Abstract
Purpose
The purpose of this clinical study was to be the first to explore whether ART-123, a recombinant human soluble thrombomodulin, prevents oxaliplatin-induced peripheral neuropathy (OIPN).
Methods
This randomized, phase IIa trial enrolled stage II/III colon cancer patients who received adjuvant mFOLFOX6 chemotherapy. Participants were randomly allocated to 3 arms in a double-blind manner: placebo (placebo: days 1–3); 1-day ART (ART-123: day 1, placebo: days 2–3); and 3-day ART (ART-123: days 1–3). ART-123 (380 U/kg/day) or placebo was infused intravenously before each 2-week cycle of mFOLFOX6. OIPN was assessed with the Functional Assessment of Cancer Therapy/Gynecological Oncology Group-Neurotoxicity-12 (FACT/GOG-Ntx-12) score by participants and the NCI Common Terminology Criteria for Adverse Events (NCI-CTCAE) by investigators.
Results
Seventy-nine participants (placebo n = 28, 1-day ART n = 27, 3-day ART n = 24) received study drugs. The least-squares mean FACT/GOG-Ntx-12 scores at cycle 12 from the mixed effect model for repeated measures were 28.9 with placebo, 36.3 with 1-day ART (vs. placebo: 7.3 [95% CI 1.9 to12.8, p = 0.009]), and 32.3 with 3-day ART (vs. placebo: 3.4 [95% CI −.1 to 9.0, p = 0.222]). The cumulative incidence of NCI-CTCAE grade ≥ 2 sensory neuropathy at cycle 12 was 64.3% with placebo, 40.7% with 1-day ART (vs. placebo: −23.5 [95% CI −48.4 to 4.0], p = 0.108), and 45.8% with 3-day ART (vs. placebo: −18.5 [95% CI −44.2 to 9.4], p = 0.264). Common adverse events were consistent with those reported with mFOLFOX6; no severe bleeding adverse events occurred.
Conclusion
ART-123 showed a potential preventive effect against OIPN with good tolerability. A larger study with 1-day ART is warranted.
NCT02792842, registration date: June 8, 2016
Electronic supplementary material
The online version of this article (10.1007/s00280-020-04135-8) contains supplementary material, which is available to authorized users.
Keywords: CIPN, Neuropathy, Oxaliplatin, Adjuvant chemotherapy, Colon cancer, Thrombomodulin
Introduction
Oxaliplatin is a key drug in the treatment of colorectal cancer and is used in combination with 5-fluorouracil/leucovorin (FOLFOX) or capecitabine for resected stage III colon cancer as adjuvant chemotherapy and for metastatic colorectal cancer as palliative chemotherapy [1–4].
Oxaliplatin-induced peripheral neuropathy (OIPN) is a well-recognized, dose-limiting toxicity. There are two types of neuropathy, acute and chronic (cumulative) neuropathy. Acute neuropathy symptoms including cold allodynia and muscle cramps are generally transient and mild, and they disappear within a few days [5]. Chronic neuropathy is problematic with the oxaliplatin-containing regimen, and its severity is correlated with the cumulative dosage of oxaliplatin [6]. Chronic OIPN is mainly a sensory neuropathy characterized by numbness, paresthesia, and allodynia; motor neuropathy is less frequent. The symptoms of OIPN often limit patients’ daily activities [5, 7, 8]. Chronic OIPN lasts for months or even years after discontinuation of oxaliplatin, and it sometimes worsens transiently for a few months [9–11]. Therefore, there is a need to prevent OIPN in clinical practice, but there are currently no effective agents for OIPN [12, 13]. To relieve only painful OIPN, duloxetine is moderately recommended in the American Society of Clinical Oncology clinical practice guideline [12].
ART-123 is a recombinant human soluble thrombomodulin composed of the extracellular domain of thrombomodulin. In Japan, ART-123 was approved for the treatment of disseminated intravascular coagulation (DIC) in 2008. In previous clinical trials of the treatment of DIC caused by infection, hematological malignancy, and solid tumors, the efficacy and safety of ART-123 at 380 U/kg/day for 6 days were confirmed [14, 15]. ART-123 has an anti-coagulation effect by accelerating the activation of protein C, as well as anti-inflammatory and anti-fibrinolytic effects through activated thrombin-activatable fibrinolysis inhibitor (TAFI) [16–18]. An additional anti-inflammatory effect of ART-123 attributed to direct binding to high-mobility group box 1 protein (HMGB1) and enhancement of its degradation by thrombin has also been reported [19, 20]. Recently, it has been reported that ART-123 prevented oxaliplatin-induced hyperalgesia and allodynia in animal models [21, 22]. The animal study suggested that ART-123 prevents the development of sensory symptoms of OIPN through activation of TAFI and protein C without affecting the anti-tumor activity of oxaliplatin [21]. Moreover, it was reported that an anti-HMGB1-neutralizing antibody prevented oxaliplatin-induced allodynia in animal models [22].
This placebo-controlled, double-blind, randomized, phase IIa clinical study was conducted to explore the preventive effect of ART-123 on OIPN.
Materials and methods
Study design and participants
This was a placebo-controlled, randomized, double-blind, phase IIa study to evaluate the efficacy and safety of ART-123 for the prevention of OIPN. This study was registered at Clinicaltrial.gov (ClinicalTrials.gov Identifier: NCT02792842).
Eligible participants had: curatively-resected and histologically-confirmed stage II or III colon cancer, including rectosigmoid cancer, as defined in the 8th edition of the Japanese Classification of Colorectal Carcinoma [23]; age 20–79 years; Eastern Cooperative Oncology Group performance status of 0 or 1; and a plan to receive 12 cycles of postoperative adjuvant chemotherapy with modified FOLFOX6 (mFOLFOX6). Participants with symptomatic peripheral neuropathy, central nervous system damage, any history of chemotherapy/radiotherapy, other malignancy, history of cerebrovascular disorder in the past one year, or major bleeding were excluded.
All participants provided their written, informed consent. This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol of this study was approved by the institutional review board of each participating site.
Randomization and masking
Study drugs consisted of placebo infusion on days 1–3 (placebo arm), ART-123 380 U/kg infusion on day 1 and placebo infusion on days 2–3 (1-day ART arm), and ART-123 380 U/kg infusion on days 1–3 (3-day ART arm). Eligible participants were randomly allocated to the placebo, 1-day ART, and 3-day ART arms in a double-blind manner (1:1:1) by an interactive web response system (IWRS) using random allocation from a computer-generated random number table with permuted blocks of 6 and stratification by site. All persons involved in the study, including participants, investigators and clinical study coordinators performing neuropathy assessments, and sponsors, were blinded to group assignments until unblinding. Lyophilized formulations of ART-123 and placebo with identical appearance were used, and both appearances were identical after dissolution formulations.
Treatment
Study drug was given intravenously for 30 min once daily on days 1, 2, and 3 in each cycle of mFOLFOX6. On day 1, study drug administration was initiated for 30–120 min before oxaliplatin administration. Study drug was suspended if oxaliplatin treatment was suspended.
The mFOLFOX6 regimen consisted of oxaliplatin 85 mg/m2 and levofolinate 200 mg/m2 for 2 h, followed by an intravenous bolus of 5-fluorouracil 400 mg/m2 and continuous intravenous infusion of 5-fluorouracil 2400 mg/m2 for 46 h. A cycle was defined as the period starting from administration of any agent from mFOLFOX6 to the subsequent administration of any agent from mFOLFOX6 and was, thus, typically a two-week period. Dose modification of oxaliplatin due to OIPN was performed based on the sensory and motor neuropathy grades of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. Criteria for suspending/reducing the dose of oxaliplatin in association with peripheral neuropathy and restricted concomitant medications, such as pregabalin or gabapentin, are shown in Online Resource 1. Participants who discontinued all agents of the FOLFOX regimen were discontinued from the study (as long as a participant continued one of oxaliplatin, levofolinate, or 5-FU, the participant continued with the remaining study activities).
Study assessments
OIPN severity was assessed by both participants and investigators. OIPN assessments were performed before administration of any agent, study drug, or chemotherapy, when done on day 1 of each cycle. Participant-reported outcomes were evaluated using the Functional Assessment of Cancer Therapy/Gynecological Oncology Group-Neurotoxicity-12 (FACT/GOG-Ntx-12) version 4.0, which measures the severity and impact of symptoms of neuropathy over the past 7 days [24]. Scores range from 0 to 48, with lower scores indicating more severe neurotoxicity. Participants completed paper questionnaires on days 1 and 8 of each cycle and on days 15 and 43 of cycle 12, with follow-up assessment on the last day (day 43). Clinical research coordinators not involved in the investigators’ evaluations collected questionnaires and entered data into case report forms. Investigators and clinical research coordinators who helped investigators evaluate peripheral neuropathy were blinded to the FACT/GOG-Ntx-12 scores. NCI-CTCAE was used for investigator-reported outcomes of sensory and motor neuropathy; both were assessed every day from day 1 to day 3 of each cycle and on days 15 and 43 of cycle 12. Other adverse events were also assessed by NCI-CTCAE.
No primary endpoint was specified due to the exploratory nature of the study. Exploratory endpoints included the FACT/GOG-Ntx-12 score, cumulative incidence of NCI-CTCAE grade 2 or higher neuropathy, cumulative oxaliplatin dosages to the first grade 2 or higher neuropathy, total cumulative oxaliplatin dosages, and the discontinuation rate of oxaliplatin due to OIPN.
Statistical analysis
Since this study was exploratory, a precision-based sample size calculation was used. A sample size of 25 participants per treatment arm was calculated to estimate a proportion with a 95% confidence interval (95% CI) whose half-width (the distance between the center of the confidence interval and the upper/lower limit of the confidence interval) is 20%.
The preventive effect of ART-123 on neuropathy was analyzed in all randomly assigned participants who received at least one dose of study drug and oxaliplatin and had a FACT/GOG-Ntx-12 or NCI-CTCAE evaluation at least once after oxaliplatin administration. Safety analyses were performed in participants who received at least one dose of study drug.
The FACT/GOG-Ntx-12 scores were analyzed based on the mixed effect model for repeated measures (MMRM) using all observations including all time points at baseline and each post-baseline visit and the observed case analysis. It was assumed that missing data were missing at random, and missing data were not imputed explicitly in MMRM. The model included the fixed, categorical effects of study treatment, cycle, and study treatment-by-cycle interaction. An unstructured covariance structure was used to model the within-participant errors. The Kenward–Roger approximation was used to estimate denominator degrees of freedom. Least-squares (LS) means were calculated from the MMRM. The p value was calculated from the MMRM and the t-test in post hoc analyses that were planned after treatment unblinding. The cumulative incidence of participants with grade 2 or higher NCI-CTCAE sensory or motor neuropathy was compared between placebo and 1-day ART arms and between placebo and 3-day ART arms using Fisher’s exact test. Once grade 2 or higher neuropathy was observed in a certain participant, that participant was categorized as grade 2 or higher even if the grade returned to 1 or lower in subsequent cycles. Participants who discontinued the study or whose evaluation data were missing without reaching grade 2 or higher neuropathy were analyzed in two ways: having and not having grade 2 or higher neuropathy. The cumulative oxaliplatin dosages to the first grade 2 or higher neuropathy were analyzed using the Kaplan–Meier method. The difference in the Kaplan–Meier curves of the first grade 2 or higher neuropathy between treatment arms was compared using the log-rank test in post hoc analyses. If there were multiple measurements within a cycle and within a follow-up period, the worst value was used in the analyses to estimate the preventive effect of ART-123 on the worst severity of OIPN. Combined-arm analyses of 1-day ART and 3-day ART arms were also performed in post hoc analyses. All analyses were performed with SAS 9.3. All statistical tests were two-sided, and a p value less than 0.05 was considered significant. No adjustment for multiplicity was made.
Results
Participants’ characteristics
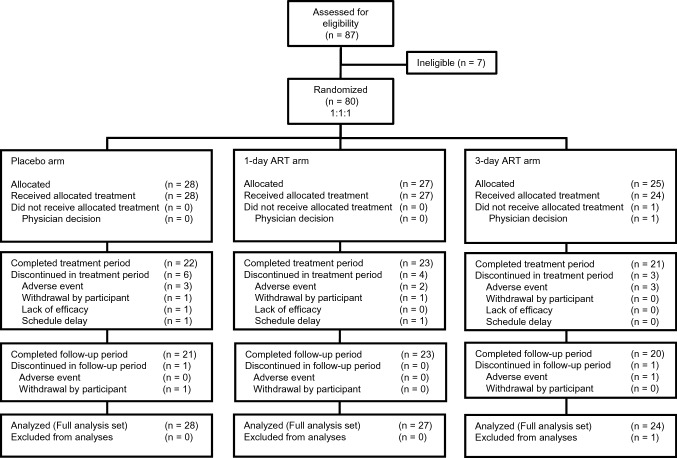
Between July 2016 and April 2017, 87 participants were recruited from 11 hospitals in Japan, and 80 participants were randomly assigned to the placebo (n = 28), 1-day ART (n = 27), and 3-day ART (n = 25) arms (Fig. 1). One participant allocated to the 3-day ART arm was unintentionally enrolled in the study due to an operational error of the IWRS and was withdrawn before study drug administration. In total, 79 participants received study drug and were included in the efficacy and safety analyses. Sixty-four participants completed follow-up (Placebo: n = 21, 1-day ART: n = 23, 3-day ART: n = 20), including 22 participants who discontinued oxaliplatin prior to cycle 12, but study assessments were continued (Placebo: n = 9, 1-day ART: n = 7, 3-day ART: n = 6). The baseline characteristics of the participants were well-balanced across arms (Table 1).
Fig. 1.
CONSORT diagram. Of the 64 participants who completed follow-up (Placebo: n = 21, 1-day ART: n = 23, 3-day ART: n = 20), 22 (Placebo: n = 9, 1-day ART: n = 7, 3-day ART: n = 6) discontinued oxaliplatin prematurely, but completed treatment and follow-up (day 43 of cycle 12)
Table 1.
Participants’ baseline characteristics
| Characteristic | Placebo n = 28 | 1-day ART n = 27 |
3-day ART n = 24 |
|---|---|---|---|
| Sex | |||
| Male | 16 (57.1) | 12 (44.4) | 11 (45.8) |
| Female | 12 (42.9) | 15 (55.6) | 13 (54.2) |
| Age (years) | |||
| < 65 | 10 (35.7) | 9 (33.3) | 11 (45.8) |
| ≥ 65 | 18 (64.3) | 18 (66.7) | 13 (54.2) |
| Median (range) | 68.0 (45–79) | 68.0 (38–78) | 66.0 (32–79) |
| Weight (kg) | |||
| Median (range) | 55.9 (37.4–82.0) | 55.3 (41.6–72.4) | 58.0 (36.1–93.3) |
| Body surface area (m2) | |||
| Median (range) | 1.6 (1.3–2.0) | 1.6 (1.3–1.8) | 1.6 (1.2–2.1) |
| Performance status | |||
| 0 | 25 (89.3) | 27 (100.0) | 24 (100.0) |
| 1 | 3 (10.7) | 0 (0.0) | 0 (0.0) |
| Colon cancer stage | |||
| II | 6 (21.4) | 3 (11.1) | 6 (25.0) |
| IIIa | 16 (57.1) | 17 (63.0) | 15 (62.5) |
| IIIb | 6 (21.4) | 7 (25.9) | 3 (12.5) |
| Diabetes mellitus | |||
| Yes | 6 (21.4) | 5 (18.5) | 4 (16.7) |
| No | 22 (78.6) | 22 (81.5) | 20 (83.3) |
| FACT/GOG-Ntx-12 | |||
| Mean (SD) score | 46.4 (2.0) | 46.7 (2.2) | 46.3 (2.9) |
Data are presented as numbers (%) unless otherwise noted
ART recombinant thrombomodulin, FACT/GOG-Ntx-12 Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity-12, SD standard deviation
Participant-reported neuropathy
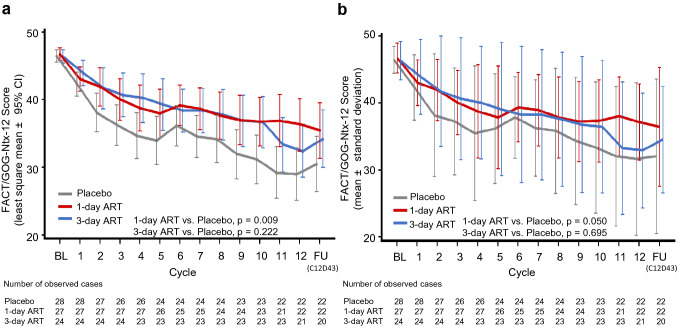
The LS means of the FACT/GOG-Ntx-12 scores decreased according to the increase of cycle numbers for mFOLFOX6 treatment. The LS means of the FACT/GOG-Ntx-12 scores were 46.4, 46.7, and 46.3 at baseline and 28.9, 36.3, and 32.3 at cycle 12 (day 15) for the placebo, 1-day ART, and 3-day ART arms, respectively (Fig. 2a). The differences in the LS means at cycle 12 were 7.3 (95% CI 1.9 to 12.8, MMRM p = 0.009) between the 1-day ART and placebo arms and 3.4 (95% CI −2.1 to 9.0; MMRM p = 0.222) between the 3-day ART and placebo arms. Results of the observed case analysis were similar to those of the MMRM analysis (Fig. 2b). These results were also comparable with those in the post hoc combined-arm analyses (Online Resource 2). The means of the FACT/GOG-Ntx-12 scores at days 1 and 8 of each cycle are shown in Online Resource 3.
Fig. 2.
a This figure presents the least-squares mean score using MMRM of FACT/GOG-Ntx-12. The p value was calculated by MMRM at cycle 12. Error bars represent the 95% confidence interval. b This figure presents the mean score using observed case analysis of FACT/GOG-Ntx-12. The p values were calculated by t-tests at cycle 12. Error bars represent standard deviations. The gray line, red line, and blue line represent the placebo arm, 1-day ART arm, and 3-day ART arm, respectively. BL baseline, FU follow-up (day 43 of cycle 12), ART recombinant thrombomodulin, FACT/GOG-Ntx-12 Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity-12
Investigator-reported neuropathy
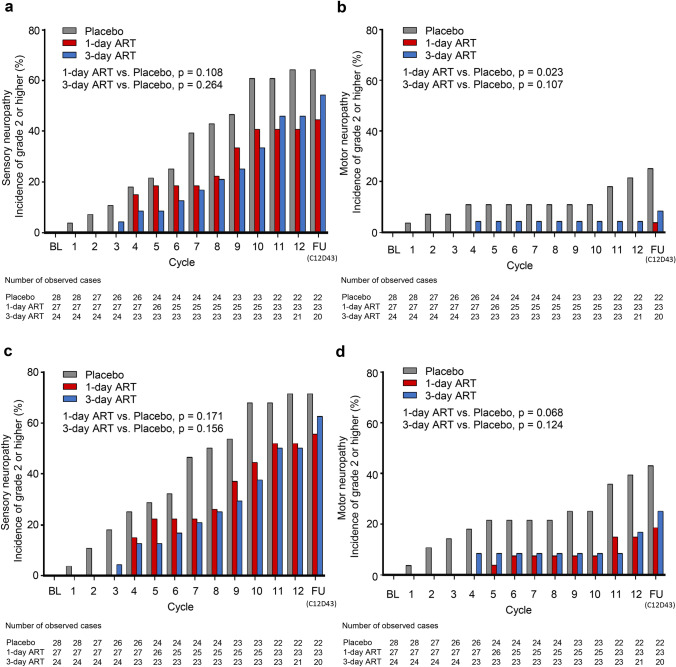
When missing data were analyzed as no grade 2 or higher neuropathy, the cumulative incidences of NCI-CTCAE grade 2 or higher sensory neuropathy at cycle 12 were 64.3% with placebo, 40.7% with 1-day ART (vs. placebo: −23.5 [95% CI − 48.4 to 4.0], p = 0.108), and 45.8% with 3-day ART (vs. placebo: −18.5 [95% CI −44.2 to 9.4], p = 0.264) (Fig. 3a). The cumulative incidences of grade 2 or higher motor neuropathy at cycle 12 were 21.4% with placebo, 0.0% with 1-day ART (vs. placebo: −21.4 [95% CI −46.0 to 4.6], p = 0.023), and 4.2% with 3-day ART (vs. placebo: −17.3 [95% CI −42.9 to 10.3], p = 0.107) (Fig. 3b). When missing data were analyzed as grade 2 or higher, both sensory neuropathy and motor neuropathy showed a similar trend (Fig. 3c, d). These results were also comparable with those in the post hoc combined-arm analyses (Online Resource 4). Throughout the entire study, from baseline to follow-up (day 43 of cycle 12), the incidence of grade 1 sensory neuropathy was 32.1%, 55.6%, and 45.8%, the incidence of grade 2 was 64.3%, 40.7%, and 41.7%, and the incidence of grade 3 was 0.0%, 3.7%, and 12.5% in the placebo, 1-day ART, and 3-day ART arms, respectively (Online resource 5a). The incidence of grade 1 motor neuropathy was 10.7%, 25.9%, and 20.8%, the incidence of grade 2 was 17.9%, 3.6%, and 8.3%, and the incidence of grade 3 was 7.1%, 0.0%, and 0.0% in the placebo, 1-day ART, and 3-day ART arms, respectively (Online resource 5b).
Fig. 3.
Cumulative incidences of NCI-CTCAE grade 2 or higher sensory neuropathy (a), (c) and motor neuropathy (b), (d). Missing grade in participants who discontinued before grade 2 or higher was analyzed as no grade 2 or higher (a), (b), or as grade 2 or higher (c), (d). The p values were calculated by Fisher’s exact test at cycle 12. The gray bar, red bar, and blue bar represent the placebo arm, 1-day ART arm, and 3-day ART arm, respectively. BL baseline, FU follow-up (day 43 of cycle 12), ART recombinant thrombomodulin
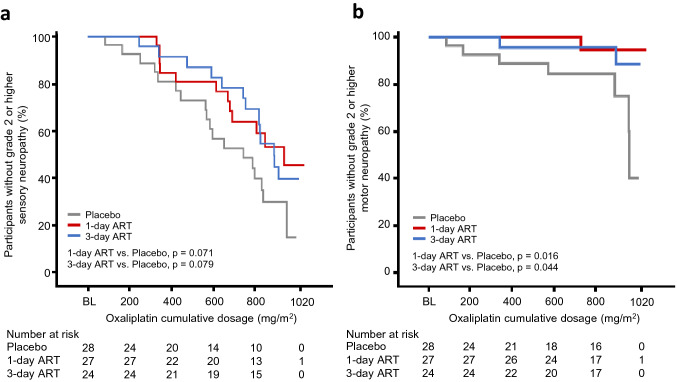
The median cumulative oxaliplatin dosages to the first grade 2 or higher sensory neuropathy were 747.2 mg/m2 (95% CI 566.9 to 840.6 mg/m2), 941.0 mg/m2 (95% CI 682.5 mg/m2 to not reached), and 893.6 mg/m2 (95% CI 757.3 mg/m2 to not reached) in the placebo, 1-day ART, and 3-day ART arms, respectively (Fig. 4a). The post hoc analysis showed a significant difference between the combined arm and the placebo arm (log-rank test, p = 0.032, Online Resource 6a). Results for motor neuropathy showed a similar trend to those of sensory neuropathy (Fig. 4b, Online Resource 6b).
Fig. 4.
Kaplan–Meier curves of cumulative oxaliplatin dosages to the first NCI-CTCAE grade 2 or higher sensory neuropathy (a) and motor neuropathy (b). The p values were calculated by the log-rank test. The gray line, red line, and blue line represent the placebo arm, 1-day ART arm, and 3-day ART arm, respectively. BL baseline, ART recombinant thrombomodulin
Administration status of oxaliplatin and use of restricted concomitant medications for OIPN
The median total dosages of oxaliplatin delivered were 819.1 mg/m2, 849.2 mg/m2, and 920.7 mg/m2 for placebo, 1-day ART, and 3-day ART, respectively. The numbers of participants who discontinued oxaliplatin were 15 (53.6%), 11 (40.7%), and 9 (37.5%), of which 9 (32.1%), 4 (14.8%), and 6 (25.0%) were due to OIPN in the placebo, 1-day ART, and 3-day ART arms, respectively. The first dose-reduction of oxaliplatin due to OIPN was observed in cycle 2, cycle 5, and cycle 8 in the placebo, 1-day ART, and 3-day ART arms, respectively (Online Resource 7).
The number of participants who used restricted concomitant medications for OIPN was 4 (14.3%), 4 (14.8%), and 3 (12.5%) in the placebo, 1-day ART, and 3-day ART arms, respectively.
Safety
The incidence of grade 3 or 4 adverse events was 53.6%, 59.3%, and 66.7% in the placebo, 1-day ART, and 3-day ART arms, respectively (Table 2). Serious adverse events were observed in 2 (7.1%), 5 (18.5%), and 3 (12.5%) in the placebo, 1-day ART, and 3-day ART arms, respectively. All serious adverse events were assessed to be unrelated to study drug. Common adverse events were consistent with those reported with mFOLFOX6. Because of the anti-coagulation effect of ART-123, bleeding-related adverse events were of particular interest. The number of bleeding adverse events is shown in Table 2. Grade 2 hematuria occurred in one participant in the 1-day ART arm, and all other bleeding adverse events were grade 1. ART-123-related bleeding adverse events as judged by investigators were epistaxis in the 3-day ART arm (n = 2) and purpura in the 1-day ART arm (n = 1).
Table 2.
Adverse events
| Placebo (n = 28) | 1-day ART (n = 27) | 3-day ART (n = 24) | ||||
|---|---|---|---|---|---|---|
| All grades | Grade 3/4 | All grades | Grade 3/4 | All grades | Grade 3/4 | |
| Overall AEs | 28 (100.0) | 15 (53.6) | 27 (100.0) | 16 (59.3) | 24 (100.0) | 16 (66.7) |
| Most common AEs | ||||||
| Neutrophil count decreased | 12 (42.9) | 9 (32.1) | 13 (48.1) | 8 (29.6) | 16 (66.7) | 11 (45.8) |
| Malaise | 17 (60.7) | 0 (0.0) | 11 (40.7) | 0 (0.0) | 12 (50.0) | 0 (0.0) |
| Nausea | 9 (32.1) | 0 (0.0) | 12 (44.4) | 1 (3.7) | 9 (37.5) | 0 (0.0) |
| Inappetence | 12 (42.9) | 1 (3.6) | 8 (29.6) | 0 (0.0) | 8 (33.3) | 0 (0.0) |
| Dysgeusia | 7 (25.0) | 0 (0.0) | 7 (25.9) | 0 (0.0) | 8 (33.3) | 0 (0.0) |
| Fatigue | 7 (25.0) | 1 (3.6) | 5 (18.5) | 0 (0.0) | 8 (33.3) | 0 (0.0) |
| Constipation | 6 (21.4) | 0 (0.0) | 7 (25.9) | 0 (0.0) | 4 (16.7) | 0 (0.0) |
| Diarrhea | 5 (17.9) | 0 (0.0) | 5 (18.5) | 0 (0.0) | 5 (20.8) | 1 (4.2) |
| Alopecia | 4 (14.3) | 0 (0.0) | 6 (22.2) | 0 (0.0) | 2 (8.3) | 0 (0.0) |
| Mucositis oral | 3 (10.7) | 1 (3.6) | 4 (14.8) | 0 (0.0) | 5 (20.8) | 0 (0.0) |
| White blood cell count decreased | 1 (3.6) | 0 (0.0) | 6 (22.2) | 1 (3.7) | 3 (12.5) | 0 (0.0) |
| Bleeding AEs | 2 (7.1) | 0 (0.0) | 3 (11.1) | 0 (0.0) | 6 (25.0) | 0 (0.0) |
| Epistaxis | 1 (3.6) | 0 (0.0) | 2 (7.4) | 0 (0.0) | 3 (12.5) | 0 (0.0) |
| Implant site hemorrhage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.3) | 0 (0.0) |
| Bronchopulmonary hemorrhage | 1 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hematuria | 0 (0.0) | 0 (0.0) | 1 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hyposphagma | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) | 0 (0.0) |
| Purpura | 0 (0.0) | 0 (0.0) | 1 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
All data are shown as Nos. (%)
AE adverse event, ART recombinant thrombomodulin
Discussion
This was the first clinical study to explore the efficacy and safety of ART-123 for the prevention of OIPN. ART-123 significantly inhibited the decrease of FACT/GOG-Ntx-12 scores in the 1-day ART arm. ART-123 tended to decrease the cumulative incidence of NCI-CTCAE grade 2 or higher sensory neuropathy caused by oxaliplatin treatment, although not significantly. No clear advantages in efficacy of the 3-day ART arm were observed compared to the 1-day ART arm. ART-123 was well-tolerated in participants who received adjuvant mFOLFOX6 chemotherapy for colon cancer.
According to ACTTION (Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities and Networks) recommendations, a participant-reported outcome should always be included in clinical studies of chemotherapy-induced peripheral neuropathy (CIPN) prevention [25]. The FACT/GOG-Ntx-12 was selected as a participant-reported outcome instrument for assessment of OIPN, because the FACT/GOG-Ntx scale is a reliable, valid, and widely-used CIPN assessment tool, including for participants with colorectal cancer treated by oxaliplatin [24, 26–28]. The changes in the FACT/GOG-Ntx scores in the placebo arm of the present study were similar to those in other reported clinical trials [29–31]. Approximately 4-point differences of the LS mean scores between the ART and placebo arms were continuously observed in the latter half of the treatment cycles. A 4-point difference in the FACT/GOG-Ntx-12 scores is reported to be clinically meaningful [24]. Moreover, considering that the median cumulative oxaliplatin dosages were around 820 mg/m2 in the placebo arm and over 840 mg/m2 in both ART arms, the higher score of the FACT/GOG-Ntx-12 of the 1-day ART arm compared to placebo was not due to less oxaliplatin delivery and suggests the possibility that ART-123 prevents OIPN.
NCI-CTCAE Grade 2 or higher sensory neuropathy was reported to occur in 40–60% of participants receiving FOLFOX at an oxaliplatin dosage level of 700–800 mg/m2 in previous studies [29, 32, 33]. The present results of the placebo arm were consistent with those reports.
Study drug was given on days 1, 2, and 3 in each cycle of mFOLFOX6. The dose regimen was determined based on a non-clinical study using a rat model of OIPN (data not shown) showing that a seven-day consecutive dosing regimen through days 1–7 provided a more potent preventive effect than a single dosing regimen on day 1 against oxaliplatin-induced hyperalgesia. Considering the inconvenience of the participants and site staff to administer study drug, the three-day dosing regimen (3-day ART arm) was established as the acceptable maximum dose frequency for sites based on a preliminary feasibility web-based survey. On the other hand, from the practical standpoint, a single dosing regimen (1-day ART) on day 1 of each cycle is preferred, given that patients generally visit one day in a cycle in clinical practice. In the present study, the preventive effects in the 3-day ART arm were not greater than those in the 1-day ART arm. These results suggest that administration of ART-123 prior to oxaliplatin on day 1 was the predominant contributor to reducing the severity and incidence of OIPN in this human study. One possible explanation for this discrepancy between the non-clinical and clinical findings is the shorter half-life of ART-123 in rats than in humans, but the details are not clear [34, 35].
The safety profile of ART-123 was generally mild and well-tolerated in participants with resected colon cancer, as expected. Although some adverse events occurred in all participants, they were similar to those reported in past studies using FOLFOX [1, 31, 36]. Particular attention was paid to bleeding adverse events because ART-123 has an anti-coagulation effect. For participants with DIC, bleeding-related adverse events, such as microscopic hematuria, were reported in the previous clinical trial [15]. In the present study, the most frequently observed bleeding adverse event was epistaxis. Only one grade 2 bleeding event, hematuria, occurred in the 1-day ART arm. In this participant, a thromboembolic event in the lower limbs occurred after 2 cycles of mFOLFOX6, and apixaban treatment was started. Then, grade 2 hematuria occurred before cycle 5, but disappeared soon after stopping apixaban. Therefore, this hematuria was assessed to be unrelated to study drugs. Further investigation is needed to demonstrate to what extent the bleeding risk of ART-123 increases when used concomitantly with anticoagulants.
The maximum plasma concentrations achieved with single- or three-day administration of ART-123 were 1001 ± 170 ng/mL and 1526 ± 269 ng/mL, respectively, in this study. According to previous reports, activation of TAFI and protein C by ART-123 was expected at a concentration range of 100–3200 ng/mL, based on data from an in vitro non-clinical study using human plasma [17, 18]. In addition, administration of ART-123, activated TAFI homolog, and exogenous human-activated protein C prevented oxaliplatin-induced hyperalgesia and allodynia in animal models [21]. Therefore, it is hypothesized that ART-123 prevents OIPN by promoting activation of TAFI and protein C. However, the exact mechanisms and the relationships between OIPN and TAFI and/or APC have not been elucidated.
This study has some limitations. First, multiple tests were performed with no adjustment for multiplicity because the study was exploratory, and the small sample size limited the power of the study. Second, the follow-up period after study drug completion was not long enough. Symptoms of OIPN are known to continue or even worsen over 3 months after completion of chemotherapy [7, 29]. Third, although it was confirmed that ART-123 did not have an impact on the anti-tumor effects of oxaliplatin in preclinical studies, the present study lacked data about whether ART-123 affected the anti-tumor activity of human chemotherapy [21]. Fourth, since no objective measurement was available, no information was obtained regarding the effect of ART-123 on sensory nerve damage or function. Last, this study was conducted only in Japan, which may limit generalization of its results. These limitations should be resolved in future global studies with larger sample sizes and longer follow-up periods.
In conclusion, this phase IIa exploratory study suggests that the recombinant human soluble thrombomodulin ART-123 has a potential preventive effect against OIPN with good tolerability. Further studies are warranted based on these results.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the participants, investigators, and institutions involved in this study.
Author contributions
Study concept and design: MK, YS, MA, GK, TS, YU, MT, SK, and IH; Acquisition and analysis, or interpretation of data: all authors; Drafting of the manuscript or critical revision of the manuscript for important intellectual content: all authors; Final approval of the version to be published: all authors.
Funding
This research was funded by Asahi Kasei Pharma Corporation (AKP). This study was designed by AKP in collaboration with M.K., Y.S., M.A. and I.H, and the data were analyzed by AKP. AKP participated in the interpretation of data, review, and approval of the report. An initial draft of the report was prepared under the authors’ guidance by AKP. Four employees of AKP helped write subsequent drafts.
Availability of data and material
The datasets generated during the current study are not publicly available because the informed consent form signed by the participants did not address an individual data sharing statement.
Compliance with ethical standards
Conflict of interest
G.K., T.S., Y.U., M.T., and S.K. are employees of Asahi Kasei Pharma. G.K., T.S., and Y.U. have a patent pending (2018–183447) with Asahi Kasei Pharma. Y.S., M.A., and I.H. were members of the advisory board of this study. M.K. reports personal fees from Chugai Pharma, Yakult Honsha, Merck Serono, Takeda Pharma, Taiho Pharmaceutical, and Eli Lilly Japan, outside the submitted work. Y.S. reports personal fees from Pfizer, Shionogi, Daiichi Sankyo, Nipponzoki, Kyowakirin, Ayumi-Pharma, Hisamitsu, Japan Vaccine, Bikken, GSK, Tsumura, Terumo, Astra-Zeneca, Maruishi, Ono Pharma, Aspen, Nestle, and Japan Blood Product, outside the submitted work. T.K. reports personal fees from Chugai Pharma, Takeda Pharma, Eli Lilly Japan, and Yakult Honsha, outside the submitted work. H.S. reports personal fees from Bayer, Bristol-Myers Squibb, Chugai Pharma, Daiichi Sankyo, Eli Lilly Japan, Merck Bio Pharma, MSD, Ono Pharma, Sanofi, Taiho Pharmaceutical, Takeda, and Yakult Honsha, outside the submitted work. A.M. reports personal fees from Eli Lilly Japan, Chugai Pharma, and Takeda, outside the submitted work. Y.T. reports personal fees from Bayer, Merck Serono, Eli Lilly Japan, Chugai Pharma, Taiho Pharmaceutical, Ono Pharma, Takeda Pharma, Medicon, and Sawai Pharmaceutical, outside the submitted work. K.S. reports personal fees from Chugai Pharma, Takeda Pharma, Mochida Pharmaceutical, Merck Biopharma, Taiho Pharmaceutical, Ono Pharma, Eisai, Shionogi, Eli Lilly Japan, Daiichi Sankyo, Nihonkayaku, Kyowakirin, and Bayer Yakuhin, outside the submitted work. T.F. reports grants and personal fees from Oncolys BioPharma, outside the submitted work. T.M. reports personal fees from Chugai Pharma, Mitsubishi Tanabe Pharma, Miyarisan Pharmaceutical, EA Pharma, AbbVie GK, Kyorin Pharmaceutical, and Sanofi K.K., and other support from Kinshukai Medical, outside the submitted work. Y.H., N.N., and N.K. have nothing to disclose. M.A. reports grants from Kyowa Kirin, outside the submitted work. I.H. reports grants and personal fees from Chugai Pharma, Taiho Pharma, Daiichi Sankyo, Yakult Honsha, and Ono Pharma, outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and with Good Clinical Practice guidelines. The protocol of this study was approved by the institutional review board of each participating site.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/2/2020
In the Original publication of the article, the authors found an error in the “Results” section under the heading “Abstract”. The corrected text is given below.
References
- 1.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 2.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 3.Schmoll HJ, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K, Haller DG. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled Phase III trial. J Clin Oncol. 2015;33:3733–3740. doi: 10.1200/JCO.2015.60.9107. [DOI] [PubMed] [Google Scholar]
- 4.Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Saltz L. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A. Oxaliplatin-safety profile: neurotoxicity. Semin Oncol. 2003;30(4 Suppl 15):5–13. doi: 10.1016/s0093-7754(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 6.Grothey A. Clinical management of oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer. 2005;5(suppl 1):S38–S46. doi: 10.3816/CCC.2005.s.006. [DOI] [PubMed] [Google Scholar]
- 7.Pachman DR, Qin R, Seisler DK, Smith EM, Beutler AS, Ta LE, Lafky JM, Wagner-Johnston ND, Ruddy KJ, Dakhil S, Staff NP, Grothey A, Loprinzi CL. Clinical course of oxaliplatin-induced neuropathy: Results from the randomized phase III trial N08CB (Alliance) J Clin Oncol. 2015;33:3416–3422. doi: 10.1200/JCO.2014.58.8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve. 2005;32:51–60. doi: 10.1002/mus.20340. [DOI] [PubMed] [Google Scholar]
- 9.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 10.Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol. 2013;31:2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 11.Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014;6:135–147. doi: 10.2147/CMAR.S44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, Kelley MR, Lavino A, Lustberg MB, Paice JA, Schneider BP, Lavoie Smith EM, Smith ML, Smith TJ, Wagner-Johnston N, Hershman DL. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Onco. 2020 doi: 10.1200/JCO.20.01399. [DOI] [PubMed] [Google Scholar]
- 13.Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther. 2011;90:377–387. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- 14.Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M, Aoki N. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5:31–41. doi: 10.1111/j.1538-7836.2006.02267.x. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Saito H, Asakura H, Okamoto K, Tagawa J, Hayakawa T, Aoki N. Recombinant human soluble thrombomodulin (thrombomodulin alfa) to treat disseminated intravascular coagulation in solid tumors: results of a one-arm prospective trial. Int J Clin Oncol. 2015;20:821–828. doi: 10.1007/s10147-014-0768-1. [DOI] [PubMed] [Google Scholar]
- 16.Mohri M, Sugimoto E, Sata M, Asano T. The inhibitory effect of recombinant human soluble thrombomodulin on initiation and extension of coagulation–a comparison with other anticoagulants. Thromb Haemost. 1999;82:1687–1693. doi: 10.1055/s-0037-1614900. [DOI] [PubMed] [Google Scholar]
- 17.Mohri M. ART-123: recombinant human soluble thrombomodulin. Cardiovasc Drug Rev. 2000;18:312–325. doi: 10.1111/j.1527-3466.2000.tb00055.x. [DOI] [Google Scholar]
- 18.Tawara S, Sakai T, Matsuzaki O. Anti-inflammatory and anti-fibrinolytic effects of thrombomodulin alfa through carboxypeptidase B2 in the presence of thrombin. Thromb Res. 2016;147:72–79. doi: 10.1016/j.thromres.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S, Yamamoto Y, Yamamoto H, Iino S, Taniguchi N, Maruyama I. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267–1274. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, Nawa Y, Meng X, Shrestha B, Hashiguchi T, Maruyama I. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol. 2008;28:1825–1830. doi: 10.1161/ATVBAHA.107.150631. [DOI] [PubMed] [Google Scholar]
- 21.Minami T, Takeda M, Sata M, Kato H, Yano K, Sakai T, Tsujita R, Kawasaki K, Ito A. Thrombomodulin alfa prevents oxaliplatin-induced neuropathic symptoms through activation of thrombin-activatable fibrinolysis inhibitor and protein C without affecting anti-tumor activity. Eur J Pharmacol. 2020;13(880):173196. doi: 10.1016/j.ejphar.2020.173196. [DOI] [PubMed] [Google Scholar]
- 22.Tsubota M, Fukuda R, Hayashi Y, Miyazaki T, Ueda S, Yamashita R, Koike N, Sekiguchi F, Wake H, Wakatsuki S, Ujiie Y, Araki T, Nishibori M, Kawabata A. Role of non-macrophage cell-derived HMGB1 in oxaliplatin-induced peripheral neuropathy and its prevention by the thrombin/thrombomodulin system in rodents: negative impact of anticoagulants. J Neuroinflammation. 2019;16:199. doi: 10.1186/s12974-019-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207–239. doi: 10.1007/s10147-015-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopec JA, Land SR, Cecchini RS, Ganz PA, Cella D, Costantino JP, Wieand HS, Smith RE, Kuebler JP, Wolmark N. Validation of a self-reported neurotoxicity scale in patients with operable colon cancer receiving oxaliplatin. J Supp Oncol. 2006;4:W1–W8. [Google Scholar]
- 25.Gewandter JS, Brell J, Cavaletti G, Dougherty PM, Evans S, Howie L, McDermott MP, O'Mara A, Smith AG, Dastros-Pitei D, Gauthier LR, Haroutounian S, Jarpe M, Katz NP, Loprinzi C, Richardson P, Lavoie-Smith EM, Wen PY, Turk DC, Dworkin RH, Freeman R. Trial designs for chemotherapy-induced peripheral neuropathy prevention: ACTTION recommendations. Neurology. 2018;91:403–413. doi: 10.1212/WNL.0000000000006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasane M, Tencer T, French A, Maro T, Beusterien KM. Patient-reported outcomes in chemotherapy-induced peripheral neuropathy: a review. J Supp Oncol. 2010;8:e15–e21. doi: 10.1016/j.suponc.2010.09.029. [DOI] [Google Scholar]
- 27.Majithia N, Temkin SM, Ruddy KJ, Beutler AS, Hershman DL, Loprinzi CL. National Cancer Institute-supported chemotherapy-induced peripheral neuropathy trials: outcomes and lessons. Support Care Cancer. 2016;24:1439–1447. doi: 10.1007/s00520-015-3063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCrary JM, Goldstein D, Boyle F, Cox K, Grimison P, Kiernan MC, Krishnan AV, Lewis CR, Webber K, Baron-Hay S, Horvath L, Park SB. Optimal clinical assessment strategies for chemotherapy-induced peripheral neuropathy (CIPN): a systematic review and Delphi survey. Support Care Cancer. 2017;25:3485–3493. doi: 10.1007/s00520-017-3772-y. [DOI] [PubMed] [Google Scholar]
- 29.Iveson TJ, Kerr RS, Saunders MP, Cassidy J, Hollander NH, Tabernero J, Haydon A, Glimelius B, Harkin A, Allan K, McQueen J, Scudder C, Boyd KA, Briggs A, Waterston A, Medley L, Wilson C, Ellis R, Essapen S, Dhadda AS, Harrison M, Falk S, Raouf S, Rees C, Olesen RK, Propper D, Bridgewater J, Azzabi A, Farrugia D, Webb A, Cunningham D, Hickish T, Weaver A, Gollins S, Wasan HS, Paul J. Three versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19:562–578. doi: 10.1016/s1470-2045(18)30093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kono T, Hata T, Morita S, Munemoto Y, Matsui T, Kojima H, Takemoto H, Fukunaga M, Nagata N, Shimada M, Sakamoto J, Mishima H. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan to prevent oxaliplatin-induced neuropathy. Cancer Chemother Pharmacol. 2013;72:1283–1290. doi: 10.1007/s00280-013-2306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T, Esaki T, Tsuji Y, Muro K, Taira K, Denda T, Funai S, Shinozaki K, Yamashita H, Sugimoto N, Okuno T, Nishina T, Umeki M, Kurimoto T, Takayama T, Tsuji A, Yoshida M, Hosokawa A, Shibata Y, Suyama K, Okabe M, Suzuki K, Seki N, Kawakami K, Sato M, Fujikawa K, Hirashima T, Shimura T, Taku K, Otsuji T, Tamura F, Shinozaki E, Nakashima K, Hara H, Tsushima T, Ando M, Morita S, Boku N, Hyodo I. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G) Ann Oncol. 2016;27:1539–1546. doi: 10.1093/annonc/mdw206. [DOI] [PubMed] [Google Scholar]
- 32.Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, Meyerhardt JA, Vernerey D, Yamanaka T, Boukovinas I, Meyers JP, Renfro LA, Niedzwiecki D, Watanabe T, Torri V, Saunders M, Sargent DJ, Andre T, Iveson T. Duration of adjuvant chemotherapy for stage III colon cancer. New Engl J Med. 2018;378:1177–1188. doi: 10.1056/NEJMoa1713709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andre T, Vernerey D, Mineur L, Bennouna J, Desrame J, Faroux R, Fratte S, Hug de Larauze M, Paget-Bailly S, Chibaudel B, Bez J, Dauba J, Louvet C, Lepere C, Dupuis O, Becouarn Y, Mabro M, Egreteau J, Bouche O, Deplanque G, Ychou M, Galais MP, Ghiringhelli F, Dourthe LM, Bachet JB, Khalil A, Bonnetain F, de Gramont A, Taieb J. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, international duration evaluation of adjuvant (IDEA) France, Phase III Trial. J Clin Oncol. 2018;36:1469–1477. doi: 10.1200/JCO.2017.76.0355. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima M, Kanamaru M, Umemura K, Tsuruta K. Pharmacokinetics and safety of a novel recombinant soluble human thrombomodulin, ART-123, in healthy male volunteers. J Clin Pharmacol. 1998;38:40–44. doi: 10.1002/j.1552-4604.1998.tb04375.x. [DOI] [PubMed] [Google Scholar]
- 35.Tsuruta K, Kodama T, Serada M, Hori K, Inaba A, Miyake T, Kohira T. Pharmacokinetics of recombinant human soluble thrombomodulin, thrombomodulin alfa in the rat. Xenobiotica. 2009;39:125–134. doi: 10.1080/00498250802604074. [DOI] [PubMed] [Google Scholar]
- 36.Kotaka M, Yoshino T, Oba K, Shinozaki K, Touyama T, Manaka D, Matsui T, Ishigure K, Hasegawa J, Inoue K, Goto K, Sakamoto J, Saji S, Ohtsu A, Watanabe T. Initial safety report on the tolerability of modified FOLFOX6 as adjuvant therapy in patients with curatively resected stage II or III colon cancer (JFMC41-1001-C2: JOIN trial) Cancer Chemother Pharmacol. 2015;76:75–84. doi: 10.1007/s00280-015-2757-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are not publicly available because the informed consent form signed by the participants did not address an individual data sharing statement.