Highlights
-
•
The severity of COVID-19 depends on the individual's immune response.
-
•
Differences between the immune responses of the lung in children and adults may be the reasons for clinical differences in the pathogenesis of COVID-19.
-
•
Impaired immune regulation lead to induction an uncontrolled local and systemic immune response, ineffective inflammation and severe COVID-19 disease.
Keywords: COVID-19, Immunopathogenesis, SARS-CoV-2, Immune dysregulation
Abstract
The coronavirus disease-2019 (COVID-19) which caused by severe acute respiratory syndrome-related coronavirus (SARS-CoV-2), is a pandemic threat to global public health. It has a wide spectrum of clinical manifestations from mild to critical illness, the most serious of which is the complications of acute respiratory distress syndrome (ARDS). SARS-CoV-2 infection appears mild in infants and children, however, in adults, it can lead to serious consequences. In this review, we highlighted the differences between the immune responses of the lung in children and adults, immune dysregulation and their possible role in clinical manifestations in COVID-19. There is a reduction in population of immunocompetent cells during aging and subsequently induced ineffective inflammation in the faces of some infections. Dysregulation in the immune system can lead to an unappropriated local and systemic immune responses and subsequently the rapid spread of the virus, leading to severe COVID-19 disease. Therefore, recognizing the differences in the immune responses of various hosts as well as to improve the immune system disorder should always be part of research and treatment protocols.
1. Introduction
Family of Coronaviridae is the second cause of the common cold and responsible for 5–10 % of acute respiratory infections and could infect both humans and animals. The family consist of two subfamilies Coronavirinae and Torovirinae in which Coronavirinae (CoV) is subclassified into four genera of alpha-CoV, beta-CoV, delta-CoV, and gamma-CoV (Beniac et al., 2006). Coronavirus is a large family of positive-sense, single-stranded RNA viruses (Delmas and Laude, 1990). Their large genome ranging from 26 to 32 kilobases in length has the properties of a mRNA, namely a 5′ cap structure and a poly adenylated 3′ end (Li et al., 2020). Coronaviruses contain three major structures: a large surface glycoprotein spike (S, 200 kDa), that forms the bulky (15–20 nm) peplomers found in the viral envelope, a matrix transmembrane glycoprotein M (20−30 kDa) and the internal phosphorylated nucleocapsid protein N (50−60 kDa). The S protein induces cell fusion and binds to the host cell receptor (Delmas and Laude, 1990). The host cell receptors for SARS-CoV and MERS-CoV are cell membrane proteins, angiotensin-converting enzyme 2 (ACE2) and dipeptidyl-peptidase IV (DPP-IV), respectively that abundantly expressed in the epithelial cells in the respiratory system (Raj et al., 2013).
So far, seven human-transmitted coronaviruses have been discovered. They are known to cause a variety of diseases including pneumonia, hepatitis, encephalomyelitis, nephritis, enteritis and other illness (Beniac et al., 2006). In the last two decades, two indigenous outbreaks of human coronavirus (HCoVs) have been reported, including coronavirus severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV) (Raj et al., 2013). Although, human coronavirus infections appear as a self-limiting respiratory infection in the most immunocompetent people, it causes lower respiratory disease in immunocompromised as well as olders. However, MERS-CoV, SARS-CoV cause pulmonary and extra-pulmonary diseases in all people (Pene et al., 2003). Howbeit, understanding the pathophysiology of SARS and MERS has been remained a debatable issue among researchers yet. Increased inflammatory cytokines in the serum of SARS and MERS patients has been shown associated with pulmonary inflammation and severe lung injury in SARS and MERS patients (Wong et al., 2004; Mahallawi et al., 2018). In December 2019, the cluster of severe acute respiratory syndrome caused by a novel beta-coronavirus emerged in Wuhan, Hubei, China. In which was named 2019 novel coronavirus (2019-nCoV). Due to an approximately 82 % genetic similarity between this strain and SARS-CoV, the new strain was named SARS-CoV-2 (Chan et al., 2020). World Health Organization (WHO) announced the disease caused by this novel virus as coronavirus disease-2019 (COVID-19). Bats, pangolins and humans are natural host, intermediate host and terminal host for SARS-CoV-2, respectively (Zhang et al., 2020a). Transmission of SARS-CoV-2 occurs human-to-human through physical contact, respiratory droplets, hospice and probably zoonotic (Cascella et al., 2020). Spike (S) glycoprotein binds to its target receptor (ACE2) on the epithelial cells of the respiratory tracts, enters and replicates. The receptor-binding domain (RBD) in the S glycoprotein directly sticks to the peptidase domain (PD) of ACE2. In addition to ACE2, SARS-CoV-2 uses the serine protease TMPRSS2 for spike protein priming to entrance the host cell (Bittmann et al., 2020) Followed by the action of TMPRSS2, metalloprotease 17 and disintegrin in human airway epithelia, SARS-CoV-2 S protein promotes ACE2 downregulating and induce the shedding of active ACE2 ectodomain to convert it as a soluble form. Increased shedding of ACE2 facilitates viral replication, vascular permeability, local inflammation and in results, exacerbation the disease (Fu et al., 2020; Peron and Nakaya, 2020; Heurich et al., 2014). Higher levels of ACE2 expression, shedding and soluble form of ACE2 have been reported in adults (Peron and Nakaya, 2020), the probable causes of development of acute respiratory distress syndrome (ARDS) and lung injury (Peron and Nakaya, 2020; Schouten et al., 2016). A recent study indicated the potential binding interaction between the spike protein of SARS−COV2 and the host glucose regulated protein (GRP78). This finding described the potency of GRP78 to facilitate the SARS−COV2 to entrance the host cell. However, this is the primary finding and needs more investigation to approve such claim, effect of that in severity of COVID-19 and the expression levels of that in adults and children (Table 1 ).
Table 1.
The main mechanism of action of potential drugs for treatment of COVID-19.
| Anti-viral activity | Immunomodulatory and anti-inflammatory properties |
|---|---|
| Chloroquine and Hydroxychloroquine | Chloroquine and Hydroxychloroquine |
| Nucleoside analogues | IL-6 inhibitor |
| Remdesivir | Tocilizumab |
| Galidesivir | Sarilumab |
| Favipiravir | Siltuximab |
| Ribavirin | |
| Anti-protease | Corticosteroid |
| Lopinavir/Rritonavir | |
| Darunavir/Cobicistat | |
| Atazanavir/Ritonavir | |
| IFN-α/IFN-β | IFN-α/IFN-β |
| Convalescent plasma | Intravenous immunoglobulin |
| Baricitinib | Baricitinib |
| Umifenovir | Thalidomide |
| Inhibition of ACE2-mediated virus entry | Fingolimod |
| Camostat mesylate | |
| mAb against S glycoprotein | Bevacizumab |
| antibodies or small molecules blocking ACE2 human recombinant soluble ACE2 (hrsACE2) | |
| Leronlimab | |
| Mesenchymal stem cell | |
| Oseltamivir | Thymosin alpha-1 |
| Nitazoxanide | Melatonin |
In contrary to adults, SARS-CoV-2 infection appears mild or asymptomatic manifestation in children and only 1–5 % worldwide prevalence (Ludvigsson, 2020). About 15–20 % of adults demonstrated severe form of the COVID-19 disease characterized by interstitial pneumonia which developed to ARDS. Differences in the immune system of children and adults may conducted to the different pathogenesis of COVID-19 in the mentioned age groups. Dysregulation and hyperactivation in the innate and acquired immune systems that present in severe cases, could accrue followed by robust virus replication. Also, SARS-CoV-2 infection impaired cellular immunity by decreasing the activated T cell markers (e.g. CD69), enhancing expression of late activation markers such as CD25 and PD-1 in both CD4+ and CD8 + T cells, reduction in the lymphocyte number (lymphopenia), and rising proinflammatory cytokines and even cytokine storm (Yang et al., 2020).
Based on defined pathophysiology of SARS−COV2, several strategies have been purposed to treat COVID-19 including drugs with potential anti-viral activity like as Chloroquine, Nucleoside analogues, Anti-protease agents, drugs targeted ACE-2 -mediated entry the virus like using the monoclonal antibody against S glycoprotein, drug with Immunomodulatory and anti- inflammatory properties, Corticosteroid, and monoclonal antibody to block inflammatory cytokines for inhibition of cytokine storm as a hallmark phenomenon in severe diseases. Despite trying these strategies, the treatment of this disease still faces challenges and surprises.
Given the importance of the immune system in predicting the response to treatment and the importance of paying attention to the differences in the immune system between adults and children, this study aims to discuss the salient differences between the immune systems of adults and children, gender, immune-dysregulation and their possible role in clinical manifestations in COVID-19.
1.1. Age-associated changes in immune responses considering immunosenescence and inflammaging
The major protective physiological system against various threats (e.g. cancer, bacteria, virus, ...) is the immune system. Immune system is in almost fully connection with the other physiological systems, lead to formation the most important axis in the body, the neuro–endocrine–immune axis. Similar to the other organs and systems, immune system could affect by the aging. According to the numerous studies, aging changes the immune agents and probably lead to reduce its potency and dysregulation. Altogether, the results and consequence of aging on immune regulation and variation is named immunosenescence. Like other organs and systems, the immune system can be affected by aging. According to several studies, aging causes changes in immune factors and may lead to a decrease in potency and dysregulation. Altogether, the results and consequence of aging on immune regulation and variations is named immunosenescence. Immunosenescence has been demonstrated to be involved in promotion of inflammatory phenomena and related factors and inflamm aging. In this section, to better understand the effect of age on COVID-19 manifestations, we discuss immune changes based on the effects of age or immunosenescence on immune cells and related mediators.
1.2. Innate immunity (the cells)
The innate immunity as the first-tier defense is stimulated after the pathogen enters the respiratory tracts and includes neutrophils, monocytes and macrophages, dendritic cells (DCs), epithelial cells, natural killer cells (NKs), mast cells and some cytokines and mediators. increased proportion of neutrophils in bronchoalveolar lavage (BAL) fluids, more tissue inflammation, increased elastase activity, and release of primary granules have were found in older compared with younger adults (Meyer et al., 1996; Sapey et al., 2014]. Nevertheless, others have found neutrophils produce less superoxide in older people (≥85 years) than younger one (Polignano et al., 1994; McLachlan et al., 1995). Lower percentage of macrophages in BAL fluid of older subjects has been shown (Meyer et al., 1996). Furthermore, aging can impair the activation of CD4 + T cells by macrophage due to low expression of major histocompatibility (MHC) class II molecules in aged mice (Herrero et al., 2001). Decreasing in the fundamental properties of macrophages including the levels of phagocytosis (Swift et al., 1999), apoptotic cells clearance (Arnardottir et al., 2014), Toll-like Receptors (TLRs) expression (Boehmer et al., 2005), proinflammatory and immunomodulatory mediators production (Mahbub et al., 2012), telomerase activity (Arai et al., 2015), superoxide anion releasing (Kelly et al., 2003), reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) have been found in relation with aging (Tasat et al., 2003).
Also, age-related changes in dendritic cells (DC) have been demonstrated in the preponderance of the evidence. It has been reported that aging cannot affect the number of DCs in the lung, although it may change the subset frequency (Wong and Goldstein, 2013). In conjunction the above, Heier et al. demonstrated that the density of antigen presenting cells (APCs), CD68+ macrophages and CD11c + DCs, are similar in the airway mucosa of children older than 2 years and adults (Heier et al., 2011). In addition, no DCs has been found in the human tracheobronchial mucosa in the first year of life, although rapid influx of them occurs by infectious stimuli (Tschernig et al., 2001). However, in some recent reports, in compare with older cases, lower densities of DC in the tracheal mucosa of infant has been detected (Tschernig et al., 2006). Furthermore, increased expression of prostaglandin D2 upon respiratory viral infection in the lungs of aged mice such as influenza A virus and SARS coronavirus infection leads to the impaired homing of lung DCs and T cell activation (Zhao et al., 2011). On the other hand, with aging, plasmacytoid dendritic cells (PDCs) showed impairment in interferon (IFN)-I production and antigen-presenting capacity after stimulation with influenza virus.
NK cell, as the other important anti-viral innate cell, has been reported to be related with higher incidence of viral infection. In older animals infected with the influenza virus, it has been shown to reduce both the number of NK cells and the ability to produce cytokines (Beli et al., 2011; Nogusa et al., 2008).
Mast cells are another player of innate immunity with ∼400 % increase in the number of activated forms compared with resting condition in aged tissues for COVID19-related pneumonia. The cytokines storms through mast cell degranulation leads to severe damage to pulmonary tissue. Rangwani demonstrated that fatal vasodilatory / interstitial macrophages are resulting from high levels of mast cells in the older population (Rangwani, 2020).
1.3. Innate immunity (soluble mediators)
In addition to the immune cells, cytokines and immune mediators of the innate immune system are affected by aging. Bronchoalveolar lavage (BAL) fluid levels of myeloperoxidase (MPO), IL-6 (inflammatory cytokine), IL-10 (anti-inflammatory cytokine), and p-selectin were significantly lower in neonates and children compared to adults; however, an opposite trend in endothelial activation marker Intercellular Adhesion Molecule 1 (ICAM-1) expression was seen with higher expression in neonates (Schouten et al., 2015).
1.4. Acquired immunity
In addition to the innate immune system, there is a difference between the acquired immune system in children and adults. In old age, insufficient production of T cells due to thymus atrophy disrupts to the effective respond to new antigens. Also, increased T helper type 2 (Th2) cytokine response, diminished CTL-mediated immunity and the granzyme B in response to influenza have been reported that is directly associated with severe form of influenza (McElhaney, 2005). Moreover, with aging, fewer naïve cells are converted to memory cells in responding to a novel pathogen (Meyer et al., 1996). However, according to the recent studies, diminished naïve T cell in elderly could be compensated by IL-7 stimulation. In addition, compared to young people, older people do not seem to have a serious problem defending themselves against new pathogens. Such finding encountered immunosenescence in adaptive immune system hypothesis with remarkable challenge. This is a controversial note about the effect of age on the severity of COVID-19 in adults and adolescents.
Interestingly, a new T cell phenotype appears in aging which called NK like T cell. This cell represents the αβ T cell receptor (TCR) and NK cell receptors. NK like T cell express two markers of senescence, including CD57 and Killer Cell Lectin Like Receptor G1(KLRG1) (Michel et al., 2016). KLRG1 is an inhibitory receptor and harnesses classical TCR signaling (Henson et al., 2009). Also, CD57 + T cells produce IFN-γ but they are unable to proliferate in response to cognate antigen (Brenchley et al., 2003). In addition, the impaired ability of B cell to differentiate into plasma cells has been detected in elderly (Bulati et al., 2017). The impact of such a finding in COVID-19 is valuable, as it is a finding that revealed the poor antibody production and un-sustainable immunity against COVID-19.
Overall, data from animal models cannot be directly translated into humans, and further human studies with different age groups are needed to better understand the age-related changes in respiratory immune cells to address these important issues. Furthermore, there is a long way to light up the immune dysregulation and immune variation in COVID-19, especially based on the severity degree of the disease and aging. Taken together, aging has been shown to affect the function and density of immune cells in the respiratory system with an outstanding role of innate immunity, which may potentially have a therapeutic effect on lung damage. The how immune responses weaken with age has been depicted in Fig. 1, Fig. 2 .
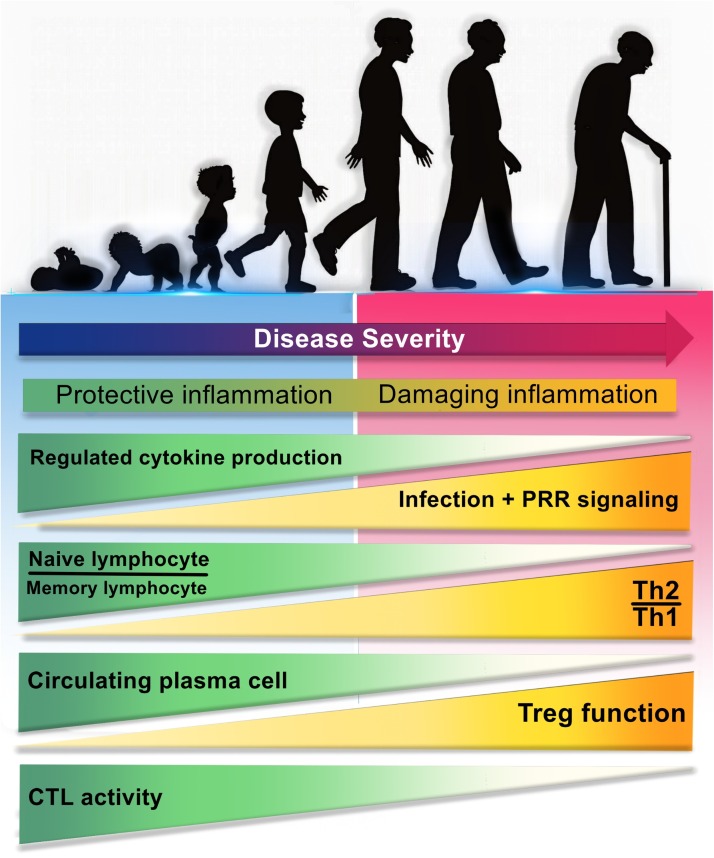
Fig. 1.
The relationship between age-dependent changes in immune responses with the severity of COVID-19 disease.
Differences in the immune system of children and adults may be the reasons for clinical differences in the severity of COVID-19. During aging, immune responses undergo changes that lead to more severe disease, some of which are include: a) depletion of well-ordinated innate immunity and regulative cytokines, b) diminished ability of the innate immune cells to recognize PAMPs, followed by strong activation of PRRs, influx of pathogenic immune cells and excessive release of proinflammatory cytokines for compensation, c) reduction ratio of naïve lymphocyte/memory lymphocyte, d) induce of negative regulation and the predominance of Th2 to Th1 responses, e) decrement of circulating plasma cells, f) increase of regulatory T cells (CD4+ CD25+ FOXP3+) function, and g) decrease of CTLs activity and diminish of CTL-mediated immunity. PAMP: Pathogen associated molecular pattern; PRR: Pathogen recognition receptor; Th: T helper; CTL: Cytotoxic T lymphocyte.
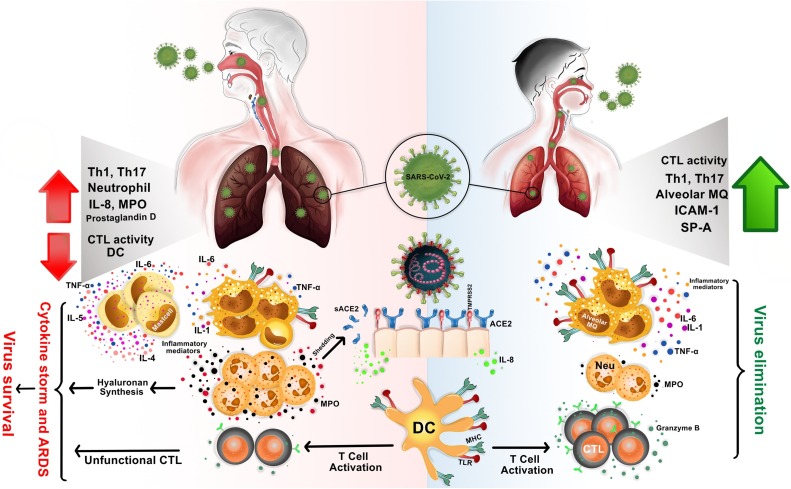
Fig. 2.
The difference in immune responses in the lungs of children and adults to SARS-CoV-2 is the reason for the different clinical manifestations.
In children, SARS−COV-2 infection may be quickly eradicated due to having less mature ACE2 receptors and rapid activation of immunocompetent immune cells (right side). In adults, the negative regulation of the immune response in the respiratory tract, late changes in the nature of the immune responses, decrease in population of immunocompetent cells, increase of ACE2 expression, ACE2 shedding and sACE2 production, all can lead to an uncontrolled immune response, widespread ineffective inflammation, immune dysregulation, cytokine storm and ARDS (left side). ACE2: Angiotensin−COnverting enzyme 2; SARS−COV2: severe acute respiratory syndrome coronavirus 2; ARDS: Acute respiratory distress syndrome.
2. Immunopathogenesis of COVID-19
The precise mechanism of COVID-19 pathogenesis and the difference between children and adults remain elusive. However, the recent studies in COVID-19 patients and previous studies in the field of SARS-CoV and MERS-CoV infections have provided some perceptions into pathogenesis of COVID-19. In addition to age and health conditions, it is not unexpected that the differences in the immune system of children and adults play a chief role in the variation observed in the severity of the COVID-19 pathogenesis.
The viral spike glycoprotein of coronavirus binds to the target cells by ACE2, merging to the membrane and release the viral RNA (Li et al., 2003). One hypothesis associated with low severity of COVID-19 in children is variations in the levels of expression of the ACE2 receptor in adults and children, a factor that may predispose adults to the disease and the high prevalence of COVID-19 among them (Brodin, 2020a). However, after the manifestation of viral RNA in the cytoplasm of infected cells, expression of viral polypeptide genes, replication, transcription, and formation of virions, they release from the host cells (Sahin et al., 2020). Viral RNA, as a pathogen-associated molecular patterns (PAMPs), evokes innate immune system. In the cytoplasm, viral RNA is sensed by cytosolic receptor melanoma differentiation-associated gene 5 (MDA5), the viral RNA receptor retinoic-acid inducible gene I (RIG-I) and nucleotidyl transferase cyclic GMP-AMP synthase (cGAS). In the endosome, viral RNA is recognized by endosomal TLRs, leading to activation of downstream cascades molecules and then cytokines production, especially IFN- I (Guo et al., 2020). Other cytokines such as IL-1, IL-2, IL-4, IL-7, IL-10, IL-12, IL-13, IL-17, macrophage colony-stimulating factor (MCSF), granulocyte-colony stimulating factor (G-CSF), MCP-1, macrophage inflammatory protein-1 alpha (MIP-1α), interferon gamma-induced protein-10 (IP-10), IFN-γ, TNF-α, and hepatocyte growth factor (HGF) have also been shown to increase in COVID-19 infection, resulting in cytokine storm and worsening the patient's condition (Huang et al., 2020; Chen et al., 2020a).
Virion particles infect a variety of immune cells and have various consequences (Lai et al., 2020). Leukopenia, neutropenia or neutrophilia, thrombocytopenia and increased infiltration of lymphocytes to the lung are the findings in the adults and infected mothers (Guo et al., 2020; Huang et al., 2020; K-q et al., 2020), while neutropenia was seen in infants (K-q et al., 2020). Because the severity of COVID-19 has been reported to be associated with T lymphopenia and possibly increased lymphocyte infiltration into the lungs (Zhou et al., 2020; Diao et al., 2020), the absence of these findings in children is likely to be associated with mild disease.
In addition to immune cells, the hepatocytes, human airway epithelial cell, and kidney tubular cells are infected by virion particles through ACE2 receptor (Lai et al., 2020; Zhang et al., 2020b; Xu et al., 2020a). ACE2 has been shown to be widely present in renal cells. In COVID-19 patients, acute kidney injury is strongly associated with the severity of diseases and increased rate of mortality and morbidity (Valizadeh et al., 2020b). In addition, overexpression of ACE2 receptor in cholangiocytes predisposes these cells to SARS-CoV-2 infection and leads to liver dysfunction. However, pathological examination of liver tissue from some dead COVID-19 patients did not shown viral inclusions in the liver (Zhang et al., 2020b). Furthermore, liver biopsy samples and histological examination showed mild lobular and portal activity in dead patient (Xu et al., 2020b). Overall, while the role of the immune system, age, endothelial system, and ACE2 expression has received considerable attention, more research is needed to better understand the exact mechanism of COVID-19 pathogenesis.
3. Immune dysregulation in COVID-19
In healthy condition, a negative regulation in the immune responses occurs in the adult lungs. However, the negative regulation declines upon respiratory virus infection (Toapanta and Ross, 2009; Sharma and Goodwin, 2006). These changes may be delayed in some adults. Decreased immunocompetent cells population during aging and induction of ineffective inflammation upon some infections may be the reason for such a delay. Robust virus replication and delayed early innate immune response (IFN-l production) lead to over-activation of immune cells to compensate. The successful treatment of either SARS-CoV or MERS-CoV infected mice by early administration of type 1interferon (IFN-I) shows that the timing of IFN-I administration has been more important to yield a protective response (Channappanavar and Perlman, 2017).
Release of Damage-Associated Molecular Patterns (DAMPs) by damaged cells through virus replication lead to over-activation of cGAS–cGAMP–STING pathway and dysregulation of IFN-I production. All of these lead to an influx of pathogenic inflammatory monocyte-macrophages (IMMs) and neutrophils, which ultimately result in inflammation and severe damage to the lung and other tissues such as seen in COVID-19 (Channappanavar et al., 2016; Motwani et al., 2019; Liu et al., 2014). In addition, large amounts of pro-inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-8, IL-18, IL-33, TNF-α, etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) release by immune effector cells that lead to cytokine storm, one of the major cause of death in COVID-19 (Huang et al., 2020). Among the inflammatory mediators mentioned, mediators produced by IMMs and neutrophils such as IL-6, IL-1β, and TNF-α, ROS and nitric oxide (NO) showed more adverse effects than others. The IL-1β and TNF-α induce hyaluronan synthase 2 (HAS2) in CD31+ endothelium, alveolar epithelial cells and fibroblasts which dramatically increase water absorption, fluid jelly formation in lung and makes breathing difficult (Bell et al., 2019). The NO and ROS can enhance endothelial permeability and extravasation of immune cells into the lungs which lead to damage of alveolar epithelium, impair efficient gas exchange and finally respiratory distress (Short et al., 2014). Activated endothelium can secrete proinflammatory cytokines and chemokines such as CCL5/RANTES and IP-10/CXCL10 which recruit of several types of leukocytes: T cells, NK cells, monocytes, DCs, and granulocytes that may exacerbate inflammation and airway hyper-reactivity (Wang et al., 2013). Excessive neutrophil response, hydrolytic enzymes and myeloperoxidase released into the extracellular space and neutrophil extracellular traps (NETs) formation are associated with severe lung pathology during infection (Short et al., 2014; Kruger et al., 2015).
Enhancing innate immune response mainly affects acquired immunity and leads to dysfunction (Diao et al., 2020). The numbers of B cells, T cells (both CD4+ and CD8 + T cells) and NK cells are more severely decrease, especially among elderly patients and in patients requiring ICU care. This finding may be due to an increased infiltration of these cells into other organs such as lung, kidney or liver which should be investigated in future pathophysiological studies of COVID-19. Interestingly, T cell numbers are negatively correlated with the serum levels of IL-6, IL-10, and TNF-α concentrations (Diao et al., 2020). Both Th cells and suppressor T cells were below normal levels, while Th and suppressor T ratio remained in the normal range. The percentage of naive helper T cells (CD3+CD4+CD45RA+) increased and memory helper T cells (CD3+CD4+CD45RO+) decreased. This imbalance between the naive and memory CD4 + T cells leads to loss an efficient immune response. Interestingly, the regulatory T cells (CD4+CD25+FOXP3+) and suppressor T cells (CD3+CD8+ CD28+) are affected and reduced, which can lead to increased inflammation and cytokine storm in COVID-19. Also, the higher neutrophil-to-lymphocyte ratio (NLR), as well as lower percentages of monocytes, eosinophils, and basophils have been indicated (Qin et al., 2020). NLR, is a well-known marker of systemic inflammation and infection.
Similar to some chronic infections, persistent virus exposure and/or inflammation along with the high levels of IL-10, as an inhibitory cytokine, lead to exhaustion T cells. Cytotoxic T cells, which play an important role against viral infections and cancers, increase the expression of some checkpoint inhibitor including PD-1 and Tim-3 and A2aR, leading to a loss of cytokine production capability, reduced cytotoxic function and T cell proliferation (Diao et al., 2020; Masoumi et al., 2020).. Interestingly, the exhausted CD94/NK group 2 member A (NKG2A)+ cytotoxic lymphocytes increase in COVID-19 patients. NK cells also get exhausted of increasing the expression of NKG2A as an inhibitory receptor. There are lower populations of CD107a + NK, IFN-γ+ NK, IL-2+ NK, TNF-α+ NK and granzyme B + NK cells in the peripheral blood of COVID-19-patients.
Humoral immunity is also thought to be involved in severity of the COVID-19. Adults are more likely to have higher levels of antibodies due to exposure to the coronavirus than young people. This results in antibody-dependent enhancement (ADE) induction. During ADE, low affinity or low concentrations antibodies due to previous exposure, instead of neutralizing, opsonize viral particles and promote FcγR mediated internalization by lung epithelial cells and infiltrating monocytes and DCs are involved in the deterioration of COVID-19(13). The phenomenon of ADE in patients with severe COVID-19 has yet to be determined and needs further investigation.
However, it should be noted that innate immune response is highly involved in the disease outcome. Rapid and regular innate immunity plays a crucial role in the first line of defense against SARS-CoV-2 infection. In children, SARS-CoV-2 infection may be quickly eradicated or at least remain mild due to having less mature ACE2 receptors and strong and well-coordinated innate immune responses. However, there is a different situation in adults. As regards different expression of ACE2, more viruses can enter the cells and infect them. Due to the negative regulation of the immune response in the adult respiratory tract, changes in the nature of responses and shifts to the effective inflammation occur with delay, especially in individuals with poor immune functions. Faster replication of the virus than the immune system's response, as well as the conversion of local inflammation into widespread lung and other organs inflammation cause the immune defense to spiral out of control and eventually exhausted. There are two complications here:
-
1
cytokine storm, acute lung and other organs injury and even death
-
2
Modulation of immune responses and exhaustion of critical and essential immune cells in viral defense.
This suggests that differences in the immune system of children and adults, especially immune homeostasis, play an important role in the clinical differences of COVID-19. Therefore, future animal and clinical studies should focus on the evaluation and efficacy of immunotherapeutic agents that directly affect host immunopathologic responses. Possible age-dependent differences in the immune system that make higher susceptibility to severe COVID-19 are summarized in Fig. 1, Fig. 2.
4. Host factors associated with mild to severe COVID-19
The wide spectrum of COVID-19 clinical manifestations varies from mild, severe to critical disease, which is including respiratory failure resulting in death (Wu and McGoogan, 2020). Not only the virus virulence factors, but some host related factors can also involve in disease outcomes. Some of the important host-associated-factors related to disease outcomes are age, gender, obesity, tobacco users/smokers, genetic variation, comorbidities like cardiovascular disease, chronic respiratory disease, hypertension, diabetes, cerebrovascular diseases, and cancer.
Older people are more likely to show the severe form of the disease (Wang et al., 2020). In addition to less accurate immune responses in aged patients, comorbidities are more frequent with aging (Wu et al., 2020). The number of leukocytes decreases and the ratio of immune cells in the peripheral blood and lungs changes with age. The number of CD8 T cells, proliferation, and granzyme + T cells have been shown to be significantly reduced in older monkeys. (Arjeyni et al., 2017). However, the compensatory effect of IL-7 stimulation on the number of T cells has recently been indicated (Gattinoni et al., 2017). B cell population decrease, followed by the reduction of neutralizing antibodies and mucosal IgA in the elderly. Decreased pulmonary DCs and macrophages in old ages are accompanied by a decrease in the frequency of costimulatory CD86 + cells through SARS infection (Clay et al., 2014). Senescence leads in the accumulation of ROS due to the attenuated antioxidative defense system. Redox imbalance activates of redox-sensitive transcription factors, such as nuclear factor kappa B (NF-κB), which promote the expression of proinflammatory genes, including IL1β, IL6, TNFα, and adhesion molecules (Chung et al., 2006). On the other hand, NF-κB activation negatively inhibits type I IFN signaling. In fact, the disease outcomes depend on the balance between the antiviral and proinflammatory responses, which are negatively influenced by aging (Smits et al., 2010). There is a correlation between ACE2 expression and COVID-19 fatality. Different expression of ACE2 during adulthood is another cause for the severity of SARS-CoV 2 disease in aged patients (Chen et al., 2020b) (Fig. 3 ).
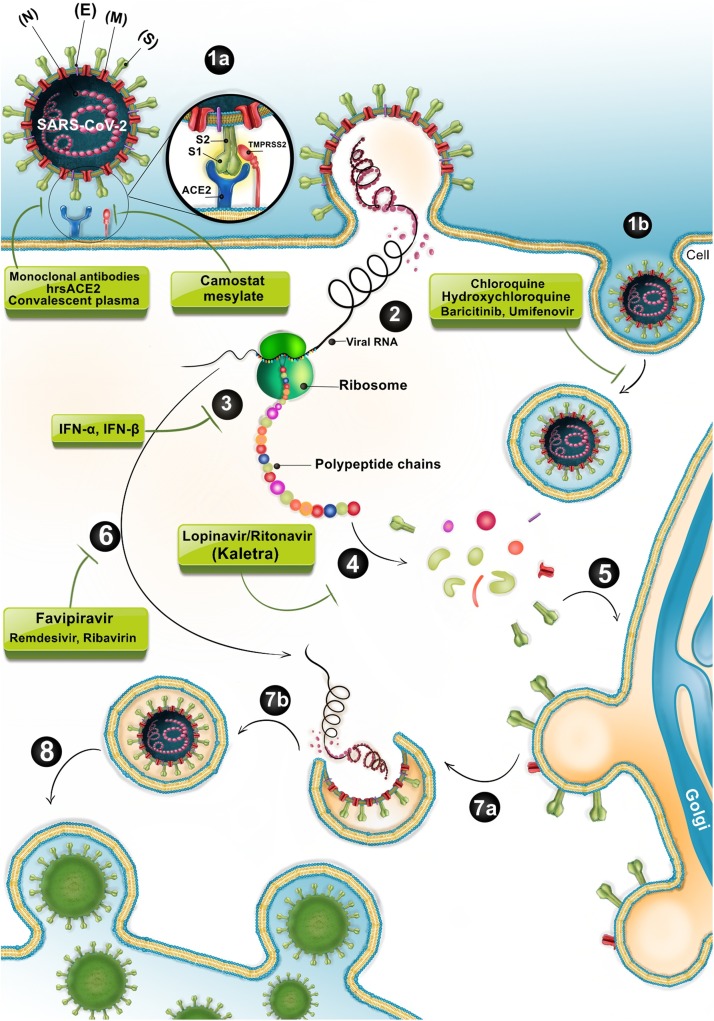
Fig. 3.
SARS-CoV-2 biogenesis cycle and probably action site of commonly used drugs in the early stage of acute infection.
TMPRSS2 cleaves and activates SARS−COV-2 -S glycoprotein for binding to its target receptor (ACE2) on the epithelial cells of the respiratory tracts. After receptor binding, the virus enters, gains access to the host cell cytosol and replicates. Following subgenomic RNA synthesis and assembly, virions are transported to the cell surface in vesicles and released by exocytosis. There are some potential approaches to prevent virus entry to the target cells and blocks several stages of life cycle of SARS−COV-2, for instance attachment, clathrin-mediated endocytosis, and replication. 1a > Fusion, 1b > Endocytosis, 2> Viral-host membrane fusion and release of viral RNA, 3> Translation, 4> Proteolysis, 5> Trafficking of newly synthesized viral proteins to the Golgi, 6> Transcription and replication of viral RNA, 7a > Assembly of mature Virion in a vesicle, 7b > Final Packaging, 8> Virion release via exocytosis. ACE2: Angiotensin−COnverting enzyme 2; SARS−COV2: severe acute respiratory syndrome coronavirus2.
Decreased sensitivity of females to COVID-19 disease could refer to sex hormones. Estrogen acts as an immunomodulatory factor in females, while testosterone is an immunosuppressor. SARS-CoV-2 replication is suppressed by estrogen signaling in females, so they have a lower viral load (Channappanavar et al., 2017). During an inflammation, estrogens repress monocyte-macrophage infiltration and NFκβ activation via suppression of special micro-RNAs such as miR125 and let7a in macrophages. Ovariectomy or estrogen receptor antagonists increase monocyte-macrophage and neutrophil recruitment into the lung of the SARS-CoV-2 infected female mice and increase disease severity and mortality (Channappanavar et al., 2017). Immune related genes on the Xchromosome play a crucial role in feminine protection. For example, overexpression of TLR-7 in women, which is located on the X chromosome, upregulates IFN-β in plasmacytoid dendritic cells and improves antiviral protection (Seillet et al., 2012). Estrogen also enhances the ACE2 mRNA levels and reduce disease fatality in women (Bukowska et al., 2017).
Single nucleotide polymorphisms (SNPs) are genetic variants between individuals. More than a hundred SNPs are identified in the ACE2 gene, in which 2 SNPs were located within the coding region. Studies on SARS are demonstrated that these polymorphisms have no effect on disease outcomes (Chiu et al., 2004). ARDS studies have been shown that the presence of SNP located within intron 16 of the ACE gene is associated with higher survival rates (Imai et al., 2007). SNPs in the oligoadenylate synthetase 1 (OAS1) and Myxovirus resistance 1 (MxA) promoter region, IL12RB1, Toll-Like Receptor Adaptor Protein Ticam2, HLA-B*0703, and HLA-DRB1*0301 are involved in SARS susceptibility (Tang et al., 2008; Gralinski et al., 2017; Ng et al., 2004).
Obesity increases the secretion of various cytokines and adipokines, such as TNF-α, IL-6, TGF-β, leptin, and adiponectin, which causes an inflammatory basal state that delays innate and acquired immune responses and allows the virus to spread (Honce and Schultz-Cherry, 2019). Suppressor of cytokine signaling (SOCS) proteins are upregulated in the peripheral blood mononuclear cell (PBMC) and lungs of the obese individuals and inhibit the production of type I and type III IFNs and pro-inflammatory cytokines (Teran-Cabanillas et al., 2014). In obese mice, alveolar macrophages are reduced and less capable to express type I IFN receptor and IFN-stimulated genes (Smith et al., 2009). CD4+ and CD8 + T cells of obese individuals produce more IL-5 and fewer IFN-γ, TNFα, granzyme B, and CD40 ligand (Paich et al., 2013). Obesity also increases the risk of many comorbidities such as type II diabetes, cancer and cardiovascular disease, leading them to severe COVID-2019 infection (Guh et al., 2009). Diabetes, hypertension and cardiovascular disease are other risk factors for COVID-19 that are associated with obesity, as these disorders are more common in people with higher BMIs. Renin–Angiotensin–Aldosterone System (RAAS) inhibitors, such as ACE inhibitors, angiotensin II type 1 receptor blockers (ARBs), or mineralocorticoid receptor antagonists (MRAs) are conventional treatments for these conditions. RAAS inhibition increases expression of ACE2, so it enhances the viral load and prone individuals to the detrimental outcomes (Kuster et al., 2020). Different mechanisms are associated with immune paralysis in diabetes include: a) decrease the secretion of IFN-γ and TNF-α by T cells, NK cells, and macrophages, b) declined MHC-І expression and also antibody biological function because of glycation (Klekotka et al., 2015). Intensive immunosuppressive drugs are used in cancer patients make them sensitive to various viral infections, including SARS-CoV-2 (Arjeyni et al., 2017).
Lung macrophages in smokers suppress the immune system more than non-smokers. These macrophages secrete low levels of IL-1, IL-6 and TNF-α (Arcavi and Benowitz, 2004). NK cell activity and serum levels of IgG and IgA are reduced in smokers (Ferson et al., 1979). ACE2 expression is also increased in tobacco users (Cai, 2020).
5. Conclusion
Not surprisingly, that the differences in the immune system of children and adults play a major role in the severity of the pathogenesis of COVID-19. During aging, the immune system changes, causing induce unfunctional cells and ineffective inflammation especially in response to infections. Therefore, because the severity of the disease varies from child to adult and depends on the severity of virus entry/replication and host immune responses, the treatment approach in COVID-19 is highly dependent on the stage of the disease. Also, future studies should focus on the evaluation and efficacy of immunotherapeutic agents that directly affect host immunopathologic responses.
Author contributions
All authors contributed to the study. Drafting of manuscript: T.S, R.A, Z.P, M.E, J.R, H.B and R.M. Creating of figures: R.M, R.A, T.S and Z.P. Revision of manuscript: R.A. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare no conflicts of interests
References
- Beniac D.R., Andonov A., Grudeski E., Booth T.F. Architecture of the SARS coronavirus prefusion spike. Nat. Struct. Mol. Biol. 2006;13(8):751–752. doi: 10.1038/nsmb1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J. Virol. 1990;64(11):5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pene F., Merlat A., Vabret A., Rozenberg F., Buzyn A., Dreyfus F. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect. Dis. 2003;37(7):929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Lam C., Wu A., Ip W., Lee N., Chan I. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020 doi: 10.1016/j.cub.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls [Internet]: StatPearls Publishing; 2020. Features, Evaluation and Treatment Coronavirus (COVID-19) [PubMed] [Google Scholar]
- Bittmann S., Luchter E., Weissenstein A., Villalon G., Moschuring-Alieva E. TMPRSS2-inhibitors play a role in cell entry mechanism of COVID-19: an insight into Camostat and Nefamostat. J. Regen. Biol. Med. 2020;2(2):1–3. [Google Scholar]
- Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-Mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020:1–6. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peron J.P.S., Nakaya H. Shedding and Antibody-dependent Enhancement (ADE) 2020. Susceptibility of the elderly to SARS-CoV-2 infection: ACE-2 overexpression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten L.R., Helmerhorst H.J., Wagenaar G., Haltenhof T., Lutter R., Roelofs J.J. Age-dependent changes in the pulmonary renin-angiotensin system are associated with severity of lung injury in a model of acute lung injury in rats. Crit. Care Med. 2016;44(12) doi: 10.1097/CCM.0000000000002008. e1226-e35. [DOI] [PubMed] [Google Scholar]
- Ludvigsson J.F. Systematic review of COVID‐19 in children show milder cases and a better prognosis than adults. Acta Paediatr. 2020 doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Dai T., Zhou X., Qian H., Guo R., Lei L. Analysis of adaptive immune cell populations and phenotypes in the patients infected by SARS-CoV-2. medRxiv. 2020 [Google Scholar]
- Meyer K.C., Ershler W., Rosenthal N.S., Lu X., Peterson K. Immune dysregulation in the aging human lung. Am. J. Respir. Crit. Care Med. 1996;153(3):1072–1079. doi: 10.1164/ajrccm.153.3.8630547. [DOI] [PubMed] [Google Scholar]
- Sapey E., Greenwood H., Walton G., Mann E., Love A., Aaronson N. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123(2):239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polignano A., Tortorella C., Venezia A., Jirillo E., Antonaci S. Age-associated changes of neutrophil responsiveness in a human healthy elderly population. Cytobios. 1994;80(322):145–153. [PubMed] [Google Scholar]
- McLachlan J.A., Serkin C.D., Morrey K.M., Bakouche O. Antitumoral properties of aged human monocytes. J. Immunol. 1995;154(2):832–843. [PubMed] [Google Scholar]
- Herrero C., Marques L., Lloberas J., Celada A. IFN-gamma-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J. Clin. Invest. 2001;107(4):485–493. doi: 10.1172/JCI11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift M.E., Kleinman H.K., DiPietro L.A. Impaired wound repair and delayed angiogenesis in aged mice. Lab. Invest. 1999;79(12):1479–1487. [PubMed] [Google Scholar]
- Arnardottir H.H., Dalli J., Colas R.A., Shinohara M., Serhan C.N. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J. Immunol. 2014;193(8):4235–4244. doi: 10.4049/jimmunol.1401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer E.D., Meehan M.J., Cutro B.T., Kovacs E.J. Aging negatively skews macrophage TLR2-and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech. Ageing Dev. 2005;126(12):1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Mahbub S., Deburghgraeve C.R., Kovacs E.J. Advanced age impairs macrophage polarization. J. Interferon Cytokine Res. 2012;32(1):18–26. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y., Martin-Ruiz C.M., Takayama M., Abe Y., Takebayashi T., Koyasu S. Inflammation, but not telomere length, predicts successful ageing at extreme old age: a longitudinal study of semi-supercentenarians. EBioMed. 2015;2(10):1549–1558. doi: 10.1016/j.ebiom.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F., Dunster C., Mudway I. Air pollution and the elderly: oxidant/antioxidant issues worth consideration. Eur. Respir. J. 2003;21(40 suppl) doi: 10.1183/09031936.03.00402903. 70s-5s. [DOI] [PubMed] [Google Scholar]
- Tasat D.R., Mancuso R., O’Connor S., Molinari B. Age‐dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages. Aging Cell. 2003;2(3):159–164. doi: 10.1046/j.1474-9728.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Wong C., Goldstein D.R. Impact of aging on antigen presentation cell function of dendritic cells. Curr. Opin. Immunol. 2013;25(4):535–541. doi: 10.1016/j.coi.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier I., Malmström K., Sajantila A., Lohi J., Mäkelä M., Jahnsen F.L. Characterisation of bronchus-associated lymphoid tissue and antigen-presenting cells in central airway mucosa of children. Thorax. 2011;66(2):151–156. doi: 10.1136/thx.2010.149591. [DOI] [PubMed] [Google Scholar]
- Tschernig T., Debertin A., Paulsen F., Kleemann W., Pabst R. Dendritic cells in the mucosa of the human trachea are not regularly found in the first year of life. Thorax. 2001;56(6):427–431. doi: 10.1136/thorax.56.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschernig T., De Vries V., Debertin A., Braun A., Walles T., Traub F. Density of dendritic cells in the human tracheal mucosa is age dependent and site specific. Thorax. 2006;61(11):986–991. doi: 10.1136/thx.2006.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhao J., Legge K., Perlman S. Age-related increases in PGD 2 expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Invest. 2011;121(12):4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beli E., Clinthorne J.F., Duriancik D.M., Hwang I., Kim S., Gardner E.M. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech. Ageing Dev. 2011;132(10):503–510. doi: 10.1016/j.mad.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogusa S., Ritz B.W., Kassim S.H., Jennings S.R., Gardner E.M. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech. Ageing Dev. 2008;129(4):223–230. doi: 10.1016/j.mad.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Rangwani C. 2020. Possible Relationship of Mast Cell Degranulation and Cytokine Storm Related COVID19 Morbidity in Young and Old Population. [Google Scholar]
- Schouten L.R., Schultz M.J., van Kaam A.H., Juffermans N.P., Bos A.P., Wosten-van Asperen R.M. Association between maturation and aging and pulmonary responses in animal models of lung injury: a systematic review. Anesthesiology. 2015;123(2):389–408. doi: 10.1097/ALN.0000000000000687. [DOI] [PubMed] [Google Scholar]
- McElhaney J.E. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23:S10–S25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Michel J.J., Griffin P., Vallejo A.N. Functionally diverse NK-like T cells are effectors and predictors of successful aging. Front. Immunol. 2016;7:530. doi: 10.3389/fimmu.2016.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson S.M., Franzese O., Macaulay R., Libri V., Azevedo R.I., Kiani-Alikhan S. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood J. Am. Soc. Hematol. 2009;113(26):6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Karandikar N.J., Betts M.R., Ambrozak D.R., Hill B.J., Crotty L.E. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood J. Am. Soc. Hematol. 2003;101(7):2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- Bulati M., Caruso C., Colonna-Romano G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by "inflamm-ageing". Ageing Res. Rev. 2017;36:125–136. doi: 10.1016/j.arr.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P. Why is COVID‐19 so mild in children? Acta Paediatrica. [DOI] [PubMed]
- Sahin A.R., Erdogan A., Agaoglu P.M., Dineri Y., Cakirci A.Y., Senel M.E. 2019 Novel Coronavirus (COVID-19) outbreak: a review of the current literature. EJMO. 2020;4(1):1–7. [Google Scholar]
- Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil. Med. Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang X., Ju Z., He W. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Chin. J. Burns. 2020;(36):E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K-q Kam, Yung C.F., Cui L., Lin Tzer Pin R., Mak T.M., Maiwald M. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Medrxiv. 2020 doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Zhang H., H-y Gong, J-x Chen, Ye J.-q, Meng T. Identification of a potential mechanism of acute kidney injury during the Covid-19 outbreak: a study based on single-cell. Transcriptome Anal. 2020 doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valizadeh R., Baradaran A., Mirzazadeh A., Bhaskar L.V. Coronavirus-nephropathy; renal involvement in COVID-19.
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toapanta F.R., Ross T.M. Impaired immune responses in the lungs of aged mice following influenza infection. Respir. Res. 2009;10(1):112. doi: 10.1186/1465-9921-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G., Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin. Interv. Aging. 2006;1(3):253. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S., editors. Seminars in Immunopathology. Springer; 2017. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motwani M., Pesiridis S., Fitzgerald K.A. DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 2019;20(11):657–674. doi: 10.1038/s41576-019-0151-1. [DOI] [PubMed] [Google Scholar]
- Liu Y., Jesus A.A., Marrero B., Yang D., Ramsey S.E., Montealegre Sanchez G.A. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 2014;371(6):507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T.J., Brand O.J., Morgan D.J., Salek-Ardakani S., Jagger C., Fujimori T. Defective lung function following influenza virus is due to prolonged, reversible hyaluronan synthesis. Matrix Biol. 2019;80:14–28. doi: 10.1016/j.matbio.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K.R., Kroeze E.J.V., Fouchier R.A., Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 2014;14(1):57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- Wang W., Yang P., Zhong Y., Zhao Z., Xing L., Zhao Y. Monoclonal antibody against CXCL-10/IP-10 ameliorates influenza A (H1N1) virus induced acute lung injury. Cell Res. 2013;23(4):577–580. doi: 10.1038/cr.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger P., Saffarzadeh M., Weber A.N., Rieber N., Radsak M., von Bernuth H. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11(3) doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. China (February 17, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoumi E., Jafarzadeh L., Mirzaei H.R., Alishah K., Fallah-Mehrjardi K., Rostamian H. Genetic and pharmacological targeting of A2a receptor improves function of anti-mesothelin CAR T cells. J. Exp. Clin. Cancer Res. 2020;39(1):1–12. doi: 10.1186/s13046-020-01546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–Infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Speiser D.E., Lichterfeld M., Bonini C. T memory stem cells in health and disease. Nat. Med. 2017;23(1):18–27. doi: 10.1038/nm.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay C.C., Donart N., Fomukong N., Knight J.B., Overheim K., Tipper J. Severe acute respiratory syndrome-coronavirus infection in aged nonhuman primates is associated with modulated pulmonary and systemic immune responses. Immun. Ageing. 2014;11(1):4. doi: 10.1186/1742-4933-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.Y., Sung B., Jung K.J., Zou Y., Yu B.P. The molecular inflammatory process in aging. Antioxid. Redox Signal. 2006;8(3-4):572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van I.W.F., Eijkemans M.J. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2) doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.J.Q., Xia X., Liu K., Yu Z., Tao W., Gong W., Han J.J. Individual variation of the SARS-CoV2 receptor ACE2 gene expression and regulation. Preprints. 2020 doi: 10.1111/acel.13168. 2020030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. (Baltimore, Md:1950) 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillet C., Laffont S., Tremollieres F., Rouquie N., Ribot C., Arnal J.F. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119(2):454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- Bukowska A., Spiller L., Wolke C., Lendeckel U., Weinert S., Hoffmann J. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp. Biol. Med. (Maywood) 2017;242(14):1412–1423. doi: 10.1177/1535370217718808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R.W.K., Tang N.L.S., Hui D.S.C., Chung G.T.Y., Chim S.S.C., Chan K.C.A. ACE2 gene polymorphisms do not affect outcome of severe acute respiratory syndrome. Clin. Chem. 2004;50(9):1683–1686. doi: 10.1373/clinchem.2004.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Penninger J.M. Angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Cell. Mol. life Sci. CMLS. 2007;64(15):2006–2012. doi: 10.1007/s00018-007-6228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F., Liu W., Zhang F., Xin Z.-T., Wei M.-T., Zhang P.-H. IL-12 RB1 genetic variants contribute to human susceptibility to severe acute respiratory syndrome infection among chinese. PLoS One. 2008;3(5):e2183. doi: 10.1371/journal.pone.0002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Menachery V.D., Morgan A.P., Totura A.L., Beall A., Kocher J. Allelic variation in the toll-like receptor adaptor protein <em>Ticam2</em> contributes to SARS-Coronavirus pathogenesis in mice. G3 (Bethesda) 2017;7(6):1653–1663. doi: 10.1534/g3.117.041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M.H., Lau K.M., Li L., Cheng S.H., Chan W.Y., Hui P.K. Association of human-leukocyte-antigen class I (B*0703) and class II (DRB1*0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. J. Infect. Dis. 2004;190(3):515–518. doi: 10.1086/421523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honce R., Schultz-Cherry S. Impact of obesity on influenza a virus pathogenesis. Immune Res. Evol. 2019;10(1071) doi: 10.3389/fimmu.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran-Cabanillas E., Montalvo-Corral M., Silva-Campa E., Caire-Juvera G., Moya-Camarena S.Y., Hernandez J. Production of interferon alpha and beta, pro-inflammatory cytokines and the expression of suppressor of cytokine signaling (SOCS) in obese subjects infected with influenza A/H1N1. Clin. Nutri. (Edinburgh, Scotland) 2014;33(5):922–926. doi: 10.1016/j.clnu.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Smith A.G., Sheridan P.A., Tseng R.J., Sheridan J.F., Beck M.A. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology. 2009;126(2):268–279. doi: 10.1111/j.1365-2567.2008.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paich H.A., Sheridan P.A., Handy J., Karlsson E.A., Schultz-Cherry S., Hudgens M.G. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring, Md). 2013;21(11):2377–2386. doi: 10.1002/oby.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh D.P., Zhang W., Bansback N., Amarsi Z., Birmingham C.L., Anis A.H. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9(1):88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster G.M., Pfister O., Burkard T., Zhou Q., Twerenbold R., Haaf P. SARS-CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19? Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klekotka R.B., Mizgala E., Krol W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol. Alergol. Pol. 2015;83(5):401–408. doi: 10.5603/PiAP.2015.0065. [DOI] [PubMed] [Google Scholar]
- Arjeyni Y., Goudarzi H., Eslami G., Faghihloo E. Viral respiratory infections in patients with Cancer. Int. J. Cancer Manag. 2017;10(2):e8084. [Google Scholar]
- Arcavi L., Benowitz N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004;164(20):2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- Ferson M., Edwards A., Lind A., Milton G.W., Hersey P. Low natural killer-cell activity and immunoglobulin levels associated with smoking in human subjects. Int. J. Cancer. 1979;23(5):603–609. doi: 10.1002/ijc.2910230504. [DOI] [PubMed] [Google Scholar]
- Cai G. 2020. Bulk and Single-cell Transcriptomics Identify Tobacco-use Disparity in Lung Gene Expression of ACE2, the Receptor of 2019-nCov. 2020.02.05.20020107. [Google Scholar]