Abstract
Endoscopic surgery of the orbit, periorbital region, and adjacent areas of the anterior and middle cranial fossae and brain has gained significant popularity over the last decade. These procedures are now being used at multiple institutions internationally with a success and safety record that has been demonstrated to be at par with or better than other techniques. The approaches provide minimally disruptive, scarless access to regions that previously required extensive open operations with significant retraction of critical neurovascular structures leading to prolonged morbidity and hospitalization.
This paper will describe the basic techniques of these approaches, how they can be used alone or in multiportal (para- and contraportal) technique and guide the reader to resources for further learning.
Keywords: orbit, skull base, endoscopic orbital, transorbital, neuroendoscopic, contraportal, paraportal, multiportal
Introduction
Endoscopic surgery of the orbit, periorbital region and adjacent skull base has undergone a period of exciting clinical development and growth in popularity over the last decade. Procedures that once required large open-field approaches are now being done endoscopically with incisions as small as 1 cm, with minimal morbidity and no visible scars ( Fig. 1 ). Through the use of these minimally disruptive, co-planar approaches we are now able to reach structures deep within the orbit and brain with minimal retraction of neurovascular structures, enabling patients to achieve a rapid return to their accustomed lives with accelerated recovery and reduced health care expenses.
Fig. 1.
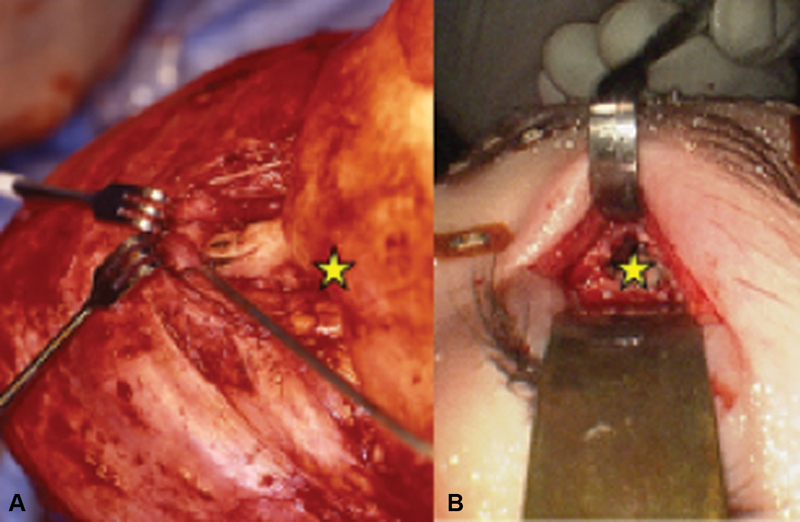
Craniofacial approach to the ACF ( A ) compared with superior transorbital endoscopic approach ( B ). Star indicates anatomical region anterior to the left frontal lobe of the brain. Note the difference in exposure and tissue retraction between the approaches. ACF, anterior cranial fossa.
Our interest in endoscopic transorbital skull base surgery arose in an attempt to overcome some of the challenges of transnasal endoscopic approaches. Among these are (1) only 20% of the anterior cranial fossa (ACF) is comprised of the apex of the sinonasal cavity (the “interorbital skull base”), while 80% of the ACF consists of the orbital roof 1 ; (2) the funnel-shaped surgical approach to the interorbital skull base becomes progressively constricted as the target is reached, leading to collisions between the endoscope and instruments, confined working space, and run-down of blood from instruments onto the lens of the endoscope; and (3) the frequent need of angled endoscopy and cross-planar technique to visualize and instrument the surgical target. In addition, transnasal endoscopic approaches necessitate placement of the endoscope and instruments in a similar vector with minimal offset angle. As a result, the instrument frequently obscures visualization of the surgical target. Furthermore, contact of the instrument with the surgical target is inferred by haptic feedback rather than direct visualization, and the parallax effect and three-dimensional viewing is lost ( Fig. 2 ).
Fig. 2.
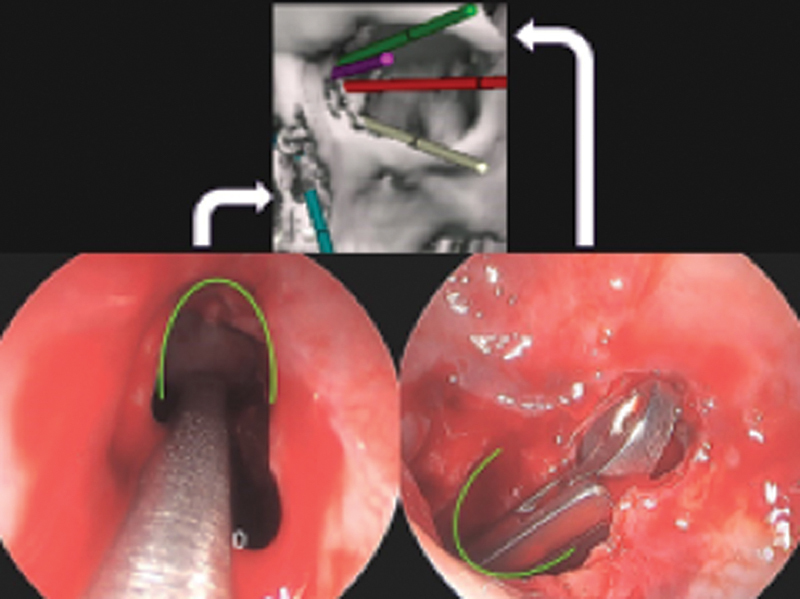
The visualization and instrumentation challenge of monoportal surgery. Top: Several transorbital vectors that can be combined with transnasal viewing and/or instrumentation. Bottom left: monoportal transnasal endoscope and instrument placed into left frontal sinus. Bottom right: View of the same instrument in identical position but with the endoscope placed through a superior orbital approach. Curved line placed in the same position in each photo for orientation. Note the superior lighting and visualization with transorbital endoscopy, and the large vector offset which allows visualization of instrument contacting and manipulating target. White arrows indicate endoscope position in photographs.
Through work on cadavers and eventually clinical research, a system of four scarless endoscopic approaches was created for access to each quadrant of the orbit and the adjacent structures. 1 2 3 We found that these techniques allow excellent surgical access, providing complementary approaches to transnasal portals that surmount some of the drawbacks of transnasal endoscopic procedures. These procedures are highly effective used alone or in multiportal technique with transnasal and other approaches to improve working space, allow coplanar surgery, and allow pseudo-parallax viewing of target manipulation with diminished reliance on haptic feedback through wide offset between the endoscope and instrument(s). 4 5 We found that these approaches allowed access to the entire ACF, including the interorbital skull base, as well as significant components of the middle cranial fossa (MCF). We and others have used these techniques for a full range of adult and pediatric indications including resection of benign and malignant tumors of soft tissue and bone, vascular and lymphatic malformations; management of cerebrospinal fluid (CSF) leaks and tension pneumocephalus; repair of trauma of the orbit, frontal sinus, and frontal bone; management of advanced mucoceles of the orbit, frontal sinus, and ACF; orbital and optic nerve decompression; foreign body resection; and drainage of abscesses of the orbit, frontal sinus, and brain. 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29
Some of the most common approaches used in combination for multiportal procedures are demonstrated in Fig. 3 . For the purposes of discussion, it is helpful to divide the craniofacial region into access zones within which the approaches are created, as demonstrated in Fig. 4 . Zone 1 is the frontal region, including the frontal sinus and posterior to this, the frontal lobes; Zone 2 , the endonasal, ethmoid, and sphenoid sinus regions; Zone 3 , the orbit; Zone 4 , the maxilla; and Zone 5 , the paramaxillary region leading to the infratemporal fossa and pterygomaxillary fissure. Multiple approaches can be created within a single zone such as the orbit, or approaches can be combined through adjacent ( paraportal ) zones with opposite ( contraportal ) zones. For example, to access Zone 1, approaches can be created through Zone 3 alone; through ipsilateral zone 2 combined with paraportal zone 3, or through zone 3 with contraportal zone 3 ( Fig. 5 ). Zone 5 may be used to reach the superior infratemporal fossa alone or in combination with zone 3, adding zone 4 and possibly zone 2 for tumors with extensive maxillary involvement.
Fig. 3.
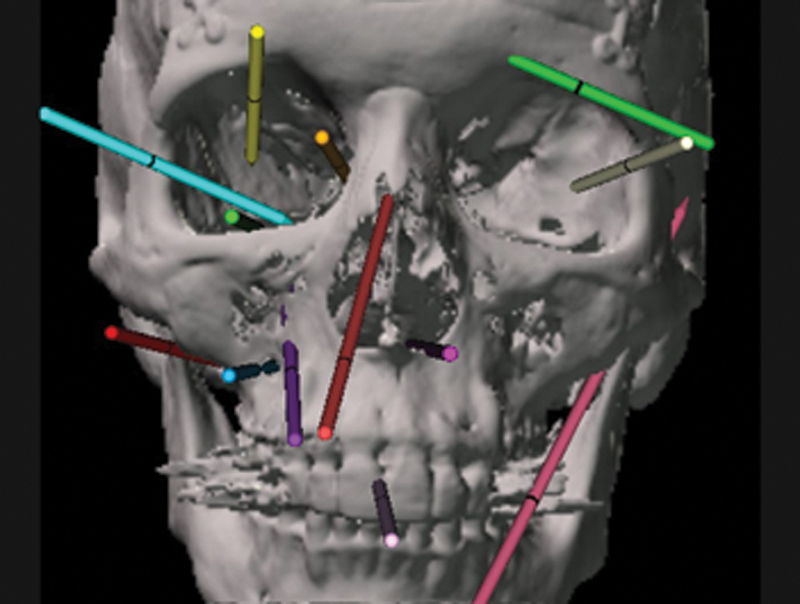
Multiple endoscopic vectors that can be used alone or in multiportal combination. Among the most common combinations in addition to transorbital are the transnasal, transmaxillary, and paramaxillary ( inferior right ) vectors.
Fig. 4.
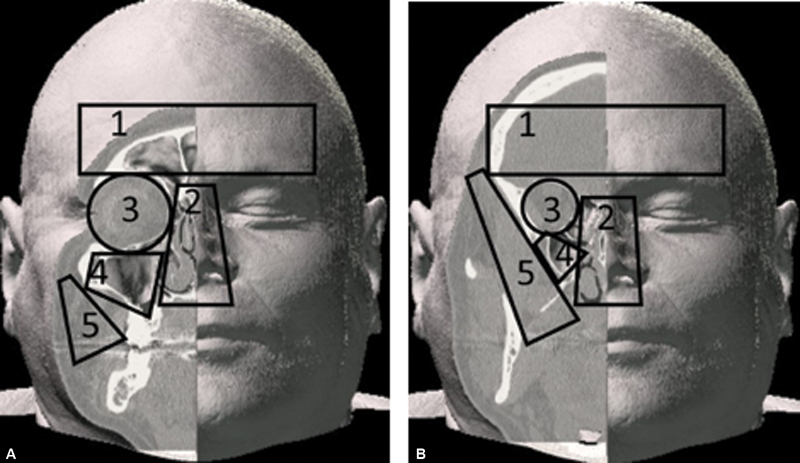
Endoscopic zones frequently combined in multiportal skull base procedures. ( A ) superficial, ( B ) deep. Zone 1, frontal; Zone 2, sinonasal; Zone 3, orbital; Zone 4, maxillary; Zone 5, paramaxillary.
Fig. 5.
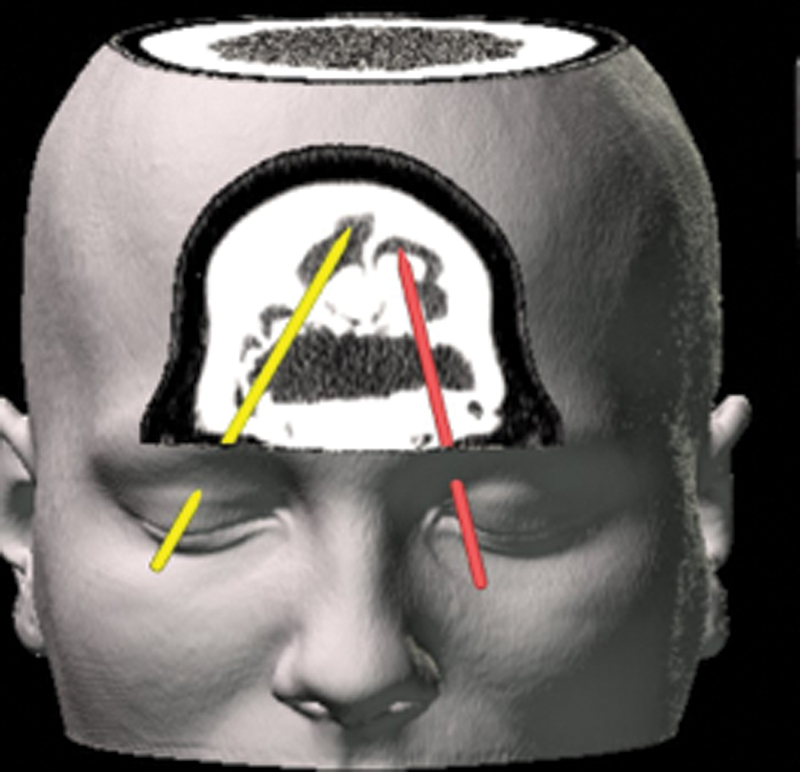
Bilateral superior transorbital approaches demonstrating contraportal access to osseous mass in superior frontal sinus (Zone 1).
Similarly, multiportal techniques can be combined with open procedures such as bifrontal craniotomy in hybrid approaches ( Fig. 6 ). This allows simultaneous contraportal viewing and instrumentation from opposing regions such as microscopic or endoscopic from above (Zone 1) and endoscopic from below (Zone 2 or 3). This technique (open combined with contraportal endoscopic) can provide increased safety and efficacy in challenging procedures such as sellar tumors with very extensive suprasellar extension.
Fig. 6.
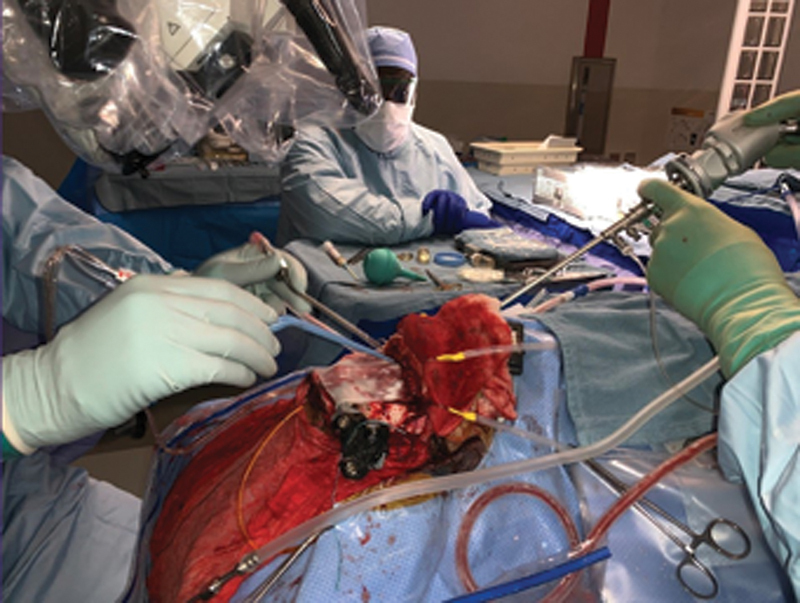
Hybrid technique. An open approach (bifrontal craniotomy) is used concurrently ( left side of image ) with an endoscopic contraportal approach from below ( right side of image ).
Endoscopic orbital and transorbital surgery has progressed to the point that it is now in common use internationally. Locatelli et al recently reported a meta-analysis of the world literature on the subject, and described their experience and techniques. 30 They concluded that “the inclusion of transorbital endoscopic approaches in the surgical armamentarium of the skull base surgeon will become crucial in the future.” Lubbe et al, also reviewed the literature and reported their experience, concluding that “numerous publications show favorable outcomes in the management of orbital, skull base, and intracranial lesions using endoscopic transorbital surgery when compared with traditional surgical approaches. Previously inaccessible or difficult-to-access lesions can now be reached using surgery which often offers superior visualization with minimal collateral tissue trauma.” 6 31 32 33 34 35 36 The following will briefly describe the various transorbital approaches that we commonly use, as well as reconstructive techniques and postoperative care.
Indications
There are over 30 defined endoscopic and open surgical approaches to reach skull base targets. 37 Extensive research has focused on determining which approach—or combination of approaches—are optimal for an individual patient. In many instances, however, the current standard relies on an individual surgeon's assessment of the cross-sectional imaging. Many factors are reflected in an expert surgeon's determination of the best surgical approach, these critical variables are listed below. The selection of surgical approach(es) to a specific lesion have massive implications on incurred morbidity. The primary objective should be to perform the surgical task while minimizing collateral tissue damage, which often correlates directly to morbidity. 38 Accurately predicting the morbidity risks will ultimately determine the risk/benefit ratio that influences not only surgical approach selection, but also global decision making of treatment modality when discussed in multidisciplinary treatment planning conferences.
Major determinants of surgical approach selection:
Pathology: The diagnosis and treatment goal define the surgical task. A benign neoplasm with a capsule may be removed without wide exposure and visualization. A narrower surgical corridor may be appropriate. In contrast, a malignant lesion that requires surgical margins will require a larger surgical corridor to carry out the surgical task and may require increased visualization. In some instances, only an incisional biopsy is required to determine pathology and treatment plan, reducing the need for surgical access.
Anatomical location: Surgical staging systems standardize the specific location of a lesion so that surgical risks can be assessed. Extension into certain regions have been shown to incur higher morbidity and other treatment modalities of radiation therapy and chemotherapy are employed as a function of stage in many instances. Staging takes into account both the pathology and involved structures. However, a caveat is that staging systems are developed based on the availability of surgical techniques at the time. As new surgical approaches are introduced and demonstrate access to specific regions of the skull base with acceptable morbidity, the stage may no longer be a reliable determinant of treatment modality.
Current state of visualization and instrumentation technology: A critical determinant of surgical access and approach planning is the availability of technology. Clearly, the endoscope is a critical tool, but the instruments that go with it are also important. Our specialty has evolved to use “four handed techniques,” becoming experts at performing complex surgical tasks through long, slender surgical corridors. Instruments that perform more than one function such as microdebriders and ultrasonic bone aspirators enable suction, tissue removal/ablation, and irrigation in one instrument. As endoscopes become smaller with higher resolution and instruments perform more and more functions, the ability to perform surgical tasks at conventionally challenging locations, becomes possible. Multiple skull base robotic platforms have been proposed and are under development. 39
Ideally, each patient and lesion are analyzed independently and systematically. This process takes into account anatomical variability. For example, the degree of aeration of the frontal sinus will determine the utility of a superior transorbital approach to treat a specific lesion, such as frontal-orbital osteoma. The above general principles are applied for the specific locations below for which transorbital surgical approaches are selected to adequately access the lesion and minimize morbidity.
Zone 1 ( Fig. 4 ) includes access to frontal sinuses and intracranial portion immediately posterior to poster table of frontal sinus. Endoscopic access to this region can be obtained through superior orbital approaches as well as transnasal approach. A superior orbit portal provides excellent access to contralateral pathology including fronto-orbital osteoma, mucoceles, and CSF leaks. This requires removing the inter-sinus septum. In our experience, this approach has made it so that osteoplastic approaches are rarely required. It can be combined with a transnasal approach from below or a Perneczky key-hole craniotomy from above. 40
Zone 2 ( Fig. 4 ) includes the ethmoid sinuses, lamina papyracea, fovea ethmoidalis and provides access to the majority of the ACF. Medial orbit portals enhance access to multiple locations within this region, augmenting transnasal visualization by widening viewing angle by 30 degrees. A sellar lesion with lateral extension can be better visualized with the addition of a contralateral medial orbit portal. Anterior and posterior ethmoid arteries can be visualized and managed within the orbit with excellent endoscopic visualization. Optic nerve decompression at the orbital apex can be performed with co-planar instrumentation/visualization, parallel to the optic nerve so that visualization of the critical structure is never obstructed by instruments. 41
Zone 3 ( Fig. 4 ) includes the orbit which can be accessed from all four transorbital approaches for a variety of orbital pathology, permitting access to a large intracranial region along the orbital roof. Zone 4 ( Fig. 4 ) includes the maxillary sinus and accesses the infratemporal fossa. Endoscopic access to this region is direct with an inferior orbital approach, often in concert with a transnasal and/or paramaxillary endoscopic approach. In many instances, particularly for juvenile nasopharyngeal angiofibroma, surgical access to safely divide blood supply to vascular lesions is one of the first steps of extirpation using these endoscopic portals. Zone 5 ( Fig. 4 ) paramaxillary space includes parapharyngeal space and mandibular ramus.
Surgical Technique
Prior to the surgery, informed consent is obtained with a clear discussion of planned approach and expected postoperative recovery including significant periorbital swelling. Once consent is obtained, the procedure is performed under general anesthesia. If goal of procedure is localization of CSF leak, intrathecal fluorescein should be placed at this time to allow for diffusion. The table is then rotated 180 degrees. To aid in relaxation of the frontal lobe from the ACF floor, the head is placed in 15 degrees of retroflexion and the bed is placed in approximately 15 to 30 degrees of reverse Trendelenburg. Local anesthetic is infiltrated into the planned incision site and intranasally, as well.
The navigation system is registered and calibrated. Accuracy should be confirmed prior to prepping and draping. When performing sterile preparation around the eye, ophthalmic betadine should be used rather than regular betadine to avoid irritation.
Transorbital approaches to the orbit and skull base are chosen with the goal of sparing critical structures with minimal function disruption to the eyelids or muscles of extraocular movement. Potential portals are primarily separated by quadrant with four primary options for approach based on target of pathology. These are the precaruncular (PC) or medial approach, preseptal (PS) lower eyelid or inferior approach, superior eyelid crease (SLC), and lateral retrocanthal approach (LRC). The superior (SLC) approach is the only one that is performed through the skin while the other three approaches (PC, PS, and LRC) are performed behind the structures of the eyelid. The PC, PS, and LRC approach are performed posterior to the supporting structures, whereas the SLC incision is superficial to the levator aponeurosis and muscle to prevent disruption of this important eyelid support system. A comprehensive understanding of the anatomy of the eyelid and canthal support system is essential prior to performing transorbital approaches. All approaches work to identify a subperiosteal plane to aid in avascular dissection, retraction of orbital contents, and provide an optical portal for visualization, retraction, and instrumentation.
The four primary approaches are presented below with a discussion on the author's technique. These techniques and caveats discussed offer a safe and effective portal for performing endoscopic transorbital surgery. We acknowledge variations reported in the literature and encourage alterations in technique while maintaining safe access and avoiding structures critical for lid and extraocular muscle movement and support.
Superior Approach: Superior Eyelid Crease
The SLC approach has classically been utilized for reconstruction of orbital roof fractures, pathology of the frontal sinus including orbital mucoceles and posterior frontal wall fractures with CSF leak, and anterior fossa pathology. 16 17 This pathology is in the superior quadrant of the orbit. Anatomical boundaries of this approach are the superior orbital fissure laterally, the orbital rim anteriorly, and the anterior and posterior ethmoid arteries medially.
The incision used for this approach is identical to that used for an upper eyelid blepharoplasty, which is placed in the crease of the upper eyelid. The incision width itself is limited laterally by the medial extent of the lacrimal gland and medially by the trochlea. Dissection following the incision is continued through the orbicularis oris muscle with skin-muscle flap elevation raised superiorly up to the orbital rim. Dissection is then performed in the PS plane to the superior orbital rim then to the superior orbit. The orbital septum and levator aponeurosis may be thin and challenging to identify, thus by keeping dissection just deep to the orbicularis oris muscle a safe approach to the orbital rim can be performed. It is essential to avoid the fat pad deep to the orbital septum to avoid damage to the levator aponeurosis, which can result in postoperative ptosis. Once the orbital rim has been identified, the supraorbital and supratrochlear neurovascular pedicles will be encountered and should be spared.
When the orbital rim is encountered, the periosteum is sharply incised at the inferior most border. Endoscopic guidance is used to aid in dissection posteriorly toward the orbital apex in the subperiosteal plane. Utilizing image guidance and after extensive exposure has been developed, the craniotomy or entrance into the frontal sinus is performed by placing gentle pressure with a periosteal elevator or with the aid of a high-speed drill. Once a small bone defect has been made, the dura or sinus mucosa is elevated and the surrounding bone is cleared either with a Kerrison rongeur, drill, or gentle down pressure. The dura is dissected off of the fragments in the typical fashion.
Fig. 7 demonstrates a potential use for a superior approach to the skull base. The patient was a 93-year-old man who presented with altered mental status. Imaging demonstrated an epidural and frontal lobe abscess. A superior lid crease incision was used to approach the frontal sinus, which contained a fungal ball. After removal, a skull base defect was evident leading to the abscess cavity. Speciation demonstrated aspergillus. The patient tolerated the procedure well and was ultimately discharged home on oral antifungals ( Fig. 8 ).
Fig. 7.
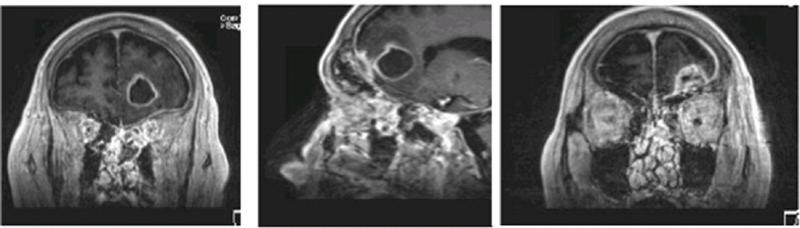
Left epidural and deep frontal lobe abscess. A 93-year-old male presented with mental status changes. MRI above demonstrated left frontal sinus mycetoma, epidural abscess, and deep frontal lobe abscess. Culture grew aspergillus fumigatus. He responded well to transorbital drainage followed by anti-fungal therapy. MRI, magnetic resonance imaging.
Fig. 8.
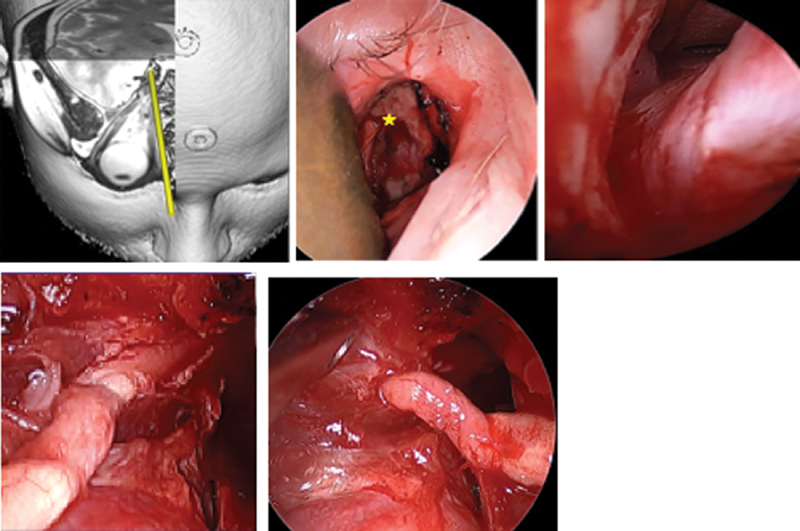
Optic nerve visualized through right medial (precaruncular) approach. Intraoperative appearance, right orbit, and computer planning image demonstrating trajectory. A 67-year-old male with history of choroidal melanoma 9 years prior treated with brachytherapy developed recurrence and underwent enucleation. Pathology showed tumor extending to optic disk, posterior globe, and subarachnoid space. Nodular areas of enhancement along the optic nerve were noted on imaging, so he was referred for endoscopic resection of the optic nerve and postoperative proton beam radiotherapy. The nerve was transected at the distal end of its intracranial course. Pathology demonstrated no further melanoma, and the patient was able to retain the same functional enucleation prosthesis. ( A ) Planning illustration of precaruncular approach. ( B ) Right precaruncular approach, superficial view. A malleable retractor is displacing orbital contents laterally. The anterior ethmoid artery ( star ) marks the frontoethmoid suture, which corresponds to the skull base. ( C ) Right optic nerve at the orbital apex exiting the optic canal. ( D ), Medial view of the optic nerve with the annulus removed and the optic canal decompressed. ( E ) Lateral aspect of the mobilized optic nerve entering the annulus prior to removal.
Medial Approach: Precaruncular
The medial approach is often the most direct option for treatment of ACF pathology and may offer the best angulation for instrumentation. Targets for this approach include pathology that involves the cavernous sinus, cavernous carotid arteries, optic nerve, and other structures of the coronal plane. The access for these structures is via a PC incision. This incision provides a direct, avascular corridor with rapid healing following the procedure. Knowledge of orbital anatomy is essential for identifying the correct plane and avoiding injury to surrounding structures, namely the lacrimal canalicula ( Fig. 9 ).
Fig. 9.
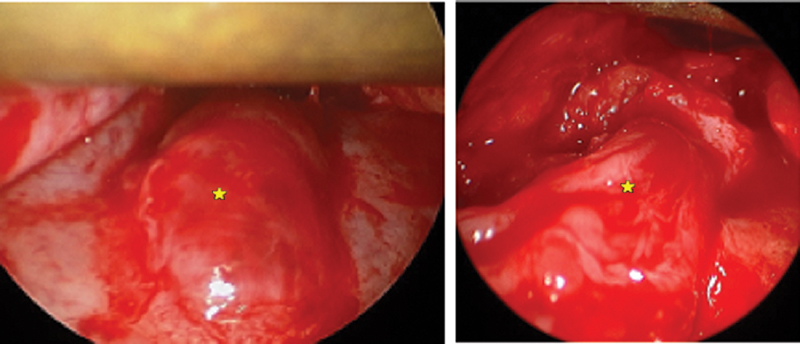
Inferior approach to orbital floor. Left, metastatic tumor in infraorbital nerve (*) causing swelling in canal of anterior floor. Right, nerve (*) lifted out of posterior canal, dissected through infraorbital fissure to foramen rotundum.
The approach begins with a lubricated corneal protector placed over the globe. Lacrimal probes may be placed through the lacrimal puncta to prevent inadvertent transection, as well as aid in retraction. The incision is made at the junction of the conjunctiva and skin, medial to the caruncle, with fine scissors. Superior and inferior extension is performed by following the posterior limb of the medial canthal tendon to the posterior lacrimal crest. Once the posterior lacrimal crest has been identified, the periorbital is sharply incised. This protects the lacrimal structures and allows for broad elevation of the periorbital off the lamina papyracea. Periosteal elevation is continued with a suction elevator under endoscopic visualization until the anterior ethmoid artery is encountered. Cauterization with bipolar forceps and ligation with scissors is performed of any ethmoid arteries that are encountered. Of note, supernumerary ethmoid arteries may be encountered beyond the traditionally described anterior and posterior vessels ( Fig. 10 ). Subperiosteal dissection continues from floor to roof until the optic nerve is identified. The nerve is located at the most posterior aspect of the medial orbit, in the same plane as the anterior ethmoid arteries.
Fig. 10.
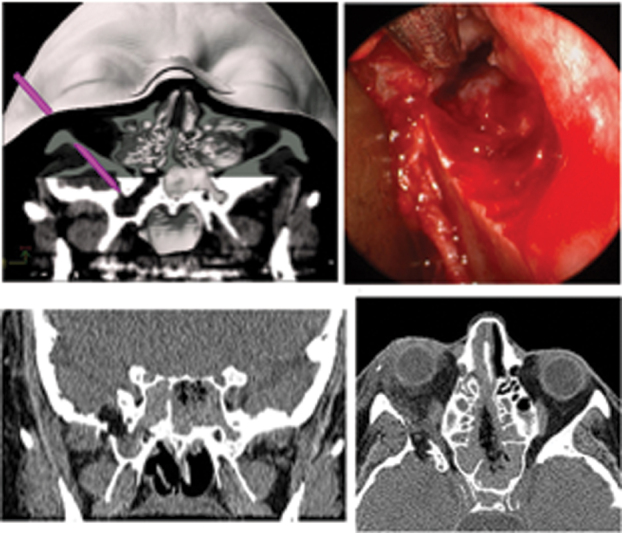
A 59-year-old woman with CSF leak from encephalocele in lateral recess right sphenoid sinus. ( A ) Preoperative plan with lateral retrocanthal approach through greater wing of the sphenoid. ( B ) Intraoperative view of pathway through greater wing of sphenoid, showing encephalocele in lateral sphenoid. ( C, D ) Coronal and axial CT scans showing postoperative appearance. Note path of bone removal and lucency of fat graft. Foramen rotundum is spared with this functional sinus-preserving approach, as seen in coronal image. CSF, cerebrospinal fluid; CT, computed tomography.
Once elevation is complete, a craniotomy can be performed based on the location of pathology. This can be performed through the medial orbital roof, through the skull base in the fovea ethmoidalis and cribriform plate. The lamina papyracea should be taken down at the level of the ethmoid arteries to help with exposure, visualization, and instrumentation, as well as define the level of the skull base. Craniotomy can be made by removing the junction of the orbital roof and fovea ethmoidalis. A vertical strut of bone should be left anterior to the optic nerve for protection if the procedure does not involve dissection of the nerve. Reconstruction of the skull base and orbit can also be performed via this corridor.
When optic nerve decompression or dissection is necessary, the medial aspect of the optic canal can be fractured toward the midline with a thin periosteal elevator taking care to avoid any traction or pressure on the nerve itself. Powered drilling with copious irrigation or an ultrasonic bone aspirator may be necessary if the bone is thick. Decompression is performed along the entire intracanalicular course of the nerve to the optic chiasm. The medial and superior aspect of the bone can be removed, and the intracranial course can be explored as indicated.
There are situations where a contralateral approach is necessary, and the medial approach can provide a direct access corridor to pathology of the lateral wall of the sphenoid sinus/medial wall of the MCF. An ipsilateral approach to these targets can be challenging without heavily angulated endoscopy and instrumentation. The contralateral PC approach is undertaken with the same technique as an ipsilateral approach. For a target within the paranasal sinuses (i.e., below the skull base), the vector from the portal to the target, as well as the extent of pathology present, dictates the amount of the lamina papyracea that needs to be removed. Removal of the posterior ethmoid cells can be performed trans nasally or through the portal as appropriate. The midline is traversed through the perpendicular plate of the ethmoid and sphenoid rostrum. Further dissection through the sphenoid and possible need for craniectomy as indicated can then be performed.
Inferior Approach (Inferior Fornix, Transconjunctival)
Access to the inferior orbit is obtained through an inferior transconjunctival approach with the same technique used for repair of orbital floor fractures. This approach can be extended medially or laterally into a PC or LRC portal, as necessary.
The benefit of the PS ITC approach is based on preservation of the orbital septum, thereby minimizing prolapse of orbital fat into the surgical path. The deep fornix ITC approach has the benefit of preserving a small amount of fat on the lower eyelid and posterior aspect of the orbital septum, which may provide a protective layer to shield the lower eyelid during surgery. Although either approach is effective, the deep fornix incision is recommended for less experienced surgeons.
For a PS approach, an incision is made 2 to 3 mm inferior to the tarsus on the conjunctival surface of the lower eyelid, corresponding to 6 to 8 mm inferior to the eyelid margin). Via this incision, the orbicularis oculi muscle will be visible. Dissection is carried inferiorly between the orbicularis and the septum until the inferior orbital rim is reached. The challenge in this approach lies in the nature of the septum to be quite thin and difficult to recognize. To prevent inadvertent violation, the dissection is performed immediately deep to the orbicularis, and superficial to the orbital fat that is retained behind the septum. For a deep fornix incision, the lower eyelid is retracted anteriorly, and the inferior orbital rim is palpated through the conjunctiva with a periosteal elevator. An incision is made directly through the conjunctival onto the orbital rim. This can be performed with a scalpel or a needle-tip electrocautery on a low setting.
Once the orbital rim has been reached and freed of periosteal attachments, a retained suture can be placed through the edge of the inferior conjunctival flap to retract superiorly over the corneal protector. The subperiosteal plane is dissected posteriorly, lifting the periorbita off the orbital floor. Once an adequate optical corridor has been created, a 0-degree endoscope can be introduced, and further dissection can be performed under endoscopic visualization. The orbital contents are gently retracted superiorly using a malleable brain elevator. The infraorbital nerve is often seen running through a canal in the orbital floor. Any fascial attachments from the nerve to the overlying orbital contents should be sharply divided. Dissection can be performed to the orbital apex with the lateral boundary of the inferior orbital fissure and medially by the lamina papyracea. To develop a path beyond the orbit to the point of the desired craniectomy, orbital bone is removed as indicated by the target vector. The bone can often be removed by gentle down-fracturing if appropriately thin. For areas of thicker bone, an ultrasonic bone aspirator or fine diamond drill can be used, although this requires care to not damage adjacent tissue. Dissection is then continued along the indicated trajectory to the MCF or infratemporal fossa. Craniectomy is performed as indicated. For this, we typically use ultrasonic aspiration, thinning the cranial bone until it is translucent, at which point it can be gently fractured and lifted off the underlying dura. The target is approached either via an intracranial subdural plane, or with intradural dissection according to the operative plan.
Lateral Approach (Lateral Retrocanthal + Optional Canthotomy and Cantholysis)
Access to the lateral orbit, lateral ACF, anterior MCF, and infratemporal fossa can be gained through a lateral corridor via the LRC. The lateral orbitotomy technique described here preserves the bone of the orbital rim, therefore not requiring extended incisions. By performing a lateral canthotomy and cantholysis, no skin incisions are required, and the integrity of the lateral canthus is maintained.
To begin, a lubricated corneal protector can be placed, and the lateral canthus is retracted laterally. An incision is made through the conjunctiva adjacent to the lateral orbital rim. Dissection follows the posterior aspect of the lateral canthal tendon to its insertion on the medial aspect of the lateral orbital wall. The incision can be extended superiorly and then dissection between the bone and periorbita is directed posteriorly. To continue dissection, the lacrimal gland and orbital contents are retracted medially. Periorbital dissection is continued on a broad front posteriorly until the superior and inferior orbital fissures are identified. The optic nerve is located medially to the fissures and so it was not encountered/visualized unless the superior fissure contents are traversed.
The sphenofrontal suture can be visualized at the superior aspect of the lateral orbital wall. For lateral ACF targets, the craniectomy will be created above this line. For MCF targets, the craniectomy is located below the suture line. If the target is within the infratemporal fossa, the thin bone lateral to the sphenozygomatic suture, posterior to the lateral orbital rim is removed. Navigation can then aid in directing dissection, which occurs between the temporalis muscle and underlying bone. Note that this approach does not place the sphenopalatine ganglion or foramen rotundum at risk, which may be a risk with transpterygoid approaches.
Reconstruction
Reconstruction of the orbit is performed based on structural requirements. In general, the orbital roof and lateral orbit are the simplest to reconstruct while the medial wall and floor are structurally integral to the function of the extraocular muscles and prevent herniation of the orbital contents into the ethmoid and maxillary sinuses.
When possible, we prefer to perform preconstruction . This refers to placing the reconstructive material within the orbit before removal of bone or tumor when possible. The implant is shaped, placed in situ, and used for retraction of orbital contents during manipulation, or to protect the orbital contents from inadvertent injury if paraportal technique is used. Preconstruction may not be possible if unfavorable anatomy such as a tumor is present.
Reconstruction is required if preconstruction could not be performed and if required to preserve orbital volume or function of the orbital contents. If the periorbital (orbital periosteum) is intact, herniation of orbital contents does not typically occur. For the orbital roof , the primary concern is to avoid abrupt bone edges over the course of the levator muscle to prevent smooth functioning. In addition, if the floor of the frontal sinus is removed, obstruction of the frontal outflow tract may occur due to herniation of the orbital fat. We thus smoothed all bone edges with a diamond drill or ultrasonic bone aspirator, and then places a layer of 0.25-mm thick polydioxanone sheet (PDS) across the defect. The PDS dissolves, leaving a thin, supple glide layer which prevents adhesion of the adjacent orbital contents. There is common concern about the need to reconstruct the orbital roof to prevent pulsatile exophthalmos. While this may occur for 1 to 2 weeks, we have not had a case in which it has failed to resolve spontaneously.
The lateral orbital wall is reconstructed to prevent dysfunction of the lateral rectus muscle, and enophthalmos if a significant amount of the greater wing of the sphenoid has been removed. In the latter case, we place a small fat graft in the defect. A PDS sheet is used as described above to prevent adhesion of the orbital contents to the temporalis muscle or impedance of muscle excursion by sharp bone edges.
The medial orbital wall and floor are more complex both in decision making and technical performance. As above, if the periorbita is intact, reconstruction is only required if bone edges pose a functional risk. If the periorbita is not intact, there is risk for herniation of orbital contents which may result in enophthalmos, hypoglobus, and muscle dysfunction. Intraoperative Hertel's or Naugle's exophthalmometry can be of great use in comparing values at the beginning and end of a case ( Fig. 11 ). Ideally at the end of a procedure the operated globe will protrude 2 to 3 mm relative to the contralateral side due to edema if the orbit has been preserved or an anatomical reconstruction has been performed. If the globes are symmetric at the end of a significant procedure that has included removal of bone, there will likely be approximately 2 mm of enophthalmos after healing, which is the point at which asymmetry becomes noticeable. If there is measurable enophthalmos at the end of an operation, this is quite likely to be significant after resolution of postoperative edema.
Fig. 11.
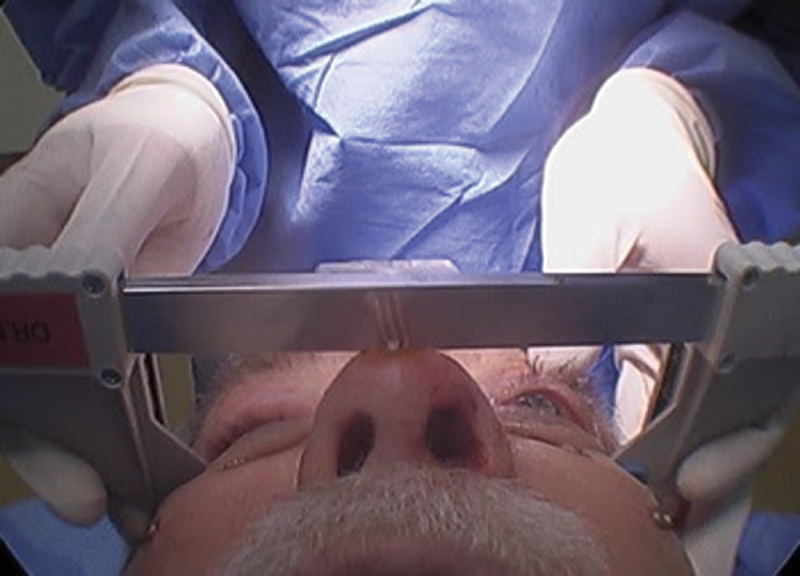
Intraoperative Hertel's exophthalmometry demonstrates globe position and helps decision to reconstruct and adequacy of reconstruction.
In the latter case, reconstruction is indicated. For isolated defects of the medial wall, PDS sheet (0.5 mm for small defects and 0.5 mm for larger repairs) alone is often adequate. The PDS should be placed on the orbital side of the bone in all regions, and endoscopic surveillance should be performed across the entire construct to ensure that the entire defect has been spanned and there is no entrapment of orbital contents between the implant and bone. For orbital floor and combined floor/medial wall defects, we usually use titanium mesh lined with 0.25-mm PDS using mirror-image overlay navigation and endoscopic in situ adjustment as described earlier. 42 We have shown a dramatic improvement in outcomes with this technique over standard methods ( Fig. 12 ). Vascularized flap reconstruction has also been proposed for this type of reconstruction.
Fig. 12.
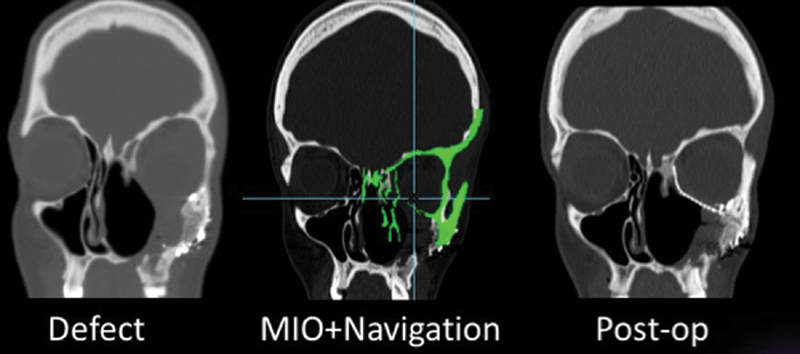
MIO + Navigation for orbit reconstruction. ( A ) Surgical defect after tumor resection. ( B ) Mirror-image overlay of normal, unaffected right side onto left side with defect. The implant is placed and shaped in situ, and navigation instruments are scanned over its surface to check conformity with the template. Any adjustments are made with the implant in place. ( C ) Note symmetry of orbital shape and volume on postoperative imaging.
Pediatric Considerations
Advanced surgical planning as mentioned above is paramount in pediatric patients. We routinely undergo complex analysis with tumor segmentation, virtual endoscopy, and computation of instrument range of motion. Based on this analysis, taking into account general principles of surgical planning (see Indications section above) to maximize visualization of the target location and minimize collateral tissue damage, an approach (or approach combination) is chosen. Instrumentation differs in patients below approximately age 12. Use of a 2.7-mm rigid telescope instead of a 4-mm scope enables more space for instrumentation. An endoscopic irrigation sheath is often not used to create more space alongside the endoscope, making the surgical task more challenging and time consuming as the endoscope needs to be removed if obstructed. Pathology in pediatric patients is more likely to be congenital or benign compared with adults. Reconstructive options are similar and nasoseptal flaps are the main reconstructive option, as in adults. Other local–regional tissue has been used by our group including frontal pericranial and temporoparietal fascia flaps. Both of these options require a partial coronal incision; a pedicled temporoparietal fascia flap can be rotated and passed through the infratemporal fossa into the posterior–lateral aspect of the maxillary sinus, allowing excellent vascularized coverage to the sella, clivus, anterior, or MCF. Postoperative care in pediatric patients is unique and may require sedation to enforce bedrest in very young patients and a planned return to the operating room to manage debridement.
Postoperative Care
Postoperative care is tailored to the procedure performed. This section will describe care specifically for the transorbital portion of the procedure. To minimize orbital edema during the procedure and immediately postoperatively, intraoperative dexamethasone (10 mg every 8 hours) is administered. If the patient is to receive steroids to decrease cerebral edema or aid in treatment of secretory tumors, an equivalent dose of methylprednisolone may be administered as indicated. Following the procedure, crushed ice is applied to the operated eye 20 min/h while awake for the first 24 to 72 hours. We have found that despite the potential for significant periorbital swelling and ecchymosis, there is typically minimal pain associated with the procedure. If significant pain does occur, this should prompt evaluation to rule out retrobulbar or ophthalmologic complications that may occur following the surgery, such as hematoma or entrapment.
Patients are seen in follow-up at 1 week to ensure appropriate healing and edema resolution, then once 2 to 4 weeks postoperatively. If all is appropriate, patients are then seen 3 months postoperatively and at the 1-year mark. It is unclear when complete resolution of edema occurs and final globe position has occurred, but exophthalmometry should be performed at each visit and documented compared with the unoperated eye.
To help with healing, patients should be encouraged to perform range of motion exercises with their eyelids, as well as their extraocular muscles. Lid squinching exercises should be encouraged to further minimize scar formation and promote optimal healing. Gentle massage of the incision or the area of access can also be performed.
Outcomes
Reporting objective outcomes in transorbital surgery can be challenging due to the varied nature of indications, wide array of pathology, and multiple distinct approach portals. We reported on our first 100 outcomes earlier. 1 6 8 12 17 We have identified no major orbital complications, though we reported one case of CSF leak. 17 Additionally, Locatelli et al performed a systematic review of published series reviewing transorbital cases to the skull base. 30 The authors concluded that transorbital endoscopic skull base approaches were not related to significant neurological or vascular complications. None of the patients experienced death, bleeding, hematoma (intraorbital or extraorbital), or infections. They noted only two cases of diplopia in the literature, both of which were transient. They found no cases of visual loss or other permanent damage to orbital function. Based on their comprehensive review of the literature, they concluded that “transorbital endoscopic skull base surgery appears to be a safe and effective technique with complication rates lower than traditional external approaches and comparable with or even better than those published for transnasal and transmaxillary approaches.”
Conclusion
Endoscopic orbital and transorbital surgery is an emerging field that has added significant surgical options for the treatment of pathology in this region. The international experience to date has been highly favorable, with rapid adoption occurring for multiple applications in the adult and pediatric populations. While there have been few notable complications reported in the literature to date, most of the reports have been from highly experienced skull base surgeons who have developed, learned, and studied the procedures in cadaver laboratories. We encourage those who intend to learn these procedures to begin by attending courses and methodical study, as the potential risk of complications in this area is significant.
Footnotes
Conflict of Interest None.
Pearls and Tips.
Comprehensive preoperative multidisciplinary analysis and planning are critical for ensuring successful outcomes.
The surgical approach should be chosen by target location with the goal of providing a coplanar vector with ample room for parallax endoscopy and contact-free instrumentation.
Approaches may be combined from multiple zones in the craniofacial skeleton to allow optimal target exposure and devascularization before manipulation; paraportal and occasionally contraportal approaches may be ideal.
Small secondary microportals can allow an additional avenue for instrumentation or visualization to overcome the challenges of monoportal single vector surgery.
Four standard transorbital approaches are based on quadrants of the orbit: Superior, inferior, lateral, and medial.
Pediatric orbital and transorbital neuroendoscopic surgery has been performed safely but requires significant preoperative planning.
References
- 1.Moe K S, Bergeron C M, Ellenbogen R G.Transorbital neuroendoscopic surgery Neurosurgery 201067(suppl operative03ons16–ons28. [DOI] [PubMed] [Google Scholar]
- 2.Moe K S. The precaruncular approach to the medial orbit. Arch Facial Plast Surg. 2003;5(06):483–487. doi: 10.1001/archfaci.5.6.483. [DOI] [PubMed] [Google Scholar]
- 3.Moe K S, Jothi S, Stern R, Gassner H G. Lateral retrocanthal orbitotomy: a minimally invasive, canthus-sparing approach. Arch Facial Plast Surg. 2007;9(06):419–426. doi: 10.1001/archfaci.9.6.419. [DOI] [PubMed] [Google Scholar]
- 4.Ciporen J N, Moe K S, Ramanathan D. Multiportal endoscopic approaches to the central skull base: a cadaveric study. World Neurosurg. 2010;73(06):705–712. doi: 10.1016/j.wneu.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Bly R A, Su D, Hannaford B, Ferreira M, Jr, Moe K S. Computer modeled multiportal approaches to the skull base. J Neurol Surg B Skull Base. 2012;73(06):415–423. doi: 10.1055/s-0032-1329623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moe K S, Kim L J, Bergeron C M. Transorbital endoscopic repair of cerebrospinal fluid leaks. Laryngoscope. 2011;121(01):13–30. doi: 10.1002/lary.21280. [DOI] [PubMed] [Google Scholar]
- 7.Balakrishnan K, Moe K S. Applications and outcomes of orbital and transorbital endoscopic surgery. Otolaryngol Head Neck Surg. 2011;144(05):815–820. doi: 10.1177/0194599810397285. [DOI] [PubMed] [Google Scholar]
- 8.Lim J H, Sardesai M G, Ferreira M, Jr, Moe K S. Transorbital neuroendoscopic management of sinogenic complications involving the frontal sinus, orbit, and anterior cranial fossa. J Neurol Surg B Skull Base. 2012;73(06):394–400. doi: 10.1055/s-0032-1329617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bly R A, Ramakrishna R, Ferreira M, Moe K S. Lateral transorbital neuroendoscopic approach to the lateral cavernous sinus. J Neurol Surg B Skull Base. 2014;75(01):11–17. doi: 10.1055/s-0033-1353363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bly R A, Morton R P, Kim L J, Moe K S. Tension pneumocephalus after endoscopic sinus surgery: a technical report of multiportal endoscopic skull base repair. Otolaryngol Head Neck Surg. 2014;151(06):1081–1083. doi: 10.1177/0194599814547502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxford R BR, Kim L, Moe K S. Transorbital neuroendoscopic surgery of the middle cranial fossa by lateral retrocanthal approach. J Neurol Surg B Skull Base. 2012;73:A197. doi: 10.1055/s-0032-1329617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakrishna R, Kim L J, Bly R A, Moe K, Ferreira M., Jr Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J Clin Neurosci. 2016;24:99–104. doi: 10.1016/j.jocn.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berens A M, Davis G E, Moe K S. Transorbital endoscopic identification of supernumerary ethmoid arteries. Allergy Rhinol (Providence) 2016;7(03):144–146. doi: 10.2500/ar.2016.7.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bly R A, Su D, Lendvay T S. Multiportal robotic access to the anterior cranial fossa: a surgical and engineering feasibility study. Otolaryngol Head Neck Surg. 2013;149(06):940–946. doi: 10.1177/0194599813509587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S A, Berens A M, Devarajan K, Whipple M E, Moe K S. Evaluation of a minimally disruptive treatment protocol for frontal sinus fractures. JAMA Facial Plast Surg. 2017;19(03):225–231. doi: 10.1001/jamafacial.2016.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicks K L, Moe K S, Humphreys I M. Bilateral transorbital and transnasal endoscopic resection of a frontal sinus osteoblastoma and orbital mucocele: a case report and review of the literature. Ann Otol Rhinol Laryngol. 2018;127(11):864–869. doi: 10.1177/0003489418798388. [DOI] [PubMed] [Google Scholar]
- 17.Miller C, Berens A, Patel S A, Humphreys I M, Moe K S. Transorbital approach for improved access in the management of paranasal sinus mucoceles. J Neurol Surg B Skull Base. 2019;80(06):593–598. doi: 10.1055/s-0038-1676982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bly R A, Berens A, Perkins J A.Surgical planning in pediatric skull base surgery Oper Tech Otolaryngol—Head Neck Surg 2019309–15.. Doi: https://doi.org/10.1016/j.otot.2019.01.005 [Google Scholar]
- 19.Balakrishnan K, Moe K. Stuttgart: Thieme; 2014. Transorbital endoscopic surgery of the skull base and sinuses. [Google Scholar]
- 20.Bly R A, Moe K S. New Delhi: Jaypee Brothers Publishing; 2013. Transorbital endoscopic skull base surgery. [Google Scholar]
- 21.Ellenbogen R G, Moe K S. Philadelphia: Walters Kluwer; 2015. Transorbital neuroendoscopic approaches to the anterior cranial fossa; pp. 151–164. [Google Scholar]
- 22.Moe K S, Ellenbogen R G. Philadelphia: Walters Kluwer; 2015. Transorbital neuroendoscopic approaches to the middle cranial fossa; pp. 343–356. [Google Scholar]
- 23.Chen H I, Bohman L E, Loevner L A, Lucas T H. Transorbital endoscopic amygdalohippocampectomy: a feasibility investigation. J Neurosurg. 2014;120(06):1428–1436. doi: 10.3171/2014.2.JNS131060. [DOI] [PubMed] [Google Scholar]
- 24.Dallan I, Castelnuovo P, Locatelli D. Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: preliminary experience. World Neurosurg. 2015;84(01):97–107. doi: 10.1016/j.wneu.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 25.Almeida J P, Omay S B, Shetty S R. Transorbital endoscopic eyelid approach for resection of sphenoorbital meningiomas with predominant hyperostosis: report of 2 cases. J Neurosurg. 2018;128(06):1885–1895. doi: 10.3171/2017.3.JNS163110. [DOI] [PubMed] [Google Scholar]
- 26.Alqahtani A, Padoan G, Segnini G. Transorbital transnasal endoscopic combined approach to the anterior and middle skull base: a laboratory investigation. Acta Otorhinolaryngol Ital. 2015;35(03):173–179. [PMC free article] [PubMed] [Google Scholar]
- 27.Dallan I, Locatelli D, Turri-Zanoni M. Transorbital endoscopic assisted resection of a superior orbital fissure cavernous haemangioma: a technical case report. Eur Arch Otorhinolaryngol. 2015;272(12):3851–3856. doi: 10.1007/s00405-015-3556-2. [DOI] [PubMed] [Google Scholar]
- 28.Di Somma A, Cavallo L M, de Notaris M. Endoscopic endonasal medial-to-lateral and transorbital lateral-to-medial optic nerve decompression: an anatomical study with surgical implications. J Neurosurg. 2017;127(01):199–208. doi: 10.3171/2016.8.JNS16566. [DOI] [PubMed] [Google Scholar]
- 29.Almeida J P, Ruiz-Treviño A S, Shetty S R, Omay S B, Anand V K, Schwartz T H. Transorbital endoscopic approach for exposure of the sylvian fissure, middle cerebral artery and crural cistern: an anatomical study. Acta Neurochir (Wien) 2017;159(10):1893–1907. doi: 10.1007/s00701-017-3296-8. [DOI] [PubMed] [Google Scholar]
- 30.Locatelli D, Pozzi F, Turri-Zanoni M. Transorbital endoscopic approaches to the skull base: current concepts and future perspectives. J Neurosurg Sci. 2016;60(04):514–525. [PubMed] [Google Scholar]
- 31.Lubbe D, Mustak H, Seayaroh K. Transorbital endoscopic surgery. Curr Otorhinolaryngol Rep. 2019;7:173–180. [Google Scholar]
- 32.Lubbe D, Mustak H, Taylor A, Fagan J. Minimally invasive endo-orbital approach to sphenoid wing meningiomas improves visual outcomes—our experience with the first seven cases. Clin Otolaryngol. 2017;42(04):876–880. doi: 10.1111/coa.12722. [DOI] [PubMed] [Google Scholar]
- 33.Cornelis M M, Lubbe D E. Pre-caruncular approach to the medial orbit and landmarks for anterior ethmoidal artery ligation: a cadaveric study. Clin Otolaryngol. 2016;41(06):777–781. doi: 10.1111/coa.12648. [DOI] [PubMed] [Google Scholar]
- 34.Lubbe D, Moe K S. Stuttgart: Thieme Medical Publishers; 2019. Transorbital approaches to the sinuses, skull base and intracranial space. [Google Scholar]
- 35.Bly R A, Chang S H, Cudejkova M, Liu J J, Moe K S. Computer-guided orbital reconstruction to improve outcomes. JAMA Facial Plast Surg. 2013;15(02):113–120. doi: 10.1001/jamafacial.2013.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alves-Belo J T, Mangussi-Gomes J, Truong H Q. Lateral transorbital versus endonasal transpterygoid approach to the lateral recess of the sphenoid sinus—a comparative anatomic study. Oper Neurosurg (Hagerstown) 2019;16(05):600–606. doi: 10.1093/ons/opy211. [DOI] [PubMed] [Google Scholar]
- 37.Liu J K, Decker D, Schaefer S D.Zones of approach for craniofacial resection: minimizing facial incisions for resection of anterior cranial base and paranasal sinus tumors Neurosurgery 200353051126–1135., discussion 1135–1137 [DOI] [PubMed] [Google Scholar]
- 38.Aghdasi N, Whipple M, Humphreys I M, Moe K S, Hannaford B, Bly R A. Automated surgical approach planning for complex skull base targets: development and validation of a cost function and semantic at-las. Surg Innov. 2018;25(05):476–484. doi: 10.1177/1553350618782287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swaney P J, Croom J M, Burgner J. 2012. pp. 387–393.
- 40.Reisch R, Perneczky A.Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision Neurosurgery 20055704242–255., discussion 242–255 [DOI] [PubMed] [Google Scholar]
- 41.Reisch R, Perneczky A, Filippi R. Surgical technique of the supraorbital key-hole craniotomy. Surg Neurol. 2003;59(03):223–227. doi: 10.1016/s0090-3019(02)01037-6. [DOI] [PubMed] [Google Scholar]
- 42.Chhabra N, Healy D Y, Freitag S K, Bleier B S. The nasoseptal flap for reconstruction of the medial and inferior orbit. Int Forum Allergy Rhinol. 2014;4(09):763–766. doi: 10.1002/alr.21351. [DOI] [PubMed] [Google Scholar]


