ABSTRACT
The vertebrate body plan is characterized by the presence of a segmented spine along its main axis. Here, we examine the current understanding of how the axial tissues that are formed during embryonic development give rise to the adult spine and summarize recent advances in the field, largely focused on recent studies in zebrafish, with comparisons to amniotes where appropriate. We discuss recent work illuminating the genetics and biological mechanisms mediating extension and straightening of the body axis during development, and highlight open questions. We specifically focus on the processes of notochord development and cerebrospinal fluid physiology, and how defects in those processes may lead to scoliosis.
KEY WORDS: Axis straightening, Reissner fiber, Notochord, Scoliosis, Spine, Vacuoles
Summary: The spine is a defining characteristic of all vertebrates. This Review examines how the spine is formed during embryonic development and defines potential etiologies underlying a common spine disorder, scoliosis.
Introduction
The vertebral column or spine is the defining feature of vertebrate animals. This structure provides axial stability, leverages movement through the environment and protects the spinal cord. Typical patterning of the spine consists of a segmented alternating pattern of intercalated vertebral bodies, or centra, and intervertebral discs (Fig. 1A,B). In amniotes (e.g. mammals and birds), centra are assembled around the notochord and the neural arches enclose the spinal cord during embryonic development, whereas in many teleosts (bony fish) and amphibians, the formation of the centra and neural arches occurs in freely swimming larvae. Owing to its axial structure and central position in the body, morphogenesis of the adult spine requires the integration of several distinct musculoskeletal tissues including bone, cartilage and connective tissue, muscles, vasculature and the nervous system. Thus, the spine physically connects axial structures, providing a scaffold for the vertebrate body plan.
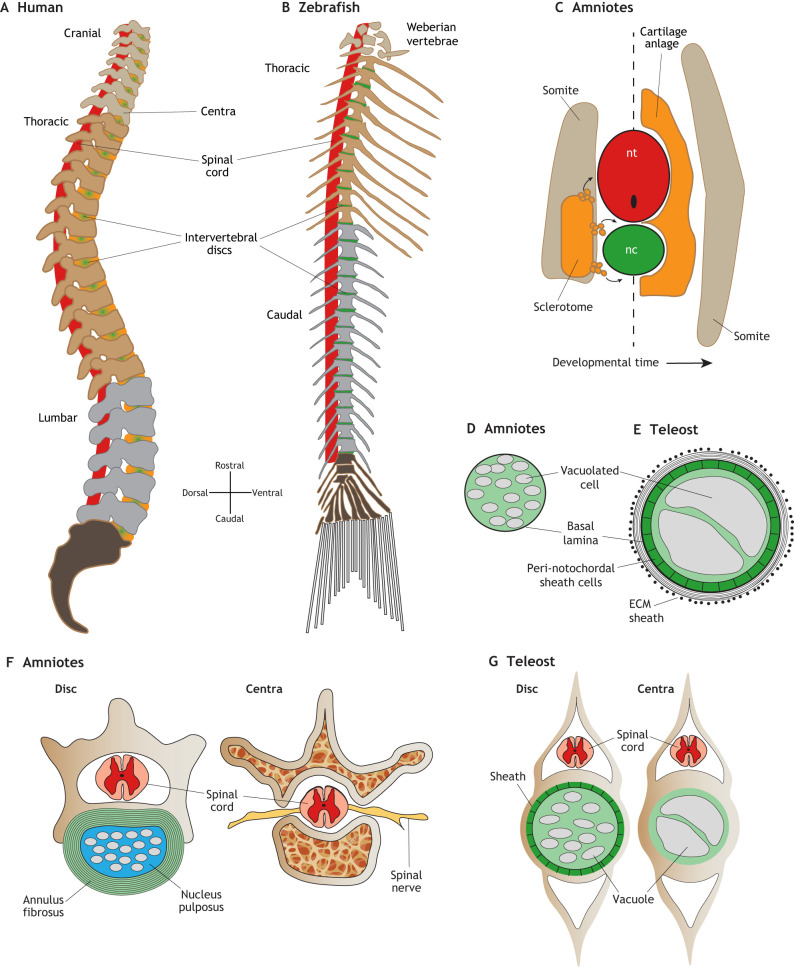
Fig. 1.
Development and morphology of the spine in amniotes and zebrafish. (A) Lateral view schematic of a human adult spine. The intervertebral discs (green/orange) are largely derived from the cartilaginous anlage and notochord in mammals. (B) Lateral view schematic of a zebrafish adult spine. The intervertebral discs (green) are largely derived from the notochord in zebrafish. (C) Transverse view schematic of re-segmentation of the somite-derived sclerotome (orange) tissue which migrates out from the somite (left side of dashed line) to form the cartilage anlage, which wraps the spinal cord and notochord (right side of dashed line). The myotome region of the somite give rise to the axial muscles (tan). (D) Schematic of an amniote notochord in cross section, showing vacuolated cells and a basal lamina sheath. (E) Schematic of a teleost notochord in cross section, showing a larger notochord with large vacuolated cells enclosed by a peri-notochordal sheath cell epithelium, and a lamellar ECM sheath. (F) Cross-sections of mouse spine highlighting the multi-lamellar annulus fibrosus fibrocartilage layer (green), surrounding the nucleus pulposus (light blue), which contains notochord-derived cell lineages, the spinal cord (pink/red) and spinal nerves (yellow), and the trabecular bony regions. (G) Cross-sections of zebrafish spine showing the intervertebral disc (Disc) region, highlighting the sheath and small fragmented vacuolated cells that form the zebrafish disc and the large vacuolated cells within the vertebral body region.
In both amniotes and bony fish, the spine shares the same metameric plan (Fleming et al., 2015) (Fig. 1A,B). Although the mechanisms controlling vertebral patterning are not fully understood, recent studies have shown clear differences between amniotes and teleost species. In amniotes, the segmented pattern of the spine is largely thought to be patterned during, and as a consequence of, somitogenesis: the process in which the presomitic mesoderm becomes compartmentalized into organized somites that flank the notochord along the midline. The molecular details of this segmentation process have been investigated intensively in a variety of vertebrate model systems (Sheeba et al., 2016; Venzin and Oates, 2020). Later in development, the myotome portion of the somites gives rise to the axial muscles, while the ventral most portion (the sclerotome) migrates from each side towards the midline and fuses, forming a cartilaginous progenitor tissue (anlage), which encircles the notochord and spinal cord (Eckalbar et al., 2012) (Fig. 1C). Sclerotome-derived tissue gives rise to the major structural elements of the spine including the osteoblast lineages forming the vertebral bones, the outer annulus fibrosus portion of the intervertebral discs, ligaments and tendons. In bony fishes, vertebral bodies are also formed from sclerotome-derived osteoblasts, but the patterning of centra/vertebral bodies is instructed via notochord-derived signals that override the somitic pattern (Peskin et al., 2020).
The dominant theory of metameric patterning of the spine in amniotes, first proposed by Remak (1855), proceeds as ‘resegmentation’ of the anterior and posterior halves of two adjacent somites, which collectively contribute to a single vertebral body (Huang et al., 2000). This theory suggests that the fidelity of somite boundaries are instructive, and directly pattern the segmentation of the spine. However, this model is controversial; for example, both zebrafish and chicken display ‘leaky’ resegmentation, where one somite can contribute to several adjacent vertebrate (Morin-Kensicki et al., 2002; Stern and Keynes, 1987). The disruption of somite boundaries in the T-box transcription factor 24 (fused somites; tbx6) mutant zebrafish does not alter the segmentation pattern of the centra, although the morphology of the neural arches is affected (Fleming et al., 2004; Wopat et al., 2018). This data suggested that, although neural arch formation follows somitic boundary cues, patterning of centra/vertebral bodies relies on cues from the notochord. Indeed, recent studies show that Notch-dependent patterning of the notochord dictates the segmentation of the vertebral column in zebrafish (Wopat et al., 2018) and when notochord segmentation cues are lost, zebrafish revert to a mechanism of vertebral patterning that relies on somite boundaries and is found in basal fishes, which gave rise to the tetrapod lineage (Peskin et al., 2020). Notochord grafting experiments in chicken provided evidence that cross talk between the notochord and the sclerotome is required for the fidelity of axial segmentation (Ward et al., 2018). Altogether, these results suggest a more complex model in which interactions between the notochord and sclerotome are necessary to refine axial patterning.
Given the tissue architecture and developmental origins of the structural elements of the spine, it is not surprising that embryonic defects of the somites and notochord are linked to the pathogenesis of spine disorders in humans and in animal models. The most common spine disorders in humans are atypical curvatures of the spine, collectively called scoliosis, a term which is derived from the ancient Greek word meaning ‘a bending’, first described generally by Hippocrates in his treatise On Articulations in 400 B.C.E. (Vasiliadis et al., 2009). In modern times, scoliosis in humans falls into three broad phenotypic groups: (1) congenital scoliosis (CS), which are observed at birth, involving vertebral malformations that directly contribute to regions of spine curvature; (2) idiopathic scoliosis (IS), which involves spine curvature without obvious vertebral malformations and in otherwise healthy children (Box 1); and (3) neuromuscular or syndromic/acquired scoliosis, which is a common co-occurring condition for a wide range of neurological or motor neuron or connective tissue diseases, Rett syndrome, amyotrophic lateral sclerosis, Ehlers-Danlos syndrome and spinal muscular atrophy (Halawi et al., 2015; Mary et al., 2018; Murphy and Mooney, 2019) or in cases of pediatric spinal cord injury (Mulcahey et al., 2013).
Box 1. The human genetics of idiopathic scoliosis.
Adolescent idiopathic scoliosis (AIS), observed as curvature of the spine ≥10° with foci of axial rotation, is the most common pediatric spine disorder worldwide, which we refer the reader to review (Cheng et al., 2015; Wise et al., 2020). Diagnosis of AIS is made after the exclusion of neuromuscular disorders, which commonly display acquired scoliosis. The majority of genetic risk in AIS has been defined by genome-wide association studies (GWAS), which utilize common single nucleotide polymorphisms to map haplotypes that segregate with the disorder. The most significant AIS risk loci, replicated in multiple ethnic backgrounds, implicate candidate genes LBX1 (Le Corre et al., 2014; Takahashi et al., 2011) and GPR126 (also known as ADGRG6 or VIGR) (Kou et al., 2013; Kou et al., 2018). LBX1, encoding the ladybird homeobox 1 protein, is required for the development of GABAergic interneurons in the dorsal spinal cord and for the migration of hypaxial muscles for the development of the musculature of the trunk in mouse (Brohmann et al., 2000; Kruger et al., 2002; Muller et al., 2002). A stable genetic model of LBX1 replicating AIS pathology has not been reported. GPR126 encoding the G-coupled protein receptor 126 protein is expressed in cartilaginous tissues of the spine in human and mouse (Karner et al., 2015; Kou et al., 2013). A conditional knockout mouse removing Gpr126 function in osteochondral progenitor cells, which give rise to cartilaginous tissues of the spine, displayed pathology consistent with AIS (Karner et al., 2015). The relevance of this animal model of AIS, together with GWAS implicating additional candidate genes (e.g. SOX6, SOX9, PAX1) known to be important for the development of cartilaginous tissues of the spine, suggests that alterations of the IVD or connective tissues of the spine may underlie pathophysiology of AIS, as has been recently reviewed (Wise et al., 2020).
The onset of IS can occur over a range of developmental stages, yet it is most common during adolescence (AIS). For the most part, CS has been attributed to defects in somite segmentation, largely attributed to disruption of NOTCH signaling in both humans and animal models (reviewed by Giampietro et al., 2009; Venzin and Oates, 2020), or after disruption of notochord biogenesis and physiology, as outlined below. In contrast, the molecular genetics and pathogenesis of IS in humans is less well understood. Recent efforts integrating human genetics and functional analysis in both mouse and fish models have begun to highlight intriguing mechanisms for this historically unresolved disorder, which are also the focus of this Review. Models of IS in amniotes have been historically driven using surgically-induced rodent or large animal models (Liu and Gray, 2018). A recent genetic model of AIS has been reported in the G-coupled receptor 126 (Gpr126; Adgrg6) conditional knockout mouse (Karner et al., 2015), which was informed by the strong association of the GPR126 locus in human AIS cohorts (Kou et al., 2013) (Box 1). Additional genetic models that disrupt the proprioceptive system in mouse also display scoliosis without vertebral malformations (Assaraf et al., 2020; Blecher et al., 2017), which may provide a rationale mechanism underlying neuromuscular acquired scoliosis or AIS. Genetic studies in zebrafish have also discovered that disruption of motile cilia in the brain and spinal cord can generate adult-viable scoliosis phenotypes, which display spine disorders akin to those observed in AIS or neuromuscular acquired scoliosis. There is also evidence that seemingly distinct spine disorders such as CS and IS may have common genetic origins (Box 2). It remains to be determined whether these zebrafish models will provide a transition of knowledge to human spine disorders. The development of more sophisticated animal models modeling human alleles associated with spine disorders will be instructive for diagnosis and potential therapeutic interventions.
Box 2. Common origins of distinct spine disorders.
Congenital scoliosis (CS) is susceptible to gene-environment interactions in mouse, shown by haploinsufficiency of several NOTCH signaling components after challenge with an environmental stressor, hypoxia, in utero (Sparrow et al., 2012). Analysis of multiple cohorts displaying familial CS have also shown increased incidence of idiopathic scoliosis (IS), suggesting that common genetic origins may underlie the expressivity of seemingly distinct spine disorders (Purkiss et al., 2002). Studies in zebrafish also support a genetic link between CS and IS, where pathology of the spine is dictated by the maternal gene dosage of the ptk7 gene (Hayes et al., 2014). Embryos which develop from eggs and sperm lacking ptk7 display disrupted patterning and expression of known somite segmentation pathway genes and defective Wnt signaling that is important for somitogenesis in vertebrates (Hubaud and Pourquie, 2014), and they develop vertebral malformations commonly associated with CS (e.g. hemivertebrae and vertebral fusions). In contrast, strict zygotic ptk7 mutant zebrafish, in which the egg contains maternally deposited Ptk7 protein but lack the zygotic expression of ptk7, develop scoliosis without vertebral malformations or somitic patterning defects, which is characteristic of IS or neuromuscular acquired scoliosis.
Here, we focus on the structural roles of the notochord that are linked to spine patterning defects such as CS. We identify unique functions of connective tissues implicated in the etiology of AIS. Finally, we discuss recent findings in zebrafish that are beginning to explain how the neural tube, cerebrospinal fluid (CSF) and the Reissner fiber contribute to axis straightening and spine morphogenesis.
The notochord
In all vertebrates, the notochord is derived from the axial mesendoderm, which transitions into a rod-like tissue comprised of vacuolated cells, surrounded by a peri-notochordal basement membrane sheath (Balmer et al., 2016; Choi et al., 2008; Choi and Harfe, 2011; Jurand, 1974) (Fig. 1D). The notochord in swimming larval animals (e.g. teleosts and amphibians) is noticeably larger in diameter in comparison with the neural tube and displays a thicker extracellular matrix (ECM) sheath with a medial layer of circumferentially aligned matrix and outer layer which is orthogonally-aligned with the medial layer (Gotz et al., 1995; Gray et al., 2014; Stemple, 2005) (Fig. 1E). In zebrafish, direct imaging of transgenic-labeled vacuolated cells demonstrate that these cells expand in volume via the pressure created by the inflation of notochord vacuoles, utilizing both lysosomal and caveolar based mechanisms (Bagwell et al., 2020; Ellis et al., 2013). Both mouse and chicken also express caveolin in the notochord (Nixon et al., 2007), suggesting that similar mechanisms of notochord vacuolation are involved in amniotes as well. The expansion or vacuolation of the chordamesoderm occurs in an anteroposterior direction concurrent with the onset of somite segmentation (Dale and Topczewski, 2011). Another unique feature of the notochord in teleost species is the presence of a peri-notochordal sheath cell epithelial (also known as chordoblast) layer surrounding the vacuolated cells (Dale and Topczewski, 2011; Grotmol et al., 2003). (Fig. 1E). This sheath cell epithelium displays abundant rough endoplasmic reticulum, common in highly secretory cell types, which likely contributes to the secretion of the thick peri-notochordal basement membrane and lamellar sheath found in fish species (Coutinho et al., 2004; Trapani et al., 2017). Recent work in zebrafish showed that the molecular signal driving the segmented pattern of the spine does not in fact originate from somite boundaries and is instead generated by Notch-dependent signals within the notochord sheath cell epithelium (Lleras Forero et al., 2018; Pogoda et al., 2018; Wopat et al., 2018).
In this model, the sheath cell epithelium serves as a blueprint, directing sclerotome-derived osteoblast migration to specific regions of vertebral body formation and eventually gives rise to the mature vertebral bone (Wopat et al., 2018). Sheath cell epithelial regions are marked by expression of the enzyme Entpd5 (Ectonucleoside triphosphate diphosphohydrolase 5) in alternating domains, promoting mineralization of the ECM sheath (Huitema et al., 2012; Pogoda et al., 2018; Wopat et al., 2018). The mineralized rings that form around the notochord sheath are called chordacentra and are fish-specific structures that may function as a primitive spine in free-swimming larvae (Fleming et al., 2015). Chordacentra mineralization is controlled by retinoic acid via a complex mechanism in which production and degradation are fine tuned to define the shape and size of these calcified rings (Pogoda et al., 2018).
As mentioned earlier, notochord sheath formation and segmentation are key innovations in teleosts driving a complete developmental shift in spine patterning away from the somite-based mechanism found in amniotes (Fleming et al., 2015). The origin of this shift is unclear, but it is linked to emergence of the ECM gene calymmin (cmn) in teleosts. Recent work showed that a dominant mutation in zebrafish cmn called spondo abrogates notochord segmentation and shifts the vertebral patterning to an ancestral somite boundary-based mechanism. In spondo mutants, osteoblasts first form arches following somitic cues, as in amniotes, and then spill over the notochord to form hemi-vertebrae in a pattern that closely resembles the spine of basal fishes from the fossil record (Peskin et al., 2020).
In all vertebrates, the notochord is essential in the patterning of midline tissues including the neural tube, gut endoderm and its derivative tissues, such as the lungs, liver, pancreas and intestine (Cleaver and Krieg, 2001; Lumsden and Graham, 1995). In addition, studies in zebrafish discussed above and recent evidence from amniotes support a model whereby the notochord provides attractive signals for the recruitment of sclerotome-derived osteoblasts that form the vertebral bones (Ward et al., 2018), a process that requires hedgehog secretion by the notochord in mouse (Choi and Harfe, 2011).
In swimming larvae such as Xenopus, zebrafish and ascidian species, the notochord also acts as pressurized rod that serves as a hydrostatic scaffold providing rigidity to the main body axis. In non-vertebrate chordates, such as ascidians, an epithelial layer surrounds a continuous lumen and internal pressure is generated by ion and water transport (Deng et al., 2013; Dong et al., 2009). In contrast, in vertebrates, the notochord interior is made up of large fluid filled vacuolated cells, which essentially compartmentalize the notochord into discrete units (Ellis et al., 2013; Norman et al., 2018) (Fig. 2A). This compartmentalization allows the teleost notochord to be built progressively and is central for the formation of a straight spine (Bagwell et al., 2020). Remarkably, although the structures of the notochord differ considerably between ascidians and zebrafish, both buffer stresses acting on the notochord cells by using caveolae as a mechanoprotective mechanism (Bhattachan et al., 2020; Garcia et al., 2017; Lim et al., 2017; Nixon et al., 2007) (Fig. 2A). Caveolae are plasma membrane invaginations that serve as membrane reservoirs which can unfold when cells are stretched, thus preventing cell rupture (Sinha et al., 2011).
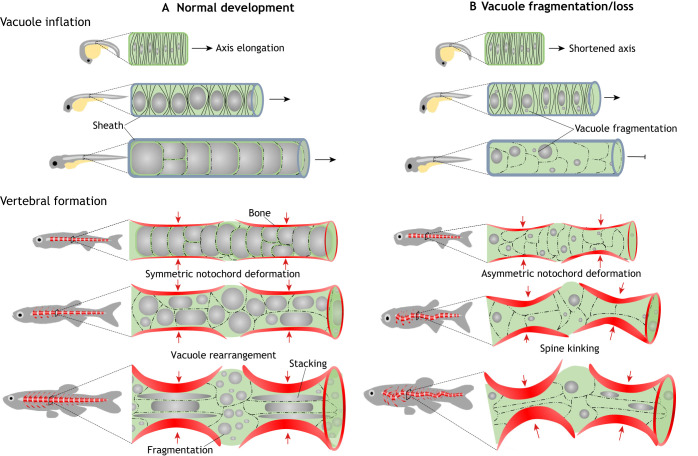
Fig. 2.
Role of notochord vacuolated cells in axis elongation and spine formation. (A) During normal development, as vacuolated cells nucleate and inflate their large fluid filled vacuole (gray spheres) in an anterior-to-posterior direction within the semi-rigid sheath they generate hydrostatic pressure that pushes axis elongation. Later in development, vertebral bone (red) is deposited around the notochord in a segmented fashion, creating an alternating pattern of vertebral and intervertebral domains. As the vertebrae grow concentrically, the large vacuoles are able to absorb the compressive force, allowing symmetrical bone growth. During this period, some vacuolated cells are stacked under the growing bone, whereas in other cells, vacuoles fragment and the cells localize to the IVD domains. This process allows for symmetric bone growth, IVD formation and a straight spine formation. (B) When vacuoles fragment prematurely or are lost, internal notochord pressure is lost, leading to shortening of the embryonic axis. Vacuole loss also causes the notochord to lose its ability to absorb the force of the growing vertebrae evenly and locally. As vertebral bone grows the notochord is deformed asymmetrically, causing vertebral malformation and kinking of the spine axis.
Much of what we know about the structural function of the notochord during vertebrate embryogenesis has come from genetic and cellular studies in zebrafish (Ellis et al., 2013; Nixon et al., 2007; Stemple et al., 1996), ex vivo experiments in Xenopus (Adams et al., 1990) and anatomical studies in salmon (Grotmol et al., 2003). In a seminal study, Adams and colleagues used dissected Xenopus notochords to probe the response of the tissue to osmotic challenge and mechanical deformation (Adams et al., 1990). This, and subsequent ex vivo and physical modeling studies (Koehl et al., 2000), led to the development of a basic model in which osmotic swelling of vacuolated cells inside a semi-rigid collagenous casing provides a hydraulic force that helps straighten and extend the embryonic axis and gives flexural stiffness to the body axis in free-swimming larvae (Adams et al., 1990). Experimental work in zebrafish also suggests that the mechanical properties of the vacuolated notochord act as a counter force against the accumulation of cortical bone, influencing the mineralization of centra and vice versa (Bagwell et al., 2020; Haga et al., 2009).
The mechanical-structural necessity of the vacuolated notochord tissue in amniotes is not known, and key developmental differences of the notochord comparing amniotes and swimming larval species exist. For example, in amniotes, the notochord transitions into segmented regions within the newly forming intervertebral disc (IVD) while the vertebral body regions are being replaced by trabecular bone in utero (Choi et al., 2008) (Fig. 1F), whereas in swimming larvae the morphogenesis of the IVD and formation of centra occurs in concert with active locomotion. Protein arginine methyltransferase 5 conditional knockout mice, display reduced trabecular bone formation in the vertebrae and scoliosis before weaning (Liu et al., 2019), which may underscore that the formation of trabecular bone in the vertebrate acts in an analogous manner to the vacuolated cells during cortical bone deposition of vertebrae in zebrafish (Bagwell et al., 2020) (Fig. 1G).
Formation and maintenance of vacuolated cells
The function of the notochord in zebrafish axis elongation is dependent on the balanced differentiation of the outer sheath epithelial and inner vacuolated cells (Fig. 1E), which occurs at the end of gastrulation and is controlled by Notch signaling. Activation of Notch by expression of the Notch intracellular domain (NICD) leads to the generation of more sheath cells and fewer vacuolated cells, whereas Notch inhibition produces more vacuolated cells (Yamamoto et al., 2010). Live imaging has revealed that these two cell types segregate rapidly along with the inflation of vacuoles within vacuolated cells (Dale and Topczewski, 2011). As vacuole inflation proceeds, vacuolated cells arrange in a stereotypical staircase pattern via a self-organization process that is governed by packing principles that are solely dependent on the number of vacuolated cells that are generated, which in turn determines the size each cell achieves, and the geometry of the notochord rod (Norman et al., 2018).
Further work in zebrafish has shown that the function of vacuolated cells in embryonic axis elongation depends on the generation and maintenance of a single vacuole per cell (Ellis et al., 2013). Notochord vacuoles are lysosome-related organelles formed by biosynthetic trafficking regulated by the Rab32 GTPase (Ellis et al., 2013). Loss or fragmentation of notochord vacuoles leads to shortening of the embryonic axis (Bagwell et al., 2020; Ellis et al., 2013; Norman et al., 2018; Yamamoto et al., 2010) (Fig. 2B). Vacuoles are formed by biosynthetic transport emanating from the Golgi complex and subsequent trafficking and fusion of membranes regulated by the homotypic fusion and vacuole protein sorting (HOPS) complex and Rab32a, whereas vacuole inflation requires the activity of the V-ATPase, which supplies the driving force for fluid accumulation (Ellis et al., 2013). More recently, it has been shown in zebrafish that fusion of large pre-vacuolar compartments with the main vacuole is a rate limiting step in vacuole formation and maintenance, which is regulated by the activity of the Dual serine/threonine and tyrosine protein kinase (Dstyk) protein (Bagwell et al., 2020). dstyk mutants show arrested fusion of Rab32a+ vesicles within the vacuole and present a shorter embryonic axis (Bagwell et al., 2020). This phenotype results from a reduction in vacuolated cell volume and tighter packing of the cells (Bagwell et al., 2020). A separate zebrafish study also implicates Dstyk in the biogenesis of notochord vacuoles and axis formation, presenting in vitro data linking Dstyk to the regulation of Transcription factor EB (Tfeb) (Sun et al., 2020). However, as zebrafish tfeb mutants do not show notochord or axial defects (Meireles et al., 2018), it is unlikely that Dstyk regulates Tfeb in vivo.
Vacuolated cells are unusually large (up to 50 µm in diameter) and have a rigid fluid filled vacuole that occupies most of their cell volume, which presents a structural challenge as the notochord is subject to mechanical stresses. Moreover, when caveolae are lost, vacuolated cells collapse during larval locomotion, and this also impairs axis elongation (Garcia et al., 2017; Lim et al., 2017). However, the effect is not as dramatic as that of vacuole fragmentation because vacuolated cell collapse or injury triggers a regenerative response in which sheath cells invade the affected areas of the notochord and differentiate into new vacuolated cells, thereby restoring notochord function (Garcia et al., 2017; Lopez-Baez et al., 2018). A similar response has been reported for the medaka notochord (Seleit et al., 2020), which is essentially identical structurally to that of zebrafish despite the long distance separating them in the phylogenetic tree.
Notochord vacuoles also play an essential role in straight spine formation in zebrafish (Ellis et al., 2013). Recent work using forward genetics and longitudinal live imaging of notochord and spine development has revealed that vacuole fragmentation causes kinking of the axis during vertebral bone growth (Bagwell et al., 2020). As vertebral bone is assembled around the notochord, the vacuoles initially resist compression thereby facilitating symmetrical centra formation (Fig. 2B). When vacuoles are fragmented prematurely or are lost, the notochord loses internal pressure and becomes more deformable, leading to asymmetrical vertebral bone growth and spine curvature (Bagwell et al., 2020). Interestingly, during late stages of normal centra formation, vacuolated cells re-arrange and stack under the central portion of each centrum, while vacuoles fragment selectively in the intervertebral areas (Bagwell et al., 2020). The stacked vacuolated cells may help to initially resist bone growth, thus facilitating centra formation, while the fragmentation later on may help absorb the compression generated by concentric mineralizing centra (Fig. 2B). The need to accommodate compressive bone growth may also explain the dramatic transition in notochord structure from the hollow lumen present in ascidians to the compartmentalization into vacuoles that is found in vertebrates.
The structural role of the peri-notochordal extracellular matrix sheath
The physical properties of the notochord and its ability to function in axis elongation also depend on the composition and crosslinking of the peri-notochordal ECM sheath (Fig. 1E), recently reviewed by Trapani et al. (2017). This was predicted by ex vivo studies of the Xenopus notochord (Adams et al., 1990) and was demonstrated by the identification of several mutations affecting the notochord sheath in zebrafish (Stemple et al., 1996). Subsequent studies have identified developmental roles for specific matrix components (Christiansen et al., 2009; Corallo et al., 2013; Gansner and Gitlin, 2008; Gray et al., 2014; Parsons et al., 2002), its secretory machinery (Coutinho et al., 2004) and collagen crosslinking (Anderson et al., 2007; Gansner et al., 2007; Mendelsohn et al., 2006), imperative for notochord morphogenesis. These genetic studies in zebrafish have revealed that when components of the ECM sheath surrounding the notochord are missing or mutated, the notochord develops an undulatory pattern (Fig. 3), owing to the inability of the peri-notochordal sheath to contain the internal pressure exerted by the highly vacuolated notochord tissue and by the deposition of cortical bone (Bagwell et al., 2020). Dorsal-ward hyperflexion of the notochord is also observed after disruption of polycystin genes pkd1a/b and pkd2 in zebrafish (Le Corre et al., 2014; Mangos et al., 2010). Loss of Pkd1/2 function leads to upregulation of collagen type-II protein deposition around the notochord, which is normally downregulated in the larval notochord, and increased expression of the endoplasmic reticulum (ER)/Golgi transport coat protein complex proteins (e.g. Sec proteins). Reduction of col2a1a expression or disruption of ER to Golgi transport, via knockdown of sec24d expression or treatment with Brefeldin A rescues the severe dorsal flexion of the notochord and body axis after loss of pkd2 function in zebrafish (Le Corre et al., 2014). Interestingly, loss of Pkd1/2 function does not alter the ultrastructure of the ECM sheath, suggesting that these receptors are aiding in the sensation of the ECM sheath in order to control the important developmental transitions of the notochord (Mangos et al., 2010).
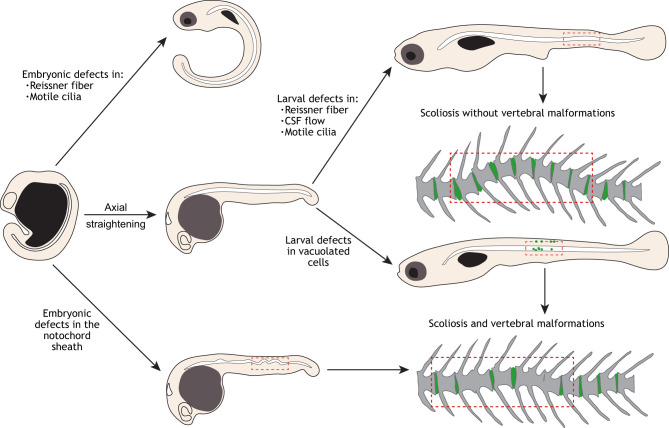
Fig. 3.
Processes regulating axial straightening and notochord integrity underlie defects of spine morphogenesis. Beginning at the curved tailbud stage, generating a straight body axis requires the function of motile cilia components, which drives the formation of the Reissner fiber (top). Once axial straightening occurs, defects of the Reissner fiber, disrupted motile cilia or CSF flow, which are revealed during larval development in zebrafish, can lead to more subtle curvatures of the notochord. Alternatively, defects in the notochord sheath or in vacuolated cell morphogenesis can contribute to severe bends or kinks of the notochord, which directly arise into regions of vertebral malformations and fusion, and sometimes scoliosis.
Work in zebrafish has also demonstrated a crucial role for the notochord in the formation of a straight spine. As the notochord acts as the template and directs the segmentation of the spinal column (Wopat et al., 2018), structural defects of the notochord during embryonic development can cause the formation of vertebral malformations and scoliosis in the adult (Ellis et al., 2013; Gray et al., 2014) (Fig. 3). The loss of Collagen, type VIII, alpha 1 (Col8a1) function leads to thinning of the outer ECM sheath, longitudinal live imaging has revealed that this leads to undulation and bending of the notochord, which later on promotes the spatial joining of normally segmented osteoblast populations, resulting in focal regions of vertebral malformations and fusions (Gray et al., 2014) (Fig. 3). Importantly, the structural role of the notochord in spine morphogenesis is distinct from the fish-specific role in vertebral patterning controlled by segmentation of the notochord sheath cells (Lleras Forero et al., 2018; Pogoda et al., 2018; Wopat et al., 2018). However, it remains unclear whether spine defects arising from impaired collagen crosslinking or deposition of the ECM sheath (Anderson et al., 2007) result from alterations of notochord segmentation, surface defects, or both.
Formation of intervertebral discs and pathology linked to scoliosis
In all vertebrates, the notochord is a transient structure, which eventually becomes completely segmented into the nucleus pulposus at the center of the IVD and typically persists throughout life (Choi et al., 2008; Choi and Harfe, 2011; Haga et al., 2009). In amniotes, the IVD is a fibrocartilaginous joint that connects two adjacent vertebrae and provides structural stability, flexibility and cushions axial loading of the spinal column (Cortes and Elliott, 2014), but the mechanical role in teleosts is less clear.
In amniotes, the disc is composed of the nucleus pulposus, a mass of notochord vacuolated cells embedded in a sulfated proteoglycan-rich matrix, which is surrounded by a multi-lamellar annulus fibrosus (Fig. 1F), derived from the sclerotome. The nucleus pulposus forms during embryonic development as vertebral bodies condense and grow into the notochord, eventually obliterating the notochord in those areas and leaving the remaining notochord vacuolated cells fated to become part of the IVD (Choi and Harfe, 2011; Jurand, 1974; Peck et al., 2017; Smith et al., 2011). The IVD is situated adjacent to the cartilaginous endplates of the vertebral bodies, which provide nutritional flux to the IVD (Urban et al., 2004; Wong et al., 2019).
Conversely, in bony fish the IVD is almost exclusively derived from the notochord, which remodels to resemble a nucleus pulposus-like tissue (Fig. 1G). Unlike in mammals, remnants of the notochord remain embedded within the vertebral bodies as large vacuolated cells in the adult spine (Bagwell et al., 2020; Haga et al., 2009) (Fig. 1G). There is no thick multi-lamellar annulus fibrosus in fish; however, there is a thin ligament that wraps circumferentially around and connects adjacent vertebrae (Inohaya et al., 2007), yet may provide a homologous function for containment of the nucleus pulposus.
Recent studies in the conditional Gpr126 knockout mouse model of AIS (Karner et al., 2015) (Box 1) have demonstrated that increased pro-inflammatory signaling and dysregulation of several anabolic and catabolic factors of the growth plate and IVD tissues, coupled with increased mechanical stiffness of the IVD unit, may underlie the pathology of scoliosis in this model (Liu et al., 2019). In the medaka fish, an undefined spontaneous scoliosis mutant has increased hypertrophy of the nucleus pulposus (Irie et al., 2016). These studies provide evidence that changes in the cellular and mechanical properties of the IVDs may drive the onset of scoliosis, particularly during the period of rapid growth, which is a common theme in AIS.
Links to scoliosis from alterations of bone and connective tissues
Additional intrinsic elements of the spine are important for spine stability. For example, low bone mineral density (osteopenia) of cortical bone is proposed as causative mechanism in a subset of AIS patients (Cheng et al., 2015). Abnormalities in bone metabolic factors, including increased adiponectin levels in circulation, which may increase bone-resorbing osteoclast activity (Luo et al., 2006), are associated with osteopenia-dependent adolescent IS (Zhang et al., 2019). Defects in the flux of terminal hypertrophic chondrocyte differentiation in the vertebral growth plate, leading to reduced trabecular bone formation in vertebra of the spine, is associated with early-onset scoliosis in mice (Liu et al., 2019). Paradoxically, the complete absence of mineralized bone in entpd5a mutant zebrafish does not affect the typical morphology of the spine (Huitema et al., 2012), suggesting that a vacuolated notochord exerts a stronger role in maintenance of a straight spine during skeletogenesis in teleosts.
A diverse array of ligaments and tendons make multiple insertions and connections with the musculature and bony spinal column. Thus, it is not surprising that connective tissue disorders have been also proposed for causing AIS in humans. For example, rare variants in the in ECM genes FIBRILLIN 1 (FBN1), FBN2 (Buchan et al., 2014), perlecan (HSPG2) (Baschal et al., 2014) and an overall increased polygenic burden of rare variants across ECM gene families have been associated with AIS (Haller et al., 2016). These genetic findings support a model whereby the accumulation of variants in ECM genes, which on their own may display low effect, can collectively synergize to compromise the typical properties or development of axial connective tissues (e.g. ligaments, tendons or annulus fibrosis) and lead to mechanical instability of the spine. Loss of fbn2 function in zebrafish causes defects in the formation of the peri-notochordal ECM sheath and undulations of the notochord (Gansner and Gitlin, 2008). Although these mutants did not survive long enough to assess spine morphology, similar defects of the notochord sheath are well-established to contribute to adult spine defects in zebrafish (Christiansen et al., 2009; Gansner and Gitlin, 2008; Gray et al., 2014) (Fig. 3). A reduction in the basal lamina sheath surrounding the notochord was observed in a global Sox6 (encoding the SRY-box transcription factor 6 protein) knockout mice, which led to defects in the development of the nucleus pulposus and kinks in the tail vertebrae (Smits and Lefebvre, 2003). However, because Sox6 is also expressed in the sclerotome-derived cartilaginous anlage, which give rise to the vertebral bodies and annulus fibrosus, a conditional Sox6 knockout approach specific to the notochord is needed to more rigorously assess the role of a reduced notochord sheath for axial stability in mouse. We refer the reader to an additional review of the role of the cartilage matrisome for scoliosis (Wise et al., 2020).
The spinal cord exerts mechanosensory control of spine morphogenesis
The neural arches of each vertebral body collectively form a cavity called the spinal canal, which encases and protects the spinal cord (Fig. 1F,G). The nerve roots of the peripheral nervous system extend out from the spinal cord at distinct points along the spine and, together with the spinal cord, serve to integrate sensory information and motor control with the brain. The stability of the spine is vitally dependent on the integration and function of the neuromuscular system. For example, muscle disorders (such as Duchenne Muscular Dystrophy and spinal muscular atrophy), neurological disorders (such as Rett syndrome, Parkinson's disease) and pediatric spinal cord injuries (Mary et al., 2018; Mulcahey et al., 2013) are all associated with scoliosis in humans (Murphy and Mooney, 2019). Recent studies in mouse have shown an essential role for the proprioceptive system for spine stability (Blecher et al., 2017).
The brain and spinal cord are chemically connected via the ventricular and central canal system, which allows for the exchange of CSF secreted from the choroid plexus in the brain with the spinal cord. In all vertebrates, multiple physiological processes control the flow of CSF including the rates of CSF production, drainage out to the circulatory system, cardiopulmonary function and body movements (Fame and Lehtinen, 2020; Olstad et al., 2019). The bulk flow of CSF provides nutrients and helps to remove waste products from the central nervous system, but also provides key signaling molecules during development of these tissues, including maintenance of adult neural stem cells in the brain (Petrik et al., 2018). Disruption of normal CSF circulation and its turnover is believed to contribute to the development of many neurodegenerative conditions, such as Alzheimer's disease, and neuroinflammatory conditions, such as multiple sclerosis (Simon and Iliff, 2016).
CSF dynamics are important for spine stability
In zebrafish, compartmentalized flows of CSF within the brain ventricles and central canal of the spinal cord require the polarized beating of motile cilia extending from the apical surface of ependymal cells which line the ventricular and spinal canal (Olstad et al., 2019; Thouvenin et al., 2020) (Fig. 4A). Several genetic models in both mouse and zebrafish that disrupt motile cilia or ependymal cell development display defects of CSF flow and hydrocephalus (Lee, 2013; Ringers et al., 2020), showing that, in addition to bulk CSF flows, motile-cilia-driven near-wall or laminar CSF flows (Spassky and Meunier, 2017) are important for homeostasis of the nervous system. Primary ciliary dyskinesia (PCD) patients commonly display pleiotropic defects in tissues, which require motile cilia function. However, hydrocephalus is rarely observed in human PCD patients (Vieira et al., 2012), suggesting that the role of motile ependymal cell cilia is not imperative for function of the human nervous system. However, recent human genetic studies suggest that the disruption of master transcriptional regulators of motile cilia development (Wallmeier et al., 2019) or of specific structural components (Morimoto et al., 2019) of the motile cilia are associated with hydrocephalus in humans. This data reinvigorates the need for understanding the role of motile cilia for the development and homeostasis of the ventricular system in humans.
Fig. 4.
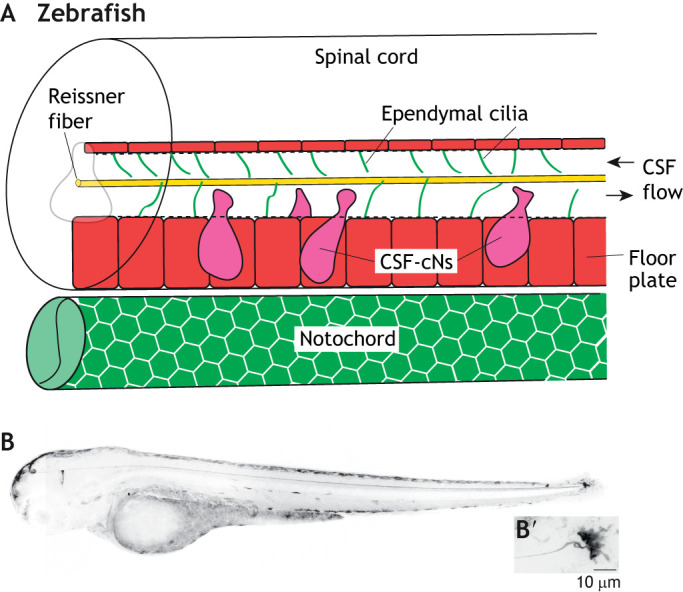
The integration of the Reissner fiber and CSF-resident components. (A) Schematic of the larval zebrafish spinal cord and notochord, showing the close associations of the Reissner fiber (yellow) with ependymal motile cilia (green lines) and CSF-cNs (magenta). The floor plate is shown in red. (B) Inverted grayscale confocal image of a 3 days post-fertilization larval scospondin-GFPut24 knock-in line in a lateral view. (B’) High magnification image of the terminal ampulla region. Taken from Troutwine et al., 2019 preprint.
Recent studies in zebrafish have uncovered a curious association between loss of ependymal motile cilia function, reduced CSF flow and scoliosis (Grimes et al., 2016; Konjikusic et al., 2018). An apparent consequence of disruption of ependymal motile cilia function in the protein tyrosine kinase 7a (ptk7a) mutant zebrafish is increased neuroinflammation in the brain and spinal cord. Moreover, stimulation of proinflammatory cues in the ependyma was sufficient to stimulate scoliosis in zebrafish, and scoliosis in zebrafish can be ameliorated after treatment with nonsteroidal anti-inflammatory drugs (Rose et al., 2020; Van Gennip et al., 2018). The coincidence of increased proinflammatory signals and scoliosis is also observed in stat3 mutant zebrafish (Liu et al., 2019), and scoliosis is observed in a rare primary immunodeficiency disease called Hyper IgE syndrome, which is attributed to STAT3 mutations in humans (Sowerwine et al., 2012). Obstruction of CSF flow in humans with syringomyelia, observed as cysts or cavity formation within the spinal cord, is strongly correlated with syndromic scoliosis (Strahle et al., 2015). Altogether these studies underscore the role of CSF physiology and neuron inflammation for the maintenance of spine stability.
An intriguing role for the Reissner fiber in the regulation of spine morphology
Recently, a CSF resident structure called the Reissner fiber has become the focus for understanding the regulation of the straight body axis and spine morphogenesis (Driever, 2018). Since its discovery over a century ago (Reissner, 1860), the Reissner fiber has been shown to be an ancient conserved structure, which has been identified in ascidian tadpoles (Olsson, 1972) and is present in numerous chordate lineages, from cephalochordates (Gobron et al., 1999) to lamprey (Reissner, 1860), teleosts (Cantaut-Belarif et al., 2018), amniotes (Vio et al., 2008) and several primates (Castenholz and Zoltzer, 1980).
In zebrafish, the Reissner fiber is an extracellular glycoprotein thread, which extends from the brain through the central canal and terminates at the base of the spinal cord (Troutwine et al., 2020) (Fig. 4B). In zebrafish, the assembly of the Reissner fiber is dependent on the secretion of the SCO-spondin protein from the subcomissural organ (SCO) in the brain, as Reissner fiber formation is completely absent in scospondin mutant zebrafish (Cantaut-Belarif et al., 2018). The Reissner fiber has long been postulated to continually move from head to tail, which was experimentally demonstrated using pulse-chase labeling of the fiber with labeled monoamines in rat (Caprile et al., 2003). Live imaging of an endogenous knock-in scospondin-GFPut24 gene fusion zebrafish line has shown that the assembly of the Reissner fiber first extends out from the subcomissural organ in the brain at early tailbud stage (∼20 h post fertilization) and travels towards the base of the spinal cord where it terminates at the terminal ampulla (Fig. 4B′) and that the fiber continually treadmills in a rostral-caudal direction throughout larval development and remains assembled in adult zebrafish (Troutwine et al., 2020).
A variety of hypotheses have proposed functions for the Reissner fiber, including regulation of CSF production and composition, circulation or detoxification. Early studies demonstrate that the Reissner fiber transports key signaling molecules, which may be important for the regulation of neurodevelopment and contribute to straightening of the body axis, from the brain to the spinal cord. For example, binding of the Reissner fiber to monoamines and catecholamines (e.g. L-DOPA, noradrenaline, adrenaline, serotonin) may provide a source-sink mechanism for transport and removal of these important neuronal signaling molecules from the brain and spinal canal (Caprile et al., 2003). Indeed, soluble SCO-spondin can also act as regulator of neurite outgrowth, neurogenesis and neurodifferentiation in chicken neural explant cultures (Gobron et al., 2000; Vera et al., 2015; Vera et al., 2013).
Interactions of the Reissner fiber and the cerebrospinal contacting neurons
A class of sensory neurons commonly called cerebrospinal contacting neurons (CSF-cNs) are a specialized GABAergic sensory neuron found in most vertebrates that, in zebrafish, line the dorsal and ventral sides of the central canal (Djenoune et al., 2017) (Fig. 4). CSF-cNs extend out into the central canal and respond to lateral and longitudinal bending of the spine through their modulation of distinct premotor circuits (Djenoune et al., 2017; Djenoune et al., 2014; Orts-Del'Immagine et al., 2020). This lead Kolmer to hypothesize more than a century ago that the SCO, the Reissner fiber and its interaction with central canal components, acts as a ‘sagittal organ’, which functions as an integrated mechanosensory system of the body (Kolmer, 1921). In support of Kolmer's hypothesis, recent studies in zebrafish have shown that CSF-cN monocilia are in direct contact with the Reissner fiber and that the loss of the Reissner fiber results in a severe reduction of CSF-cN ability to respond to mechanical stimulus, such as axial bending (Orts-Del'Immagine et al., 2020). These data suggest an intriguing role for the Reissner fiber acting as a physical cue of the axial midline that can help signals to the CSF-cNs and help refine sensory-motor control of the body axis.
CSF-cN signaling helps to maintain a straight body axis
At a molecular level, CSF-cNs express mechanosensitive channel Polycystic kidney disease1-like 1 (Pkd2l1) localized within the apical microvilli extensions that interact with CSF (Bohm et al., 2016). pdk2l1 mutant CSF-cNs display a marked reduction of spontaneous calcium activity in vivo or in response to mechanical stimulus in vitro, and adult pkd2l1 mutant fish display an exacerbated kyphosis of the spine (Sternberg et al., 2018). Genetic loss of motile cilia activity or the Reissner fiber also reduces the spontaneous activity (assayed by calcium imaging) of CSF-cNs without affecting Pkd2l1 channel activity (Orts-Del'Immagine et al., 2020; Sternberg et al., 2018), suggesting that physical interaction of the Reissner fiber and the CSF-cNs is required to stimulate Pkd2l1 channels.
CSF-cNs also express the urotensin II-related neuropeptides Urp1/2 (Quan et al., 2015). Motile-cilia mutant zebrafish with severe ventral curvatures show diminished expression of urp1/2 and the ectopic expression of Urp1 is sufficient to straighten the body axis in these animals or lead to dorsal ward flexion in wild-type animals (Zhang et al., 2018). A variety of monoamines and catecholamines, such as dopamine, epinephrine or L-DOPA, which have been shown to bind the Reissner fiber in rat (Caprile et al., 2003), are sufficient to stimulate urp1/2 expression in the CSF-cNs of motile cilia mutant zebrafish and rescue body axis morphogenesis defects in zebrafish (Lu et al., 2020; Zhang et al., 2018). The putative Urp1 receptor Uts2ra (Uts2r3) is expressed in the dorsal slow-twitch muscle fibers in zebrafish and uts2ra mutant zebrafish display larval-onset scoliosis, without vertebral malformations (Zhang et al., 2018). As mentioned above, severe dorsal flexion in Pkd1/2 loss-of-function zebrafish models is also observed as a consequence of increased ectopic secretion of collagen type-II protein in the larval notochord (Le Corre et al., 2014; Mangos et al., 2010). However, the genetic ablation of the CSF-cN mechanosensitive receptor Pdk2l1 gene only results in mild kyphoscoliosis in adult viable zebrafish (Sternberg et al., 2018). It remains to be determined how severe dorsal flexion phenotypes related to ectopic secretion of embryonic collagens in the notochord and alterations in Urp regulation of dorsal musculature are related.
In addition to its role in straightening the body axis during embryonic development, recent data show the Reissner fiber is continuously required for maintenance of a straight body axis and spine morphogenesis in larval zebrafish. In contrast to the complete loss-of-function of scospondin, recent studies show that hypomorphic recessive (Troutwine et al., 2020) or a dominant heterozygous (Rose et al., 2020) mutations in scospondin are not associated with defects of Reissner fiber assembly or body axis straightening during embryonic development. Instead, these mutants display progressive disassembly of the Reissner fiber during larval development, concurrent with the onset of axial curvatures in zebrafish. The onset of axial curvatures in these larval fish leads to scoliosis without vertebral malformations in the adult mutant fish (Troutwine et al., 2020) (Fig. 3). How might this maintenance be achieved? One hypothesis is that CSF-cNs synapse onto glutamatergic pre-motor interneurons to modulate spinal pre-motor circuits (Fidelin et al., 2015), which may facilitate neuromuscular stability of the body axis and subsequently maintain straightness of the body axis, and which indirectly controls the straightness of the notochord, which is the direct template for embryonic spine development (Wopat et al., 2018) (Fig. 1C). Interestingly, deficiencies in postural and balance control on the basis of proprioceptive cues are associated with a subset of adolescent IS patients (Cheng et al., 2015; Guo et al., 2006). Another, potentially non-exclusive, model supported by recent studies in zebrafish suggests that the loss of Reissner fiber leads to dysregulation of Urp signaling, leading to overexpression (Vesque et al., 2019 preprint) or underexpression (Lu et al., 2020; Zhang et al., 2018) of urotensin peptide from the CSF-cNs. This imbalance results in body axis instability from defects of the dorsal musculature during spine morphogenesis (Lu et al., 2020; Zhang et al., 2018). Finally, the loss of Reissner fiber has been associated with increased neuroinflammation of the brain and spinal cord (Rose et al., 2020); however, whether the loss of Reissner fiber assembly is stimulating or is a consequence of increased neuroinflammatory signaling remains undetermined. Together, these data suggest several complementary modes of action downstream of the Reissner fiber and CSF-cNs for spine morphogenesis: (1) as an instructive signaling modality involving direct stimulation of the CSF-cN activity by physical interactions with the Reissner fiber for downstream sensory motor modulation of neuromuscular muscle tone; and (2) to promote dorsal muscle contraction to facilitate straightening of the body axis, which has been recently reviewed (Grimes, 2019; Ringers and Jurisch-Yaksi, 2020).
The role of the Reissner fiber and CSF-cNs in human spine morphogenesis
The Reissner fiber and CSF-cNs are found in hundreds of chordate lineages (Djenoune and Wyart, 2017), including primates (Castenholz and Zoltzer, 1980; Djenoune et al., 2014). The extent to which the Reissner fiber instructs human development remains unclear. The SCO, which secretes the Reissner fiber in the brain, is regressive during postnatal development in human (Rodriguez et al., 2001). Although the Reissner fiber was historically observed in humans (Agduhr, 1922), neither the SCO nor the Reissner fiber are observed to immuno-react with any of the numerous polyclonal and monoclonal antibodies raised against Reissner fiber glycoproteins of animal origin in human or in several anthropoid apes (Rodriguez et al., 1990; Rodríguez et al., 1993; Rodriguez et al., 2001). The current Human Genome Organization Gene Nomenclature Committee now classifies the human SCO-spondin (HGNC:21998) as a pseudogene, a consequence of deleterious mutations common in the population. In addition, we can find no evidence of CSF-cNs being reported in human spinal cord. Altogether, this may suggest that anthropoid apes and humans represent a unique primate lineage that lost the ability to form or sustain the formation of Reissner fibers and utilize other mechanisms to maintain postural control. It is interesting to note that primates (Castenholz and Zoltzer, 1980) and other animals which have a Reissner fiber all display a relatively straight spinal column (Fig. 1B). In contrast, typical human spine morphology follows a natural cervical kyphosis and lumbar lordosis (Fig. 1A). Perhaps the loss of Reissner fiber underscores the establishment of natural spine morphology in humans. It also remains an open question as to the function of the Reissner fiber and CSF-cNs in experimental mammalian models that are known to display both components: it will be interesting to define how physiological control of posture has been innovated or coopted in humans.
Conclusions and perspectives
Recent progress in our understanding of the multiple processes involved in embryonic axis elongation and straightening, spine development and scoliosis in a variety of model systems may be summarized in the following three emerging concepts. First, the notochord plays a key role as organizing center and template for the spine. Early defects affecting the structural integrity of the notochord can translate to severe notochord curves, leading to vertebral malformations with scoliosis (Fig. 3). Perhaps if the notochord is only slightly curved, as a function of neuromuscular defects of the body axis during larval development, then this may give rise to scoliosis without vertebral malformations akin to pathology commonly observed in IS or neuromuscular/syndromic scoliosis and in zebrafish models outlined in this review (Fig. 3). Although these principles have mostly been defined in zebrafish and other swimming larvae species, it is less clear whether this principle has a strong role in mammalian development because notochord and early spine development are difficult to study in these models. Second, notochord vacuoles play a key structural role in the vertebrate axis. As vacuoles fill with fluid and vacuolated cells expand within the casing of the sheath, the notochord becomes a pressurized rod that can provide a hydrostatic scaffold for embryonic axis elongation. Then, as vertebral bone grows it facilitates symmetrical centra formation by first resisting and then absorbing compression. Loss of vacuole integrity can lead to the formation of asymmetrical vertebrae that can cause CS-phenotypes in zebrafish and perhaps also in humans, although trabecular bone formation in the vertebrae may help to overcome mechanical defects of the notochord in these animals. Finally, the Reissner fiber has a crucial role in straightening the zebrafish body axis during embryogenesis and is required for the maintenance of a straight body axis during larval development to facilitate normal spine morphology. The role of the Reissner fiber for spine morphogenesis or nervous system physiology in mammals is unknown and warrants further study. Although it is unclear how the Reissner fiber might be related to scoliosis observed in IS or neuromuscular/syndromic scoliosis phenotypes, it is clearly linked to CSF sensing and influences neuromuscular postural control, which in turn can cause bending of the axis if it is inappropriately regulated. An open question in the field remains to understand how loss of motile cilia function in the central canal aids in the assembly of the Reissner fiber and how this translates to other amniote models.
Acknowledgements
We thank Jennifer Bagwell for comments on the manuscript and for the illustrations of Fig. 2.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by National Institutes of Health grant R01 AR065439-04 (to M.B.) and R01 AR072009 to (R.S.G.). M.B. is a Howard Hughes Medical Institute Faculty Scholar. Deposited in PMC for release after 12 months.
References
- Adams D. S., Keller R. and Koehl M. A. (1990). The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 110, 115-130. [DOI] [PubMed] [Google Scholar]
- Agduhr E. (1922). Über ein zentrales Sinnesorgan bei den Vertebraten. Zeitschrift für Anatomie und Entwicklungsgeschichte 66, 223-360. 10.1007/BF02593586 [DOI] [Google Scholar]
- Anderson C., Bartlett S. J., Gansner J. M., Wilson D., He L., Gitlin J. D., Kelsh R. N. and Dowden J. (2007). Chemical genetics suggests a critical role for lysyl oxidase in zebrafish notochord morphogenesis. Mol. BioSyst. 3, 51-59. 10.1039/B613673G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaraf E., Blecher R., Heinemann-Yerushalmi L., Krief S., Carmel Vinestock R., Biton I. E., Brumfeld V., Rotkopf R., Avisar E., Agar G. et al. (2020). Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity. Nat. Commun. 11, 3168 10.1038/s41467-020-16971-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagwell J., Norman J., Ellis K. L., Peskin B., Hwang J., Ge X., Nguyen S., McMenamin S. K., Stainier D. Y. and Bagnat M. (2020). Notochord vacuoles absorb compressive bone growth during zebrafish spine formation. eLife 9, e51221. 10.7554/eLife.51221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer S., Nowotschin S. and Hadjantonakis A.-K. (2016). Notochord morphogenesis in mice: Current understanding & open questions. Dev. Dyn. 245, 547-557. 10.1002/dvdy.24392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschal E. E., Wethey C. I., Swindle K., Baschal R. M., Gowan K., Tang N. L., Alvarado D. M., Haller G. E., Dobbs M. B., Taylor M. R. et al. (2014). Exome sequencing identifies a rare HSPG2 variant associated with familial idiopathic scoliosis. G3; Genes|Genomes|Genetics 5, 167-174. 10.1534/g3.114.015669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattachan P., Rae J., Yu H., Jung W., Wei J., Parton R. G. and Dong B. (2020). Ascidian caveolin induces membrane curvature and protects tissue integrity and morphology during embryogenesis. FASEB J. 34, 1345-1361. 10.1096/fj.201901281R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecher R., Krief S., Galili T., Biton I. E., Stern T., Assaraf E., Levanon D., Appel E., Anekstein Y., Agar G. et al. (2017). The proprioceptive system masterminds spinal alignment: insight into the mechanism of scoliosis. Dev. Cell. 42, 388-399e383. 10.1016/j.devcel.2017.07.022 [DOI] [PubMed] [Google Scholar]
- Böhm U. L., Prendergast A., Djenoune L., Nunes Figueiredo S., Gomez J., Stokes C., Kaiser S., Suster M., Kawakami K., Charpentier M. et al. (2016). CSF-contacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits. Nat. Commun. 7, 10866 10.1038/ncomms10866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohmann H., Jagla K. and Birchmeier C. (2000). The role of Lbx1 in migration of muscle precursor cells. Development 127, 437-445. [DOI] [PubMed] [Google Scholar]
- Buchan J. G., Alvarado D. M., Haller G. E., Cruchaga C., Harms M. B., Zhang T., Willing M. C., Grange D. K., Braverman A. C., Miller N. H. et al. (2014). Rare variants in FBN1 and FBN2 are associated with severe adolescent idiopathic scoliosis. Hum. Mol. Genet. 23, 5271-5282. 10.1093/hmg/ddu224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantaut-Belarif Y., Sternberg J. R., Thouvenin O., Wyart C. and Bardet P. L. (2018). The Reissner fiber in the cerebrospinal fluid controls morphogenesis of the body axis. Curr. Biol. 28, 2479-2486e2474. 10.1016/j.cub.2018.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprile T., Hein S., Rodriguez S., Montecinos H. and Rodriguez E. (2003). Reissner fiber binds and transports away monoamines present in the cerebrospinal fluid. Mol. Brain Res. 110, 177-192. 10.1016/S0169-328X(02)00565-X [DOI] [PubMed] [Google Scholar]
- Castenholz A. and Zoltzer H. (1980). Formation and morphology of Reissner's fibre in primates. A scanning electron microscopic study. Cell Tissue Res. 207, 43-53. [DOI] [PubMed] [Google Scholar]
- Cheng J. C., Castelein R. M., Chu W. C., Danielsson A. J., Dobbs M. B., Grivas T. B., Gurnett C. A., Luk K. D., Moreau A., Newton P. O. et al. (2015). Adolescent idiopathic scoliosis. Nat. Rev. Dis. Primers 1, 15030 10.1038/nrdp.2015.30 [DOI] [PubMed] [Google Scholar]
- Choi K.-S. and Harfe B. D. (2011). Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc. Natl. Acad. Sci. USA 108, 9484-9489. 10.1073/pnas.1007566108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. S., Cohn M. J. and Harfe B. D. (2008). Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev. Dyn. 237, 3953-3958. 10.1002/dvdy.21805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen H. E., Lang M. R., Pace J. M. and Parichy D. M. (2009). Critical early roles for col27a1a and col27a1b in zebrafish notochord morphogenesis, vertebral mineralization and post-embryonic axial growth. PLoS One 4, e8481 10.1371/journal.pone.0008481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O. and Krieg P. A. (2001). Notochord patterning of the endoderm. Dev. Biol. 234, 1-12. 10.1006/dbio.2001.0214 [DOI] [PubMed] [Google Scholar]
- Corallo D., Schiavinato A., Trapani V., Moro E., Argenton F. and Bonaldo P. (2013). Emilin3 is required for notochord sheath integrity and interacts with Scube2 to regulate notochord-derived Hedgehog signals. Development 140, 4594-4601. 10.1242/dev.094078 [DOI] [PubMed] [Google Scholar]
- Cortes D. H. and Elliott D. M. (2014). The Intervertebral Disc: Overview of Disc Mechanics. In The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease (ed. Shapiro I. M. and Risbud M. V.), pp. 17-31. Vienna: Springer Vienna. [Google Scholar]
- Coutinho P., Parsons M. J., Thomas K. A., Hirst E. M., Saude L., Campos I., Williams P. H. and Stemple D. L. (2004). Differential requirements for COPI transport during vertebrate early development. Dev. Cell 7, 547-558. 10.1016/j.devcel.2004.07.020 [DOI] [PubMed] [Google Scholar]
- Dale R. M. and Topczewski J. (2011). Identification of an evolutionarily conserved regulatory element of the zebrafish col2a1a gene. Dev. Biol. 357, 518-531. 10.1016/j.ydbio.2011.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Nies F., Feuer A., Bocina I., Oliver D. and Jiang D. (2013). Anion translocation through an Slc26 transporter mediates lumen expansion during tubulogenesis. Proc. Natl Acad. Sci. USA 110, 14972-14977. 10.1073/pnas.1220884110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djenoune L. and Wyart C. (2017). Light on a sensory interface linking the cerebrospinal fluid to motor circuits in vertebrates. J. Neurogenet. 31, 113-127. 10.1080/01677063.2017.1359833 [DOI] [PubMed] [Google Scholar]
- Djenoune L., Khabou H., Joubert F., Quan F. B., Nunes Figueiredo S., Bodineau L., Del Bene F., Burckle C., Tostivint H. and Wyart C. (2014). Investigation of spinal cerebrospinal fluid-contacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Front. Neuroanat. 8, 26 10.3389/fnana.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djenoune L., Desban L., Gomez J., Sternberg J. R., Prendergast A., Langui D., Quan F. B., Marnas H., Auer T. O., Rio J.-P. et al. (2017). The dual developmental origin of spinal cerebrospinal fluid-contacting neurons gives rise to distinct functional subtypes. Sci. Rep. 7, 719 10.1038/s41598-017-00350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B., Horie T., Denker E., Kusakabe T., Tsuda M., Smith W. C. and Jiang D. (2009). Tube formation by complex cellular processes in Ciona intestinalis notochord. Dev. Biol. 330, 237-249. 10.1016/j.ydbio.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W. (2018). Developmental biology: Reissner’s fiber and straightening of the body axis. Curr. Biol. 28, R833-R835. 10.1016/j.cub.2018.05.080 [DOI] [PubMed] [Google Scholar]
- Eckalbar W. L., Fisher R. E., Rawls A. and Kusumi K. (2012). Scoliosis and segmentation defects of the vertebrae. Wiley Interdiscip. Rev. Dev. Biol. 1, 401-423. 10.1002/wdev.34 [DOI] [PubMed] [Google Scholar]
- Ellis K., Bagwell J. and Bagnat M. (2013). Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J. Cell Biol. 200, 667-679. 10.1083/jcb.201212095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fame R. M. and Lehtinen M. K. (2020). Emergence and developmental roles of the cerebrospinal fluid system. Dev. Cell 52, 261-275. 10.1016/j.devcel.2020.01.027 [DOI] [PubMed] [Google Scholar]
- Fidelin K., Djenoune L., Stokes C., Prendergast A., Gomez J., Baradel A., Del Bene F. and Wyart C. (2015). State-dependent modulation of locomotion by GABAergic spinal sensory neurons. Curr. Biol. 25, 3035-3047. 10.1016/j.cub.2015.09.070 [DOI] [PubMed] [Google Scholar]
- Fleming A., Keynes R. and Tannahill D. (2004). A central role for the notochord in vertebral patterning. Development 131, 873-880. 10.1242/dev.00952 [DOI] [PubMed] [Google Scholar]
- Fleming A., Kishida M. G., Kimmel C. B. and Keynes R. J. (2015). Building the backbone: the development and evolution of vertebral patterning. Development 142, 1733-1744. 10.1242/dev.118950 [DOI] [PubMed] [Google Scholar]
- Gansner J. M. and Gitlin J. D. (2008). Essential role for the alpha 1 chain of type VIII collagen in zebrafish notochord formation. Dev. Dyn. 237, 3715-3726. 10.1002/dvdy.21779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansner J. M., Mendelsohn B. A., Hultman K. A., Johnson S. L. and Gitlin J. D. (2007). Essential role of lysyl oxidases in notochord development. Dev. Biol. 307, 202-213. 10.1016/j.ydbio.2007.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., Bagwell J., Njaine B., Norman J., Levic D. S., Wopat S., Miller S. E., Liu X., Locasale J. W., Stainier D. Y. R. et al. (2017). Sheath cell invasion and trans-differentiation repair mechanical damage caused by loss of caveolae in the zebrafish notochord. Curr. Biol. 27, 1982-1989e1983. 10.1016/j.cub.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampietro P. F., Dunwoodie S. L., Kusumi K., Pourquié O., Tassy O., Offiah A. C., Cornier A. S., Alman B. A., Blank R. D., Raggio C. L. et al. (2009). Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Ann. N. Y. Acad. Sci. 1151, 38-67. 10.1111/j.1749-6632.2008.03452.x [DOI] [PubMed] [Google Scholar]
- Gobron S., Creveaux I., Meiniel R., Didier R., Dastugue B. and Meiniel A. (1999). SCO-spondin is evolutionarily conserved in the central nervous system of the chordate phylum. Neuroscience 88, 655-664. 10.1016/S0306-4522(98)00252-8 [DOI] [PubMed] [Google Scholar]
- Gobron S., Creveaux I., Meiniel R., Didier R., Herbet A., Bamdad M., El Bitar F., Dastugue B. and Meiniel A. (2000). Subcommissural organ/Reissner's fiber complex: characterization of SCO-spondin, a glycoprotein with potent activity on neurite outgrowth. Glia 32, 177-191. [DOI] [PubMed] [Google Scholar]
- Gotz W., Osmers R. and Herken R. (1995). Localisation of extracellular matrix components in the embryonic human notochord and axial mesenchyme. J. Anat. 186, Pt 1, 111-121. [PMC free article] [PubMed] [Google Scholar]
- Gray R. S., Wilm T. P., Smith J., Bagnat M., Dale R. M., Topczewski J., Johnson S. L. and Solnica-Krezel L. (2014). Loss of col8a1a function during zebrafish embryogenesis results in congenital vertebral malformations. Dev. Biol. 386, 72-85. 10.1016/j.ydbio.2013.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes D. T. (2019). Developmental biology: go with the flow to keep the body straight. Curr. Biol. 29, R101-R103. 10.1016/j.cub.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Grimes D. T., Boswell C. W., Morante N. F., Henkelman R. M., Burdine R. D. and Ciruna B. (2016). Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science 352, 1341-1344. 10.1126/science.aaf6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotmol S., Kryvi H., Nordvik K. and Totland G. K. (2003). Notochord segmentation may lay down the pathway for the development of the vertebral bodies in the Atlantic salmon. Anat. Embryol. 207, 263-272. 10.1007/s00429-003-0349-y [DOI] [PubMed] [Google Scholar]
- Guo X., Chau W. W., Hui-Chan C. W., Cheung C. S., Tsang W. W. and Cheng J. C. (2006). Balance control in adolescents with idiopathic scoliosis and disturbed somatosensory function. Spine 31, E437-E440. 10.1097/01.brs.0000222048.47010.bf [DOI] [PubMed] [Google Scholar]
- Haga Y., Dominique V. J. 3rd and Du S. J. (2009). Analyzing notochord segmentation and intervertebral disc formation using the twhh:gfp transgenic zebrafish model. Transgenic Res. 18, 669-683. 10.1007/s11248-009-9259-y [DOI] [PubMed] [Google Scholar]
- Halawi M. J., Lark R. K. and Fitch R. D. (2015). Neuromuscular Scoliosis: Current Concepts. Orthopedics 38, e452-e456. 10.3928/01477447-20150603-50 [DOI] [PubMed] [Google Scholar]
- Haller G., Alvarado D., McCall K., Yang P., Cruchaga C., Harms M., Goate A., Willing M., Morcuende J. A., Baschal E. et al. (2016). A polygenic burden of rare variants across extracellular matrix genes among individuals with adolescent idiopathic scoliosis. Hum. Mol. Genet. 25, 202-209. 10.1093/hmg/ddv463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M., Gao X., Yu L. X., Paria N., Henkelman R. M., Wise C. A. and Ciruna B. (2014). ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated Wnt signalling in disease. Nat. Commun. 5, 4777 10.1038/ncomms5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Zhi Q., Brand-Saberi B. and Christ B. (2000). New experimental evidence for somite resegmentation. Anat. Embryol. 202, 195-200. 10.1007/s004290000110 [DOI] [PubMed] [Google Scholar]
- Hubaud A. and Pourquié O. (2014). Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 15, 709-721. 10.1038/nrm3891 [DOI] [PubMed] [Google Scholar]
- Huitema L. F., Apschner A., Logister I., Spoorendonk K. M., Bussmann J., Hammond C. L. and Schulte-Merker S. (2012). Entpd5 is essential for skeletal mineralization and regulates phosphate homeostasis in zebrafish. Proc. Natl Acad. Sci. USA 109, 21372-21377. 10.1073/pnas.1214231110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohaya K., Takano Y. and Kudo A. (2007). The teleost intervertebral region acts as a growth center of the centrum: in vivo visualization of osteoblasts and their progenitors in transgenic fish. Dev. Dyn. 236, 3031-3046. 10.1002/dvdy.21329 [DOI] [PubMed] [Google Scholar]
- Irie K., Kuroda Y., Mimori N., Hayashi S., Abe M., Tsuji N., Sugiyama A. and Furukawa S. (2016). Histopathology of a wavy medaka. J. Toxicol. Pathol. 29, 115-118. 10.1293/tox.2015-0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurand A. (1974). Some aspects of the development of the notochord in mouse embryos. J. Embryol. Exp. Morphol. 32, 1-33. [PubMed] [Google Scholar]
- Karner C. M., Long F., Solnica-Krezel L., Monk K. R. and Gray R. S. (2015). Gpr126/Adgrg6 deletion in cartilage models idiopathic scoliosis and pectus excavatum in mice. Hum. Mol. Genet. 24, 4365-4373. 10.1093/hmg/ddv170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M. A. R., Quillin K. J. and Pell C. H. (2000). Mechanical design of fiber-wound hydraulic skeletons: the stiffening and straightening of embryonic notochords. Am. Zool. 40, 28-41. [Google Scholar]
- Kolmer W. (1921). Das “Sagittalorgan” der Wirbeltiere. Zeitschrift für Anatomie und Entwicklungsgeschichte 60, 652-717. 10.1007/BF02593657 [DOI] [Google Scholar]
- Konjikusic M. J., Yeetong P., Boswell C. W., Lee C., Roberson E. C., Ittiwut R., Suphapeetiporn K., Ciruna B., Gurnett C. A., Wallingford J. B. et al. (2018). Mutations in Kinesin family member 6 reveal specific role in ependymal cell ciliogenesis and human neurological development. PLoS Genet. 14, e1007817 10.1371/journal.pgen.1007817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou I., Takahashi Y., Johnson T. A., Takahashi A., Guo L., Dai J., Qiu X., Sharma S., Takimoto A., Ogura Y. et al. (2013). Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat. Genet. 45, 676-679. 10.1038/ng.2639 [DOI] [PubMed] [Google Scholar]
- Kou I., Watanabe K., Takahashi Y., Momozawa Y., Khanshour A., Grauers A., Zhou H., Liu G., Fan Y.-H., Takeda K. et al. (2018). A multi-ethnic meta-analysis confirms the association of rs6570507 with adolescent idiopathic scoliosis. Sci. Rep. 8, 11575 10.1038/s41598-018-29011-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger M., Schäfer K. and Braun T. (2002). The homeobox containing gene Lbx1 is required for correct dorsal-ventral patterning of the neural tube. J. Neurochem. 82, 774-782. 10.1046/j.1471-4159.2002.01078.x [DOI] [PubMed] [Google Scholar]
- Le Corre S., Eyre D. and Drummond I. A. (2014). Modulation of the secretory pathway rescues zebrafish polycystic kidney disease pathology. J. Am. Soc. Nephrol. 25, 1749-1759. 10.1681/ASN.2013101060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. (2013). Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J. Neurosci. Res. 91, 1117-1132. 10.1002/jnr.23238 [DOI] [PubMed] [Google Scholar]
- Lim Y. W., Lo H. P., Ferguson C., Martel N., Giacomotto J., Gomez G. A., Yap A. S., Hall T. E. and Parton R. G. (2017). Caveolae protect notochord cells against catastrophic mechanical failure during development. Curr. Biol. 27, 1968-1981e1967. 10.1016/j.cub.2017.05.067 [DOI] [PubMed] [Google Scholar]
- Liu Z. and Gray R. S. (2018). Animal Models of Idiopathic Scoliosis. In The Genetics and Development of Scoliosis (ed. Kusumi K. and Dunwoodie S. L.), pp. 107-138. Cham: Springer International Publishing. [Google Scholar]
- Liu Z., Ramachandran J., Vokes S. A. and Gray R. S. (2019). Regulation of terminal hypertrophic chondrocyte differentiation in Prmt5 mutant mice modeling infantile idiopathic scoliosis. Dis. Models Mech.12, dmm041251. 10.1242/dmm.041251 [DOI]
- Liu Z., Easson G. W. D., Zhao J., Makki N., Ahituv N., Hilton M. J., Tang S. Y. and Gray R. S. (2019). Dysregulation of STAT3 signaling is associated with endplate-oriented herniations of the intervertebral disc in Adgrg6 mutant mice. PLoS Genet. 15, e1008096 10.1371/journal.pgen.1008096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleras Forero L., Narayanan R., Huitema L. F., VanBergen M., Apschner A., Peterson-Maduro J., Logister I., Valentin G., Morelli L. G., Oates A. C. et al. (2018). Segmentation of the zebrafish axial skeleton relies on notochord sheath cells and not on the segmentation clock. eLife 7, e33843. 10.7554/eLife.33843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Baez J. C., Simpson D. J., Forero L. L. L., Zeng Z., Brunsdon H., Salzano A., Brombin A., Wyatt C., Rybski W., Huitema L. F. A. et al. (2018). Wilms Tumor 1b defines a wound-specific sheath cell subpopulation associated with notochord repair. eLife 7, e30657. 10.7554/eLife.30657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Shagirova A., Goggi J. L., Yeo H. L. and Roy S. (2020). Reissner fibre-induced urotensin signalling from cerebrospinal fluid-contacting neurons prevents scoliosis of the vertebrate spine. Biol. Open 9, bio052027. 10.1242/bio.052027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A. and Graham A. (1995). Neural patterning: A forward role for hedgehog. Curr. Biol. 5, 1347-1350. 10.1016/S0960-9822(95)00266-1 [DOI] [PubMed] [Google Scholar]
- Luo X. H., Guo L. J., Xie H., Yuan L. Q., Wu X. P., Zhou H. D. and Liao E. Y. (2006). Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J. Bone Miner. Res. 21, 1648-1656. 10.1359/jbmr.060707 [DOI] [PubMed] [Google Scholar]
- Mangos S., Lam P. Y., Zhao A., Liu Y., Mudumana S., Vasilyev A., Liu A. and Drummond I. A. (2010). The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis. Model. Mech. 3, 354-365. 10.1242/dmm.003194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary P., Servais L. and Vialle R. (2018). Neuromuscular diseases: Diagnosis and management. Orthop. Traumatol. Surg. Res. 104, S89-S95. 10.1016/j.otsr.2017.04.019 [DOI] [PubMed] [Google Scholar]
- Meireles A. M., Shen K., Zoupi L., Iyer H., Bouchard E. L., Williams A. and Talbot W. S. (2018). The lysosomal transcription factor TFEB represses myelination downstream of the Rag-Ragulator complex. Dev. Cell 47, 319-330e315. 10.1016/j.devcel.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn B. A., Yin C., Johnson S. L., Wilm T. P., Solnica-Krezel L. and Gitlin J. D. (2006). Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 4, 155-162. 10.1016/j.cmet.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Morimoto Y., Yoshida S., Kinoshita A., Satoh C., Mishima H., Yamaguchi N., Matsuda K., Sakaguchi M., Tanaka T., Komohara Y. et al. (2019). Nonsense mutation in CFAP43 causes normal-pressure hydrocephalus with ciliary abnormalities. Neurology 92, e2364-e2374. 10.1212/WNL.0000000000007505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki E. M., Melancon E. and Eisen J. S. (2002). Segmental relationship between somites and vertebral column in zebrafish. Development 129, 3851-3860. [DOI] [PubMed] [Google Scholar]
- Mulcahey M. J., Gaughan J. P., Betz R. R., Samdani A. F., Barakat N. and Hunter L. N. (2013). Neuromuscular scoliosis in children with spinal cord injury. Top. Spinal Cord Inj. Rehabil. 19, 96-103. 10.1310/sci1902-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T., Brohmann H., Pierani A., Heppenstall P. A., Lewin G. R., Jessell T. M. and Birchmeier C. (2002). The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron 34, 551-562. 10.1016/S0896-6273(02)00689-X [DOI] [PubMed] [Google Scholar]
- Murphy R. F. and Mooney J. F. 3rd (2019). Current concepts in neuromuscular scoliosis. Curr. Rev. Musculoskelet. Med. 12, 220-227. 10.1007/s12178-019-09552-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon S. J., Carter A., Wegner J., Ferguson C., Floetenmeyer M., Riches J., Key B., Westerfield M. and Parton R. G. (2007). Caveolin-1 is required for lateral line neuromast and notochord development. J. Cell Sci. 120, 2151-2161. 10.1242/jcs.003830 [DOI] [PubMed] [Google Scholar]
- Norman J., Sorrell E. L., Hu Y., Siripurapu V., Garcia J., Bagwell J., Charbonneau P., Lubkin S. R. and Bagnat M. (2018). Tissue self-organization underlies morphogenesis of the notochord. Philos. Trans. R Soc. Lond. B Biol. Sci. 373, 20170320. 10.1098/rstb.2017.0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson R. (1972). Reissner's fiber in ascidian tadpole larvae. Acta Zool 53, 17-21. 10.1111/j.1463-6395.1972.tb00568.x [DOI] [Google Scholar]
- Olstad E. W., Ringers C., Hansen J. N., Wens A., Brandt C., Wachten D., Yaksi E. and Jurisch-Yaksi N. (2019). Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Curr. Biol. 29, 229-241e226. 10.1016/j.cub.2018.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orts-Del'Immagine A., Cantaut-Belarif Y., Thouvenin O., Roussel J., Baskaran A., Langui D., Koeth F., Bivas P., Lejeune F. X., Bardet P. L. et al. (2020). Sensory neurons contacting the cerebrospinal fluid require the Reissner fiber to detect spinal curvature in vivo. Curr. Biol. 30, P827-839.E4. 10.1016/j.cub.2019.12.071 [DOI] [PubMed] [Google Scholar]
- Parsons M. J., Pollard S. M., Saude L., Feldman B., Coutinho P., Hirst E. M. and Stemple D. L. (2002). Zebrafish mutants identify an essential role for laminins in notochord formation. Development 129, 3137-3146. [DOI] [PubMed] [Google Scholar]
- Peck S. H., McKee K. K., Tobias J. W., Malhotra N. R., Harfe B. D. and Smith L. J. (2017). Whole transcriptome analysis of notochord-derived cells during embryonic formation of the nucleus pulposus. Sci. Rep. 7, 10504 10.1038/s41598-017-10692-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin B., Henke K., Cumplido N., Treaster S., Harris M. P., Bagnat M. and Arratia G. (2020). Notochordal signals establish phylogenetic Identity of the teleost spine. Curr. Biol. 30, P2805-2814.E3. 10.1016/j.cub.2020.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik D., Myoga M. H., Grade S., Gerkau N. J., Pusch M., Rose C. R., Grothe B. and Gotz M. (2018). Epithelial sodium channel regulates adult neural stem cell proliferation in a flow-dependent manner. Cell Stem Cell 22, 865-878e868. 10.1016/j.stem.2018.04.016 [DOI] [PubMed] [Google Scholar]
- Pogoda H. M., Riedl-Quinkertz I., Lohr H., Waxman J. S., Dale R. M., Topczewski J., Schulte-Merker S. and Hammerschmidt M. (2018). Direct activation of chordoblasts by retinoic acid is required for segmented centra mineralization during zebrafish spine development. Development 145, dev159418. 10.1242/dev.159418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkiss S. B., Driscoll B., Cole W. G. and Alman B. (2002). Idiopathic scoliosis in families of children with congenital scoliosis. Clin. Orthop. Relat. Res. 27-31. [DOI] [PubMed] [Google Scholar]
- Quan F. B., Dubessy C., Galant S., Kenigfest N. B., Djenoune L., Leprince J., Wyart C., Lihrmann I. and Tostivint H. (2015). Comparative distribution and in vitro activities of the urotensin II-related peptides URP1 and URP2 in zebrafish: evidence for their colocalization in spinal cerebrospinal fluid-contacting neurons. PLoS One 10, e0119290 10.1371/journal.pone.0119290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner E. (1860). Beiträge zur Kenntnis vom Bau des Rückenmarkes von Petromyzon fluviatilis L. Arch. Anat. Physiol. Wiss. Med., 545-588. [Google Scholar]
- Remak R. (1855). Untersuchungen über die Entwicklung der Wirbelthiere. Berlin: G. Reimer.
- Ringers C. and Jurisch-Yaksi N. (2020). Development: How the Reissner Fiber Keeps Our Back Straight. Curr. Biol. 30, R705-R708. 10.1016/j.cub.2020.04.073 [DOI] [PubMed] [Google Scholar]
- Ringers C., Olstad E. W. and Jurisch-Yaksi N. (2020). The role of motile cilia in the development and physiology of the nervous system. Philos. Trans. R Soc. Lond. B Biol. Sci. 375, 20190156 10.1098/rstb.2019.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez E. M., Garrido O. and Oksche A. (1990). Lectin histochemistry of the human fetal subcommissural organ. Cell Tissue Res. 262, 105-113. 10.1007/BF00327751 [DOI] [PubMed] [Google Scholar]
- Rodríguez E. M., Jara P., Richter H., Montecinos H., Flández B., Wiegand R. and Oksche A. (1993). Evidence for the Release of CSF-Soluble Secretory Material from the Subcommissural Organ, with Particular Reference to the Situation in the Human, pp. 121-131. Berlin, Heidelberg: Springer. [Google Scholar]
- Rodríguez E. M., Oksche A. and Montecinos H. (2001). Human subcommissural organ, with particular emphasis on its secretory activity during the fetal life. Microsc. Res. Tech. 52, 573-590. [DOI] [PubMed] [Google Scholar]
- Rose C. D., Pompili D., Henke K., Van Gennip J. L. M., Meyer-Miner A., Rana R., Gobron S., Harris M. P., Nitz M. and Ciruna B. (2020). SCO-Spondin defects and neuroinflammation are conserved mechanisms driving spinal deformity across genetic models of idiopathic scoliosis. Curr. Biol. 30, 2363-2373e2366. 10.1016/j.cub.2020.04.020 [DOI] [PubMed] [Google Scholar]
- Seleit A., Gross K., Onistschenko J., Woelk M., Autorino C. and Centanin L. (2020). Development and regeneration dynamics of the Medaka notochord. Dev. Biol. 463, 11-25. 10.1016/j.ydbio.2020.03001. [DOI] [PubMed] [Google Scholar]
- Sheeba C. J., Andrade R. P. and Palmeirim I. (2016). Mechanisms of vertebrate embryo segmentation: Common themes in trunk and limb development. Semin. Cell Dev. Biol. 49, 125-134. 10.1016/j.semcdb.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Simon M. J. and Iliff J. J. (2016). Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta 1862, 442-451. 10.1016/j.bbadis.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B., Köster D., Ruez R., Gonnord P., Bastiani M., Abankwa D., Stan R. V., Butler-Browne G., Vedie B., Johannes L. et al. (2011). Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402-413. 10.1016/j.cell.2010.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. J., Nerurkar N. L., Choi K.-S., Harfe B. D. and Elliott D. M. (2011). Degeneration and regeneration of the intervertebral disc: lessons from development. Dis. Model. Mech. 4, 31-41. 10.1242/dmm.006403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P. and Lefebvre V. (2003). Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development 130, 1135-1148. 10.1242/dev.00331 [DOI] [PubMed] [Google Scholar]
- Sowerwine K. J., Holland S. M. and Freeman A. F. (2012). Hyper-IgE syndrome update. Ann. N. Y. Acad. Sci. 1250, 25-32. 10.1111/j.1749-6632.2011.06387.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow D. B., Chapman G., Smith A. J., Mattar M. Z., Major J. A., O'Reilly V. C., Saga Y., Zackai E. H., Dormans J. P., Alman B. A. et al. (2012). A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell 149, 295-306. 10.1016/j.cell.2012.02.054 [DOI] [PubMed] [Google Scholar]
- Spassky N. and Meunier A. (2017). The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 18, 423-436. 10.1038/nrm.2017.21 [DOI] [PubMed] [Google Scholar]
- Stemple D. L. (2005). Structure and function of the notochord: an essential organ for chordate development. Development 132, 2503-2512. 10.1242/dev.01812 [DOI] [PubMed] [Google Scholar]
- Stemple D. L., Solnica-Krezel L., Zwartkruis F., Neuhauss S. C., Schier A. F., Malicki J., Stainier D. Y., Abdelilah S., Rangini Z., Mountcastle-Shah E. et al. (1996). Mutations affecting development of the notochord in zebrafish. Development 123, 117-128. [DOI] [PubMed] [Google Scholar]
- Stern C. D. and Keynes R. J. (1987). Interactions between somite cells: the formation and maintenance of segment boundaries in the chick embryo. Development 99, 261-272. [DOI] [PubMed] [Google Scholar]
- Sternberg J. R., Prendergast A. E., Brosse L., Cantaut-Belarif Y., Thouvenin O., Orts-Del'Immagine A., Castillo L., Djenoune L., Kurisu S., McDearmid J. R. et al. (2018). Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat. Commun. 9, 3804 10.1038/s41467-018-06225-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahle J., Smith B. W., Martinez M., Bapuraj J. R., Muraszko K. M., Garton H. J. and Maher C. O. (2015). The association between Chiari malformation Type I, spinal syrinx, and scoliosis. J. Neurosurg. Pediatr. 15, 607-611. 10.3171/2014.11.PEDS14135 [DOI] [PubMed] [Google Scholar]
- Sun X., Zhou Y., Zhang R., Wang Z., Xu M., Zhang D., Huang J., Luo F., Li F., Ni Z. et al. (2020). Dstyk mutation leads to congenital scoliosis-like vertebral malformations in zebrafish via dysregulated mTORC1/TFEB pathway. Nat. Commun. 11, 479 10.1038/s41467-019-14169-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kou I., Takahashi A., Johnson T. A., Kono K., Kawakami N., Uno K., Ito M., Minami S., Yanagida H. et al. (2011). A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nat. Genet. 43, 1237-1240. 10.1038/ng.974 [DOI] [PubMed] [Google Scholar]
- Thouvenin O., Keiser L., Cantaut-Belarif Y., Carbo-Tano M., Verweij F., Jurisch-Yaksi N., Bardet P. L., van Niel G., Gallaire F. and Wyart C. (2020). Origin and role of the cerebrospinal fluid bidirectional flow in the central canal. eLife 9, e47699 10.7554/eLife.47699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani V., Bonaldo P. and Corallo D. (2017). Role of the ECM in notochord formation, function and disease. J. Cell Sci. 130, 3203-3211. 10.1242/jcs.175950 [DOI] [PubMed] [Google Scholar]
- Troutwine B. R., Gontarz P., Konjikusic M. J., Minowa R., Monstad-Rios A., Sepich D. S., Kwon R. Y., Solnica-Krezel L. and Gray R. S. (2019). The Reissner fiber is highly dynamic in vivo and controls morphogenesis of the spine. bioRxiv. 10.1101/847301v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutwine B. R., Gontarz P., Konjikusic M. J., Minowa R., Monstad-Rios A., Sepich D. S., Kwon R. Y., Solnica-Krezel L. and Gray R. S. (2020). The Reissner fiber is highly dynamic in vivo and controls morphogenesis of the spine. Curr. Biol. 30, 2353-2362e3. 10.1016/j.cub.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. P., Smith S. and Fairbank J. C. (2004). Nutrition of the intervertebral disc. Spine 29, 2700-2709. 10.1097/01.brs.0000146499.97948.52 [DOI] [PubMed] [Google Scholar]
- Van Gennip J. L. M., Boswell C. W. and Ciruna B. (2018). Neuroinflammatory signals drive spinal curve formation in zebrafish models of idiopathic scoliosis. Sci. Adv. 4, eaav1781 10.1126/sciadv.aav1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliadis E. S., Grivas T. B. and Kaspiris A. (2009). Historical overview of spinal deformities in ancient Greece. Scoliosis 4, 6 10.1186/1748-7161-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venzin O. F. and Oates A. C. (2020). What are you synching about? Emerging complexity of Notch signaling in the segmentation clock. Dev. Biol. 460, 40-54. 10.1016/j.ydbio.2019.06.024 [DOI] [PubMed] [Google Scholar]
- Vera A., Stanic K., Montecinos H., Torrejon M., Marcellini S. and Caprile T. (2013). SCO-spondin from embryonic cerebrospinal fluid is required for neurogenesis during early brain development. Front. Cell. Neurosci. 7, 80 10.3389/fncel.2013.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera A., Recabal A., Saldivia N., Stanic K., Torrejon M., Montecinos H. and Caprile T. (2015). Interaction between SCO-spondin and low density lipoproteins from embryonic cerebrospinal fluid modulates their roles in early neurogenesis. Front. Neuroanat. 9, 72 10.3389/fnana.2015.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesque C., Anselme I., Pezeron G., Cantaut-Belarif Y., Eschstruth A., Djebar M., Santos D. L., Ribeuz H. L., Jenett A., Khoury H. et al. (2019). Loss of the Reissner Fiber and increased URP neuropeptide signaling underlie scoliosis in a zebrafish ciliopathy mutant. bioRxiv, 10.1101/2019.12.19.882258 [DOI] [Google Scholar]
- Vieira J. P., Lopes P. and Silva R. (2012). Primary ciliary dyskinesia and hydrocephalus with aqueductal stenosis. J. Child Neurol. 27, 938-941. 10.1177/0883073811429856 [DOI] [PubMed] [Google Scholar]
- Vio K., Rodriguez S., Yulis C. R., Oliver C. and Rodriguez E. M. (2008). The subcommissural organ of the rat secretes Reissner's fiber glycoproteins and CSF-soluble proteins reaching the internal and external CSF compartments. Cerebrospinal Fluid Res. 5, 3 10.1186/1743-8454-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmeier J., Frank D., Shoemark A., Nöthe-Menchen T., Cindric S., Olbrich H., Loges N. T., Aprea I., Dougherty G. W., Pennekamp P. et al. (2019). De novo mutations in FOXJ1 result in a motile ciliopathy with hydrocephalus and randomization of left/right body asymmetry. Am. J. Hum. Genet. 105, 1030-1039. 10.1016/j.ajhg.2019.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L., Pang A. S. W., Evans S. E. and Stern C. D. (2018). The role of the notochord in amniote vertebral column segmentation. Dev. Biol. 439, 3-18. 10.1016/j.ydbio.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise C. A., Sepich D., Ushiki A., Khanshour A. M., Kidane Y. H., Makki N., Gurnett C. A., Gray R. S., Rios J. J., Ahituv N. et al. (2020). The cartilage matrisome in adolescent idiopathic scoliosis. Bone Res. 8, 13 10.1038/s41413-020-0089-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Sampson S. L., Bell-Briones H., Ouyang A., Lazar A. A., Lotz J. C. and Fields A. J. (2019). Nutrient supply and nucleus pulposus cell function: effects of the transport properties of the cartilage endplate and potential implications for intradiscal biologic therapy. Osteoarthritis Cartilage 27, 956-964. 10.1016/j.joca.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wopat S., Bagwell J., Sumigray K. D., Dickson A. L., Huitema L. F. A., Poss K. D., Schulte-Merker S. and Bagnat M. (2018). Spine Patterning Is Guided by Segmentation of the Notochord Sheath. Cell Rep. 22, 2026-2038. 10.1016/j.celrep.2018.01.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Morita R., Mizoguchi T., Matsuo H., Isoda M., Ishitani T., Chitnis A. B., Matsumoto K., Crump J. G., Hozumi K. et al. (2010). Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions. Development 137, 2527-2537. 10.1242/dev.051011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Jia S., Chen Z., Chong Y. L., Xie H., Feng D., Wu X., Song D. Z., Roy S. and Zhao C. (2018). Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat. Genet. 50, 1666-1673. 10.1038/s41588-018-0260-3 [DOI] [PubMed] [Google Scholar]
- Zhang H. Q., Wang L. J., Liu S. H., Li J., Xiao L. G. and Yang G. T. (2019). Adiponectin regulates bone mass in AIS osteopenia via RANKL/OPG and IL6 pathway. J. Transl. Med. 17, 64 10.1186/s12967-019-1805-7 [DOI] [PMC free article] [PubMed] [Google Scholar]