Abstract
Longevity differs between sexes, with females being longer-lived in most mammals, including humans. One hallmark of aging is the functional decline of stem cells. Thus, a key question is whether the aging of stem cells differs between males and females and whether this has consequences for disease and lifespan.
A glance at the list of the human individuals currently living over the age of 110—supercentenarians—reveals a surefire strategy for achieving such exceptional longevity: be female. Out of the 53 living supercentenarians, 51 are female. No other demographic factor comes remotely close to sex in predicting the likelihood of achieving such an advanced age. Sexual dimorphism with respect to longevity is a characteristic of most mammals and has been recorded in human populations since at least the mid-18th century. This dichotomous capacity for resilience has inspired wide-ranging hypotheses to explain the underlying mechanisms. It also raises questions regarding the sexual dimorphism of processes known to sustain tissue regeneration and function throughout life, including adult stem cell renewal. Most adult stem cell populations undergo an age-related decline, leading to dysfunctional tissue homeostasis, which most likely participates in defining the ultimate lifespan of the organism. Interestingly, sex-specific regulation of stem cell populations has been demonstrated for several stem cell types, and it has long been appreciated that many canonical aging pathways exhibit sex specificity. However, despite the seeming interrelationship between sex, stem cell maintenance, and aging, few studies have sought to directly explore the interaction of these three variables. Here we discuss the sexual dimorphism of adult stem cell populations and how processes regulating the aging of stem cells may also be modified by sex.
Sexual Dimorphism of Longevity
Hypotheses to explain the sexual imbalance in longevity have come from divergent fields, including genetics, evolutionary biology, and physiology. One hypothesis is that the single X chromosome of males makes them functionally homozygous at all loci on that chromosome, potentially rendering them more susceptible to deleterious recessive traits. The maternal inheritance of mitochondria could also result in sexual dimorphism in the function of mitochondria, which could in turn impact metabolism and longevity. Evolutionary hypotheses have postulated that the sexes have adapted to be fit for different needs—for example, females put more investment in progeny production and care than males in most species—and that these different adaptive pressures could result in antagonistic selection at longevity associated loci. While these genetic hypotheses are difficult to test experimentally, the evolutionary forces at play have resulted in differential sex-associated phenotypes that may determine lifespan. Most notably, the differential utilization of steroid hormones, estrogen and testosterone (prominent determinants of sex-specific phenotypes), has been proposed to contribute to lifespan. Indeed, studies of human eunuchs (castrated males) have shown that their lifespan is about 14 years longer than non-castrated males (Min et al., 2012). Furthermore, supplementation with estrogen increases the lifespan of male mice specifically (Fontana and Partridge, 2015). However, the mechanisms by which estrogen and testosterone modify lifespan have remained elusive.
Stem Cells in Many Adult Niches Are Regulated in a Sex-Specific Manner
The sex of an organism can modify the behavior of its stem cell populations in a way that may be adaptive. A recent study has shown that hematopoietic stem cells (HSCs) are more abundant and more proliferative in female mice than in male mice, an effect that is dependent on estrogen signaling (Nakada et al., 2014). The dependency on estrogen results in a further increase in HSC number and proliferation during pregnancy (Nakada et al., 2014). A similar paradigm has been described in neural stem cells (NSCs), where estrogen increases the proliferation of NSCs in a transient manner that fluctuates throughout the estrus cycle (Pawluski et al., 2009). NSC proliferation is highest during the proestrus phase of the estrus cycle, when estrogen levels are particularly high. Exogenous administration of estrogen is also sufficient to increase the proliferation of NSCs in vivo (Pawluski et al., 2009). In muscle, the resident stem cells, termed satellite cells (SCs), exhibit greater self-renewal and regenerative capacity in females than in their male counterparts (Deasy et al., 2007). Interestingly, the enhanced regenerative capacity of female SCs does not appear to be related to estrogen signaling as is observed in other stem cell niches (Deasy et al., 2007), suggesting that estrogen signaling is not the sole contributor to differences in stem cell regulation between the sexes. Other studies have shown that females also exhibit increased capacity for rapid wound healing and liver regeneration, processes that are likely dependent on resident stem cell populations (Deasy et al., 2007).
Thus, females tend to exhibit increased stem cell self-renewal, regeneration potential, and in some cases, proliferation. However, the question remains: does this tendency toward increased self-renewal in females alter the capacity of stem cells to regenerate tissues throughout aging, and does it influence longevity (Figure 1)?
Figure 1. Potential Interactions between Stem Cells, Aging, and Sex.
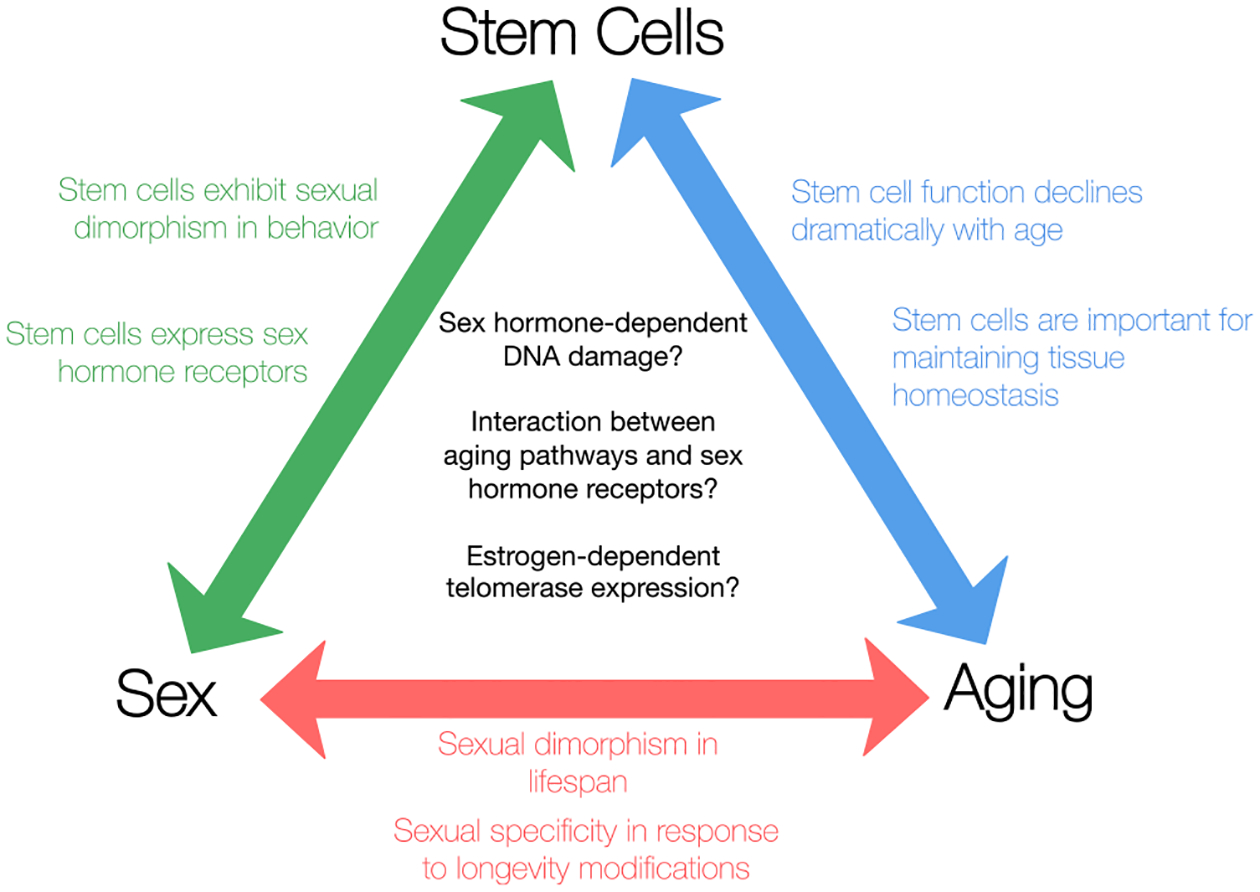
Though interactions between stem cells, aging, and sex have been topics of great interest, the intersection of all three—the effect of sex on the aging of stem cells—has not been well studied. However, several mechanisms could be involved in establishing and perpetuating sexual dimorphism during the aging of stem cells.
The Ability of Stem Cells to Regenerate Tissue Declines with Age and May Be Dependent on Sex
Many stem cell niches experience a decline in self-renewal potential with age (Signer and Morrison, 2013). However, it is unclear whether age-associated changes in stem cells differ between the sexes. Interestingly, many canonical aging pathways have been shown to have a sex-specific effect on lifespan. For example, heterozygous knockout of insulin-like growth factor type 1 receptor (Igfr1) increases the lifespan of female mice only (Fontana and Partridge, 2015). In contrast, transgenic overexpression of Sirt6 increases the lifespan of male mice only (Fontana and Partridge, 2015). What are the effects of sexual differences in aging pathways on stem cells? At this point few studies have addressed this question. However, the limited work in this field does point to potentially fascinating differences between the aging of male and female stem cells. With regards to HSCs, a study of a pair of dizygotic twins—a male and female—with hematopoietic chimerism demonstrated that in the male environment, both genotypically male and female HSCs had shorter telomeres as compared to the same cells in the female (Brüderlein et al., 2008). This finding is consistent with the idea that a female organism provides an environment that is more conducive to sustained self-renewal. This study also suggests an intriguing non-cell-autonomous mechanism of differential aging patterns in male and female stem cells, mediated by the environment of the stem cells. There is undoubtedly a dearth of data addressing the differences in the aging of male and female stem cells, and this will likely be an active area for future investigation.
Potential Mechanisms Linking Stem Cell Aging and Sex
Though the sexual dimorphism of aging stem cells has been largely uncharacterized, there are strong links between sexually dimorphic characteristics—most notably the sex steroid hormones—and classical aging pathways known to be important for stem cell maintenance. These links suggest potential mechanisms that may be involved in generating sex-dependent aging phenotypes in stem cells (Figure 1).
One such mechanism is the sex-specific regulation of DNA damage and reactive oxygen species (ROS). Several recent studies have explored the role of DNA damage and ROS accumulation in stem cell aging, processes which evidence suggests may be modulated by sex. One such study demonstrated that, in muscle, quiescent SCs accumulate DNA damage with age (Sousa-Victor et al., 2014). The accumulation of DNA damage over time in this stem cell population is associated with irreversible senescence (Sousa Victor et al., 2014), suggesting that DNA damage accumulation can lead to loss of regenerative potential. While the influence of sex cannot be determined in this particular case because the aforementioned study used only males, there is good reason to believe that sex may play a role. Estrogen is known to induce the expression of anti-oxidant genes and reduce ROS, while in contrast testosterone increases oxidative stress. Thus, DNA damage may be bidirectionally regulated by estrogen and testosterone in an oxidative tug-of-war, which may bear consequences for the health and regenerative potential of stem cells throughout the aging process.
Another potential mechanism involves the conserved “pro-longevity” transcription factor FOXO3. FOXO3 is inhibited by insulin signaling and is necessary for increased longevity in insulin signaling mutants (Signer and Morrison, 2013), a pathway that has a greater impact on female than male lifespan (Fontana and Partridge, 2015). Moreover, FOXO3 has been shown to be vitally important for stem cell maintenance in both HSCs and NSCs by preventing premature stem cell exhaustion (Signer and Morrison, 2013). Interestingly, FOXO3 can interact with estrogen receptor (ER-a) (Sisci et al., 2013). This interaction is dependent on estrogen and profoundly alters the cellular effects of FOXO3 (Sisci et al., 2013). These data suggest a fascinating link between a known determinant of stem cell maintenance during aging and sex.
A final prospective mechanism involves telomerase, which is expressed by many stem cell populations and is important for their regenerative potential. Intriguingly, estrogen itself is capable of activating telomerase directly through ER-mediated transcription of TERT, the protein component of the telomerase complex (Kyo et al., 1999). This hints at an intriguing mechanism by which cells in a female environment could delay the aging process through the maintenance of telomeres.
While there are several tantalizing links between known mediators of aging stem cells and sexual dimorphism, their relationships need to be further explored. This investigation may lead to new insights about the aging of stem cell populations and the sexual dimorphism of lifespan.
Conclusions
Emerging evidence indicates that adult stem cells in non-sexual tissues are regulated in a sexually dimorphic manner and are responsive to sex hormones. Because stem cell maintenance is important for tissue regeneration throughout life, sex-associated differences in stem cell aging may be associated with sexual dimorphism in lifespan. At this point, however, very limited work has been done to directly address this question.
So what does this mean for the field of stem cell aging? At the very least it should emphasize the importance of controlling for sex in studies in which age is a variable, as most recent work in the field has done. However, beyond that, it suggests a productive line of investigation assessing the effects of sex on the aging of stem cells. It will also be important to understand how sexual dimorphism in aging stem cells predisposes individuals to sex-associated age-related pathologies such as osteoporosis and cardiovascular disease. Furthermore, in any discussion of aging, it is also important to consider not only the lifespan, but also the portion of healthy life or “healthspan” an individual experiences. It is likely that sex plays a role in defining both lifespan and healthspan, and the effects of sex may not be identical for these two variables. As the search continues for ways to ameliorate the aging process and maintain the regenerative capacity of stem cells, let us not forget one of the most effective aging modifiers: sex.
REFERENCES
- Brüderlein S, Müller K, Melzner J, Högel J, Wiegand P, and Möller P (2008). Aging Cell 7, 663–666. [DOI] [PubMed] [Google Scholar]
- Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, et al. (2007). J. Cell Biol 177, 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, and Partridge L (2015). Cell 161, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, and Inoue M (1999). Cancer Res. 59, 5917–5921. [PubMed] [Google Scholar]
- Min KJ, Lee CK, and Park HN (2012). Curr. Biol 22, R792–R793. [DOI] [PubMed] [Google Scholar]
- Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, and Morrison SJ (2014). Nature 505, 555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, and Galea LA (2009). Front. Neuroendocrinol 30, 343–357. [DOI] [PubMed] [Google Scholar]
- Signer RAJ, and Morrison SJ (2013). Cell Stem Cell 12, 152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisci D, Maris P, Cesario MG, Anselmo W, Coroniti R, Trombino GE, Romeo F, Ferraro A, Lanzino M, Aquila S, et al. (2013). Cell Cycle 12, 3405–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardí M, Ballestar E, González S, Serrano AL, et al. (2014). Nature 506, 316–321. [DOI] [PubMed] [Google Scholar]


