Abstract
Background
Unprecedented lockdown measures have been introduced in countries worldwide to mitigate the spread and consequences of COVID-19. Although attention has been focused on the effects of these measures on epidemiological indicators relating directly to the infection, there is increased recognition of their broader health implications. However, assessing these implications in real time is a challenge, due to the limitations of existing syndromic surveillance data and tools.
Objective
The aim of this study is to explore the added value of mobile phone app–based symptom assessment tools as real-time health insight providers to inform public health policy makers.
Methods
A comparative and descriptive analysis of the proportion of all self-reported symptoms entered by users during an assessment within the Ada app in Germany and the United Kingdom was conducted between two periods, namely before and after the implementation of “Phase One” COVID-19 measures. Additional analyses were performed to explore the association between symptom trends and seasonality, and symptom trends and weather. Differences in the proportion of unique symptoms between the periods were analyzed using a Pearson chi-square test and reported as log2 fold changes.
Results
Overall, 48,300-54,900 symptomatic users reported 140,500-170,400 symptoms during the Baseline and Measures periods in Germany. Overall, 34,200-37,400 symptomatic users in the United Kingdom reported 112,100-131,900 symptoms during the Baseline and Measures periods. The majority of symptomatic users were female (Germany: 68,600/103,200, 66.52%; United Kingdom: 51,200/71,600, 72.74%). The majority were aged 10-29 years (Germany: 68,500/100,000, 68.45%; United Kingdom: 50,900/68,800, 73.91%), and about one-quarter were aged 30-59 years (Germany: 26,200/100,000, 26.15%; United Kingdom: 14,900/68,800, 21.65%). Overall, 103 symptoms were reported either more or less frequently (with statistically significant differences) during the Measures period as compared to the Baseline period, and 34 of these were reported in both countries. The following mental health symptoms (log2 fold change, P value) were reported less often during the Measures period: inability to manage constant stress and demands at work (–1.07, P<.001), memory difficulty (–0.56, P<.001), depressed mood (–0.42, P<.001), and impaired concentration (–0.46, P<.001). Diminished sense of taste (2.26, P<.001) and hyposmia (2.20, P<.001) were reported more frequently during the Measures period. None of the 34 symptoms were found to be different between the same dates in 2019. In total, 14 of the 34 symptoms had statistically significant associations with weather variables.
Conclusions
Symptom assessment apps have an important role to play in facilitating improved understanding of the implications of public health policies such as COVID-19 lockdown measures. Not only do they provide the means to complement and cross-validate hypotheses based on data collected through more traditional channels, they can also generate novel insights through a real-time syndromic surveillance system.
Keywords: epidemiology; participatory epidemiology; participatory surveillance; COVID-19 symptom assessment apps; symptom checker apps; syndromic surveillance; COVID-19 measures; COVID-19 lockdown; digital public health; health effects of COVID-19 measures, infoveillance
Introduction
Background
Since its emergence at the end of 2019, COVID-19 has had an enormous and wide-ranging impact, with millions of confirmed cases and hundreds of thousands of deaths reported worldwide [1]. Governments have introduced a series of measures ranging from work and school closures to social distancing and lockdowns to mitigate the spread and consequences of infection. The measures have been unprecedented on many levels: in how disruptive they are to daily life, by the proportions of populations affected, in the duration of implementation, and by their global reach. As a result, the daily lives of millions have been upended.
The extent of the countermeasures to the pandemic raises questions as to their impact on public and individual health. Although much of the initial focus in the medical and scientific community has been on understanding the virus and infection itself [2], as well as the effects of various policy measures on epidemiological indicators relating directly to COVID-19 [3,4], there has also been increased recognition of the broader health effects [5].
Some of the broader implications concern the direct and immediate consequences of lockdown. For instance, as people are distressed due to social isolation and the economic fallout of the crisis, an upsurge in the incidence and severity of mental health problems has been predicted [5-11]. Increased handwashing (a primary recommendation to reduce transmission of COVID-19) is expected to result in increased skin irritation and dermatitis [12,13]. Other implications are related to more indirect factors such as delays and cancellations of surgeries and nonurgent treatments for patients with cancer and other diseases [14-16], interruptions in drug and commodities supply chains [17,18], and drops in vaccination rates for vaccine-preventable diseases [19,20]. The consequences of these interruptions to medical services during this pandemic will likely create a higher morbidity, but the impact may not be visible for several years to come [17].
Assessing the health implications of lockdown policies in real-time is a challenge. Apart from the time lag, evidence cited in support of purported health effects is often anecdotal or based on surveys conducted among medical professionals. The latter provide important insights into changes in health symptoms identified at the point of contact, but may not account for the effects of social distancing guidelines on health-seeking behavior. Traditional syndromic surveillance data [21] provides valuable information for a defined set of indicators over time, but is unable to distinguish whether the trends reflect changes in disease incidence or in the uptake of health services. More significant efforts toward data collection among the general public are currently being undertaken [22]. However, these are missing baseline data from before the emergence of COVID-19 that would facilitate a clearer understanding of the impact. In general, surveys can provide snapshots of a highly dynamic situation, but tend to be restricted in scope, meaning that changes in health beyond predefined indicators risk being overlooked.
In the past years, a growing number of studies have shown the benefits of mobile phone app–based symptom assessment tools for improved health outcomes (eg, in lowering the barrier to seek help) [23-29]. If the literature on symptom assessment tools has primarily focused on individual health benefits (eg, early detection of rare diseases [30]), recent research has also demonstrated their potential contribution to public health. Specifically with regard to COVID-19, symptom tracker apps have helped flag anosmia (loss of the sense of smell) as a potentially relevant symptom of infection [31,32], and have challenged common understandings of presenting symptoms of COVID-19 [33].
Goal of This Study
In the present study, we add to this body of literature by exploring the added value of symptom assessment tools for the analysis of broader health implications resulting from public health interventions such as lockdown measures. We were particularly interested in symptoms not related to COVID-19 that could be potentially overlooked as an unintended consequence of the measures. A tool like Ada can provide real-time insights for policy makers, informing their decisions as they aim for the right balance of such measures. This study is based on self-reported symptom data from Ada, a digital symptom assessment app that uses a probabilistic reasoning engine that collects demographic information, symptoms, and medical history to suggest possible conditions and then guides individuals to the most appropriate care. The Ada app is available in seven languages and has over ten million users. It is described in Gilbert et al [34] in further detail. Using an inductive approach and focusing on the immediate impact of COVID-19 control measures, we compared symptom data reported by users in Germany and the United Kingdom before and during the first phase of COVID-19 lockdown measures to identify changes and continuities in the incidence of self-reported symptoms.
The aim of this study was to explore the potential of the Ada symptom assessment app to generate real-time health insights to inform public health policy makers.
Methods
Study Focus
This analysis is a comparative descriptive study of self-reported symptoms entered by users in an Ada assessment completed during the “Phase One” COVID-19 interventions (the Measures period) compared to a Baseline period in Germany and the United Kingdom. The Measures periods started when all five major nonpharmaceutical interventions described in Flaxman et al [4] were implemented in the respective countries. Specifically, these interventions are school closures, case-based measures (strong recommendation of self-isolation when showing COVID-19–like symptoms), banning of public events, encouragement of social distancing, and lockdowns. The Measures period lasted until April 22, 2020, when data was extracted. The end of the Baseline period was defined as the day before any of the five interventions were implemented in the respective countries, and the length of the Baseline period was equal to the Measures period in the same country. The period between the Baseline and Measures periods is excluded from the analysis due to the partial implementation of “Phase One” COVID-19 measures. This is an exploratory analysis of all symptoms (not only COVID-19–related) reported by symptomatic users during the defined periods. In this study, we consider a symptomatic user as one who completed at least one assessment during the periods of analysis, whether the assessment was self-reported or reported by someone on their behalf (ie, a legal guardian if under 16 years of age). We considered symptom patterns to be trends if in both countries, the proportion that a specific symptom was reported (out of all reported symptoms) was significantly different between the two periods.
Additional Analyses
Ad hoc analyses were later performed to test identified trends for symptoms against selected potential confounders, such as seasonality and weather. To explore the potential impact of seasonality on trends, the results were compared to the results of the same analysis conducted for the same dates in 2019. To explore the impact of weather on trends, associations between the monthly proportion of reported symptoms and weather variables (average monthly temperature [°C], monthly precipitation [mm], and monthly hours of sunshine) were investigated during the period from January 2019 to March 2020. The weather analysis was restricted to Germany.
Participants
All assessments completed by Ada users in Germany and the United Kingdom during the Baseline and Measures periods were included in the analysis. We analyzed pseudonymized health data for public health purposes, according to the European General Data Protection Regulation (GDPR), and users are duly informed of such use of their data (information available at any time in Ada’s Privacy Policy). Additionally, users maintain their right to object to such processing for reasons arising from their particular situation, as required by the GDPR.
Variables
An Ada assessment consists of several different parts: (1) the user enters an unlimited number of symptoms, (2) the user is asked about other potential symptoms they could have, and (3) an assessment result is provided, with conditions that could potentially explain the reported symptoms and adequate triage. This analysis only includes symptoms that are self-reported by a user in the first part of the assessment: that is, responses to the initial question “Let’s start with the symptom that’s troubling you the most,” followed by “Do you have any other symptoms?” Upon entering free text, the user is then given a range of medically-curated options (based on linguistic relevance) to select from.
The variable of interest, Si,k, representing the proportion a symptom i is self-reported during the period k is defined as
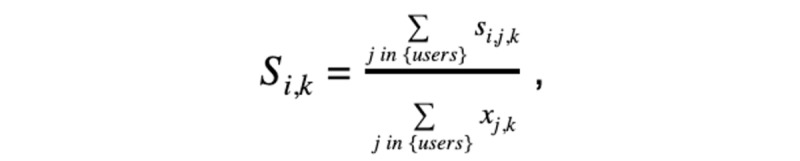
Where si,j,k equals 1 if the user j self-reported the symptom i during the period k, and equals 0 otherwise; and xj,k equals 1 if the user j has completed one assessment during the period k, and equals 0 otherwise.
The age variable was grouped using the following categories (years): 0-9, 10-19, 20-29, 30-39, 40-59 and ≥60.
Weather data (average monthly temperature [°C], monthly precipitation [mm], and monthly hours of sunshine) for Germany was extracted from the Deutscher Wetter Dienst database for the period from January 2019 to March 2020 [35].
For ease of reporting and to aid interpretation, symptoms that were reported in significantly different proportions during the Baseline and Measures periods were grouped using the International Classification of Diseases, Version 2019 (ICD-10) of the World Health Organization [36]. ICD-10 R subgroups, named “Symptoms, signs and abnormal clinical and laboratory symptoms, not elsewhere classified” were used when possible. Similar categories were grouped together later, as presented in Table 1.
Table 1.
List of groups based on the International Classification of Diseases, Version 2019 classification.
| Group name | International Classification of Diseases, Version 2019 classification |
| Circulatory and respiratory systems | R00-R09, J |
| Digestive system and abdomen | R10-R19 |
| Skin and subcutaneous tissue | R20-R23, L |
| Musculoskeletal | M |
| Genitourinary system | R30-R39, N |
| Cognition, perception, emotional state, and behavior | R40-R46, F |
| Speech and voice | R47-R49 |
| General symptoms and signs | R50-R69 |
| Nervous system | G |
| Eye and adnexa | H |
Bias
As Ada’s medical model and databases are continuously updated, we defined the Baseline period to be as close as possible to the Measures period to limit the impact of these changes on the data. All modeled symptoms that were added, deleted, significantly modified, or significantly affected by the modification of any other symptom from the first day of the Baseline period until the end of the Measures period were removed from this study.
Statistical Methods
Sex and age groups of symptomatic users were reported as percentages and tested for differences between the periods with a Pearson chi-square test. Differences in the proportion of symptoms between the periods were reported as log2 fold changes and were analyzed with a Pearson chi-square test. A log2 fold change of 0.5 means that the proportion of that reported symptom was 1.41 times as large during the Measures period compared to the Baseline. A log2 fold change of 1 is interpreted as being twice as large during the Measures period compared to the Baseline, and a log2 fold change of 2 is four times as large. Conversely, a log2 fold change of –1 means that the proportion of the reported symptom was twice as large during the Baseline period compared to the Measures period. In general, log2 fold change calculations are helpful in understanding relative differences in the proportions of users reporting each symptom between the two periods, but do not reflect how common reporting of that symptom was overall. Associations between weather variables and the proportion of symptoms were tested based on the Spearman Rank correlation coefficient.
When required, P values were adjusted for multiple testing using the false discovery rate method. P values ≤.05 were considered statistically significant. Statistical analyses and figures were executed using R (Version 3.6.1; R Foundation for Statistical Computing).
The analysis was done using exact numbers, but results representing user numbers are presented rounded to the closest hundred, to ensure a fully anonymized presentation of the results.
Results
Principal Results
An overview of the Baseline and Measures periods in Germany and the United Kingdom (numbers of Ada users, numbers of symptoms reported) are shown in Table 2.
Table 2.
Key parameters.
| Key parameters | Germany | United Kingdom | |||
|
|
Baseline | Measures | Baseline | Measures | |
| Dates of period (MM/DD/YY) | 02/03/20-03/05/20 | 03/22/20-04/22/20 | 02/11/20-03/11/20 | 03/24/20-04/22/20 | |
| Number of days per period | 32 | 32 | 30 | 30 | |
| Total number of usersa | 467,000 | 483,100 | 488,800 | 501,300 | |
| Number of symptomatic usersa | 54,900 | 48,300 | 37,400 | 34,200 | |
| Number of reported symptoms by symptomatic usersa | 170,400 | 140,500 | 131,900 | 112,100 | |
aNumbers were rounded to the nearest hundred.
Demographic characteristics of users are shown in Table 3. During both the Baseline and Measures periods, the majority of symptomatic users in both countries were female (Germany Baseline: 36,300/54,900, 66.19%; Germany Measures: 32,300/48,300, 66.90%; United Kingdom Baseline: 26,600/37,400, 71.17%; United Kingdom Measures: 24,600/34,200, 71.94%). The majority were aged 10-29 years (Germany Baseline: 37,000/53,200, 69.51%; Germany Measures: 31,400/46,800, 67.13%; United Kingdom Baseline: 27,200/35,800, 75.76%; United Kingdom Measures: 23,700/32,900, 71.89%). Those aged 30-59 years represented roughly one-quarter of symptomatic users (Germany Baseline: 13,300/53,200, 24.94%; Germany Measures: 12,900/46,800, 27.53%; United Kingdom Baseline: 7,100/35,800, 19.92%; United Kingdom Measures: 7,700/32,900, 23.54%). The number of symptomatic users in the Baseline period (Germany: 54,900; United Kingdom: 37,400) was slightly higher than in the Measures period (Germany: 48,300; United Kingdom: 34,200).
Table 3.
Demographic characteristics of the study population.
| Demographic characteristics |
Germany | United Kingdom | |||||
|
|
Baseline, na (%) | Measures, na (%) | P valueb | Baseline, na (%) | Measures, na (%) | P valueb | |
| Sex | |||||||
|
|
Female | 36,300 (66.2) | 32,300 (66.9) | .02 | 26,600 (72.2) | 24,600 (72.9) | .02 |
| Agec (years) | |||||||
|
|
0-9 | 1300 (2.4) | 700 (1.6) | <.001 | 900 (2.4) | 700 (2.0) | <.001 |
|
|
10-19 | 17,100 (32.1) | 13,700 (29.2) | <.001 | 16,200 (45.1) | 13,600 (41.2) | <.001 |
|
|
20-29 | 20,000 (37.5) | 17,800 (37.9) | .28 | 11,000 (30.7) | 10,100 (30.7) | .95 |
|
|
30-39 | 6400 (12.0) | 6000 (12.9) | <.001 | 3100 (8.7) | 3300 (9.9) | <.001 |
|
|
40-59 | 6900 (13.0) | 6800 (14.6) | <.001 | 4000 (11.2) | 4500 (13.6) | <.001 |
|
|
≥60 | 1700 (3.2) | 1800 (3.9) | <.001 | 700 (2.1) | 900 (2.7) | <.001 |
aNumbers were rounded to the nearest hundred.
bValues were obtained from Pearson chi-square tests for differences between Baseline and Measures data.
cUsers who did not report a birth year were excluded from the analysis.
In total, 21 symptoms were excluded from the analysis as they had been added to or deleted from the medical model during either the Baseline or Measures period. In addition, three other symptoms were excluded from the analysis as there were significant changes in the associated terms (text entered by users to match the description of a symptom to the term used in the model), which could affect the number of times a symptom is reported. A list of these symptoms is included in Table 1 of Multimedia Appendix 1.
Main Analyses
Out of 1328 symptoms investigated in Germany and 1294 symptoms investigated in the United Kingdom, 103 symptoms were reported either more or less frequently, in either country, during the Measures period as compared to the Baseline period. The complete results can be found in Tables 2 and 3 of Multimedia Appendix 1.
Figure 1 presents the 34 symptoms that showed a statistically significant difference in both countries. Overall, 24 symptoms were reported less often and 10 were reported more often during the Measures period than during the Baseline period.
Figure 1.
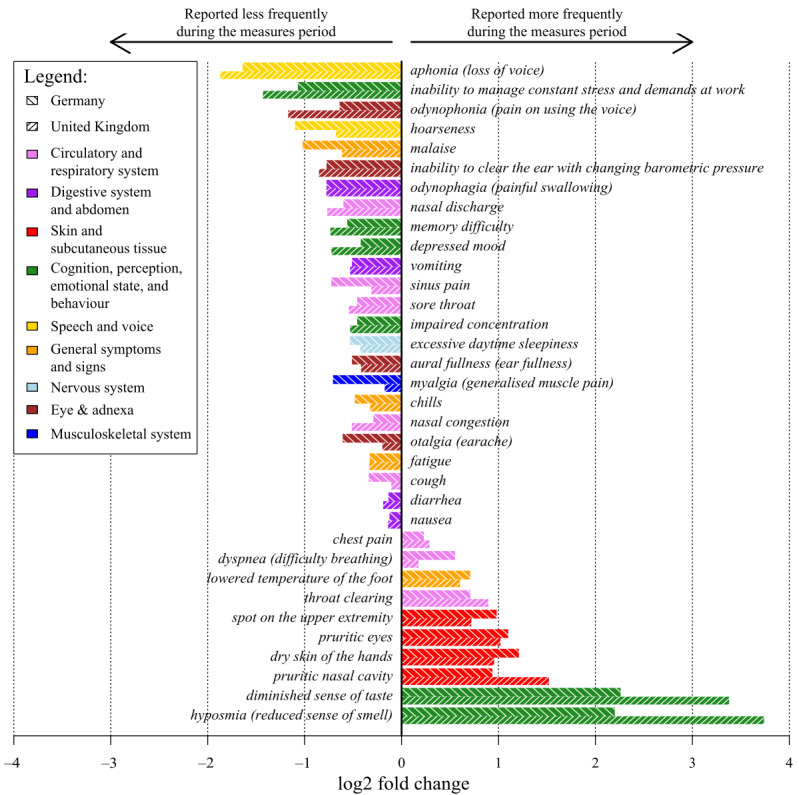
Relative difference (log2 fold change values) in the proportions of Ada users' reported symptoms with statistically significant differences between the baseline and COVID-19 measures periods in Germany and the United Kingdom.
Out of the 34 significant symptoms, all skin- and tissue-related symptoms (pruritic nasal cavity, spot on the upper extremity, dry skin of the hands, and pruritic eyes) were reported more frequently during the Measures period. In contrast, all speech and voice symptoms (aphonia and hoarseness), all eye and adnexa symptoms (odynophonia, inability to clear the ear with changing barometric pressure, aural fullness, and otalgia), all digestive system and abdomen symptoms (odynophagia, diarrhea, and nausea), and all musculoskeletal and nervous systems symptoms (excessive daytime sleepiness and myalgia) were reported less frequently during the Measures period.
In the cognition, perception, emotional state, and behavior symptoms group, two perception symptoms (diminished sense of taste and hyposmia) were reported more frequently during the Measures period, whereas the mental health symptoms (inability to manage constant stress and demands at work, memory difficulty, depressed mood, and impaired concentration) were reported less frequently during the Measures period. Out of the circulatory and respiratory symptoms, three were reported more frequently during the Measures period (throat clearing, dyspnea, and chest pain) and four were reported less frequently (nasal discharge, sinus pain, sore throat, and cough). One general symptom was reported more frequently (lowered temperature of the foot) and three (malaise, chills, and fatigue) were reported less frequently during the Measures period.
Additional Analyses
Out of the 34 symptoms found to be different between the Baseline and Measures periods in both Germany and the United Kingdom in 2020, none were found to be different between the same periods in 2019 in both countries. However, looking at the countries separately, in Germany, the data shows that 8 of the 34 significant symptoms were also reported less frequently during the Measures period in 2019: cough, chills, nasal discharge, myalgia, sore throat, malaise, fatigue, and sinus pain. The complete results can be found in Tables 4 and 5 of Multimedia Appendix 1.
The monthly weather report in February 2020 (which corresponds to most of the Baseline period) differed from that of April 2020 (which corresponds to most of the Measures period) in Germany. The average temperature increased by 5.1 °C (from 5.3 °C to 10.4 °C), monthly precipitation decreased by 107.8 mm (from 124.1 mm to 16.3 mm), and the number of hours of sunshine per month increased by 228.5 hours (from 63.9 to 292.4 hours). In total, 14 of the 34 significant symptoms had statistically significant associations with weather. Increased temperature was positively associated with reporting spot on the upper extremity and negatively associated with chest pain, lowered temperature of the foot, odynophagia, malaise, myalgia, cough, otalgia, chills, vomiting, sinus pain, and nasal congestion. Increased hours of sunshine were positively associated with pruritic eyes, spot on the upper extremity, and pruritic nasal cavity, and negatively associated with lowered temperature of the foot and sinus pain. The complete results can be found in Table 6 and Figure 1 of Multimedia Appendix 1.
Discussion
Principal Findings
The results presented above show significant differences in the frequency and proportion of self-reported symptoms in Ada assessments before and after the implementation of measures aimed at reducing the transmission of COVID-19. Importantly, the same differences were found in both Germany and the United Kingdom, despite the divergent trajectories of these countries in the lead-up to the implementation of lockdown policies [37], as well as other national differences such as those relating to health systems [38]. Furthermore, these differences were not found during the same time periods in 2019, suggesting that the lockdown measures could have contributed to the results.
Many of the observed differences were to be expected. The reduced frequency of reported respiratory symptoms (nasal discharge, sore throat, cough, sinus pain, nasal congestion, hoarseness, odynophonia, aphonia) and influenza-like illness (ie, malaise, fatigue, chills, myalgia) following the measures is understandable as the cold and flu season has also waned exceptionally rapidly during this period [39], likely facilitated by reduced contact resulting from lockdown. To support this interpretation, cough, chills, nasal discharge, myalgia, sore throat, malaise, fatigue, and sinus pain were also reported less frequently during the Measures period in 2019 in Germany, suggesting that seasonal changes are reflected in the data. The increased reporting of dry hands following the measures is consistent with more frequent handwashing during the Measures period, as expected by dermatologists [12,13]. Increased reports of pruritic eyes and pruritic nasal cavity following the measures could be a consequence of seasonal hay fever (known to be worse in spring than during winter months due to increased pollen in the air [40]), as these symptoms were found to be associated with increased sunshine (presumably when people spend more time outdoors).
The reduction of gastrointestinal symptoms could be associated with the closing of preschool/day care settings (as they are known to contribute to the spread of these diseases [41]), restaurant closures, or improved hand hygiene. The decrease in ear problems could be a result of the sharp reduction in air travel, resulting in fewer people experiencing pressure adjustment problems called “airplane ear” [42], or related to the end of the cold and flu season. More research is needed to explore these hypotheses.
We also observed an increase in the reporting of hyposmia, diminished sense of taste, and dyspnea during the Measures period. These are less frequent but also typical COVID-19 symptoms and were increasingly recognized in the general public [31,43,44]. They were not found to be associated with seasonality or weather. This increase could be related to COVID-19 infections, or an artefact resulting from increased awareness of these symptoms due to media coverage.
A more surprising result is that depressed mood, inability to manage constant stress and demands at work, impaired concentration, memory difficulty, and excessive daytime sleepiness were reported in a lower proportion during the Measures period. This is not only contrary to conventional wisdom, but also runs counter to what was observed during previous infectious disease outbreaks (such as severe acute respiratory syndrome [SARS], Middle East respiratory syndrome [MERS], and Ebola [45,46]), as well as to the literature reporting on the effects of COVID-19 on mental health [6-11,47,48]. Despite the fact that the temperature was warmer during the Measures period and there was more sunshine than usual, the analysis on weather data for Germany did not show a significant impact on the changed mental health symptoms. Our findings are supported by a growing body of evidence from ongoing studies [49] and recently published research [50], as well as anecdotal evidence reported in various mainstream media reports [51-53] that suggest that, at least in the short term, the mental health effects of the COVID-19 measures may not be as negative as expected. One factor may be that the stress of everyday life and work/study is reduced during lockdown and when working or studying from home. In addition, the reduction of excessive daytime sleepiness may be due to the fact that people may sleep better or more during lockdown as there are fewer opportunities to go out and socialize in the evenings. Ongoing studies into the mental health effects of COVID-19 and its countermeasures may shed further light on these questions.
Limitations
It is important to interpret the study results taking into consideration the characteristics of the study population and the normal use case for Ada. Due to the specific age and sex distribution of users (predominantly young and female), the results are not generalizable to other population groups, especially the elderly. Furthermore, this analysis was limited to Germany and the United Kingdom (due to sufficient user numbers), and represents a two-month snapshot of reported symptoms. Users who know they have a disease might not use Ada if their symptoms deteriorate, as the cause is already known. In addition, this is an analysis of patient-reported symptoms, which are not validated. The impact of user acquisition strategies is not known. However, the similarity in trends observed across two countries (and for respiratory symptoms, over the same period of time in 2019) adds weight to our findings.
Despite these limitations, the analysis presented in this paper has a number of unique strengths. First, the analysis was conducted using a large, existing data set that updates in real time and covers over 1400 unique self-reported symptoms since November 2016, allowing the monitoring of changes in trends over time. Second, the data is user-driven as a user self-reports their symptoms during an assessment on their own initiative. This allows for identification of changes that would not be detected in traditional studies focusing on specific and/or predefined areas. Third, the large number of symptoms presented in the results that are consistent with expectations, the observation of seasonal differences, and the observation that the results were similar in both Germany and the United Kingdom indicate that the Ada data is reliable. Fourth, the app covers a large range of symptoms that are not captured by traditional surveillance systems. For example, during the COVID-19 pandemic, we were able to compare the occurrence of hyposmia and diminished sense of taste during the outbreak with data from previous years, as those symptoms were already covered by the app before the pandemic brought them into focus. Although the clinical soundness of Ada’s model at the level of individual diagnostics has already been demonstrated in other studies [54,55], the presented results build confidence that the data collected through the Ada app can also detect health changes in a population in real time.
Future Research
Future research can build on these strengths, focusing on the reasons for some of the detected changes and expanding the analysis to more countries. Of particular interest are countries from the Global South and low- and middle-income countries, given the comparative paucity of up-to-date health data in these countries and the differentials in the burden of disease. In addition, investigating changes in trends over time as the implementation of the COVID-19 measures changes according to the reality in different countries (ie, as individuals return to work) will offer meaningful insights into the effects of policy changes.
Conclusions
Our findings suggest that symptom assessment tools might have a role to play in improving understanding of the implications of public health measures. In this analysis, we have shown an innovative use of an existing data set that would enable policy makers to inform and monitor public health measures with a real-time, low-resource syndromic surveillance system that is relevant both during the COVID-19 pandemic and in the future.
Acknowledgments
Stephen Gilbert (Director of Clinical Evaluation), Vincent Jörres (Communications & Public Affairs Lead), Vanessa Lemarié (Director of Business Development & Program Owner of Rare Disease Initiative in Business Development), Nicola Vona (Director of Reasoning Technology in Product), and Paul Wicks (Scientific Advisor) reviewed the manuscript and provided feedback. This study was funded by Ada Health GmbH.
Abbreviations
- GDPR
General Data Protection Regulation
- ICD-10
International Classification of Diseases, Version 2019
Appendix
Supplementary material.
Footnotes
Authors' Contributions: AM and FB contributed to the planning of this study (study conception, protocol development), which was revised by CC and AG. AM and FB contributed to the data collection and analysis. AM, FB, CC, and AG contributed to the reporting (report writing). All authors contributed comments on drafts of the report. AM is the guarantor for this work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Conflicts of Interest: AM, FB, CC, and AG are employees or company directors of Ada Health GmbH.
References
- 1.COVID-19 Map. Johns Hopkins Coronavirus Resource Center. [2020-05-11]. https://coronavirus.jhu.edu/map.html.
- 2.EPPI-Mapper. [2020-05-13]. http://eppi.ioe.ac.uk/COVID19_MAP/covid_map_v10.html.
- 3.Prem K, Liu Y, Russell TW, Kucharski AJ, Eggo RM, Davies N, Jit M, Klepac P, Flasche S, Clifford S, Pearson CAB, Munday JD, Abbott S, Gibbs H, Rosello A, Quilty BJ, Jombart T, Sun F, Diamond C, Gimma A, van Zandvoort K, Funk S, Jarvis CI, Edmunds WJ, Bosse NI, Hellewell J. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. The Lancet Public Health. 2020 May;5(5):e261–e270. doi: 10.1016/s2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaxman S, Mishra S, Gandy A, Unwin HJT, Coupland H, Mellan TA, Zhu H. Estimating the number of infections and the impact of nonpharmaceutical interventions on COVID-19 in 11 European countries. 2020. Mar 30, [2020-10-02]. https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-03-30-COVID19-Report-13.pdf.
- 5.Douglas M, Katikireddi SV, Taulbut M, McKee M, McCartney G. Mitigating the wider health effects of covid-19 pandemic response. BMJ. 2020 Apr 27;369:m1557. doi: 10.1136/bmj.m1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United Nations Policy Brief: COVID-19 and the Need for Action on Mental Health Internet. 2020. May, [2020-10-02]. https://www.un.org/sites/un2.un.org/files/un_policy_brief-covid_and_mental_health_final.pdf.
- 7.Torales J, O'Higgins M, Castaldelli-Maia JM, Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry. 2020 Jun 31;66(4):317–320. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 8.Shigemura J, Ursano R, Morganstein J, Kurosawa M, Benedek D. Public responses to the novel 2019 coronavirus (2019-nCoV) in Japan: Mental health consequences and target populations. Psychiatry Clin Neurosci. 2020 Apr;74(4):281–282. doi: 10.1111/pcn.12988. http://europepmc.org/abstract/MED/32034840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moccia L, Janiri D, Pepe M, Dattoli L, Molinaro M, De Martin V, Chieffo D, Janiri L, Fiorillo A, Sani G, Di Nicola M. Affective temperament, attachment style, and the psychological impact of the COVID-19 outbreak: an early report on the Italian general population. Brain Behav Immun. 2020 Jul;87:75–79. doi: 10.1016/j.bbi.2020.04.048. http://europepmc.org/abstract/MED/32325098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, Ho RC. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Public Health. 2020 Mar 06;17(5):1729. doi: 10.3390/ijerph17051729. https://www.mdpi.com/resolver?pii=ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galea S, Merchant RM, Lurie N. The Mental Health Consequences of COVID-19 and Physical Distancing: The Need for Prevention and Early Intervention. JAMA Intern Med. 2020 Jun 01;180(6):817–818. doi: 10.1001/jamainternmed.2020.1562. [DOI] [PubMed] [Google Scholar]
- 12.Beiu C, Mihai M, Popa L, Cima L, Popescu M. Frequent Hand Washing for COVID-19 Prevention Can Cause Hand Dermatitis: Management Tips. Cureus. 2020 Apr 02;12(4):e7506. doi: 10.7759/cureus.7506. http://europepmc.org/abstract/MED/32373409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacGibeny MA, Wassef C. Preventing adverse cutaneous reactions from amplified hygiene practices during the COVID-19 pandemic: how dermatologists can help through anticipatory guidance. Arch Dermatol Res. 2020 May 09; doi: 10.1007/s00403-020-02086-x. http://europepmc.org/abstract/MED/32388643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Lancet Oncology COVID-19: global consequences for oncology. Lancet Oncol. 2020 Apr;21(4):467. doi: 10.1016/S1470-2045(20)30175-3. http://europepmc.org/abstract/MED/32240603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan KK, Lau J. Cessation of cancer screening: An unseen cost of the COVID-19 pandemic? Eur J Surg Oncol. 2020 May 11; doi: 10.1016/j.ejso.2020.05.004. http://europepmc.org/abstract/MED/32409112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laupacis A. Working together to contain and manage COVID-19. CMAJ. 2020 Mar 30;192(13):E340–E341. doi: 10.1503/cmaj.200428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan AB, Jewell B, Sherrard-Smith E, Vesga JF, Watson OJ, Whittaker C, Hamlet A, Smith JA, Winskill P, Verity R, Baguelin M, Lees JA, Whittles LK, Ainslie KEC, Bhatt S, Boonyasiri A, Brazeau NF, Cattarino L, Cooper LV, Coupland H, Cuomo-Dannenburg G, Dighe A, Djaafara BA, Donnelly CA, Eaton JW, van Elsland S, FitzJohn RG, Fu H, Gaythorpe KAM, Green W, Haw DJ, Hayes S, Hinsley W, Imai N, Laydon DJ, Mangal TD, Mellan TA, Mishra S, Nedjati-Gilani G, Parag KV, Thompson HA, Unwin HJT, Vollmer MAC, Walters CE, Wang H, Wang Y, Xi X, Ferguson NM, Okell LC, Churcher TS, Arinaminpathy N, Ghani AC, Walker PGT, Hallett TB. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. The Lancet Global Health. 2020 Sep;8(9):e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riley T, Sully E, Ahmed Z, Biddlecom A. Estimates of the Potential Impact of the COVID-19 Pandemic on Sexual and Reproductive Health In Low- and Middle-Income Countries. Int Perspect Sex Reprod Health. 2020 Apr 16;46:73–76. doi: 10.1363/46e9020. [DOI] [PubMed] [Google Scholar]
- 19.Measles Rubella Initiat. More than 117 million children at risk of missing out on measles vaccines, as COVID-19 surges. [2020-05-11]. https://measlesrubellainitiative.org/measles-news/more-than-117-million-children-at-risk-of-missing-out-on-measles-vaccines-as-covid-19-surges/
- 20.Hoffman J. Vaccine Rates Drop Dangerously as Parents Avoid Doctor's Visits. The New York Times. 2020. Apr 23, [2020-05-11]. https://www.nytimes.com/2020/04/23/health/coronavirus-measles-vaccines.html.
- 21.Emergency Department Syndromic Surveillance System: England. Public Health England Real-time Syndromic Surveillance Team. 2020. Apr, [2020-10-02]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/882234/EDSSSBulletin2020wk17.pdf.
- 22.Longitudinal studies | COVID-MINDS. [2020-05-11]. https://www.covidminds.org/longitudinalstudies.
- 23.Laranjo L, Dunn A, Tong H, Kocaballi A, Chen J, Bashir R, Surian D, Gallego B, Magrabi F, Lau A, Coiera E. Conversational agents in healthcare: a systematic review. J Am Med Inform Assoc. 2018 Sep 01;25(9):1248–1258. doi: 10.1093/jamia/ocy072. http://europepmc.org/abstract/MED/30010941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas GM, Gratch J, King A, Morency L. It’s only a computer: Virtual humans increase willingness to disclose. Computers in Human Behavior. 2014 Aug;37:94–100. doi: 10.1016/j.chb.2014.04.043. [DOI] [Google Scholar]
- 25.Miner AS, Milstein A, Schueller S, Hegde R, Mangurian C, Linos E. Smartphone-Based Conversational Agents and Responses to Questions About Mental Health, Interpersonal Violence, and Physical Health. JAMA Intern Med. 2016 May 01;176(5):619–25. doi: 10.1001/jamainternmed.2016.0400. http://europepmc.org/abstract/MED/26974260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinhubl SR, Topol EJ. Now we're talking: bringing a voice to digital medicine. The Lancet. 2018 Aug;392(10148):627. doi: 10.1016/s0140-6736(18)31803-8. [DOI] [Google Scholar]
- 27.Nobles AL, Leas EC, Caputi TL, Zhu S, Strathdee SA, Ayers JW. Responses to addiction help-seeking from Alexa, Siri, Google Assistant, Cortana, and Bixby intelligent virtual assistants. NPJ Digit Med. 2020 Jan 29;3(1):11. doi: 10.1038/s41746-019-0215-9. doi: 10.1038/s41746-019-0215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kocaballi AB, Quiroz JC, Rezazadegan D, Berkovsky S, Magrabi F, Coiera E, Laranjo L. Responses of Conversational Agents to Health and Lifestyle Prompts: Investigation of Appropriateness and Presentation Structures. J Med Internet Res. 2020 Feb 09;22(2):e15823. doi: 10.2196/15823. https://www.jmir.org/2020/2/e15823/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bickmore TW, Trinh H, Olafsson S, O'Leary TK, Asadi R, Rickles NM, Cruz R. Patient and Consumer Safety Risks When Using Conversational Assistants for Medical Information: An Observational Study of Siri, Alexa, and Google Assistant. J Med Internet Res. 2018 Sep 04;20(9):e11510. doi: 10.2196/11510. https://www.jmir.org/2018/9/e11510/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronicke S, Hirsch MC, Türk E, Larionov K, Tientcheu D, Wagner AD. Can a decision support system accelerate rare disease diagnosis? Evaluating the potential impact of Ada DX in a retrospective study. Orphanet J Rare Dis. 2019 Mar 21;14(1):69. doi: 10.1186/s13023-019-1040-6. https://ojrd.biomedcentral.com/articles/10.1186/s13023-019-1040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, Ganesh S, Varsavsky T, Cardoso MJ, El-Sayed Moustafa JS, Visconti A, Hysi P, Bowyer RCE, Mangino M, Falchi M, Wolf J, Ourselin S, Chan AT, Steves CJ, Spector TD. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020 Jul 11;26(7):1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denis F, Galmiche S, Dinh A, Fontanet A, Scherpereel A, Benezit F, Lescure F. Epidemiological Observations on the Association Between Anosmia and COVID-19 Infection: Analysis of Data From a Self-Assessment Web Application. J Med Internet Res. 2020 Jun 11;22(6):e19855. doi: 10.2196/19855. https://www.jmir.org/2020/6/e19855/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drew DA, Nguyen LH, Steves CJ, Menni C, Freydin M, Varsavsky T, Sudre CH, Cardoso MJ, Ourselin S, Wolf J, Spector TD, Chan AT, COPE Consortium Rapid implementation of mobile technology for real-time epidemiology of COVID-19. Science. 2020 Jun 19;368(6497):1362–1367. doi: 10.1126/science.abc0473. http://europepmc.org/abstract/MED/32371477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert S, Mehl A, Baluch A, Cawley C, Challiner J, Fraser H, Millen E, Multmeier J, Pick F, Richter C, Tuerk E, Upadhyay S, Virani V, Vona N, Wicks P, Novorol C. Original research: How accurate are digital symptom assessment apps for suggesting conditions and urgency advice?: a clinical vignettes comparison to GPs. MedRxiv. 2020 May 13;:1. doi: 10.1101/2020.05.07.20093872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wetter und Klima - Deutscher Wetterdienst - Klimaüberwachung - Deutschland - Zeitreihen und Trends. [2020-05-12]. https://www.dwd.de/DE/leistungen/zeitreihen/zeitreihen.html?nn=480164#buehneTop.
- 36.ICD-10 Version 2019. [2020-05-12]. https://icd.who.int/browse10/2019/en.
- 37.Gibney E. Whose coronavirus strategy worked best? Scientists hunt most effective policies. Nature. 2020 May;581(7806):15–16. doi: 10.1038/d41586-020-01248-1. https://www.nature.com/articles/d41586-020-01248-1. [DOI] [PubMed] [Google Scholar]
- 38.Papanicolas I, Mossialos E, Gundersen A, Woskie L, Jha AK. Performance of UK National Health Service compared with other high income countries: observational study. BMJ. 2019 Nov 27;367:l6326. doi: 10.1136/bmj.l6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert Koch Institut Epidemiologisches Bulletin 16/2020. [2020-10-05]. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2020/Ausgaben/16_20.html.
- 40.Institute for Quality and Efficiency in Health Care (IQWiG) Allergies: Overview. 2020. Apr 23, [2020-10-05]. https://www.ncbi.nlm.nih.gov/books/NBK447112/
- 41.Hebbelstrup Jensen B, Jokelainen P, Nielsen ACY, Franck KT, Rejkjær Holm D, Schønning K, Petersen AM, Krogfelt KA. Children Attending Day Care Centers are a Year-round Reservoir of Gastrointestinal Viruses. Sci Rep. 2019 Mar 01;9(1):3286. doi: 10.1038/s41598-019-40077-9. doi: 10.1038/s41598-019-40077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharya S, Singh A, Marzo RR. "Airplane ear"-A neglected yet preventable problem. AIMS Public Health. 2019;6(3):320–325. doi: 10.3934/publichealth.2019.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bénézit F, Le Turnier P, Declerck C, Paillé C, Revest M, Dubée V, Tattevin P, RAN COVID Study Group Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis. 2020 Sep;20(9):1014–1015. doi: 10.1016/S1473-3099(20)30297-8. http://europepmc.org/abstract/MED/32304632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lao WP, Imam SA, Nguyen SA. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS-CoV-2 infection. World J Otorhinolaryngol Head Neck Surg. 2020 Apr 17; doi: 10.1016/j.wjorl.2020.04.001. https://linkinghub.elsevier.com/retrieve/pii/S2095-8811(20)30033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sommer IE, Bakker PR. What can psychiatrists learn from SARS and MERS outbreaks? Lancet Psychiatry. 2020 Jul;7(7):565–566. doi: 10.1016/S2215-0366(20)30219-4. http://europepmc.org/abstract/MED/32437680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Bortel T, Basnayake A, Wurie F, Jambai M, Koroma AS, Muana AT, Hann K, Eaton J, Martin S, Nellums LB. Psychosocial effects of an Ebola outbreak at individual, community and international levels. Bull World Health Organ. 2016 Mar 01;94(3):210–4. doi: 10.2471/BLT.15.158543. http://europepmc.org/abstract/MED/26966332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmes EA, O'Connor RC, Perry VH, Tracey I, Wessely S, Arseneault L, Ballard C, Christensen H, Cohen Silver R, Everall I, Ford T, John A, Kabir T, King K, Madan I, Michie S, Przybylski AK, Shafran R, Sweeney A, Worthman CM, Yardley L, Cowan K, Cope C, Hotopf M, Bullmore E. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020 Jun;7(6):547–560. doi: 10.1016/S2215-0366(20)30168-1. http://europepmc.org/abstract/MED/32304649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Agteren J, Bartholomaeus J, Fassnacht D, Iasiello M, Ali K, Lo L, Kyrios M. Using Internet-Based Psychological Measurement to Capture the Deteriorating Community Mental Health Profile During COVID-19: Observational Study. JMIR Ment Health. 2020 Jun 11;7(6):e20696. doi: 10.2196/20696. https://mental.jmir.org/2020/6/e20696/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Covid Social Study. [2020-05-20]. https://www.covidsocialstudy.org/
- 50.Jacobson NC, Lekkas D, Price G, Heinz MV, Song M, O'Malley AJ, Barr PJ. Flattening the Mental Health Curve: COVID-19 Stay-at-Home Orders Are Associated With Alterations in Mental Health Search Behavior in the United States. JMIR Ment Health. 2020 Jun 01;7(6):e19347. doi: 10.2196/19347. https://mental.jmir.org/2020/6/e19347/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farr M. Australians are as happy as before coronavirus lockdown, Guardian Essential poll finds. The Guardian. 2020. Apr 13, [2020-05-21]. https://www.theguardian.com/australia-news/2020/apr/14/australians-are-as-happy-as-before-coronavirus-lockdown-guardian-essential-poll-finds.
- 52.Vu V. Wie geht es Ihnen heute: Geht es Ihnen seit Corona besser? Zeit. 2020. Apr 29, [2020-05-21]. https://www.zeit.de/gesellschaft/zeitgeschehen/2020-04/wie-geht-es-ihnen-heute-warum-besser.
- 53.Jarral F. The lockdown paradox: why some people?s anxiety is improving during the crisis. The Guardian. 2020. Apr 29, [2020-05-21]. https://www.theguardian.com/commentisfree/2020/apr/29/coronavirus-lockdown-anxiety-mental-health.
- 54.Jungmann SM, Klan T, Kuhn S, Jungmann F. Accuracy of a Chatbot (Ada) in the Diagnosis of Mental Disorders: Comparative Case Study With Lay and Expert Users. JMIR Form Res. 2019 Oct 29;3(4):e13863. doi: 10.2196/13863. https://formative.jmir.org/2019/4/e13863/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nateqi J, Lin S, Krobath H, Gruarin S, Lutz T, Dvorak T, Gruschina A, Ortner R. [From symptom to diagnosis-symptom checkers re-evaluated : Are symptom checkers finally sufficient and accurate to use? An update from the ENT perspective] HNO. 2019 May 16;67(5):334–342. doi: 10.1007/s00106-019-0666-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


