Abstract
Determination of phytohormones have attracted increasing attentions in food safety field. In this study, an efficient and quantitative method was developed which can simultaneously determinate thirteen phytohormones in fruits and vegetables using solid phase extraction (SPE) combined with high performance liquid chromatography–diode array detection (HPLC–DAD). The samples were extracted with 80% methanol containing 0.5% (V/V) formic acid, and the extracts were then concentrated and purified using primary secondary amine (PSA) and C18 tandem dual SPE cartridges. The analytes were separated on a Waters XBridge™ C18 column and eluated utilizing a gradient elution program of water and methanol. Mean recoveries of the thirteen analytes varied from 74.69 to 92.40%, with relative standard deviations < 3.57%. The limits of detection and quantitation were 0.005–0.018 mg/kg and 0.02–0.10 mg/kg, respectively. The phytohormones in kiwi fruit, strawberry, bean sprout, and green pepper were detected using the above method, respectively. Only the IAA content of 0.14 mg/kg was detected for the strawberry from a supermarket, which was lower than the prescribed limit in food safety standards (0.2 mg/kg).
Keywords: Phytohormone, High performance liquid chromatography–diode array detection, Simultaneous analysis, Fruit and vegetable
Introduction
Phytohormones are nonnutritious chemicals that artificial synthesized or extracted from microorganisms and applied externally in plants to regulate the growth of plants. The use of lower concentrations of phytohormones can promote or inhibit the growth and development of plants (Hou et al., 2008; Santner et al., 2009). The fruits and vegetables with phytohormone residues may have little effect on human body in the short term, but after long-term consumption, it will cause endocrine disorder and affect the metabolic balance in the body (Cho et al., 2013; Oulkar et al., 2011). The abuse or improper use of phytohormones has resulted in increasing food safety problems. In recent years, the government and legislative setup have stipulated maximum residue limits of phytohormones to mitigate its potential risks for the people’s health (Li et al., 2015). Therefore, it is imperative and urgent to formulate and validate a simple and generic pretreatment method which is applicable to different classes of phytohormones.
The methods used to analyze phytohormones are varied, owing to the significant differences in the chemical structures of the phytohormone varieties. The determination methods including spectral analysis (Li et al., 2009; Zhang et al., 2010), enzyme-linked immune sorbent assay (ELISA) (Wang et al., 2001), capillary electrophoresis (CE) (Chen et al., 2011), gas chromatography (GC) (Royer et al., 2006). high-performance liquid chromatography (HPLC) (Aral et al., 2013), and liquid chromatography-mass spectrometry (LC–MS) (Cai et al., 2015; Luo et al., 2017) have been used to detect phytohormones. In general, the accuracy of spectral analysis techniques is relatively low, and ELISA methods require further improvement to reduce the cross-reaction and improve accuracy. The derivatization methods for GC analysis are different for different phytohormones. HPLC can overcome the defects that need to be derived using GC analysis, and moreover, it can be used to detect the multiple residues of different phytohormones. The LC–MS has used for the identification of residues in excess of established limits. Unfortunately, the determination of multiple phytohormones is still a challenging issue. In contrast, a potential alternative for the determination and confirmation of phytohormone residues is the use of HPLC–DAD methods, which are cost-effective and can be used for the simultaneous detection of many analytes.
Sample pretreatment is an important step in chromatography. It is a time-consuming procedure, and also greatly affects the accuracy and precision of the analysis. There has been progress in sample preparation technology for the analysis of phytohormones, including the use of liquid–liquid extraction (LLE) (Wells et al., 2013), solid-phase extraction (SPE) (Dobrev et al., 2005; Ma et al., 2008; Pan et al., 2008), magnetic solid-phase extraction (MSPE) (Cai et al., 2014), vapor-phase extraction (VPE) (Engelberth et al., 2003), solid-phase microextraction (SPME) (Liu et al., 2010), and molecularly imprinted solid-phase extraction (MISPE) (Yan et al., 2012). Compared with other techniques, SPE has emerged as an eminent tool for sample pretreatment with less organic solvents involved, and matrix interferences can be removed and target analytes can be pre-concentrated simultaneously. However, single SPE process is usually inadequate to remove lipids and lipophilic matrix substances. Hence, tandem dual SPE processes have been adopted to purify various classes of phytohormones in complex plant matrices, but some of them suffer from low recovery and reproducibility (Dobrev and Kamínek, 2002; Susawaengsup et al., 2011). The challenges in the simultaneously determination of multi-class phytohormones originate are not only from their low abundance and the complex matrix of plants, but also from the difficulties of simultaneous extraction and purification of multi-class phytohormones owing to their chemical diversity.
This study developed a method for simultaneously determination of thirteen phytohormones: Gibberellic acid (GA), zeatin (Z), paclobutrazol (PBZ), 4-fluorophenoxyacetic acid (4-FPA), 4-chlorophenoxyacetic acid (4-CPA), 3-indolyl-acetic acid (IAA), 3-indolyl-butyric acid (IBA), 6-benzylaminopurine (6-BA), abscisic acid (ABA), 1-naphthyl acetic acid (NAA), forchlorfenuron (CPPU), 2.4-dichlorophenoxyacetic acid (2,4-D), and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in fruits and vegetables. The method involved sample pretreatment with multiple SPE and analytical determination with HPLC–DAD, which was an uncomplicated instrumentation system available in most laboratories. The objectives of this study were to optimize and compare, in terms of efficiency, the sample pretreatment involved in the extraction and purification of multiple phytohormones and develop an efficient analytical method for their simultaneous determination in fruits and vegetables. This method could be applied for the rapid detection of phytohormones at a limit of detection (LOD) low enough for quantitative determination below established limits.
Materials and methods
Reagents and chemicals
GA (98.0%), Z (98.5%), PBZ (98.5%), 4-FPA (98.5%), 4-CPA (98.0%), IAA (99.5%), IBA (99.9%), 6-BA (99.0%), ABA (99.5%), NAA (98.5%), CPPU (98.7%), 2,4-D (99.0%), and 2,4,5-T (99.0%) were obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). The chemical structures of the phytohormones are shown in Fig. 1. HPLC-grade methanol was obtained from Burdick & Jackson (Muskegon, MI, USA) and HPLC-grade acetic acid from E. Merck (Darmstadt, Germany). Throughout the study, water from a Milli-Q system from Millipore (Billerica, MA, USA) was used.
Fig. 1.
Chemical structures of the thirteen phytohormone compounds
Oasis HLB (60 mg, 3 mL) and PSA (60 mg, 3 mL) SPE cartridges were purchased from Waters (Milford, MA, USA). C18 (60 mg, 3 mL) SPE cartridges were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Kiwi fruit, strawberry, bean sprout, and green pepper were purchased local supermarket.
Standard solutions
Using 50% methanol as solvent, standard stock solutions of GA, Z, PBZ, 4-FPA, 4-CPA, IAA, IBA, 6-BA, ABA, NAA, CPPU, 2,4-D, and 2,4,5-T were prepared to a final concentration of 1.0 mg/mL. These solutions were stored under refrigeration (− 20 °C). Diluted aliquots of the standard stock solution with 50% methanol and prepared the working solution daily.
Sample extraction
The plant hormones were extracted from plant samples according to the method of Dobrev et al. (2005) with modifications. All plant samples were cut into pieces, and then homogenized with high speed homogenizer. The homogenized plant samples (5 g) were added to a 50 mL centrifuge tube and extracted twice with 40 mL of the extractants, followed by mixing after ultrasonic extraction for 15 min. The mixture was centrifuged (2960×g) for 20 min, and the supernatant were combined. The combined extracts were evaporated and diluted with methanol/water/formic acid (10/95/0.1, v/v/v) to 5 mL for further analysis.
Purification using C18 cartridge
The extracted supernatants were dissolved in 5.0 mL of methanol/water/formic acid (10/95/0.1, v/v/v) and purified by C18 cartridges. The cartridges were activated and conditioned with 6.0 mL of methanol and equilibrated with 3.0 mL of methanol/water/formic acid (10/95/0.1, v/v/v). Each sample was loaded on a cartridge and washed with 3.0 mL of methanol/water/formic acid (10/95/0.1, v/v/v). The phytohormones were eluted with 2 × 3.0 mL of methanol/water/formic acid (80/20/0.1, v/v/v). The obtained eluates were dried using nitrogen evaporators and dissolved with 1 mL 80% methanol, and then filtered using a 0.45 μm microfiltration membrane prior to HPLC analysis.
Purification using Oasis HLB cartridge
The purification method was carried out according to Urbanová et al. (2013) with minor modifications. The extracted supernatants were dissolved in 5.0 mL of methanol/water/formic acid (10/95/0.1, v/v/v), and then loaded on Oasis HLB cartridges pre-conditioned with 6.0 mL of MeOH and allowed to equilibrate with 3.0 mL of methanol/water/formic acid (10/95/0.1, v/v/v). After washing the cartridges with 3.0 mL of methanol/water/formic acid (10/95/0.1, v/v/v), the target phytohormones were eluted with 3.0 mL of methanol/water/formic acid (80/20/0.1, v/v/v) twice. The obtained eluates were dried using nitrogen evaporators and dissolved with 1 mL 80% methanol, and then filtered using a 0.45 μm microfiltration membrane prior to HPLC analysis.
Purification using PSA and C18 cartridges
The extracted supernatants were dissolved in 5.0 mL of methanol/water/formic acid (10/95/0.1, v/v/v) and purified using PSA and C18 tandem dual cartridges. The SPE procedure was as follows: The PSA SPE column was connected in series above C18 SPE column with a string joint. The cartridges were activated and conditioned with 12.0 mL of methanol and equilibrated with 6.0 mL of methanol/water/formic acid (10/95/0.1, v/v/v). Each sample was loaded on tandem dual cartridge and washed with 6.0 mL of methanol/water/formic acid (10/95/0.1, v/v/v). The target phytohormones were eluted with 3.0 mL of methanol/water/formic acid (80/20/0.1, v/v/v) twice. The obtained eluates were dried using nitrogen evaporators and dissolved with 1 mL 80% methanol, and then filtered using a 0.45 μm microfiltration membrane prior to HPLC analysis.
HPLC–DAD analysis
HPLC was performed with a Waters 1525 (Waters, Milford, MA, USA) system, including Waters 1525 secondary pump, Waters 2998 DAD, and Waters 2707 autosampler. The Waters Empower3 software controlled the HPLC parameters and processed the data. The XBridge™ C18 column (250 mm × 4.6 mm, 5 μm) (Waters, Milford, MA, USA) was used for separation. Eluent A (water/acetic acid = 99.9/0.1) and eluent B (methanol) were combined in a linear gradient as follows: 0–40 min, 20–70% B; 40–50 min, 70–95% B; 50–54 min, 95% B. The flow rate was 0.9 mL/min, and the column heater was set at 30 °C. The DAD absorption spectrum of the compounds were recorded between 210 nm and 500 nm.
Method validation
The analytical method validation was conducted according to AOAC guidelines (Han et al., 2017). The following validation parameters were established: linearity range, LOD, limit of quantitation (LOQ), repeatability, reproducibility, recovery and stability. Relative standard deviation (RSD) of repeated experiment was used to evaluate the precision. The recoveries of the phytohormones in the samples were assessed by spiking a standard mixture at three concentrations (0.1 mg/kg, 0.3 mg/kg and 0.5 mg/kg). The intra- and inter-day accuracies and precisions were determined by samples spiked with high, medium and low levels of phytohormones on five consecutive days, and on each day six replicates. Standard calibration was performed by plotting analyte concentrations versus peak areas. LOD and LOQ were determined by signal-to-noise ratios of 3 and 10, respectively.
Statistical analysis
All data were analyzed using the Statistical Package for Social Science 23 (SPSS 23) and all charts were produced using Origin 9.0. Significant differences were evaluated using Statistix 9 (p < 0.05). All values are expressed as the mean ± standard deviation (SD).
Results and discussion
Evaluation of chromatographic conditions
The chromatographic conditions for simultaneous separation and quantification of thirteen phytohormones were optimized by evaluating the variables that affect the chromatographic process. According to the literature, phytohormones are usually separated through acid mobile phases on reverse phase column (Kantiani et al., 2009). Different systems were evaluated in which various types of column and mobile phases were employed. Once the column and mobile phase were selected, the separation of the analytes was optimized with different gradient programs.
In this study, various reverse-phase C18 columns were tested, including XBridge™ C18, 250 mm × 4.6 mm, 5 μm (Waters, Milford, MA, USA), Zorbax Eclipse XDB-C18, 250 mm × 4.6 mm, 5 μm (Agilent Technologies, Santa Clara, CA, USA), and GraceSmart RP18, 250 mm × 4.6 mm, 5 μm (Grace, Columbia, MD, USA). It was found that the peaks of the thirteen phytohormones were well separated in 50 min on the XBridge™ C18 columns.
The effect of mobile phase composition on chromatographic separation was investigated using acetonitrile-0.2% acetic acid, acetonitrile-0.05% phosphoric acid, and methanol-0.05% phosphoric acid. Several elution gradient procedures using acetic acid (0.2%) as eluent A and acetonitrile with acetic acid (0.2%) as eluent B were studied. The chromatograms obtained cannot resolve all the signals satisfactorily. It was observed form Fig. 2, the mobile phase of methanol-0.1% acetic acid (v/v) with gradient program yielded the most effective separation.
Fig. 2.
HPLC chromatograms of standard mixtures of phytohormones. Peak identities: 1, Z; 2, GA; 3, PBZ; 4, 4-FPA; 5, 4-CPA; 6, IAA; 7, IBA; 8, 6-BA; 9, ABA; 10, NAA; 11, CPPU; 12, 2,4-D; 13, 2,4,5-T
According to the above results, the optimal chromatographic conditions were XBridge™ C18 column kept at 30 °C, eluent A (methanol/water/acetic acid = 10/89.9/0.1) and eluent B (methanol/water/acetic acid = 90/9.9/0.1) were selected. Further improvement in separation was obtained by mobile phase gradient. The gradient condition is as follows: 0–40 min, 20–70% B; 40–50 min, 70–95% B; 50–54 min, 95% B. As a result, resolution improved considerably, and narrow and symmetrical shape peaks were achieved.
The wavelengths of the target analytes were selected by a DAD full-wavelength scan (190–500 nm). As the thirteen phytohormones showed different UV absorption properties, different detection wavelengths were simultaneously set to monitor these compounds in a single run: 220 nm (for 4-FPA, IBA, and 2,4,5-T), 240 nm (for Z, GA, PBZ, IAA, 6-BA, CPPU and 2,4-D), and 290 nm (for 4-CPA, ABA and NAA). The obtained results are presented in the chromatogram in Fig. 2.
Study on extraction methods
According to the physicochemical properties of the phytohormones and previous reports (Aral et al., 2013; Cai et al., 2015), acetonitrile–formic acid (99.5/0.5, v/v), acetonitrile–water–formic acid (80/19.5/0.5, v/v/v), acetonitrile–water (80/20, v/v/v), and methanol–water–formic acid (80/19.5/0.5, v/v/v) for extracting phytohormones from fruits or vegetables were investigated, using the samples with the added target phytohormones of 0.5 mg/kg. Non-spiked fresh fruits or vegetables samples were also processed in parallel as sample blanks.
The results showed that the most efficient extractions of the target phytohormones with the least amount of interfering substances including pigments, lipids, and other lipophilic matrix substances were obtained using the methanol systems (Table 1). Whereas 4-FPA, IAA, and 2,4,5-T showed slightly lower extraction efficiencies than acetonitrile. This may be due to the good solubilities of 4-FPA, IAA, and 2,4,5-T in acetonitrile. The comparison between acidified and non-acidified extraction solvents showed that slightly higher recoveries of the phytohormones were achieved using 80% methanol with 0.5% formic acid. In terms of molecular structure of the phytohormones, 4-FPA, ABA, 4-CPA, IBA, NAA, IAA, 2,4-D, and 2,4,5-T all possess carboxyl groups. The ionic form of the carboxyl groups will be inhibited in an acidic environment. The addition of 0.5% formic acid to the extracting solution can hence improve the recovery. In general, methanol–water–formic acid (80/19.5/0.5, v/v/v) was more suitable for the extraction of the target phytohormones. The recovery of phytohormones was in the range of 86.37–110.57%.
Table 1.
Comparison of the recoveries (%) of the extraction solvents (n = 3)
| Analyte | Methanol–formic acid (99.5/0.5, v/v) | Methanol–water–formic acid (80/19.5/0.5, v/v/v) | Methanol–water (80/20, v/v/v) | Acetonitrile–formic acid (99.5/0.5, v/v) | Acetonitrile–water–formic acid (80/19.5/0.5, v/v/v) |
|---|---|---|---|---|---|
| Z | 79.35 ± 7.52abc | 89.35 ± 7.35c | 86.38 ± 6.27bc | 72.35 ± 4.28a | 75.34 ± 4.58ab |
| GA | 68.37 ± 4.57a | 90.24 ± 6.74 cd | 92.37 ± 4.53d | 81.27 ± 5.37bc | 79.58 ± 5.27b |
| PBZ | 86.42 ± 5.34a | 103.47 ± 5.37b | 89.57 ± 5.27a | 86.37 ± 5.46a | 90.27 ± 4.20a |
| 4-FPA | 91.57 ± 6.31a | 91.27 ± 6.58a | 90.25 ± 4.39a | 102.57 ± 5.38b | 95.31 ± 5.21ab |
| 4-CPA | 75.67 ± 5.28a | 86.37 ± 5.41b | 81.27 ± 5.69ab | 75.38 ± 4.39a | 89.37 ± 4.38b |
| IAA | 101.24 ± 6.37bc | 95.37 ± 4.37b | 106.37 ± 5.64c | 69.87 ± 5.68a | 101.24 ± 5.38bc |
| IBA | 74.28 ± 7.27a | 105.37 ± 4.27b | 76.37 ± 8.67a | 73.68 ± 7.21a | 75.21 ± 4.38a |
| 6-BA | 68.27 ± 6.51a | 110.57 ± 6.87c | 81.29 ± 5.61b | 85.31 ± 4.65b | 85.37 ± 5.21b |
| ABA | 84.37 ± 5.64a | 87.64 ± 5.34a | 102.37 ± 7.14b | 89.68 ± 7.27a | 85.37 ± 5.27a |
| NAA | 84.27 ± 4.27bc | 90.38 ± 6.18 cd | 75.29 ± 4.29ab | 95.07 ± 7.08d | 72.38 ± 5.74a |
| CPPU | 68.31 ± 5.64a | 103.57 ± 5.87c | 80.37 ± 6.28b | 86.17 ± 4.50b | 106.37 ± 5.21c |
| 2,4-D | 75.14 ± 6.38a | 92.74 ± 7.27b | 81.67 ± 5.79a | 76.38 ± 4.89a | 81.14 ± 4.35a |
| 2,4,5-T | 77.38 ± 3.28a | 89.27 ± 4.16b | 83.10 ± 6.47ab | 86.47 ± 4.27ab | 98.24 ± 5.20c |
Values in the same line with different letters were significantly different at p < 0.05
Optimization of SPE conditions
Several SPE cartridges with various sorbents have been widely applied for the removal of the co-extracted interferences in the analysis of phytohormones due to their convenience and simple method. The commonly used solid phase extraction columns are C18 and hydrophilic-lipophilic balance column (Oasis HLB) according to the polarity of weak acidic phytohormones (Hou et al., 2008; Kojima et al., 2009). The nonpolar substance like chlorophyll can be removed by C18, and some strongly polar components can also be washed out by adjusting eluent. Ma et al. (2008) purified coconut juice using C18 column, and IBA, ABA, GA can be detected using HPLC combined with mass spectrometer.
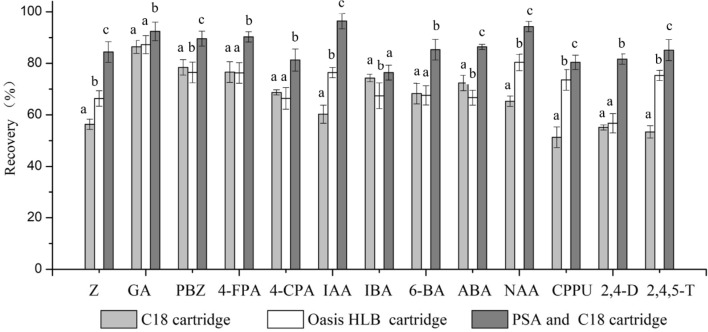
Figure 3 shows the recoveries of the 0.5 mg/kg addition standards with extracts from strawberry obtained using the different purification methods. When the sample was purified using C18 SPE alone, it was found that the recovery rate of the phytohormones was not satisfied yet. The separation ability of the C18 SPE was limited when the plants containing numerous polar and medium polar substances, as many interfering peaks appeared. Oasis HLB is a hydrophilic-lipophilic balance column. Oasis HLB cartridges produced slightly improved recoveries compared to the C18 cartridges. For IAA, NAA, CPPU and 2,4,5-T, improved recoveries were observed on Oasis HLB cartridges, but this cartridge showed poor recoveries (< 70%) for the other phytohormones (Z, 4-CPA, IBA, 6-BA, ABA and 2,4-D). Although the polarity of this column is high, the separation is unsatisfied using a single column, as it cannot purify complex plant extracts. This result is consistent with previous study (Ge et al., 2007).
Fig. 3.
Recovery rates of addition standards with extracts from strawberry by different purification procedures (n = 3). Bars by different purification procedures with different letters were significantly different at p < 0.05
PSA is a polar adsorbent, which has good adsorption effect on polar compounds. At present, it is widely used in the analysis of fruits and vegetables residue matrix dispersion solid phase pretreatment (Huang et al., 2015). After adsorption on PSA SPE, the recovery rate of the target compound was obviously increased (Fig. 3). During the process of experiment, it was found that PSA SPE effectively adsorbed pigments in fruits and vegetables extracts, but the adsorption of target compounds was less. After the purification process using PSA and C18 tandem dual SPE cartridges, almost all the standard recoveries reached > 80.37% and higher than those of the samples purified using C18 SPE (or Oasis HLB SPE) alone. Considering the recoveries of the target phytohormones and their separation efficiencies from the plant samples, a two-step cleanup method using PSA and C18 SPE was developed and used for the analysis of multiple phytohormones in fruits and vegetables.
Validation of the method
The standard solutions containing thirteen analytes were prepared and diluted to appropriate concentrations to plot the calibration curves. The curves between peak area and concentration of each analyte were plotted at six concentrations of the thirteen analytes. The linear relationships and good coefficients of determination (R2 ≥ 0.9924) were obtained. This showed good linearity comparable to that reported in the study of different tissues of model plants (Cai et al., 2015). As shown in Table 2, the LOQs and LODs were in the range of 0.005–0.018 mg/kg and 0.02–0.10 mg/kg, respectively, which were lower than previously reported value (Aral et al., 2013).
Table 2.
Linear regression data, LODs, and LOQs of the thirteen analytes
| Analyte | Regression equationa | Linear range (μg/mL) | R2 | LOD (mg/kg) | LOQ (mg/kg) |
|---|---|---|---|---|---|
| Z | Y = 5203.1X + 124.6 | 0.50–80 | 0.9987 | 0.018 | 0.06 |
| GA | Y = 7025.8X − 257.6 | 0.05–90 | 0.9958 | 0.009 | 0.05 |
| PBZ | Y = 1147.2X + 102.8 | 0.50–80 | 0.9924 | 0.015 | 0.07 |
| 4-FPA | Y = 3352.9X + 121.7 | 0.30–90 | 0.9951 | 0.014 | 0.06 |
| 4-CPA | Y = 7620.7X + 68.9 | 0.05–90 | 0.9959 | 0.013 | 0.07 |
| IAA | Y = 8627.9X − 25.9 | 0.05–90 | 0.9984 | 0.009 | 0.02 |
| IBA | Y = 9863.8X + 152.9 | 0.07–90 | 0.9980 | 0.009 | 0.03 |
| 6-BA | Y = 7104.9X + 42.7 | 0.07–90 | 0.9985 | 0.007 | 0.02 |
| ABA | Y = 5012.7X − 40.9 | 0.05–90 | 0.9996 | 0.012 | 0.10 |
| NAA | Y = 7453.9X + 78.6 | 0.05–90 | 0.9957 | 0.010 | 0.07 |
| CPPU | Y = 5809.7X − 42.9 | 0.04–90 | 0.9968 | 0.005 | 0.02 |
| 2,4-D | Y = 8573.9X + 135.0 | 0.07–90 | 0.9984 | 0.007 | 0.03 |
| 2,4,5-T | Y = 22,478.9X − 59.3 | 0.20–90 | 0.9958 | 0.010 | 0.07 |
aY is the peak area, X is the concentration injected
The intra-day and inter-day precisions were determined by analyzing known concentrations of the thirteen phytohormones in six replicates every day for three consecutive days. To verify the repeatability of the method, six different concentrations of thirteen phytohormones prepared from the same sample were analyzed. Table 3 shows that the intra- and inter-day precisions ranged from 1.25 to 4.07% and 0.57 to 2.37%, respectively. The repeatability RSD values of thirteen phytohormones were all < 1.68%. These results indicated that the developed method had high reproducibility.
Table 3.
Precision, repeatability, stability, and recovery of the thirteen analytes
| Analyte | Precision (RSD, %) | Repeatability (RSD, %, n = 6) | Stability (RSD, %, n = 6) | Recovery (%, n = 3) | ||
|---|---|---|---|---|---|---|
| Intra-day (n = 6) | Inter-day (n = 5) | Mean | RSD, % | |||
| Z | 2.37 | 0.57 | 0.58 | 2.28 | 74.69 | 3.54 |
| GA | 1.98 | 1.58 | 1.27 | 3.57 | 88.52 | 2.89 |
| PBZ | 2.27 | 1.57 | 1.68 | 2.27 | 82.78 | 3.12 |
| 4-FPA | 3.27 | 1.17 | 0.58 | 1.89 | 83.45 | 2.57 |
| 4-CPA | 2.89 | 2.37 | 0.68 | 1.57 | 81.64 | 2.68 |
| IAA | 2.57 | 1.27 | 0.98 | 2.57 | 79.44 | 1.57 |
| IBA | 1.89 | 1.98 | 0.47 | 3.51 | 89.84 | 1.74 |
| 6-BA | 3.6 | 0.57 | 0.59 | 2.54 | 78.58 | 2.57 |
| ABA | 4.07 | 2.37 | 0.59 | 1.82 | 84.64 | 2.15 |
| NAA | 3.87 | 2.14 | 0.68 | 1.58 | 92.40 | 3.57 |
| CPPU | 1.25 | 1.29 | 1.59 | 3.27 | 84.76 | 2.58 |
| 2,4-D | 2.47 | 2.34 | 0.97 | 0.98 | 80.04 | 2.91 |
| 2,4,5-T | 1.57 | 1.57 | 1.27 | 2.51 | 83.40 | 3.17 |
The accuracy of the method was further investigated by the recovery test using three concentration levels of the mixed standard solutions. The standards were added into the samples for extraction and purification, and then analyzed. The average recoveries were calculated as the ratio of the detected amount to the added amount. As shown in Table 3, the recovery of the method was in the range of 74.69–92.40%, and the RSD was < 3.57%, indicating that the established method was accurate for the determination of thirteen phytohormones in fruits and vegetables. Thirteen phytohormones were added into strawberry samples at 0.1 mg/kg. There was no interference in the retention time of the phytohormones, indicating this method has good selectivity.
Sample analysis
Kiwi fruit, strawberry, bean sprout, and green pepper were detected using the above method, with the samples of 5 g each. The IAA content was 0.14 mg/kg for the strawberry sample from a supermarket, which was lower than the prescribed limit in food safety standards (0.2 mg/kg). None of the thirteen phytohormones were detected from other samples. In conclusion,the proposed method is suitable for trace phytohormones determination and analysis in fruits and vegetables with the merits of simplicity, sensitivity, reliability and cost-effective.
The detection of phytohormones has become increasingly imperative for food safety. This study established the extraction and purification methods for different phytohormones in fruits and vegetables, and optimized a sensitive HPLC–DAD method to simultaneously determinate of these phytohormones. Through methodology investigation, the established analysis method was successfully applied for the determination of thirteen phytohormones in fruits and vegetables. This method was proved suitable for the rapid simultaneous analysis of different classes of phytohormones in agriculture.
Acknowledgements
This work was financially supported by the Natural Science Foundation of China (Nos. 31871794, 21676122), the Innovative Project of State Key Laboratory of Food Science and Technology, and Fundamental Research Funds for the Central Universities (JUSRP21905).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Song Zhu, Email: zhusong@jiangnan.edu.cn.
Shang-Wei Chen, Email: 15497162@qq.com.
Yue Li, Email: liyue@jiangnan.edu.cn.
References
- Aral H, Aral T, Ziyadanoğulları B, Ziyadanoğulları R. Development of a novel amide-silica stationary phase for the reversed-phase HPLC separation of different classes of phytohormones. Talanta. 2013;116:155–163. doi: 10.1016/j.talanta.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Cai BD, Ye EC, Yuan BF, Feng YQ. Sequential solvent induced phase transition extraction for profiling of endogenous phytohormones in plants by liquid chromatography-mass spectrometry. J. Chromatogr. B. 2015;1004:23–29. doi: 10.1016/j.jchromb.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Cai BD, Zhu JX, Gao Q, Luo D, Yuan BF, Feng YQ. Rapid and high-throughput determination of endogenous cytokinins in Oryza sativa by bare Fe3O4 nanoparticles-based magnetic solid-phase extraction. J. Chromatogr. A. 2014;1340:146–150. doi: 10.1016/j.chroma.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Chen H, Guo XF, Zhang HS, Wang H. Simultaneous determination of phytohormones containing carboxyl in crude extracts of fruit samples based on chemical derivatization by capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2011;879:1802–1808. doi: 10.1016/j.jchromb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Cho SK, AbdElAty AM, Park KH, Park JH, Assayed ME, Jeong YM, Park YS, Shim JH. Simple multiresidue extraction method for the determination of fungicides and plant growth regulator in bean sprouts using low temperature partitioning and tandem mass spectrometry. J. Agric. Food Chem. 2013;136:1414–1420. doi: 10.1016/j.foodchem.2012.09.068. [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Havlíček L, Vágner M, Malbeck J, Kamínek M. Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatography. J. Chromatogr. A. 2005;1075:159–166. doi: 10.1016/j.chroma.2005.02.091. [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Kamínek M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A. 2002;950:21–29. doi: 10.1016/S0021-9673(02)00024-9. [DOI] [PubMed] [Google Scholar]
- Engelberth J, Schmelz EA, Alborn HT, Cardoza YJ, Huang J, Tumlinson JH. Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor-phase extraction and gas chromatography-chemical ionization-mass spectrometry. Anal. Biochem. 2003;312:242–250. doi: 10.1016/S0003-2697(02)00466-9. [DOI] [PubMed] [Google Scholar]
- Ge L, Peh CYC, Yong JWH, Tan SN, Hua L, Ong ES. Analyses of gibberellins by capillary electrophoresis-mass spectrometry combined with solid-phase extraction. J. Chromatogr. A. 2007;1159:242–249. doi: 10.1016/j.chroma.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Han Z, Lu L, Wang L, Yan Z, Wang X. Development and validation of an HPLC method for simultaneous determination of ibuprofen and 17 related compounds. Chromatographia. 2017;80:1353–1360. doi: 10.1007/s10337-017-3358-3. [DOI] [Google Scholar]
- Huang ZH, Wang ZL, Shi BL, Wei D, Chen JX, Wang SL, Gao BJ. Simultaneous determination of salicylic acid, jasmonic acid, methyl salicylate, and methyl jasmonate from ulmus pumila leaves by GC-MS. Int. J. Anal. Chem. 2015 doi: 10.1155/2015/698630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Zhu J, Ding M, Lv G. Simultaneous determination of gibberellic acid, indole-3-acetic acid and abscisic acid in wheat extracts by solid-phase extraction and liquid chromatography-electrospray tandem mass spectrometry. Talanta. 2008;76:798–802. doi: 10.1016/j.talanta.2008.04.041. [DOI] [PubMed] [Google Scholar]
- Kantiani L, Farré M, Sibum M, Postigo C, López AM, Barceló D. Fully automated analysis of beta-lactams in bovine milk by online solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Anal. Chem. 2009;81:4285–4295. doi: 10.1021/ac9001386. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kamada-Nobusada T, Komatsu H, Takei K, Kuroha T, Mizutani M, Ashikari M, Ueguchi-Tanaka M, Matsuoka M, Suzuki K, Sakakibara H. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in oryza sativa. Plant Cell Physiol. 2009;50:1201–1214. doi: 10.1093/pcp/pcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GL, Liu SC, Sun ZW, Xia L, Chen G, You J. A simple and sensitive HPLC method based on pre-column fluorescence labelling for multiple classes of plant growth regulator determination in food samples. Food Chem. 2015;170:123–130. doi: 10.1016/j.foodchem.2014.07.146. [DOI] [PubMed] [Google Scholar]
- Li YN, Wu HL, Zhu SH, Nie JF, Yu YJ, Wang XM, Yu RQ. Determination of indole-3-acetic acid in soil using excitation-emission matrix fluorescence with trilinear decomposition-based calibration methods. Anal. Sci. 2009;25:83–88. doi: 10.2116/analsci.25.83. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wei F, Feng YQ. Determination of cytokinins in plant samples by polymer monolith microextraction coupled with hydrophilic interaction chromatography-tandem mass spectrometry. Anal. Methods. 2010;2:1676–1685. doi: 10.1039/c0ay00334d. [DOI] [Google Scholar]
- Luo XT, Cai BD, Chen X, Feng YQ. Improved methodology for analysis of multiple phytohormones using sequential magnetic solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta. 2017;983:112–120. doi: 10.1016/j.aca.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Ma Z, Ge L, Lee AS, Yong JW, Tan SN, Ong ES. Simultaneous analysis of different classes of phytohormones in coconut (Cocos nucifera L.) water using high-performance liquid chromatography and liquid chromatography-tandem mass spectrometry after solid-phase extraction. Anal. Chim. Acta. 2008;610:274–281. doi: 10.1016/j.aca.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Oulkar DP, Banerjee K, Ghaste MS, Ramteke SD, Naik DG, Patil SB, Jadhav MR, Adsule PG. Multiresidue analysis of multiclass plant growth regulators in grapes by liquid chromatography/tandem mass spectrometry. J. AOAC Int. 2011;94:968–977. doi: 10.1093/jaoac/94.3.968. [DOI] [PubMed] [Google Scholar]
- Pan X, Welti R, Wang X. Simultaneous quantification of major phytohormones and related compounds in crude plant extracts by liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry. 2008;69:1773–1781. doi: 10.1016/j.phytochem.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Royer A, Laporte F, Bouchonnet S, Communal PY. Determination of ethephon residues in water by gas chromatography with cubic mass spectrometry after ion-exchange purification and derivatisation with N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide. J. Chromatogr. A. 2006;1108:129–135. doi: 10.1016/j.chroma.2005.12.078. [DOI] [PubMed] [Google Scholar]
- Santner A, Calderonvillalobos LI, Estelle M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009;5:301–307. doi: 10.1038/nchembio.165. [DOI] [PubMed] [Google Scholar]
- Susawaengsup C, Rayanakorn M, Wongpornchai S, Wangkarn S. Investigation of plant hormone level changes in shoot tips of longan (Dimocarpus longan Lour.) treated with potassium chlorate by liquid chromatography–electrospray ionization mass spectrometry. Talanta. 2011;85:897–905. doi: 10.1016/j.talanta.2011.04.073. [DOI] [PubMed] [Google Scholar]
- Urbanová T, Tarkowská D, Novák O, Hedden P, Strnad M. Analysis of gibberellins as free acids by ultra performance liquid chromatography-tandem mass spectrometry. Talanta. 2013;112:85–94. doi: 10.1016/j.talanta.2013.03.068. [DOI] [PubMed] [Google Scholar]
- Wang SC, Li GJ, Xia K, Xu LL, Chen PY, Zhou X. Preparation and application of monoclonal antibodies specific for salicylic acid. Acta Bot. Sin. 2001;43:1207–1210. [Google Scholar]
- Wells DM, Laplaze L, Bennett MJ, Vernoux T. Biosensors for phytohormone quantification: challenges, solutions, and opportunities. Trends Plant Sci. 2013;18:244–249. doi: 10.1016/j.tplants.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Yan H, Wang F, Han D, Yang G. Simultaneous determination of four plant hormones in bananas by molecularly imprinted solid-phase extraction coupled with high performance liquid chromatography. Analyst. 2012;137:2884–2890. doi: 10.1039/c2an35362h. [DOI] [PubMed] [Google Scholar]
- Zhang L, Guan HL, He ZK. Determination of abscisic acid based on the fluorescent quenching of quantum dots. Sci. China Chem. 2010;53:245–249. doi: 10.1007/s11426-010-0001-7. [DOI] [Google Scholar]