ABSTRACT
Salivary glands exert exocrine secretory function to provide saliva for lubrication and protection of the oral cavity. Its epithelium consists of several differentiated cell types, including acinar, ductal and myoepithelial cells, that are maintained in a lineage-restricted manner during homeostasis or after mild injuries. Glandular regeneration following a near complete loss of secretory cells, however, may involve cellular plasticity, although the mechanism and extent of such plasticity remain unclear. Here, by combining lineage-tracing experiments with a model of severe glandular injury in the mouse submandibular gland, we show that de novo formation of acini involves induction of cellular plasticity in multiple non-acinar cell populations. Fate-mapping analysis revealed that, although ductal stem cells marked by cytokeratin K14 and Axin2 undergo a multipotency switch, they do not make a significant contribution to acinar regeneration. Intriguingly, more than 80% of regenerated acini derive from differentiated cells, including myoepithelial and ductal cells, that appear to dedifferentiate to a progenitor-like state before re-differentiation into acinar cells. The potential of diverse cell populations serving as a reserve source for acini widens the therapeutic options for hyposalivation.
KEY WORDS: Salivary gland, Regeneration, Stem cells, Lineage tracing, Lineage plasticity, Dedifferentiation
Summary: Salivary glands rely on recruitment of differentiated cell populations as well as stem cells to ensure rapid regeneration and recovery of secretory cells.
INTRODUCTION
The salivary gland (SG) epithelium is a slowly renewing tissue, organized into a highly branched tubular structure composed of saliva-secreting terminal units or acini, a ductal network of intercalated, granular (in rodents), striated and excretory ducts for modifying and transporting saliva, and a contractile myoepithelium to aid in expulsion of saliva from acini through ducts (Redman, 1994; Amano et al., 2012). In the mouse submandibular gland (SMG), which is the most complex among salivary glands, acinar, myoepithelial and granular duct (GD) cells are highly specialized and well-differentiated cell populations that can be easily be distinguished by their unique morphology and expression of cell-specific biomarkers (Fig. 1A). The intercalated duct (ID) that connects several acini to a GD is the smallest epithelial compartment, composed of two distinct subsets of cuboidal cells with a relatively undifferentiated morphology (Denny et al., 1997). These cells are distinguished based on the expression of Kit and cytokeratin K14, which are known to mark multipotent embryonic progenitors during SG development (Kwak et al., 2016; Lombaert et al., 2013). However, cell proliferation in the adult SMG is not limited to ID cells, as well-differentiated cells in various epithelial compartments undergo mitosis, thus contributing to the slow cellular turnover during homeostasis (Denny et al., 1997; Kwak and Ghazizadeh, 2015). Furthermore, recent genetic lineage-tracing studies have shown that various cell types in the mature SMG are maintained in a lineage-restricted manner, either by contribution from unipotent stem cells or by self-duplication of differentiated cells (Kwak et al., 2016, 2018; Aure et al., 2015; Emmerson et al., 2017; May et al., 2018). To date, only a single cell population in the SMG has been shown to display characteristics assigned to tissue stem cells, including the capacity for long-term clonal self-renewal and for the generation of at least one differentiated cell progeny during homeostasis (Kwak et al., 2016; Clevers and Watt, 2018). This cell population was initially identified as a small subset of ductal cells residing at the ID/GD junction and expressing the basal cell markers K14/K5 but not α-smooth muscle actin (SMA), which is specific to myoepithelial cells (MECs), or Kit, which marks ID cells (Fig. 1A) (Kwak et al., 2016). Long-term lineage-tracing studies showed that K14+ ductal cells function as an actively cycling, long-term self-renewing cell population that generates a progeny destined to differentiate into K19+ cells in the GD (Kwak et al., 2016). Subsequence studies using other biomarkers, including cytokeratin K5 and transcription factor TP63, or Axin2, a target of WNT signaling, have confirmed these initial findings (Weng et al., 2018; May et al., 2018; Song et al., 2018). Moreover, ex vivo studies have verified the regenerative capacity of K14+ cells in organoid cultures and upon intra-glandular transplantation, which are often used to assess stem cell function (Kwak et al., 2018; Xiao et al., 2014). On the other hand, although Kit has long been considered a marker for the adult salivary gland tissue stem cells (Hisatomi et al., 2004; Lombaert et al., 2008), recent in vivo and ex vivo genetic labeling and lineage-tracing studies have not been supportive of this hypothesis (Kwak et al., 2018).
Fig. 1.
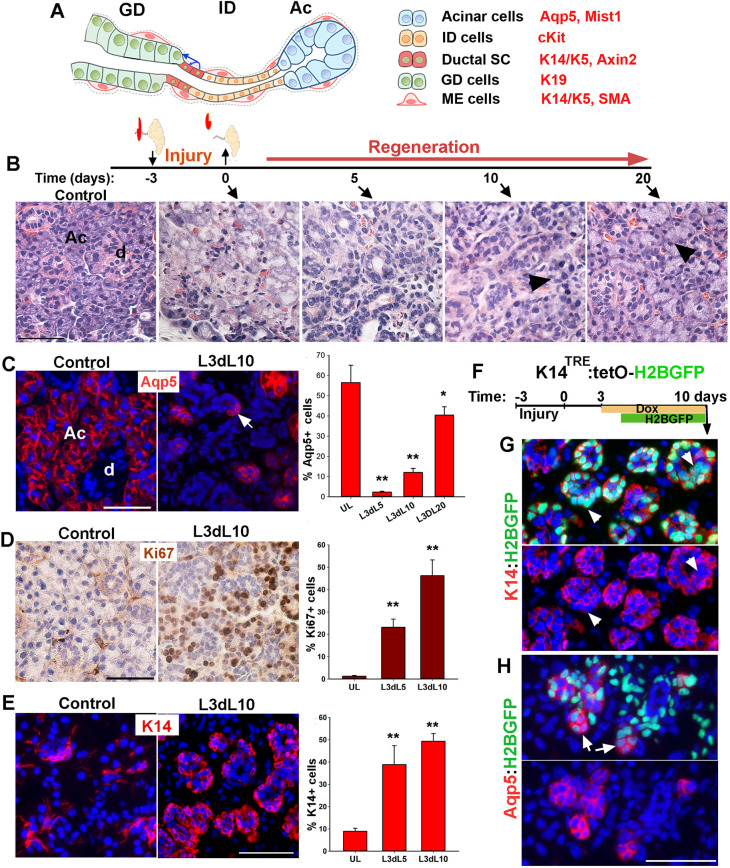
Characterization of salivary gland regeneration in a model of severe injury. (A) Schematic representation of secretory complex in mouse SMG and specific markers used to identify each cell type. Ac, acini; ID, intercalated duct; GD, granular duct. (B) Timeline of duct ligation/deligation injury and images of Hematoxylin and Eosin-stained sections of SMG at the indicated time points. Arrowheads indicate acini. (C) Distribution and quantification of Aqp5+ cells in control (UL) and injured gland collected at 5, 10 and 20 days post-de-ligation (L3dL). Arrow indicates proacinar cells budding from tubular structures. d, duct. (D,E) Distribution and quantification of Ki67 proliferation marker and K14 at days 5 and 10 post-ligation when tubular structures are present. Image shown is from day 10 post-de-ligation (L3dL10). Data are mean±s.e.m (n=3 females), one-way ANOVA and post-hoc Tukey's test (*P<0.05 and **P<0.001). (F) Timeline of nuclear tracking of K14-H2BGFP. (G,H) Images of regenerative gland stained for K14 or Aqp5. Arrowheads in G indicate nuclear GFP in K14neg cells. Arrows in H indicate acini-like structures with dim green fluorescence in their nuclei. Images are representative of n=3 female mice. Scale bars: 50 µm.
Cell behavior and mechanisms maintaining cell lineages during homeostasis often change dramatically in response to injury (Merrell and Stanger, 2016). Like many other tissues, SG regeneration after a severe tissue damage may involve dedifferentiation of various cell types (reviewed by Denny et al., 1997). Two classic injury models in rodents have been used extensively to study functional recovery and regeneration in SGs. In these models, injury is induced either by exposure of the neck to ionizing radiation or by unilateral ligation of the main excretory duct. Irradiation of the gland with a moderate dose (15 Gy) of radiation induces a bi-phasic injury with the initial cell loss being compensated for by rapid cell proliferation within 10 days, followed by extensive infiltration of inflammatory cells and acinar degeneration during the late phase of injury (>90 days) (Urek et al., 2005; Bralic et al., 2005). Lineage tracing of ductal and acinar cells in this model of injury have shown that the gland recovers from the early phase of injury in a lineage-restricted manner (Weng et al., 2018; May et al., 2018). During the chronic phase of injury, however, the progeny of ductal stem cells (marked by K5 or Axin2) partially contribute to the limited number of atypical acini detected in the degenerative gland, suggesting that, under some conditions, ductal stem cells can exhibit plasticity and multipotency (Weng et al., 2018).
Contrary to irradiation, ligation of the main excretory duct results in almost a complete loss of acinar cells within a few days (Takahashi et al., 2000; Martinez et al., 1982). Removal of the ligature induces a robust regenerative response that begins with emergence of embryonic-like tubular structures followed by regeneration of acini from these structures (Takahashi et al., 2004b; Cotroneo et al., 2008). However, genetic lineage tracing of ductal stem cells using K5-or Axin2-Cre drivers has shown that regeneration of acini after duct ligation/de-ligation is independent of ductal stem cells (Weng et al., 2018). The lack of plasticity of ductal stem cells was attributed to the survival of a substantial number of acinar cells and their rapid proliferation after de-ligation (Aure et al., 2015; Weng et al., 2018). In this model of injury, however, the severity and extent of acinar cell damage appears to be dependent on the degree to which the duct-associated blood and nerve supplies are constricted by the ligature (Denny et al., 1997). Despite the severity of injury, as long as the autonomic innervation is not permanently damaged, acinar regeneration proceeds rapidly after de-ligation, suggesting contribution from non-acinar cells (Walker and Gobé, 1987; Proctor and Carpenter, 2007).
Here, to investigate the cellular mechanisms contributing to acini regeneration after a severe and reversible glandular injury, we combined inducible lineage tracing of diverse cell populations with the model of severe obstruction-induced injury. Inclusion of the periductal tissues within the ligature in this model of injury has been shown to induce extensive acinar cell death, rather than atrophy (Walker and Gobé, 1987). Our analysis revealed a remarkable cellular plasticity of not only ductal stem cells but also differentiated epithelial cells, including myoepithelial and ID cells.
RESULTS
Regeneration of acini after severe injury is initiated from K14-expressing progenitors
To assess the extent of injury and dynamics of recovery of acini in a model of severe and reversible injury, adult FVB mice (8 weeks old, females) were subjected to unilateral ligation of Wharton's duct and periductal tissues for 3 days (hereafter referred to as severe ligature-induced injury); the ligature was removed and both SMGs were collected either immediately (time 0) or at 5, 10 and 20 days after de-ligation for tissue processing and analysis (Fig. 1B). SMGs collected from non-injured mice were used as controls. Histological analysis of SMGs at the time of de-ligation demonstrated a necrotic tissue associated with an acute loss of acini and GDs, with only a few acini remaining at the periphery of lobules, indicating a severe damage to the secretory complex within 3 days of ligation (Fig. 1B and Fig. S1A). The removal of ligature induced a progressive regenerative response, highlighted by rapid formation of tubular structures in the lobules, which continued with a slow reappearance of acini-like structures and subsequent transition into a nearly normal histology by 20 days post-de-ligation (Fig. 1B and Fig. S1A). Immunofluorescent staining of the control and injured SMGs with acinar cell markers, including aquaporin 5 (Aqp5) and Mist1, verified a nearly complete loss of acinar cells at the time of de-ligation (Fig. S1B,C). Analysis of SMG at later time points showed a gradual reappearance of small Aqp5+ cell clusters that were often associated with the tubular structures (Fig. 1C, arrow and Fig. S1B). Although the number of Aqp5-expressing cells returned close to the pre-injury level by day 20 post-de-ligation (Fig. 1C and Fig. S1B), the gland weight remained at about 76±13% (n=3, Fig. S1D) of normal glands, which is consistent with a previous report indicating a smaller size of the injured glands even after 8 weeks of recovery (Cotroneo et al., 2010). Evaluation of Ki67 proliferation marker showed a significant increase in the number of proliferating cells at 5 and 10 days after deligation (Fig. 1D), consistent with the rapid expansion of tubular and acini-like structures. Overall, these data validated a model of severe but reversible glandular injury in the adult SMG. It is worth noting that a similar regenerative response was observed when ligature was removed after 7 days (Fig. S1E); however, the ligation period was limited to 3 days throughout the remaining studies for consistency.
The robust proliferative response in tubular structures suggested progenitor cell activity. As K14 is a marker of embryonic and adult progenitor cells in the SG (Kwak et al., 2016; Lombaert et al., 2013), we assessed the expression of K14 at day 5 and 10 post-ligation, when tubular structures dominate the tissue. Analysis of SMG sections stained for K14 revealed a four- to fivefold increase in the number of K14+ cells (Fig. 1E). Moreover, these cells displayed a cuboidal morphology and localized mostly to the basal layer of these multilayered tubular structures (Fig. 1E). To determine whether K14+ cells directly contribute to acinar regeneration, we performed a short-term pulse-chase experiment in the K14TRE:tetOH2BGFP mouse model, which we have previously used to study proliferation dynamics of epithelial cells in the SMG (Kwak and Ghazizadeh, 2015). In this model, administration of doxycycline (Dox) induces expression of Histone2B-GFP (H2BGFP) fusion protein in K14-expressing cells. Incorporation of H2BGFP in the nucleosome of K14+ cells allows monitoring of cell division as nuclear GFP is passed on to their progenies, albeit at reduced fluorescent intensities (Tumbar et al., 2004). Adult bi-transgenic mice (8- to 10-week-old females) were subjected to severe ligature-induced injury and Dox was administered from 3-10 days post-de-ligation in order to label K14+ cells in tubular structures (Fig. 1F). The non-injured contralateral gland was used as a control. Analysis of SMGs collected at 10 days post-de-ligation showed robust expression of nuclear H2BGFP in tubular structures (Fig. 1G and Fig. S2A). Immunostaining for K14 protein verified selective expression of H2BGFP by K14-expressing cells, including ductal stem cells and MECs in the control glands (Fig. S2B-E, control). In the regenerated gland, however, nuclear GFP was detected not only in the K14+ cells but also in other cells within the tubular structures devoid of K14 protein (Fig. 1G, arrowheads). This was consistent with active cycling of K14+ cells and the passage of H2BGFP to their K14neg progeny. Interestingly, immunostaining for Aqp5+ showed low-intensity nuclear GFP in about 60% of newly formed clusters of Aqp5+ cells (Fig. 1H, arrows; Fig. S2D,F), suggesting that they were progeny of GFP+K14+ cells. In addition, about 20% of Kit+ luminal cells in tubular structures were also labeled with low-intensity nuclear GFP (Fig. S2E,F, arrowheads). Overall, these data demonstrate that K14-expressing cells in the transitory tubular structures function as progenitor cells for a subset of the newly regenerated acini in this model of severe injury.
K14+ cells in the adult mouse SMG survive and contribute to acinar regeneration after severe injury
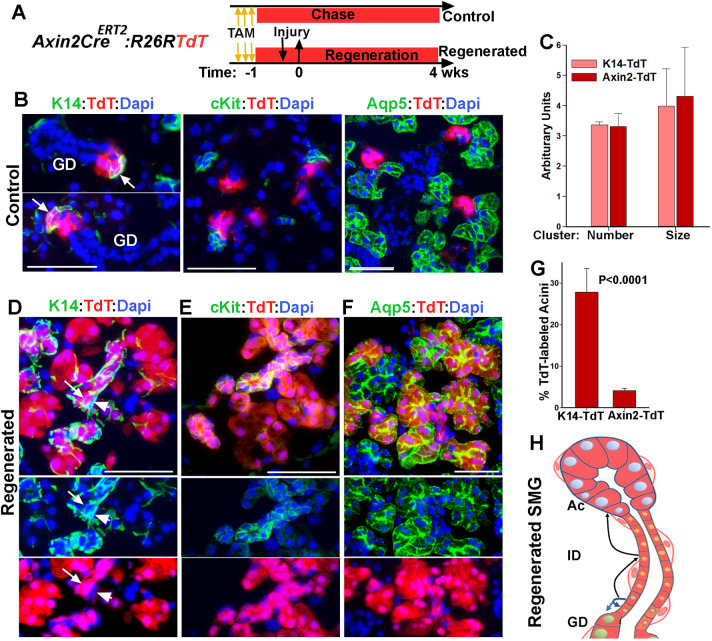
The broad expression of K14 in regenerative glands could be either due to disproportionate survival of K14+ cells and their subsequent expansion, or upregulation of K14 expression in other cell types. To distinguish between these two possibilities, an inducible genetic lineage-tracing approach in the K14CreTRE:R26RYFP mouse model (hereafter referred to as K14-YFP) was used to permanently and selectively mark K14+ cells before the injury and map their fate after recovery. We have previously shown that Dox administration for 4 consecutive days in these mice results in yellow fluorescent protein (YFP) labeling of K14-expressing cells, including ductal stem cells and MECs, with an efficiency of about 50% (Kwak et al., 2016). A similar strategy was used to label cells in the adult K14-YFP mice (8-10 weeks old) and 4 days later, before significant expansion of ductal stem cell progeny, mice were subjected to severe ligature-induced injury and allowed to recover for 4 weeks before both SMGs were collected for lineage-tracing analysis (Fig. 2A). For every mouse, the contralateral gland served as a control to assess the labeling efficiency and map the YFP+ cells in the absence of severe injury. In control SMG sections co-stained for K14 and YFP, 49±3.4% (mean±s.d., n=3 glands, >150 cells/gland) of K14-expressing cells were labeled with YFP. Furthermore, after a 5-week chase period, the progeny of YFP-labeled K14+ cells formed small cell clusters in the GD (Fig. 2B), consistent with our previous lineage-tracing studies in unperturbed glands (Kwak et al., 2016). Remarkably, analysis of the regenerated SMG revealed vast expansion of YFP-labeled cells into large cell clusters often encompassing the entire secretory complex (Fig. 2C-D). Fate-mapping analysis using phenotypic markers demonstrated that, in addition to the K14+ stem cells and their expected progeny of K19+ cells in the GD (Fig. S3A-B), numerous acini (Aqp5+) and their associated IDs (Kit+) were also expressing YFP (Fig. 2D-F), suggesting multilineage contribution from the initially labeled K14-YFP cells. Quantitative analysis showed that all lineage-labeled acini were uniformly labeled with YFP and the majority (92±5%, n=181 acini) were contiguous with YFP-labeled Kit+ ID cells (Fig. 2D-F and Fig. S3C). The latter cells, however, did not always expand into the ID/GD junction where ductal stem cells reside (Fig. 2F and Fig. S3C). This suggests that some of the lineage-labeled acinar and ID cells are not the progeny of ductal stem cells, instead they might have been derived from MECs, which were also labeled with YFP prior to the injury. However, it was difficult to establish a lineal relationship between MECs and acini in this mouse model, as solitary YFPneg MECs were often found surrounding YFP-labeled acini and YFP+MECs were surrounding the YFPneg acini (Fig. 2G and Fig. S3C, arrows). This was likely due to sporadic distribution of the initially labeled MECs and migration of MECs during regeneration (Takahashi et al., 1999).
Fig. 2.
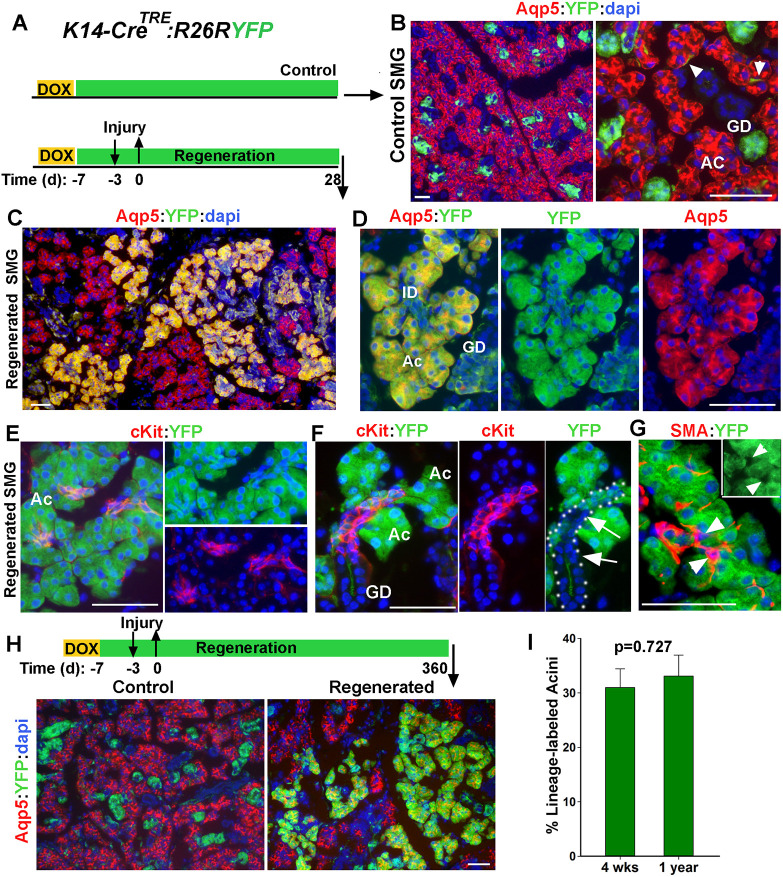
Lineage tracing of K14+ cells during injury and recovery of SMG. (A) Timeline of lineage tracing of K14-expressing cells during injury in transgenic K14CreTRE;R26RYFP female mice. (B) YFP expression in control SMG stained for Aqp5. Arrowheads indicate the YFP-labeled MECs. (C-F) Distribution of YFP-labeled cells in regenerated SMGs co-stained for YFP and Aqp5 or Kit. Dotted line in F outlines ID and GD with arrows indicating YFPneg ID cells. (G) Distribution of YFP− SMA+ cells in labeled cell clusters. Arrowheads indicate YFP−SMA+ cells. (H) Images of lineage-traced cells in control and regenerated gland 1 year after injury stained for Aqp5. Ac, acini; GD, granular duct. Scale bars: 50 µm. (I) Percentage of YFP-labeled Aqp5+ in regenerated SMG collected at 4 weeks or 1 year after injury. Data are mean±s.e.m (n=3 glands), two-tailed Student's t-test.
Quantification of Aqp5+YFP+/total Aqp5+ cells indicated that, at 4 weeks post-injury, the proportion of YFP-labeled acini in the regenerated SMGs was about 32.5±3% (mean±s.d., n=3 glands). Given the initial labeling efficiency of about 50% for K14+ cells, this implies that nearly 65% of the regenerated acini were derived from the surviving K14+ cells. Previous studies in skin epithelia have suggested that lineage reversion by committed progenitors is not sustained long term (Mascre et al., 2012). To determine whether lineage-labeled acini derived from K14+ cells persist over a long period of time, the chase period was extended to 1 year post-injury (Fig. 2H). Remarkably, there was no significant change in the pattern of YFP-labeled cell clusters or the frequency of YFP-labeled Aqp5+ cells from 4 weeks to 1 year post-injury (Fig. 2H-I), demonstrating persistence of multi-lineage YFP-labeled cell clusters in the regenerated gland. Taken together, these data indicate that K14+ cells in the adult SMG survive the severe ligature-induced injury, expand their differentiation potential, and contribute to the repair and regeneration of multiple cell lineages in the secretory complex.
Myoepithelial cells are the primary K14+ cell population contributing to acinar regeneration after injury
In the K14-YFP mouse model, both ductal stem cells and MECs were labeled prior to the injury and could have potentially contributed to de novo formation of acini after injury. To determine whether MECs contribute to acinar regeneration after injury, we used αSMACreERT2;R26R-tdTomato (SMA-TdT) in which tamoxifen (TAM)-inducible Cre expression is driven by the Acta2 promoter, to induce specific and permanent expression of the red fluorescence protein tdTomato (TdT) in MECs (Grcevic et al., 2012). Unlike ductal stem cells, MECs do not contribute to any other cell lineages during SMG development or adulthood (May et al., 2018). Therefore, TAM was administered at least 4 weeks before injury to prevent any residual drug from acting in response to injury. Adult transgenic mice (8-10 weeks old) were subjected to severe ligature-induced injury and both SMGs were collected 4 weeks later for lineage-tracing analysis (Fig. 3A). Immunostaining of sections from control SMGs for phenotypic markers, including SMA, K14, Kit and Aqp5 verified exclusive expression of TdT by SMA+ cells with an efficiency of 50.5±4.8% (mean±s.d., n=3 glands) (Fig. 3B and Fig. S4A). On the other hand, TdT-labeled cells in the regenerated SMG had expanded into large cell clusters composed of Aqp5+ and Kit+ cells (Fig. 3B,C and Fig. S4B-D), indicating contribution of the initially labeled MECs to regeneration of both acinar and ID cells, likely through the process of transdifferentiation. Interestingly, TdT-labeled acini and ducts were often surrounded by MECs that did not express TdT (Fig. 3D, arrowheads), suggesting that repopulation of MECs is not driven by the same progenitor cell that replenishes acinar and ID cells. More importantly, TdT expression was limited to acini and their adjacent region of the ID but did not advance further towards K14+ ductal stem cells (Fig. 3E, arrows; Fig. S4E). Quantification of TdT+Aqp5+ cells indicated that 24.4±1.1% (mean±s.d., n=3 glands) of all acini in the regenerated glands derived from the initially labeled MECs. The proportion of lineage-labeled acini in SMA-TdT mice was comparable with that in K14-YFP mice (Fig. 3F), identifying MECs as the primary source of regenerated acini in this model of injury.
Fig. 3.
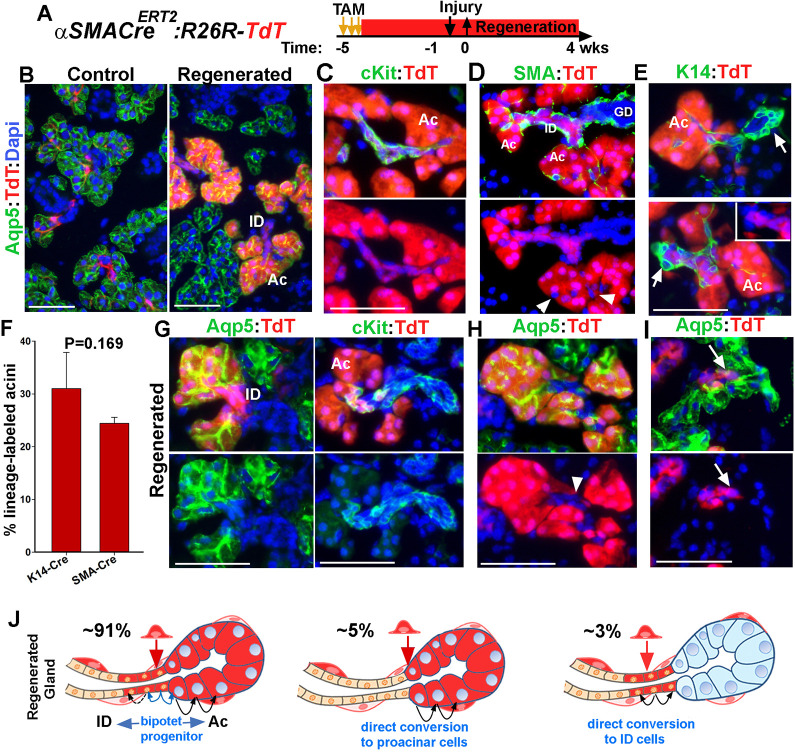
Contribution of myoepithelial cells to glandular regeneration after injury. (A) Timeline of lineage tracing of MECs with tamoxifen (TAM) and injury in αSMACreERT2;R26RTdT mice. (B) Expression of TdT in control and regenerated SMGs stained for Aqp5. (C,D) TdT distribution in regenerated SMGs stained for Kit and SMA. Green channel is not shown in lower panels to clearly show TdT− myoepithelial cells (arrowheads) surrounding the lineage-labeled acini. (E) Images of regenerated gland stained for K14. Arrows indicate the location of ductal stem cells. (F) Quantification of TdT-labeled Aqp5+ in the regenerated glands of K14-YFP and SMA-TdT mice at 4 weeks post-injury (mean±s.d., n=3 females, two-tailed Student's t-test). (G-I) TdT expression in acini and their associated ID. Sections are stained for Aqp5 or Kit; arrowhead indicates TdT+ acini connected to unlabeled IDs. Arrows indicate a TdT+ duct connected to unlabeled acini. ID, intercalated duct; Ac, acini; GD, granular duct. Blue nuclear staining is DAPI. Scale bars: 50 µm. (J) Schematics showing the three patterns of lineage-labeled cells at Ac/ID junctions, their frequencies and suggested mechanisms. Blue arrows indicate bipotent ductal progenitors that redifferentiate into proacinar and Kit+ ID cells. Black arrows indicate self-duplication.
Transdifferentiation is referred to a change in cellular identity from one differentiated cell type to an alternative differentiated state. This may result either from dedifferentiation to a progenitor-like state followed by redifferentiation to a distinct cell type, or by direct conversion from one cell fate to another (Merrell and Stanger, 2016). Analysis of the pattern of TdT-labeled acinar and ID cells within the regenerated SMGs of SMA-TdT mice showed that all acinar cells within the lineage-labeled acini (n=478 from 3 glands) were uniformly expressing TdT, suggesting their clonal origin (Fig. 3 and Fig. S4). Analysis of acini/ID junctions showed that although a small number of TdT-labeled acini were connected to unlabeled IDs (Fig. 3H and Fig. S4E) or a small number of TdT-labeled ID cells were connected to unlabeled acini (Fig. 3I and Fig. S4G), the vast majority (91.1%±5.6%; mean±s.d.; n=3 glands, a total of 158 acini/ID junctions) were contiguous with at least a few TdT+Kit+ cell in the ID (Fig. 3C-G, Fig. S4F). Examination of SMG sections where the entire longitudinal dimension of IDs was visible, showed that in 61.1%±5% of the IDs (mean±s.d., n=3 glands, a total of 97 IDs), the lineage-labeled Kit+ cells did not span the entire length of the duct (Fig. 3G and Fig. S4F). This was comparable with the distribution pattern of some of the lineage-labeled acini and IDs in the regenerated gland of K14-YFP mice in which YFP-labeled cells did not expand toward the position of ductal stem cells (Fig. 2F, arrows; Fig. S3C). The contiguity of lineage-labeled acini and their associated IDs suggests that injury induces transdifferentiation of the initially labeled MEC into a bi-potent progenitor cell that gives rise to both Kit+ ductal cells and proacinar cells; the latter subsequently self-replicate to repopulate an acinus, although a small proportion of MECs may directly convert to ID cells or proacinar cells (Fig. 3J).
SMG myoepithelial cells have a limited proliferative capacity in culture
The robust response of MECs to injury could be attributed to activation of slowly dividing stem cells rather than trans- or dedifferentiation. In the mammary gland, MECs are maintained as self-sustained cell population but display functional properties of stem cells when placed at clonal density in culture (Prater et al., 2014). However, whether salivary MECs display similar characteristics have not been determined. To address this, we first sought to compare the colony-forming ability of MECs and ductal stem cells purified from the same gland in adherent cultures. Therefore, we genetically labeled K14-expressing cells in K14TRE:H2BGFP mice (6 weeks old) and then differentially isolated MECs and ductal stem cells based on the expression levels of integrin α6 by FACS (Fig. 4A) (Ogawa, 2003; Lu et al., 2012). Cytospin and immunofluorescent staining of the sorted cell populations for K14 and SMA verified enrichment of SMA+ cells with a star-shaped morphology in the α6highGFP+ cell fraction and those of SMAneg cuboidal-shaped cells in the α6medGFP+ cell fraction (Fig. 4B). When sorted cells were cultured at clonal density and grown for 2 weeks, ductal stem cells formed large colonies (≥0.5 mm in diameter) with a colony-forming efficiency of 4.5±0.36% (Fig. 4C), consistent with their function as tissue stem cells. On the contrary, the colony-forming efficiency of MECs was 0.15±0.1%, as the majority of colonies formed by MECs were abortive (Fig. 4C), indicative of their low proliferative capacity. In addition to clonogenicity, stem cells are assessed by their ability to form organoids in culture (Kopp et al., 2016). When SG cell suspensions are placed in three-dimensional cultures, they form small number of organoids (Lombaert et al., 2008). More recent studies have shown that organoids originate from K14+ cells, although no distinction was made between ductal stem cells and MECs (Kwak et al., 2018). To assess the capacity of MECs to form organoids, SMG cells were isolated from TAM-induced SMA-TdT mice (6-8 weeks old) and the fate of TdT-labeled MECs was tracked in organoid cultures (Fig. 4D). SMG cells from Dox-induced K14CreTRE:R26RTdT mice (7-8 weeks old; K14-TdT) were used as a control. Interestingly, despite a comparable TdT-labeling efficiency in these two transgenic lines (49.5±4.7% for SMA-TdT and 47±1.9% for K14-TdT, n=4 mice), traced MECs formed very few TdT+ organoids (3.2±1.1%, n=4 glands, >1100 spheres/gland) when compared with traced K14+ cells (47.6±7.6%, mean+s.d., n=4 glands, >850 spheres/gland) (Fig. 4E,F). The direct correlation between the TdT-labeling efficiency of K14+ cells in vivo and that of organoids in culture indicates that almost all organoids originate from ductal stem cells. Overall, functional analysis of MECs in two culture systems indicates that salivary MECs have low proliferative capacity in culture and may not function as quiescent stem cells. Therefore, the robust regenerative response of MECs to injury is likely due to transdifferentiation of MECs rather than stem cell activation.
Fig. 4.
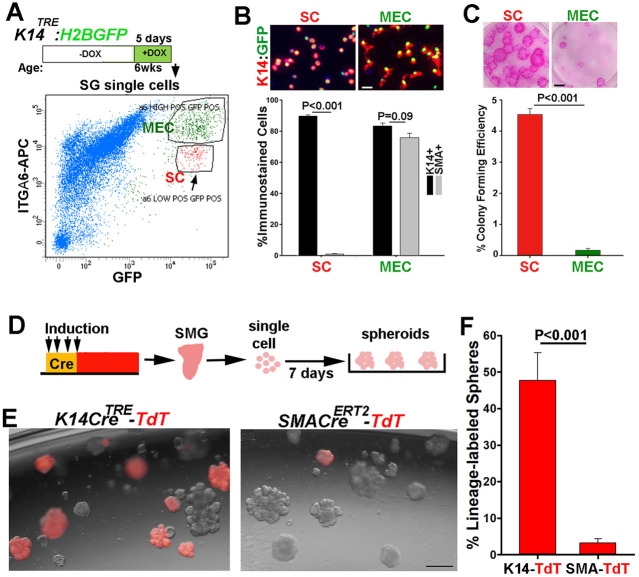
MECs do not contain highly proliferative progenitors. (A) FACS profile of Dox-induced K14-H2BGFP-labeled cells stained with APC-anti-integrin α6 antibody. Arrow indicates the ductal stem cell population expressing lower levels of integrin α6. (B) Cytospin analysis of sorted GFP+α6hi (MEC) and GFP+α6med (SC) cells. Scale bar: 20 μm. Graph shows percentages of K14+ (black bars) and SMA+ (gray bars) cells in each population (n=200 cells/population). (C) Colony formed from sorted cells seeded at 500 cell/well, grown for 2 weeks and stained with rhodamine. Scale bar: 1 mm. Graph shows colony-forming efficiency (diameter ≥0.5 mm). (D) Strategy used to induce label in either SMA+ or K14+ cells in vivo and analyze their fate in organoid cultures. (E) Images of organoids established from SMG cells of K14-TdT and SMA-TdT mice after 7 days in culture. Scale bar: 200 µm. (F) Percentage of TdT-labeled organoids in culture (>1100 organoids counted/gland). Values are mean±s.e.m, from at least two independent experiments. Two-tailed Student's t-test, P-values are indicated.
Ductal stem cells undergo a unipotency to multipotency switch in response to injury
To determine whether K14+ ductal stem cells make any contribution to acinar regeneration in this injury model, we traced the lineage of ductal stem cells in Axin2CreERT2;R26RTdT (Axin2-TdT) mice (Fig. 5A). K14-TdT mice were treated in parallel and used for comparative analysis. Axin2Cre driver has been shown to target a subset of K5+/K14+ ductal cells but is not active in MECs (Weng et al., 2018). In the control SMG of Axin2-TdT mice, TdT-labeled cells were localized to small cell clusters near the ID/GD junctions, which consisted of K14+ ductal stem cells and their progeny (Fig. 5B, arrows), but excluded cells expressing Kit, Aqp5 or SMA (Fig. 5B). Moreover, the size and distribution frequency of TdT-labeled cell clusters in Axin2-TdT mice were comparable with those in K14-TdT mice, with the exception that TdT-labeled MECs appeared only in the latter (Fig. 5C and Fig. S5A,B). In the regenerated SMGs, however, a few but relatively large TdT-labeled cell clusters composed of ductal and acinar cells were detected (Fig. 5D-F and Fig. S5B). In these cell clusters, TdT could be traced from K14+ ductal cells (Fig. 5D, arrow) along the length of IDs (Kit+) into acini (Fig. 5D,E), indicating multi-lineage contribution from ductal stem cells. However, SMA+ cells (or K14+ basal cells) juxtaposed to the TdT-labeled cell clusters were completely clear of TdT expression (Fig. 5D, arrowheads; Fig. S5D, arrows), supporting the independence of MEC lineage from ductal stem cells. Quantification of TdT+Aqp5+ cells showed a significantly lower frequency for lineage-labeled acini in the regenerated SMGs of Axin2-TdT mice (4.1%±0.5%) when compared with those in K14-TdT mice (28±5%) (Fig. 5G and Fig. S5B). This is consistent with the dominant contribution of MECs to acinar regeneration in this model of injury. Taken together, these data indicate that the multilineage differentiation capacity of ductal stem cells is induced in this model of injury (Fig. 5H). However, their contribution to acinar regeneration is not as significant as the contribution made by MECs, possibly owing to the scarcity of ductal stem cells in the adult SMG when compared with MECs (∼2.5% versus ∼8% of total parenchymal cells, respectively) (Kwak, et. al. 2016).
Fig. 5.
Severe injury induces a switch to multipotency in ductal stem cells. (A) Timeline of lineage tracing of ductal stem cells in Axin2-CreERT2;R26RTdT in control and injured glands. (B) TdT expression in control SMG stained for K14, Kit and Aqp5. Arrows indicate K14+ ductal stem cells. (C) Comparative analysis of TdT-labeled clusters in control SMGs of Axin2-TdT and K14-TdT mice after a 5-week chase period. (D-F) TdT expression in the regenerated SMG stained for K14, Kit and Aqp5. Arrow and arrowheads in D indicate ductal stem cells and MECs, respectively. Nuclear blue is DAPI. Scale bars: 50 µm. (G) The percentage of TdT-labeling in acinar cells in the regenerated glands K14-TdT and Axin2-TdT mice. Data are mean±s.d. (n=3 females), two-tailed Student's t-test. (H) Summary of results of lineage tracing of ductal stem cells. Blue arrows indicate normal fate of stem cells; black arrows indicate expansion of their differentiation potential.
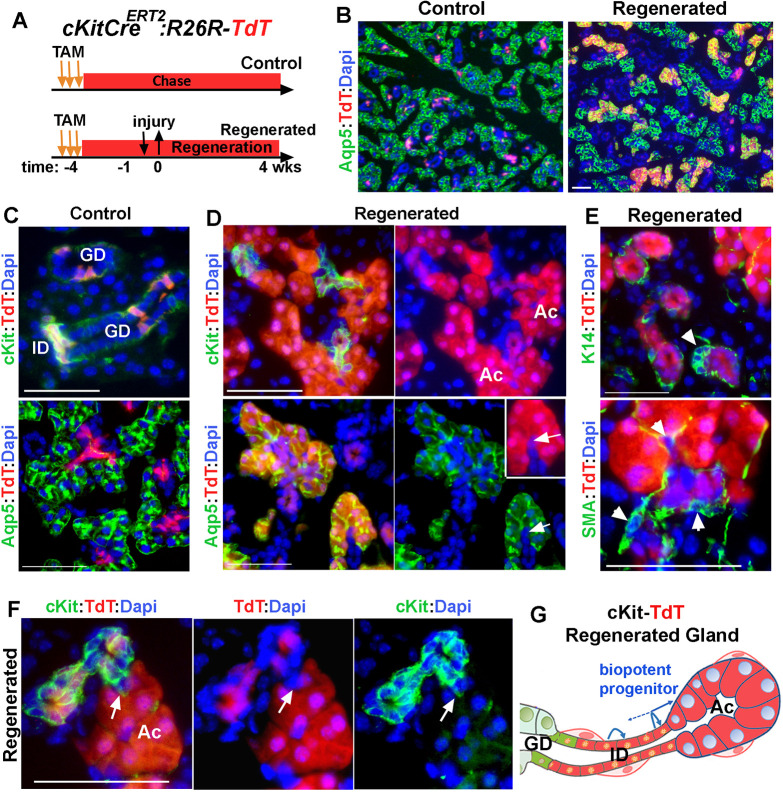
Kit+ ductal cells function as reserve progenitors for acinar cells
The injury-induced plasticity of MECs toward acinar cell differentiation appeared to transition through Kit+ bipotent progenitors as an intermediate cell population. In the adult SMG, Kit marks the ID cells as well as tuft cells that are scattered in the larger ducts; however, neither cell gives rise to any other cell types within the gland (Kwak et al., 2018). More importantly, when placed in culture, Kit+ cells do not form organoids, indicating that they do not function as progenitor cells in the adult gland (Kwak et al., 2018). During SMG development, however, Kit+ marks progenitors that differentiate into ID and acinar cells (May et al., 2018). Therefore, we hypothesized that severe injury provokes dedifferentiation of Kit+ ID cells to an earlier progenitor-like state that will generate acinar cells. To test this, we traced the lineage of Kit+ cells in this model of injury using KitCreERT2/+; R26RTdT (Kit-TdT) mice (Fig. 6A). The specificity of TdT expression in Kit+ cells were verified in control SMG (Fig. 6B,C and Fig. S6A,B) and the TdT-labeling efficiency of Kit+ ID cells was estimated to be at 71%±7.4% (Fig. S6B; mean±s.d., n=3 glands), which was consistent with our previous studies (Kwak et al., 2018). Fate mapping of Kit-lineage cells in the regenerated SMGs revealed expansion of the TdT-labeled cells from IDs into acini (Fig. 6B and D and Fig. S6C). This expansion was directed only toward acini, as we did not detect any TdT-expressing K14+ ductal cells or MECs in the regenerated gland (Fig. 6E, arrowheads and Fig. S6D,E). These data suggested that, in response to this injury, Kit+ ID cells function as reserve acinar progenitor cells (Fig. 6G). Quantitative analysis of Aqp5+ cells indicated that 26±8.9% (mean±s.d., n=3 glands) of acinar cells were expressing TdT. Given the initial labeling efficiency of about 70% for Kit+ ID cells, these results indicated that ∼37% of the total regenerated acini derived from Kit+ ID cells (Fig. 7).
Fig. 6.
Contribution of Kit+ ductal cells to regeneration of acini. (A) Timeline of label induction and lineage tracing of Kit+ cells during injury and regeneration. (B-D) TdT-lineage traced cells in control and regenerated glands stained for Aqp5 or Kit. Arrows in D indicate a single TdT+ ID cell contiguous with TdT+ acini. (E) Images of TdT-labeled cell clusters stained for K14 and SMA. Arrowheads indicate ductal stem cells. (F) A small number of TdT-labeled acini are not contiguous with TdT-labeled Kit+ cells (arrow). Nuclear blue staining is DAPI. ID, intercalated duct; AC, acini; GD, granular duct. Scale bars: 50 µm. Images are representative of three glands from female mice. (G) Summary of results of lineage tracing of ID cells, indicating dedifferentiation of Kit+ cells to a bi-potent progenitor cell population that re-differentiated to both ID and acinar cells. Distal ID cells may self-replicate.
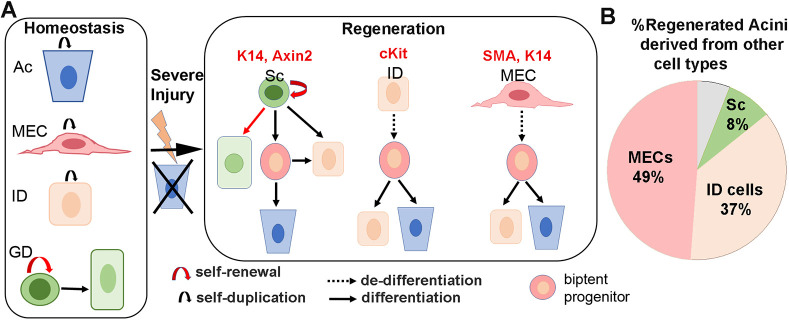
Fig. 7.
Severe injury provokes lineage plasticity of ductal and myoepithelial cells toward acinar cell differentiation. (A) The cell lineages in the adult SMG are maintained in lineage-restricted manner under homeostasis. Ac, acini; MEC, myoepithelial cells; ID, intercalated duct; Sc, ductal stem cell; GD, granular duct cell. Severe injury induces multipotency in ductal SCs and promote dedifferentiation of Kit and MECs into a bi-potent progenitor-like state that re-differentiates to ID and Ac. The gene derivers used for lineage tracing of each cell lineage are indicated on the top. (B) The contribution of each cell lineage to acinar regeneration after correcting for the initial labeling efficiency.
The expression pattern of TdT in regenerated glands of Kit-TdT mice was almost identical to that in SMA-TdT mice. Although all acinar cells within an acinus appeared to be clonal in origin (Fig. 6D-F and Fig. S6C-G), TdT expression in the ID was more heterogeneous, with about 55% of the IDs (n=142) composed of a mixture of TdT+ and TdTneg patches of Kit+ cells (Fig. 6F and Fig. S6G). Moreover, analysis of Acini/ID junctions demonstrated that, despite the presence of a few TdT+ acini connected to non-labeled IDs (Fig. 6F, arrow and Fig. S6G), the majority of TdT+ acini (91.6%±5.7%, mean±s.d.; n=100 from three glands) were traced to the TdT+ IDs (Fig. 6D and Fig. S6F). This pattern is consistent with dedifferentiation of Kit+ cells to an earlier progenitor-state when Kit+ cells function as bi-potent progenitor cells (May et al., 2018). The similarity in organization of lineage-labeled cells in the regenerated glands of Kit-TdT and SMA-TdT mice suggests that both myoepithelial and ID cells revert into a common bipotent progenitor like-state before redifferentiation to acinar cells (Fig. 7).
DISCUSSION
The robust regenerative responses of SGs to injury suggest that there are efficient and effective mechanisms in place to maintain the secretory component and gland function. Here, we used a model of reversible glandular injury in which acinar cells are severely depleted and therefore cannot undergo compensatory self-replication to reveal the cellular mechanisms underlying de novo formation of acini in the adult gland. By tracing the lineage of three distinct non-acinar cell populations that reside in the secretory complex, we uncovered a remarkable cell plasticity and reversibility in epithelial cells within this compartment. Our findings reveal that both stem cells and differentiated cells contribute to regeneration of secretory units following injury to allow rapid recovery of glandular tissue. We show that in this model of injury, K14+/Axin2+ ductal stem cells expand their differentiation capacity to include Kit+ ductal and acinar cell lineages, suggesting the induction of a unipotency to multipotency switch (Fig. 7). However, this is not the major mechanism by which acini are regenerated. Intriguingly, our findings demonstrate that most of the regenerated acini derive from differentiated cells, including MECs and Kit+ ductal cells. Contrary to ductal stem cells, these cells display a very slow turnover rate and normally do not contribute to any other cell lineages in the adult gland (Kwak et al., 2018; Kwak and Ghazizadeh, 2015; May et al., 2018). However, in response to a severe insult, they appear to participate in tissue repair and regeneration by reverting into a bi-potent progenitor-like state and subsequently re-differentiate to acinar cells (Fig. 7). Therefore, at least in the model of severe glandular injury used in our studies, plasticity of differentiated parenchymal cells is the predominant mechanism of acini regeneration.
We used a severe but reversible model of injury to investigate the degree of cellular plasticity in the secretory complex of adult SMGs when acinar cells are severely damaged. Although this injury model has been used extensively to study salivary gland repair and regeneration, the source of acinar regeneration that follows de-ligation has been a subject of much speculation. Whereas some studies found that acinar regeneration occurred mainly by self-duplication of surviving acinar cells (Burgess and Dardick, 1998; Aure et al., 2015), others suggested that ductal cells differentiate to acinar cells (Cotroneo et al., 2010; Takahashi et al., 1998; Walker and Gobé, 1987). The compelling evidence provided by our fate-mapping analyses of two distinct ductal cell populations (Kit+ cells and K14+/Axin2+ cells) are consistent with the latter and underlines the plasticity of ductal cells toward acinar cell differentiation during the repair of secretory complex. Our data, however, contradict previous lineage-tracing studies that did not find any contribution from K5+ or Axin2+ ductal cells to regeneration of acini following duct ligation and de-ligation (Weng et al., 2018). This is likely related to the severity of obstruction-induced injury and the extent of acinar cell damage (i.e. atrophy versus cell death) which appears to be dependent on the degree to which the duct-associated blood and nerve supplies were obstructed by the ligature (Denny et al., 1997). In our study, the inclusion of periductal tissue within the ligature induced a severe damage to secretory cells within 3 days of ligation, while in the study reported by Weng et al. (Weng et al., 2018), periductal tissues was not included in the ligature and a substantial number of acinar cells were present even after 2 weeks of duct ligation. Indeed, in a parallel comparison between obstructing salivary duct with and without periductal tissue, we confirmed survival of a substantial number of acini and a lack of contribution from Kit+ cells to regenerated acini in the latter model of injury (Fig. S7). Clearly, the type of injury and the resulting wound environment induced by obstruction of duct and associated vascular and neural tissues is very different from those induced by the isolated duct obstruction and further investigation will be needed to elucidate key differences that provoke plasticity of non-acinar cells toward acinar cells.
Our analyses provide the first direct evidence demonstrating the injury-induced plasticity of MECs to other cell types in the SG. Although previous studies have described the presence of proliferating MECs covering newly formed acini during regeneration of SMG after duct deligation (Takahashi et al., 2004a), their role in acinar regeneration was not known. MECs are found on the basement membrane of all exocrine glands and have been known to display functional properties of stem/progenitor cells in some organs such as mammary gland and submucosal glands of the lung (Tata et al., 2018; Lynch et al., 2018; Prater et al., 2014). Although mammary MECs represent a long-lived lineage-restricted cell population in vivo, when placed in adherent cultures more than half of MECs are clonogenic, indicating their robust proliferative potential (Prater et al., 2014). Salivary MECs are also a self-sustained cell lineage during development and throughout adulthood (Kwak and Ghazizadeh, 2015; May et al., 2018; Song et al., 2018), and their robust regenerative response to injury suggested that salivary MECs may also contain quiescent stem cells. However, using two well-established culture systems that have been used to assess stemness in various tissues, we found no evidence for the existence of cells with high proliferative potential within the salivary MECs. Given that MECs do not appear to function as progenitors in the normal SMG, their multilineage contribution to the regeneration of secretory complex can be best explained by reversion of a differentiated cell into a progenitor-like state (Mannik et al., 2010; Blanpain and Fuchs, 2014). The distribution pattern of MEC-derived lineage cells in the regenerated secretory complex supports this conclusion. The uniform fluorescence labeling (YFP or TdT) of cells within regenerated acini suggests that each newly formed acinus originated from a single labeled proacinar cell that was a product of the initially labeled MEC. Given that the efficiency of Cre recombination in the K14-CreTRE or SMA-CreERT2 mice was about 50%, contribution from more than one proacinar would have resulted in a mosaic pattern of labeled and unlabeled acinar cells, at least within some acini. Furthermore, more than 90% of the lineage-labeled acini were contiguous with at least one lineage-labeled Kit+ cell in the associated ID. More importantly, a similar pattern was observed in Kit-lineage-labeled cell clusters, suggesting that both Kit+ ID cells and MECs revert to a common bi-potent progenitor-like state to generate a Kit+ ductal cell and a proacinar cell that subsequently self-replicates to repopulate/regenerate the acinus. Interestingly, the presence of transitory tubular structures during the regenerative phase (5-10 days after deligation) of recovery in this model of injury recapitulates the perinatal stage of gland development, both morphologically and chronologically (Fig. 1) (Cotroneo et al., 2010). During SMG development, the differentiation of proacinar, ID and myoepithelial cells begins at the same time (E15-E16) from a common K14+/Kit+/Sox10+ progenitor and Sox10 has been identified as key transcription factor controlling plasticity and multipotency of this cell population (Lombaert et al., 2013; Athwal et al., 2019). Although this supports our conclusion that both Kit+ cells and MECs in the injured adult SMG may revert back to a common progenitor-like state, Sox10 is broadly expressed in acini, their associated ID cells and MECs, even in the unperturbed adult mouse SMG (Fig. S8) (Ohtomo et al., 2013). The identity of the major constituents of the embryonic-like phenotype/microenvironment promoting the plasticity of ductal and myoepithelial cells, and how Sox10 and many other factors, including inflammation, may function in this model of injury await further investigation.
In summary, our findings reveal a remarkable plasticity of ductal and myoepithelial cells toward acinar cell differentiation provoked by a severe injury to secretory complex in the adult salivary gland. Salivary glands rely on the recruitment of differentiated cell populations as well as stem cells to ensure rapid regeneration and recovery of secretory cells. Further studies in this model of injury will provide greater clarity on environmental signals that control fate decisions in the context of severe glandular injury. Such studies are likely to yield important information regarding epithelial tissue plasticity that could be employed to develop new effective therapies aimed at reversing hyposalivation.
MATERIALS AND METHODS
Animal studies
The care and experimental use of animals including the surgical procedures were approved by the Institutional Animal Care and Use Committee (IACUC protocol 287271) of the Stony Brook University, Stony Brook, New York, USA. Animals were maintained in the Stony Brook University-Health Science Center Division of Laboratory Animal Resources, an AAALAC-accredited center, and housed in groups of up to five mice per cage in a pathogen-free facility on a 12 h light/dark cycle provided with food and water ad libitum. All animal experiments were performed in accordance with institutional guidelines set forth by the State University of New York. Adult mice (>8 weeks of age) of the following transgenic strains were used: transgenic tetO-H2BGFP (stock 005104), K14rtTA (stock 008099), Axin2-CreERT2+/− (stock 018867), Cre reporter lines R26R-EYFP (stock 006148) and R26R-tdTomato (stock 007914), all purchased from Jackson Laboratory. K14CreTRE and Kit-CreERT2+/− transgenic lines have been described previously (Kwak et al., 2016; Klein et al., 2013). αSMA-CreERT2+/− has bee described previously and was generously provided by Dr Ivo Kalajzic's lab (Grcevic et al., 2012). Kit-CreERT2+/− and αSMA-CreERT2+/− were maintained on a C57BL/6 background. Mice were genotyped by polymerase chain reaction using mouse genomic DNA from tail biopsy specimens. For lineage-tracing experiments Cre-mediated recombination was induced in mice by replacing regular diet with doxycycline- (1 g/kg pellet) or tamoxifen-containing (250 mg/kg pellet) diet for 4-5 days. Mice were returned to regular diet for 4 days to 4 weeks before subjected to injury. Surgical procedures were performed under anesthesia and using aseptic techniques. A small incision was made on the lateral side of the neck and the main excretory duct and associated vessels on one side of the neck were dissected using a surgical stereoscope. A small plastic microtube (2 mm in length; sterile intramedic polyethelene tubing, 427400, Becton Dickinson) was placed along the duct and they were tied together using 6-0-black-silk suture (Thermo Fisher Scientific); the incision was closed with surgical sutures. When ligating Wharton's duct by itself, the duct was carefully separated from accompanied periductal tissues before placement of the microtube and ligature. At the time of de-ligation, mice were anesthetized and external sutures were removed to expose the ligated duct; microtube was removed and suture was cut to release the obstruction. The area was rinsed with saline and the incision was closed. Animals were left to recover for the indicated period of time before both ligated and contralateral glands were harvested and processed.
Tissue processing and immunofluorescent staining
SMGs were harvested and the attached fat and connective tissues were dissected. SMGs were either fixed in 10% formalin for 24 h for paraffin embedding or in 4% paraformaldehyde at 4°C for 1 h followed by 30% sucrose before cryopreservation. Cryosections (5 μm) were prepared, dried for at least 2 h, rehydrated and immunostained as described previously (Kwak and Ghazizadeh, 2015). The following primary antibodies were used: anti-GFP/YFP (chicken, 1:2000, ab13970, Abcam) for the R26R-YFP reporter strain, anti-Kit (rat, 1:100, 14-1171-82, eBioscience), anti-Aqp5 (rabbit, 1:100, 178615, EMD Millipore), anit-Mist1 (rabbit, 1:50, 14896, Cell Signaling Technologies), anti K19 (rat, 1:10, TROMA-III, DHSB), anti-K14 (rabbit, 1:2000, RB-155P, Covance) and anti α-SMA (rabbit, 1:100, ab5694, Abcam) (Kwak et al., 2016). Alexa-594- or Alexa-488-conjugated secondary antibodies were from Molecular Probes (1:500, Invitrogen). TdT was detected by direct fluorescence. Sections were mounted in Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Fluorescent staining was visualized using a Nikon E800 fluorescent microscope and images were captured using NIS-Elements (Nikon Instrument). ImageJ was used for quantification of labeled cells using images from a pool of at least five images taken at 100× or 15 images taken 400× from multiple sections stained for each control and regenerated gland.
SG cell preparation, cell sorting and cultures
SMGs were dissected, dissociated by mechanical and enzymatic digestion (0.025% collagenase, 0.05% hyaluronidase, 1 U/ml dispase) to single cell suspensions as described previously (Kwak and Ghazizadeh, 2015). For adherence cultures, single cell suspensions prepared from H2BGFP-labeled SMGs were re-suspended in PBS-1% bovine serum albumin and stained with APC-conjugated antibody to integrin α6 (clone GoH3, from eBioscience) for 20 min at 4°C in dark. Cells were washed with PBS-1% BSA and re-suspended in PBS-2% fetal bovine serum. Cell sorting was performed on FACSAria-III with FACSDiva software (BD Biosciences). Sorted cells were plated at 50 cells/cm2 in the presence of gamma-irradiated 3T3 fibroblasts as described previously (Kwak and Ghazizadeh, 2015). Colonies were visualized and counted after staining with 1% rhodamine B. For organoid cultures, single cell suspensions of SMGs were re-suspended to 1×104 cells in 40 µl media, mixed with 60 µl Matrigel (BD Biosciences) on ice, seeded in the periphery of a 12-well tissue culture plate and transferred to a 37°C incubator for 20 min to solidify. Gels were covered in a modified salisphere growing media containing epidermal growth factor (20 ng/ml), fibroblast growth factor 2 (20 ng/ml), N2 supplement, insulin (10 μg/ml), dexamethasone (1 μM), 5% fetal bovine serum and 10 µM ROCK inhibitor [Y-27632 (Kwak et al., 2018)], and cultured for 7 days before analysis by fluorescent phase-contrast microscopy. Images were captured from the entire periphery of the wells and used to quantify the number and percentage of labeled spheres in culture.
Quantification and statistical analyses
Typically, three or four sections taken at least 50 µm apart were analyzed for each animal, and the average values for each animal were used to calculate the mean±s.d. For analysis of lineage-labeled acini, 100× images were used to measure the ratio of the lineage labelled Aqp5+ cells to total Aqp5+ areas expressed in pixel density. For quantification of labeling efficiency in the targeted cell population and fate mapping analysis, 400× images were used and the numbers of labeled cells within a specific population or location were quantified. All statistics were performed using SPW12 software (Systat software). Differences among means were evaluated using unpaired two-sided Student's t-test when comparing two groups, and one-way analysis of variance and the Tukey's HSD post-hoc comparison when comparing several groups. Significance was set at P<0.05.
Supplementary Material
Acknowledgements
We thank Dr Lucille London for critically reviewing this manuscript, Dr Ivo Kalajzic for donating the aSMACreERT2 mice and Laurie Levine for technical assistance with our mouse colonies.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.G.; Methodology: N.N.; Validation: N.N.; Formal analysis: S.G.; Investigation: N.N., M.K., S.G.; Writing - original draft: S.G.; Writing - review & editing: N.N., M.K.; Visualization: M.K., S.G.; Supervision: S.G.; Project administration: S.G.; Funding acquisition: S.G.
Funding
This work was supported by the National Institutes of Health-National Institute of Dental and Craniofacial Research (R21DE022959 to S.G.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.192807.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.192807.reviewer-comments.pdf
References
- Amano O., Mizobe K., Bando Y. and Sakiyama K. (2012). Anatomy and histology of rodent and human major salivary glands: -overview of the Japan salivary gland society-sponsored workshop. Acta Histochem. Cytochem. 45, 241-250. 10.1267/ahc.12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athwal H. K., Murphy G. III, Tibbs E., Cornett A., Hill E., Yeoh K., Berenstein E., Hoffman M. P. and Lombaert I. M. A. (2019). Sox10 Regulates plasticity of epithelial progenitors toward secretory units of exocrine glands. Stem Cell Reports 12, 366-380. 10.1016/j.stemcr.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure M. H., Konieczny S. F. and Ovitt C. E. (2015). Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev. Cell 33, 231-237. 10.1016/j.devcel.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C. and Fuchs E. (2014). Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281 10.1126/science.1242281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bralic M., Muhvic-Urek M., Stemberga V., Golemac M., Jurkovic S., Borcic J., Braut A. and Tomac J. (2005). Cell death and cell proliferation in mouse submandibular gland during early post-irradiation phase. Acta Med. Okayama 59, 153-159. [DOI] [PubMed] [Google Scholar]
- Burgess K. L. and Dardick I. (1998). Cell population changes during atrophy and regeneration of rat parotid gland. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 85, 699-706. 10.1016/S1079-2104(98)90038-5 [DOI] [PubMed] [Google Scholar]
- Clevers H. and Watt F. M. (2018). Defining adult stem cells by function, not by phenotype. Annu. Rev. Biochem. 87, 1015-1027. 10.1146/annurev-biochem-062917-012341 [DOI] [PubMed] [Google Scholar]
- Cotroneo E., Proctor G. B., Paterson K. L. and Carpenter G. H. (2008). Early markers of regeneration following ductal ligation in rat submandibular gland. Cell Tissue Res. 332, 227-235. 10.1007/s00441-008-0588-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotroneo E., Proctor G. B. and Carpenter G. H. (2010). Regeneration of acinar cells following ligation of rat submandibular gland retraces the embryonic-perinatal pathway of cytodifferentiation. Differentiation 79, 120-130. 10.1016/j.diff.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny P. C., Ball W. D. and Redman R. S. (1997). Salivary glands: a paradigm for diversity of gland development. Crit. Rev. Oral Biol. Med. 8, 51-75. 10.1177/10454411970080010301 [DOI] [PubMed] [Google Scholar]
- Emmerson E., May A. J., Nathan S., Cruz-Pacheco N., Lizama C. O., Maliskova L., Zovein A. C., Shen Y., Muench M. O. and Knox S. M. (2017). SOX2 regulates acinar cell development in the salivary gland. Elife 6, e26620 10.7554/eLife.26620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grcevic D., Pejda S., Matthews B. G., Repic D., Wang L., Li H., Kronenberg M. S., Jiang X., Maye P., Adams D. J. et al. (2012). In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells 30, 187-196. 10.1002/stem.780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatomi Y., Okumura K., Nakamura K., Matsumoto S., Satoh A., Nagano K., Yamamoto T. and Endo F. (2004). Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology 39, 667-675. 10.1002/hep.20063 [DOI] [PubMed] [Google Scholar]
- Klein S., Seidler B., Kettenberger A., Sibaev A., Rohn M., Feil R., Allescher H. D., Vanderwinden J. M., Hofmann F., Schemann M. et al. (2013). Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat. Commun. 4, 1630 10.1038/ncomms2626 [DOI] [PubMed] [Google Scholar]
- Kopp J. L., Grompe M. and Sander M. (2016). Stem cells versus plasticity in liver and pancreas regeneration. Nat. Cell Biol. 18, 238-245. 10.1038/ncb3309 [DOI] [PubMed] [Google Scholar]
- Kwak M. and Ghazizadeh S. (2015). Analysis of histone H2BGFP retention in mouse submandibular gland reveals actively dividing stem cell populations. Stem Cells Dev. 24, 565-574. 10.1089/scd.2014.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M., Alston N. and Ghazizadeh S. (2016). Identification of stem cells in the secretory complex of salivary glands. J. Dent. Res. 97, 776-783. 10.1177/0022034516634664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M., Ninche N., Klein S., Saur D. and Ghazizadeh S. (2018). c-Kit+ cells in adult salivary glands do not function as tissue stem cells. Sci. Rep. 8, 14193 10.1038/s41598-018-32557-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I. M., Brunsting J. F., Wierenga P. K., Faber H., Stokman M. A., Kok T., Visser W. H., Kampinga H. H., De Haan G. and Coppes R. P. (2008). Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE 3, e2063 10.1371/journal.pone.0002063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I. M., Abrams S. R., Li L., Eswarakumar V. P., Sethi A. J., Witt R. L. and Hoffman M. P. (2013). Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Reports 1, 604-619. 10.1016/j.stemcr.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. P., Polak L., Rocha A. S., Pasolli H. A., Chen S.-C., Sharma N., Blanpain C. and Fuchs E (2012). Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150, 136-150. 10.1016/j.cell.2012.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch T. J., Anderson P. J., Rotti P. G., Tyler S. R., Crooke A. K., Choi S. H., Montoro D. T., Silverman C. L., Shahin W., Zhao R. et al. (2018). Submucosal gland myoepithelial cells are reserve stem cells that can regenerate mouse tracheal epithelium. Cell Stem Cell 22, 653-667.e5. 10.1016/j.stem.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannik J., Alzayady K. and Ghazizadeh S. (2010). Regeneration of multilineage skin epithelia by differentiated keratinocytes. J. Invest. Dermatol. 130, 388-397. 10.1038/jid.2009.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J. R., Bylund D. B. and Cassity N. (1982). Progressive secretory dysfunction in the rat submandibular gland after excretory duct ligation. Arch. Oral Biol. 27, 443-450. 10.1016/0003-9969(82)90082-6 [DOI] [PubMed] [Google Scholar]
- Mascre G., Dekoninck S., Drogat B., Youssef K. K., Brohee S., Sotiropoulou P. A., Simons B. D. and Blanpain C. (2012). Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489, 257-262. 10.1038/nature11393 [DOI] [PubMed] [Google Scholar]
- May A. J., Cruz-Pacheco N., Emmerson E., Gaylord E. A., Seidel K., Nathan S., Muench M. O., Klein O. D. and Knox S. M. (2018). Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage. Development 145, dev166363 10.1242/dev.166363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell A. J. and Stanger B. Z. (2016). Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 17, 413-425. 10.1038/nrm.2016.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y. (2003). Immunocytochemistry of myoepithelial cells in the salivary glands. Prog. Histochem. Cytochem. 38, 343-426. 10.1016/S0079-6336(03)80001-3 [DOI] [PubMed] [Google Scholar]
- Ohtomo R., Mori T., Shibata S., Tsuta K., Maeshima A. M., Akazawa C., Watabe Y., Honda K., Yamada T., Yoshimoto S. et al. (2013). SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod. Pathol. 26, 1041-1050. 10.1038/modpathol.2013.54 [DOI] [PubMed] [Google Scholar]
- Prater M. D., Petit V., Alasdair Russell I., Giraddi R. R., Shehata M., Menon S., Schulte R., Kalajzic I., Rath N., Olson M. F. et al. (2014). Mammary stem cells have myoepithelial cell properties. Nat. Cell Biol. 16, 942-950. 10.1038/ncb3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor G. B. and Carpenter G. H. (2007). Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 133, 3-18. 10.1016/j.autneu.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Redman R. S. (1994). Myoepithelium of salivary glands. Microsc. Res. Tech. 27, 25-45. 10.1002/jemt.1070270103 [DOI] [PubMed] [Google Scholar]
- Song E.-A. C., Min S., Oyelakin A., Smalley K., Bard J. E., Liao L., Xu J. and Romano R.-A. (2018). Genetic and scRNA-seq analysis reveals distinct cell populations that contribute to salivary gland development and maintenance. Sci. Rep. 8, 14043 10.1038/s41598-018-32343-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Schoch E. and Walker N. I. (1998). Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int. J. Exp. Pathol. 79, 293-301. 10.1046/j.1365-2613.1998.710405.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nakamura S., Suzuki R., Domon T., Yamamoto T. and Wakita M. (1999). Changing myoepithelial cell distribution during regeneration of rat parotid glands. Int. J. Exp. Pathol. 80, 283-290. 10.1046/j.1365-2613.1999.00124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nakamura S., Suzuki R., Islam N., Domon T., Yamamoto T. and Wakita M. (2000). Apoptosis and mitosis of parenchymal cells in the duct-ligated rat submandibular gland. Tissue Cell 32, 457-463. 10.1016/S0040-8166(00)80002-6 [DOI] [PubMed] [Google Scholar]
- Takahashi S., Shinzato K., Domon T., Yamamoto T. and Wakita M. (2004a). Mitotic proliferation of myoepithelial cells during regeneration of atrophied rat submandibular glands after duct ligation. J. Oral Pathol. Med. 33, 430-434. 10.1111/j.1600-0714.2004.00234.x [DOI] [PubMed] [Google Scholar]
- Takahashi S., Shinzato K., Nakamura S., Domon T., Yamamoto T. and Wakita M. (2004b). Cell death and cell proliferation in the regeneration of atrophied rat submandibular glands after duct ligation. J. Oral Pathol. Med. 33, 23-29. 10.1111/j.1600-0714.2004.00191.x [DOI] [PubMed] [Google Scholar]
- Tata A., Kobayashi Y., Chow R. D., Tran J., Desai A., Massri A. J., Mccord T. J., Gunn M. D. and Tata P. R. (2018). Myoepithelial cells of submucosal glands can function as reserve stem cells to regenerate airways after injury. Cell Stem Cell 22, 668-683.e6. 10.1016/j.stem.2018.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T., Guasch G., Greco V., Blanpain C., Lowry W. E., Rendl M. and Fuchs E. (2004). Defining the epithelial stem cell niche in skin. Science 303, 359-363. 10.1126/science.1092436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urek M. M., Bralic M., Tomac J., Borcic J., Uhac I., Glazar I., Antonic R. and Ferreri S. (2005). Early and late effects of x-irradiation on submandibular gland: a morphological study in mice. Arch. Med. Res. 36, 339-343. 10.1016/j.arcmed.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Walker N. I. and Gobé G. C. (1987). Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J. Pathol. 153, 333-344. 10.1002/path.1711530407 [DOI] [PubMed] [Google Scholar]
- Weng P.-L., Aure M. H., Maruyama T. and Ovitt C. E. (2018). Limited regeneration of adult salivary glands after severe injury involves cellular plasticity. Cell Rep. 24, 1464-1470.e3. 10.1016/j.celrep.2018.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N., Lin Y., Cao H., Sirjani D., Giaccia A. J., Koong A. C., Kong C. S., Diehn M. and Le Q. T. (2014). Neurotrophic factor GDNF promotes survival of salivary stem cells. J. Clin. Invest. 124, 3364-3377. 10.1172/JCI74096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.