Abstract
Orbital schwannomas are rare neoplasms of the orbit. The presenting symptoms are often nonspecific. Classic imaging characteristics seen on magnetic resonance imaging (MRI) and orbital ultrasound can be useful to help aid in the diagnosis of orbital schwannoma. When diagnosed, the goal of treatment is complete surgical excision. The location of the tumor within the orbit dictates which surgical approach would provide the best exposure. When complete excision is achieved, recurrence rates are very low.
This article addresses the etiology, patient population, presentation, natural history, and differential diagnosis of orbital schwannomas. Imaging characteristics and histopathologic subtypes are reviewed. Treatment goals, approaches, and specialties involved in the management of these patients is discussed. Finally, a representative case is presented.
Keywords: orbit, orbital neoplasm, schwannoma, orbital schwannoma, nerve sheath tumor
Introduction
Schwannomas are benign, slow-growing nerve sheath tumors that originate from the Schwann cells of the perineurium of peripheral nerves. 1 They are the most common benign peripheral nerve tumors in adults. While the exact etiology of schwannomas is not known, genetic mutations that result in the Schwann cell hyperplasia, similar to those of the neurofibromatoses syndromes, are thought to be responsible. 2 From the study of neurofibromatosis type 1, it is known that the biallelic loss of tumor suppressor gene neurofibromin on 17q11.2 leads to unregulated ras signal transduction and subsequent Schwann's cell hyperplasia. Similarly, in neurofibromatosis type 2, loss of the merlin gene on 22q11.2 promotes Schwann's cell hyperplasia. 3 While the exact etiology is not understood on the sporadic level, similar pathways are thought to be responsible.
Any nerve with the Schwann cell sheath can be affected, including all cranial nerves (except for optic and olfactory), spinal nerves, and the autonomic nervous system. 4 As such, 25 to 45% of all schwannomas are found in the head and neck. 5 Despite this, they are rare to occur in the orbit. Orbital schwannomas account for only 1% of all orbitaltumors. 1
Natural History
Orbital schwannomas have an insidious growth pattern. They most commonly present as painless, nonpulsatile ocular proptosis, displacement of the globe, or a palpable mass in the orbit. 6 7 Occasionally, lid swelling has been reported. 7 Due to the complexity of nerve fibers traversing the orbit and the volume of orbital fat, the specific nerve of origin is often not identified. 8 However, orbital schwannomas commonly arise from sensory nerves, most frequently the supraorbital and supratrochlear nerves. 8 Accordingly, they are primarily found in the supraorbital region. For this reason, orbital schwannomas rarely result in extraocular movement or vision deficits. However, if the tumor is located at the orbital apex and is large enough to compress the optic nerve, diminished visual acuity, or compressive symptoms including scotomas, dyschromatopsia, and impaired contrast sensitivity, may be appreciated. 9 Likewise, in the rare circumstance that a motor nerve is affected, the functional deficit may result in diplopia and can indicate the originating nerve in the preoperative setting. 10
Orbital schwannomas typically present in patients between the third and sixth decades of life, but can present at any age. 1 They have no predilection for specific eye or gender. 1 11 12 In patients with multiple schwannomas, it is critical to evaluate for neurofibromatosis or schwannomatosis syndromes.
Indications for Treatment
Because of the often nonspecific presenting symptoms, the differential diagnosis is broad, including malignant peripheral nerve sheath tumors, neurofibromas, leiomyoma, solitary fibrous tumor, meningiomas, cavernous hemangiomas, lymphangioma, fibrous histiocytoma, lymphoma, dermoid cyst, hemangiopericytoma, or pleomorphic adenoma of the lacrimal gland. 8
It is critical to differentiate schwannomas from other intraorbital tumors as the prognosis and treatment approach may differ. As a result of the nonspecific symptoms, it is difficult to differentiate orbital schwannomas from other intraorbital tumors clinically. When a schwannoma is predicted, surgical resection is nearly always indicated. Certain imaging characteristics can be suggestive of orbital schwannoma, particularly on magnetic resonance imaging (MRI) and ultrasound.
On MRI, orbital schwannomas are classically hypointense (although can be isointense) on T1-weighted imaging and hyperintense on T2-weighted imaging. On postgadolinium MRI, schwannomas enhance, either homogenously or heterogeneously. These regions correlate with histologic and morphologic features of the tumor. Antoni's A regions have intermediate intensities with T1- and T2-weighted imaging, while Antoni's B regions are hypointense on T1 and hyperintense on T2. Areas of cystic degeneration may correlate to Antoni's B regions. 1
On ultrasound, orbital schwannomas most often are oval or lobulated masses with heterogeneous middle-to-low internal reflectivity. The Doppler ultrasound shows flow signals inside the tumor. This rich blood signal is characteristic of schwannomas. In a purely cystic schwannoma, a double wall sign will be appreciated on ultrasound. 1
While computed tomography (CT) has less diagnostic value than the aforementioned imaging modalities, CT is helpful for assessing bony expansion, or erosion and for surgical planning. Orbital schwannomas are smooth, elongated, and oval to spindle shaped on CT imaging. They are homogeneous lesions with a density similar to extraocular muscle. CT may show characteristic expansion into bone, without bony erosion. 1
Histopathology
There are five histologic subtypes of schwannomas: conventional, cellular, melanotic, plexiform, and neuroblastoma. 13 In the orbit, the majority of schwannomas are conventional subtype; less commonly they are the cellular subtype, and very infrequently any of the other variants. Conventional and cellular subtypes are identified by a fibrous, smooth capsule from the perineurium. 2
On light microscopy, there are two patterns of cell morphology: Antoni's types A and B. Antoni's type-A patches are characterized by closely packed spindle cells with fusiform nuclei and eosinophilic cytoplasm ( Figs. 1 and 2 ). Antoni's A regions stain strongly for Periodic Acid Schiff and immunoperoxidase assay for laminin. Antoni's type-B patches are characterized by haphazardly distributed cells with distinct cytoplasmic margins in a loose myxoid matrix ( Fig. 1 ). 14 15
Fig. 1.
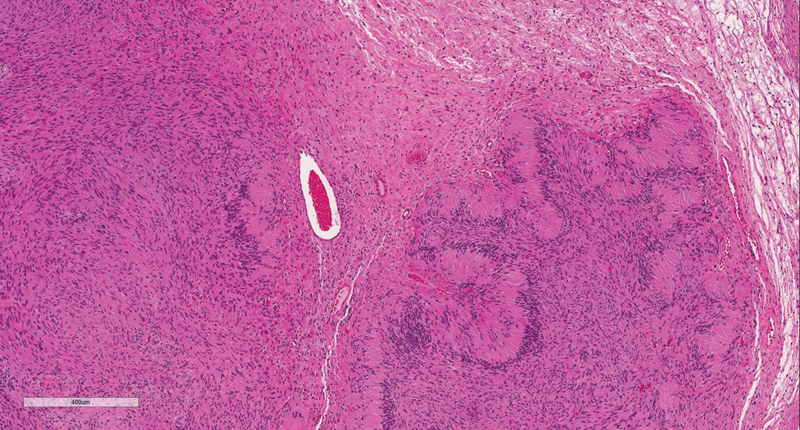
H&E stain demonstrating Antoni's A and Antoni B regions. H&E, hematoxylin and eosin.
Fig. 2.
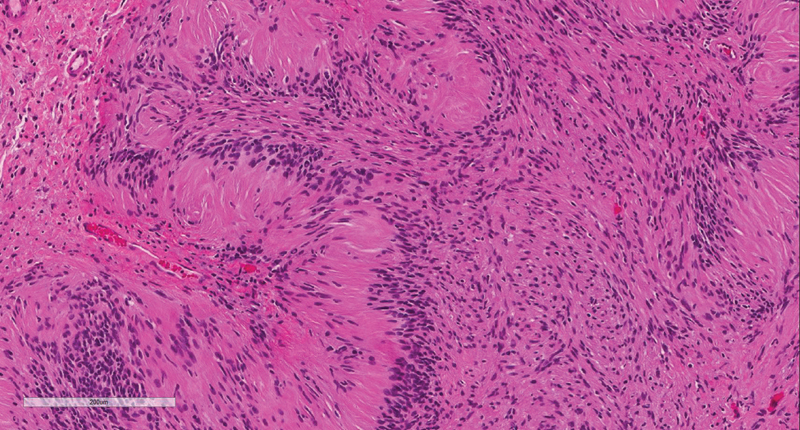
H&E stain demonstrating Antoni A regions with nuclear palisading. H&E, hematoxylin and eosin.
Schwannomas characteristically stain strongly for S-100, SOX10, p16, vimentin, and neurofibromin ( Fig. 3 ). A stain for type-4 collagen classically shows pericellular collagen deposition. They do not stain for epidermal growth factor receptor. 2
Fig. 3.
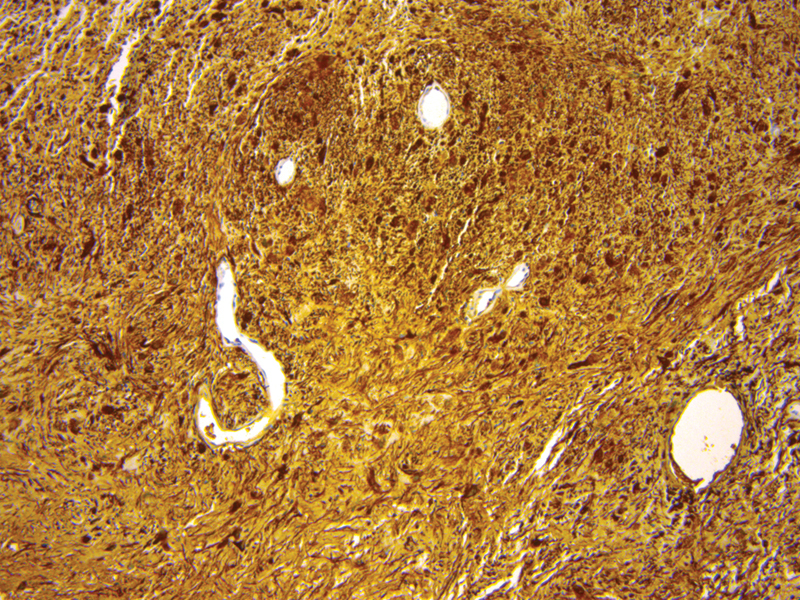
Diffusely positive S100 stain.
Goals of Treatment
Complete surgical resection is the treatment of choice. Although the capsule is very thin, particularly in tumors with cystic degeneration, in the absence of capsule violation recurrence rate is near zero. Depending on surgical approach, tumor location and extent of dissection, persistent decreased visual acuity, and restricted eye motion have been reported. 16 Long-term follow-up with repeat MRI is recommended to evaluate for recurrence. 17
Typical Locations and Approaches
While orbital schwannomas are most often located in the superior orbit, they have been reported in all quadrants. The location of the tumor dictates which surgical approach is chosen. A maxim of orbital surgery dictates the best approach to intraorbital lesions is to avoid surgical manipulation of the optic and oculomotor nerves.
For schwannomas superior to the optic nerve and anterior in the orbit, a superior orbitotomy or a supraorbital approach via an eyebrow incision is often the ideal approach. An incision is made in an upper eyelid crease or hidden in the brow. Dissection is carried through the orbicularis and subcutaneous soft tissue to the superior orbital rim without violating the septum. A subperiosteal dissection is performed and the subperiosteal potential space opened. The periorbita is then incised. Herniating orbital fat can be carefully shrunk with bipolar cautery. 8 18
The superior posterior orbit is best accessed via a frontotemporal craniotomy. This can be extended to the superolateral orbit with a zygomatic osteotomy. A curvilinear incision from tragus to the midline apex of the anterior hairline is usually sufficient but can be extended to a modified bicoronal incision for more medial exposure. A subperiosteal dissection is performed onto the orbit and orbital rim. A frontotemporal craniotomy is then performed with removal of the lateral bone of the greater wing of the sphenoid. Frontal lobe dura is dissected free from the external aspect of the roof and periorbita dissected free from the inner aspect of the roof. The medial osteotomy can then be made at, or just lateral to, the supraorbital notch. The lateral osteotomy is made from the inferior orbital fissure to just above the zygomatic prominence for a superolateral orbital osteotomy or just below the zygomatic prominence for an orbitozygomatic osteotomy. For the latter, an additional posterior osteotomy is made across the zygomatic arch. It is critical to complete the posterior bony removal to ensure adequate dural retraction and posterior exposure. 19
The lateral orbit is best accessed via a lateral orbitotomy. In this approach, an incision is made either via a small brow or cantholysis incision. The anterior temporalis is dissected and the lateral wall of the orbit is exposed. A subperiosteal dissection is continued within the orbit to allow medial retraction of orbital contents including the lacrimal gland. Once the bone is cleanly exposed, a first transverse osteotomy superior to the zygomaticofrontal suture and a second transverse osteotomy just superior to the origin of the zygomatic arch can be made. The lateral orbital bone can then be drilled or fractured exposing the sphenoid wing; and the sphenoid wing can then be drilled as needed to expose the orbital apex. The periorbita is opened parallel to the lateral rectus muscle. 19
The anterior medial orbit is best accessed through a medial transconjunctival approach. A conjunctival peritomy is performed. The medial rectus is controlled at its insertion into the globe. The muscle is freed from its intermuscular septa and distal ligaments are cut from the insertion site. After tumor resection, the medial rectus is reattached at the insertion site. 19
The mid- and posterior medial orbit and orbital apex can often be accessed via an endoscopic endonasal approach. In this approach, a 0-degree endoscope is used to expose the entire medial (and sometimes inferior) orbital walls via an uncinectomy, wide maxillary antrostomy, total ethmoidectomy, and sphenoidotomy. For intraconal tumors or orbital apex tumors, a limited posterior septectomy may be performed when a binarial approach is required. The lamina papyracea is taken down to provide access to the medial orbit. It is critical at this step to identify the optic nerve and carotid artery posteriorly. The periorbita is incised parallel to the medial rectus. Herniated extraconal fat can be cauterized with bipolar cautery if necessary and the rectus muscles identified. When working endonasally in the intraconal space, external anterior retraction of the rectus muscles or retraction of the orbital fat is often required with the assistance of an ophthalmologist. Alternatively, the medial rectus can be detached at its insertion on the globe, passed intranasally, so that it is pedicled posteriorly on the annulus of Zinn, and then reattached to the globe after tumor resection. 19
In cases of unresectable tumor, tumor debulking with serial imaging surveillance may be considered. Alternatively, orbital decompression may be suitable, particularly in apical schwannomas with optic nerve compression. This approach is most appropriate for cases of slow-growing tumors in older patients with rapidly declining vision who are reliable for serial imaging in follow-up. 2 20
While surgical resection is the mainstay of treatment, radiation treatment has evolved in recent years. This therapy still does not have well established indications, but has been proposed to treat small, unresectable, or recurrent orbital schwannomas. Optic neuropathy has been observed with radiation doses as low as 8 to 12 gray. As such, this treatment modality has not gained much traction. However, more recently, multisession gamma knife therapy has shown to provide tumor stability or reduction. 21
Specialties Involved in Treatment
Management of these tumors often requires an interdisciplinary approach with ophthalmology, otolaryngology, neurosurgery, and radiation oncology.
Case Example
A 68-year-old male presents with an 8-month history of right eye proptosis and progressive visual acuity deficits. On presentation, he had diminished abduction and elevation. He had right eye ptosis, which he developed only after an open biopsy by an outside provider. Fundoscopic exam showed right optic disc pallor. Visual fields were intact. Visual acuity was 20/400.
On MRI, there was a lobular, heterogeneously enhancing mass centered within the intraconal superior orbit extending to the orbital apex. The mass demonstrated isointense T1 and very hyperintense T2 signal ( Fig. 4 ). Both imaging and histopathology from the open biopsy were consistent with an orbital schwannoma.
Fig. 4.
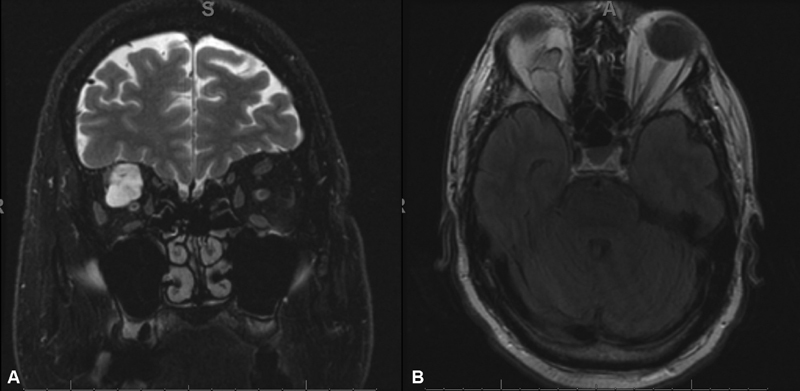
( A ) Hyperintense lesion on fast spin echo fat suppressed T2-weighted magnetic resonance imaging (MRI), ( B ) Isointense lesion on T1-weighted MRI.
The tumor was approached via frontotemporal craniotomy. An epidural dissection was performed along the right superior orbital rim and roof, mobilizing the cranial base dura. The frontal lobe was retracted to allow for complete visualization of the orbital roof. Osteotomies were then made and the superior orbital rim and roof were removed using a combination of cutting burrs and Kerrison's rongeurs. The superior orbital fissure was drilled and decompressed. The periorbita was incised and orbital exploration performed. Displaced intraorbital structures, including the superior rectus levator complex and oblique muscle, were identified and preserved. Orbital soft tissue was gently retracted and the tumor identified, a combination of sharp and blunt microdissection around the tumor capsule was utilized to mobilize the tumor from surrounding muscle and nerve structures. The tumor was removed en bloc.
The periorbita was reconstructed with sheets of a porous collagen matrix dressing. The orbital roof and anterior cranial base osteotomies were repaired with titanium mesh, careful to estimate the appropriate volume of the orbit. The patient recovered in the neurosurgical intensive care unit.
At 40-month follow-up, he had mild right eye ptosis and mild diplopia on extreme superior rightward gaze. There was no evidence of recurrence on MRI.
Footnotes
Conflict of Interest None declared.
Pearls and Tips.
Typically present as painless, nonpulsatile ocular proptosis, displacement of the globe, or a palpable mass in the orbit.
The specific nerve of origin is often not identified. However, orbital schwannomas commonly arise from sensory nerves, most frequently from the supraorbital and supratrochlear nerves.
Classically appears as hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.
The location of the tumor dictates which surgical access approach is chosen.
Surgical resection often requires one or a combination of the following approaches: superior orbitotomy, transconjunctival, lateral orbitotomy, endoscopic endonasal, or frontotemporal craniotomy.
Depending on surgical approach, ophthalmology, otolaryngology, and neurosurgery may all be involved.
Complete surgical resection is the treatment of choice. In the absence of capsule violation, recurrence rate is near zero.
Long-term follow-up with repeat magnetic resonance imaging (MRI) is recommended.
It is critical to evaluate for neurofibromatosis and schwannomatosis syndromes in patients with multiple schwannomas.
References
- 1.Chen M H, Yan J H. Imaging characteristics and surgical management of orbital neurilemmomas. Int J Ophthalmol. 2019;12(07):1108–1115. doi: 10.18240/ijo.2019.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweeney A, Burkat C N, Stewart K, Lee V. www.eyewiki.org/Orbital_schwannoma 2019. www.eyewiki.org/Orbital_schwannoma
- 3.Kurtkaya-Yapicier O, Scheithauer B, Woodruff J M. The pathobiologic spectrum of Schwannomas. Histol Histopathol. 2003;18(03):925–934. doi: 10.14670/HH-18.925. [DOI] [PubMed] [Google Scholar]
- 4.Ansari I, Ansari A, Graison A A, Patil A J, Joshi H. Head and neck schwannomas: a surgical challenge-a series of 5 cases. Case Rep Otolaryngol. 2018;2018:4.074905E6. doi: 10.1155/2018/4074905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batsakis J G. Baltimore, MD: Williams and Wilkins; 1979. Tumours of the Peripheral Nervous System. 2nd ed. [Google Scholar]
- 6.Rootman J, Goldberg C, Robertson W. Primary orbital schwannomas. Br J Ophthalmol. 1982;66(03):194–204. doi: 10.1136/bjo.66.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh M, Singh U, Zadeng Z, Pathak A, Sukhija J. Clinico-radiological spectrum and management of orbital schwannomas: a tertiary care institute study. Orbit. 2013;32(03):171–177. doi: 10.3109/01676830.2013.788661. [DOI] [PubMed] [Google Scholar]
- 8.Kim K S, Jung J W, Yoon K C, Kwon Y J, Hwang J H, Lee S Y. Schwannoma of the Orbit. Arch Craniofac Surg. 2015;16(02):67–72. doi: 10.7181/acfs.2015.16.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behbehani R. Clinical approach to optic neuropathies. Clin Ophthalmol. 2007;1(03):233–246. [PMC free article] [PubMed] [Google Scholar]
- 10.Young S M, Kim Y D, Hwang S S, Woo K I. Orbital schwannoma with atypical presentation. J Craniofac Surg. 2018;29(03):e224–e226. doi: 10.1097/SCS.0000000000004177. [DOI] [PubMed] [Google Scholar]
- 11.Pointdujour-Lim R, Lally S E, Shields J A, Eagle R C, Jr, Shields C L. Orbital schwannoma: radiographic and histopathologic correlation in 15 cases. Ophthlamic Plast Reconstr Surg. 2018;34(02):162–167. doi: 10.1097/IOP.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 12.Kron M, Bohnsack B L, Archer S M, McHugh J B, Kahana A. Recurrent orbital schwannomas: clinical course and histopathologic correlation. BMC Ophthalmol. 2012;12:44. doi: 10.1186/1471-2415-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez F J, Folpe A L, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123(03):295–319. doi: 10.1007/s00401-012-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wippold F J, II, Lubner M, Perrin R J, Lämmle M, Perry A. Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. AJNR Am J Neuroradiol. 2007;28(09):1633–1638. doi: 10.3174/ajnr.A0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pushker N, Khurana S, Kashyap S. Orbital schwannoma: a clinicopathologic study. Int Ophthalmol. 2015;35(04):481–486. doi: 10.1007/s10792-014-9973-1. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian N, Rambhatia S, Mahesh L. Cystic schwannoma of the orbit-a case series. Orbit. 2005;24(02):125–129. doi: 10.1080/01676830590922020. [DOI] [PubMed] [Google Scholar]
- 17.Kapur R, Mafee M F, Lamba R, Edward D P. Orbital schwannoma and neurofibroma: role of imaging. Neuroimaging Clin N Am. 2005;15(01):159–174. doi: 10.1016/j.nic.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Karcioglu Z A. New York, NY: Springer/Cham; 2019. Surgical techniques for orbital tumors. [Google Scholar]
- 19.Paluzzi A, Gardner P A, Fernandez-Miranda J C. “Round-the-Clock” surgical access to the orbit. J Neurol Surg B Skull Base. 2015;76(01):12–24. doi: 10.1055/s-0033-1360580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pförtner R, Mohr C, Daamen J, Metz A. Orbital tumors: operative and therapeutic strategies. Facial Plast Surg. 2014;30(05):570–577. doi: 10.1055/s-0034-1395210. [DOI] [PubMed] [Google Scholar]
- 21.Kim M S, Park K, Kim J H, Kim Y D, Lee J I. Gamma knife radiosurgery for orbital tumors. Clin Neurol Neurosurg. 2008;110(10):1003–1007. doi: 10.1016/j.clineuro.2008.06.008. [DOI] [PubMed] [Google Scholar]


