Abstract
Sphenoid wing meningiomas are benign tumors that can result in proptosis, visual impairment, and pain. Traditional open surgical approaches are associated with significant morbidity. Transorbital endoscopic surgery has been developed as a minimally invasive approach to gain access to these tumors and address the main presenting symptoms. Case series reporting transorbital endoscopic resection of sphenoid wing meningiomas using combined endonasal, pre-caruncular, and extended superior eyelid approaches have demonstrated stable and/or improved short- and medium-term visual outcomes. Earlier medial optic nerve decompression appears to result in more favorable long-term visual outcomes. Transorbital endoscopic surgery therefore represents an emerging minimally invasive alternative to deal with these challenging lesions.
Keywords: meningiomas, sphenoid wing meningiomas, Transorbital
Introduction
Meningiomas are the most common benign tumors of the central nervous system, comprising between 12 and 15% of all intracranial neoplasms. 1 2 3 4 They are of mesenchymal origin and arise from arachnoid cells of neurovascular structures in close proximity to cranial sutures. 3 Sphenoid wing meningiomas are the most frequently occurring tumor of the sphenoid wing making up approximately 18% of all meningiomas. 2 4 Cushing and Eisenhardt were the first to classify sphenoid wing meningiomas into globoid, nodular and en plaque subtypes. 2 The globoid entity has been subclassified according to the anatomical location of the lesser sphenoid wing, that is, medial, middle, and lateral. 2 This classification was further modified into medial third and lateral two-thirds by Fohanno and Bitar, as the medial third corresponds to the posterior margin of the lesser wing and the lateral two-thirds corresponds to the posterior margin of the greater wing. 4 5 En plaque meningiomas are rare and mostly occur in the sphenoid wing. 6 Sphenoid wing meningiomas may spread secondarily into the orbit, at which point they are classified as sphenoorbital meningiomas. 4 Sphenoorbital meningiomas characteristically present with an en plaque meningeal component and hyperostosis. 7 Sphenoorbital meningiomas are slow growing with an annual growth rate of 0.3 cm 3 per year. 8 Faster growth rates have been associated with younger age and a larger soft tissue component compared with bony involvement. 8
Sphenoid wing meningiomas occur between the ages of 36 and 70 years. They occur more frequently in females with a female to male ratio of 2.8:1. 4 9 Patients with neurofibromatosis type 2 (NF-2) and multiple endocrine neoplasia type 1 (MEN-1) are at a greater risk of developing meningiomas. 10 Sphenoid wing en plaque meningiomas may be associated with hyperostosis of the sphenoid wing, a phenomenon well-characterized occurring in 25 to 49% of cases. 6 9 Hyperostosis is thought to occur as a result of direct invasion of tumor into bone via the Haversian canals and commonly occurs in the lateral third of the sphenoid; however, the exact cause of hyperostosis remains unclear. 3 4 6 The hyperostotic bone is typically large and out of proportion compared with the relatively adjacent small tumor. Sphenoid wing meningiomas most commonly occur in the lesser sphenoid wing, orbital roof, and lateral orbital wall. 3 The tumor may spread to the optic canal, superior orbital fissure, and anterior clinoid process. 3 The average size of tumors measured on computed tomography (CT) scan at presentation has been reported to be 3.4 cm. Chaichana et al found that medial sphenoid wing meningiomas involved critical neurovascular structures, the optic nerve being the most common (40% of cases). 11 The presenting symptoms of patients with sphenoid wing meningiomas are determined by the tumor location on the sphenoid wing. 2 Lateral third tumors commonly present with proptosis and medially situated tumors are more likely to present with visual disturbances due to the close proximity to the optic nerve. 2 Proptosis is the most common presenting symptom and is likely attributed to several causes including periorbital tumor invasion, intraorbital tumor, ophthalmic vein thrombosis, and hyperostotic bony change of the sphenoid bone. 6 8 12 Visual loss, occurring for 40 to 60% of cases, is related to tumor infiltration into the optic canal and compression of the optic nerve resulting in a compressive optic neuropathy rather than intraorbital tumor spread. 12 Patients may also present with diplopia, ptosis, and orbital pain, all of which may impact quality of life. 6
Meningioma tumor growth has been postulated to be influenced by female sex hormones. 13 The higher incidence of meningiomas in females, an increased tumor growth rate during pregnancy and a possible association between breast cancer and meningioma may support tumor hormone responsiveness. 13 It is well established that most meningiomas express progesterone receptors and generally lack estrogen receptors. 13 It has been demonstrated that the presence of progesterone receptors is a good prognostic tumor indicator and is associated with improved progression free survival. 13 Meningiomas that show negative progesterone expression are more likely to be malignant and have a high mitotic index. 13 There is no correlation between estrogen receptors and prognostic outcomes. 13
Interestingly, sphenoorbital meningiomas have been reported to have a greater female predominance and recurrence risk compared with meningiomas in other sites. 8 The identification of progesterone receptor expression in meningioma has led to the use of hormonal therapy as part of the medical management. Mifepristone, an antiprogesterone agent, has been used in the treatment of meningioma and was found to be of benefit in diffuse meningiomatosis. 14 However, mifepristone has not been shown to reduce tumor size and provide symptom relief in patients with meningioma. 14
The management of sphenoid wing meningioma includes observation, surgery, and radiation therapy. 10 Small incidental tumors that are asymptomatic may be observed due to their slow growing benign nature. However symptomatic tumors are most commonly treated by surgical excision. 10 In patients undergoing surgery for sphenoid meningioma, the standard of care has been complete tumor resection. 10 However, due to the nature of tumor spread and close proximity to the optic nerve and dura, complete resection is not always possible and is often associated with significant morbidity. 10 Champagne et al showed that gross total resection is less likely with large meningiomas of more than 6 cm and involving major cerebral blood vessels. The study showed that total resection via an open approach was only achieved in a minority of cases and was associated with a higher recurrence rate. 15 Therefore, the paradigm of meningioma surgical resection has evolved with time due to the understanding of the biology of the tumor. Although benign, they are locally invasive into surrounding bone and dura. Thus, complete surgical resection is challenging, associated with an increased risk of postoperative complications, high residual tumor, and recurrence rates postsurgery. 16 Tumors that partly encase or invade multiple cerebral vessels are not resectable and have an increased risk of postoperative cerebrovascular accident. 15 The Simpson grading system is typically used as the standard for grading tumor resection. 17 This grading system is used to predict the recurrence rates as it correlates directly with the extent of the tumor resection. 17 Grades I to V range from gross-total resection (I) to simple decompression (V). The rates of recurrence for grades I, II, III, IV are 9, 16, 29, and 39%, respectively. 17
Standard open surgical approaches include variations of the pterional approach, lateral orbitotomy, and open extradural optic nerve decompression followed by orbital reconstruction using autologous split calvarial bone grafts, titanium mesh, or nonmetallic orbital prostheses. 4 18 However, open surgical approaches are associated with significant morbidity, new onset cranial nerve neuropathy, and a recurrence rate of 10%. 4
Gross-total resection may not always be possible due to the intimate anatomical relationship to underlying neurovascular structures necessitating subtotal resection. 2 It is interesting to note that tumor recurrence has been shown to be unlikely in cases where subtotal resection has been performed despite the presence of an aggressive histological tumor grade and in the absence of additional therapy. 2 This has led to the hypothesis that it is possible that tumor remnants may remain dormant and cease to grow. Nonetheless, further research is necessary to determine the key factors and molecular mechanisms involved in the process. 2 It has been demonstrated that adequate control, defined as an 80% control rate over a period of 8 years postoperatively, can be achieved of residual tumor using adjuvant radiotherapy or radiosurgery, supporting the efficacy of a more conservative subtotal resection. 2
A novel minimally invasive endoscopic surgical approach for sphenoid wing meningiomas has recently been described. 19 20 The above procedure addresses the most common symptoms namely, proptosis, visual loss, and pain. Proptosis is reduced by using a lateral orbital wall approach to address the lateral tumor component and to remove hyperostotic bone. Furthermore, stabilization of visual loss is achieved by early decompression of the optic canal via a medial optic canal decompression. The surgery involves a combined method utilizing both an endoorbital, as well as an endonasal transsphenoidal approach. 19 20
Rationalization for a Multiportal Approach to Sphenoid Wing Meningiomas
A major advantage of this approach is the ability to stabilize and improve vision by performing a medial optic canal decompression prior to addressing the main intracranial and orbital tumor components. 3
Our hypothesis is that a medial optic canal decompression allows for manipulation of the main tumor component during the second stage of the surgery, without the risk of compressive damage to the optic nerve, which may occur during traditional approaches and may cause worse visual outcomes. Removal of the medial sphenoid sinus tumor component is also only possible using an endonasal or precaruncular approach. The superior eyelid approach gives excellent and direct access to the superior and lateral hyperostotic orbital walls and orbit itself, structures that are often infiltrated by tumor. Other presenting symptoms, such as proptosis and pain, can therefore be addressed at the same time by resecting the orbital tumor and removing the lateral and superior walls of the orbit, up to temporalis muscle, and anterior and middle fossa dura. The corridor thus created provides access to the intracranial component of the tumor. This multiportal approach potentially allows for resection of all components of the tumor during a single surgery with no brain retraction required and minimal collateral tissue damage with no need for reconstruction.
Step-Wise Surgical Approach
Medial optic canal decompression (endonasal or multiportal precaruncular and endonasal): medial sphenoid wing meningiomas often involve the sphenoid sinus itself and a uni- or binostril approach can be used to resect this component. The binostril approach allows for four-handed surgery but requires a posterior septectomy as is done for transsphenoidal pituitary surgery. Using the precaruncular approach in combination with the above approach has some advantages. First, these patients often have proptosis and with removal of the posterior lamina papyracea, periorbital fascia can herniate into the ethmoid cavity, obscuring good visualization of the optic canal. The precaruncular approach allows for a ribbon (dural) retractor to be inserted through this portal to protect the orbit and make visualization of the bony canal easier ( Fig. 1A and B ). A second advantage is the slight difference in angulation obtained, allowing for the endoscope and instruments to be manipulated and to converge at the target area. It is convenient to keep the endoscope and orbital retractor within the precaruncular portal while powered instruments are used at the target area.
-
Resection of superior and lateral hyperostotic orbital wall bone through an extended superior eyelid approach: sphenoid wing meningiomas often involve the superior and lateral orbital walls and optic canal, with tumor directly infiltrating bone, causing bony hyperostosis leading to compression of the orbital apex and optic canal ( Fig. 2 ). The superior portal is accessed through an extended superior eyelid approach, using the same crease line as for blepharoplasty surgery ( Fig. 3 ). It is our opinion that it is the best practice to use this incision rather than a retrocanthal or lateral canthotomy incision, since sparing of the lateral canthus allows for wider surgical access, quicker healing, and less patient discomfort. Crucial steps need to be followed to avoid postoperative sequalae such as ptosis and dystopia. 21 22
Use a natural upper eyelid crease line as for blepharoplasty surgery.
Dissect through skin and orbicularis oculi muscle only and remain just deep to the orbicularis muscle until the bony orbital roof is reached. Remain superficial to orbital septum and avoid exposing orbital fat.
Once deep to the orbicularis oculi muscle, dissection starts at the lateral aspect of the incision, going directly onto bone just lateral to the lateral orbital rim ( Fig. 4 ).
Dissection is initiated at the lateral aspect of the orbit, using scissors to dissect in a subperiosteal plane, moving from the lateral orbital rim superiorly, dissecting tissue above the superior orbital rim to avoid damage to the levator muscle ( Fig. 5 ). This technique allows for quick access to the superior orbital rim. The limit of the dissection is usually the supraorbital nerve although this nerve can be mobilized if required.
The orbital periosteum is elevated in a lateral-to-medial fashion starting at the lateral orbital rim, thereby “rolling” the periosteum over the orbital rim, keeping the ligamentous attachments to Whitnall's tubercle attached to the periosteum. The periosteum is resutured at the end of the procedure to prevent dystopia due to lateral canthal tendon displacement.
Deep within the orbital cavity, periosteum can easily be elevated off the lateral orbital wall to first identify a recurrent branch of the middle meningeal artery ( Figs. 6 , 7 ). This artery is an important landmark for the superior orbital fissure (SOF). The limit of dissection along the lateral wall is the SOF, found 1-cm posterior to this artery ( Fig. 8 ). It is important not to injure the structures entering the SOF and to avoid excessive traction, especially in patients who have no neurological fallout. Excessive tissue retraction could cause a superior orbital fissure syndrome.
Superiorly, the lacrimal gland is protected by the periorbital fascia that covers it and will not be damaged if dissection remains in the subperiosteal plane. Once the gland is elevated out of its fossa, this bony area can be drilled away to provide wider access, the so called “lacrimal keyhole” area ( Fig. 9 ). This is a good space to rest the endoscope during surgery.
The superior orbital wall can now be resected using a high speed drill, the extent of resection depending on bony tumor involvement. The anterior cranial fossa dura is exposed from the frontal sinus to the orbital apex if needed. No reconstruction is required and any dural pulsations felt should settle within 2 weeks.
The inferior limit of dissection is the inferior orbital fissure (IOF). The amount of bone requiring resection depends on the extent of the hyperostosis. The area of bone between the SOF and the IOF is called the orbital door jamb. It is important to resect all this bone until the temporalis muscle is seen anterolaterally, the middle cranial fossa posterolaterally, and the anterior cranial fossa dura superiorly ( Fig. 10 ). The entire lateral orbital wall therefore can be resected, leaving 5 mm of orbital rim intact laterally for cosmetic purposes.
Resection of the orbital component through the lateral transorbital portal: it can initially be difficult to differentiate between orbital tumor and orbital fat, muscle and fibrotic tissue. It is relatively easy to resect the periorbital fascia up to the SOF but if patients have no neurological fallout, it is important to preserve the neurovascular structures to prevent significant complications such as diplopia related to injury to cranial nerve III, IV, or VI. More research is needed with the use of ultrasound and nerve monitoring to assist with achieving more complete resection of the orbital component.
Resection of the intracranial component through the lateral orbital portal: a wide surgical corridor is created once the lateral hyperostotic bone has been drilled away, allowing the intracranial component to be resected using either an endoscope or microscope ( Fig. 10 ). Standard neurosurgical principles apply with regards to the extent of resection, preservation of important neurovascular structures, and repair of the ensuing cerebrospinal fluid (CSF) leak using fat, fascia, or synthetic materials. The authors do not use any sealants within the orbit. The lateral orbital wall does not require reconstruction as the orbital rim is left intact and the aim in most patients is a reduction in proptosis. Access is gained to the anterior and lateral aspects of the temporal lobe.
Fig. 1.
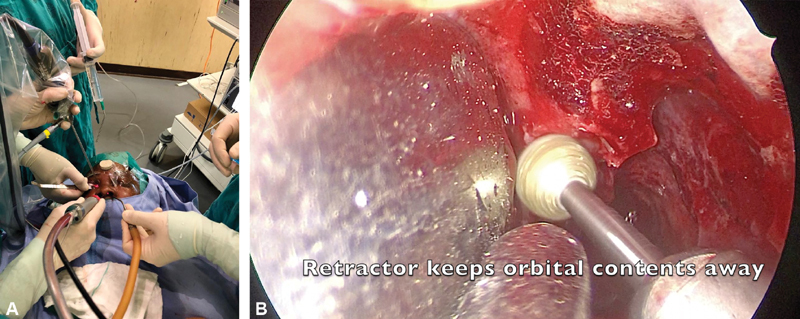
( A, B ) Multiportal surgery ( A ) using a precaruncular and unilateral endonasal approach to decompress the medial optic nerve canal (right eye). ( B ) Instruments converge on the target area.
Fig. 2.
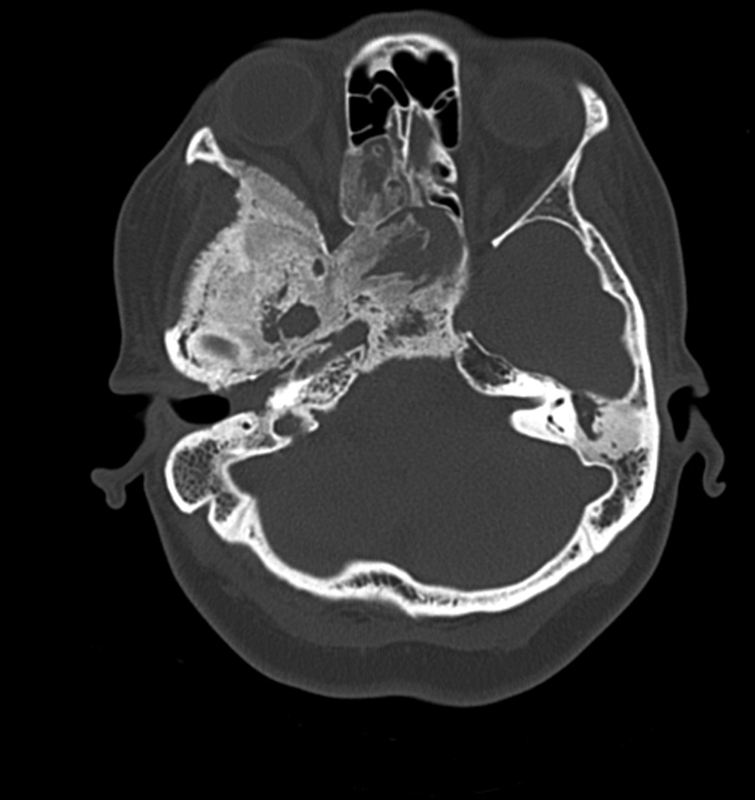
Sphenoid wing meningioma with bony hyperostosis causing compression at the optic canal and orbital apex.
Fig. 3.
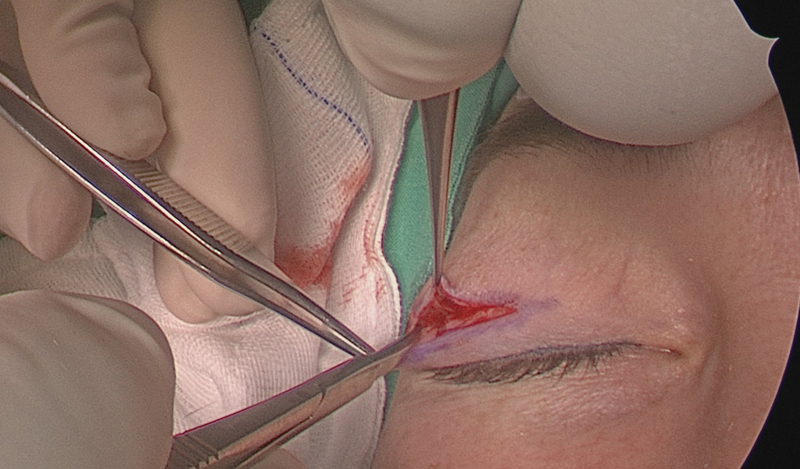
The superior eyelid incision is made as for blepharoplasty surgery using a natural crease line. The incision can be extended laterally to spare the lateral canthus of the eye.
Fig. 4.
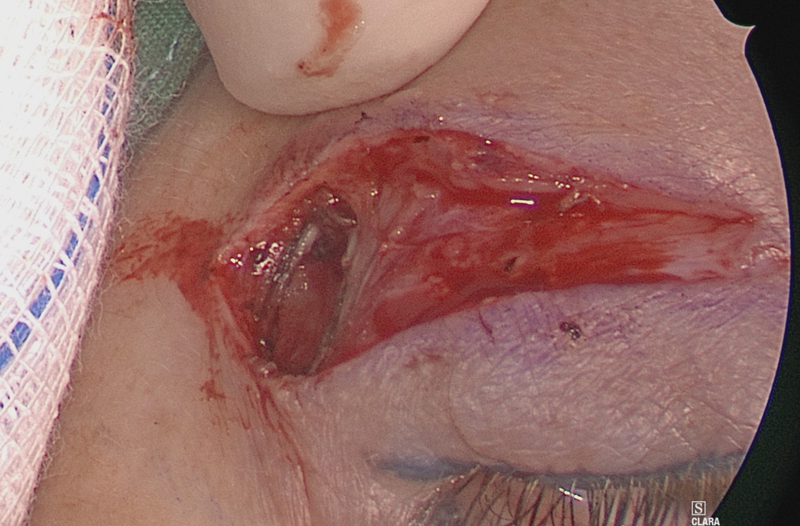
Dissection starts on the lateral orbital rim to prevent damage to the levator muscle.
Fig. 5.
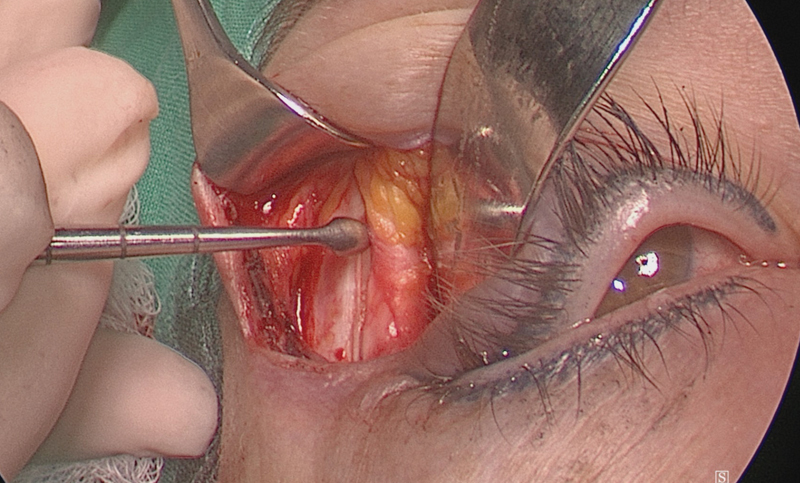
The periosteum is elevated off the orbital rim from laterally to medially around the orbital rim and then off the superior orbital rim.
Fig. 6.
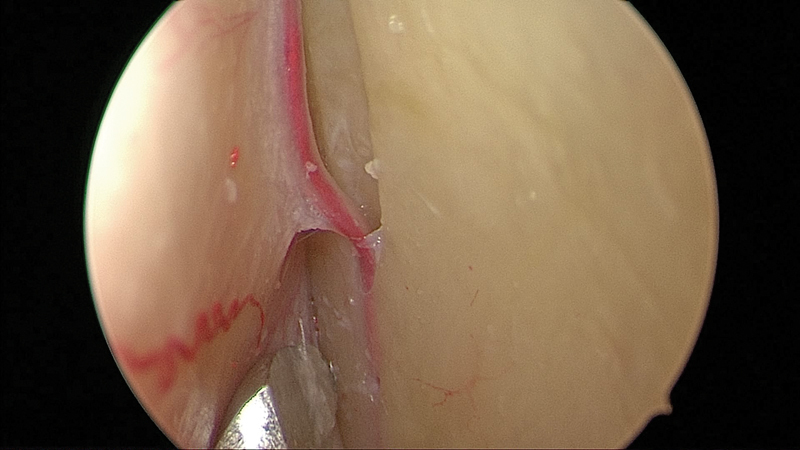
A recurrent branch of the middle meningeal artery in a cadaveric specimen (left eye).
Fig. 7.
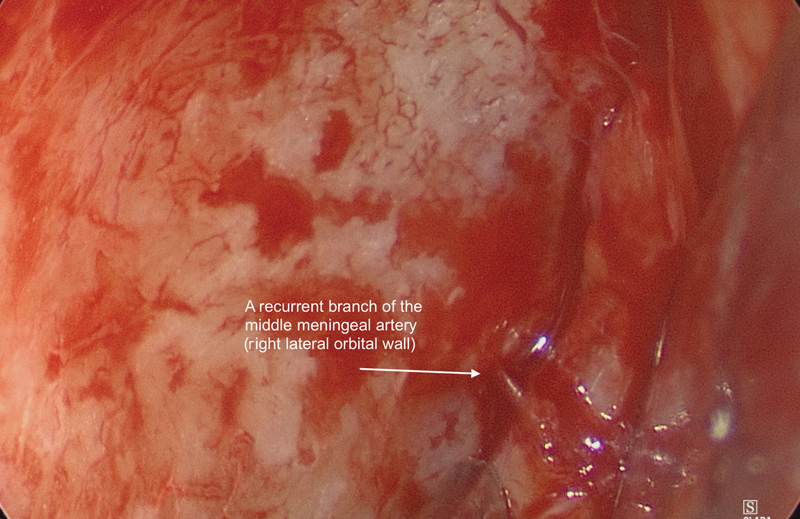
Recurrent branch of the middle meningeal artery (right eye), 1 cm anterior to the superior orbital fissure.
Fig. 8.
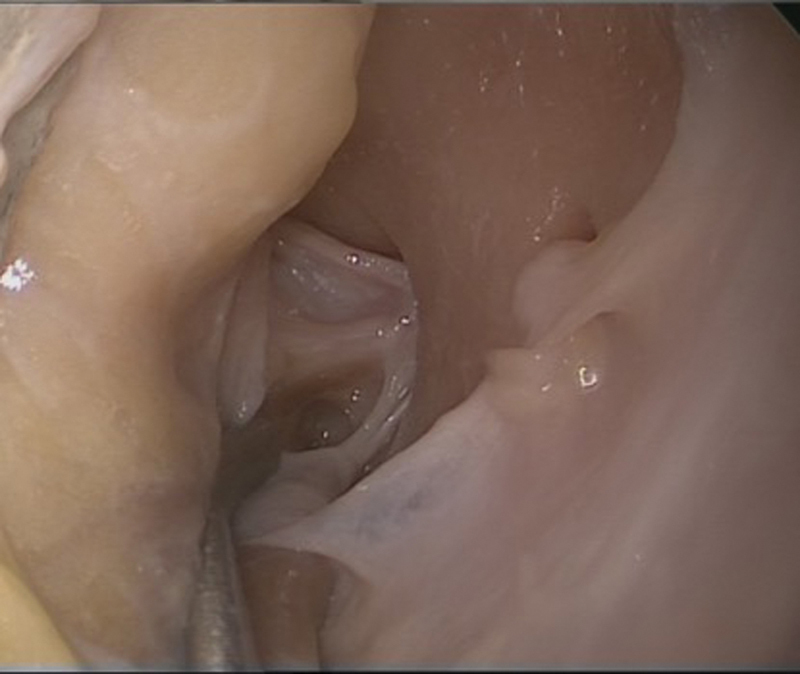
The SOF is 1 cm posterior to the recurrent branch of the middle meningeal artery (left eye in cadaveric specimen). SOF, superior orbital fissure.
Fig. 9.
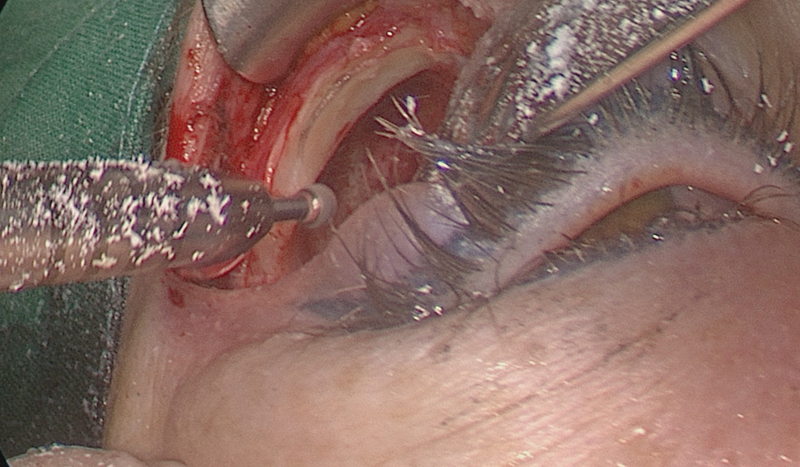
Lacrimal keyhole area is drilled away once the lacrimal gland is dissected out of its fossa.
Fig. 10.
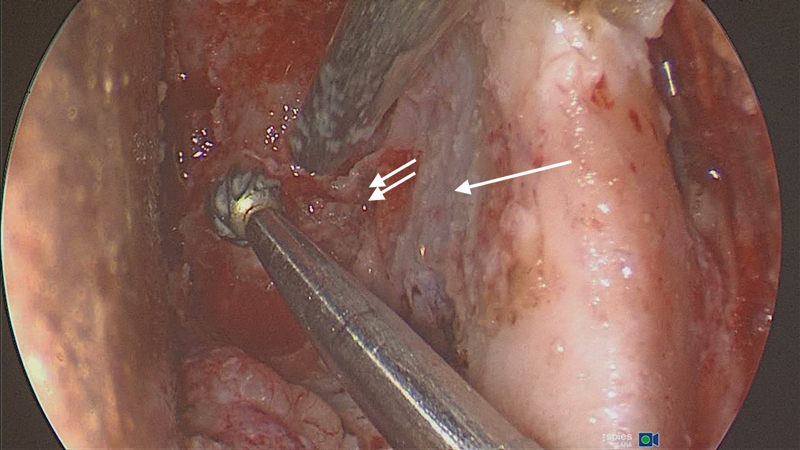
The lateral orbital corridor: thick area of hyperostotic bone requiring removal to expose the temporalis muscle anterolaterally (arrow), the middle cranial fossa posteriolaterally (double arrow) and the anterior cranial fossa dura superiorly (Left eye).
Currently, there is a lack of published data in the English literature regarding the level of reduction in proptosis, visual improvement and gross-total resection achieved in patients that have undergone the above procedure for sphenoid wing meningiomas. 16
This study retrospectively determined the extent of reduction in proptosis, as well as the improvement in visual outcomes in patients routinely undergoing a minimally invasive endoscopic transorbital approach for the treatment of sphenoid wing meningiomas. Most of the patients underwent initial multiportal surgery in an attempt to salvage vision and reduce proptosis while awaiting a craniotomy as definitive surgery for resection of the intracranial component.
Methods
Ethics approval was obtained from the Human Ethic Committee, University of Cape Town (HREC 345/2017). A retrospective folder review was conducted in 21 patients who had undergone transorbital endoscopic resection for sphenoid wing meningioma at Groote Schuur Hospital and Cape Town Mediclinic from 2015 to 2019. All patients were operated on by the same multidisciplinary surgical team which included otolaryngology, ophthalmology, and neurosurgery. All patients had preoperative CT and MRI scans. The procedure included a combined endonasal and lateral orbitotomy using a superior eyelid approach.
The following data were collected: age, sex, presenting symptoms, comorbidities, history of previous radiation, and craniotomy. Visual acuity, using a Snellen's chart, and proptosis measurements, using a Hertel exophthalmometer, were recorded by ophthalmology and were recorded preoperatively, at 6 weeks, 6 months, and 1 year postoperatively. Patients were categorized according to World Health Organization (WHO) classification of vision into “blind,” “low vision,” and “normal vision.” Tumor specimens were histopathologically analyzed.
Statistical Analysis
Nonparametric test analysis (performed in R) were used. The Wilcoxon's signed-rank test was employed to evaluate visual acuity (Snellen's fractions converted to LogMAR values) and proptosis in millimeter. Preoperative values were compared with values obtained at 6 weeks, 6 months, and 1 year postoperatively. Patients who were in the blind category preoperatively (group A) were excluded from analysis. Only patients who were in the category of low or normal vision (groups B and C) were included in the analysis. A p -value of < 0.05 was considered to be statistically significant.
Results
Patient and Clinical Data
A total of 21 patients had undergone transorbital endoscopic tumor resection along with medial orbital decompression for sphenoid wing meningioma. A subtotal resection was performed in all cases (Simpson's grades II–IV). The mean age at initial presentation was 48.8 years (range: 34–79 years). Of them, 20 (95%) patients were female and 1 (5%) was male. Of the 21 patients, 6 (28%) had hypertension, 4 (19%) had type-2 diabetes mellitus, and 3 (14%) had asthma.
The most common presenting symptom was decreased visual acuity reported by 21 patients (100%), followed by proptosis and headache, that is, 20 (95%) and 16 (76%) patients, respectively (see Table 1 for summary of symptoms). The mean duration of symptoms prior to presentation was 9 months (range: 3–24 months). In the 12 (57%) patients, tumors were located on the left side and in 9 (43%) patients, they occurred on the right. In the cohort of 21 patients, 3 (14%) had had a previous craniotomy which included pterional and lateral orbitotomy approaches and 2 (10%) had received previous radiation therapy. All patients had pre-and postoperative CT scans. All patients had lateral en plaque sphenoid wing meningiomas, with evidence of hyperostosis. CT scan identified intracranial extension in 6 (29%), intraorbital extension in 11 (52%), and diffuse meningiomatosis in 1 (5%). One resection was complicated by superior orbital fissure syndrome postoperatively that resolved spontaneously. This patient had had a previous craniotomy and radiation therapy. There was one mortality unrelated to the surgery. There were no other major surgical complications and the mean hospital stay was 2.7 (range: 2–4) days.
Table 1. Presenting symptoms.
| Symptom | n (%) |
|---|---|
| Visual impairment | 21 (100) |
| Proptosis | 20 (95) |
| Headache | 16 (76) |
| Facial Pain | 15 (71) |
| Diplopia | 3 (14) |
| Ptosis | 1 (5) |
| Blocked nose | 1 (5) |
| Epiphora | 1 (5) |
Visual Acuity
Improvement in visual acuity was defined as stabilization of vision or a single line increase on the Snellen chart. The average line improvement of vision was 1.5 at 6 weeks, 0.8 at 6 months, and 1.4 at 1 year postoperatively ( Table 2 ). Patients were placed into categories according to the WHO classification for vision, which was determined by their preoperative visual acuity ( Tables 2 and 3 ). In group A (blind), 6 (60%) patients remained unchanged, 1 (10%) improved, and 2 (20%) had further decline in vision by 1 year postoperatively. In group B (low vision), 3 (75%) had improvement in vision and 1 (25%) remained stable. In group C, 5 (71%) patients had improvement in vision and 2 (29%) remained stable. No patients in groups B and C experienced deterioration in vision.
Table 2. Visual acuity fractions according to Snellen's chart preoperatively, and 6 weeks, 6 months, and at 1 year postoperatively.
| Patient | VA Pre op |
VA 6-week post op |
VA 6-month post op |
VA 1 year post op |
Proptosis (mm) Pre op |
Proptosis (mm) 6-week post op |
Proptosis (mm) 6-month post op |
Proptosis (mm) 1 year post op |
|---|---|---|---|---|---|---|---|---|
| 1 | NLP | NLP | NLP | NLP | 28 | 28 | 26 | 28 |
| 2 | CF | 6/36 | NLP | NLP | 22 | 19 | 19 | 26 |
| 3 | HM | 6/9 | HM | NLP | 24 | 22 | 22 | 24 |
| 4 | 6/6 | 6/5 | 6/5 | 6/5 | 24 | 22 | 23 | 24 |
| 5 | NLP | NLP | NLP | NLP | 25 | 21 | 21 | 27 |
| 6 | CF | 6/12 | 6/12 | 6/12 | 23 | 21 | 21 | 21 |
| 7 | 6/12 | 6/9 | 6/9 | 6/6 | 24 | 23 | 20 | 20 |
| 8 | 6/18 | 6/18 | 6/18 | 6/18 | 18 | 18 | 18 | 18 |
| 9 | NLP | NLP | NLP | D | 24 | 21 | 21 | D |
| 10 | 6/12 | 6/12 | 6/12 | 6/12 | 23 | 20 | 21 | 23 |
| 11 | 6/12 | 6/9 | 6/9 | 6/6 | 22 | 19 | 18 | 22 |
| 12 | NLP | NLP | NLP | NLP | 29 | 27 | 18 | 29 |
| 13 | 6/12 | 6/9 | 6/9 | 6/9 | 24 | 22 | 22 | 23 |
| 14 | 6/18 | 6/18 | 6/18 | 6/5 | 21 | 20 | 20 | 25 |
| 15 | 6/36 | 6/24 | 6/18 | 6/18 | 22 | 20 | 19 | 20 |
| 16 | NLP | NLP | NLP | NLP | 28 | 26 | 26 | 28 |
| 17 | HM | HM | HM | HM | 24 | 20 | 19 | 22 |
| 18 | 6/9 | 6/6 | 6/6 | 6/6 | 22 | 19 | 20 | 22 |
| 19 | CF | CF | CF | CF | 25 | 21 | 21 | 23 |
| 20 | 6/60 | 6/24 | 6/24 | 6/24 | 28 | 22 | 23 | 28 |
| 21 | 6/12 | 6/12 | 6/12 | 6/12 | 21 | 18 | 27 | 22 |
Abbreviations: CF, counting fingers; HM, hand movements; NLP, no light perception; post op, postoperative; pre op, preoperative; VA, visual acuity.
Note: proptosis (mm) measured by Hertel exophthalmometer preoperatively, and 6 weeks, 6–8 months, and 1 year post-operatively.
Table 3. Category of VA preoperative and number of patients in which vision had either remained unchanged or stabilized, improved or deteriorated by 1 year postoperatively.
| Category | VA | Pre op | Number of patients | |
|---|---|---|---|---|
| A (blind) | NLP/HM/CF | 10 (48%) | Unchanged/stabilized | 6 (60%) |
| Deteriorated | 2 (20%) | |||
| Improved | 1 (10%) | |||
| B (low vision) | 6/18–6/60 | 4 (19%) | Unchanged/stabilized | 1 (25%) |
| Deteriorated | 0 | |||
| Improved | 3 (75%) | |||
| C (normal vision) | 6/6–6/12 | 7 (33%) | Unchanged/stabilized | 2 (29%) |
| Deteriorated | 0 | |||
| Improved | 5 (71%) | |||
Abbreviations: CF, counting fingers; HM, hand movements; NLP, no light perception; pre op, preoperative; VA, visual acuity.
Proptosis
Twenty (95%) patients presented with proptosis. The mean proptosis preoperatively was 23.8 (range: 18–28) mm. The mean proptosis at 6 weeks, 6 months, and 1 year was 21.3, 21.1, and 23.8 mm, respectively. Even though the average improvement in proptosis was 2.4 mm at 6 weeks, proptosis trended toward preoperative values at 1 year.
Statistical Analysis
The nonparametric data model shows a statistically significant improvement in visual acuity at 6 weeks (95% confidence interval [CI]: p = 0.021). This trend extends to long-term follow-up at 6 months (95% CI; p = 0.021), and 1 year (95% CI; p = 0.0054) postoperatively. Proptosis was shown to decrease initially proving statistical significance at 6 weeks (95% CI; p = 0.0054) postoperatively. The decrease at 6 months ( p = 0.08) was not statistically significant and trended toward an increase in proptosis by 1 year ( p = 0.78) postoperatively.
Histology
Nineteen tumors (95%) were WHO grade I and 1 (5%) was WHO grade II. Histological examination of hyperostotic bone showed tumor infiltration in 14 (70%) patients. Biopsies of periorbita were positive for meningioma in 6 (28%) patients.
Discussion
Sphenoid wing meningiomas are complex and challenging to manage. Traditional open neurosurgical approaches have aimed for gross-total resection and is associated with significant morbidity, mostly related to traction of the brain and surgical manipulation, 23 and increases the risk of new-onset cranial nerve palsy, stroke, and mortality. 15 The open approach may inadequately address the primary symptoms of proptosis, decreased visual acuity, and pain. Thus a move to subtotal tumor resection has evolved due to the benign nature of the disease and the risk of damage to underlying vital neurovascular structures. 11
The advent of multiportal transorbital pathways introduced by Moe et al has permitted a minimally invasive approach to resect sphenoid wing meningiomas. 24 A superior eyelid approach utilizing the lateral corridor to resect the lateral hyperostotic bone has been reported. 19 20
Among the patients in this study, the goal of surgery was to address the presenting symptoms, namely, loss of vison and proptosis, while they were awaiting definitive neurosurgical management. In all cases, a subtotal resection was performed (Simpson's grades II–IV). Complete microscopic surgical clearance was not possible due to extensive dural involvement, and in most cases, the dura was cauterized with bipolar.
It is well documented that meningiomas occur more commonly in females compared with males. In our study, 95% of patients were female between the ages of 34 and 79 years. This correlates with other studies showing a similar demographic and reports that >90% of patients with sphenoorbital menigioma are female. 4 9 10
In the current study, the most common presenting symptom was decline in vision (100%) followed by proptosis (95%). This differs from other studies that report a higher percentage of proptosis as the most common symptom and visual impairment ranging between 40 and 60% in lateral sphenoid wing tumors. 6 8 12 Dallan et al reported proptosis to be the most common presenting symptom (100% of their cases), while 42.8% presented with visual impairment. 16 Our findings could be explained by the delayed presentation, extended duration of symptoms, extensive disease, and large number (52%) of patients with intraorbital tumor extension.
There was a statically significant improvement in visual acuity in groups B and C at all postoperative time intervals, that is, 6 weeks, 6 months, and 1 year (95% CI; p = 0.0054). If patients were category A or blind preoperatively, it was less likely that their vision would improve and their long-term outcomes were poor. Two category-A patients had further deterioration in vision, one had diffuse meningiomatosis, and the other WHO type-II meningioma, which are both aggressive forms of the disease. It is possible that subsequent tumor growth had occurred postresection which resulted in further decline in vision. In addition, patients in this group were older, had a longer duration of symptoms and evidence of optic nerve atrophy compared with groups B and C. One patient had improved vision postoperatively despite presenting with blindness. This patient was young (36 years), had a short duration of symptoms (3 months), and had no evidence of optic atrophy. These factors may have played a role in the ability of the optic nerve to recover from a compressive optic neuropathy following decompression.
In addition, it was noted in our study population that the poorer the preoperative vision, the less likely vision would improve in the long term (group A). The trend showed that the better the preoperative vision, the more favorable the long-term vision outcomes, with most patients showing an improvement in visual acuity in group C. Additionally, some patients showed a further improvement in vision between 6 weeks and 1 year that would suggest a long-term recovery in optic nerve function. Vision was also shown to stabilize in groups B and C with no further decline at 1 year. These findings were consistent with the literature.
In a systematic review and meta-analysis of visual outcomes following optic nerve decompression for chronic compressive optic neuropathy, it was reported that at least 60% of patients will show some improvement in vision. The factors that were statistically significant in predicting improvement were clinical factors, that is, absence of optic disc atrophy and small tumor size. Positive surgical factors included primary resection, a soft tumor consistency, a clear dissection plane, absence of tumor in the cavernous sinus, and a complete tumor excision. 25 Age and gender were not shown to have predictive value in determining visual outcomes. The duration of symptoms was shown to play a role in visual outcomes, that is, the longer the duration of symptoms, the greater the chance of poor postoperative vision. Finally, preoperative vision status was noted to be of value to predict outcomes, that is, the worse the preoperative vision, the less likely vision will improve postoperatively. However, if vision is on the poorer side preoperatively, there is a small chance of improvement. 25
Shrivastava et al reported an improvement in visual acuity in only 28% and vision remained unchanged in 72% of cases when employing optic nerve decompression for sphenoid wing meningioma using an open surgical approach. 18 On review of the literature, there is a wide range of improvement in visual outcomes in patients with sphenoid wing meningioma using an open craniotomy approach, that is, 28 to 70 %. 9 26 27 28 29 Mariniello et al reported that vision improved in 50% of cases, remained the same in 36%, and deteriorated in 14% of cases in 36 patients undergoing open optic nerve decompression via a supraorbital–pterional approach. Outcomes were thought to be predicted by extent of optic canal invasion, tumor grade and Simpson's grade of tumor resection. The intraorbital portion of the tumor is thought to be a less significant factor in visual outcomes. They therefore advocated for early optic nerve decompression. 12
There is less published data on visual outcomes using a transorbital endoscopic approach for sphenoid wing meningioma. Our surgical unit previously reported an average line increase of 2.7 in the Snellen chart and an average reduction in proptosis of 3.5 mm at 6 weeks postoperatively. 20 Almeida et al reported two cases using a endoscopic transorbital technique via superior eyelid approach for sphenoid wing meningioma and showed an improvement in visual acuity and proptosis at 1 month postoperatively. 30 Dallan et al reported 14 cases of sphenoorbital meningioma treated via transorbital endoscopic and superior eyelid approach and demonstrated that the approach was feasible and safe. In three (21.4%) patients, complete tumor resection was achieved. The tumor volume removed as measured by CT scan ranged between 55 and 100%. 16
Proptosis is caused by tumor infiltration of bone resulting in a hyperostotic reaction of the sphenoid wing. Hyperostosis displaces the globe from medially and by mass effect may stretch the optic nerve and has the potential to compromise vision. 20 27 Our results for proptosis were promising in the initial postoperative period with an average reduction of 2 mm which is statistically significant (95% CI; p = 0.0054). However, it was noted that proptosis recurred by 1 year. This may be due to several factors, that is, subtotal tumor resection, growth of residual tumor, and intraconal tumor involving the periorbita. Open craniotomy or lateral orbitotomy approaches have been shown to reduce proptosis. Scarone et al studied 30 patients with sphenoid wing meningiomas who had undergone open craniotomy to remove hyperostotic bone. In their series, proptosis improved in the short term (average 1 year) but in the long term (61 months), up to 50% of patients showed a worsening of proptosis despite Simpson's grade I and II resections. 31 Little has been published on the reduction in proptosis using the transorbital approach. Research performed by our surgical unit demonstrated a reduction in proptosis in the early postoperative period of 3.5 mm on average. 19
Conclusion
This is the first report of long-term outcomes of vision and proptosis using a transorbital endoscopic approach for the treatment of lateral sphenoid wing meningioma. Our results show that medial optic nerve decompression dramatically improves vision in patients who present with low-to-normal vision with minimal morbidity. This benefit is maintained for at least 1 year postoperatively. Patients with evidence of optic nerve atrophy and who are blind preoperatively did not benefit from optic nerve decompression. The earlier one performs optic nerve decompression the more favorable the long term vision outcomes. Proptosis was shown to improve postoperatively but recurred with time, returning to preoperative measurements by 1 year if a subtotal resection was performed. Of interest, vision remained stable or further improved despite an increase in proptosis over time. This supports the indication of optic nerve decompression as it buys time while awaiting definitive craniotomy or a more extensive resection using the transorbital route.
These results are valuable in selecting patients who would benefit from surgery and for preoperative counselling.
Based on our results, we therefore recommend early medial optic nerve decompression to preserve or improve vision. It is however clear that these complex tumors require multidisciplinary teams for effective management, as they may require multimodal treatment, that is, medial optic nerve decompression to improve vision, craniotomy to address lateral hyperostosis, and postoperative radiation for residual disease.
Limitations and Future Research
We acknowledge that the retrospective nature and small sample size are limiting factors. These are however rare tumors, and this represents the largest sample of patients reported in the English literature. Future research is required to determine the role of progesterone in sphenoid wing meningioma. As technology and expertise advance, more likely total resection will become possible via a transorbital approach. A prospective trial comparing craniotomy and transorbital approach is needed.
Acknowledgments
Prof Allan Taylor, Department of Neurosurgery, Groote Schuur Hospital, Cape Town, South Africa.
Dr. Hamzah Mustak, Division of Ophthalmology, Groote Schuur Hospital, Cape Town, South Africa.
Mark Webb and Duncan Brett for Statistical analysis, Research LampPost, Cape Town, South Africa.
Footnotes
Conflict of Interest None declared.
Pearls and Tips.
Early medial optic nerve decompression improves visual outcomes and prevents visual deterioration.
-
For tumors with a large intracranial component, a staged procedure may be indicated:
Firstly a medial optic nerve decompression and resection of the sphenoid sinus component +/− lateral transorbital approach for the orbital component.
Secondly, a craniotomy for resection of the intracranial component.
The orbital component must be addressed to reduce proptosis permanently.
Multiportal transorbital surgery has advantages in the management of SWM - Decreased morbidity, decreased hospital stay & access to the sphenoid sinus component of the tumor.
References
- 1.Inamura A, Ideguchi M, Sadahiro H, Suzuki M. Sphenoid ridge meningioma with increased intracranial pressure caused by venous congestion. Acta Neurochir (Wien) 2012;154(10):1945–1946. doi: 10.1007/s00701-012-1477-z. [DOI] [PubMed] [Google Scholar]
- 2.Cushing H, Eisenhardt L. Meningiomas: Their Classification, Regional Behavior, Life History, and Surgical End Results. Springfield, IL: Charles C Thomas, 1938 [Google Scholar]
- 3.Luetjens G, Krauss J K, Brandis A, Nakamura M. Bilateral sphenoorbital hyperostotic meningiomas with proptosis and visual impairment: a therapeutic challenge. Report of three patients and review of the literature. Clin Neurol Neurosurg. 2011;113(10):859–863. doi: 10.1016/j.clineuro.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Honig S, Trantakis C, Frerich B, Sterker I, Schober R, Meixensberger J. Spheno-orbital meningiomas: outcome after microsurgical treatment: a clinical review of 30 cases. Neurol Res. 2010;32(03):314–325. doi: 10.1179/016164109X12464612122614. [DOI] [PubMed] [Google Scholar]
- 5.Fohanno D, Bitar A. Sphenoidal ridge meningioma. Adv Tech Stand Neurosurg. 1986;14:137–174. doi: 10.1007/978-3-7091-6995-7_4. [DOI] [PubMed] [Google Scholar]
- 6.Boari N, Gagliardi F, Spina A, Bailo M, Franzin A, Mortini P. Management of spheno-orbital en plaque meningiomas: clinical outcome in a consecutive series of 40 patients. Br J Neurosurg. 2013;27(01):84–90. doi: 10.3109/02688697.2012.709557. [DOI] [PubMed] [Google Scholar]
- 7.De Rosa A, Pineda J, Cavallo L M. Endoscopic endo- and extra-orbital corridors for spheno-orbital region: anatomic study with illustrative case. Acta Neurochir (Wien) 2019;161(08):1633–1646. doi: 10.1007/s00701-019-03939-9. [DOI] [PubMed] [Google Scholar]
- 8.Saeed P, van Furth W R, Tanck M. Natural history of spheno-orbital meningiomas. Acta Neurochir (Wien) 2011;153(02):395–402. doi: 10.1007/s00701-010-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107(05):905–912. doi: 10.3171/JNS-07/11/0905. [DOI] [PubMed] [Google Scholar]
- 10.Rogers L, Barani I, Chamberlain M. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(01):4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaichana K L, Jackson C, Patel A. Predictors of visual outcome following surgical resection of medial sphenoid wing meningiomas. J Neurol Surg B Skull Base. 2012;73(05):321–326. doi: 10.1055/s-0032-1321510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariniello G, Bonavolontà G, Tranfa F, Maiuri F. Management of the optic canal invasion and visual outcome in spheno-orbital meningiomas. Clin Neurol Neurosurg. 2013;115(09):1615–1620. doi: 10.1016/j.clineuro.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Hsu D W, Efird J T, Hedley-Whyte E T. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86(01):113–120. doi: 10.3171/jns.1997.86.1.0113. [DOI] [PubMed] [Google Scholar]
- 14.Cossu G, Levivier M, Daniel R T, Messerer M. The role of mifepristone in meningiomas management: a systematic review of the literature. BioMed Res Int. 2015;2015:267831. doi: 10.1155/2015/267831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Champagne P O, Lemoine E, Bojanowski M W. Surgical management of giant sphenoid wing meningiomas encasing major cerebral arteries. Neurosurg Focus. 2018;44(04):E12. doi: 10.3171/2018.1.FOCUS17718. [DOI] [PubMed] [Google Scholar]
- 16.Dallan I, Sellari-Franceschini S, Turri-Zanoni M. Endoscopic transorbital superior eyelid approach for the management of selected spheno-orbital meningiomas: preliminary experience. Oper Neurosurg (Hagerstown) 2018;14(03):243–251. doi: 10.1093/ons/opx100. [DOI] [PubMed] [Google Scholar]
- 17.Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg. 2012;117(01):121–128. doi: 10.3171/2012.3.JNS111945. [DOI] [PubMed] [Google Scholar]
- 18.Shrivastava R K, Sen C, Costantino P D, Della Rocca R. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103(03):491–497. doi: 10.3171/jns.2005.103.3.0491. [DOI] [PubMed] [Google Scholar]
- 19.Lubbe D, Seayaroyh K, Goncalves N, Fagan J. Transorbital Endoscopic Surgery Current Otorhinolaryngological Reports. 2019;7(02):173–180. [Google Scholar]
- 20.Lubbe D, Mustak H, Taylor A, Fagan J. Minimally invasive endo-orbital approach to sphenoid wing meningiomas improves visual outcomes - our experience with the first seven cases. Clin Otolaryngol. 2017;42(04):876–880. doi: 10.1111/coa.12722. [DOI] [PubMed] [Google Scholar]
- 21.Stamm A. Chapter 18: Optic nerve decompression. In: Transorbital Endoscopic Skull Base & Brain Surgery. 2nd ed. Stuttgart, Germany: Thieme Publishers; 2019 [Google Scholar]
- 22.Bleier BS. Chapter 16: Transorbital approaches to the sinuses, skull base & intracranial space. In: Endoscopic Surgery of the Orbit-Anatomy, Pathology & Management. Stuttgart, Germany: Thieme Publishers; 2019 [Google Scholar]
- 23.Simas N M, Farias J P. Sphenoid Wing en plaque meningiomas: Surgical results and recurrence rates. Surg Neurol Int. 2013;4:86. doi: 10.4103/2152-7806.114796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moe K S, Bergeron C M, Ellenbogen R G.Transorbital neuroendoscopic surgery Neurosurgery 201067(3 suppl operative):ons16–28. [DOI] [PubMed] [Google Scholar]
- 25.Carlson A P, Stippler M, Myers O. Predictive factors for vision recovery after optic nerve decompression for chronic compressive neuropathy: systematic review and meta-analysis. J Neurol Surg B Skull Base. 2013;74(01):20–38. doi: 10.1055/s-0032-1329624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schick U, Bleyen J, Bani A, Hassler W. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006;104(02):208–214. doi: 10.3171/jns.2006.104.2.208. [DOI] [PubMed] [Google Scholar]
- 27.Ringel F, Cedzich C, Schramm J.Microsurgical technique and results of a series of 63 spheno-orbital meningiomas Neurosurgery 2007600402214–221., discussion 221–222 [DOI] [PubMed] [Google Scholar]
- 28.Sandalcioglu I E, Gasser T, Mohr C, Stolke D, Wiedemayer H. Spheno-orbital meningiomas: interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005;33(04):260–266. doi: 10.1016/j.jcms.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Cannon P S, Rutherford S A, Richardson P L, King A, Leatherbarrow B. The surgical management and outcomes for spheno-orbital meningiomas: a 7-year review of multi-disciplinary practice. Orbit. 2009;28(06):371–376. doi: 10.3109/01676830903104645. [DOI] [PubMed] [Google Scholar]
- 30.Almeida J P, Omay S B, Shetty S R. Transorbital endoscopic eyelid approach for resection of sphenoorbital meningiomas with predominant hyperostosis: report of 2 cases. J Neurosurg. 2018;128(06):1885–1895. doi: 10.3171/2017.3.JNS163110. [DOI] [PubMed] [Google Scholar]
- 31.Scarone P, Leclerq D, Héran F, Robert G. Long-term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. Clinical article. J Neurosurg. 2009;111(05):1069–1077. doi: 10.3171/2009.1.JNS081263. [DOI] [PubMed] [Google Scholar]


