecular size of SCF from 21 kDa to 35 kDa, though the (SP)20 tag added only ~4.7 kDa to the SCF molecular size. The fully Hyp-O-glycosylated SCF-(SP)20 or (SP)20-SCF were detected only in the culture media, implying that the extensive Hyp-O-glycosylation of the (SP)20 tag was essential for its function as a molecular carrier in facilitating the secretion of the fused SCF protein.
Although the designer (SP)20 tag could dramatically increase the secreted yields of SCF to 2.5 μg/mL, this number was still low compared with what was achieved during the transient expression of the cytokine in N. benthamiana [1]. This is partially due to the much lower copy number of target genes occurring in the stably transformed BY-2 cells (usually 1 to 2 copy) as compared to the transient expression system (several hundred copies) [33]. In addition, as a cytokine, SCF is prone to proteolytic degradation during intracellular transport and after secretion into culture media. Considering the much higher secreted yields of other proteins (35 to 200 μg/ml) achieved in BY-2 cells with the same technology [13, 15, 16], we think it is possible to further improve SCF expression levels by optimizing the gene expression cassette and culture conditions.
Due to the extracellular secretion of the (SP)20-tagged SCF, the fusion protein could be easily purified with the two-step HIC and nickel affinity chromatography. Obviously, the polysaccharides-decorated (SP)20 tag did not interfere with the binding of the 6×His tag to the Ni-NTA resins and the subsequent elution (Fig. 4a). High purity of the purified (SP)20-SCF and SCF-(SP)20 was confirmed by both the Coomassie Blue staining and silver staining (Fig. 4b). Compared with the isolation of recombinant proteins from plant leaf tissues that involves tissue grounding, ammonium sulfate precipitation, and two or three steps of chromatography [24, 34], purification of the proteins from culture media saved time, energy, and cost. More importantly, pure proteins were readily obtained.
The Hyp-O-glycosylated (SP)20 tag, particularly its orientation, was found to affect the biological activity of the SCF to promote the proliferation of TF-1 cells. As seen in Fig. 5, while the EC50 SCF-(SP)20 (8.5 ng/mL) was close to that of the SCF standard (6.0 ng/mL), the EC50 of (SP)20-SCF (80 ng/mL) was up to 13.4-fold more than that of the SCF standard. The dramatically increased EC50 value (decreased potency) when the (SP)20 was attached at the N-terminus of SCF instead of the C-terminus implied that the functional site of SCF in binding its receptor is located at the N-terminus, which was blocked or interfered with by the N-terminal (SP)20 tag. This concurred with some previous reports indicating that the N-terminus of SCF is crucial for full SCF activity, and deletions of residues 1–3 from the N-terminus reduces the binding of SCF to c-kit by 50% while the deletion of Cys-4 inactivates SCF [35–37].
In our previous studies, engineering a HypGP tag comprised of a (SP)20, (SP)10, (SP)5, or (AP)20 tag did not show significant impact on the bioactivities of the fused proteins, such as interferon α2, human growth hormones, and α1-antitrypsin [15–17]. This was due to the linear structure and high hydrophilicity of the HypGP tags, which unlikely interacted with the tagged proteins and interfered with the protein activities. However, the Hyp- glycan attached (SP)20 tag could block the receptor binding domain at the N-terminus of SCF, leading to the drastic loss of the proteins’ potency. As a comparison, the C-terminal (SP)20 tag did not substantially reduce the potency of the fused SCF (Fig. 5).
SCF has been reported to play an important role in the differentiation of erythroid progenitor cells and facilitating the growth and survival of HSCs, especially at the early stage [18]. Therefore, it was important to demonstrate that the plant cell produced SCF with the (SP)20 tag was capable of promoting the expansion and differentiation of HSCs. The results from the flow cytometric analysis, cell morphology examination, and hemoglobin development assay have demonstrated that BY-2 cell secreted SCF-(SP)20 and (SP)20-SCF could exhibit a similar function as the commercial SCF standard in stimulating the expansion and differentiation of the human umbilical cord blood CD34+ cells (Fig. 6 to 8). Particularly, SCF plays a critical role in the early stage proliferation and survival of the CD34+ cells because without the SCF or SCF derivatives in the cell culture media, the CD34+ cells did not proliferate, though they still showed differentiation to some extent (Fig. 7).
Although the SCF with a N-terminal or C-terminal (SP)20 tag showed a 8.4-fold difference (EC50) in its potency to promote the differentiation of TF-1 cells (Fig. 5), the orientation of the (SP)20 tag did not have as much impact on the SCF’s function in stimulating the expansion and differentiation of the CD34+ cells. This could be due to the high dose of (SP)20-tagged SCF used in the CD34+ cell differentiation experiment. This SCF dosage (100 ng/mL) was reported previously [25] and in other literature [38, 39]. The high dose of the SCF molecules possibly saturated the SCF receptors at the surface of the cultured CD34+ cells, thus concealing the difference in potency between the SCF-(SP)20 and (SP)20-SCF proteins. Multiple doses of the SCF derivatives, for example 1 to 100 ng/ml, need be tested in the CD34+ cell differentiation experiment to differentiate the activity of between SCF-(SP)20 and (SP)20-SCF. However, this did not affect our conclusion that the plant cell produced SCF with a (SP)20 tag exhibited the desired function in stimulating the expansion and differentiation of the HSCs towards RBCs.
To conclude, this work demonstrates that HypGP engineering can be applied for the improved production of functional eryGFs in plant cell cultures, though further improvements of expression levels would be necessary to make it a viable commercial alternative. Our research thus provides a promising plant cell-based platform to produce large quantities of eryGFs to facilitate HSCs research and their clinical applications.
Highlights.
Plant cell culture is a promising production platform for high-quality growth factors
Hyp-O-glycosylated (SP)20 tag increases the secreted yields of SCF up to 2.5 μg/ml
The orientation of the (SP)20 tag substantially impacts the potency of SCF
(SP)20-tagged SCF stimulates the differentiation of hematopoietic stem cell CD34+ cells
Acknowledgements
This work was supported by the Arkansas IDeA Network of Biomedical Research Excellence - Research Development Grant under Grant No. P20GM103429, the National Science Foundation under Grant No. 1605564, and the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
None
References
- [1].Musiychuk K, Sivalenka R, Jaje J, Bi H, Flores R, Shaw B, Jones RM, Golovina T, Schnipper J, Khandker L, Sun R, Li C, Kang L, Voskinarian-Berse V, Zhang X, Streatfield S, Hambor J, Abbot S, Yusibov V, Plant-produced human recombinant erythropoietic growth factors support erythroid differentiation in vitro, Stem Cells Dev. 22(16) (2013) 2326–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shah SN, Gelderman MP, Lewis EM, Farrel J, Wood F, Strader MB, Alayash AI, Vostal JG, Evaluation of stem cell-derived red blood cells as a transfusion product using a novel animal model, PloS One 11(12) (2016) e0166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rousseau GF, Giarratana MC, Douay L, Large-scale production of red blood cells from stem cells: what are the technical challenges ahead?, Biotechnol. J 9(1) (2014) 28–38. [DOI] [PubMed] [Google Scholar]
- [4].Madrigal M, Rao KS, Riordan NH, A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods, J. Transl. Med 12(1) (2014) 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Audet J, Zandstra PW, Eaves CJ, Piret JM, Advances in hematopoietic stem cell culture, Curr. Biotechnol 9(2) (1998) 146–151. [DOI] [PubMed] [Google Scholar]
- [6].Youngblood BA, Alfano R, Pettit SC, Zhang D, Dallmann HG, Huang N, Macdonald CC, Application of recombinant human leukemia inhibitory factor (LIF) produced in rice (Oryza sativa L.) for maintenance of mouse embryonic stem cells, J. Biotechnol 172 (2014) 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Akuta T, Kikuchi-Ueda T, Imaizumi K, Oshikane H, Nakaki T, Okada Y, Sultana S, Kobayashi K, Kiyokawa N, Ono Y, Expression of bioactive soluble human stem cell factor (SCF) from recombinant Escherichia coli by coproduction of thioredoxin and efficient purification using arginine in affinity chromatography, Protein Expr. Purif 105C (2014) 1–7. [DOI] [PubMed] [Google Scholar]
- [8].Xu J, Zhang N, On the way to commercializing plant cell culture platform for biopharmaceuticals: present status and prospect, Pharma. Bioprocess 2(6) (2014) 499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xu J, Ge X, Dolan MC, Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures, Biotechnol. Adv 29(3) (2011) 278–299. [DOI] [PubMed] [Google Scholar]
- [10].Xu J, Dolan MC, Medrano G, Cramer CL, Weathers PJ, Green factory: Plants as bioproduction platforms for recombinant proteins, Biotechnol. Adv 30(5) (2012) 1171–1184. [DOI] [PubMed] [Google Scholar]
- [11].Paul M, Ma JK, Plant-made pharmaceuticals: leading products and production platforms, Biotechnol. Appl. Biochem 58(1) (2011) 58–67. [DOI] [PubMed] [Google Scholar]
- [12].Yusibov V, Streatfield SJ, Kushnir N, Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond, Hum. Vacc 7(3) (2011) 313–321. [DOI] [PubMed] [Google Scholar]
- [13].Zhang N, Gonzalez M, Savary B, Xu J, High-yield secretion of recombinant proteins expressed in tobacco cell culture with a designer glycopeptide tag: Process development, Biotechnol. J 11(4) (2016) 497–506. [DOI] [PubMed] [Google Scholar]
- [14].Zhang N, Wright T, Wang X, Karki U, Savary B, Xu J, Engineering “designer” glycomodules for boosting recombinant protein secretion in tobacco hairy root culture and studying hydroxyproline-O-glycosylation process in plants, Plant. Biotechnol. J 17 (2019) 1130–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu J, Tan L, Goodrum KJ, Kieliszewski MJ, High-yields and extended serum half-life of human interferon alpha2b expressed in tobacco cells as arabinogalactan-protein fusions, Biotechnol. Bioeng 97(5) (2007) 997–1008. [DOI] [PubMed] [Google Scholar]
- [16].Xu J, Okada S, Tan L, Goodrum KJ, Kopchick JJ, Kieliszewski MJ, Human growth hormone expressed in tobacco cells as an arabinogalactan-protein fusion glycoprotein has a prolonged serum life, Transgenic. Res 19(5) (2010) 849–867. [DOI] [PubMed] [Google Scholar]
- [17].Zhang N, Wright T, Caraway P, Xu J, Enhanced secretion of human alpha1-antitrypsin expressed with a novel glycosylation module in tobacco BY-2 cell culture, Bioengineered 10(1) (2019) 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Broudy VC, Stem cell factor and hematopoiesis, Blood 90(4) (1997) 1345–1364. [PubMed] [Google Scholar]
- [19].Toksoz D, Zsebo KM, Smith KA, Hu S, Brankow D, Suggs SV, Martin FH, Williams DA, Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor, Proc. Natl. Acad. Sci. U S A 89(16) (1992) 7350–7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shpak E, Leykam JF, Kieliszewski MJ, Synthetic genes for glycoprotein design and the elucidation of hydroxyproline-O-glycosylation codes, Proc. Natl. Acad. Sci. U S A 96(26) (1999) 14736–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu J, Tan L, Lamport DTA, Showalter AM, Kieliszewski MJ, The O-Hyp glycosylation code in tobacco and Arabidopsis and a proposed role of Hyp-glycans in secretion, Phytochem. 69(8) (2008) 1631–1640. [DOI] [PubMed] [Google Scholar]
- [22].De Loose M, Gheysen G, Tire C, Gielen J, Villarroel R, Genetello C, Van Montagu M, Depicker A, Inze D, The extensin signal peptide allows secretion of a heterologous protein from protoplasts, Gene 99(1) (1991) 95–100. [DOI] [PubMed] [Google Scholar]
- [23].Schenk RU, Hildebrandt AC, Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures, Can. J. Bot 50 (1972) 199–204. [Google Scholar]
- [24].Fang H, Wright T, Jinn JR, Guo W, Zhang N, Wang X, Wang YJ, Xu J, Engineering hydroxyproline-O-glycosylated biopolymers to reconstruct the plant cell wall for improved biomass processability, Biotechnol. Bioeng 117(4) (2020) 945–958. [DOI] [PubMed] [Google Scholar]
- [25].Kim HO, In-vitro stem cell derived red blood cells for transfusion: are we there yet?, Yonsei Med. J 55(2) (2014) 304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tan L, Varnai P, Lamport DT, Yuan C, Xu J, Qiu F, Kieliszewski MJ, Plant O-hydroxyproline arabinogalactans are composed of repeating trigalactosyl subunits with short bifurcated side chains, J. Biol. Chem 285(32) (2010) 24575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mandal MK, Fischer R, Schillberg S, Schiermeyer A, Inhibition of protease activity by antisense RNA improves recombinant protein production in Nicotiana tabacum cv. Bright Yellow 2 (BY-2) suspension cells, Biotechnol. J 9(8) (2014) 1065–73. [DOI] [PubMed] [Google Scholar]
- [28].Gross S, Helm K, Gruntmeir JJ, Stillman WS, Pyatt DW, Irons RD, Characterization and phenotypic analysis of differentiating CD34+ human bone marrow cells in liquid culture, Eur. J. Haematol 59(5) (1997) 318–326. [DOI] [PubMed] [Google Scholar]
- [29].Wu JM, Borowitz MJ, Weir EG, The usefulness of CD71 expression by flow cytometry for differentiating indolent from aggressive CD10+ B-cell lymphomas, Am. J. Clin. Pathol 126(1) (2006) 39–46. [DOI] [PubMed] [Google Scholar]
- [30].Cynshi O, Satoh K, Higuchi M, Imai N, Kawaguchi T, Hirashima K, Effects of recombinant human erythropoietin on anaemic W/Wv and Sl/Sld mice, Br. J. Haematol 75(3) (1990) 319–24. [DOI] [PubMed] [Google Scholar]
- [31].Chen Q, Davis KR, The potential of plants as a system for the development and production of human biologics, F1000Res 19 (2016) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang N, Dolan M, Wu D, Phillips GC, Xu J, Dramatic secretion of recombinant protein expressed in tobacco cells with a designer glycopeptide tag is highly impacted by medium composition, Plant Cell Rep. 35(12) (2016) 2513–2522. [DOI] [PubMed] [Google Scholar]
- [33].Komarova TV, Baschieri S, Donini M, Marusic C, Benvenuto E, Dorokhov YL, Transient expression systems for plant-derived biopharmaceuticals, Expert Rev. Vaccines 9(8) (2010) 859–76. [DOI] [PubMed] [Google Scholar]
- [34].Acosta W, Ayala J, Dolan MC, Cramer CL, RTB Lectin: a novel receptor-independent delivery system for lysosomal enzyme replacement therapies, Sci. Rep 5 (2015) 14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhang Z, Zhang R, Joachimiak A, Schlessinger J, Kong XP, Crystal structure of human stem cell factor: implication for stem cell factor receptor dimerization and activation, Proc. Natl. Acad. Sci. U S A 97(14) (2000) 7732–7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Langley KE, Mendiaz EA, Liu N, Narhi LO, Zeni L, Parseghian CM, Clogston CL, Leslie I, Pope JA, Lu HS, et al. , Properties of variant forms of human stem cell factor recombinantly expressed in Escherichia coli, Arch. Biochem. Biophys 311(1) (1994) 55–61. [DOI] [PubMed] [Google Scholar]
- [37].Jiang X, Gurel O, Mendiaz EA, Stearns GW, Clogston CL, Lu HS, Osslund TD, Syed RS, Langley KE, Hendrickson WA, Structure of the active core of human stem cell factor and analysis of binding to its receptor kit, EMBO J. 19(13) (2000) 3192–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boehm D, Murphy WG, Al-Rubeai M, The potential of human peripheral blood derived CD34+ cells for ex vivo red blood cell production, J. Biotechnol 144(2) (2009) 127–134. [DOI] [PubMed] [Google Scholar]
- [39].Zhang Y, Wang C, Wang L, Shen B, Guan X, Tian J, Ren Z, Ding X, Ma Y, Dai W, Jiang Y, Large-scale ex vivo generation of human red blood cells from cord blood CD34(+) cells, Stem Cells Transl. Med 6(8) (2017) 1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]


