Abstract
Phthalates are chemical esters used as additives in common consumer goods, such as plastics, household cleaners, and personal care products. Phthalates are not chemically bound to the items to which they are added and can easily leach into the surrounding environment. Anthropogenic drivers, such as coastal plastic pollution and wastewater runoff, increase the exposure potential for coastal marine fauna. Phthalate exposure in free-ranging bottlenose dolphins has been the focus of recent study, with indications of heightened exposure to certain phthalate compounds. The objective of this study was to compare urinary phthalate metabolite concentrations among bottlenose dolphins (Tursiops truncatus) sampled in Sarasota Bay, FL, to levels reported in human samples collected as part of the Centers for Disease Control and Prevention’s (CDC) National Health and Nutrition Examination Survey (NHANES). Monoethyl phthalate (MEP) and mono-(2-ethylhexyl) phthalate (MEHP) were the most prevalent metabolites detected in dolphin urine (n = 51; MEP = 29.41%; MEHP = 54.90%). The geometric mean (GM) concentration of MEP was significantly lower for dolphins (GM = 4.51 ng/mL; 95% CI: 2.77–7.34 ng/mL) compared to humans (p<0.05), while dolphin concentrations of MEHP (GM = 4.57 ng/mL; 95% CI: 2.37–8.80 ng/mL) were significantly higher than levels reported in NHANES (p<0.05). Health impacts to bottlenose dolphins resulting from elevated exposure to the MEHP parent compound (diethyl-2-ethylhexyl phthalate, DEHP) are currently unknown. However, given the evidence of endocrine disruption, reproductive impairment, and abnormal development in humans, pursuing investigations of potential health effects in exposed bottlenose dolphins would be warranted.
Introduction
Phthalates are a class of manmade chemicals added to plastics, personal care products, cleaning solutions, cosmetics, and pesticides to enhance various properties such as lubrication, flexibility, and fragrance [1–3]. These chemicals are concerning to public and wildlife health because of their ubiquitous use in common goods and their potential for endocrine disruption [4–6]. Endocrine disrupting chemicals (EDCs) interfere with the normal production, secretion, or transport of hormones through the body by either mimicking naturally produced compounds or interfering with hormone receptors [7]. Ultimately, this hormone disruption can impact reproduction, development, and/or growth [4, 5, 8–12]. Humans are exposed to phthalates intravenously through the use of medical tubing or via dermal absorption, inhalation, or ingestion resulting from the use of products and materials containing phthalates [13–15]. Because of the environmental ubiquity of phthalates, humans seem particularly at risk of adverse health impacts resulting from chronic exposure.
The Centers for Disease Control and Prevention’s (CDC) National Health and Nutrition Examination Survey (NHANES) is a health assessment of randomly selected individuals across the United States. NHANES data are collected annually and use surveys and specimen collection to evaluate markers of behavioral, mental, physical, and biological health. Blood, urine, and fecal samples are collected to evaluate exposure to environmental chemicals, including phthalate metabolites [16]. Urine is the preferred sampling matrix to evaluate exposure due to the rapid hydrolysis and conjugation of diester phthalate parent compounds, thereby resulting in detectable monoester metabolites [17–20]. Phthalate exposure has been assessed in NHANES urine samples since 1999 [16], and these national surveys often serve as a comparison for studies investigating populations with heightened exposure and risk of adverse health impacts [21–23].
In contrast to persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs), dioxins, and furans, phthalates are not considered persistent environmental contaminants, but ongoing release of phthalates into the environment may lead to a chronic exposure risk to wildlife. Marine and aquatic organisms may be exposed through inhalation or ingestion of phthalate-contaminated air, water, sediment, and prey, as well as ingestion of plastic [24–27]. Studies of phthalate exposure have been widespread among marine and aquatic fauna, including the harbor porpoise (Phocoena phocoena; [28]), fin whale (Balaenoptera physalus; [26, 29]), Risso’s dolphin (Grampus griseus; [29]), striped dolphin (Stenella coeruleoalba; [29]), common bottlenose dolphin (Tursiops truncatus) [29, 30], Atlantic bluefin tuna (Thunnus thynnus; [31]), basking shark (Cetorhinus maximus; [26]), American alligator (Alligator mississippiensis; [32]), European eel (Anguilla anguilla; [33]), as well as crustacean, mollusc, and fish species [27, 34]. These studies have detected phthalate parent compounds and/or metabolites in a variety of matrices (e.g., skin, blubber, muscle, urine), with variable concentrations reported within and across species (Table 1).
Table 1. Mean concentrations (solid mass reported as wet weight (w.w.) or dry weight (d.w.)) of monoethyl phthalate (MEP) and mono-(2-ethylhexyl) phthalate (MEHP) with corresponding ranges and standard deviations as reported in varying matrices from other marine species.
| Species | N | Sampling Pd. | MEP mean (s.d.) | MEP range | MEHP mean (s.d.) | MEHP range | Matrix |
|---|---|---|---|---|---|---|---|
| Harbor porpoise (Phocoena phocoena; [28]) | 100 | 2016–2017 | 5.99 ng/g w.w. | 2.62–17.4 ng/g w.w. | - | - | Liver |
| Fin whale (Balaenoptera physalus; [26]) | 5 | 2007–2013 | - | - | Approximately 190 ng/g l.b. reported graphically | - | Blubber |
| Fin whale (Balaenoptera physalus; [29]) | 3 | 2014 | - | - | <LOD | - | Blubber |
| Risso’s dolphin (Grampus griseus; [29]) | 1 | 2014 | - | - | 463.7 ng/g d.w. | - | Blubber |
| Common Bottlenose dolphin (Tursiops truncatus; [29]) | 1 | 2014 | - | - | 1770 ng/g d.w. | - | Blubber |
| Striped dolphin (Stenella coeruleoalba; [29]) | 2 | 2014 | - | - | 1720 ng/g d.w. | - | Blubber |
| Atlantic Bluefin tuna (Thunnus thynnus; [31]) | 23 | 2012 | - | - | 2.13 (1.52) ng/g w.w. | 1.58–6.30 ng/g w.w. | Muscle |
| Basking shark (Cetorhinus maximus; [26]) | 6 | 2006–2013 | - | - | Approximately 90 ng/g l.b. reported graphically | Muscle | |
| American alligator (Alligator mississippiensis; [32]) Everglades | 9 | Sampling year not reported | - | - | 4,540 (11,800) ng/mL | ND-35,700 ng/mL | Urine |
| American alligator (Alligator mississippiensis; [32]) Okeechobee–Belle Glade | 10 | Sampling year not reported | - | - | 1,490 (1,290) ng/mL | ND-11,500 ng/mL | Urine |
| American alligator (Alligator mississippiensis; [32]) Okeechobee–Moonshine Bay | 10 | Sampling year not reported | - | - | 1,290 (3,470) ng/mL | ND-11,100 ng/mL | Urine |
| American alligator (Alligator mississippiensis; [32]) Woodruff | 9 | Sampling year not reported | - | - | 56.4 ng/mL | ND-506 ng/mL | Urine |
| American alligator (Alligator mississippiensis; [32]) Apopka | 12 | Sampling year not reported | - | - | - | - | Urine |
| European eels (Anguilla Anguilla; [33]) | 117 | 2010 | 33 (108) ng/g d.w. | - | 282 (206) ng/g d.w | - | Fillet muscle |
| Roach (Rutilus rutilus; [34]) | 4 | Sampling year not reported | 53 (15.3) ng/mL | - | 15.5 ng/mL | - | Bile |
| Roach (Rutilus rutilus; [34]) | 4 | Sampling year not reported | 28.6 (4.9) ng/mL | - | 122 (7.7) ng/mL | - | Plasma |
| Roach (Rutilus rutilus; [34]) | 4 | Sampling year not reported | 263 (154) ng/g d.w. | - | 237 (81) ng/g d.w. | - | Liver |
| Prawn [27] | 20 | 2013 | ND-6.82 ng/g w.w. | - | ND-61.6 ng/g w.w. | - | Edible tissue |
| Mollusc [27] | 6 | 2013 | 0.42–3.31 ng/g w.w. | - | 7.50–11.6 ng/g w.w. | - | Edible tissue |
| Fish [27] | 69 | 2013 | 0.06–4.70 ng/g w.w. | - | ND-24.8 ng/g w.w. | - | Edible tissue |
A recent study of free-ranging common bottlenose dolphins sampled in Sarasota Bay, Florida, USA during 2016 and 2017 (n = 17) detected phthalate metabolite concentrations among 70% of dolphins sampled, suggesting prevalent environmental exposure to these man-made chemicals [30]. As top-level predators with a long lifespan (>60 years; [35]), year-round resident bottlenose dolphins serve as sensitive gauges to detect disturbances in their local environment [36]. This has been demonstrated in epidemiologic studies of dolphin health impacts linked with PCBs [37, 38], harmful algal blooms [39], and oil-associated toxin exposure [38, 40]. Unfortunately, the extent, sources, and impacts of widespread phthalate exposure in bottlenose dolphins are not yet understood. The objective of this study was to compare bottlenose dolphin phthalate metabolite concentrations to levels reported for human reference populations (i.e., NHANES) and use a One Health approach to develop hypotheses for phthalate-associated health impacts for exposed bottlenose dolphins. “One Health” is a term used to describe gained knowledge of wildlife health by studying humans, and vice versa [41]. In this study, phthalate-associated health effects reported in human epidemiological studies were used to predict potential dolphin health impacts that will be explored in future investigations.
Materials and methods
Dolphin study population
Bottlenose dolphins have been the subject of population and health studies in Sarasota Bay, FL since 1970 [42]. The study area includes inshore waters between southern Tampa Bay (approximately 27.56°N) and Venice Inlet (approximately 27.10°N), and offshore of the barrier islands to approximately 82.75°W. Using well-established techniques developed and refined over 50 years, free-ranging dolphins were encircled by a net and temporarily restrained to collect biological samples indicative of an individual’s health [36]. The sex of each dolphin was determined by physical examination, and age was estimated by either known birth year or the observation of dentinal growth layers [43]. A combination of factors were used to determine maturity status including age (≥ 10 years; [44]), calving history, pregnancy diagnosis via ultrasonography, or sex hormone concentrations [45, 46]. Bottlenose dolphin health assessments were conducted under scientific research permit #522–1785, #15543, and #20455 from the National Oceanic and Atmospheric Administration’s (NOAA) National Marine Fisheries Service (NMFS), and research studies were reviewed and approved annually by Mote Marine Laboratory’s Institutional Animal Care and Use Committee (IACUC).
Urinary phthalate metabolite detection and quantification
This study relied upon bottlenose dolphin urinary metabolite concentrations reported in Hart et al. [30] (sample years 2016–2017), as well as results from analyses conducted on samples collected 2010–2015 and 2018–2019. Urine samples were opportunistically collected aseptically via catheterization from bottlenose dolphins during routine health assessments conducted under permit from the National Oceanic and Atmospheric Administration’s (NOAA) National Marine Fisheries Service (NMFS) between 2010 and 2019, as described in Wells [42] and Hart et al. [30]. Archived urine samples for the years 2010–2015 were retrieved from the Sarasota Dolphin Research Program’s specimen bank, where urine samples were stored frozen at -80°C. The protocol for sample collection and storage for years 2016–2019 are described in Hart et al. [30]. All urine samples were screened for eight phthalate metabolites including: monomethyl phthalate (MMP), monoethyl phthalate (MEP), monoisobutyl phthalate (MiBP), monobutyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP). Methods for analyzing phthalate metabolite concentrations in bottlenose dolphin urine were based on protocols established in Hart et al. [30] and conducted at the NOAA National Centers for Coastal Ocean Science (NCCOS) lab in Charleston, SC, USA. Briefly, urine samples were spiked with 13C-labeled internal standards prior to an enzymatic de-glucuronidation step. Phthalate metabolites were isolated by solid phase extraction (SPE) using an Agilent Bond Elute Nexus SPE. Male samples that had excess sperm were centrifuged (1,000 rpm for 10 minutes) prior to extraction to separate the urine and prevent the SPE cartridge from becoming clogged. Analytical separation, detection, and quantification of phthalate metabolites in urine were performed using high performance liquid chromatography (HPLC; Agilent 1100) paired with electrospray ionization (ESI) tandem mass spectrometry (AB Sciex API 4000; ESI- mode). Compounds were separated using a C18 analytical column (Waters XBridge 50 mm x 2.1 mm; 2.5 μm particle size) with a gradient mobile phase of HPLC water with 0.1% acetic acid and acetonitrile with 0.1% acetic acid. Analytes of interest were identified using multiple reaction monitoring (MRM; S2 Table) and quantified against standard calibration curves [17, 30], and the limit of detection (LOD) was determined for each metabolite based on the lowest point of the calibration curve that could be detected on the instrument, divided by the volume of urine extracted. Complete details of the instrumental methodology is detailed in Hart et al. [30]. As reported in Hart et al. [30] quality assurance and control methods included laboratory spikes, laboratory blanks, field blanks, a standard reference material (SRM 3672), and matrix spikes. Both lab and field blanks were used to correct urine samples for equipment contamination. Urine samples were run in batches of 10–12, with one corresponding blank per batch. Field blanks were taken in triplicate for each week where sampling occurred and used to correct all samples in the corresponding year. In addition, calibration verification was conducted for each batch of urine samples to ensure the integrity of the calibration curve.
Human reference population data
Human geometric mean concentrations (ng/mL) were retrieved from NHANES for the following study periods: 2009–2010; 2011–2012; 2013–2014; 2015–2016; concentrations used for analyses were not corrected for creatinine [16]. These study periods were selected due to the temporal overlap with our bottlenose dolphin samples (2010–2019), although NHANES concentrations were unavailable for years following 2016. Phthalate metabolite concentrations were measured in randomly selected individuals from the total NHANES sample population for a given study period (n~2,500; [16]). Metabolite concentrations were quantified from urine samples using mass spectrometry in the CDC’s Environmental Health Laboratory [17], and non-creatine corrected measurements were used for comparisons to bottlenose dolphins [16].
Statistical analysis
Minimum, maximum, and geometric mean (GM) concentrations were calculated for all phthalate metabolites where concentrations exceeded the limit of detection (LOD) for at least 10% of the bottlenose dolphin study sample. Geometric means were calculated for all detectable concentrations across the entire study period (2010–2019). Concentrations were natural log-transformed prior to testing for correlation between major metabolites. Geometric mean concentrations of detectable phthalate metabolites (i.e., concentrations >LOD) were compared between bottlenose dolphins and human reference populations by evaluating overlap of the 95% confidence intervals for each pairwise comparison (e.g., dolphin GM (2010–2019) vs. NHANES GM (2009–2010) [47]. For each NHANES study period (2009–2010; 2011–2012; 2013–2014; 2015–2016), comparisons between bottlenose dolphins and humans relied on reported geometric mean concentrations for the total NHANES sample (i.e., all age groups, gender, and race/ethnicity). Statistical significance was evaluated using α = 0.05 and all analyses were performed using Statistica (v. 13.3, Tibco Software Inc., Palo Alto, CA) or R (v. 3.6.1, R Foundation for Statistical Computing, Vienna, Austria) computing software.
Results
Study sample details
Between 2010 and 2019, 69 urine samples were screened for detectable concentrations of phthalate metabolites. These samples included 17 dolphin specimens reported in Hart et al. [30]. Thirteen dolphins were repeatedly evaluated in Sarasota Bay health assessments during the study period, but only the most recent specimens were used for the analysis herein (n = 51 unique individuals). More than half of the dolphins were female (58.82%), and 66.67% were considered adults.
Overall phthalate metabolite detection in sampled bottlenose dolphins
Detectable concentrations (i.e., concentrations > LOD) of at least one metabolite were measured in 74.51% of individual dolphins sampled (n = 51; 2010–2019). Limits of detection are provided in Table 2. In addition to data reported by Hart et al. [30], the most commonly detected metabolites were MEHP (n = 28) and MEP (n = 15; S1 Table).
Table 2. Range of limits of detection for phthalate metabolites (ng/mL) measured in urine samples from common bottlenose dolphins sampled during health assessments conducted in Sarasota Bay, FL, USA (2010–2019).
| MMP | MEP | MEHP | MEOHP | MEHHP | MBzP | MBP | MiBP | |
|---|---|---|---|---|---|---|---|---|
| LOD | 0.10–0.167 | 1.0–3.43 | 0.24–0.60 | 0.10–0.70 | 0.20–0.90 | 0.10–0.80 | 0.50–0.85 | 0.50–0.983 |
This table includes limits of detection reported in Hart et al. [30].
Findings by major metabolites
MEHP was the most commonly detected metabolite among sampled bottlenose dolphins (54.90%), with a geometric mean concentration of 4.57 ng/mL (95% CI:2.37–8.80 ng/mL) and overall range of <LOD to 76.60 ng/mL (Table 3). Approximately 29% of screened dolphins had detectable concentrations of MEP, with a geometric mean of 4.51 ng/mL (95% CI: 2.77–7.34 ng/mL) and range of <LOD to 33.40 ng/mL (Table 3). Natural-log transformed concentrations of MEP and MEHP were not significantly correlated (r = -0.12; p = 0.41). In fact, only nine of the 28 dolphins (32.14%) with detectable concentrations of MEHP had detectable concentrations of MEP.
Table 3. Summary of common phthalate metabolites (ng/mL) detected in common bottlenose dolphins sampled during health assessments conducted in Sarasota Bay, FL, USA (2010–2019).
| MEP | MEHP | |
|---|---|---|
| Number >LOD | 15 | 28 |
| % Above Limit of Detection | 29.41 | 54.90 |
| Minimum1 | 1.30 | 0.26 |
| Maximum1 | 33.40 | 76.60 |
| GM among detects (95% CI)1 | 4.51 (2.77–7.34) | 4.57 (2.37–8.80) |
1reported for dolphins with concentrations >LOD for metabolite.
GM = geometric mean.
Comparisons between bottlenose dolphin and human concentrations
Comparisons to human urinary phthalate metabolite concentrations were conducted for detectable concentrations of MEP and MEHP. Geometric mean concentrations of MEP ranged between 33.60 and 64.40 ng/mL for NHANES study samples (2009–2016; [16]; Table 4). The geometric mean concentration of MEP for bottlenose dolphins (4.51 ng/mL; 95% CI: 2.77–7.34 μg/L) was significantly lower than NHANES for all study years (p<0.05, Table 4 and Fig 1). For MEHP, NHANES geometric mean concentrations were only reported for 2009 (1.59 ng/mL) and 2010 (1.36 ng/mL) because of the high prevalence of non-detectable concentrations in subsequent years ([16]; Table 4). In our study, the geometric mean concentration of detectable MEHP (4.57 ng/mL; 95% CI:2.37–8.80 ng/mL) was significantly higher than NHANES 2009 and 2010 (p<0.05, Table 4 and Fig 1).
Table 4. Comparison of detectable concentrations of urinary phthalate metabolites between bottlenose dolphins sampled in Sarasota Bay, FL, USA (2010–2019) and human reference populations (NHANES; 2009–2010, 2011–2012, 2013–2014, 2015–2016).
| Bottlenose Dolphins | NHANES 2009–2010 | p3 | NHANES 2011–2012 | p3 | NHANES 2013–2014 |
p3 | NHANES 2015–2016 | p3 | |
|---|---|---|---|---|---|---|---|---|---|
| MEHP | <0.05 | <0.05 | - | - | |||||
| N | 28 | 2,749 | 2,489 | 2,685 | 2,975 | ||||
| GM_detects1 (95% CI) | 4.57 (2.37–8.80) | 1.59 (1.41–1.79) | 1.36 (1.25–1.49) | NA2 | - | NA2 | - | ||
| MEP | |||||||||
| N | 15 | 2,749 | <0.05 | 2,489 | <0.05 | 2,685 | <0.05 | 2,975 | <0.05 |
| GM_detects1 (95% CI) | 4.51 (2.77–7.34) | 64.40 (58.30–71.20) | 37.90 (33.00–43.50) | 35.70 (32.10–39.80) | 33.60 (29.30–38.40) |
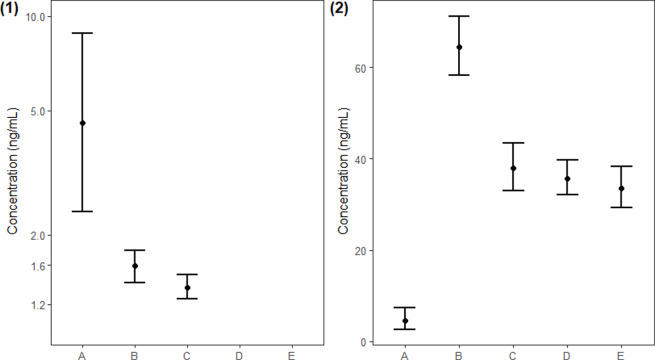
Fig 1.
Geometric mean concentrations and 95% confidence intervals for detectable phthalate metabolites for Sarasota Bay bottlenose dolphins (“A”) and NHANES human reference populations (“B-E”: 1) MEHP (n = 28); 2) MEP (n = 15). Bottlenose Dolphin (A); NHANES 2009–2010 (B); NHANES 2011–2012 (C); NHANES 2013–2014 (D); NHANES 2015–2016 (E).
Discussion
This study revealed prevalent exposure (74.51%, n = 51) to phthalates among free-ranging bottlenose dolphins sampled in Sarasota Bay, FL (2010–2019). MEHP was detected in over half of the individuals sampled (n = 28), while MEP was detected in nearly one-third (n = 15) of bottlenose dolphins. Detectable concentrations of these metabolites were highly variable for bottlenose dolphins in this sample (MEHP: 0.39–76.60 ng/mL; MEP: 1.60–33.40 ng/mL), which has been observed in other species (e.g., American alligator, Mediterranean fin whale, harbor porpoise; Table 1), and could be attributed to diet, metabolism, or spatiotemporal exposure differences [25, 32]. Environmental introduction of phthalates can occur via wastewater contamination, groundwater intrusion via landfill leakage, surface runoff, direct application (e.g., agricultural, residential, and industrial pesticides and fertilizers), or atmospheric evaporation [1, 3, 48, 49]. Removal of phthalate metabolites from wastewater is variable, ranging between 29% and 100% depending on the compound, treatment methodology, and treatment time [48, 49]. Once in the environment, persistence in different substrates can be affected by temperature, precipitation, microbial conditions, and chemical properties [49].
Dolphin-human comparisons: MEP and MEHP
Bottlenose dolphin MEP concentrations were significantly lower than reported exposure in human reference populations [16]. MEP is the monoester metabolite of diethyl phthalate (DEP), which is added to a wide range of commercial goods including plastic items, food packaging, personal care products, cosmetics, pesticides, and some medications [1, 50–52]. Humans are directly exposed via inhalation, ingestion, or dermal absorption of products made with DEP or contaminated by DEP, albeit at variable concentrations [1, 14]. Epidemiologic studies of human exposure to DEP demonstrate higher risk among users of certain personal care products, especially cosmetics and products containing fragrance [53–58]. Given the primary sources for DEP exposure in humans, it is not surprising that bottlenose dolphin concentrations of MEP were significantly lower than NHANES levels. Compared to other commonly used phthalate esters, DEP has a lower molecular weight, is more quickly degraded by microbes, and has a shorter half-life in marine environments (< 1 day to 2 weeks; [49, 59]). As a result, DEP may be less bioavailable than other phthalate parent compounds for free-ranging bottlenose dolphins.
The geometric mean concentration of MEHP for bottlenose dolphins was significantly higher than NHANES concentrations [16]. MEHP is a metabolite of di-(2-ethylhexyl) phthalate (DEHP), which is primarily used to increase the flexibility of plastic (e.g., polyvinyl chloride, food packaging, wire covering, toys, medical tubing; [3, 50, 60]). DEHP can also be found in cosmetics, personal care products, oil and paint [3], although studies have suggested this is due to migration from the plastic containers housing these products [14, 61]. Because of weak bonds with the corresponding substrate, DEHP can be easily leached into food and the environment [3, 60].
Human exposure occurs primarily through the ingestion of dust and food stored in packaging containing DEHP, particularly dairy, meats, and other fatty foods as DEHP is lipophilic [3, 14, 60–62]. The highest doses of exposure, however, occur among people requiring transfusions or dialysis due to DEHP-containing medical equipment [3]. Because DEHP is one of the most abundantly used phthalates, contamination of the natural environment is expected. DEHP introduction into the marine environment can occur via agricultural [63] and non-permeable surface runoff [48], as well as wastewater effluent, although removal can be quite high depending on treatment methods (e.g., up to 95%, [48]). DEHP is the most prevalent phthalate detected in freshwater, soil, atmospheric and landfill leachate samples, and this environmental pervasiveness is likely due to many factors including high production, increased urbanization, and chemical properties that slow down the biodegradation process [49]. Resident dolphins in Sarasota Bay, FL are considered selective feeders, choosing soniferous fish disproportionately relative to their availability [64, 65]. While diet may be a source of exposure to DEHP for bottlenose dolphins, evidence from Staples et al. [59] suggests that prey ingestion is not likely the primary exposure route. In fact, Staples et al. [59] demonstrated that phthalate ester concentrations actually decrease with increasing trophic levels and suggested that higher-order metabolic biotransformation might outpace bioaccumulation.
Plastics are of increasing concern to environmental health because of their ubiquitous use in industrial settings and in the production of many commercial goods [66], combined with the fact that plastic waste materials are slow to degrade and therefore persist in the environment [67]. Geyer et al. [68] estimated a global plastic production of 380 million tons in 2015, of which 11% was polyvinyl chloride. Plastic additives, composed primarily of plasticizers such as phthalates, accounted for 7% of non-fiber plastic mass [68]. Approximately 60% of plastics are disposed of in landfills or the natural environment [68], so it seems reasonable that plastic pollution could be a source of phthalate exposure for marine fauna. Marine plastic debris is often categorized by size [67]; macro- and mesoplastics (≥ 5mm diameter) enter the marine environment directly as waste, while microplastics may not be filtered out by water treatment facilities or result from fragmentation of larger plastic items [24, 67]. Eriksen et al. [69] estimated that our oceans contain over 5.25 trillion plastic particles, of which 92.4% are microplastics. Previous cetacean studies provide evidence of a link between environmental microplastic contamination and phthalate exposure, based on blubber samples of stranded cetaceans and water/plankton samples from nearby seas and estuaries [25, 26, 29]. MEHP concentrations measured in blubber and muscle samples from stranded fin whales and bycaught basking sharks were higher in animals sampled in regions with significantly higher water and plankton microplastic concentrations [25, 26]. While the potential exposure sources of DEHP and other phthalate compounds are not yet understood for Sarasota Bay bottlenose dolphins, the detection of high concentrations of MEHP warrants further investigation.
Potential implications for bottlenose dolphin exposure and health impacts
Between 2010 and 2019, Sarasota Bay bottlenose dolphin urinary concentrations of MEHP ranged between <LOD and 76.60 μg/L, with a geometric mean concentration of 4.57 μg/L (95% CI: 2.37–8.80 μg/L). Upon exposure, DEHP is hydrolyzed into MEHP and conjugated before urinary excretion. Hydrolysis occurs in the liver through mechanisms involving the alpha-mediated enzymes of the peroxisome proliferator-activated receptor (PPAR⍺) and efficiency can vary among individuals. Metabolites of DEHP (e.g., MEHP, MEOHP, MEHHP) can bind to PPAR⍺ and disrupt normal kidney, liver, heart, and reproductive function [60]. In humans, higher MEHP concentrations have been associated with myriad health impacts including decreased oocyte counts [70], early pregnancy loss [71], reduced sperm quality [72], and abnormal reproductive development [73], among others. These phthalate-associated health impacts may be due to interference in steroid, sex, or thyroid hormone circulation, but the occurrence and magnitude of endocrine disruption seem to vary by phthalate ester type, degree of exposure, sex, pregnancy, and age [3–5, 8–12, 60, 74–79]. While epidemiological studies in humans and experimental studies in laboratory rodents indicate potential health risks for bottlenose dolphins, we found that direct comparisons to human studies were hindered as most papers reported concentrations were adjusted for creatinine or specific gravity. Additionally, NHANES concentrations of MEHP have declined in recent years [16], suggesting reduced exposure and inhibiting studies to identify and validate health impacts. Thus, continued study of Sarasota Bay bottlenose dolphins will enable epidemiological investigations of hormonal, physiological, and reproductive correlates with phthalate exposure, due in large part to long-term monitoring efforts and regularly conducted health assessments.
Study strengths and limitations
This study relied upon well-established analytical methods developed by the CDC to screen for phthalate metabolites in mammalian urine samples. Urinary phthalate metabolites are considered the most reliable indicators of exposure in human populations due to the rapid metabolism of these chemicals, and because sampling equipment can be contaminated with parent compounds during analysis [18, 80, 81]. Additionally, several methods were used to avoid sample and analytical contamination and validate reported phthalate metabolite data measured in these dolphin samples. The cross-sectional study design, however, reflects prevalent, rather than cumulative, exposure. Studies in humans suggest urinary phthalate metabolite concentrations measured in spot urine samples can reflect prior exposure to parent compounds of a time spanning months to a year (3–6 months; [17, 82]); however, this information is not yet available for dolphins.
To our knowledge, this is the largest assessment of phthalate exposure within any wild marine mammal species. The large sample size provided statistical power to facilitate comparisons between dolphin and human concentrations. To facilitate comparability between study samples, we selected NHANES years that overlapped the timing of our dolphin sample collection, thereby helping to control for decreasing trends in human exposure to MEP and MEHP over time [16]. We observed significant differences in exposure to MEP (lower in dolphins) and MEHP (higher in dolphins), but despite having different routes and sources for exposure, metabolic differences between dolphins and humans should also be considered as a possible explanation for divergent metabolite concentrations. Resting metabolic rate (RMR) for a 150 kg bottlenose dolphin in Sarasota Bay is estimated to be 3.9 mL O2 min-1 kg-1 [83], while McMurray et al. (2014) [84] estimate an average RMR between 2.8 mL O2 min-1 kg-1 and 3.0 mL O2 min-1 kg-1 for men and women. Physiologic adaptations to help dolphins dive, thermoregulate, and forage are likely to impact the biotransformation of phthalate parent compounds; however, these mechanisms, as they relate to phthalate metabolism, are not currently understood.
Finally, laboratory and human epidemiological studies investigating phthalate-related health impacts have demonstrated mixed findings [3, 72, 85] likely due to biases in study design, data collection, or sampling demographic. As such, future studies to investigate bottlenose dolphin phthalate exposure and health impacts should consider the influence of potential confounding variables.
Conclusions
Findings from this study indicate higher exposure to MEHP among bottlenose dolphins inhabiting Sarasota Bay, FL, compared to U.S. human reference populations; however, the significance of these results is uncertain. For decades, bottlenose dolphins have been considered sentinels of environmental health and indicators of potential health risks to human users of coastal resources [86]. For example, studies of bottlenose dolphins inhabiting waters near Brunswick, GA and Miami, FL, documented unprecedented exposure to toxic, polychlorinated biphenyl compounds (PCBs; [87–90]), which corresponded with high exposure risks for humans living in the same area [91]. Backer et al. [91] suggest that because of trophic concurrence, dolphins can gauge and even predict environmental pollution risks for coastal human populations (and vice versa). Similarly, Rabinowitz et al. [92] state that ‘shared health risks’ can facilitate the comparison of exposure risks and corresponding health outcomes between wildlife and human populations. Health impacts to bottlenose dolphins resulting from elevated exposure to the MEHP parent compound (DEHP) are currently unknown. Accordingly, studies relying on long-term reproductive health data collected from Sarasota Bay bottlenose dolphins to investigate associations between phthalate exposure and indicators of endocrine disruption, reproductive impairment, or abnormal growth and development are underway.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
A special thanks to Dr. Barbara Beckingham, Kerry Wischusen, and Moriah Alten Flagg for their contributions to method development and sample analysis for the preliminary study. We also greatly appreciate Amanda Moors, Brian Balmer, Sunnie Brenneman, and Christina Toms for their assistance with protocol development, sample collection, and urine processing. We acknowledge and sincerely appreciate the contributions from staff, collaborators, and volunteers of the Sarasota Dolphin Research Program for ensuring the safe capture, sampling, and release of the dolphins. Finally, we thank Allisan Beck, Wayne McFee, and Paul Pennington, for their review of this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Funding to support this work was provided by an anonymous donor ([LH], no grant number), the College of Charleston’s School of Education, Health, and Human Performance Dean's Discretionary Funding, ([LH], no grant number), the College of Charleston’s Department of Health and Human Performance Research and Development Funds ([LH], no grant number), and the Masters of Environmental and Sustainability Studies’ Student Association ([MD], student travel funds, no grant number). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. CSS Inc. provided support in the form of salaries for authors [EP], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dolphin Quest Inc. provided support for bottlenose dolphin health assessments but had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.ATSDR Toxicological Profile for Diethyl Phthalate. Agency for Toxic Substances and Disease Registry. 1995. [PubMed]
- 2.Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006. February;29(1):134–9. 10.1111/j.1365-2605.2005.00567.x [DOI] [PubMed] [Google Scholar]
- 3.ATSDR Toxicological Profile for Di(2-Ethylhexyl)Phthalate (DEHP) Draft for Public Comment. Agency for Toxic Substances and Disease Registry. 2019. [PubMed]
- 4.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008. October;108(2):177–84. 10.1016/j.envres.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc B Biol Sci. 2009. July 27;364(1526):2097–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang XF, Li Y, Gu YH, Liu M, Xu Y, Yuan Y, et al. the effects of di-(2-ethylhexyl)-phthalate exposure on fertilization and embryonic development in vitro and testicular genomic mutation in vivo. PLoS One. 2012. November 30;7(11). 10.1371/journal.pone.0050465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Coster S, Van Larebeke N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J Enviro and Public Health. 2012. May 10; 10.1155/2012/713696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell FP. Effects of phthalate esters on lipid metabolism in various tissues, cells and organelles in mammals. Environ Health Perspect. 1982. November;45:41–50. 10.1289/ehp.824541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latini G, Verrotti A, De Felice C. Di-2-ethylhexyl phthalate and endocrine disruption: a review. Curr Drug Targets Immune Endoc Metabol Disord. 2004. March 4 4(1):37–40. 10.2174/1568008043340017 [DOI] [PubMed] [Google Scholar]
- 10.Meeker JD, Ferguson KK. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J Clin Endocrinol Metab. 2014. November 1;99(11):4346–52. 10.1210/jc.2014-2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathieu-Denoncourt J, Wallace SJ, de Solla SR, Langlois VS. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen Comp Endocrinol. 2015. August 1;219:74–88. 10.1016/j.ygcen.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 12.Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, et al. Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J Clin Endocrinol Metab. 2017. June 1;102(6):1870–8. 10.1210/jc.2016-3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendiola J, Meeker JD, Jørgensen N, Andersson AM, Liu F, Calafat AM, et al. Urinary concentrations of Di(2-ethylhexyl) phthalate metabolites and serum reproductive hormones: Pooled analysis of fertile and infertile men. J Androl. 2012. May;33(3):488–98. 10.2164/jandrol.111.013557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Kannan K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol. 2013. December 17;47(24):14442–9. 10.1021/es4042034 [DOI] [PubMed] [Google Scholar]
- 15.Giulivo M, Lopez de Alda M, Capri E, Barceló D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res. 2016. November;151:251–264. 10.1016/j.envres.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 16.CDC. Fourth National Report on Human Exposure to Environmental Chemicals Update. Center for Disease Control and Prevention. 2019; Available from: https://www.cdc.gov/nchs/nhanes/.
- 17.Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, et al. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000. October;108(10):979–82. 10.1289/ehp.00108979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frederiksen H, Skakkebæk NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007. July 51(7):899–911. 10.1002/mnfr.200600243 [DOI] [PubMed] [Google Scholar]
- 19.Wittassek M, Angerer J. Phthalates: Metabolism and exposure. Int J Androl. 2008. April;31(2):131–8. 10.1111/j.1365-2605.2007.00837.x [DOI] [PubMed] [Google Scholar]
- 20.CDC. Laboratory Procedure Manual: Metabolites of phthalates and phthalate alternatives (Method No. 6306.07). Centers for Disease Control and Prevention. 2012.
- 21.Koch HM, Rüther M, Schütze A, Conrad A, Pälmke C, Apel P, et al. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int J Hyg Environ Health. 2017. March 1;220(2):130–41. 10.1016/j.ijheh.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 22.Rahbar M, Swingle H, Christian M, Hessabi M, Lee M, Pitcher M, et al. environmental exposure to dioxins, dibenzofurans, bisphenol a, and phthalates in children with and without autism spectrum disorder living near the Gulf of Mexico. Int J Environ Res Public Health [Internet]. 2017. November 21;14(11):1425 10.3390/ijerph14111425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varshavsky J, Morello-Frosch R, Harwani S, Snider M, Petropoulou S-S, Park J-S, et al. a pilot biomonitoring study of cumulative phthalates exposure among Vietnamese american nail salon workers. Int J Environ Res Public Health. 2020. November 12;17(1):325 10.3390/ijerph17010325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole M, Lindeque P, Halsband C, Galloway TS. Microplastics as contaminants in the marine environment: A review. Mar Pollut Bull. 2011;62:2588–97. 10.1016/j.marpolbul.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 25.Fossi MC, Panti C, Guerranti C, Coppola D, Giannetti M, Marsili L, et al. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar Pollut Bull. 2012. November 1;64(11):2374–9. 10.1016/j.marpolbul.2012.08.013 [DOI] [PubMed] [Google Scholar]
- 26.Fossi MC, Coppola D, Baini M, Giannetti M, Guerranti C, Marsili L, et al. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: The case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus). Mar Environ Res. 2014. September;100:17–24. 10.1016/j.marenvres.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Gu Y, Huang W, Yin D. Phthalate monoesters as markers of phthalate contamination in wild marine organisms. Environ Pollut. 2016. November 1;218:410–8. 10.1016/j.envpol.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 28.Rian MB, Vike-Jonas K, Gonzalez SV, Ciesielski TM, Venkatraman V, Lindstrøm U, et al. Phthalate metabolites in harbor porpoises (Phocoena phocoena) from Norwegian coastal waters. Environ Int. 2020. April 1;137:105525 10.1016/j.envint.2020.105525 [DOI] [PubMed] [Google Scholar]
- 29.Baini M, Martellini T, Cincinelli A, Campani T, Minutoli R, Panti C, et al. First detection of seven phthalate esters (PAEs) as plastic tracers in superficial neustonic/planktonic samples and cetacean blubber. Anal Methods. 2017. March 7;9(9):1512–20. 10.1039/C6AY02674E [DOI] [Google Scholar]
- 30.Hart LB, Beckingham B, Wells RS, Alten Flagg M, Wischusen K, Moors A, et al. Urinary Phthalate Metabolites in Common Bottlenose Dolphins (Tursiops truncatus) From Sarasota Bay, FL, USA. GeoHealth. 2018. October 1;2(10):313–26. 10.1029/2018GH000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerranti C, Cau A, Renzi M, Badini S, Grazioli E, Perra G, et al. Phthalates and perfluorinated alkylated substances in Atlantic bluefin tuna (Thunnus thynnus) specimens from Mediterranean Sea (Sardinia, Italy): Levels and risks for human consumption. J Environ Sci B. 2016. October 2;51(10):661–7. 10.1080/03601234.2016.1191886 [DOI] [PubMed] [Google Scholar]
- 32.Brock JW, Bell JM, Guillette LJ. Urinary phthalate metabolites in american alligators (alligator mississippiensis) from selected florida wetlands. Arch Environ Contam Toxicol. 2016. July 1;71(1):1–6. 10.1007/s00244-015-0260-6 [DOI] [PubMed] [Google Scholar]
- 33.Fourgous C, Chevreuil M, Alliot F, Amilhat E, Faliex E, Paris-Palacios S, et al. Phthalate metabolites in the European eel (Anguilla anguilla) from Mediterranean coastal lagoons. Sci Total Environ. 2016. November 1;569–570:1053–9. 10.1016/j.scitotenv.2016.06.159 [DOI] [PubMed] [Google Scholar]
- 34.Valton AS, Serre-Dargnat C, Blanchard M, Alliot F, Chevreuil M, Teil MJ. Determination of phthalates and their by-products in tissues of roach (Rutilus rutilus) from the Orge river (France). Environ Sci Pollut Res. 2014. June 26;21(22):12723–30. 10.1007/s11356-014-3213-0 [DOI] [PubMed] [Google Scholar]
- 35.Wells RS. Social Structure and Life History of Bottlenose Dolphins Near Sarasota Bay, Florida: Insights from Four Decades and Five Generations In: Yamagiwa J. & Karczmarski L, editors. Primates and Cetaceans. Tokyo, Japan: Springer; 2014. pp. 149–72. [Google Scholar]
- 36.Wells R, Rhinehart H, Hansen L, Sweeney J, Townsend F, Stone R, et al. Bottlenose Dolphins as Marine Ecosystem Sentinels: Developing a Health Monitoring System. Ecohealth. 2004. September 28;1(3):246–54. 10.1007/s10393-004-0094-6 [DOI] [Google Scholar]
- 37.Wells RS, Tornero V, Borrell A, Aguilar A, Rowles TK, Rhinehart HL, et al. Integrating life-history and reproductive success data to examine potential relationships with organochlorine compounds for bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Sci Total Environ. 2005. October 15;349(1–3):106–19. 10.1016/j.scitotenv.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 38.Schwacke LH, Smith CR, Townsend FI, Wells RS, Hart LB, Balmer BC, et al. Health of common bottlenose dolphins (Tursiops truncatus) in Barataria Bay, Louisiana, following the Deepwater Horizon oil spill. Environ Sci Technol. 2014. January 7;48(1):93–103. 10.1021/es403610f [DOI] [PubMed] [Google Scholar]
- 39.Twiner MJ, Fire S, Schwacke L, Davidson L, Wang Z, Morton S, et al. Concurrent exposure of bottlenose dolphins (Tursiops truncatus) to multiple algal toxins in Sarasota Bay, Florida, USA. PLoS One. 2011;6(3). 10.1371/journal.pone.0017394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith C, Rowles T, Hart L, Townsend F, Wells R, Zolman E, et al. Slow recovery of Barataria Bay dolphin health following the Deepwater Horizon oil spill (2013–2014), with evidence of persistent lung disease and impaired stress response. Endanger Species Res [Internet]. 2017. January 31;33(1):127–42. 10.3354/esr00778 [DOI] [Google Scholar]
- 41.Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev Vet Med. 2011. September 1;101(3–4):148–56. 10.1016/j.prevetmed.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells RS. Learning from nature: Bottlenose dolphin care and husbandry. Zoo Biol. 2009;28(6):635–51. 10.1002/zoo.20252 [DOI] [PubMed] [Google Scholar]
- 43.Hohn AA, Scott MD, Wells RS, Sweeney JC, Irvine AB. Growth layers in teeth from known‐age, free‐ranging bottlenose dolphins. Mar Mammal Sci. 1989. October;5(4):315–42. 10.1111/j.1748-7692.1989.tb00346.x [DOI] [Google Scholar]
- 44.Read AJ, Wells RS, Hohn AA, Scott MD. Patterns of growth in wild bottlenose dolphins, Tursiops truncatus. J Zool. 1993. September 1;231(1):107–23. 10.1111/j.1469-7998.1993.tb05356.x [DOI] [Google Scholar]
- 45.Wells RS, Scott MD, Irvine AB. The Social Structure of Free-Ranging Bottlenose Dolphins. Curr Mammal. 1987;247–305. [Google Scholar]
- 46.Wells RS, Smith CR, Sweeney JC, Townsend FI, Fauquier DA, Stone R, et al. Fetal survival of common bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Aquat Mamm. 2014. January 1;40(3):252–9. [Google Scholar]
- 47.Cumming G, Finch S. Inference by eye confidence intervals and how to read pictures of data. Am Psychol. 2005. Fem Mar; 60(2):170–80. 10.1037/0003-066X.60.2.170 [DOI] [PubMed] [Google Scholar]
- 48.Clara M, Windhofer G, Hartl W, Braun K, Simon M, Gans O, et al. Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere. 2010. February 1;78(9):1078–84. 10.1016/j.chemosphere.2009.12.052 [DOI] [PubMed] [Google Scholar]
- 49.Gao DW, Wen ZD. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci Total Environ. 2016. January 15;541:986–1001. 10.1016/j.scitotenv.2015.09.148 [DOI] [PubMed] [Google Scholar]
- 50.Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect. 2012. July;120(7)935–43. 10.1289/ehp.1104052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helm JS, Nishioka M, Brody JG, Rudel RA, Dodson RE. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018. August 1;165:448–58. 10.1016/j.envres.2018.03.030 [DOI] [PubMed] [Google Scholar]
- 52.Gao CJ, Kannan K. Phthalates, bisphenols, parabens, and triclocarban in feminine hygiene products from the United States and their implications for human exposure. Environ Int. 2020. March;136:105465 10.1016/j.envint.2020.105465 [DOI] [PubMed] [Google Scholar]
- 53.Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(5):459–66. 10.1038/jes.2013.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallner P, Kundi M, Hohenblum P, Scharf S, Hutter HP. Phthalate metabolites, consumer habits and health effects. Int J Environ Res Public Health. 2016. July 15;13(7). 10.3390/ijerph13070717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nassan FL, Coull BA, Gaskins AJ, Williams MA, Skakkebaek NE, Ford JB, et al. Personal care product use in men and urinary concentrations of select phthalate metabolites and parabens: Results from the environment and reproductive health (EARTH) study. Environ Health Perspect. 2017. August 1;125(8). 10.1289/EHP1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher M, Arbuckle TE, MacPherson S, Braun JM, Feeley M, Gaudreau É. Phthalate and BPA Exposure in Women and Newborns through Personal Care Product Use and Food Packaging. Environ Sci Technol. 2019. September 17;53(18):10813–26. 10.1021/acs.est.9b02372 [DOI] [PubMed] [Google Scholar]
- 57.Husøy T, Andreassen M, Hjertholm H, Carlsen MH, Norberg N, Sprong C, et al. The Norwegian biomonitoring study from the EU project EuroMix: Levels of phenols and phthalates in 24-hour urine samples and exposure sources from food and personal care products. Environ Int. 2019. November;132:105103 10.1016/j.envint.2019.105103 [DOI] [PubMed] [Google Scholar]
- 58.Koppen G, Govarts E, Vanermen G, Voorspoels S, Govindan M, Dewolf MC, et al. Mothers and children are related, even in exposure to chemicals present in common consumer products. Environ Res. 2019. August 1;175:297–307. 10.1016/j.envres.2019.05.023 [DOI] [PubMed] [Google Scholar]
- 59.Staples CA, Peterson DR, Parkerton TF, Adams WJ. The environmental fate of phthalate esters: A literature review. Chemosphere. 1997. August;35(4):667–749. 10.1016/S0045-6535(97)00195-1 [DOI] [Google Scholar]
- 60.Ito Y, Kamijima M, Nakajima T. Di(2-ethylhexyl) phthalate-induced toxicity and peroxisome proliferator-activated receptor alpha: A review. Environ Health Prev Med. 2019; 24 10.1186/s12199-019-0802-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pacyga DC, Sathyanarayana S, Strakovsky RS. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv Nutr. 2019. September 1;10(5):803–15. 10.1093/advances/nmz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ Health. 2014. June 2;13(1):43 10.1186/1476-069X-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu XY, Wen B, Shan XQ. Survey of phthalate pollution in arable soils in China. J Environ Monit. 2003. August;5(4):649–53. 10.1039/b304669a [DOI] [PubMed] [Google Scholar]
- 64.McCabe EJB, Gannon DP, Barros NB, Wells RS. Prey selection by resident common bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Mar Biol. 2010;157(5):931–42. 10.1007/s00227-009-1385-9 [DOI] [Google Scholar]
- 65.Wells RS, McHugh KA, Douglas DC, Shippee S, McCabe EB, Barros NB, et al. Evaluation of potential protective factors against metabolic syndrome in bottlenose dolphins: Feeding and activity patterns of dolphins in Sarasota Bay, Florida. Front Endocrinol. 2013. October 10;4:139 10.3389/fendo.2013.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Autian J. Toxicity and health threats of phthalate esters: review of the literature. Environ Health Perspect. 1973. June;4:3–26. 10.1289/ehp.73043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andrady AL. Microplastics in the marine environment. Mar Pollut Bull. 2011. August;62(8):1596–605. 10.1016/j.marpolbul.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 68.Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017. July 19;3(7):e1700782 10.1126/sciadv.1700782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, et al. plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One. 2014. December 10;9(12): e111913 10.1371/journal.pone.0111913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, et al. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: Results from the EARTH study. Environ Health Perspect. 2016. June;124(6):831–9. 10.1289/ehp.1509760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toft G, Jönsson BAG, Lindh CH, Jensen TK, Hjollund NH, Vested A, et al. Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ Health Perspect. 2012. March;120(3):458–63. 10.1289/ehp.1103552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radke EG, Braun JM, Meeker JD, Cooper GS. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ Int. 2018. December;121(Pt 1):764–93. 10.1016/j.envint.2018.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RHN, et al. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 2015. April;30(4):963–72. 10.1093/humrep/deu363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Przybyla J, Geldhof GJ, Smit E, Kile ML. A cross sectional study of urinary phthalates, phenols and perchlorate on thyroid hormones in US adults using structural equation models (NHANES 2007–2008). Environ Res. 2018. May;163:26–35. 10.1016/j.envres.2018.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romano ME, Eliot MN, Zoeller RT, Hoofnagle AN, Calafat AM, Karagas MR, et al. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Int J Hyg Environ Health. 2018. May;221(4):623–31. 10.1016/j.ijheh.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim MJ, Moon S, Oh BC, Jung D, Choi K, Park YJ. Association between Diethylhexyl Phthalate Exposure and Thyroid Function: A Meta-Analysis. Thyroid [Internet]. 2019. February;29(2):183–92. 10.1089/thy.2018.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao M, Pan W, Shen X, Li C, Zhou J, Liu J. Urinary levels of phthalate metabolites in women associated with risk of premature ovarian failure and reproductive hormones. Chemosphere. 2020. March;242:125206 10.1016/j.chemosphere.2019.125206 [DOI] [PubMed] [Google Scholar]
- 78.Woodward MJ, Obsekov V, Jacobson MH, Kahn LG, Trasande L. Phthalates and sex steroid hormones among men from NHANES, 2013–2016. J Clin Endocrinol Metab. 2020. April 1;105(4). 10.1210/clinem/dgaa039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nuttall JR, Kucera HR, Supasai S, Gaikwad NW, Oteiza PI. Combined Effects of Gestational Phthalate Exposure and Zinc Deficiency on Steroid Metabolism and Growth. Toxicol Sci. 2017;156(2):469–79. 10.1093/toxsci/kfx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calafat AM, McKee RH. Integrating biomonitoring exposure data into the risk assessment process: Phthalates [diethyl phthalate and di(2-ethylhexyl) phthalate] as a case study. Environ Health Perspect. 2006. November;114(11):1783–9. 10.1289/ehp.9059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Högberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect. 2008. March;116(3):334–9. 10.1289/ehp.10788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008. February;106(2):257–69. 10.1016/j.envres.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 83.Fahlman A, McHugh K, Allen J, Barleycorn A, Allen A, Sweeney J, et al. Resting metabolic rate and lung function in Wild Offshore common bottlenose dolphins, Tursiops truncatus, near Bermuda. Front Physiol. 2018. July 17;9(886). 10.3389/fphys.2018.00886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McMurray RG, Soares J, Caspersen CJ, McCurdy T. Examining variations of resting metabolic rate of adults: A public health perspective. Med Sci Sports Exerc. 2014. July;46(7):1352–8. 10.1249/MSS.0000000000000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rowdhwal SSS, Chen J. Toxic Effects of di-2-ethylhexyl phthalate: An overview. Biomed Res Int. 2018. February 22;2018:1750368 10.1155/2018/1750368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bossart GD. Marine mammals as sentinel species for oceans and human health. Vet Pathol. 2011. May;48(3):676–90. 10.1177/0300985810388525 [DOI] [PubMed] [Google Scholar]
- 87.Litz JA, Garrison LP, Fieber LA, Martinez A, Contillo JP, Kucklick JR. Fine-scale spatial variation of persistent organic pollutants in bottlenose dolphins (Tursiops truncatus) in Biscayne Bay, Florida. Environ Sci Technol. 2007. November 1;41(21):7222–8. 10.1021/es070440r [DOI] [PubMed] [Google Scholar]
- 88.Balmer BC, Schwacke LH, Wells RS, George RC, Hoguet J, Kucklick JR, et al. Relationship between persistent organic pollutants (POPs) and ranging patterns in common bottlenose dolphins (Tursiops truncatus) from coastal Georgia, USA. Sci Total Environ. 2011. May 1;409(11):2094–101. 10.1016/j.scitotenv.2011.01.052 [DOI] [PubMed] [Google Scholar]
- 89.Kucklick J, Schwacke L, Wells R, Hohn A, Guichard A, Yordy J, et al. Bottlenose dolphins as indicators of persistent organic pollutants in the western North Atlantic Ocean and northern Gulf of Mexico. Environ Sci Technol. 2011. May 15;45(10):4270–7. 10.1021/es1042244 [DOI] [PubMed] [Google Scholar]
- 90.Schwacke LH, Zolman ES, Balmer BC, De Guise S, Clay George R, Hoguet J, et al. Anaemia, hypothyroidism and immune suppression associated with polychlorinated biphenyl exposure in bottlenose dolphins (Tursiops truncatus). Proc R Soc B Biol Sci. 2011;279(1726):48–57. 10.1098/rspb.2011.0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Backer LC, Bolton B, Litz JA, Trevillian J, Kieszak S, Kucklick J. Environmental contaminants in coastal populations: Comparisons with the National Health and Nutrition Examination Survey (NHANES) and resident dolphins. Sci Total Environ. 2019. December 15;696:134041 10.1016/j.scitotenv.2019.134041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rabinowitz P, Scotch M, Conti L. Human and animal sentinels for shared health risks. Vet Ital. 2009. Jan-Mar;45(1):23–4. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.