Abstract
Background
The ongoing COVID-19 pandemic warrants accelerated efforts to test vaccine candidates. We aimed to assess the safety and immunogenicity of an inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine candidate, BBIBP-CorV, in humans.
Methods
We did a randomised, double-blind, placebo-controlled, phase 1/2 trial at Shangqiu City Liangyuan District Center for Disease Control and Prevention in Henan Province, China. In phase 1, healthy people aged 18–80 years, who were negative for serum-specific IgM/IgG antibodies against SARS-CoV-2 at the time of screening, were separated into two age groups (18–59 years and ≥60 years) and randomly assigned to receive vaccine or placebo in a two-dose schedule of 2 μg, 4 μg, or 8 μg on days 0 and 28. In phase 2, healthy adults (aged 18–59 years) were randomly assigned (1:1:1:1) to receive vaccine or placebo on a single-dose schedule of 8 μg on day 0 or on a two-dose schedule of 4 μg on days 0 and 14, 0 and 21, or 0 and 28. Participants within each cohort were randomly assigned by stratified block randomisation (block size eight) and allocated (3:1) to receive vaccine or placebo. Group allocation was concealed from participants, investigators, and outcome assessors. The primary outcomes were safety and tolerability. The secondary outcome was immunogenicity, assessed as the neutralising antibody responses against infectious SARS-CoV-2. This study is registered with www.chictr.org.cn, ChiCTR2000032459.
Findings
In phase 1, 192 participants were enrolled (mean age 53·7 years [SD 15·6]) and were randomly assigned to receive vaccine (2 μg [n=24], 4 μg [n=24], or 8 μg [n=24] for both age groups [18–59 years and ≥60 years]) or placebo (n=24). At least one adverse reaction was reported within the first 7 days of inoculation in 42 (29%) of 144 vaccine recipients. The most common systematic adverse reaction was fever (18–59 years, one [4%] in the 2 μg group, one [4%] in the 4 μg group, and two [8%] in the 8 μg group; ≥60 years, one [4%] in the 8 μg group). All adverse reactions were mild or moderate in severity. No serious adverse event was reported within 28 days post vaccination. Neutralising antibody geometric mean titres were higher at day 42 in the group aged 18–59 years (87·7 [95% CI 64·9–118·6], 2 μg group; 211·2 [158·9–280·6], 4 μg group; and 228·7 [186·1–281·1], 8 μg group) and the group aged 60 years and older (80·7 [65·4–99·6], 2 μg group; 131·5 [108·2–159·7], 4 μg group; and 170·87 [133·0–219·5], 8 μg group) compared with the placebo group (2·0 [2·0–2·0]). In phase 2, 448 participants were enrolled (mean age 41·7 years [SD 9·9]) and were randomly assigned to receive the vaccine (8 μg on day 0 [n=84] or 4 μg on days 0 and 14 [n=84], days 0 and 21 [n=84], or days 0 and 28 [n=84]) or placebo on the same schedules (n=112). At least one adverse reaction within the first 7 days was reported in 76 (23%) of 336 vaccine recipients (33 [39%], 8 μg day 0; 18 [21%], 4 μg days 0 and 14; 15 [18%], 4 μg days 0 and 21; and ten [12%], 4 μg days 0 and 28). One placebo recipient in the 4 μg days 0 and 21 group reported grade 3 fever, but was self-limited and recovered. All other adverse reactions were mild or moderate in severity. The most common systematic adverse reaction was fever (one [1%], 8 μg day 0; one [1%], 4 μg days 0 and 14; three [4%], 4 μg days 0 and 21; two [2%], 4 μg days 0 and 28). The vaccine-elicited neutralising antibody titres on day 28 were significantly greater in the 4 μg days 0 and 14 (169·5, 95% CI 132·2–217·1), days 0 and 21 (282·7, 221·2–361·4), and days 0 and 28 (218·0, 181·8–261·3) schedules than the 8 μg day 0 schedule (14·7, 11·6–18·8; all p<0·001).
Interpretation
The inactivated SARS-CoV-2 vaccine, BBIBP-CorV, is safe and well tolerated at all tested doses in two age groups. Humoral responses against SARS-CoV-2 were induced in all vaccine recipients on day 42. Two-dose immunisation with 4 μg vaccine on days 0 and 21 or days 0 and 28 achieved higher neutralising antibody titres than the single 8 μg dose or 4 μg dose on days 0 and 14.
Funding
National Program on Key Research Project of China, National Mega projects of China for Major Infectious Diseases, National Mega Projects of China for New Drug Creation, and Beijing Science and Technology Plan.
Research in context.
Evidence before this study
There are currently no licensed vaccines to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We searched PubMed for research articles published between database inception and Oct 7, 2020, using various combinations of the terms “COVID-19” or “SARS-CoV-2”, “vaccine”, and “clinical trial”. No language or date restrictions were applied. Three adenovirus-vectored vaccine clinical trials have been published. A phase 1 study of a recombinant adenovirus type-5 vectored vaccine against SARS-CoV-2, using a one-dose vaccination schedule of intramuscular injection, was done in China. The vaccine was well tolerated and neutralising antibodies were detected in 63 (58%) of 108 participants by day 28. Additionally, a chimpanzee adenovirus-vectored vaccine phase 1/2 study was done in the UK, and a combination of recombinant adenovirus type-26 and type-5 vectored vaccine phase 1/2 study was done in Russia. Another published clinical trial described a phase 1 study done in the USA, of an mRNA vaccine, using a two-dose vaccination schedule (28 days apart). The vaccine was well tolerated and neutralising antibodies were detected in all 45 participants. We also searched the ClinicalTrials.gov and Chictr.org.cn registry for unpublished trials of COVID-19 vaccines, up to July 12, 2020. In addition to the inactivated vaccine reported here, another 20 candidate SARS-CoV-2 vaccines are in ongoing clinical trials, including DNA plasmid vaccines, inactivated vaccine, adenovirus-vectored vaccine, RNA vaccine, protein subunit vaccine, and virus-like particle vaccine.
Added value of this study
This is the first report of an inactivated SARS-CoV-2 vaccine tested on human participants. This trial showed that the inactivated SARS-CoV-2 vaccine BBIBP-CorV was safe, tolerable, and immunogenic in healthy people. Two-dose immunisations (on days 0 and 28) at all doses (2 μg, 4 μg, and 8 μg) in two age groups (18–59 years and ≥60 years) induced neutralising antibodies in 100% of vaccine recipients. Mild adverse reactions, including pain and fever, were observed but no severe adverse reaction was reported in all groups.
Implications of all the available evidence
A vaccine against SARS-CoV-2 is urgently needed to prevent further waves of COVID-19. Immunisation with BBIBP-CorV results in rapid induction of immune responses against SARS-CoV-2, and would be valuable in preventing or limiting the COVID-19 pandemic. Further clinical studies are warranted to evaluate the potential of this vaccine in clinical application.
Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),1, 2, 3, 4, 5, 6 has caused 35 million infections and more than 1 000 000 deaths worldwide as of Oct 5, 2020, according to the WHO COVID-19 Dashboard. People aged 60 years and older and people with pre-existing respiratory or cardiovascular diseases have a high risk of severe disease and death if infected with SARS-CoV-2.7
Compared with other coronaviruses, SARS-CoV-2 appears to undergo more rapid transmission,1, 2 leading to the urgent demand for a vaccine for control and prevention of COVID-19. According to WHO's draft landscape of COVID-19 candidate vaccines,8 42 candidate vaccines are in clinical evaluation and 151 candidate vaccines are in preclinical evaluation. The candidate vaccines in clinical trials include DNA plasmid vaccines, inactivated vaccines, adenovirus-vectored vaccines, RNA vaccines, protein subunit vaccines, and virus-like particle vaccines. The safety and efficacy of some of these candidates have been shown in preclinical trials, and the safety and immunogenicity in clinical trials.9, 10, 11, 12, 13, 14, 15 The development of inactivated vaccines is a mature technology, which is widely used for the prevention and control of emerging infectious diseases, including influenza virus and poliovirus. To date, two inactivated vaccine candidates have been reported to protect non-human primates against SARS-CoV-2, with good safety in preclinical trials.9, 13 Here, we report the safety, tolerability, and immunogenicity of an inactivated vaccine candidate, called BBIBP-CorV,13 in healthy people in China.
Methods
Study design and participants
We did a dose-escalation, randomised, double-blind, placebo-controlled, phase 1/2 trial of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV, in a single centre (Center for Disease Control and Prevention, Liangyuan District, Shangqiu City, Henan Province, China). Participants were screened for SARS-CoV-2 infection by serology only before enrolment. Eligible participants were healthy people aged 18–80 years, who were negative for serum-specific IgM/IgG antibodies against SARS-CoV-2, as measured by a commercial kit (Innovita, China) at the time of screening. Exclusion criteria were a history of travelling to Hubei Province (China), regions outside of China, or regions with reported COVID-19 cases from December, 2019; a history of infection with SARS-CoV; fever, cough, runny nose, sore throat, diarrhoea, dyspnoea, or tachypnoea in the 14 days before vaccination; abnormalities in laboratory tests; pregnancy or lactation; allergy to any ingredient included in the vaccine; a history of seizures or mental illness; and being unable to comply with the study schedule. Laboratory tests included measurement of alanine aminotransferase, aspartate aminotransferase, serum total bilirubin, creatinine, blood urea nitrogen, white blood cell count, haemoglobin, blood glucose, urinary protein, and urinary glucose.
To minimise the possibility of recruiting participants infected with SARS-CoV-2 or of patients becoming infected with SARS-CoV-2 during the trials, despite serology testing during screening, volunteers were also recruited from COVID-19-free communities and follow-up monitoring for COVID-19 was done after vaccination.
Criteria for early suspension of trials are outlined in the protocol (www.chictr.org.cn, ChiCTR2000032459).
The protocol and informed consent were approved by the Medical Ethics Committee of Henan Provincial Center for Disease Control and Prevention. Written informed consent from all participants was obtained before screening. This study was undertaken by Henan Provincial Center for Disease Control and Prevention, and implemented in Liangyuan District, Shangqiu City, in accordance with the Declaration of Helsinki and Good Clinical Practice.
Randomisation and masking
In phase 1, participants were separated into two groups—ages 18–59 years and 60 years and older—before being randomly assigned (1:1:1:1) to receive the first dose (of a two-dose schedule) of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV) at 2 μg, 4 μg, or 8 μg, or placebo via intramuscular injection in the arm.
In phase 2, participants in each immunisation schedule were randomly assigned (3:1) to receive two intramuscular injections of the vaccine or placebo. The participants were sequentially assigned a randomisation number generated by Stata, version 12.0, and stratified block randomisation (block size eight) by subgroups was used, generated by an independent statistician. Individuals involved in randomisation and masking had no involvement in the rest of the trial. Participants, investigators, and staff undertaking laboratory analyses were masked to group allocation.
Within each randomisation block, the ratio of vaccine versus placebo was 3:1. The safety evaluation was masked for all participants. Vaccine and placebo were distributed in identical packages with serial numbers. Group allocation was concealed from participants, investigators, and outcome assessors.
Procedures
BBIBP-CorV was developed by the Beijing Institute of Biological Products (Beijing, China), and manufactured as previously described.13 At the time of the SARS-CoV-2 outbreak, we isolated three SARS-CoV-2 strains from the bronchoalveolar lavage samples or throat swabs of three patients admitted to hospital (Wuhan Jinyintan Hospital, Chongqing Wushan County People's Hospital, or Qingdao Binhai New District Hospital). We selected strain 19nCoV-CDC-Tan-HB02 (HB02) to develop an inactivated vaccine because of its optimal replication and high virus yields in Vero cells, when compared with the other two strains. The HB02 strain was purified and passaged in Vero cells to generate the stock for vaccine production by using a novel carrier in a basket reactor. The stock virus replicated efficiently and reached a peak titre over 7·0 log10 cell culture infectious dose 50% assay by 48–72 h post infection at multiplicities of infection of 0·01–0·3. To inactivate virus production, β-propionolactone was thoroughly mixed with the harvested viral solution at a ratio of 1:4000 at 2–8°C. The vaccine was manufactured as a liquid formulation containing 2 μg, 4 μg, or 8 μg total protein with aluminium hydroxide adjuvant (0·45 mg/mL) per 0·5 mL.
In phase 1, on days 0 and 28, vaccine recipients received BBIBP-CorV containing 2 μg, 4 μg, or 8 μg total protein, and placebo recipients received saline containing aluminium hydroxide adjuvant. Adverse events were self-reported by participants, and verified by investigators each day for the first 7 days after each vaccination. Adverse events were subsequently recorded by participants on contact cards in the following 4 weeks. Laboratory tests were done before the first vaccination, and on day 4 after both the first and second vaccinations. Adverse events and abnormal changes in laboratory tests were graded according to the scale issued by the China State Food and Drug Administration (version 2019). Blood samples were taken from participants for serology tests at the scheduled site visits before the vaccination (on the same day), and on days 7, 14, 28, 32, and 42 after the vaccination. The neutralising antibody responses induced by vaccination were established for all blood samples using the infectious SARS-CoV-2 virus (strain 19nCoV-CDC-Tan-Strain04, QD01). In six participants (manually selected by staff) vaccinated with 4 μg, 2 weeks after the second dose (ie, day 42), the neutralising antibody responses against multiple SARS-CoV-2 strains were determined. Four of these isolated strains (35T, 56Y, 834Y, BJ01) are natural variants with spike mutant (D614G), which have been reported in the current pandemic (appendix 2 p 22).
In phase 2, four immunisation schedules were tested: three schedules of two doses of BBIBP-CorV at 4 μg total protein each or placebo, and one schedule of one shot of BBIBP-CorV at 8 μg total protein or placebo. In the 4 μg schedules, inoculations were given to participants on days 0 and 14, days 0 and 21, or days 0 and 28. For the 8 μg schedule, the inoculation was given on day 0. For the 8 μg schedule and the 4 μg days 0 and 28 schedule, neutralising antibody titres were measured on day 28 after the last inoculation. For the 4 μg days 0 and 14 and days 0 and 21 schedules, neutralising antibody titres were measured 2 weeks (ie, on day 14) after the second inoculation in half of the participants, and 4 weeks (ie, on day 28) after the second inoculation in the other half of participants. Adverse events were recorded, but laboratory safety tests were not included in phase 2. The neutralising antibody responses elicited by vaccination were determined by using the infectious SARS-CoV-2 virus (strain 19nCoV-CDC-Tan-Strain04, QD01).
Outcomes
The primary endpoint for safety was the occurrence of adverse reactions within 7 days after the first and second vaccinations. Any abnormal changes in laboratory measures at day 4 post inoculations, and adverse events within 28 days after the first and the second vaccinations across the treatment groups were analysed as secondary safety endpoints. The secondary humoral immunogenicity outcomes were measured using an infectious SARS-CoV-2 neutralising assay and expressed as neutralising antibody geometric mean titre (GMT). Seroconversion was defined as an increase in post-vaccination titre of four-fold or more from baseline. To directly demonstrate antibody neutralising efficacy and avoid the situation in which antibody binds to the receptor-binding domain, but fails to neutralise SARS-CoV-2 infection,16 IgG binding to specific virus protein (eg, S protein) assay was not included in the trial.
Statistical analysis
The sample size was not determined based on the statistical power calculation. Both phases 1 and 2 were designed at the same time. When 84 participants in the 2 μg, 4 μg, and 8 μg schedules of each age subgroup were enrolled (phase 1 n=24; phase 2 n=60), we had 80% power to detect a 15% rate difference in immunogenicity, with a 10% dropout rate at a significance level of 0·05 using a Z test for two independent proportions in PASS13-NCSS10. Planned sample sizes of 24 participants for each vaccination group and eight for each placebo group in phase 1, and 84 participants for each vaccination group and 28 for each placebo group in phase 2 (appendix 2 p 24) were determined.
The safety analysis was based on the safety set consisting of participants who received at least one dose of the vaccine after randomisation and had any safety evaluation information. Humoral immunogenicity analysis was based on the full analysis set consisting of enrolled participants who had randomly received vaccination with blood collection before and after each inoculation, and the compliance set consisting of enrolled participants who had randomly received vaccination with blood collection before and after each inoculation and did not violate the trial protocol. From the trial design perspective, for both age groups considered, the ratio of vaccination to placebo was 3:1 within the dose escalating schedule in phase 1 and vaccination timepoint in phase 2. All analyses were done using GraphPad Prism (version 8.0.1). All statistical tests were two-sided and the significance level was set at p values of 0·05 or less for inferential analyses. No formal statistical analysis was planned for unsolicited adverse events. For normal distributed data, differences between dose groups at a specific timepoint were tested with a two-sample t test with 95% CIs. All data were included in the analyses. Data from the two phases with 0-day, 28-day, and 56-day procedures and the same doses were pooled. Because the participants in the two phases came from the same regions and were randomised to the vaccine group and placebo group with the same lots, the safety and immunogenicity of two phases should be theoretically comparable. Pooled analysis is not included in the current report, because we have not obtained the results of phase 2 with the 0-day, 28-day, and 56-day procedures. This study is registered with www.chictr.org.cn, ChiCTR2000032459.
Role of the funding source
The funders of the study had no role in data collection, data analysis, data interpretation, or writing of the Article. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
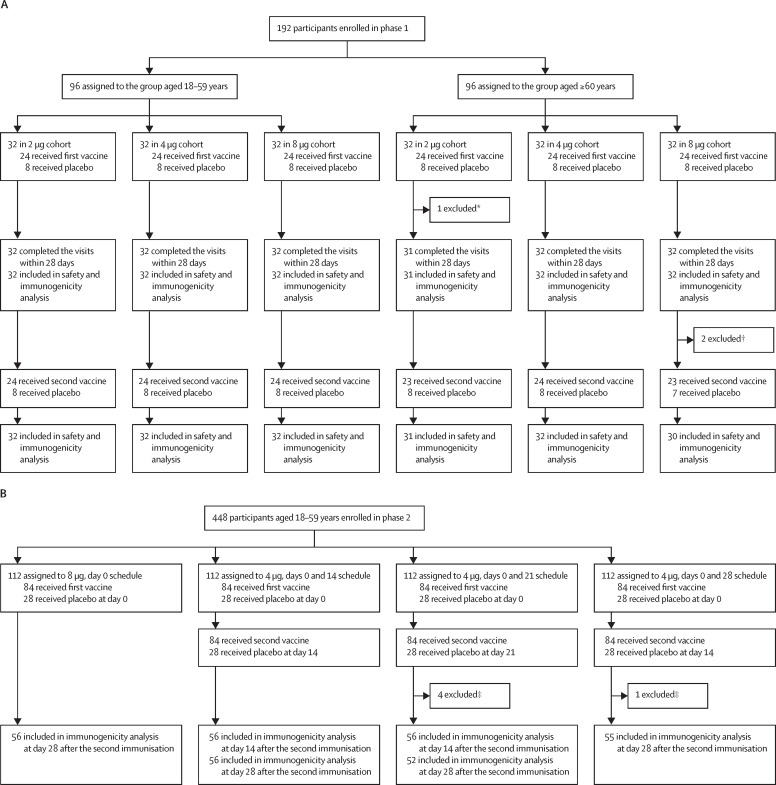
In phase 1, between April 29 and June 28, 2020, 466 participants were screened, and 192 were enrolled (47% male, 53% female; 50% aged 18–59 years, 50% aged ≥60 years; mean age 53·7 years). 96 participants were included in the group aged 18–59 years and 96 in the group aged 60 years and older. Within these groups, 32 participants were each randomly assigned to receive two doses of 2 μg, 4 μg, or 8 μg of vaccine or placebo in a 3:1 ratio; thus, 144 participants received vaccine and 48 participants received placebo (figure 1 ; appendix 2 p 24). One participant in the group aged 60 years and older receiving the 2 μg dose received the first vaccination, finished all safety visits, but did not have blood samples taken on personal request. One vaccine recipient and one placebo recipient in the group aged 60 years and older receiving 8 μg quit on day 28 before the second vaccination on personal request. Their safety data and existing immunogenicity data were included in the analysis. None of the enrolled patients had underlying disease. Baseline characteristics of the participants enrolled in phase 1 are shown in table 1 .
Figure 1.
Trial profile for phase 1 (A) and phase 2 (B)
*Participant received the first vaccination, finished all safety visits, but did not have blood sample taken on personal request. †Two participants quit the trial at day 28 before the second vaccination on personal request. ‡Four participants in the days 0 and 21 schedule and one participant in the days 0 and 28 schedule quit or did not finish taking blood sample at day 28 on request.
Table 1.
Baseline characteristics in phase 1
|
18–59 years |
≥60 years |
Total (n=192) | |||||
|---|---|---|---|---|---|---|---|
| 2 μg (n=32) | 4 μg (n=32) | 8 μg (n=32) | 2 μg (n=32) | 4 μg (n=32) | 8 μg (n=32) | ||
| Age, years | |||||||
| 18–29 | 2 (6%) | 10 (31%) | 3 (9%) | NA | NA | NA | 15 (8%) |
| 30–39 | 9 (28%) | 8 (25%) | 12 (38%) | NA | NA | NA | 29 (15%) |
| 40–49 | 15 (47%) | 8 (25%) | 11 (34%) | NA | NA | NA | 34 (18%) |
| 50–59 | 6 (19%) | 6 (19%) | 6 (19%) | NA | NA | NA | 18 (9%) |
| 60–69 | NA | NA | NA | 26 (81%) | 22 (69%) | 28 (88%) | 76 (40%) |
| 70–79 | NA | NA | NA | 6 (19%) | 10 (31%) | 3 (9%) | 19 (10%) |
| ≥80 | NA | NA | NA | 0 | 0 | 1 (3%) | 1 (1%) |
| Mean | 42·7 (8·1) | 37·7 (12·2) | 40·1 (8·6) | 65·90 (4·1) | 67·5 (4·1) | 67·5 (4·0) | 53·7 (15·6) |
| Sex | |||||||
| Male | 11 (34%) | 16 (50%) | 17 (53%) | 20 (62%) | 17 (53%) | 9 (28%) | 90 (47%) |
| Female | 21 (66%) | 16 (50%) | 15 (47%) | 12 (38%) | 15 (47%) | 23 (72%) | 102 (53%) |
Data are n (%) or mean (SD). NA=not applicable.
42 (29%) of 144 vaccine recipients had at least one adverse reaction within 7 days of either vaccination, compared with eight (17%) of 48 placebo recipients (Table 2, Table 3 ). In the group aged 18–59 years, at least one adverse reaction occurred within the first 7 days after either vaccination in 11 (46%) of 24 vaccine recipients in the 2 μg cohort (compared with three [38%] of eight placebo recipients; p>0·99), eight (33%) of 24 vaccine recipients in the 4 μg cohort (compared with two [25%] of eight placebo recipients; p>0·99), and 11 (46%) of 24 vaccine recipients in the 8 μg cohort (compared with one [13%] of eight placebo recipients; p=0·2). In the group aged 60 years and older, at least one adverse reaction occurred within the first 7 days of either vaccination in one (4%) of 24 vaccine recipients in the 2 μg cohort (compared with one [13%] of eight placebo recipients; p=0·44), six (25%) of 24 vaccine recipients in the 4 μg cohort (compared with zero placebo recipients; p=0·3), and five (21%) of 24 vaccine recipients in the 8 μg cohort (compared with one [13%] of eight placebo recipients; p>0·99).
Table 2.
Adverse reactions within 7 days and overall adverse events within 28 days after the first and the second vaccinations for the group aged 18–59 years in phase 1
|
2 μg cohort (n=32) |
4 μg cohort (n=32) |
8 μg cohort (n=32) |
Total (n=96) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination (n=24) | Placebo (n=8) | p value | Vaccination (n=24) | Placebo (n=8) | p value | Vaccination (n=24) | Placebo (n=8) | p value | Vaccination (n=72) | Placebo (n=24) | p value | ||
| All adverse reactions within 0–7 days | |||||||||||||
| Any | 11 (46%) | 3 (38%) | >0·99 | 8 (33%) | 2 (25%) | >0·99 | 11 (46%) | 1 (13%) | 0·20 | 30 (42%) | 6 (25%) | 0·22 | |
| Grade 1 | 10 (42%) | 3 (38%) | .. | 8 (33%) | 2 (25%) | .. | 11 (46%) | 1 (13%) | .. | 29 (40%) | 6 (25%) | .. | |
| Grade 2 | 1 (4%) | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 2 (3%) | 0 | .. | |
| Injection site adverse reactions within 0–7 days | |||||||||||||
| Pain | 9 (38%) | 1 (13%) | 0·38 | 7 (29%) | 1 (13%) | 0·64 | 9 (38%) | 0 | 0·07 | 25 (35%) | 2 (8%) | 0·017 | |
| Grade 1 | 9 (38%) | 1 (13%) | .. | 7 (29%) | 1 (13%) | .. | 9 (38%) | .. | .. | 25 (35%) | 2 (8%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Swelling | 0 | 0 | .. | 0 | 1 (13%) | 0·25 | 2 (8%) | 0 | >0·99 | 2 (3%) | 1 (4%) | >0·99 | |
| Grade 1 | 0 | 0 | .. | 0 | 1 (13%) | .. | 1 (4%) | 0 | .. | 1 (1%) | 1 (4%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (1%) | 0 | ||
| Itch | 0 | 0 | .. | 1 (4%) | 0 | >0·99 | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 1 (4%) | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Redness | 1 (4%) | 0 | >0·99 | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 1 (4%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Systemic adverse reactions within 0–7 days | |||||||||||||
| Fever | 1 (4%) | 2 (25%) | 0·15 | 1 (4%) | 0 | >0·99 | 2 (8%) | 0 | >0·99 | 4 (6%) | 2 (8%) | 0·64 | |
| Grade 1 | 1 (4%) | 2 (25%) | .. | 1 (4%) | 0 | .. | 2 (8%) | 0 | .. | 4 (6%) | 2 (8%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Fatigue | 2 (8%) | 0 | >0·99 | 0 | 0 | .. | 0 | 1 (13%) | 0·25 | 2 (3%) | 1 (4%) | >0·99 | |
| Grade 1 | 2 (8%) | 0 | .. | 0 | 0 | .. | 0 | 1 (13%) | .. | 2 (3%) | 1 (4%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Inappetence | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | >0·99 | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Nausea | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | >0·99 | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Constipation | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | >0·99 | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Mucocutaneous abnormalities | 1 (4%) | 0 | >0·99 | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Grade 2 | 1 (4%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | |
| Headache | 1 (4%) | 0 | >0·99 | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 1 (4%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Vomiting | 0 | 0 | .. | 1 (4%) | 0 | >0·99 | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 0 | .. | 1 (4%) | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | ||
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Itch (non-injection site) | 1 (4%) | 0 | >0·99 | 0 | 0 | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | ||
| Grade 1 | 1 (4%) | 0 | .. | .. | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | .. | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Overall adverse events within 0–28 days | |||||||||||||
| Any | 12 (50%) | 3 (38%) | 0·69 | 11 (46%) | 2 (25%) | 0·42 | 11 (46%) | 2 (25%) | 0·42 | 34 (47%) | 7 (29%) | 0·16 | |
| Grade 1 | 10 (42%) | 3 (38%) | .. | 8 (33%) | 1 (13%) | .. | 8 (33%) | 1 (8%) | .. | 26 (36%) | 5 (21%) | .. | |
| Grade 2 | 2 (8%) | 0 | .. | 3 (13%) | 1 (13%) | .. | 3 (13%) | 1 (8%) | .. | 8 (11%) | 2 (8%) | .. | |
Data are n (%). Any refers to all the participants with any grade adverse reactions or events. Adverse reactions and events were graded according to the scale issued by the China State Food and Drug Administration.
Table 3.
Adverse reactions within 7 days and overall adverse events within 28 days after the first and the second vaccinations for the group aged 60 years or older in phase 1
|
2 μg cohort (n=32) |
4 μg cohort (n=32) |
8 μg cohort (n=32) |
Total (n=96) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination (n=24) | Placebo (n=8) | p value | Vaccination (n=24) | Placebo (n=8) | p value | Vaccination (n=24) | Placebo (n=8) | p value | Vaccination (n=24) | Placebo (n=8) | p value | ||
| All adverse reactions within 0–7 days | |||||||||||||
| Any | 1 (4%) | 1 (13%) | 0·44 | 6 (25%) | 0 | 0·30 | 5 (21%) | 1 (13%) | >0·99 | 12 (17%) | 2 (8%) | 0·51 | |
| Grade 1 | 1 (4%) | 1 (13%) | .. | 6 (25%) | 0 | .. | 4 (17%) | 1 (13%) | .. | 11 (15%) | 2 (8%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (1%) | 0 | .. | |
| Injection site adverse reactions within 0–7 days | |||||||||||||
| Pain | 1 (4%) | 1 (13%) | 0·44 | 4 (17%) | 0 | 0·55 | 4 (17%) | 0 | 0·55 | 9 (13%) | 1 (4%) | 0·44 | |
| Grade 1 | 1 (4%) | 1 (13%) | .. | 4 (17%) | 0 | .. | 4 (17%) | 0 | .. | 9 (13%) | 1 (4%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Induration | 0 | 0 | .. | 0 | 0 | .. | 2 (8%) | 0 | >0·99 | 2 (3%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (1%) | 0 | .. | |
| Systemic adverse reactions within 0–7 days | |||||||||||||
| Fever | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | >0·99 | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Fatigue | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | >0·99 | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Headache | 0 | 0 | .. | 1 (4%) | 0 | >0·99 | 1 (4%) | 0 | >0·99 | 2 (3%) | 0 | .. | |
| Grade 1 | 0 | 0 | .. | 1 (4%) | 0 | .. | 1 (4%) | 0 | .. | 2 (3%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Diarrhoea | 0 | 0 | .. | 2 (8%) | 0 | >0·99 | 0 | 0 | .. | 2 (3%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 2 (8%) | 0 | .. | 0 | 0 | .. | 2 (3%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Joint pain | 0 | 0 | .. | 1 (4%) | 0 | >0·99 | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | |
| Grade 1 | 0 | 0 | .. | 1 (4%) | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Muscle pain | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (13%) | 0·25 | 0 | 1 (4%) | 0·26 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (13%) | .. | 0 | 1 (4%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Overall adverse events within 0–28 days | |||||||||||||
| Any | 2 (8%) | 1 (13%) | >0·99 | 7 (29%) | 0 | 0·15 | 5 (21%) | 2 (25%) | >0·99 | 14 (19%) | 3 (13%) | 0·55 | |
| Grade 1 | 1 (4%) | 1 (13%) | .. | 7 (29%) | 0 | .. | 5 (21%) | 1 (13%) | .. | 13 (18%) | 2 (8%) | .. | |
| Grade 2 | 1 (4%) | 0 | .. | 0 | 0 | .. | 1 (4%) | 0 | .. | 2 (4%) | 0 | .. | |
| Grade 3 | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (13%) | .. | 0 | 1 (4%) | .. | |
Data are n (%). Any refers to all the participants with any grade adverse reactions or events. Adverse reactions and events were graded according to the scale issued by the China State Food and Drug Administration. Grade 3=severe.
The most common injection site adverse reaction was pain, which was reported in 34 (24%) of 144 vaccine recipients after either vaccination, compared with three (6%) of 48 placebo recipients (Table 3, Table 4 ). For vaccine recipients in the group aged 18–59 years (n=72), besides pain (nine [38%] in the 2 μg group, seven [29%] in the 4 μg group, and nine [38%] in the 8 μg group), additional injection site adverse reactions included swelling (two [3%] of 72) and itch (one [1%] of 72). For the vaccine recipients in the group aged 60 years and older (n=72), besides pain (one [4%] in the 2 μg group, four [17%] in the 4 μg group, and four [17%] in the 8 μg group), an additional injection site adverse reaction was induration (two [3%] of 72). We observed statistically higher reports for pain in the group aged 18–59 years than the placebo group (two [8%] of 24; p=0·017).
Table 4.
Baseline characteristics in phase 2 (participants aged 18–59 years)
| 8 μg day 0 (n=112) | 4 μg days 0and 14 (n=112) | 4 μg days 0 and 21 (n=112) | 4 μg days 0and 28 (n=112) | Total (n=448) | |
|---|---|---|---|---|---|
| Age, years | |||||
| 18–29 | 13 (12%) | 14 (13%) | 13 (12%) | 11 (10%) | 51 (11%) |
| 30–39 | 36 (32%) | 37 (33%) | 39 (35%) | 27 (24%) | 139 (31%) |
| 40–49 | 42 (38%) | 33 (29%) | 32 (29%) | 36 (32%) | 143 (32%) |
| 50–59 | 21 (19%) | 28 (25%) | 28 (25%) | 38 (34%) | 115 (26%) |
| Mean | 40·8 (10·0) | 41·0 (10·0) | 41·7 (9·6) | 43·7 (9·9) | 41·7 (9·9) |
| Sex | |||||
| Male | 52 (46%) | 50 (45%) | 53 (47%) | 48 (43%) | 203 (45%) |
| Female | 60 (54%) | 62 (55%) | 59 (53%) | 64 (57%) | 245 (55%) |
Data are n (%) or mean (SD).
The most commonly reported systematic adverse reaction overall after either vaccination was fever, which was reported in five (4%) of 144 vaccine recipients, compared with three (6%) of 48 placebo recipients (Table 3, Table 4). For the group aged 18–59 years, fever was reported in all three dose cohorts of vaccine recipients: one (4%) of 24 in the 2 μg cohort, one (4%) of 24 in the 4 μg cohort, and two (8%) of 24 in the 8 μg cohort. For this same age group (n=72), besides fever, the systematic adverse reactions included fatigue (two [3%]), inappetence (one [1%]), nausea (one [1%]), constipation (one [1%]), mucocutaneous abnormalities (two [3%]), headache (one [1%]), vomiting (one [1%]), and itch (non-injection site; one [1%]). For the vaccine recipients in the cohort aged 60 years or older (n=72), fever (one [1%]) and fatigue (one [1%]) were reported in the 8 μg cohort; and headache (one [1%]), diarrhoea (one [1%]), and joint pain (one [1%]) in the 4 μg cohort. One placebo recipient was reported to have muscle pain. All adverse reactions were mild or moderate in severity. No serious adverse event was reported within 28 days post vaccination for all cohorts.
At day 4 after either vaccination, in the group aged 18–59 years, a small proportion of vaccine recipients (n=72) had mild to moderate abnormal haemoglobin (one [1%]), blood urea nitrogen (one [1%]), blood glucose (seven [10%]), serum total bilirubin (14 [19%]), urinary protein (one [1%]), or urinary glucose (one [1%]; appendix 2 pp 3–4). In the group aged 60 years and older (n=72), a small proportion of vaccine recipients had mild to moderate abnormal white blood cells (one [1%]), haemoglobin (three [4%]), alanine aminotransferase (two [3%]), blood urea nitrogen (four [6%]), aspartate aminotransferase (four [6%]), blood glucose (ten [14%]), serum total bilirubin (nine [12%]), and urinary protein (three [4%]; appendix 2 pp 5–6). No instances were considered as clinically significant.
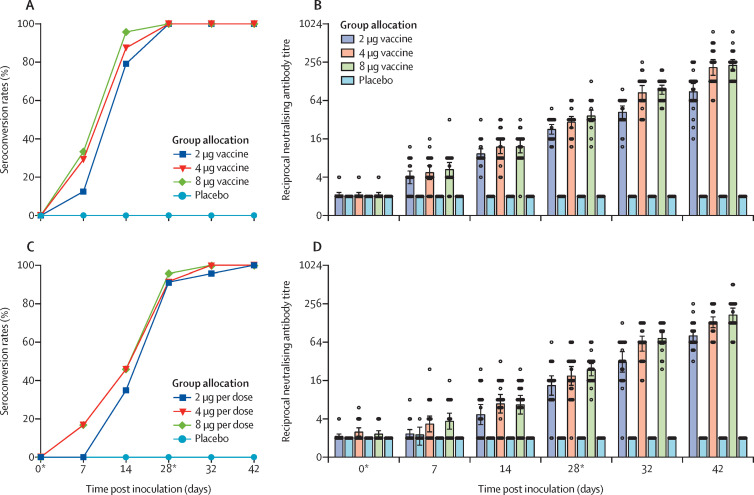
For vaccine recipients in the group aged 18–59 years, 19 (79%) of 24 in the 2 μg group, 21 (87%) of 24 in the 4 μg group, and 23 (96%) of 24 in the 8 μg group seroconverted by day 14 (figure 2 ). Seroconversion rates reached 100% in all three cohorts on day 28. For vaccine recipients in the group aged 60 years and older, eight (4%) of 23 in the 2 μg group and 11 (46%) of 24 in each of the 4 μg and 8 μg groups seroconverted by day 14. 21 (91%) of 23 in the 2 μg group, 22 (92%) of 24 in the 4 μg group, and 22 (96%) of 23 in the 8 μg group seroconverted by day 28. The neutralising antibodies in all placebo recipients were negative throughout the trials.
Figure 2.
Seroconversion ratios and neutralising antibody titres for and 60 years and older
Seroconversion rates (A) and neutralising antibody titres (B) for participants aged 18–59 years; and seroconversion rates (C) and neutralising antibody titres (D) for participants aged 60 years and older. We defined the seroconversion as at least a four-fold increase in post-vaccination titre from baseline. The negative in neutralisation antibody detection is represented as a GMT of 2. *Days of vaccination.
The neutralising antibodies against infectious SARS-CoV-2 were detected in 26 (18%) of 143 vaccine recipients on day 7 after the first inoculation, and increased to 100% on day 42 after the second inoculation (figure 2; appendix 2 pp 7–21). In the group aged 18–59 years, by day 28, the neutralising antibody GMTs in the three vaccine recipient cohorts were greater than the placebo cohort (2 μg 22·6, 95% CI 18·9–27·0; 4 μg 29·3, 23·8–36·0; 8 μg 36·7, 29·8–45·2; placebo 2·0, 2·0–2·0). By day 28, neutralising antibody GMT in the 2 μg cohort was significantly lower than the 8 μg cohort (p=0·0093), and the neutralising antibody GMT in the 4 μg cohort was not significantly different than in the 8 μg cohort (p=0·58). By day 42, the neutralising antibody GMT in the 2 μg cohort (87·7, 95% CI 64·9–118·6) was significantly lower than the 8 μg cohort (228·7, 186·1–281·1; p<0·001), and the neutralising antibody GMT in the 4 μg cohort (211·2, 158·9–280·6) was statistically comparable to that in the 8 μg cohort (p>0·99).
In the group aged 60 years and older, by day 28, the neutralising antibody GMT was greater in the vaccine recipients (2 μg 13·4, 95% CI 9·4–19·0; 4 μg 18·9, 13·4–26·6; 8 μg 23·7, 19·0–29·6) than the placebo cohort (2·0, 2·0–2·0). By day 28, the neutralising antibody GMTs in the 2 μg (p=0·087) and 4 μg (p=0·96) cohorts were not significantly different than that in the 8 μg cohort. By day 42, the neutralising antibody GMT in the 2 μg cohort (80·7, 95% CI 65·4–99·6) was significantly lower than that in the 8 μg cohort (170·9, 133·0–219·5; p<0·001), and the neutralising antibody GMT in the 4 μg cohort (131·5, 108·2–159·7) was not significantly different than that in the 8 μg cohort (p=0·30).
In the six randomly selected participants vaccinated with 4 μg, by day 14 after the second dose (day 42), the neutralising antibody GMTs against multiple SARS-CoV-2 strains were 279·2 (95% CI 192·6–404·7) against 35C, 234·8 (122·2–450·8) against 56Y, 181·0 (105·9–309·5) against 834Y, 304·4 (202·1–485·6) against HN97, 117·4 (61·1–225·4) against F13, 193·3 (141·4–264·0) against HB01, 210·7 (120·3–369·1) against BJ01, 146·8 (93·8–230·0) against CQ01, 218·5 (125·3–380·8) against QD01, and 394·8 (256·5–607·6) against passage 7 virus stock for vaccine manufacture (appendix 2 p 2).
In phase 2, between May 18 and July 30, 2020, 546 participants aged 18–59 years were screened, and 448 were enrolled (45% male, 55% female; mean age 41·7 years). Baseline characteristics of the participants enrolled in phase 2 are shown in table 4. None of the enrolled patients had underlying disease. To test the impact of 8 μg of antigen in different combinations, either via a single dose with the full amount or two doses each with 4 μg of antigen, and based on the results from preclinical and phase 1 studies, participants were randomly assigned to receive 8 μg of vaccine or placebo on day 0 (n=112), or 4 μg of vaccine or placebo on days 0 and 14 (n=112), 0 and 21 (n=112), or 0 and 28 (n=112; figure 1; appendix 2 p 24). Four participants in the 4 μg days 0 and 21 group, and one participant in the 4 μg days 0 and 28 group quit or did not finish providing a blood sample on day 28; their existing data were included in the analysis. In phase 2, at least one adverse reaction within the first 7 days after either vaccination was reported in 76 (23%) of 336 vaccine recipients (table 5 ). The most common injection site adverse reaction in the vaccine recipient group was pain (53 [16%] of 336), and was higher than the placebo group (four [4%] of 112; p=0·008). The most common systematic adverse reaction in the vaccine recipient group was fever (seven [2%] of 336). One placebo recipient in the 4 μg days 0 and 21 group reported grade 3 fever, but was self-limited and recovered. All other adverse reactions were mild or moderate in severity.
Table 5.
Adverse reactions within 7 days and overall adverse events within 30 days after the first and the second vaccinations for all schedules in phase 2
|
8 μg day 0 (n=112) |
4 μg days 0 and 14 (n=112) |
4 μg days 0 and 21 (n=112) |
4 μg days 0 and 28 (n=112) |
Total (n=448) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination (n=84) | Placebo (n=28) | p value | Vaccination (n=84) | Placebo (n=28) | p value | Vaccination (n=84) | Placebo (n=28) | p value | Vaccination (n=84) | Placebo (n=28) | p value | Vaccination (n=336) | Placebo (n=112) | p value | ||
| All adverse reactions within 0–7 days | ||||||||||||||||
| Any | 33 (39%) | 3 (11%) | 0·0049 | 18 (21%) | 5 (18%) | 0·79 | 15 (18%) | 5 (18%) | >0·99 | 10 (12%) | 6 (21%) | 0·22 | 76 (23%) | 19 (17%) | 0·20 | |
| Grade 1 | 31 (37%) | 3 (11%) | .. | 18 (21%) | 3 (11%) | .. | 13 (15%) | 4 (14%) | .. | 8 (10%) | 2 (7%) | .. | 70 (21%) | 12 (11%) | .. | |
| Grade 2 | 2 (2%) | 0 | .. | 0 | 2 (7%) | .. | 2 (2%) | 0 | .. | 2 (2%) | 4 (14%) | .. | 6 (2%) | 6 (5%) | .. | |
| Grade 3 | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (4%) | .. | 0 | 0 | .. | 0 | 1 (1%) | .. | |
| Injection site adverse reactions within 0–7 days | ||||||||||||||||
| Pain | 25 (30%) | 2 (7%) | 0·02 | 12 (14%) | 0 | 0·035 | 10 (12%) | 1 (4%) | 0·29 | 6 (7%) | 1 (4%) | 0·68 | 53 (16%) | 4 (4%) | 0·0008 | |
| Grade 1 | 25 (30%) | 2 (7%) | .. | 12 (14%) | 0 | .. | 10 (12%) | 1 (4%) | .. | 6 (7%) | 1 (4%) | .. | 53 (16%) | 4 (4%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Swelling | 2 (2%) | 0 | >0·99 | 0 | 0 | .. | 3 (4%) | 0 | 0·57 | 1 (1%) | 0 | >0·99 | 6 (2%) | 0 | 0·15 | |
| Grade 1 | 2 (2%) | 0 | .. | 0 | 0 | .. | 3 (4%) | 0 | .. | 1 (1%) | 0 | .. | 6 (2%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Itch | 2 (2%) | 0 | >0·99 | 1 (1%) | 1 (4%) | 0·44 | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | 4 (1%) | 1 (1%) | 0·80 | |
| Grade 1 | 2 (2%) | 0 | .. | 1 (1%) | 1 (4%) | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | 4 (1%) | 1 (1%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Redness | 1 (1%) | 0 | >0·99 | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | 1 (1%) | 0 | >0·99 | 3 (1%) | 0 | 0·32 | |
| Grade 1 | 1 (1%) | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | 1 (1%) | 0 | .. | 3 (1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Rash | 1 (1%) | 0 | >0·99 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (<1%) | 0 | 0·58 | |
| Grade 1 | 1 (1%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (<1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Systemic adverse reactions within 0–7 days | ||||||||||||||||
| Fever | 1 (1%) | 1 (4%) | 0·44 | 1 (1%) | 0 | >0·99 | 3 (4%) | 1 (4%) | >0·99 | 2 (2%) | 3 (11%) | 0·099 | 7 (2%) | 5 (4%) | 0·18 | |
| Grade 1 | 1 (1%) | 1 (4%) | .. | 1 (1%) | 0 | .. | 2 (2%) | 0 | .. | 1 (1%) | 2 (7%) | .. | 5 (1%) | 3 (3%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | 1 (1%) | 1 (4%) | .. | 2 (1%) | 1 (1%) | .. | |
| Grade 3 | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (4%) | .. | 0 | 0 | .. | 0 | 1 (1%) | .. | |
| Fatigue | 5 (6%) | 0 | 0·33 | 2 (2%) | 1 (4%) | >0·99 | 1 (1%) | 3 (11%) | 0·048 | 1 (1%) | 1 (4%) | 0·44 | 9 (3%) | 5 (4%) | 0·35 | |
| Grade 1 | 5 (6%) | 0 | .. | 2 (2%) | 1 (4%) | .. | 1 (1%) | 2 (7%) | .. | 1 (1%) | 0 | 9 (3%) | 5 (4%) | .. | ||
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (4%) | .. | 0 | 1 (4%) | 0·25 | 0 | 0 | .. | |
| Nausea | 0 | 1 (4%) | 0·25 | 0 | 0 | .. | 2 (2%) | 1 (4%) | >0·99 | 0 | 1 (4%) | 0·25 | 2 (1%) | 3 (3%) | 0·07 | |
| Grade 1 | 0 | 1 (4%) | .. | 0 | 0 | .. | 1 (1%) | 1 (4%) | .. | 0 | 0 | .. | 1 (<1%) | 3 (3%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | 0 | 1 (4%) | .. | 1 (<1%) | 0 | .. | |
| Headache | 1 (1%) | 0 | >0·99 | 2 (2%) | 0 | >0·99 | 1 (1%) | 2 (7%) | 0·15 | 0 | 1 (4%) | 0·25 | 4 (1%) | 3 (3%) | 0·27 | |
| Grade 1 | 1 (1%) | 0 | .. | 2 (2%) | 0 | .. | 1 (1%) | 2 (7%) | .. | 0 | 0 | .. | 4 (1%) | 2 (2%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 1 (4%) | .. | 0 | 1 (1%) | .. | |
| Itch (non-injection site) | 0 | 0 | .. | 2 (2%) | 0 | >0·99 | 0 | 1 (4%) | 0·25 | 1 (1%) | 0 | >0·99 | 3 (1%) | 1 (1%) | >0·99 | |
| Grade 1 | 0 | 0 | .. | 2 (2%) | 0 | .. | 0 | 1 (4%) | .. | 1 (1%) | 0 | .. | 3 (1%) | 1 (1%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Cough | 0 | 0 | .. | 0 | 1 (4%) | 0·25 | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | 1 (<1%) | 1 (1%) | 0·41 | |
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 1 (4%) | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | 1 (<1%) | 1 (1%) | .. | |
| Diarrhoea | 2 (2%) | 0 | >0·99 | 2 (2%) | 1 (4%) | >0·99 | 0 | 2 (7%) | 0·061 | 0 | 0 | .. | 4 (1%) | 3 (3%) | 0·27 | |
| Grade 1 | 2 (2%) | 0 | .. | 2 (2%) | 1 (4%) | .. | 0 | 2 (7%) | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Muscle pain | 1 (1%) | 0 | >0·99 | 1 (1%) | 0 | >0·99 | 0 | 0 | .. | 0 | 0 | .. | 1 (<1%) | 1 (1%) | 0·41 | |
| Grade 1 | 1 (1%) | 0 | .. | 1 (1%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (<1%) | 1 (1%) | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Anaphylaxis | 1 (1%) | 0 | >0·99 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (<1%) | 0 | 0·58 | |
| Grade 1 | 1 (1%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (<1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Other adverse reactions within 0–7 days | ||||||||||||||||
| Drowsiness | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | >0·99 | 0 | 0 | 1 (<1%) | 0 | 0·58 | ||
| Grade 1 | 0 | 0 | .. | 0 | 0 | .. | 1 (1%) | 0 | .. | 0 | 0 | .. | 1 (<1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Dizziness | 1 (1%) | 0 | >0·99 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (<1%) | 0 | 0·58 | |
| Grade 1 | 1 (1%) | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 1 (<1%) | 0 | .. | |
| Grade 2 | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | 0 | 0 | .. | |
| Overall adverse events within 0–30 days | ||||||||||||||||
| Any | 33 (39%) | 3 (11%) | 0·015 | 19 (23%) | 6 (21%) | >0·99 | 15 (18%) | 6 (21%) | 0·78 | 11 (13%) | 6 (21%) | 0·36 | 78 (23%) | 21 (19%) | 0·32 | |
| Grade 1 | 30 (36%) | 3 (11%) | .. | 17 (20%) | 3 (11%) | .. | 13 (15%) | 4 (14%) | .. | 5 (6%) | 2 (7%) | .. | 65 (19%) | 12 (11%) | .. | |
| Grade 2 | 2 (2%) | 0 | .. | 2 (2%) | 3 (11%) | .. | 2 (2%) | 1 (4%) | .. | 6 (7%) | 4 (14%) | .. | 12 (4%) | 8 (7%) | .. | |
| Grade 3 | 1 (1%) | 0 | .. | 0 | 0 | .. | 0 | 1 (4%) | .. | 0 | 0 | .. | 1 (<1%) | 5 (1%) | .. | |
Data are n (%). Any refers to all the participants with any grade adverse reactions or events. Adverse reactions and events were graded according to the scale issued by the China State Food and Drug Administration. One placebo recipient in the days 0 and 21 schedule reported grade 3 fever (38·5°C), but was self-limited and recovered. Grade 3=severe.
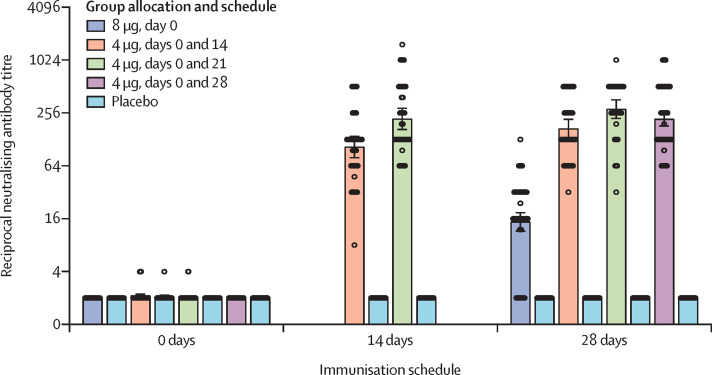
In phase 2, the neutralising antibodies against infectious SARS-CoV-2 were detected in all vaccine recipients after inoculation in the 8 μg day 0 group, and the 4 μg days 0 and 14, 0 and 21, and 0 and 28 schedules (figure 3 ; appendix 2 p 18–21). In all four vaccination schedules (8 μg day 0, 14·7 [95% CI 11·6–18·8]; 4 μg days 0 and 14, 169·5 [132·2–217·1]; 4 μg days 0 and 21, 282·7 [221·2–361·4]; 4 μg days 0 and 28, 218·0 [181·8–261·3]), the neutralising antibody GMT was greater than the placebo cohort (2·0 [2·0–2·0]) by day 28 after the inoculation. By day 14 after the second inoculation, neutralising antibody GMT in the 4 μg days 0 and 14 cohort (104·9 [79·0–139·1]) was significantly lower than that in the 4 μg days 0 and 21 cohort (218·9 [165·6–289·5]; p=0·0051). By day 28 after the last inoculation, neutralising antibody GMT in the 8 μg day 0 cohort was significantly lower than that in the 4 μg days 0 and 14, 0 and 21, and 0 and 28 cohorts (all p<0·001). By day 28 after the second inoculation, the neutralising antibody GMT in the 4 μg days 0 and 14 cohort was lower than in the days 0 and 21 (p=0·0081) and days 0 and 28 (p=0·084) cohorts, which showed comparable GMT volume (p=0·34).
Figure 3.
Neutralising antibody titres for different immunisation schedules
The negative in neutralisation antibody detection is represented as a GMT of 2. 0 days is pre-immunisation. 14 days and 28 days refers to day 14 and day 28 after the second inoculation, with the exception of the 8 μg, day 0 group (in which it refers to day 28 after the single inoculation). The measurement of neutralising antibody at day 14 was not designed for the 4 μg days 0 and 14 or days 0 and 28 groups. For the 4 μg days 0 and 14 and days 0 and 21 groups, samples from day 14 were collected from half of the participants in the group and day 28 from the other half.
Discussion
In this phase 1/2 trial, the BBIBP-CorV inactivated vaccine, given as a two-dose immunisation, was safe and well tolerated at all three doses in both age groups. A robust humoral immune response was observed in 100% of vaccine recipients. In preclinical studies, we showed that immunisation with BBIBP-CorV can induce high levels of neutralising antibody titres in mice, rats, guinea pigs, rabbits, and non-human primates (cynomolgus monkeys and rhesus macaques) to provide protection against SARS-CoV-2.13
The most common adverse reactions were pain and fever, which were reported in small proportions of vaccine recipients and with no significant difference across the groups. All adverse events were mild or moderate in severity. Notably, there was a higher number of systemic adverse events in the placebo group, but during the follow-up monitoring of respiratory symptoms, no upper respiratory tract infections were detected. There were also no clinically significant abnormal changes in laboratory measurements, nor were any changes considered to be related to the vaccine. Evidence to indicate antibody-dependent enhancement in SARS-CoV infection has emerged;17, 18 however, in preclinical studies of BBIBP-CorV immunisation and SARS-CoV-2 challenge, no antibody-dependent enhancement was observed in rhesus macaques.13 In our ongoing phase 2 clinical trial, we use BBIBP-CorV at 2 μg, 4 μg, and 8 μg, in one-dose, two-dose, and three-dose immunisation schedules to profile vaccine safety and immunogenicity in children and adolescents (aged 3–17 years), adults (18–59 years), and older people (≥60 years).
BBIBP-CorV was immunogenic and induced robust humoral responses rapidly. For patients aged 60 years and older, and patients with pre-existing respiratory or cardiovascular disease, COVID-19 presents a remarkably high risk of severe disease and death.7 In this trial, we aimed to evaluate the safety and tolerability of BBIBP-CorV in patients aged 60 years and older. The 100% seroconversion rate was reached earlier in the group aged 18–59 years than in the group aged 60 years and older. More than 75% of vaccine recipients in the group aged 18–59 years seroconverted after the first vaccine dose (day 14). The remaining vaccine recipients seroconverted on day 28. For the group aged 60 years and older, the seroconversion rate of the 4 μg and 8 μg dose recipients reached 100% on day 28, and the 2 μg group was 100% seroconverted by day 42. The magnitude of neutralising antibodies in the group aged 60 years and older was lower than in the group aged 18–59 years.
The neutralising antibodies induced by BBIBP-CorV can neutralise multiple SARS-CoV-2 strains (appendix 2 pp 2, 22). These findings indicate the potential of BBIBP-CorV to provide cross-protection against other SARS-CoV-2 strains.
In the test of different immunisation schedules, neutralising antibody titres of the 8 μg, day 0 single-dose immunisation schedule were significantly lower than those of all three two-dose immunisation schedules. The neutralising antibody titres after the second inoculation in the 4 μg days 0 and 21 schedule were comparable to those in the 4 μg days 0 and 28 schedule, but were significantly greater than that in the 4 μg days 0 and 14 schedule. These results suggest that the boost inoculation is necessary to achieve more efficient protection, providing useful information for a phase 3 trial.
While this Article was under revision, the results of a similar trial of SARS-CoV-2 inactivated vaccine were reported.19 Despite similar findings in safety and immunogenicity, the study claimed no notable changes in lymphocyte subset or cytokines, which is consistent with our unpublished findings.
Interpretation of the results of this study is limited by the short duration of follow-up. Another limitation of our study is the absence of safety and immunogenicity testing in children and adolescents. Although it was part of the original plan for this trial, we will not begin testing in people aged younger than 18 years until the full analysis of the adult groups is finalised. We will investigate younger ages in the ongoing phase 1 trial. As our study was, to our knowledge when the trial began, the first trial of an inactivated SARS-CoV-2 vaccine, it was not designed to measure vaccine efficacy.
In conclusion, we found that the inactivated SARS-CoV-2 vaccine BBIBP-CorV is tolerable and immunogenic in healthy people. Rapid humoral responses against SARS-CoV-2 were noted from day 4 after the first inoculation and 100% seroconversion was found in all participants on day 42. The days 0 and 21 and days 0 and 28 two-immunisation schedules elicited significantly greater neutralising antibody titres than the days 0 and 14 schedule and the single-immunisation schedule. There is potential for further investigation of this inactivated vaccine for the control and prevention of COVID-19. The ongoing phase 1/2 and phase 3 trials will provide more information on the safety and immunogenicity, dose, and immunisation schedule of BBIBP-CorV.
Data sharing
We support data sharing of the individual participant data. The individual participant data that underlie the results reported in this Article, after deidentification (ie, text, tables, figures, and appendix 2) will be shared. Individual participant data will be available beginning 3 months and ending 1 year after publication. Supporting clinical documents, including the study protocol, statistical analysis plan, and the informed consent form, will be available immediately following publication for at least 1 year. Researchers who provide a scientifically sound proposal will be allowed access to the individual participant data. Proposals should be directed to wangyanxia99@163.com or nvsiclinicaltrials@163.com. These proposals will be reviewed and approved by the funder, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.
Acknowledgments
Acknowledgments
This work was supported by the National Program on Key Research Project of China (2020YFA0707500, 2016YFD0500301, 2017YFC0840300, 2020YFC0842100), National Mega projects of China for Major Infectious Diseases (2016ZX10004001–003), National Mega Projects of China for New Drug Creation (2018ZX09734–004), and Beijing Science and Technology Plan (Z201100005420014). The China National Biotec Group and the Beijing Institute of Biological Products provided the study product, and oversaw all trial operations. The sponsors used contract clinical research organisations to coordinate interactions with regulatory authorities and oversee clinical site operations. Data were collected by the clinical site research staff, managed by a masked contract research organisation data management team, monitored by a contract research organisation, and overseen by the sponsor and an independent data and safety monitoring board. The analysis was done by an independent statistician who was not involved in the trial after the data were collected, checked, and locked for the specific groups before unblinding.
Contributors
The study was designed by SX, YZhan, YuY, and YW. WZ, ZX, and WY collected study data and oversaw participant visits. LH and YoY provided regulatory oversight. XiY and WG provided project management. Safety data analysis and interpretation were done by MX, WeiW, XuY, YL, QW, GX, and ZL. Immunogenicity testing was done by WH, BH, HuijW, PL, and WenW. Immunogenicity data were collected and analysed by NL, LD, and YZhao, and were interpreted by GFG, HuiW, WX, GW, WT, GX, and ZL. Clinical trial data management was done by BL and HuiW. GX and ZL wrote the Article. All authors contributed to the reviewing and editing of the Article, and approved the final version. Article preparation was done by all study authors and the decision to submit the Article for publication was made by all study authors.
Declaration of interests
XiY, YZhan, YuY, HuiW, WeiW, NL, LD, YZhao, XuY, YL, and QW are employees of the Beijing Institute of Biological Products. All other authors declare no competing interests.
Supplementary Material
References
- 1.Chan JF, Yuan S, Kok KH. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan WJ, Zhao X, Ma XJ. A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019–2020. China CDC Weekly. 2020;2:61–62. [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO DRAFT landscape of COVID-19 candidate vaccines–7. July 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 9.Gao Q, Bao L, Mao H. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Tostanoski LH, Peter L. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Doremalen N, Lambe T, Spencer A. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020 doi: 10.1101/2020.05.13.093195. published online May 13. (preprint) [DOI] [PubMed] [Google Scholar]
- 12.Lurie N, Saville M, Hatchett R, Halton J. Developing COVID-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Zhang Y, Huang B. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182:713–721. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbett KS, Flynn B, Foulds KE. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020 doi: 10.1056/NEJMoa2024671. published online July 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson LA, Anderson EJ, Rouphael NG. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. published online July 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi X, Yan R, Zhang J. A neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang ZY, Werner HC, Kong WP. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc Natl Acad Sci USA. 2005;102:797–801. doi: 10.1073/pnas.0409065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Zhang L, Kuwahara K. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2016;2:361–376. doi: 10.1021/acsinfecdis.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia S, Duan K, Zhang Y. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We support data sharing of the individual participant data. The individual participant data that underlie the results reported in this Article, after deidentification (ie, text, tables, figures, and appendix 2) will be shared. Individual participant data will be available beginning 3 months and ending 1 year after publication. Supporting clinical documents, including the study protocol, statistical analysis plan, and the informed consent form, will be available immediately following publication for at least 1 year. Researchers who provide a scientifically sound proposal will be allowed access to the individual participant data. Proposals should be directed to wangyanxia99@163.com or nvsiclinicaltrials@163.com. These proposals will be reviewed and approved by the funder, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.