Abstract
We investigated the prevalence and clinical outcomes of COVID-19 in recipients of kidney transplants in the Bronx, New York, one of the epicenters of the pandemic. Between March 16 and June 2, 2020, 132 kidney transplant recipients tested positive by SARS-CoV-2 RT-PCR. From May 3 to July 29, 2020, 912 kidney transplant recipients were screened for SARS-CoV-2 IgG antibodies during routine clinic visits, of which 16.6% tested positive. Fifty-five of the 152 patients had previously tested positive by RT-PCR, while the remaining 97 did not have significant symptoms and had not been previously tested by RT-PCR. The prevalence of SARS-CoV-2 infection was 23.4% in the 975 patients tested by either RT-PCR or SARS-CoV-2 IgG. Older patients and patients with higher serum creatinine levels were more likely diagnosed by RT-PCR compared to SARS-CoV-2 IgG. Sixty-nine RT-PCR positive patients were screened for SARS-CoV-2 IgG antibodies at a median of 44 days post-diagnosis (Inter Quartile Range 31-58) and 80% were positive. Overall mortality was 20.5% but significantly higher (37.8%) in the patients who required hospitalization. Twenty-three percent of the hospitalized patients required kidney replacement therapy and 6.3% lost their allografts. In multivariable analysis, older age, receipt of deceased-donor transplantation, lack of influenza vaccination in the previous year and higher serum interleukine-6 levels were associated with mortality. Thus, 42% of patients with a kidney transplant and with COVID-19 were diagnosed on antibody testing without significant clinical symptoms; 80% of patients with positive RT-PCR developed SARS-CoV-2 IgG and mortality was high among patients requiring hospitalization.
Keywords: COVID-19, kidney transplantation, mortality, SARS-CoV-2 IgG antibody
Graphical abstract
Editor’s Note.
This is one of several articles we think you will find of interest that are part of our special issue of Kidney International addressing the challenges of dialysis and transplantation during the COVID-19 pandemic. Please also find additional material in our commentaries and letters to the editor sections. We hope these insights will help you in the daily care of your own patients.
Following the first case of coronavirus disease 2019 (COVID-19) in the United States recorded in January 2020, New York quickly became the epicenter of the pandemic in March and April 2020. Initial results from 3 centers in New York reported a high mortality rate of 20%–39% in hospitalized patients.1, 2, 3 Kidney transplant recipients are expected to be at an increased risk of complications from COVID-19 owing not only to their chronic immunosuppression, but also to frequently associated comorbidities, including older age, hypertension, diabetes mellitus, chronic kidney disease, and cardiovascular disease. We previously reported 28% mortality at 3 weeks of follow-up in a cohort of 36 kidney transplant recipients with COVID-19.4 Other kidney transplantation centers in New York have reported similar mortality rates of 13%–30%,5, 6, 7, 8 as did an international multicenter registry, including our center and 12 others in the USA, Italy, and Spain, with an initial mortality of 32% in kidney transplant recipients.9 Another multicenter cohort study of 482 solid organ transplant recipients (318 kidney or kidney/pancreas) from more than 50 transplantation centers in the USA reported 20.5% mortality.10
In addition to transplant recipients, patients with end-stage renal disease (ESRD) also appear to be at increased risk for severe COVID-19 illness. Early reports from European centers showed a mortality rate of 20%–30% among patients receiving chronic dialysis who were hospitalized for COVID-19.11, 12, 13 The mortality rate in 114 patients with ESRD admitted to our center was 28%,14 and it was 31% at another New York center.15
Based on these early studies, there is a concern that kidney transplantation may be an independent risk factor for mortality compared with both the general population and patients with ESRD, prompting questions regarding the safety of transplantation during the pandemic. However, the mortality of COVID-19 in renal transplant recipients is difficult to determine without understanding the actual prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which has been hampered owing to limited polymerase chain reaction (PCR) analysis early in the pandemic and lack of widespread SARS-CoV-2 antibody testing in this population. Furthermore, given their immunosuppression, whether kidney transplant recipients mount an antibody response to SARS-CoV-2 similar to that in the general population is unknown, and thus SARS-CoV-2 may be underdiagnosed by antibody testing in transplant recipients.
Accurate serologic tests for IgG antibodies to SARS-CoV-2 are imperative to better understand the immune response in the setting of SARS-CoV-2 infection. Zhao et al. found that RNA detectability by PCR decreased over time to 45.5% by 15–39 days after onset, whereas the presence of IgG increased to nearly 80% by 15–39 days, and concluded that diagnostic sensitivity may be improved with a combination of RNA and antibody detection.16 Reverse-transcription (RT)–PCR for diagnosis of SARS-CoV-2 infection has been associated with a high rate of false-negative results.17 Recent reports have shown that the use of SARS-CoV-2 IgG and IgM antibodies can help diagnose patients who present with COVID-like symptoms despite negative RT-PCR swabs.18 Furthermore, screening for antibody testing in the general population has determined that SARS-CoV-2 infections are 6–24 times more prevalent than initially thought.19 Notably, the reported seropositivity in patients receiving hemodialysis has been as high as 36.2%.20 Data on the use of serologic testing for diagnosing SARS-CoV-2 in kidney transplant recipients are lacking. Moreover, differences in the clinical presentation and course of disease among kidney transplant recipients may differ between those diagnosed by serology and those diagnosed by RT-PCR, although this has not yet been evaluated.
The objectives of the present study were (i) to determine the seroprevalence of SARS-CoV-2 IgG in our kidney transplant recipients, (ii) to compare clinical and demographic features of patients diagnosed by SARS-CoV-2 IgG to those diagnosed by RT-PCR, (iii) to identify the antibody response rate in kidney transplant recipients with confirmed SARS-CoV-2 on RT-PCR, and (iv) to determine predictors of mortality, including inflammatory markers, blood type, and HLA types.
Results
COVID-19 diagnosis by RT-PCR and SARS-CoV-2 IgG
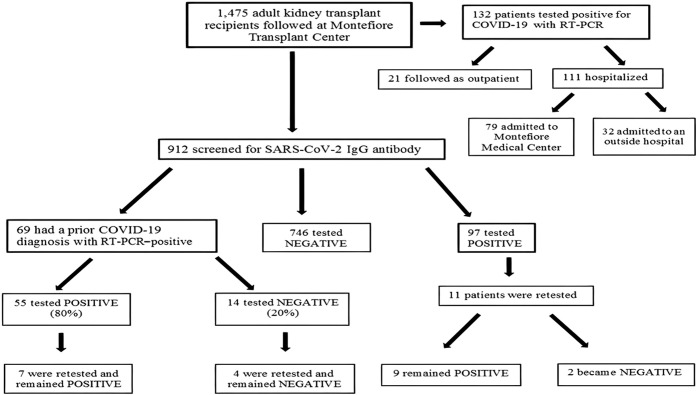
Figure 1 summarizes the study design involving the 1475 adult kidney transplant recipients currently followed by our center. Between March 16 and June 2, 2020, 132 patients tested positive for SARS-CoV-2 by RT-PCR. Between May 3 and July 29, 2020, 912 patients were screened for SARS-CoV-2 IgG antibodies, and 152 (16.6%) tested positive. Fifty-five of the 152 patients had COVID-19 confirmed by previous RT-PCR. The remaining 97 COVID-19 IgG–positive patients did not have significant symptoms, did not seek medical attention, and were not tested for SARS-CoV-2 by RT-PCR. The prevalence of SARS-CoV-2 infection was 23.4% in the 975 patients tested by either RT-PCR or SARS-CoV-2 IgG.
Figure 1.
Study design. COVID-19, coronavirus disease 2019; RT-PCR, reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
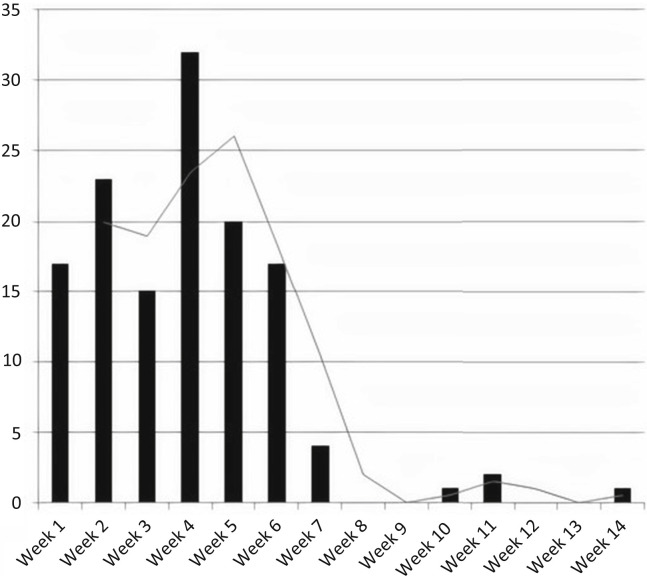
Of the 132 patients with a positive SARS-CoV-2 RT-PCR, 21 were followed as outpatients with close monitoring of their symptoms by the transplantation center. The remaining 111 patients were hospitalized, 79 at Montefiore Medical Center and 32 at outside facilities. Figure 2 shows the number of cases of SARS-CoV-2 RT-PCR positivity over the first 14 weeks of the pandemic. Our first confirmed case of COVID-19 was diagnosed on March 16, 2020, which corresponds to the start date of week 1. RT-PCR–positive cases peaked at week 4 and declined thereafter.
Figure 2.
Weekly number of kidney transplant recipients diagnosed by coronavirus disease 2019 reverse-transcription polymerase chain reaction starting on March 16, 2020.
Table 1 summarizes the demographic data and clinical presentation of the 229 patients with COVID-19 and compares those diagnosed by RT-PCR and those diagnosed by SARS-CoV-2 antibodies. The cohort was 62% male, 55% Hispanic, and 32% African American, and the median patient age was 59 years (IQR, 49 to 68 years). Seventy-three percent were deceased donor graft recipients, and diagnosis of COVID-19 occurred at a median of 58.2 months (IQR, 25.4–127.6 months) after transplantation. Only 9% and 7% underwent kidney transplantation within the preceding 12 months and 6 months, respectively. Most patients were on triple immunosuppression with tacrolimus (97%), mycophenolate mofetil/mycophenolic acid (87%), and prednisone (95%). Patients had multiple additional medical comorbidities, including hypertension in 98%, diabetes mellitus in 61%, heart disease in 22%, and history of smoking in 36%. The median body mass index (BMI) was 28.5 (IQR, 24.2–32.6).
Table 1.
Clinical characteristics of patients by type of COVID-19 diagnosis and mortality
| Characteristics | Total patients (N = 229) | COVID-19 diagnosis |
Mortality |
||||
|---|---|---|---|---|---|---|---|
| COVID-19 RT-PCR–positive (N = 132) | SARS-CoV-2 IgG antibody–positive (N = 97) | P value | Survivors (N = 182) | Nonsurvivors (N = 47) | P value | ||
| Sex | 0.84 | ||||||
| Male | 141 (62) | 82 (62) | 59 (61) | 113 (62) | 28 (60) | 0.75 | |
| Female | 88 (38) | 50 (38) | 38 (39) | 69 (38) | 19 (40) | ||
| Age, yr | 59 [49–68] | 62.5 [51–71] | 57 [46–65] | 0.0024 | 58 [46–66] | 70 [58–74] | < 0.001 |
| Race | 0.87 | 0.53 | |||||
| Hispanic | 125 (55) | 74 (56) | 51 (53) | 74 (56) | 51 (53) | ||
| African American | 74 (32) | 41 (31) | 33 (34) | 41 (31) | 33 (34) | ||
| Other | 30 (13) | 17 (13) | 13 (13) | 17 (13) | 13 (13) | ||
| Type of transplant | 0.039 | ||||||
| Deceased donor | 165 (73) | 101 (77) | 64 (66) | 124 (69) | 41 (89) | 0.0058 | |
| Living donor | 61 (27) | 28 (21) | 33 (34) | 56 (31) | 5 (11) | ||
| Time after transplantation, mo | 58.2 [25.4–127.6] | 60.8 [20–128.5] | 57.7 [28.7–124.6] | 0.9 | 57.7 [27.3–123.7] | 65.2 [16.3–134.1] | 0.82 |
| Transplantation at <6 mo | 13 (7) | 9 (9) | 4 (4) | 0.49 | 10 (6) | 3 (6) | 0.21 |
| Transplantation at <12 mo | 18 (9) | 11 (11) | 7 (8) | 0.97 | 13 (7) | 5 (11) | 0.43 |
| Etiology of ESRD | 0.005 | ||||||
| Diabetes mellitus | 106 (47) | 72 (55) | 34 (35) | 73 (40) | 33 (70) | 0.0065 | |
| Hypertension | 49 (22) | 21 (16) | 28 (29) | 45 (25) | 4 (9) | ||
| Glomerulonephritis | 52 (23) | 23 (18) | 29 (30) | 44 (24) | 8 (17) | ||
| Polycystic kidney disease | 9 (4) | 2 (2) | 7 (5) | 8 (4) | 1 (2) | ||
| Others | 12 (5) | 8 (6) | 4 (4) | 11 (6) | 1 (2) | ||
| Body mass index, kg/m2 | 28.5 [24.2–32.6] | 28.7 [23.7–32.5] | 28.1 [24.7–32.6] | 0.76 | 28.3 [24.2–32.3] | 29.1 [23.7–34.3] | 0.66 |
| History of smoking | 81 (36) | 48 (37) | 33 (34) | 0.68 | 64 (35) | 17 (36) | 0.92 |
| Influenza vaccination | 193 (89) | 102 (86) | 91 (94) | 0.055 | 162 (93) | 31 (66) | 0.0015 |
| Comorbidities | |||||||
| Hypertension | 224 (98) | 128 (98) | 96 (99) | 0.47 | 178 (98) | 46 (98) | 0.83 |
| Diabetes mellitus | 140 (61) | 89 (68) | 51 (53) | 0.019 | 104 (58) | 36 (77) | 0.016 |
| Heart disease | 49 (22) | 28 (21) | 21 (22) | 0.96 | 38 (21) | 11 (23) | 0.72 |
| Lung disease | 16 (7) | 11 (8) | 5 (5) | 0.34 | 10 (6) | 6 (13) | 0.083 |
| Cancer | 23 (10) | 12 (9) | 11 (11) | 0.59 | 18 (10) | 5 (11) | 0.89 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use | 60 (26) | 33 (25) | 27 (28) | 0.65 | 47 (26) | 13 (28) | 0.81 |
| Statin use | 143 (63) | 84 (64) | 59 (61) | 0.61 | 113 (62) | 30 (64) | 0.86 |
| Baseline serum creatinine, mg/dl | 1.4 [1.0–1.7] | 1.4 [1.1–1.8] | 1.2 [1.0–1.5] | 0.0048 | 1.3 [1.0–1.6] | 1.5 [1.2–1.8] | 0.032 |
| Blood type | 0.73 | ||||||
| A | 84 (38) | 47 (37) | 37 (39) | 64 (36) | 20 (43) | 0.68 | |
| B | 44 (20) | 28 (22) | 16 (17) | 35 (20) | 9 (19) | ||
| AB | 6 (3) | 4 (3) | 2 (2) | 4 (2) | 2 (4) | ||
| O | 90 (40) | 49 (38) | 41 (43) | 74 (42) | 16 (34) | ||
COVID-19, coronavirus disease 2019; ESRD, end-stage renal disease; IQR, interquartile range; RT-PCR, reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are n (%) or median [IQR], unless otherwise noted.
Patients diagnosed by RT-PCR tend to be older (median age, 62.5 years vs. 57 years; P < 0.0024) and were more likely to have received a deceased donor kidney transplant (77% vs. 66%; P = 0.039). These patients were also more likely to have diabetes (68% vs. 53%; P = 0.019), have diabetic nephropathy as the cause of renal failure (55% vs. 35%; P = 0.027), and had a higher baseline creatinine concentration (median, 1.4 mg/dl vs. 1.2 mg/dl; P = 0.039) compared with patients diagnosed by a positive SARS-CoV-2 IgG only. On multivariate analysis, the odds of being diagnosed by RT-PCR compared with SARS-CoV-2 IgG were estimated to increase by 21% for every 5-year increase in age (odds ratio [OR], 1.2; 95% confidence interval [CI], 1.09–1.35; P < 0.001) and by 7% for every 0.1 unit increase in baseline creatinine level (OR, 1.07; 95% CI, 1.02–1.12; P = 0.0082).
Thirty-five of the 97 patients (36.1%) diagnosed by SARS-CoV-2 IgG did not recall having any symptoms suggestive of COVID-19 during the peak of the pandemic, and the remaining patients had mild symptoms that did not necessitate medical evaluation. Compared with patients diagnosed by SARS-CoV-2 IgG, patients who were diagnosed by positive RT-PCR were more likely to have fever (61% vs. 30%), dyspnea (60% vs. 28%), cough (62.5% vs. 23%), and diarrhea (28% vs. 15%). Interestingly, anosmia was more common in those diagnosed by antibody testing (14.8%).
A total of 69 RT-PCR–positive patients were tested for SARS-CoV-2 IgG antibodies during routine follow-up. Fifty-five patients (80%) were positive for SARS-CoV-2 IgG antibodies, measured at a median of 44 days after initial diagnosis (IQR, 31–58 days). Only 7 of the 55 IgG-positive patients were retested at a later date, and their antibodies remained positive. Fourteen patients (20%) with confirmed positive SARS-CoV-2 RT-PCR were negative for SARS-CoV-2 antibodies. Four of these patients were retested within 1 month after the initial test, and their IgG remained negative.
Of the 97 patients who were diagnosed by SARS-CoV-2 IgG only, 11 were retested at 1–2 months after the initial test. Nine patients showed sustained IgG positivity, and 2 tested negative. Thirty patients underwent repeat RT-PCR testing 14–90 days after their initial positive RT-PCR, and 5 patients (16.7%) showed sustained positivity. Of the 25 patients with a repeat negative RT-PCR test, 16 were retested a third time and 8 were retested a fourth time, and all tests remained negative except for 1 patient.
Mortality
The 229 COVID-19 patients were followed for a median of 140 days (IQR, 86–164). Forty-seven patients (20.5%) died at a median of 10 days (IQR, 6–16 days) after diagnosis. Patient sex, race, time after transplantation, immunosuppressive medications, BMI, history of smoking, angiotensin-converting enzyme inhibitor or receptor blocker use, and statin use were not associated with mortality. Risk factors associated with mortality included older age (70 years vs. 58 years; P < 0.001), receipt of a deceased donor renal transplant (89% vs. 69%; P = 0.0058), diabetic nephropathy as a cause of renal disease (70% vs. 40%; P = 0.0065), diabetes mellitus (77% vs. 58%; P = 0.016), non-receipt of influenza vaccination the previous year (76% vs. 93%; P = 0.0015), and higher baseline serum creatinine levels (1.5 mg/dl vs. 1.3 mg/dl; P = 0.032) in univariate analysis. In multivariate analysis, age, type of transplant, and receipt of influenza vaccination were statistically significant. The odds of mortality were estimated to increase by 48% for every 5-year increase in age (OR, 1.48; 95% CI, 1.22–1.79; P < 0.001). Compared with living donor graft recipients, deceased donor graft recipients had an estimated OR of mortality of 1.22 (95% CI, 1.067–1.74; P = 0.015), and those who did not receive influenza vaccine the previous year had an estimated OR of 1.13 of mortality compared with those who did receive the vaccine (95% CI, 1.040–1.43; P < 0.001).
There was no statistically significant association between blood group type and mortality. In terms of HLA typing, there was no association between any HLA type and mortality. The smallest adjusted P value for the 75 alleles assessed was 0.973.
Clinical features and inflammatory markers of hospitalized patients
Among the 111 patients who required hospitalization, mortality was 37.8%. Of these 111 patients, 79 were admitted to Montefiore Medical Center and underwent specific monitoring of inflammatory markers and T-cell subtypes. Supplementary Table S1 presents the demographic data of those 79 patients, as well as demographic data for the subgroup of patients who survived the hospital admission (n = 51) and those who died (n = 28). There was no difference between survivors and nonsurvivors in terms of demographics; however, nonsurvivors tend to have a higher BMI (30.4 vs. 26.7; P = 0.057) and had a lower oxygen saturation on room air on presentation (93% vs. 96%; P = 0.037).
On admission, 67 patients (85%) were lymphopenic, 54 (68%) had a low CD3 cell count, 52 (66%) had a low CD4 cell count, and 22 (28%) had a low CD8 cell count. The C-reactive protein (CRP) level was >10 mg/dl in 38 patients (48%), procalcitonin level was >0.2 ng/ml in 41 patients (52%), ferritin level was >900 ng/ml in 50 patients (63%), and 66 patients (84%) had a D-dimer level >0.5 μg/ml, and 20 (25%) had a level >3 μg/ml. Thirty-two patients (41%) had a serum interleukin-6 (IL-6) level >60 pg/ml. Fifty-three (67%) patients had a lactic dehydrogenase level >1.5 times the upper range of normal, 19 (24.1%) had a creatine kinase level >200 U/l, 49 (62%) had a fibrinogen level >500 mg/dl, and 43 (54.4%) had an N-terminal pro-brain natriuretic peptide (pro-BNP) level >900 pg/ml. Admission laboratory values and inflammatory markers are presented in Table 2 . When comparing survivors and nonsurvivors, initial IL-6 levels (47 pg/ml vs. 101 pg/ml; P = 0.036) and initial pro-BNP levels (1278 pg/ml vs. 2380 pg/ml; P = 0.031) were significantly higher in nonsurvivors compared with survivors. There were no other statistically significant differences in admission laboratory values or inflammatory markers between the 2 groups.
Table 2.
Laboratory values and inflammatory markers on admission of the patients admitted to Montefiore Medical Center
| Laboratory values and inflammatory markers on admission | Total patients (N = 79) | Survivors (N = 51) | Nonsurvivors (N = 28) | P value |
|---|---|---|---|---|
| Hemoglobin, g/dl | 12.1 [10.6–13.2] | 12.2 [10.6–13.3] | 11.8 [11.1–13] | 0.94 |
| WBC count, k/μl | 6.2 [4.4–8.0] | 5.8 [4.1–7.7] | 6.4 [5.4–8.1] | 0.23 |
| WBC count <4 k/μl | 12 (15) | 11 (22) | 1 (4) | |
| Lymphocytes, k/μl | 0.6 [0.4–0.8] | 0.6 [0.4–0.8] | 0.7 [0.4–0.8] | 0.96 |
| Lymphocyte count <1 k/μl | 67 (85) | 42 (82) | 25 (89) | |
| Platelets, k/μl | 178 [132–240] | 189 [132–241] | 162 [118.5–205.5] | 0.22 |
| Platelets count <150 k/μl | 30 (38) | 18 (35) | 12 (43) | |
| CD3 cell count, cells/μl | 319 [205–552] | 390 [226.5–574] | 243 [158–529] | 0.12 |
| CD3 count <706 cells/μl | 54 (68) | 33 (65) | 21 (75) | |
| CD4 cell count, cells/μl | 147 [88–304] | 178 [117–305] | 120 [74–252] | 0.085 |
| CD4 count <344 cells/μl | 52 (66) | 31 (61) | 21 (75) | |
| CD8 cell count, cells/μl | 126 [83–272] | 147 [87.5–263] | 123 [71–272] | 0.4 |
| CD8 count <104 cells/μl | 22 (28) | 13 (26) | 9 (32) | |
| CRP, mg/dl | 9.9 [4.9–16.2] | 7.2 [4.6–14.8] | 11.3 [5.7–18.1] | 0.25 |
| CRP >10 mg/dl | 38 (48) | 23 (45) | 15 (54) | |
| Procalcitonin, ng/ml | 0.3 [0.1–1.7] | 0.2 [0.1–1.6] | 0.4 [0.2–2.9] | 0.065 |
| Procalcitonin >0.2 ng/ml | 41 (52) | 22 (43) | 19 (68) | |
| Ferritin, ng/ml | 1345 [681–2397] | 1516 [713–3179] | 1029 [629–1939] | 0.16 |
| Ferritin >900 ng/ml | 50 (63) | 35 (69) | 15 (54) | |
| D-dimer, μg/ml | 1.7 [0.8–3.3] | 1.8 [0.7–3.5] | 1.7 [1.1–2.2] | 0.99 |
| D-dimer >0.5 μg/ml | 66 (84) | 42 (82) | 24 (86) | |
| D-dimer >3 μg/ml | 20 (25) | 15 (29) | 5 (18) | |
| IL-6, pg/ml | 54 [25–154] | 47 [26–98] | 101 [22–335] | 0.036 |
| IL-6 >60 pg/ml | 32 (41) | 15 (29) | 17 (61) | |
| LDH, U/l | 356 [274–414] | 350 [271–406] | 364 [286.5–433] | 0.42 |
| LDH >1.5 times upper limit of normal | 53 (67) | 33 (65) | 20 (71) | |
| Creatine kinase, U/l | 103 [56–204] | 91 [55–143] | 140 [68–362] | 0.095 |
| Creatine kinase >200 U/l | 19 (24) | 8 (16) | 11 (39) | |
| Fibrinogen, mg/dl | 605.5 [504.5–728.5] | 606 [511–754] | 605 [459–666] | 0.46 |
| Fibrinogen >500 mg/dl | 49 (62) | 33 (65) | 16 (57) | |
| Pro-BNP, pg/ml | 1785 [740–4987] | 1278 [450–3234] | 2380 [1152–9342] | 0.031 |
| Pro-BNP >900 pg/ml | 43 (54) | 24 (47) | 29 (68) | |
| Serum creatinine, mg/dl | 2.2 [1.5–3.0] | 1.9 [1.3–3.0] | 2.3 [1.7–2.9] | 0.33 |
CRP, C-reactive protein; IL, interleukin; IQR, interquartile range; LDH, lactate dehydrogenase; Pro-BNP, pro-brain natriuretic peptide; WBC, white blood cell.
Data are n (%) or median [IQR], unless otherwise noted.
Inflammatory markers were checked frequently during the patients’ hospitalizations at our institution. When peak laboratory values and inflammatory markers were compared (Table 3 ), nonsurvivors had lower median lymphocyte counts (300 cells/μl vs. 500 cells/μl; P = 0.021) and platelet counts (135 k/μl vs. 170 k/μl; P = 0.045), and higher levels of median CRP (22.8 mg/dl vs. 14.3 mg/dl; P = 0.0032), procalcitonin (1.9 ng/ml vs. 0.3 ng/ml; P = 0.006), lactate dehydrogenase (LDH) (612 U/l vs. 389 U/l; P = 0.0017), creatine kinase (194 U/l vs. 106 U/l; P = 0.022) and IL-6 (182 pg/ml vs. 48 pg/ml; P = 0.0004). In the multivariate analysis, every 10-unit increase in serum IL-6 levels was associated with a 3.6% increase in the odds of death (OR, 1.036; 95% CI, 1.008–1.065; P = 0.01).
Table 3.
Peak values of laboratory values and inflammatory markers of the patients during hospitalization
| Peak laboratory values and inflammatory markers | Total patients (N = 79) | Survivors (N = 51) | Nonsurvivors (N = 28) | P value |
|---|---|---|---|---|
| Lowest hemoglobin, g/dl | 10.2 [8.2–11.9] | 9.9 [8.2–11.8] | 10.9 [7.9–11.9] | 0.19 |
| Lowest WBC count, k/μl | 4.7 [3.6–6.2] | 4.6 [3.0–5.9] | 5.8 [4.1–6.4] | 0.052 |
| Lowest lymphocyte count, k/μl | 0.4 [0.3–0.6] | 0.5 [0.3–0.6] | 0.3 [0.2–0.4] | 0.021 |
| Lowest platelet count, k/μl | 154 [111–214] | 170 [124–222] | 135 [102–170] | 0.045 |
| Highest CRP, mg/dl | 16.2 [10.2–27.8] | 14.3 [5.9–25.6] | 22.8 [17.4–31.9] | 0.0032 |
| Highest procalcitonin, ng/ml | 0.6 [0.1–2.7] | 0.3 [0.1–1.7] | 1.9 [0.4–3.9] | 0.006 |
| Highest ferritin, ng/ml | 1908 [936–4489] | 2079 [1057–4489] | 1568 [675.5–5493] | 0.59 |
| Highest D-dimer, μg/ml | 3.5 [1.4–8.7] | 3.3 [1.0–5.2] | 4.4 [2.3–16.2] | 0.06 |
| Highest IL-6, pg/ml | 64 [32–208] | 48 [28–98] | 182 [83–498] | 0.0004 |
| Highest LDH, U/l | 448 [337–683] | 389 [303–578] | 612 [446–868] | 0.0017 |
| Highest creatine kinase, U/l | 138 [69–318] | 105.5 [64.5–182.5] | 194 [107–481] | 0.022 |
CRP, C-reactive protein; IL, interleukin; IQR, interquartile range; LDH, lactate dehydrogenase; WBC, white blood cell.
Data are median [IQR], unless otherwise noted.
The clinical outcomes of both survivors and nonsurvivors are summarized in Table 4 . Twenty-eight patients (35%) required intubation and mechanical ventilation, and an additional 7 patients opted to not be resuscitated/intubated. Acute renal failure necessitating renal replacement therapy occurred in 18 patients (23%), and 5 patients (6.3%) lost their allografts. Ten patients (13%) developed new thromboembolic events, and 3 patients (4%) sustained a cerebrovascular accident. In terms of secondary infections, 7 patients developed bacteremia, 9 developed urinary tract infections, and 4 had concurrent bacterial pneumonia. In terms of opportunistic infections, 12 patients developed low-grade cytomegalovirus viremia, with viral loads of 50–559 copies, likely reflecting reactivation in the setting of acute illness and decreased lymphocyte numbers and function. Four patients developed fungal infections.
Table 4.
Clinical outcomes of the hospitalized patients
| Clinical outcomes | Total patients (N = 79) | Survivors (N = 51) | Nonsurvivors (N = 28) | P value |
|---|---|---|---|---|
| Intubation | 28 (35) | 5 (10) | 23 (82) | <0.001 |
| Acute kidney injury requiring renal replacement therapy | 18 (23) | 9 (18) | 9 (32) | 0.15 |
| Bacteremia | 7 (9) | 4 (8) | 3 (6) | 0.67 |
| Urinary tract infection | 9 (11) | 5 (10) | 4 (14) | 0.55 |
| Bacterial pneumonia | 4 (5) | 0 (0) | 4 (14) | 0.014 |
| Fungal infection | 4 (5) | 1 (2) | 3 (11) | 0.12 |
| Cytomegalovirus viremia | 12 (15) | 8 (16) | 4 (14) | 0.87 |
| Deep venous thrombosis | 10 (13) | 6 (12) | 4 (14) | 0.75 |
| Cerebrovascular accident | 3 (4) | 1 (2) | 2 (7) | 0.29 |
Data are n (%) unless otherwise noted.
Treatment
Treatment modalities are summarized in Table 5 . Seventy-four patients (93.7%) had their antimetabolite withdrawn at the time of diagnosis, and calcineurin inhibitors were withdrawn in 11 patients (13.9%), mostly after clinical deterioration (7.8% of survivors and 25% of nonsurvivors). Sixty-five patients (82.3%) were treated with antibiotics (ceftriaxone with doxycycline or azithromycin) for prevention of secondary infections. Initially all the patients were started on hydroxychloroquine; however, after the first 59 patients, this practice was discontinued at our institution owing to lack of efficacy. Eighty six percent of nonsurvivors were treated with hydroxychloroquine, compared with 69% of survivors (P = 0.067). Anticytokine agents were used in patients with moderate to severe clinical pictures; 35 received increased doses of corticosteroids (44%), 11 received tocilizumab (14%), and 6 received leronlimab (7.6%), an experimental CCR-5 inhibitor. Seven patients received convalescent plasma (13.7%), 3 of whom survived. An anti–IL-1 agent, i.v. Igs, and a tyrosine kinase inhibitor were used in 1 patient each. Eight patients were enrolled in clinical trials, 6 in a remdesivir trial and 2 in a sarilumab trial. Forty-four patients (55.7%) received anticoagulation with apixaban and/or heparin for prevention or treatment.
Table 5.
Therapeutics of patients hospitalized at Montefiore Health System
| Treatment | Total patients (N = 79) | Survivors (N= 51) | Nonsurvivors (N = 28) |
|---|---|---|---|
| Antimetabolite withdrawal | 74 (94) | 48 (94) | 26 (93) |
| Calcineurin inhibitor withdrawal | 11 (14) | 4 (8) | 7 (25) |
| Antibiotics | 65 (82) | 38 (75) | 27 (96) |
| Hydroxychloroquine | 59 (75) | 35 (69) | 24 (86) |
| Remdesivira | 6 (8) | 5 (10) | 1 (4) |
| High-dose corticosteroids | 35 (44) | 14 (28) | 21 (75) |
| Tocilizumab | 11 (14) | 5 (10) | 6 (21) |
| Sarilumaba | 2 (3) | 0 (0) | 2 (7) |
| Leronlimab | 6 (8) | 3 (6) | 3 (11) |
| Convalescent plasma | 7 (9) | 3 (6) | 4 (14) |
| i.v. Ig | 1 (1) | 0 (0) | 1 (4) |
| Anakira | 1 (1) | 0 (0) | 1 (4) |
| Anticoagulation | 44 (56) | 26 (51) | 18 (64) |
Data are n (%).
Patients enrolled in a randomized clinical trial; the arms to which patients were randomized are unknown.
Discussion
The present study includes the largest number of kidney transplant recipients tested for SARS-CoV-2 IgG antibodies reported to date in a predominantly Hispanic and African American population. Seropositivity was 16.6% in our cohort of renal transplant recipients. The prevalence of SARS-CoV-2 infection was 23.4% among 975 patients who underwent testing by either RT-PCR and/or SARS-CoV-2 IgG. Forty-two percent of SARS-CoV-2 diagnoses were made by antibody testing, and one-third of those patients were asymptomatic, whereas the remainder did not have severe enough symptoms to warrant medical attention. These patients were younger, were less likely to be diabetic, and had better renal allograft function compared with those diagnosed by positive RT-PCR.
Our in-house SARS-CoV-2 IgG assay is performed with an Abbott Architect I immunoassay analyzer (Abbott, Abbott Park, IL). The manufacturer-reported sensitivity by day 14 after symptom onset in RT-PCR–positive patients is 100% (96.8% when 5 specimens from 1 immunocompromised patient are included). The specificity was 99.6% from >1000 specimens presumed to be SARS-CoV-2–negative, including pre–COVID-19 samples as well as specimens collected in 2020 from subjects who were exhibiting signs of respiratory illness but were negative for SARS-CoV-2 by RT-PCR. Bryan et al. also evaluated the Abbott SARS-CoV-2 assay and found similar performance specifications with a specificity of 99.9% from 1020 pre–COVID-19 serum specimens and a sensitivity of 100% 17 days after symptom onset.21 On internal validation of the assay, our laboratory found a specificity of 100% for pre–COVID-19 specimens, PCR-negative patient samples, and remnant samples from patients who tested positive for other coronavirus strains on respiratory panels from January or February 2020.
Whether measured antibodies are protective against reinfection and if so, for how long, remain unknown. In addition to antibodies, CD4 and CD8 responses to the virus are also potentially important in assessing immunity. Using HLA class I and II predicted peptide “megapools,” circulating SARS-CoV-2–specific CD8+ and CD4+ T cells were identified in ∼70% and 100% of COVID-19 convalescent patients, respectively.22 Interestingly, the authors detected SARS-CoV-2–reactive CD4+ T cells in ∼40%–60% of unexposed individuals, suggesting cross-reactive T-cell recognition between circulating “common cold” coronaviruses and SARS-CoV-2. Although antibody titers might decrease overtime, memory T and B cells may allow for enhanced antibody response on reexposure to the virus.
In a study by Long et al., asymptomatic cases, defined as those with positive RT-PCR but no symptoms suggestive of COVID-19, were hospitalized for observation. These patients developed SARS-CoV-2 IgG at 4 weeks postdiagnosis at rate of 81%; however, in the convalescent phase (8 weeks postdischarge), only 60% were still positive for antibodies. Interestingly, symptomatic patients had a higher positivity rate of antibodies in the convalescent phase than in acute phase (87.1% vs. 83.8%).23 In our cohort, 80% of patients with positive RT-PCR tested positive for SARS-CoV-2 IgG antibodies. Only 7 of these patients were retested at a later date, but all remained positive. Several of the 20% of patients who did not mount an antibody response were also retested later and remained negative. Seroprevalence in our patient population was lower than that reported by the New York City Department of Health, which reported 26% antibody positivity (33% in Bronx) among 1.8 million people who were tested (https://www1.nyc.gov/site/doh/covid/covid-19-data-testing.page).
In our cohort of kidney transplant recipients, overall mortality was 20.5% and in-hospital mortality was 37.8%. Our inpatient mortality rate is similar to that reported in hospitalized patients in New York during the peak of the pandemic1, 2, 3 as well as in ESRD patients11 , 13 , 15 and in some reports of kidney transplant recipients.5 , 9 Our results should be evaluated in the context of our patient population in the Bronx, the majority of which are Hispanic or African American, have lower incomes, and live in more densely populated areas. Compared with other New York City boroughs, COVID-19–related hospitalization and death rates are higher in the Bronx.24 Other factors associated with higher mortality include older age,1 , 2 , 9, 10, 11 , 25 , 26 diabetes mellitus,26 obesity,10 frailty,25 chronic heart2 , 10 , 11 and lung disease,2 , 10 and longer duration of dialysis.13 In terms of laboratory values, lymphopenia and higher levels of CRP, ferritin, procalcitonin, IL-6, D-dimer, and LDH are reported predictors of mortality.2 , 9 , 11 , 25 , 27 In our entire cohort, older age, receipt of deceased donor transplant, and nonreceipt of flu vaccination were associated with increased risk of mortality. The findings regarding flu vaccination are particularly interesting given a recent analysis of immunization records of individuals receiving non–COVID-19 vaccinations that found a decreased rate of COVID-19 infection in the population receiving non–COVID-19 vaccinations.28 These results might suggest the importance of annual influenza vaccination, but further studies are needed.
Our hospitalized patients were lymphopenic, had low CD3/CD4/CD8 cell counts, and had high levels of CRP, procalcitonin, ferritin, D-dimer, and IL-6. Elevated IL-6 level on admission was associated with an increased risk of mortality, indicating the importance of monitoring IL-6 levels in hospitalized patients. Early observational cohort studies of tocilizumab, a monoclonal antibody that blocks the IL-6 receptor, found that patients receiving tocilizumab had reduced mortality compared with standard of care,29 although larger randomized trials have failed to show a benefit with IL-6 therapy.
Among genetic factors that may influence the susceptibility to and clinical outcomes of SARS-CoV-2 infection, HLA genes are attractive candidates owing to their high diversity and key roles in shaping the adaptive immune responses against viruses.22 , 30 Currently there are limited reports of HLA gene variation in COVID-19–infected patients. Nguyen et al. analyzed the binding affinity of SARS-CoV-2–derived peptides to HLA class I alleles.31 Their model predicted that some peptide-HLA complexes may be shared across different SARS viruses. The B∗46:01 allele was found to bind the lowest number of peptides derived from SARS proteins, suggesting that this allele may be associated with a weaker immune response to SARS viruses. In our study, we did not identify any HLA alleles associated with COVID-19–related death. Large-scale studies are warranted to analyze the full impact of HLA gene diversity on the immune responses to SARS-CoV-2.
Our study has multiple strengths. It is the first study screening for SARS-CoV-2 IgG antibodies in a large cohort of renal transplant recipients to determine the seroprevalence of SARS-CoV-2 in this population. It is also the largest single-center study documenting antibody response in kidney transplant recipients with COVID-19 along with a detailed analysis of predictors of mortality, including inflammatory markers and HLA types. A limitation of our study is that only a minority of our cohort underwent repeat antibody testing, and as such, we are unable to assess the durability of these antibodies in this patient population. In addition, only qualitative IgG testing was performed; quantitative values may have provided further information. We also did not check for SARS-CoV-2 IgM antibodies.
In summary, a significant number of kidney transplant recipients (42%) were identified to be SARS-CoV-2 IgG–positive without significant symptoms or testing for SARS-CoV-2 by RT-PCR. Among those with confirmed diagnosis of COVID-19 by RT-PCR, the majority (80%) developed an antibody response. Older age, receipt of a deceased donor transplant, and lack of flu vaccination were associated with mortality. Increased IL-6 levels were the most predictive inflammatory biomarker for mortality in hospitalized patients.
Materials and Methods
COVID-19 diagnosis and study design
This prospective cohort study of 2 groups of kidney transplant recipients is summarized in Figure 1. The first group was kidney transplant recipients who presented to healthcare facilities with COVID-19–like symptoms and tested positive for SARS-CoV-2 by RT-PCR (nasopharyngeal/oral swab). The second group was asymptomatic kidney transplant recipients who were screened as part of routine care for SARS-CoV-2 IgG antibodies during routine post-transplantation clinic visits. Patient demographics and clinical information were obtained through routine patient care and chart review. Patients who were hospitalized at Montefiore Medical Center underwent frequent monitoring of inflammatory markers including CRP, ferritin, procalcitonin, IL-6, D-dimer, and T-cell subtypes (CD3, CD4, and CD8) as part of our programmatic treatment protocol. The study was approved by the Albert Einstein Medical College Institutional Review Board.
COVID-19 RT-PCR and SARS-CoV-2 IgG antibody methods
Nasopharyngeal and/or oropharyngeal swabs were collected in 3 ml of viral transport media, and RNA extraction followed by real-time RT PCR was performed using 1 of 3 commercial methods at our institution. IgG antibody testing was performed using the Abbott SARS-CoV-2 IgG antibody test on the Abbott Architect immunoassay analyzer. Testing was performed on serum samples following the manufacturer’s instructions. The assay is a chemiluminescent microparticle immunoassay intended for the qualitative detection of IgG antibodies to SARS-CoV-2.
HLA typing
HLA genes were typed by low-resolution DNA methods using sequence-specific oligonucleotide probes and sequence-specific primers. Typing results of HLA-A, -B, -DRB1, and -DQB1 loci were available in 220 patients who tested positive by SARS-CoV-2 RT-PCR and/or IgG antibody tests.
Statistical analysis
The characteristics of the sample were described using frequencies and relative frequencies for categorical variables and median and IQR for continuous variables. Significance tests of associations between categorial variables and outcomes were based on the χ2 test or Fisher exact test. Significance tests comparing outcomes for continuous variables were based on the Wilcoxon rank-sum test. No adjustments were made for the multiplicity of comparisons performed, and thus the nominal P values are primarily descriptive. Multivariable logistic regression models were used to test whether significant univariate effects (at P < 0.010) remained significant after adjustment for other significant variables. The associations between the independent variables in these models and outcome were quantified using ORs and their respective 95% CIs. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC). A total of 75 alleles were assessed to determine whether expression was associated with increased risk of mortality. The association between expression and mortality was assessed by the χ2 test or Fisher exact test. The Benjamini–Hochberg procedure was used to control the false discovery rate of claiming significance to be at most 20%.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank Diane Rampersaud and Jennifer Lapponese for helping obtain patient data.
Footnotes
see commentary on page 1404
Table S1. Clinical characteristics of the patients hospitalized at Montefiore Medical Center.
Supplementary Material
References
- 1.Argenziano M.G., Bruce S.L., Slater C.L. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akalin E., Azzi Y., Bartash R. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair V., Jandovitz N., Hirsch J.S. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubetzky M., Aull M.J., Craig-Schapiro R. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020;35:1250–1261. doi: 10.1093/ndt/gfaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta SA, Leonard J, Labella P, et al. Outpatient management of kidney transplant recipients with suspected COVID-19—single-center experience during the New York City surge [e-pub ahead of print]. Transpl Infect Dis.https://doi.org/10.1111/tid.13383. Accessed November 1, 2020. [DOI] [PMC free article] [PubMed]
- 9.Cravedi P., Mothi S.S., Azzi Y. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study [e-pub ahead of print]. Clin Infect Dis.https://doi.org/10.1093/cid/ciaa1097. Accessed November 1, 2020.
- 11.Alberici F., Delbarba E., Manenti C. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett R.W., Blakey S., Nitsch D. Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol. 2020;31:1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goicoechea M., Sánchez Cámara L.A., Macias N. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher M., Yunes M., Mokrzycki M.H. Chronic hemodialysis patients hospitalized with COVID-19: short-term outcomes in Bronx, New York. Kidney360. 2020;1:755–762. doi: 10.34067/KID.0003672020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valeri A.M., Robbins-Juarez S.Y., Stevens J.S. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31:1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [e-pub ahead of print]. Clin Infect Dis.https://doi.org/10.1093/cid/ciaa344. Accessed November 1, 2020. [DOI] [PMC free article] [PubMed]
- 17.Liu R., Han H., Liu F. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172–175. doi: 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie J., Ding C., Li J. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol. 2020;92:2004–2010. doi: 10.1002/jmv.25930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020 [e-pub ahead of print]. JAMA Intern Med.https://doi.org/10.1001/jamainternmed.2020.4130. Accessed November 1, 2020. [DOI] [PMC free article] [PubMed]
- 20.Clarke C., Prendecki M., Dhutia A. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol. 2020;31:1969–1975. doi: 10.1681/ASN.2020060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryan A., Pepper G., Wener M.H. Performance characteristics of the Abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grifoni A., Weiskopf D., Ramirez S.I. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long Q.X., Tang X.J., Shi Q.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 24.Wadhera R.K., Wadhera P., Gaba P. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawlowski C, Puranik A, Bandi H, et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. medRxiv. 10.1101/2020.07.27.20161976. Accessed November 1, 2020. [DOI] [PMC free article] [PubMed]
- 29.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19 [e-pub ahead of print]. Clin Infect Dis.https://doi.org/10.1093/cid/ciaa954. Accessed November 1, 2020. [DOI] [PMC free article] [PubMed]
- 30.Le Bert N., Tan A.T., Kunasegaran K. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen A., David J.K., Maden S.K. Human leukocyte antigen susceptibility map for severe acute respiratory syndrome coronavirus 2. J Virol. 2020;94:e00510–e00520. doi: 10.1128/JVI.00510-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.