Abstract
In recent months, the parasitology research community has been tasked with investigation of the influence of parasite coinfection on coronavirus disease 2019 (COVID-19) outcomes. Herein, we share our approach to analyze the effect of the trematode Fasciola hepatica as a modulator of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and of COVID-19 pathology.
Keywords: COVID-19, helminth parasites, Fasciola hepatica, pathology modulation, infection modulation
Helminth parasites have adapted to their hosts during long coevolution processes, which usually result in chronic disease with low mortality and variable morbidity. During this evolutionary coadaptation with their hosts, including vertebrate hosts, parasites have contributed to the modulation of several molecular and physiological host mechanisms, for example, the immune system. Thereby, helminth parasites trigger a modulated T helper (Th)2 response in their vertebrate hosts, resulting in an immune reaction with a tightly controlled inflammatory component, including the inhibition of proinflammatory cytokines and the induction of a hyporesponsive state involving interleukin-10 (IL-10)-producing T regulatory (Treg) cell populations [1]. In addition, the hygiene hypothesis proposes that the absence of helminth infections in the population of developed countries, thus, the lack of immunological stimuli of helminth parasites during childhood, has propitiated the rise of autoimmune diseases with an exacerbated inflammatory component (e.g., allergy, asthma, and rheumatoid arthritis, among others) [2].
We have read with interest the comment by Bradbury et al. [3] in which the authors discuss the potential role of helminth coinfections in the modulation of hyperinflammatory responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Severe cases of coronavirus disease 2019 (COVID-19) show an exacerbated immune response affecting the lungs. The innate immune response to tissue damage caused by the virus could result in an acute respiratory distress syndrome, characterized by the rapid onset of generalized inflammation in the lungs and subsequent death by respiratory distress [4]. This is due to a 'cytokine storm' in which proinflammatory cytokines, for example IL-6, dominate [5]. One of the compounds showing efficacy against this hyperinflammation in COVID-19 is Tocilizumab, a monoclonal antibody targeting the IL-6 receptor that is usually administered to patients with autoimmune rheumatoid arthritis. Tocilizumab causes general immunosuppression and thus, could be of limited use in the current pandemic. Similarly, a variety of immunosuppressive drugs have shown beneficial impacts on COVID-19, but the risk of higher viral replication and secondary infections should be carefully evaluated in patients treated with immunosuppressive compounds [6].
Helminth parasites could change the outcome of COVID-19 infections, in areas of the world where helminthic infections are still prevalent, by inducing a modified Th2 response with a controlled inflammatory component. Notably, in countries of Africa and Latin America, where helminth infections are still common, the numbers of reported COVID-19 deaths are substantially lower than those reported in high-income countriesi.
The use of specific helminth derivatives as therapeutic tools of autoimmune diseases has been proposed. Specifically, the helminth parasite Fasciola hepatica has shown immunomodulatory properties, and several molecules from this parasite have been described as potent immunomodulators [7]. Derivatives of these molecules in their synthetic, safe formats have given rise to promising results; for example, a 68 mer peptide of helminth defense molecule (HDM) has been shown to inhibit inflammation and airway hyper-reactivity in murine experimental asthma [8], and the fatty-acid-binding recombinant protein Fh15 blocked the lipopolysaccharide (LPS)-induced cytokine storm in a murine model [9]. As a result, recombinant safe-to-use forms of the above-mentioned molecules are available.
Devoid of our chronic helminthic infections, humans could be more susceptible to not only develop hyperinflammatory pathology related to different stimuli, including viruses, but also be more susceptible to infection by emerging viruses. Helminth schistosome infection has been used as a protective anti-inflammatory strategy against viruses such Type A influenza or murine pneumonia virus by Scheer et al. [10]. Additionally, these authors also showed that mice with schistosomiasis were significantly protected against respiratory viral infections [10]. Furthermore, in mice infected with the nematodes Heligmosomoides polygyrus or Trichinella spiralis, these organisms enhance and reactivate enteric viral infections by limiting innate and adaptive immune responses against viruses [11]. These differences are attributed to the different immunomodulatory and immunosuppressive responses induced by infection with Schistosoma mansoni or with H. polygyrus and T. spiralis [10], mainly in relation to the antiviral Th1 responses; for example, S. mansoni elicits a biased Th1 response in early stages of infection. The influence of helminth infections on viral coinfections in relation to mechanisms deployed by juveniles of trematode parasites have not been explored. Interestingly, helminth parasites not entering or residing in the lung, and derived products, modulate pulmonary responses protecting against airway inflammation [12]. Although helminth coinfections could be linked to a lower morbi-mortality due to SARS-Cov-2 in endemic areas, further studies on the COVID-19 severity in helminth-endemic areas are needed to support this hypothesis. Since the ambiguous animal coinfection studies showing both favorable and unfavorable effects [10,11] are insufficient to define the role of helminths and, more importantly, of specific helminth-derived molecules, on viral coinfections, studies on the impact of helminth products in the infection and pathology of COVID-19 should be explored. The in vitro and in vivo models of F. hepatica developed by our group for the study of host–parasite cross-interactions and modulations [13] have allowed the identification and production of several F. hepatica molecules with potential modulatory properties. Based on this previous work, our group, together with a team specializing in virology, has now been given a national Spanish project grant to explore the influence of these parasite molecules on viral infection and on the modulation of hyperinflammation reactions in lungs (Figure 1 ). Our results could help to define the role of the coevolutionary adaptation of parasites and humans with regard to human interaction with pathogenic viruses (Box 1 ). In addition, we call on the research community to propose and conduct more studies on the potential influence of the immunomodulatory capacity of helminth parasites on COVID-19 pathology.
Figure 1.
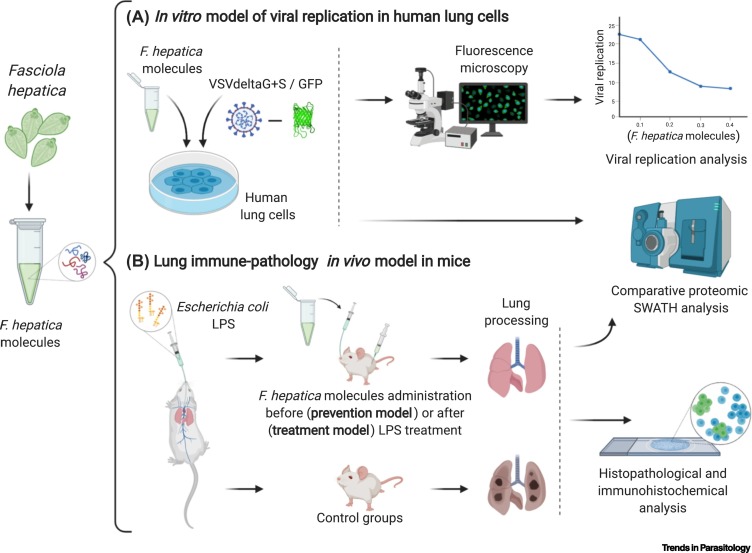
Testing the Role of the Helminth Parasite Fasciola hepatica as a Modulator of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection and of Coronavirus Disease 2019 (COVID-19) Pathology.
F. hepatica molecules will be tested in vitro and in vivo to assess their influence on viral entry and replication in lung cells, and on the modulation of lung hyperinflammation driven by a cytokine storm. (A) The in vitro model consists of primary human lung cells that will be stimulated with defined parasite recombinant proteins and peptides or left unstimulated, and subsequently infected with a vesicular stomatitis virus (VSV) carrying the GFP reporter and pseudotyped with the SARS-CoV-2 envelope protein S (VSVdeltaG+S). Differences in reporter gene expression between stimulated and unstimulated cells will be quantified to assess the antiviral effect of different parasite molecules at different concentrations. Stimulated and unstimulated lung cells will be subjected to comparative proteomic analysis by SWATH to identify the modifications induced by the parasite molecules in treated cells and link these changes to the modulation of viral replication. Parasite molecules showing an antiviral effect in the VSVdeltaG+S system will be further evaluated in primary human lung cell cultures infected with SARS-CoV-2. (B) The in vivo model will be established as follows. The mouse model of sterile lung hyperinflammation progressing with a cytokine storm, consisting of the intranasal administration of bacterial lipopolysaccharide (LPS), will be subjected to intranasal or intraperitoneal administration of F. hepatica recombinant antigens and peptides before LPS administration to check the preventive potential of the parasite compounds on the lung hyperinflammation (prevention assay) or after LPS treatment to check the treatment potential of the parasite molecules against the lung hyperinflammation triggered by LPS (treatment assay), compared with the control group (treated with LPS alone). Lungs from treated and control groups will be studied comparatively, by histopathology and immunohistochemistry, and subjected to protein extraction for comparative proteomic SWATH analysis. Parasite molecules showing an anti-inflammatory activity in this model will be tested in an in vivo model of COVID-19. Created with BioRender.com.
Box 1. Hypothesis on the Potential Modulation by Helminth Parasites of Human Susceptibility to SARS-CoV-2 Infections and Pathogenesis.
As a result of an intricate evolutionary coadaptation process, helminth parasites usually drive a Th2-modified immune response in infected individuals devoid of the proinflammatory component. In addition, and according to the hygiene hypothesis, the lack of helminth infections during childhood may result in unmodulated inflammatory responses against other stimuli, including viral infections. Furthermore, it has been demonstrated that helminth parasites are capable of regulating host mechanisms related to viral infection. Consequently, it could be hypothesized that helminth parasites and their derivatives might influence SARS-CoV-2 entry into host cells and exert an antipathological (anti-inflammatory) effect in COVID-19 patients.
Alt-text: Box 1
Acknowledgments
The authors want to thank the Ministry MINECO, Spain (Projects AGL2015-67023-C2 and PID2019-108782RB-C22) and Agencia Estatal Consejo Superior de Investigaciones Científicas, Spain (Project CSIC-COV19-104) for financial support. The authors also want to acknowledge the support of the CSIC Thematic Platform 'Global Health'ii and their participants that have enabled the building of our interdisciplinary team. J.G.M. is supported by the JIN project 'ULYSSES' (RTI2018-093463-J-100) (MCIU/AEI/FEDER, UE).
Resources
ihttps://hgis.uw.edu/virus/iihttps://pti-saludglobal-covid19.corp.csic.es/References
- 1.Maizels R.M., McSorley H.J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 2016;138:666–675. doi: 10.1016/j.jaci.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson J.A. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradbury R.S. Will helminth co-infection modulate COVID-19 severity in endemic regions? Nat. Rev. Immunol. 2020;20:342. doi: 10.1038/s41577-020-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intens. Care Med. 2020;8:1–2. doi: 10.1007/s00134-020-06065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell B. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson M.W. Helminth defence molecules – immunomodulators designed by parasites! Front. Microbiol. 2013;4:296. doi: 10.3389/fmicb.2013.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka A. The parasitic 68-mer peptide FhHDM-1 inhibits mixed granulocytic inflammation and airway hyperreactivity in experimental asthma. J. Allergy Clin. Immunol. 2018;141:2316–2319. doi: 10.1016/j.jaci.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Ramos-Benitez M.J. Fh15 blocks the lipopolysaccharide-induced cytokine storm while modulating peritoneal macrophage migration and CD38 expression within spleen macrophages in a mouse model of septic shock. mSphere. 2018;3 doi: 10.1128/mSphere.00548-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheer S. S. mansoni bolsters anti-viral immunity in the murine respiratory tract. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maizels R.M., Gause W.C. Immunology. How helminths go viral. Science. 2014;345:517–518. doi: 10.1126/science.1258443. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz C. Helminth modulation of lung inflammation. Trends Parasitol. 2018;34:388–403. doi: 10.1016/j.pt.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 13.González-Miguel J. Set up of an in vitro model to study early host–parasite interactions between newly excysted juveniles of Fasciola hepatica and host intestinal cells using a quantitative proteomics approach. Vet. Parasitol. 2020;278:109028. doi: 10.1016/j.vetpar.2020.109028. [DOI] [PubMed] [Google Scholar]