Summary
Muscle satellite cells are normally quiescent but are rapidly activated following muscle damage. Here, we investigated whether damaged myofibers influence the activation of satellite cells. Our findings revealed that satellite cells are directly activated by damaged-myofiber-derived factors (DMDFs). DMDFs induced satellite cells to enter the cell cycle; however, the cells stayed at the G1 phase and did not undergo S phase, and these cells were reversible to the quiescent-like state. Proteome analysis identified metabolic enzymes, including GAPDH, as DMDFs, whose recombinant proteins stimulated the activation of satellite cells. Satellite cells pre-exposed to the DMDFs demonstrated accelerated proliferation ex vivo. Treatment with recombinant GAPDH prior to muscle injury promoted expansion of the satellite cell population in vivo. Thus, our results indicate that DMDFs are not only a set of biomarkers for muscle damage, but also act as moonlighting proteins involved in satellite cell activation at the initial step of muscle regeneration.
Keywords: muscle damage, satellite cells, muscle stem cells, skeletal muscle, myokines, muscle regeneration, moonlighting, GAPDH, PAX7, MYOD
Graphical Abstract
Highlights
-
•
Damaged-myofiber-derived factors (DMDFs) activate satellite cells
-
•
DMDFs induce activation, but not proliferation, of satellite cells
-
•
Satellite cells activated by DMDFs are reversible to the quiescent-like state
-
•
DMDFs play an important role in the initial step of muscle regeneration
In this article, Tsuchiya et al. demonstrate that muscle satellite cells are directly activated by damaged-myofiber-derived factors (DMDFs). DMDFs promote population expansion of satellite cells during muscle regeneration. DMDFs are not only a set of biomarkers for muscle damage, but also act as functional proteins involved in satellite cell activation at the initial step of muscle regeneration.
Introduction
Resident muscle stem cells (also known as satellite cells), located between the plasmalemma of myofiber and the basal lamina (Mauro, 1961), possess a remarkable potential for regeneration after muscle damage. Following muscle damage, the satellite cells are rapidly activated from their quiescent state and become myoblasts to proliferate and fuse with one another and/or with existing myofibers in order to regenerate muscle. A subpopulation of these activated cells reverts to the quiescent state to self-renew and maintain the stem cell pool (Brack and Rando, 2012; Kuang and Rudnicki, 2008; Relaix and Zammit, 2012). In adult muscle, quiescent satellite cells express the paired box protein-7 (PAX7), while the activated cells upregulate the myogenic regulatory factor MYOD and undergo proliferation. In myogenic differentiation, most satellite cells downregulate PAX7 and maintain MYOD expression to initiate myogenesis through upregulation of myogenin (Halevy et al., 2004; Zammit et al., 2004). MyoD-null mice exhibit reduced regeneration ability after muscle injury due to defects in population expansion and differentiation, indicating that MYOD plays an important role in the activation of satellite cells at the initial stages of regeneration (Cornelison et al., 2000; Megeney et al., 1996; Yablonka-Reuveni et al., 1999; Yamamoto et al., 2018).
The mechanism of regulation underlying the activation of satellite cells is one of the fundamental questions in the field of muscle biology (Baghdadi et al., 2018; Bjornson et al., 2012; Chakkalakal et al., 2012; Cheung et al., 2012; Crist et al., 2012; Fujimaki et al., 2018; Fukada et al., 2007; Mourikis et al., 2012; Rodgers et al., 2014; Verma et al., 2018; Yamaguchi et al., 2015). In the later years of the 20th century, researchers found that muscle extracts can stimulate activation and proliferation of cultured muscle cells (Haugk et al., 1995; Kardami et al., 1985; Mezzogiorno et al., 1993; Vandenburgh and Lent, 1984), which prompted them to explore factors in extracts from intact or crushed muscle tissues that contribute to muscle regeneration. Consequently, several growth factors, including hepatocyte growth factor (HGF), that promote activation and proliferation of satellite cells were identified in muscle tissues (Li, 2003; Tatsumi et al., 1998; Zeng et al., 2010). Satellite cell activity is also modulated through growth factors secreted from a variety of cells in interstitial spaces, such as macrophages (Du et al., 2017; Lescaudron et al., 1999; Segawa et al., 2008), fibroblasts (Mackey et al., 2017; Murphy et al., 2011), and mesenchymal progenitors (Joe et al., 2010; Uezumi et al., 2010). Recent findings have demonstrated that the regulatory mechanisms underlying the activation of satellite cells are rather complicated. For instance, in muscle injury in mice, the satellite cells are stimulated in the contralateral intact muscles and transition into the GAlert stage, which is a primed cellular state preceding activation and entrance into the S phase of the cell cycle (Rodgers et al., 2014, 2017). These findings suggest that satellite cells are stimulated by damaged-muscle-tissue-derived factors. However, it is impossible to track the exact source of these factors, as whole muscle tissues contain not only myofibers but also interstitial cells capable of influencing satellite cells.
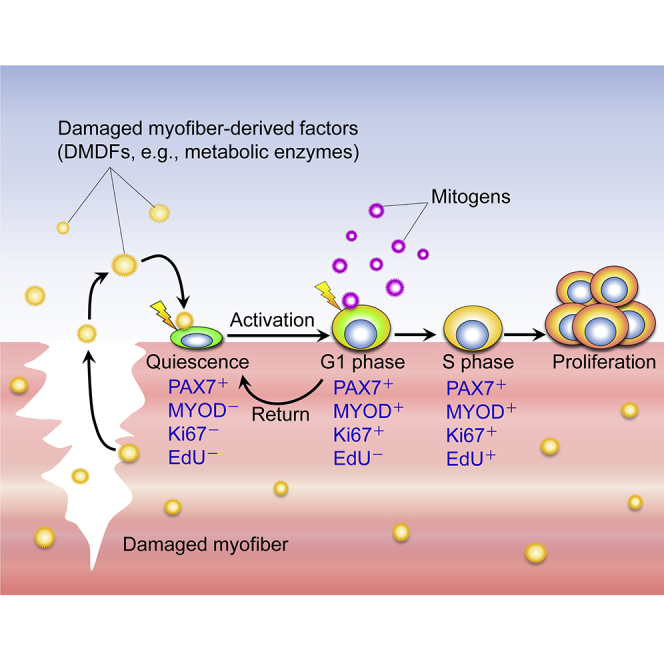
In the present study, we investigated how satellite cell activation is regulated, using isolated individual myofibers in a floating culture model. This strategy has allowed us to analyze both purified myofiber extracts and quiescent satellite cells associated with myofibers without contamination by interstitial cells. Our study has revealed that damaged-myofiber-derived factors (DMDFs) stimulate satellite cells to transition from the G0 to the G1 stage. In addition, we have identified metabolic enzymes as DMDFs that promote satellite cell activation ex vivo and facilitate population expansion of satellite cells during muscle regeneration in vivo. Our findings, thus, indicate that damaged myofibers provide a direct signal to resident stem cells to accelerate the initial step of tissue regeneration.
Results
Muscle Extracts Promote Activation of Satellite Cells
Although extracts from muscle tissues stimulate proliferation of myoblasts (Bischoff, 1986; Haugk et al., 1995; Kardami et al., 1985), the effect of these extracts on quiescent satellite cells remains unclear. In combination with appropriate markers, the isolated myofiber model allows us to investigate the satellite cell fate decision, ranging from quiescence to activation, proliferation, differentiation, and self-renewal on the myofibers (Ono et al., 2011; Zammit et al., 2004). Accordingly, we examined how muscle extracts influence the satellite cells, using the isolated myofiber culture model.
To examine the effect of extracts on satellite cell activation, we treated the satellite cells associated with myofibers with tibialis anterior (TA) muscle extracts (Figure 1A). PAX7 is uniformly expressed in quiescent to proliferative state satellite cells, whereas MYOD is a marker for satellite cell activation. Ki67 marks the cells that enter the cell cycle (G1, S, G2, and M phases), while incorporation of EdU into genomic DNA is detected in S phase of the cell cycle. The immunofluorescence studies revealed that the proportions of PAX7+MYOD+, PAX7+Ki67+, and PAX7+EdU+ satellite cells increase after adding muscle extracts for 24 h in the floating culture condition compared with the control myofibers maintained in Dulbecco's modified Eagle medium (DMEM) alone (Figures 1B–1G). Thus, these results suggest that muscle extracts accelerate the activation and progression into S phase of satellite cells.
Figure 1.
Muscle Extracts Accelerate Satellite Cell Activation
(A) A schematic illustrating the experimental procedure. TA muscle homogenates were filtered and used as muscle extracts.
(B–G) Individual myofibers associated with satellite cells were freshly isolated from EDL and cultured in DMEM with or without muscle extracts for 24 h and then immunostained for (B) PAX7 and MYOD (quantified in C), (D) PAX7 and Ki67 (quantified in E), or (F) PAX7 and EdU (quantified in G). Data represent means ± SEM. (C) Control n = 3 mice, extract n = 5 mice; (E) n = 3 mice per condition; (G) control n = 3 mice, extract n = 4 mice; >15 individual myofibers per mouse were counted. ∗p < 0.05. Scale bars, 50 μm.
DMDFs Induce Activation, but Not Proliferation, of Satellite Cells
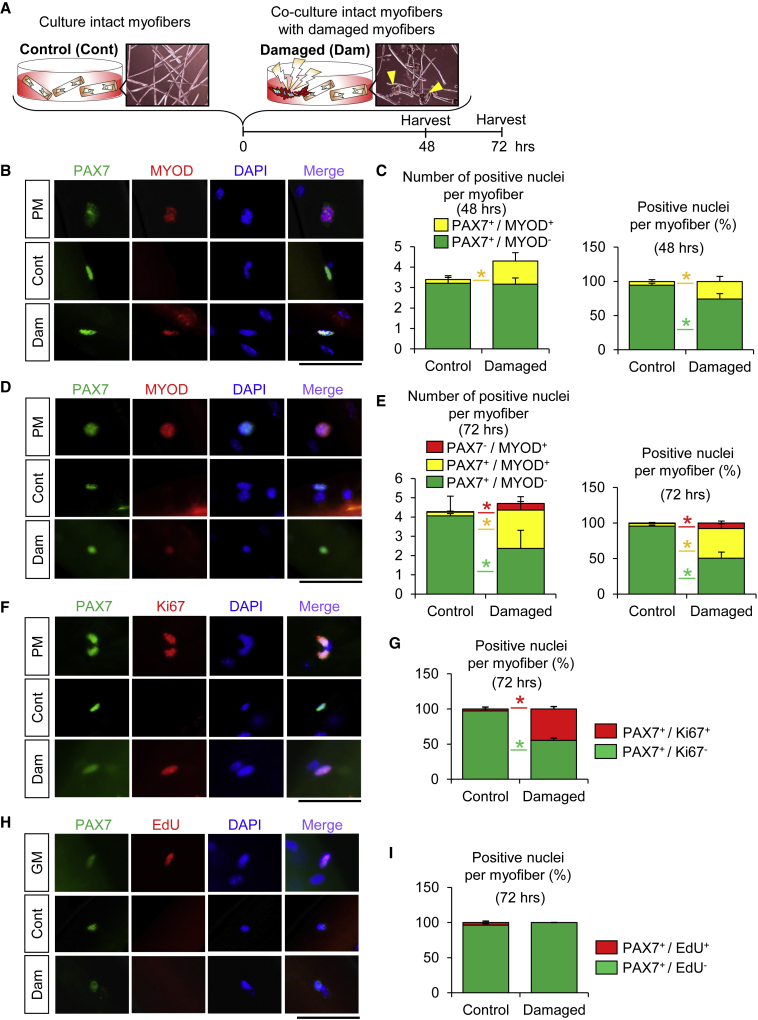
Because skeletal muscle tissues comprise not only myofibers but also various types of other cells, such as endothelial cells and interstitial mesenchymal cells (Evano and Tajbakhsh, 2018), there exists a possibility that such non-muscle-cell-derived factors may influence the activation of satellite cells, as shown in Figure 1. To exclude this possibility, we evaluated satellite cell activation using isolated healthy intact and damaged individual myofibers (Figures 2A and S1A). This co-culture of intact myofibers with damaged myofibers allowed us to examine whether DMDFs directly stimulate the activation and proliferation of satellite cells associated with the healthy intact myofibers.
Figure 2.
Damaged-Myofiber-Derived Factors Activate Satellite Cells
(A) A schematic illustrating the experimental procedure. Individual myofibers were freshly isolated from EDL muscles and mechanically damaged with a Pasteur pipette. Damaged myofibers shrank immediately (arrowheads). A growth-factor-rich medium (plating medium: PM) condition was used for a positive control. Intact (undamaged) myofibers were co-cultured with damaged myofibers (co-cultured, intact myofibers:damaged myofibers = 1:1).
(B–E) Individual myofibers associated with satellite cells were cultured in DMEM with or without damaged myofibers for (B) 48 h or (D) 72 h and then immunostained for PAX7 and MYOD (quantified in C or E, respectively). (n = 4 mice per condition; >15 individual myofibers per mouse were counted.) ∗p < 0.05. Scale bars, 50 μm.
(F–I) Individual myofibers associated with satellite cells were cultured in DMEM with or without damaged myofibers for 72 h and then immunostained for PAX7 and Ki67 or PAX7 and EdU (quantified in G or I, respectively). (n = 4 mice per condition; >15 individual myofibers per mouse were counted.) ∗p < 0.05. Scale bars, 50 μm.
To obtain mechanically damaged myofibers, healthy intact myofibers freshly isolated from extensor digitorum longus (EDL) muscles were mechanically damaged with a Pasteur pipette in a culture dish (Figure 2A) and the damage was confirmed based on shrunken morphology. Immunofluorescence analysis revealed the presence of a higher proportion of MYOD+ satellite cells on intact myofibers when they were co-cultured with damaged myofibers at both 48 (Figures 2B and 2C) and 72 h (Figures 2D and 2E) of culture compared with control conditions, although the total number of satellite cells per myofiber remained unaltered (Figures 2B–2E). Interestingly, the proportion of Ki67+ satellite cells was increased (Figures 2F and 2G), but that of cells positive for EdU, an S-phase cell-cycle entry marker, was unchanged under the co-culture conditions (Figures 2H and 2I). These results, therefore, imply that DMDFs induce satellite cells to enter the cell cycle; however, the cells stay in the G1 phase of the cell cycle and do not undergo the S phase.
To exclude the possibility that intact myofibers themselves were responding to DMDFs and then indirectly stimulating their associated satellite cells, we tested whether DMDFs activate satellite cells on damaged (shrunken/dying) myofibers, whose myonuclei are disappearing and so are unable to respond to DMDFs and induce gene expression. We confirmed that satellite cells associated with damaged myofibers enter into the G1 phase (Figures S1B–S1D) as well as satellite cells on healthy intact myofibers, indicating that satellite cells can be directly activated by DMDFs but not through their associated intact myofibers.
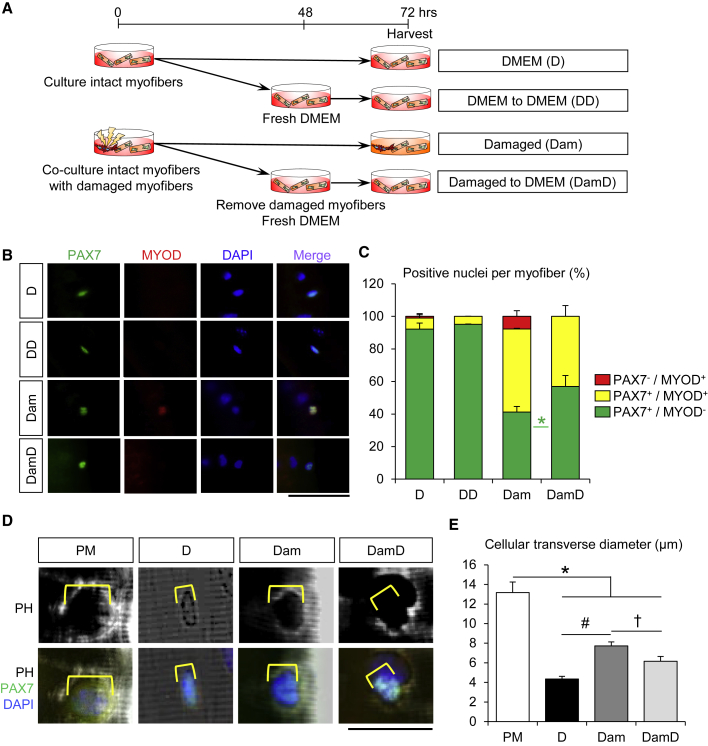
Satellite Cells in the G1 Phase Are Reversible to the Quiescent-like State
Following activation, satellite cells asymmetrically generate both self-renewed cells that maintain the satellite cell pool for future requirement and myogenic cells that undergo transient amplification followed by terminal differentiation to form new myofibers (Dhawan and Rando, 2005; Kuang and Rudnicki, 2008; Zammit et al., 2006). Interestingly, we noticed that approximately 10% of the cells were MYOD+ or Ki67+ satellite cells under DMEM control conditions at 24 h (Figure 1), but these populations were decreased to only ∼2% at 48–72 h (Figure 2) after isolation. These results indicate that activated cells may revert to the quiescent-like state without cell division. To validate this hypothesis, we tested whether the activated satellite cells in the G1 phase return to a quiescent state in the co-culture model as shown in Figure 2. Consequently, isolated satellite cells associated with intact myofibers were stimulated by co-culturing with damaged myofibers. The damaged myofibers and the culture media containing DMDFs were then washed out and replaced with fresh DMEM and cultured for an additional 24 h (Figure 3A). Further immunofluorescence analysis showed that the proportion of PAX7+MYOD− quiescent satellite cells per myofiber was decreased upon co-culture of damaged myofibers, whereas this population was increased when the damaged myofibers and culture media were washed out (Figures 3B and 3C). Recent studies on muscle injury mouse models have reported that satellite cells could be slightly activated even in contralateral intact muscles and undergo transition into GAlert stage (Rodgers et al., 2014, 2017). Hallmarks of GAlert stage include increased cell size concomitant with upregulation of mtDNA and intracellular ATP contents (Rodgers et al., 2014, 2017). In the present study, we therefore evaluated cell size of satellite cells stimulated by damaged myofibers. The transverse diameter of satellite cells associated with intact myofibers was evidently increased upon co-culture with damaged myofibers and became smaller when the damaged myofibers and culture media were washed out (Figures 3D and 3E). Taken together, our findings indicate that DMDFs stimulate activation but not the proliferation of satellite cells, while the activated cells may remain biochemically and morphologically reversible to quiescent-like cells.
Figure 3.
Satellite Cells in the G1 Phase Revert to the Quiescent-like State
(A) A schematic illustrating the experimental procedure. To determine whether activated satellite cells are reversible to the quiescent-like state without cell division, individual myofibers were freshly isolated from EDL muscles and cultured in DMEM with or without damaged myofibers for 48 h, as shown in Figure 2. Damaged myofibers and culture media were then removed and intact myofibers were maintained in fresh DMEM for a further 24 h.
(B and C) (B) Immunostaining of satellite cells associated with myofibers for PAX7 and MYOD was performed (quantified in C). (D, DD, Dam, and DamD: n = 3, 3, 5, and 5 mice, respectively; >15 individual myofibers per mouse were counted.) Scale bars, 50 μm. Asterisk (∗) indicates differences among Dam and DamD conditions (p < 0.05).
(D and E) (D) Immunostaining of satellite cells associated with myofibers for PAX7 was performed (transverse diameter of satellite cells on myofiber quantified in E). Yellow lines indicate transverse diameter of satellite cells. (n = 3 mice per condition; at least 11 cells per mouse were counted.) Scale bars, 25 μm. Asterisk (∗), pound sign (#), and dagger (†) represent differences among plating medium (PM) and either D, Dam or DamD; D and Dam; or Dam and DamD, respectively (p < 0.05).
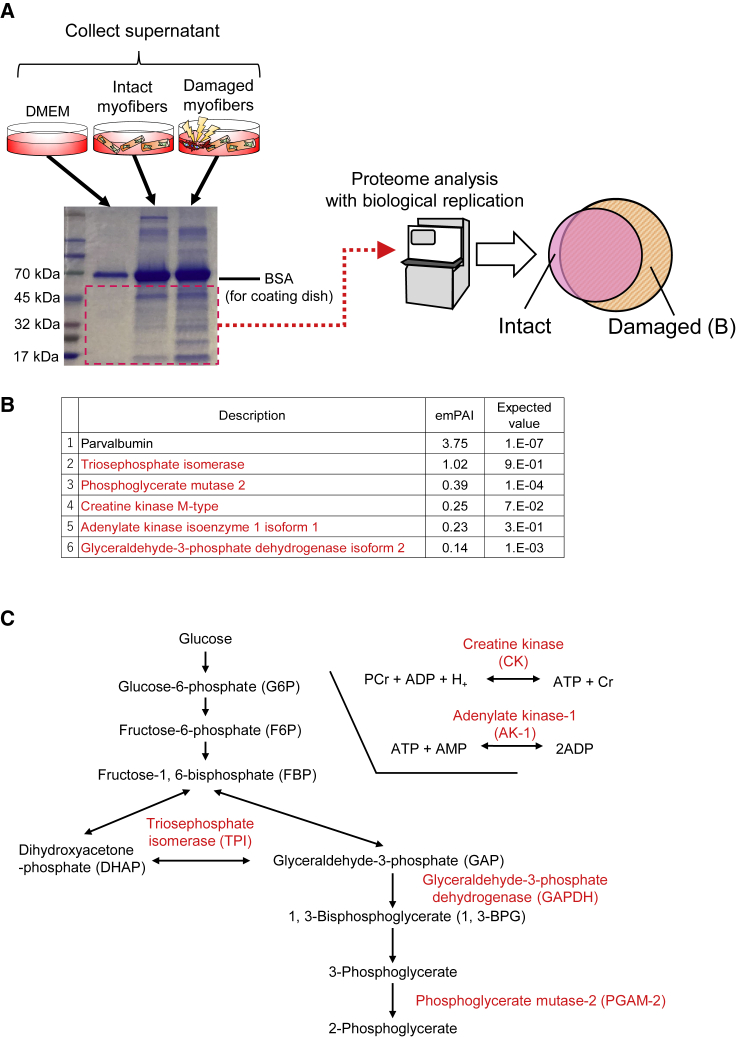
Metabolic Enzymes Identified as DMDFs by Proteome Analysis
To identify DMDFs that stimulate activation of satellite cells, proteome analysis was performed with biological replicates (Figure 4A). Proteins ranging from 10 to 50 kDa were screened on SDS-PAGE gels because they exhibited apparent differences in the supernatant between intact and damaged myofiber conditions (Figure 4A). Consequently, five protein candidates were identified as DMDFs: adenylate kinase-1 (AK-1), creatine kinase (CK), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerate mutase-2 (PGAM-2), and triose phosphate isomerase (TPI) (Figure 4B). AK-1 is one of the major AK isoforms expressed in the cytoplasm of skeletal muscles (Tanabe et al., 1993). CK is composed of two isoforms, namely CK-MB and CK-MM, of which CK-MM is predominantly expressed in skeletal muscle (Fredericks et al., 2001). PGAM is composed of muscle-specific (M) and non-muscle-specific (B) subunits, existing as a dimer. Skeletal muscles express M-type isozymes of PGAM-2 (Uchida et al., 1995). TPI is a glycolytic enzyme that is abundantly expressed in skeletal muscles (Puigjaner et al., 1997). These proteins are known as metabolic enzymes for ATP production except parvalbumin (Maughan et al., 2005) (Figure 4C).
Figure 4.
Proteome Analysis to Identify Metabolic Enzymes as Damaged-Myofiber-Derived Factors
(A) A schematic illustrating the proteome analysis. To distinguish damaged-myofiber-derived proteins from supernatant, MALDI-TOF/MS analysis was performed. Individual myofibers were freshly isolated from EDL muscles and mechanically damaged as shown in Figure 2. Supernatant from damaged myofibers was collected at 72 h following myofiber injury. Supernatant from intact myofibers was used as control.
(B and C) (B) Top six enriched proteins are listed. These enriched proteins were confirmed by their biological replication. (C) Proteins highlighted with red font are the metabolic enzymes that are involved in the pathways for glycolysis and production of ATP.
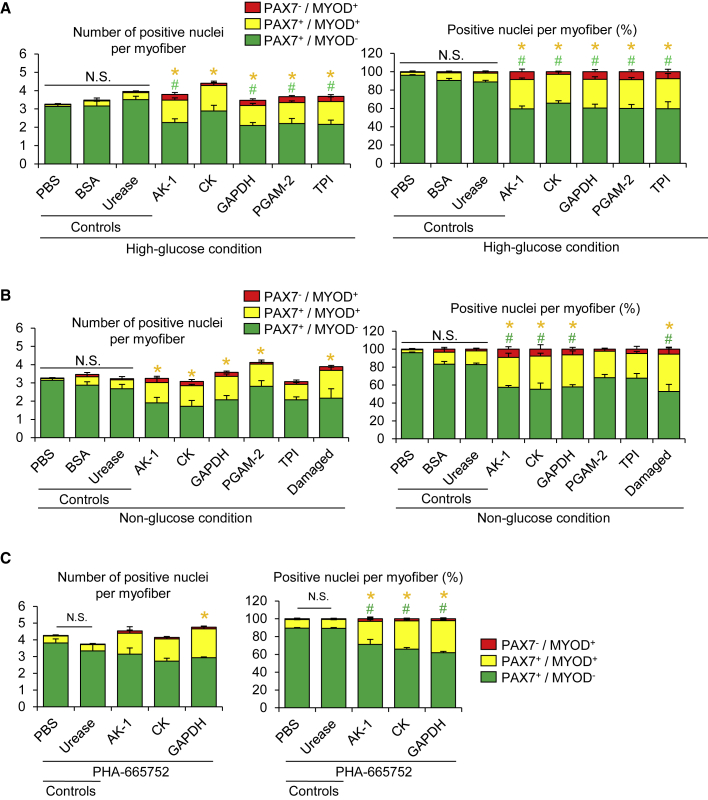
Extracellular Treatment with Metabolic Enzymes Stimulates the Entry of Satellite Cells into the G1 Phase
Recent studies have reported that metabolic enzymes such as GAPDH have a variety of roles in addition to, in this case, its function in glycolysis (and the phenomenon is termed as protein moonlighting, where a single protein has more than one distinct function) (Henderson and Martin, 2014; Sirover, 2018). The phenomenon of protein moonlighting prompted us to further investigate if extracellular metabolic enzymes leaked from damaged myofibers act as moonlighting proteins that stimulate satellite cell activation using a mechanism that is independent of glucose metabolism. To test this likelihood, isolated myofibers associated with satellite cells were treated with recombinant proteins of AK-1, CK, GAPDH, PGAM-2, and TPI in a floating culture condition for 72 h and immunostained for MYOD and PAX7. PBS and BSA were used as a negative control and a non-specific protein control, respectively (Figures 5A and 5B). The recombinant protein of urease that is expressed in prokaryotes but not in mammals (Carlini and Ligabue-Braun, 2016) was used as a non-muscle enzymatic control (Figures 5A–5C). In our preliminary experiments, the optimal concentrations of recombinant DMDF proteins, putative physiological concentrations of AK-1, CK, GAPDH, PGAM-2, and TPI in the floating culture model, were determined by taking into reference the enzymatic activity (U) levels in the mammalian skeletal muscle tissues as follows: AK-1, 1.25–12.5 U/mL; CK, 0.2–10 mU/mL; GAPDH, 15–300 mU/mL; PGAM-2, 0.2–10 U/mL; and TPI, 1–10 U/mL (Beisswenger et al., 2003; Durany and Carreras, 1996; Eber and Krietsch, 1980; Ge et al., 2003; Kaczor et al., 2005). Each concentration of recombinant proteins for satellite cell activation was determined by a dose-ranging study (data not shown). We showed that the number of PAX7+MYOD+ satellite cells is increased by extracellular treatment with metabolic enzyme proteins compared with PBS, BSA, or urease-treated conditions (Figure 5A). We confirmed that the number of PAX7+Ki67+ satellite cells is significantly increased by treatment with recombinant GAPDH compared with urease-treated conditions, although treatment with AK-1 or CK proteins did not statistically significantly increase the number of PAX7+Ki67+ cells (Figures S2A and S2B). Consistent with the co-culture conditions (Figures 2H and 2I), the number of PAX7+EdU+ satellite cells was also unchanged in the DMDF-treated conditions (Figures S2C and S2D).
Figure 5.
Extracellular Treatment with Metabolic Enzymes Induces the Entry of Satellite Cells into the G1 Phase
(A and B) To test whether metabolic enzymes identified in Figure 4 as DMDFs activate satellite cells, individual myofibers were freshly isolated from EDL muscles and treated with recombinant metabolic enzyme proteins in DMEM for 72 h. Immunostaining of satellite cells associated with individual myofibers for PAX7 and MYOD was performed (A, high-glucose condition; B, non-glucose condition). (Left, absolute numbers of positive cells; right, relative ratio of positive cells.) PBS, BSA, and urease were used as a negative control, a non-specific protein control, and non-muscle enzymatic control, respectively. Damaged myofibers (Damaged) were used as a positive control. All scale bars, 50 μm. Values are means ± SE (n = 3–4 mice per condition). Asterisk (∗) and pound sign (#) indicate differences compared with urease control (p < 0.05).
(C) To examine whether the HGF signaling pathway is involved in the DMDF-induced satellite cell activation, satellite cells associated with individual myofibers were treated with recombinant metabolic enzyme proteins in the presence of PHA-665752 (c-MET inhibitor) in DMEM for 72 h. Immunostaining for PAX7 and MYOD was performed (left, absolute numbers of positive cells; right, relative ratio of positive cells). Values are means ± SE (n = 4 mice per condition). Asterisk (∗) and pound sign (#) indicate differences compared with urease control (p < 0.05).
As most of the DMDFs identified in our proteome analysis are involved in glucose metabolism, we anticipated that glucose-rich media might influence metabolites in the media, resulting in satellite cell activation. To exclude this possibility, we treated satellite cells associated with myofibers with metabolic enzymes in a non-glucose medium. The proportion of PAX7+MYOD+ activated satellite cells per myofiber was increased even in non-glucose DMEM in the presence of AK-1, CK, or GAPDH (Figure 5B).
HGF was the first growth factor identified in muscle extracts, that stimulates activation and proliferation of cultured satellite cells via its receptor c-MET (Anderson, 2016; Li, 2003; Tatsumi et al., 1998; Zeng et al., 2010). Since HGF is a potent activator of satellite cells in muscle extracts and is also shown to stimulate the transition of satellite cells to GAlert via the c-MET/mTORC1 pathway in contralateral intact muscles (Rodgers et al., 2017), we determined whether HGF signaling is necessary for satellite cell activation induced by DMDFs. We showed that PHA-665752, a c-MET inhibitor, does not attenuate the activation induced by recombinant DMDFs (Figure 5C). We next investigated whether more major signaling pathways controlling the satellite cell fate (Dumont et al., 2015) are involved in the DMDF-induced entry of satellite cells into the G1 phase. To this end, several concentrations of chemical compounds were tested in the floating culture model and the optimal concentrations were determined in our preliminary experiments: Rapamycin (mTOR inhibitor, 100 nM to 1 μM), SU5402 (FGFR tyrosine kinase inhibitor, 1–10 μM), Wortmannin (PI3K inhibitor, 100 nM to 1 μM), PD98059 (MEK inhibitor, 1–10 μM), and Dorsomorphin (BMP-R inhibitor, 100 nM to 1 μM). We showed that the upregulation of MYOD in GAPDH-treated cells is suppressed by Dorsomorphin, concomitant with downregulation of the protein levels of p-SMAD1/5 (Figures S2E–S2H), which are the BMP signaling downstream targets. Taken together, these results suggest that damaged myofiber-derived metabolic enzymes act as moonlighting proteins that stimulate the satellite cell transition into the G1 phase, probably through the BMP signaling pathway.
Pre-treatment with DMDFs Accelerates Proliferation of Satellite Cells Ex Vivo
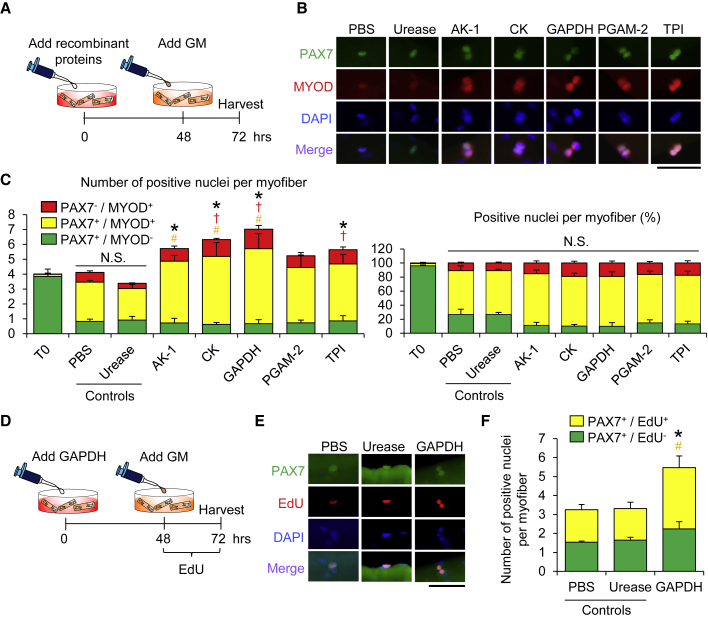
Having shown that extracellular metabolic enzymes identified as DMDFs stimulated the entry of satellite cells into the G1 phase, we next investigated whether these metabolic enzymes promote mitosis and myogenic progression under growth-factor-rich conditions. Satellite cells associated with myofibers were pre-exposed to the recombinant enzyme proteins for 48 h under floating culture conditions and then stimulated with growth-factor-rich medium (GM) for a further 24 h (Figure 6A). The total numbers of satellite cells were increased upon pre-treatment with AK-1, CK, GAPDH, and TPI, but not in the case of PGAM-2, compared with the urease pre-treated condition (Figures 6B and 6C). We also confirmed that pre-treatment with GAPDH as a DMDF increases both the total number of PAX7+ cells and the number of EdU+ satellite cells per myofiber in the GM condition (Figures 6D–6F). These data indicate that satellite cells are activated by DMDFs to enter into the G1 phase, where an additional mitogen stimulation permits a prompt population expansion of satellite cells.
Figure 6.
Pre-treatment with DMDFs Promotes Proliferation of Satellite Cells
(A) A schematic illustrating the experimental procedure. To evaluate the effect of pre-treated metabolic enzymes on population expansion of satellite cells, individual myofibers were freshly isolated from EDL muscles and treated with recombinant metabolic enzyme proteins in DMEM for 48 h, and then the culture media were replaced with growth-factor-rich medium (GM) to stimulate activation for a further 24 h. PBS and urease were used as a negative control and a non-muscle enzymatic control, respectively.
(B and C) (B) Immunostaining of satellite cells associated with individual myofibers for PAX7 and MYOD (quantified in C). (Left, absolute numbers of positive cells; right, relative ratio of positive cells.) Scale bars, 50 μm. Values are means ± SE (n = 4 in each condition). Asterisk (∗), dagger (†), and pound sign (#) indicate differences in the total, differentiated (PAX7−MYOD+), and activated (PAX7+MYOD+) cell populations, respectively, compared with urease control (p < 0.05).
(D–F) (D) A schematic illustrating the experimental procedure for EdU incorporation. (E) Immunostaining of satellite cells associated with individual myofibers for PAX7 and EdU. (F) Absolute numbers of positive cells are shown. PBS and urease were used as a negative control and a non-muscle enzymatic control, respectively. Scale bars, 50 μm. Values are means ± SE (n = 4 mice). Asterisk (∗) and pound sign (#) indicate differences in the total and activated/proliferative (PAX7+EdU+) cell populations, respectively, compared with urease control (p < 0.05).
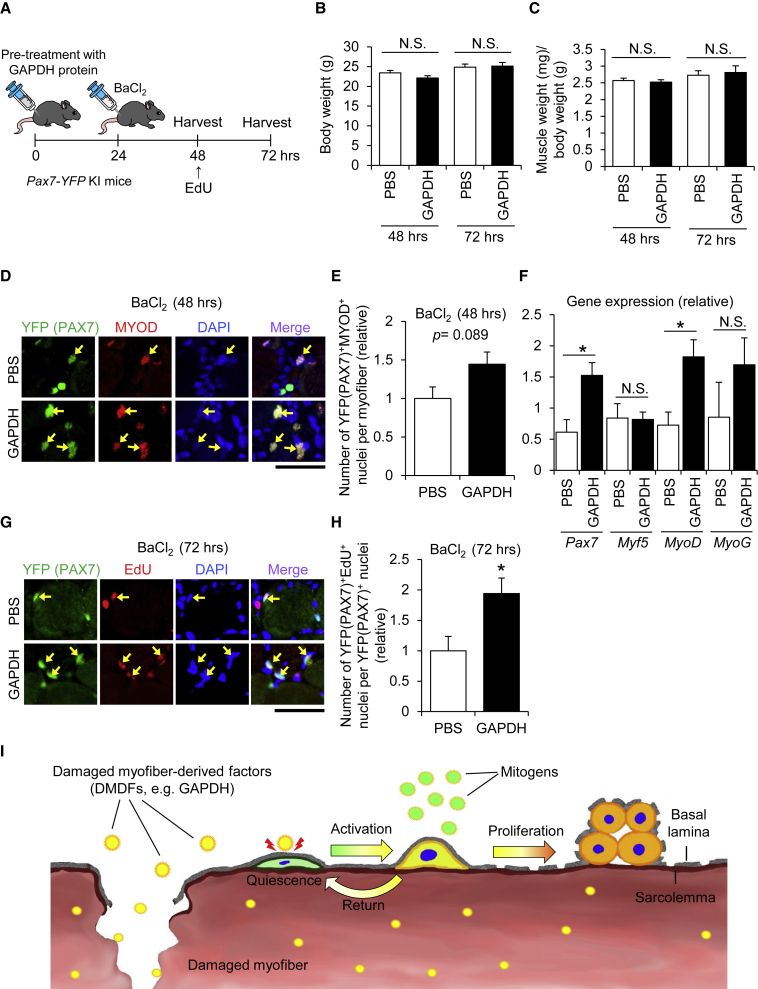
GAPDH as a DMDF Accelerates Activation and Proliferation of Satellite Cells In Vivo
Finally, we sought to examine whether DMDFs accelerate satellite cell activation and population expansion in vivo. GAPDH recombinant protein was selected as a DMDF for this experiment because it efficiently stimulated population expansion of cells, as shown in Figures 5 and 6. The recombinant protein was administered into TA muscles at 24 h prior to injection of BaCl2 to induce muscle regeneration (Figure 7A). PBS was used as a control. Both the body and the TA muscle weights remained unchanged among groups (Figures 7B and 7C), whereas the number of PAX7+MYOD+ activated satellite cells was slightly, but not statistically significantly, increased 48 h following treatment with GAPDH protein compared with PBS control (Figures 7D and 7E). Correspondingly, qPCR analysis demonstrated that the expression of Pax7 and MyoD genes in GAPDH-pre-treated mice is significantly higher than that of PBS controls (Figure 7F). Further, immunohistochemistry revealed that pre-treatment with GAPDH remarkably increases the number of PAX7+EdU+ proliferative satellite cells in regenerating muscles (Figures 7G and 7H). These results suggest that GAPDH acts as a DMDF, promoting activation and proliferation of satellite cells during muscle regeneration in vivo.
Figure 7.
GAPDH as a DMDF Accelerates Population Expansion of Satellite Cells In Vivo
(A) A schematic illustrating the experimental procedure. To investigate the effect of DMDFs on population expansion of satellite cells in vivo, TA muscles were pre-treated with GAPDH recombinant protein, followed by BaCl2 injection to induce muscle regeneration. EdU was intraperitoneally injected into mice 24 h before sacrifice. PBS was used as a control.
(B and C) (B) Body weight and (C) TA muscle weight of mice at 48 and 72 h after treatment with GAPDH recombinant protein.
(D and E) (D) Immunohistochemistry of TA cross sections for yellow fluorescent protein (YFP) (PAX7) and MYOD (quantified in E). Arrows indicate YFP (PAX7)+/MYOD+ nuclei. Scale bars, 50 μm. Values are means ± SE (n = 3–5 mice per condition).
(F) qPCR analysis for the expression of Pax7, Myf5, MyoD, and Myogenin (MyoG) mRNAs in TA muscle tissues. Values are means ± SE (n = 3–5 mice per condition). Asterisk (∗) indicates differences compared with PBS control (p < 0.05).
(G and H) (G) Immunohistochemistry of TA cross sections for YFP (PAX7) and EdU (quantified in H). Arrows indicate YFP (PAX7+)/EdU+ nuclei. Scale bars, 50 μm. Values are means ± SE (n = 5 mice per condition). Asterisk (∗) indicates differences compared with PBS control (p < 0.05).
(I) Postulated roles of DMDFs in muscle damage and regeneration. Skeletal muscles abundantly contain metabolic enzymes such as GAPDH. Theoretically, these metabolic enzymes leak from damaged myofibers and immediately stimulate the entry of satellite cells into the G1 phase. The satellite cells are then able to proliferate extensively when subsequently stimulated by mitogens in the regenerating niche. Meanwhile, activated satellite cells also return to a quiescent-like state in the absence of mitogens, which is a multi-step protective mechanism that avoids unnecessary cell division of satellite cells.
Discussion
In the present study, we revealed that quiescent satellite cells associated with myofibers could be activated by factors leaked from damaged myofibers (i.e., DMDFs) that promote activation and proliferation in coordination with mitogens during muscle regeneration. Of note, DMDFs did not induce satellite cells to enter the S phase of the cell cycle even with the increased expression of MYOD and Ki67. More interestingly, satellite cells activated with DMDFs were reversible to the quiescent-like state without mitosis. A study has reported that endurance exercise training does not always result in improvement of myofiber size and satellite cell pool (Snijders et al., 2011), and even non-hypertrophic exercise can stimulate satellite cell activation without mitosis (Joanisse et al., 2015). Thus, our observations suggest that satellite cells activated by upregulation of Ki67 and MYOD are not necessarily expected to undergo S phase of the cell cycle and subsequent mitosis. In other words, inappropriate proliferation could be prevented by a multi-step process, which is probably much more complex than assumed previously (Arora et al., 2017; Dumont et al., 2015; Evano and Tajbakhsh, 2018; Sousa-Victor et al., 2018).
Our ex vivo culture study revealed that growth-factor-enriched media accelerate proliferation of satellite cells post treatment with DMDFs. Treatment with recombinant GAPDH, which is one of the DMDFs, prior to muscle injury also promoted satellite cell proliferation during muscle regeneration in vivo. Accumulating evidence has revealed that, while GAPDH is well known to play an important role in glycolysis, it also has moonlighting roles (Garcin, 2019). For instance, GAPDH acts to mediate the immune reaction in lymphocytes and peripheral blood leukocytes (Sirover, 2018). It is possible to speculate that GAPDH as a DMDF not only activates satellite cells but also recruits inflammatory immune cells during muscle regeneration. Based on our findings, we propose a cascade model from muscle damage to regeneration as follows: theoretically, damaged myofibers first leak their intracellular contents (DMDFs), including metabolic enzymes, out to the interstitial spaces, which in turn triggers immediate activation of satellite cells as well as migration and infiltration of inflammatory cells such as neutrophils and macrophages. Subsequently, these inflammatory cells produce a variety of growth factors and cytokines such as FGF, EGF, IGF-1, and Wnts in the regenerating niche, further prompting activated satellite cells to undergo population expansion for efficient tissue regeneration after injury. Collectively, our results indicate that DMDFs in the blood not only are biomarkers of muscle damage but also play an important role in the initial step of muscle regeneration, and are therefore suited to be also defined as damaged-myofiber-derived myokines (Pedersen and Febbraio, 2012).
In response to muscle damage, satellite cells can be stimulated even in the contralateral intact muscles (Rodgers et al., 2014). A subsequent study elucidated the injury-induced regulation of the HGF activator that stimulates the transition of satellite cells and mesenchymal progenitors to GAlert in contralateral intact muscles (Rodgers et al., 2017). In the present study, we have also identified that metabolic enzymes leaked extracellularly act as activators of satellite cells. However, it is unlikely that HGF signaling is involved in this mechanism, because metabolic-enzyme-induced activation of satellite cells was not blocked by treatment with the c-MET inhibitor. We previously reported that BMP signaling is important to maintain the proliferative state of activated satellite cells by preventing premature differentiation (Ono et al., 2011). In the present study, we showed that inhibition of the BMP signaling pathway blocked the GAPDH-induced upregulation of MYOD, suggesting that BMP signaling is, in part, involved in the DMDF-induced activation of satellite cells.
In conclusion, our study has revealed that quiescent satellite cells can be directly induced into the G1 phase by DMDFs, including GAPDH, which supports muscle regeneration by accelerating population expansion of the satellite cells. However, it remains unclear how extracellular metabolic enzymes activate the satellite cells. It is also unknown why not all satellite cells could be activated by co-culture with damaged myofibers or treatment with metabolic enzyme proteins. Presumably, some cells are more sensitive to the extracellular stimuli than others, which indicates a functional heterogeneity in the satellite cell population (Chakkalakal et al., 2012; Der Vartanian et al., 2019; Kuang et al., 2007; Ono, 2014; Ono et al., 2010, 2012; Rocheteau et al., 2012; Scaramozza et al., 2019). Further investigations are necessary to elucidate the mechanisms and functional significance of DMDFs in muscle regeneration. This study has the potential of extending the window of opportunity for developing efficient regeneration therapies for muscle diseases as well as for establishing a strategy for rapid recovery from severe muscle injury in elderly patients or athletes.
Experimental Procedures
Animals
All experiments were performed using 10- to 16-week-old male C57BL/6 wild-type mice and Pax7-yellow fluorescent protein (Pax7-YFP) knock-in mice (Kitajima and Ono, 2018). Individual myofibers were isolated from EDL muscle of wild-type mice and plated in a floating culture ex vivo. Pax7-YFP knock-in mice were used for muscle damage experiments in vivo. The Ethical Committee for Animal Care and Use (no. 1203190970) of Nagasaki University and Kumamoto University (A30-098) approved all experimental procedures.
Cell Culture
To assay satellite cells associated with myofibers, we used a floating culture method using individual myofibers (Ono et al., 2015) that allowed determination of satellite cell fate from the quiescent to the activation state. Individual myofibers associated with satellite cells were isolated from EDL muscles using 0.2% type I collagenase (Worthington Biochemical, Lakewood, NJ) in DMEM (Thermo Fisher Scientific, MA) for 90 min at 37°C and 5% CO2. Following purification of myofibers, isolated myofibers were further incubated in DMEM for 3 h at 37°C under 5% CO2 to eliminate dying contracted myofibers during isolation (Figure S1). For a co-culture assay, equal numbers of isolated myofibers were cultured with or without damaged myofibers under floating conditions (Figure 2A). DMEM and non-glucose DMEM (Wako, Osaka, Japan) were used. The volume of medium was determined by a ratio of 50 myofibers/mL volume. GM (DMEM supplemented with 30% fetal bovine serum, 1% chicken-embryo extract, 10 ng/mL basic fibroblast growth factor, and 1% penicillin-streptomycin) and plating medium (DMEM supplemented with 10% horse serum, 0.5% chicken-embryo extract, and 1% penicillin-streptomycin) were used for satellite cell activation (Figure 6) and as a positive control (Figures 2 and 3), respectively.
To obtain muscle tissue extracts, TA muscle tissues of adult mice were isolated and crushed in a bead crusher. Tissue homogenates were then filtered with a 0.45 μm filter before use as muscle extracts. Isolated myofibers associated with satellite cells were treated with muscle tissue extracts in DMEM under floating culture conditions (Figure 1A). To obtain mechanically damaged myofibers, healthy intact myofibers were directly damaged with a Pasteur pipette in the culture dish. The damaged myofibers were detected by shrunken morphology (Figure 2A). The ratio of intact to damaged myofibers was 1:1 in the co-culture condition and the total numbers of myofibers were equivalent between conditions (Figures 2 and 3). Cells were labeled with EdU (Thermo Fisher Scientific) in the culture medium for 6 h prior to fixation. Reagents were obtained from the following sources: PHA-665752, Cayman Chemical, MI; Rapamycin, LC Laboratories, MA; SU5402, Merck Millipore, MA; Wortmannin, AdipoGen, CA; PD98059, ALEXIS Biochemicals, CA; Dorsomorphin, FUJIFILM Wako Pure Chemical Corp., Osaka, Japan; and SB216763, Merck Millipore, MA.
Statistical Analysis
All experimental data are shown as mean ± SE. The comparison between two conditions was done by unpaired t test. A one-way repeated-measures ANOVA was applied to identify significant differences among conditions or groups. When a significant difference was observed, the data were subjected to post hoc analysis. A p < 0.05 was considered significant.
Author Contributions
Y.T. designed and performed the experiments, interpreted and analyzed the data, and wrote the manuscript. Y.K. and H.M. performed the experiments and interpreted and analyzed the data. Y.O. designed and performed the experiments, interpreted the data, assembled the input data, and wrote the manuscript. All authors discussed the results and implications and commented on the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank all the lab members for technical support. This work was supported by the Japan Agency for Medical Research and Development (AMED, 16bm0704010h0001, 18ek0109383h0001, and 19bm0704036h0001), and the Grant-in-Aid for Scientific Research KAKENHI (17K13138, 18H03193, and 18K19749). This work was also supported, in part, by the Takeda Science Foundation. Y.T. is funded by a JSPS Research Fellowship.
Published: September 3, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.08.002.
Supplemental Information
References
- Anderson J.E. Hepatocyte growth factor and satellite cell activation. Adv. Exp. Med. Biol. 2016;900:1–25. doi: 10.1007/978-3-319-27511-6_1. [DOI] [PubMed] [Google Scholar]
- Arora R., Rumman M., Venugopal N., Gala H., Dhawan J. Mimicking muscle stem cell quiescence in culture: methods for synchronization in reversible arrest. Methods Mol. Biol. 2017;1556:283–302. doi: 10.1007/978-1-4939-6771-1_15. [DOI] [PubMed] [Google Scholar]
- Baghdadi M.B., Castel D., Machado L., Fukada S.I., Birk D.E., Relaix F., Tajbakhsh S., Mourikis P. Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche. Nature. 2018;557:714–718. doi: 10.1038/s41586-018-0144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisswenger P.J., Howell S.K., Smith K., Szwergold B.S. Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochim. Biophys. Acta. 2003;1637:98–106. doi: 10.1016/s09254439(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev. Biol. 1986;115:140–147. doi: 10.1016/0012-1606(86)90235-6. [DOI] [PubMed] [Google Scholar]
- Bjornson C.R., Cheung T.H., Liu L., Tripathi P.V., Steeper K.M., Rando T.A. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack A.S., Rando T.A. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10:504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini C.R., Ligabue-Braun R. Ureases as multifunctional toxic proteins: a review. Toxicon. 2016;110:90–109. doi: 10.1016/j.toxicon.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Chakkalakal J.V., Jones K.M., Basson M.A., Brack A.S. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung T.H., Quach N.L., Charville G.W., Liu L., Park L., Edalati A., Yoo B., Hoang P., Rando T.A. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison D.D., Olwin B.B., Rudnicki M.A., Wold B.J. MyoD(-/-) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev. Biol. 2000;224:122–137. doi: 10.1006/dbio.2000.9682. [DOI] [PubMed] [Google Scholar]
- Crist C.G., Montarras D., Buckingham M. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell. 2012;11:118–126. doi: 10.1016/j.stem.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Der Vartanian A., Quetin M., Michineau S., Aurade F., Hayashi S., Dubois C., Rocancourt D., Drayton-Libotte B., Szegedi A., Buckingham M. PAX3 confers functional heterogeneity in skeletal muscle stem cell responses to environmental stress. Cell Stem Cell. 2019;24:958–973.e9. doi: 10.1016/j.stem.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J., Rando T.A. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Du H., Shih C.H., Wosczyna M.N., Mueller A.A., Cho J., Aggarwal A., Rando T.A., Feldman B.J. Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat. Commun. 2017;8:669. doi: 10.1038/s41467-017-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont N.A., Wang Y.X., Rudnicki M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development. 2015;142:1572–1581. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durany N., Carreras J. Distribution of phosphoglycerate mutase isozymes in rat, rabbit and human tissues. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996;114:217–223. doi: 10.1016/0305-0491(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Eber S.W., Krietsch W.K. The isolation and characterization of the multiple forms of human skeletal muscle triosephosphate isomerase. Biochim. Biophys. Acta. 1980;614:173–184. doi: 10.1016/0005-2744(80)90178-3. [DOI] [PubMed] [Google Scholar]
- Evano B., Tajbakhsh S. Skeletal muscle stem cells in comfort and stress. NPJ Regen. Med. 2018;3:24. doi: 10.1038/s41536-018-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks S., Merton G.K., Lerena M.J., Heining P., Carter N.D., Holt D.W. Cardiac troponins and creatine kinase content of striated muscle in common laboratory animals. Clinica Chim. Acta Int. J. Clin. Chem. 2001;304:65–74. doi: 10.1016/s0009-8981(00)00409-5. [DOI] [PubMed] [Google Scholar]
- Fujimaki S., Seko D., Kitajima Y., Yoshioka K., Tsuchiya Y., Masuda S., Ono Y. Notch1 and Notch2 coordinately regulate stem cell function in the quiescent and activated states of muscle satellite cells. Stem Cells. 2018;36:278–285. doi: 10.1002/stem.2743. [DOI] [PubMed] [Google Scholar]
- Fukada S., Uezumi A., Ikemoto M., Masuda S., Segawa M., Tanimura N., Yamamoto H., Miyagoe-Suzuki Y., Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–2459. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- Garcin E.D. GAPDH as a model non-canonical AU-rich RNA binding protein. Semin. Cell Dev. Biol. 2019;86:162–173. doi: 10.1016/j.semcdb.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Ge Y., Molloy M.P., Chamberlain J.S., Andrews P.C. Proteomic analysis of mdx skeletal muscle: Great reduction of adenylate kinase 1 expression and enzymatic activity. Proteomics. 2003;3:1895–1903. doi: 10.1002/pmic.200300561. [DOI] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Allouh M.Z., Rosser B.W., Rinkevich Y., Reshef R., Rozenboim I., Wleklinski-Lee M., Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Haugk K.L., Roeder R.A., Garber M.J., Schelling G.T. Regulation of muscle cell proliferation by extracts from crushed muscle. J. Anim. Sci. 1995;73:1972–1981. doi: 10.2527/1995.7371972x. [DOI] [PubMed] [Google Scholar]
- Henderson B., Martin A.C. Protein moonlighting: a new factor in biology and medicine. Biochem. Soc. Trans. 2014;42:1671–1678. doi: 10.1042/BST20140273. [DOI] [PubMed] [Google Scholar]
- Joanisse S., McKay B.R., Nederveen J.P., Scribbans T.D., Gurd B.J., Gillen J.B., Gibala M.J., Tarnopolsky M., Parise G. Satellite cell activity, without expansion, after nonhypertrophic stimuli. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309:R1101–R1111. doi: 10.1152/ajpregu.00249.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe A.W., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M.A., Rossi F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczor J.J., Ziolkowski W., Popinigis J., Tarnopolsky M.A. Anaerobic and aerobic enzyme activities in human skeletal muscle from children and adults. Pediatr. Res. 2005;57:331–335. doi: 10.1203/01.PDR.0000150799.77094.DE. [DOI] [PubMed] [Google Scholar]
- Kardami E., Spector D., Strohman R.C. Selected muscle and nerve extracts contain an activity which stimulates myoblast proliferation and which is distinct from transferrin. Dev. Biol. 1985;112:353–358. doi: 10.1016/0012-1606(85)90406-3. [DOI] [PubMed] [Google Scholar]
- Kitajima Y., Ono Y. Visualization of PAX7 protein dynamics in muscle satellite cells in a YFP knock-in-mouse line. Skeletal Muscle. 2018;8:26. doi: 10.1186/s13395-018-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S., Kuroda K., Le Grand F., Rudnicki M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S., Rudnicki M.A. The emerging biology of satellite cells and their therapeutic potential. Trends Mol. Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Lescaudron L., Peltekian E., Fontaine-Perus J., Paulin D., Zampieri M., Garcia L., Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul. Disord. 1999;9:72–80. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Li Y.P. TNF-alpha is a mitogen in skeletal muscle. Am. J. Physiol. Cell Physiol. 2003;285:C370–C376. doi: 10.1152/ajpcell.00453.2002. [DOI] [PubMed] [Google Scholar]
- Mackey A.L., Magnan M., Chazaud B., Kjaer M. Human skeletal muscle fibroblasts stimulate in vitro myogenesis and in vivo muscle regeneration. J. Physiol. 2017;595:5115–5127. doi: 10.1113/JP273997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan D.W., Henkin J.A., Vigoreaux J.O. Concentrations of glycolytic enzymes and other cytosolic proteins in the diffusible fraction of a vertebrate muscle proteome. Mol. Cell Proteom. 2005;4:1541–1549. doi: 10.1074/mcp.M500053-MCP200. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megeney L.A., Kablar B., Garrett K., Anderson J.E., Rudnicki M.A. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- Mezzogiorno A., Coletta M., Zani B.M., Cossu G., Molinaro M. Paracrine stimulation of senescent satellite cell proliferation by factors released by muscle or myotubes from young mice. Mech. Ageing Dev. 1993;70:35–44. doi: 10.1016/0047-6374(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Mourikis P., Sambasivan R., Castel D., Rocheteau P., Bizzarro V., Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- Murphy M.M., Lawson J.A., Mathew S.J., Hutcheson D.A., Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y. Satellite cell heterogeneity and hierarchy in skeletal muscle. J. Phys. Fitness Sports Med. 2014;3:229–234. [Google Scholar]
- Ono Y., Boldrin L., Knopp P., Morgan J.E., Zammit P.S. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev. Biol. 2010;337:29–41. doi: 10.1016/j.ydbio.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Calhabeu F., Morgan J.E., Katagiri T., Amthor H., Zammit P.S. BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Differ. 2011;18:222–234. doi: 10.1038/cdd.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Masuda S., Nam H.S., Benezra R., Miyagoe-Suzuki Y., Takeda S. Slow-dividing satellite cells retain long-term self-renewal ability in adult muscle. J. Cell Sci. 2012;125:1309–1317. doi: 10.1242/jcs.096198. [DOI] [PubMed] [Google Scholar]
- Ono Y., Urata Y., Goto S., Nakagawa S., Humbert P.O., Li T.S., Zammit P.S. Muscle stem cell fate is controlled by the cell-polarity protein Scrib. Cell Rep. 2015;10:1135–1148. doi: 10.1016/j.celrep.2015.01.045. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Puigjaner J., Rais B., Burgos M., Comin B., Ovadi J., Cascante M. Comparison of control analysis data using different approaches: modelling and experiments with muscle extract. FEBS Lett. 1997;418:47–52. doi: 10.1016/s0014-5793(97)01347-1. [DOI] [PubMed] [Google Scholar]
- Relaix F., Zammit P.S. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- Rocheteau P., Gayraud-Morel B., Siegl-Cachedenier I., Blasco M.A., Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Rodgers J.T., King K.Y., Brett J.O., Cromie M.J., Charville G.W., Maguire K.K., Brunson C., Mastey N., Liu L., Tsai C.R. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J.T., Schroeder M.D., Ma C., Rando T.A. HGFA is an injury-regulated systemic factor that induces the transition of stem cells into GAlert. Cell Rep. 2017;19:479–486. doi: 10.1016/j.celrep.2017.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramozza A., Park D., Kollu S., Beerman I., Sun X., Rossi D.J., Lin C.P., Scadden D.T., Crist C., Brack A.S. Lineage tracing reveals a subset of reserve muscle stem cells capable of clonal expansion under stress. Cell Stem Cell. 2019;24:944–995.e5. doi: 10.1016/j.stem.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa M., Fukada S., Yamamoto Y., Yahagi H., Kanematsu M., Sato M., Ito T., Uezumi A., Hayashi S., Miyagoe-Suzuki Y. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp. Cell Res. 2008;314:3232–3244. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Sirover M.A. Pleiotropic effects of moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in cancer progression, invasiveness, and metastases. Cancer Metastasis Rev. 2018;37:665–676. doi: 10.1007/s10555-018-9764-7. [DOI] [PubMed] [Google Scholar]
- Snijders T., Verdijk L.B., Hansen D., Dendale P., van Loon L.J. Continuous endurance-type exercise training does not modulate satellite cell content in obese type 2 diabetes patients. Muscle Nerve. 2011;43:393–401. doi: 10.1002/mus.21891. [DOI] [PubMed] [Google Scholar]
- Sousa-Victor P., Garcia-Prat L., Munoz-Canoves P. New mechanisms driving muscle stem cell regenerative decline with aging. Int. J. Dev. Biol. 2018;62:583–590. doi: 10.1387/ijdb.180041pm. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Yamada M., Noma T., Kajii T., Nakazawa A. Tissue-specific and developmentally regulated expression of the genes encoding adenylate kinase isozymes. J. Biochem. 1993;113:200–207. doi: 10.1093/oxfordjournals.jbchem.a124026. [DOI] [PubMed] [Google Scholar]
- Tatsumi R., Anderson J.E., Nevoret C.J., Halevy O., Allen R.E. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev. Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Uchida K., Kondoh K., Matuo Y. Recombinant M-, B- and MB-type isozymes of human phosphoglyceric acid mutase: their large-scale production and preparation of polyclonal antibodies specific to M- and B-type isozymes. Clinica Chim. Acta. 1995;237:43–58. doi: 10.1016/0009-8981(95)06063-j. [DOI] [PubMed] [Google Scholar]
- Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Vandenburgh H.H., Lent C.M. Relationship of muscle growth in vitro to sodium pump activity and transmembrane potential. J. Cell Physiol. 1984;119:283–295. doi: 10.1002/jcp.1041190306. [DOI] [PubMed] [Google Scholar]
- Verma M., Asakura Y., Murakonda B.S.R., Pengo T., Latroche C., Chazaud B., McLoon L.K., Asakura A. Muscle satellite cell cross-talk with a vascular niche maintains quiescence via VEGF and notch signaling. Cell Stem Cell. 2018;23:530–543.e9. doi: 10.1016/j.stem.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z., Rudnicki M.A., Rivera A.J., Primig M., Anderson J.E., Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev. Biol. 1999;210:440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Watanabe Y., Ohtani T., Uezumi A., Mikami N., Nakamura M., Sato T., Ikawa M., Hoshino M., Tsuchida K. Calcitonin receptor signaling inhibits muscle stem cells from escaping the quiescent state and the niche. Cell Rep. 2015;13:302–314. doi: 10.1016/j.celrep.2015.08.083. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Legendre N.P., Biswas A.A., Lawton A., Yamamoto S., Tajbakhsh S., Kardon G., Goldhamer D.J. Loss of MyoD and Myf5 in skeletal muscle stem cells results in altered myogenic programming and failed regeneration. Stem Cell Rep. 2018;10:956–969. doi: 10.1016/j.stemcr.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P.S., Golding J.P., Nagata Y., Hudon V., Partridge T.A., Beauchamp J.R. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P.S., Partridge T.A., Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zeng L., Akasaki Y., Sato K., Ouchi N., Izumiya Y., Walsh K. Insulin-like 6 is induced by muscle injury and functions as a regenerative factor. J. Biol. Chem. 2010;285:36060–36069. doi: 10.1074/jbc.M110.160879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.