Abstract
Background:
Few states have published statewide epidemiology of abusive head trauma (AHT).
Objective:
To examine the statewide epidemiology of AHT in West Virginia (WV), with the primary objective of establishing AHT incidence for comparison to national data, and to use as a baseline for comparison to incidence post-implementation of a statewide AHT prevention program.
Participants and setting:
AHT cases in children less than 2 years old were identified from the 3 tertiary pediatric centers in WV.
Methods:
Cases were identified by using ICD-9 codes for initially identifying those with injuries which might be consistent with AHT, followed by medical record review to determine which of these met the criteria for inclusion as a case. Medical examiner data was used to find additional cases of AHT. Using the number of cases identified along with relevant census data, incidence of AHT was calculated.
Results:
There were 120 cases of AHT treated in WV hospitals from 2000 to 2010, 100 of which were WV residents. The incidence was 36.1/100,000 children < 1 year of age and was 21.9 cases per 100,000 children < 2 years of age. Incidence in infants increased during the latter years (2006–2010) of the study to 51.8/100,000 compared to the incidence during 2000–2005, which was 24.0/100,000 (p < .01).
Conclusions:
Compared to US national, state and regional figures, the WV incidence of AHT was among the highest. In addition, the incidence of AHT increased significantly over the study period. Possible factors contributing to the rise in incidence are discussed.
Keywords: Abusive head trauma, Child abuse, Infants, Incidence
1. Introduction
Homicide is the leading cause of injury-related death in infants, often in the form of Abusive Head Trauma (AHT, previously known as Shaken Baby Syndrome, or SBS) (Shanahan, Zolotor, Parrish, Barr, & Runyan, 2013). AHT can cause severe injuries to the brain and can be associated with other injuries, including retinal hemorrhages and long bone and rib fractures (Shanahan et al., 2013). The average age of the victims is reported to be between 2.2 months to 11.7 months (Barlow & Minns, 2000; Douglas & Archer, 2004; King & MacKay, 2003; Starling, Holden, & Jenny, 1995).
Prior studies have examined the incidence of AHT on national, state, and hospital levels. Recent work in other states indicates the usefulness of hospital record review as a means of ongoing surveillance of AHT, though the methods of case identification have varied. The most comprehensive approach to date was the North Carolina study, which used not only medical record review, but also identified cases prospectively over a two-year period (Keenan et al., 2003). A California study performed retrospective, passive surveillance based on hospital inpatient data to look for AHT from a seven-year period (Wirtz & Trent, 2008). Their methods were less comprehensive than the North Carolina study; cases were identified solely on discharge ICD-9 codes. Two incidences were determined; one was based on the strict ICD-9 code for SBS (995.55), the other on a broader case definition that included ICD-9 codes from the constellation of symptoms associated with AHT (Wirtz & Trent, 2008). Shanahan et al. determined the national AHT incidence by applying the Centers for Disease Control and Prevention (CDC) coding algorithm to a national database sample of pediatric discharges (Parks, Annest, Hill, & Karch, 2012; Shanahan et al., 2013). Similar to Shanahan et al., Parrish et al. applied the CDC coding algorithm to multiple statewide databases, including Bureau of Vital Statistics death records, Alaska Maternal Infant Mortality Review – Child Death Review (MIMR-CDR), Medicaid, Hospital Discharge Database (HDD), Alaska Trauma Registry (ATR) and Alaska Violent Death Reporting System databases, to calculate the statewide incidence of AHT (Parrish, Baldwin-Johnson, Volz, & Goldsmith, 2013). The California, Alaska, and national incidences were determined from ICD-9 codes only; there was no chart review to better determine whether or not the case met the definition of AHT. An incidence study in Pennsylvania included substantiated AHT cases that were reported to a state-wide registry (Kesler et al., 2008).
Accurately understanding incidence is essential to evaluating prevention programs. Prevention programs, while effective in helping parents understand normal infant crying behavior, have to date had mixed results in reducing incidence of AHT. A hospital-based intervention was effective in reducing the incidence of AHT in western New York (Dias et al., 2005); however, a similar intervention did not decrease AHT hospitalization rates in Pennsylvania (Dias et al., 2017). In both Dias studies, most parents reported learning from the intervention, and they retained the knowledge for 7 months, through the peak age of crying. A study of the Period of PURPLE Crying intervention, developed by the National Center on Shaken Baby Syndrome, did not reduce the incidence of AHT in North Carolina over the two years after its implementation, though parent phone calls related to crying to a nurse line were reduced (Zolotor et al., 2015). In British Columbia, Canada, implementation of the Period of PURPLE Crying intervention over an eight-year period led to a 35% reduction in the incidence of AHT in children ≤24 months old (Barr et al., 2018).
The main objective of this study, which used both an ICD-9 code search and medical record review, was to determine the incidence of AHT in West Virginia (WV) children < 24 months of age from 2000 through 2010 in order to compare the WV incidence with the national incidence, and to establish a baseline incidence as part of a statewide prevention program. In addition to incidence, other epidemiologic aspects of AHT in WV children were examined.
2. Methods
The AHT Incidence Monitoring (AIM) team of WV is a collaboration between the three tertiary pediatric centers in WV, the only centers that have pediatric intensive care units (PICU) in the state. Team members included: 1) a hospital-based primary investigator from each institution, each trained in pediatric critical care with experience in diagnosing AHT, one of whom was also the principal investigator; 2) two board-certified Child Abuse Pediatricians; 3) pediatric trainees from each institution; 4) and a research coordinator from the lead institution. Our purpose was to define the epidemiology of AHT in WV and to establish the baseline incidence in order to inform prevention efforts. This project was reviewed and approved by the Institutional Review Board for the Protection of Human Subjects at Charleston Area Medical Center, Marshall University, and West Virginia University (Office of Research Integrity and Compliance).
2.1. Case identification
The first step in our case identification was finding potential cases using ICD-9 codes. All children < 24 months of age brought to the pediatric tertiary care centers in WV from 2000 to 2010, including cases treated in the emergency room, pediatric floor, and Pediatric Intensive Care Unit were included in the initial search. Diagnostic codes used to find potential cases included not only the isolated, strict ICD-9 code for SBS (995.55), but also codes for other diagnoses that might include part or all of the constellation of injuries associated with AHT (Table 1). Such an approach addresses the concern that if cases were only identified by the strict ICD-9 code for SBS, then this may underestimate the actual number of cases since hospital coders can only assign this code when the physician clearly specifies the diagnosis of SBS/AHT in the chart. Our code search was modeled after the codes used in the North Carolina incidence study. In one of the three pediatric centers, cases were identified from the PICU admission log as well as by using ICD-9 codes to identify additional cases.
Table 1.
ICD-9 codes used to identify potential cases of AHT.
| ICD-9 | Definition |
|---|---|
| 362.81 | Retinal hemorrhage |
| 800.1-800.49 | Closed skull vault fracture with intracranial injury |
| 800.6-800.99 | Open skull vault fracture with intracranial injury |
| 801.1-801.49 | Closed fracture of base of skull with intracranial injury |
| 801.6-801.99 | Open fracture base of skull with intracranial injury |
| 803.1-803.49 | Other closed skull fractures with intracranial injury |
| 803.6-803.99 | Other open skull fractures with intracranial injury |
| 804.1-804.49 | Multiple closed fractures to skull or face with intracranial injury |
| 804.6-804.99 | Multiple open fractures to skull or face with intracranial injury |
| 850.0-850.99 | Concussion (violent jar or shock or the condition that results from such injury) |
| 851.0-851.99 | Cerebral laceration and concussion |
| 852.0-852.59 | Subarachnoid, subdural, and extradural hemorrhage |
| 853.0-853.19 | Other and unspecified intracranial hemorrhage |
| 854.0-854.19 | Other and unspecified intracranial injury |
| 959.01 | Other and unspecified injury to head |
| 959.8-959.9 | Other and unspecified injury to other sites |
| E960.0-E969.9 | Assault |
The next step in case identification was medical record review of every potential case identified from the code search. Any cases that had a clear cause of injury other than abuse (such as motor vehicle accident) were excluded. Falls were not excluded in the initial review since a history of a short fall may be reported in cases that turn out to be AHT. From the remaining medical records, relevant data was collected such as demographics, presenting history, any inconsistencies in the history, types and extent of injuries (as recorded in the physical exam, in imaging and/or in other ancillary tests), social work and/or child protective services (CPS) evaluations, length of hospitalization, outcome, and disposition, were collected. The data abstraction was done by pediatric trainees, under the supervision of each hospital-based physician investigator, using a data collection instrument specifically designed for the study. All completed forms were reviewed by the PI to confirm or deny the designation of AHT, in consultation with a board-certified Child Abuse Pediatrician. Consistent with the narrow definition of AHT of the CDC and with the criteria used in the NC study, intracranial injury had to be documented for inclusion as a case. (Keenan, 2008; Parks et al., 2012; Shanahan et al., 2013) The minimum criteria for inclusion as a case was the presence of intracranial injury in addition to a history incompatible to explain the injury (Reece, 2008). An additional board-certified Child Abuse Pediatrician performed a second review of potential cases to ensure interrater reliability.
While the great majority of potential cases were identified using the surveillance of hospital ICD-9 codes for all children < 24 months of age brought to the pediatric tertiary care centers in WV from 2000 to 2010, including cases treated in the emergency room, pediatric floor, and Pediatric Intensive Care Unit, we also obtained information from the medical examiner (ME), mainly provided in death certificates. Fatal cases included from the ME were those which listed SBS or AHT in the diagnosis.
2.2. Statistical analysis
For infants, incidence was calculated using the number of live births in the state for each year under study in the denominator. For children 12 month to 23 months old, incidence was calculated using the number of live births, minus the number of infant deaths for the preceding year, as the denominator. For descriptive statistics, means, standard deviations, and confidence intervals, were typically reported for continuous variables. Frequencies and/or percentages were reported for categorical variables. For comparative statistics, student’s t-test was used for continuous variables, and either Chi-square or Fischer’s exact was used for categorical variables. For purposes of describing the clinical and demographic picture of AHT from WV, all cases treated in WV hospitals, including a small minority from neighboring states, were included in the analyses of the data, whereas calculation of incidence of AHT in WV was based only on the number of cases that were WV residents, excluding non-residents. Cohen’s kappa and percent agreement were used to assess interrater reliability in identifying cases.
3. Results
One hundred twenty cases of AHT in children < 24 months of age were identified. Of these, 104 cases (86.7%) involved infants (< 12 months old). Cohen’s kappa was 0.79 (95% confidence interval 0.62–0.95); the percent agreement between the two case reviews was 95.3%. Demographic characteristics of the cases are presented in Table 2. There was a slight male predominance. Identified victims were Caucasian in 90.7% of cases. The mean age of victims was 6.25 months; the median age was 4 months. The victim was younger than or equal to 6 months of age in 65.8% of cases. The peak occurrence of AHT was at 2 months, accounting for 18.3% of cases (Fig. 1).
Table 2.
Demographics.
| Variable | Percent (n) |
|---|---|
| Gender | |
| Male | 55.8 (67) |
| Female | 44.2 (53) |
| Race | |
| Caucasian | 90.7 (98) |
| Other | 9.3 (10) |
| Unknown | (12) |
| State of Residence | |
| West Virginia | 83.3 (100) |
| Adjacent states (Maryland, Ohio, Kentucky, Pennsylvania) | 16.7 (20) |
| Age | |
| <12 months | 86.7 (104) |
| 12–24 months | 13.3 (16) |
| Facility where patient initially presented | |
| Emergency Room of study hospital | 38.1 (45) |
| Outlying facility | 53.4 (63) |
| Primary Care | 7.6 (9) |
| Death at home | 0.9 (1) |
| Unknown | (2) |
Fig. 1.
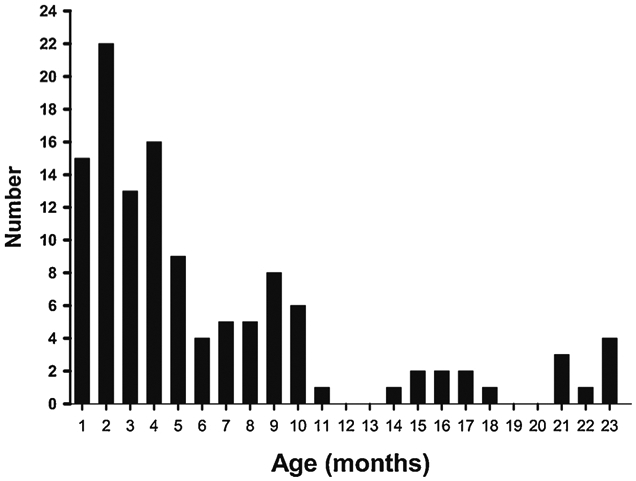
Age distribution of AHT cases.
The presenting history was inconsistent with the injuries in all but 2 cases; in those cases, the perpetrator confessed at the time of admission. In most cases, caregivers reported no history of injury. In the others, short falls were reported in 39.1% of cases, including 16 from a bed or couch and 14 from a caregiver’s arms.
Five fatal cases (21.7% of fatal cases and 4.2% of all cases) were never admitted to one of the three tertiary pediatric centers in WV; of those, 4 died at an outlying hospital prior to transfer and one died at home. Thus, hospital data, including presenting symptoms, were available in the remaining 115 patients (Table 3).
Table 3.
Presenting symptoms in AHT cases that presented to AIM team hospital.
| Symptom | Percent (n) |
|---|---|
| Seizures | 43.5 (50) |
| Unresponsive | 40.0 (46) |
| Vomiting | 38.3 (44) |
| Irritability | 27.6 (32) |
| Apnea | 18.3 (21) |
| Poor feeding | 16.5 (19) |
| Upper respiratory symptoms | 14.8 (17) |
| Diarrhea | 7.8 (9) |
| Cyanosis | 2.6 (3) |
Injuries found in AHT cases are presented in Table 4. Intracranial injury, as evidenced by intracranial hemorrhage or cerebral edema, was noted in all cases. Intracranial hemorrhage was present in 96.5%; cerebral edema was found in 22.6%. Subdural hematomas were present in 88.7% at admission. Fractures were found in 44.3% of all patients: skull fractures in 27.8%, long bone fractures in 19.1%, and rib fractures in 15.7%. Retinal hemorrhages were present in 70.4% of patients. Impact injury was present in 55.7% as evidenced by either skull fracture or swelling, bruising, or abrasions on the face or scalp.
Table 4.
Injuries.
| Injury | Percent (n) |
|---|---|
| History inconsistent with injuries at admission | |
| Yes | 98.3 (113) |
| Perpetrator confessed | 1.7 (2) |
| Intracranial injury | 100 (115) |
| Intracranial Hemorrhage | 96.5 (111) |
| Cerebral Edema | 22.6 (26) |
| Retinal Hemorrhage | 70.4 (81) |
| Impact Trauma | 55.7 (64) |
| Fractures | |
| Skull | 27.8 (32) |
| Rib | 15.7 (18) |
| Long Bone | 19.1 (22) |
| Any Fracture | 44.3 (51) |
Of the 120 cases identified, 23 were fatalities (19.2%). Of the nonfatal cases with documentation of discharge status, an additional 44 patients had evident morbidity, such as blindness (6), seizures (31), and ventriculoperitoneal shunt placement (7) at the time of initial hospital discharge.
Disposition data were available in 92 of 97 (94.8%) of the nonfatal cases. Of these, 80 (87%) were discharged to a custody placement other than their admission custodian. Only 17 (18.5%) children were discharged home with the parent or parents who had custody at the time of the injury.
With 85 cases of AHT identified in WV infants (< 12 months old), the average annual incidence for this age group was 36.6/100,000 live births (95% Confidence Interval (CI) 24.3–49.0) measured over the study period of 2000–2010. The incidence was higher in the latter part of the study period; the average annual incidence in of AHT in infants in the years 2000–2005 was 24.0/100,000 live births (95% CI 15.7–32.4) but increased to 51.8/100,000 live births (95% CI 32.4–71.2) during the years 2006–2010 (p < 0.01) (Table 5, Fig. 2). This latter WV incidence significantly exceeds that reported in NC infants (29.7/100,000, 95% CI 22.9–36.7, p < 0.05).
Table 5.
The WV AHT incidence (per 100,000 live births) increased over time.
| 2000–2005 | 2006–2010 | p-value | |
|---|---|---|---|
| Incidence < 12 months old | 24.0 (15.7–32.4) | 51.8 (32.4–71.2) | < 0.01 |
| Incidence < 24 months old | 14.5 (10.3–18.7) | 30.3 (16.3–44.2) | < 0.05 |
Fig. 2.
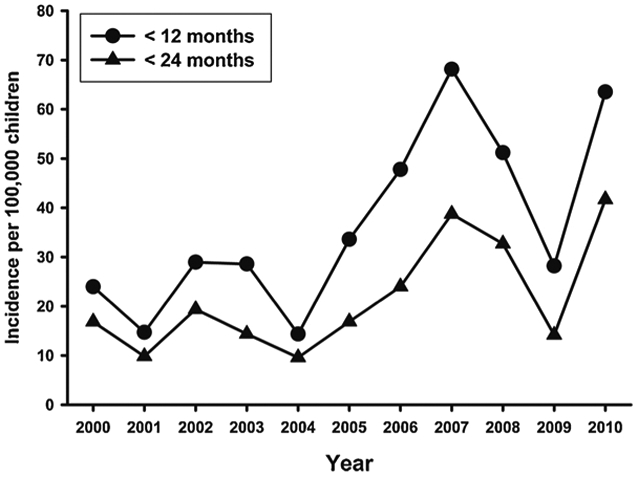
WV AHT rates by year.
Including all children with AHT < 24 months old, the average annual incidence was 21.6/100,000 live births (95% CI 14.1–29.2). Again, the incidence was higher in the latter part of the study period with 14.5 cases per 100,000 live births from 2000 to 2005 (95% CI 10.3–18.7) versus 30.3/100,000 from 2006 to 2010 (95% CI 16.3–44.2) (p < 0.05). The elevated incidence of AHT in WV children < 24 months old during the years 2006–2010 significantly exceeds the NC incidence in children < 24 months old (17.0/100,000, 95% CI 13.3–20.7, p < 0.01).
4. Discussion
This is the first study to evaluate the incidence of AHT in a predominantly Appalachian state. Acknowledging the differences in methodology, the WV incidence of AHT over the entire study period is among the highest reported to date, only exceeded by that of the Midwest region reported by Shanahan et al. (Table 6). In addition, the WV incidence appears to be rising, more than doubling over the study period (Table 5) to a level which was shown to significantly exceed that in NC (p < 0.05), a state in the same region as WV. This latter finding is concerning but should be interpreted with caution at it is based on a smaller sample size than the incidence figure based on the entire study period (2000–2010).
Table 6.
WV AHT incidence compared to other studies.
| Incidence < 1 year old (95% Confidence Interval) |
Incidence < 2 years old (95% Confidence Interval) |
|
|---|---|---|
|
West Virginia Passive surveillance using hospital codes and chart review plus medical examiner data |
36.6 (24.3–49.0) | 21.6 (14.1-29.21) |
|
California (Wirtz & Trent, 2007) Passive surveillance using hospital codes |
22.4 | 14.0 |
|
North Carolina (Keenan et al., 2003) Prospective identification plus medical examiner data |
29.7 (22.9–36.7) | 17.0 (13.3-20.7) |
|
National (Shanahan et al., 2013) Application of CDC Narrow Definition to Kids’ Inpatient Databases |
33.4 (31.4–35.4) | (Not reported) |
|
South Region (Shanahan et al., 2013) Application of CDC Narrow Definition to Kids’ Inpatient Databases |
32.0 (28.4–35.9) | (Not reported) |
|
Midwest Region (Shanahan et al., 2013) Application of CDC Narrow Definition to Kids’ Inpatient Databases |
41.9 (35.9–48.5) | (Not reported) |
The peak occurrence of AHT was at 2 months of age, which coincides with the peak incidence of normal infant crying (Brazelton, 1962). The most common presenting complaints were seizures and unresponsiveness, each present in more than 40% of cases. Retinal hemorrhages were seen in the majority of cases. More than 40% of cases had fractures, including skull fractures, rib, and long bone fractures. Roughly one in five children died.
Our study did not specifically explore why AHT incidence had an apparent increase in the latter years of the study period (2005–2010), but two factors bear mention. The first is the 2008 recession which was well studied by Berger in neighboring states as a possible contributing factor to AHT (Berger et al., 2011). While the recession did occur during the period of elevated incidence in our study, the rise in incidence in WV seemed to slightly antedate the 2008 recession (Fig. 2), bringing any clear temporal association into question. The second societal factor worth mentioning in this regard is the rising incidence of drug abuse in WV over the study period, as evidenced by a nearly 5-fold increase in drug-related fatalities in WV from 2000 to 2008 (Kaplan, Richards-Waugh, Bailey, & Kraner, 2010), which was further confirmed through 2010 by the Office of the State Registrar. While our study provides no direct evidence to link either of the above factors to rising AHT incidence, the contemporaneous rise of drug abuse to such a degree is suggestive of a contributory role. Another potential explanation for the increase over time could be related to improved diagnosis of mild cases of AHT due to increased screening of well-appearing children with a fracture or other sentinel injury.
Optimizing the measurement and interpretation of incidence requires a clear case definition and an understanding of how study methodologies can be compared. We used ICD-9 codes to identify potential cases of AHT. Our codes were similar to those used in the North Carolina study and to the narrow definition of AHT proposed by the CDC, both of which required the presence of intracranial injury in the case definition (Keenan, 2008; Parks et al., 2012; Shanahan et al., 2013). When examining cases by ICD-9 codes alone, the narrow definition gives more specificity than the broad definition since the latter allows for cases such as apparently abusive skull fracture without intracranial injury to be included. Our approach of using the CDC “narrow” set of ICD-9 codes for AHT case finding in addition to medical record review allowed for greater specificity than by using diagnostic codes alone. This helped to ensure that we did not overestimate the AHT incidence in WV.
Our study did have some limitations. Because of the medical and multidisciplinary resources available at the three children’s hospitals in WV, we believe that the vast majority of children with suspected AHT were referred to one of these centers, but it is possible that some subclinical and/or mild cases were treated in an outside facility rather than being transferred. Another limitation of our study was that WV children treated for AHT at hospitals in neighboring states were not identified, which could lead to an underestimate of the WV incidence. To better understand the scope of this phenomenon, we reached out to three large tertiary children’s hospitals potentially serving WV children, with one hospital in Pennsylvania reporting approximately 1–2 WV children per year with acute AHT in recent years (which was estimated to be consistent with previous referral patterns), one in the DC area reporting only 2 cases of possible AHT from WV over the entire study period (2000–2010), and one in Ohio reporting no cases from WV in the past year. We also reached out to the Ohio and Virginia Trauma Registries, and neither registry identified any cases of AHT from WV treated during the study period (2000–2010). Considering the information from this limited post-hoc analysis, as well as considering the fact that the percentage of out-of-state children treated within West Virginia hospitals during the study period was 20 percent, we estimate that the percentage of West Virginia children with AHT who may have been treated outside of West Virginia over the study period to be between 10 and 20 percent. One way to capture affected children treated at out-of-state facilities in the future might involve working with Child Protective Services and/or WV Medicaid, although an attempt to identify additional cases by the latter means was of low yield for the years under study. Our case-retrieval via ICD-9 codes also had its limitations, with one children’s hospital relying more on the PICU logbook than ICD-9 codes for identification of cases. This institution did, in fact, report the fewest cases of AHT of the three centers, and, therefore, in future studies this will need to be considered. Despite these limitations, each of these would err on the side of a conservative estimate of incidence.
While the retrospective approach used in our study has its limitations, such an approach was necessary to establish an estimate of baseline incidence in an expeditious way in preparation for initiation of a prevention program for AHT. This approach might prove more feasible than a prospective approach for states faced with limited resources, but still wishing to establish a baseline incidence of AHT.
5. Conclusions
The average annual incidence of AHT over the study period (2000–2010) in young WV children was among the highest compared to relevant state, regional and national data (Table 6). In addition, the incidence of AHT increased significantly in WV in both infants (age < 12 months) and young toddlers (age 12–23 months) over the study period, rising to levels both in infants and in all children less than 24 months of age that exceeded the respective incidence figures in NC, a well-studied state in the same region as WV (Keenan et al., 2003). Changes in the occurrence of drug abuse and in economic factors occurred contemporaneously to the rise in AHT incidence in WV, suggesting contributory factors. (Kaplan et al., 2010, Berger et al., 2011). The epidemiologic picture of AHT in WV which emerged from medical record review was consistent with national data, showing an egregiously morbid and often fatal syndrome with peak occurrence at 2 months of age, with nearly 90% of cases occurring in those less than 12 months of age. Our study provided impetus for the initiation of a prevention program for AHT in 2011 and established a baseline incidence of AHT (2000–2010) to compare to post-implementation incidence (2011–2021). Our methods may help other states/regions wishing to examine the baseline incidence and epidemiology of AHT in their population.
Acknowledgements
The Martha Gaines and Russell Schilling Wehrle Pediatric Research Endowment supported presentation of an earlier version of the paper. The authors would like to acknowledge Matt Morris, MD, and Bethany Woomer, MD, who assisted in data collection. We also would like to thank the Ohio and Virginia trauma Registries and the three out-of-state Childrens Hospitals who helped to look for WV cases of AHT treated outside of WV.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Declarations of Interest
None.
References
- Barlow KM, & Minns RA (2000). Annual incidence of shaken impact syndrome in young children. Lancet, 356(9241), 1571. [DOI] [PubMed] [Google Scholar]
- Barr RG, Barr M, Rajabali F, Humphreys C, Pike I, Brant R, et al. (2018). Eight-year outcome of implementation of abusive head trauma prevention. Child Abuse & Neglect, 84, 106–114. 10.1016/j.chiabu.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Berger RP, Fromkin JB, Stutz H, Makoroff K, Scribano PV, Feldman K, et al. (2011). Abusive head trauma during a time of increased unemployment: A multicenter analysis. Pediatrics, 128(4), 637–643. 10.1542/peds.2010-2185. [DOI] [PubMed] [Google Scholar]
- Brazelton TB (1962). Crying in infancy. Pediatrics, 29(4), 579–588. [PubMed] [Google Scholar]
- Dias MS, Rottmund CM, Cappos KM, Reed ME, Wang M, Stetter C, et al. (2017). Association of a postnatal parent education program for abusive head trauma with subsequent pediatric abusive head trauma hospitalization rates. JAMA Pediatrics, 171(3), 223–229. 10.1001/jamapediatrics.2016.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MS, Smith K, DeGuehery K, Mazur P, Li V, & Shaffer ML (2005). Preventing abusive head trauma among infants and young children: A hospital-based, parent education program. Pediatrics, 115(4), e470–477. 10.1542/peds.2004-1896. [DOI] [PubMed] [Google Scholar]
- Douglas M, & Archer P (2004). Shaken baby syndrome-related traumatic brain injuries: Statewide surveillance findings. The Journal of the Oklahoma State Medical Association, 97(11), 487–490. [PubMed] [Google Scholar]
- Kaplan JA, Richards-Waugh LL, Bailey KM, & Kraner JC (2010). Epidemic: fatal pharmaceutical abuse in West Virginia 1991–2008. West Virginia Medical Journal, 106(4 Spec No), 88–90. [PubMed] [Google Scholar]
- Keenan HT (2008). Practical aspects of conducting a prospective statewide incidence study. American Journal of Preventive Medicine, 34(4), S120–S125. 10.1016/j.amepre.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, & Sinal SH (2003). A population-based study of inflicted traumatic brain injury in young children. JAMA, 290(5), 621–626. 10.1001/jama.290.5.621. [DOI] [PubMed] [Google Scholar]
- Kesler H, Dias MS, Shaffer M, Rottmund C, Cappos K, & Thomas NJ (2008). Demographics of abusive head trauma in the Commonwealth of Pennsylvania. Journal of Neurosurgery Pediatrics, 1(5), 351–356. 10.3171/PED/2008/1/5/351. [DOI] [PubMed] [Google Scholar]
- King WJ, & MacKay M (2003). Shaken baby syndrome in Canada: Clinical characteristics and outcomes of hospital cases. CMAJ: Canadian Medical Association Journal, 168(2), 155. [PMC free article] [PubMed] [Google Scholar]
- Parks SE, Annest JL, Hill HA, & Karch DL (2012). Pediatric abusive head trauma: Recommended definitions for public health surveillance and research. Atlanta, GA: Centers for Disease Control and Prevention5–16. [Google Scholar]
- Parrish J, Baldwin-Johnson C, Volz M, & Goldsmith Y (2013). Abusive head trauma among children in Alaska: A population-based assessment. International Journal of Circumpolar Health, 72 10.3402/ijch.v72i0.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece R (2008). What Are We Trying to Measure? The Problems of Case Ascertainment. American Journal Of Preventive Medicine, 34, S116–S118. [DOI] [PubMed] [Google Scholar]
- Shanahan ME, Zolotor AJ, Parrish JW, Barr RG, & Runyan DK (2013). National, regional, and state abusive head trauma: Application of the CDC algorithm. Pediatrics, 132(6), e1546–1553. 10.1542/peds.2013-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling SP, Holden JR, & Jenny C (1995). Abusive head trauma: The relationship of perpetrators to their victims. Pediatrics, 95(2), 259–262. [PubMed] [Google Scholar]
- Wirtz SJ, & Trent RB (2008). Passive surveillance of shaken baby syndrome using hospital inpatient data. American Journal of Preventive Medicine, 34(4), S134–S139. 10.1016/j.amepre.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Zolotor AJ, Runyan DK, Shanahan M, Durrance CP, Nocera M, Sullivan K, et al. (2015). Effectiveness of a statewide abusive head trauma prevention program in North Carolina. JAMA Pediatrics, 169(12), 1126–1131. 10.1001/jamapediatrics.2015.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]


