Abstract
Determinations of 66 pesticide residues in different matrices including beef, pork, chicken, eggs, and milk were conducted using GC–MS/MS combined with the quick easy cheap effective rugged safe (QuEChERS) method for sample extraction. A high linearity was achieved in the concentration range from 2.5 to 1000 µg/L (R2 ≥ 0.99), and the limit of quantification for multi-class pesticides ranged from 0.74 to 23.1 µg/kg. The recovery ranged from 70.0 to 120%, while the reproducibility of the measurements was between 0.23 and 19.9%. Monitoring was conducted for livestock products purchased from local markets. Chlorpyrifos and fenitrothion in beef and chlorpyrifos in pork were detected below the maximum residue limits for the respective samples. No detectable residues were found in the other samples. Due to its high efficiency, reproducibility, and simple analytical operation, the proposed method can be applied to the regular monitoring of multi-residue pesticides in livestock products.
Keywords: Pesticide, Meat, Eggs, Milk, QuEChERS, GC–MS/MS
Introduction
The use of pesticides in modern agriculture has played a major role in boosting agricultural productivity; however, their misuse is a growing problem. Such misuse, including the application of pesticides to livestock and to improve the farm environment, can lead to the transfer of pesticide residues to livestock products (LeDoux, 2011). As such, pesticides are ubiquitous in the environment and are commonly found in livestock products, leading to their incorporation into the food chain. For example, in 2017 in Korea, the metabolites of fipronil, a pesticide used to control mites, were found to have contaminated chickens and eggs, resulting in the death of a cow that consumed contaminated rice straw feed (Ko et al., 2015; Rahman et al., 2016). The pathways by which livestock are exposed to pesticides include skin absorption, inhalation, and ingestion. The absorbed substances can then be metabolized or accumulate within the body and subsequently serve as an interim host for consumption by other animals (Covaci et al., 2004; Pagliuca et al., 2005). Due to such issues, the appropriate management of pesticide residues is crucial. Many countries therefore operate inspection programs to manage the pesticide residues present in livestock products, with examples including the National Residue Survey of the Australian Ministry of Agriculture and Fisheries, the National Chemical Residues Programme of the New Zealand Food Safety Agency, the National Chemical Residue Monitoring Program of the Canadian Food Inspection Agency, and the National Residue Program of the US Department of Agriculture (USDA, 2019). In addition, Korea has a pesticide monitoring and management system through the National Residue Program for domestic and imported products. Since the maximum residue limits (MRLs) were established for 16 pesticides in livestock products in 1995, new standard limits have been continuously added by 2019 to become 99 pesticides (MFDS, 2019).
Currently, the majority of organochlorine pesticides are banned, but they are still found in the environment (Surma et al., 2014). For example, aldrin and dieldrin are known as persistent organic pollutants, as categorized by the UN Environment Programme. Although organophosphorus pesticides are degradable, they tend to be used in large quantities, and so continuous monitoring is required, and method development based on the use of mass spectrometry (MS) is necessary for the determination of low concentration multi-residue pesticides in livestock products. Indeed, the development and verification of analytical methods for pesticide residues in various foodstuffs are essential to ensure food safety. Such methods involve the monitoring of pesticide residues in livestock products according to changes in the pesticide residual acceptance criteria for different livestock products (Oh et al., 2009). Continued improvements to analytical methods are also required as the number of pesticides increases and the MRLs decrease. To date, pesticide residues have typically been analyzed by multi-component gas chromatography (GC) (Molina-Ruiz et al., 2015; Yang et al., 2011) and liquid chromatography (LC) (da Costa Morais et al., 2018; Stachniuk and Fornal, 2016), with various MS techniques also enabling the simultaneous analysis of hundreds of pesticides (Facco et al., 2015; He et al., 2015; Huang et al., 2010; Hunter et al., 2010; Samadi et al., 2012; Zhang et al., 2013). Although liquid–liquid extraction and solid-phase extraction are the most commonly used processes in terms of sample preparation for pesticide analysis, these processes are time-consuming, and the high volumes of solvent required can be detrimental to the environment (Bidari et al., 2011; Cho et al., 2008). Thus, the quick, easy, cheap, effective, rugged, and safe (QuEChERS) method is a popular alternative for the pre-treatment of pesticide analytical samples and veterinary drugs (Anastassiades et al., 2003; Qin et al., 2016; Xu et al., 2019). We therefore consider that the QuEChERS method could be applied to the analysis of pesticides in livestock products, especially for different matrices, such as meat, eggs, and milk. Several studies have been conducted to analyze pesticides in livestock products (Hercegová et al., 2007; Juhler, 1997; Rimkus et al., 1996). However, there are still complex steps and difficulties in extracting the fat compartment. In this study, we investigated methods of simultaneous analysis of multi-component pesticides in livestock products that contain a large amount of fat and a high level of interference such as proteins. Thus, we herein report the simultaneous analysis of 66 pesticides, including 24 insecticides, 15 fungicides, and 27 acaricides, in livestock products through the development of a combined QuEChERS and GC–MS/MS method to achieve simple and effective quantitation within the MRL criteria.
Materials and methods
Chemicals and reagents
Standards of the 66 pesticides were purchased from AccuStandard (New Haven, CT, USA), Chem Service (West Chester, PA, USA), Dr. Ehrenstorfer (Augsburg, Germany), Sigma-Aldrich (St. Louise, MO, USA), Toronto Research Chemicals (North York, ON, Canada), and Wako Pure Chemical Industries (Osaka, Japan). Acetonitrile, hexane, and acetone were purchased from Burdick & Jackson (Muskegon, MI, USA). All reagents were either of analytical or HPLC grade. Standard solutions were prepared by dilution of the stock solutions with acetone, and stored in amber bottles at 4 °C. Ultrapure water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA; resistivity ≥ 18.2 MΩ cm at 25 °C). A HyperSep™ florisil solid phase extraction (SPE) cartridge (6 mL, 1 g, ThermoFisher Scientific, Waltham, MA, USA) was used for sample purification.
GC–MS/MS analysis
GC–MS/MS (TSQ EVO 8000, Thermo Scientific, Waltham, MA, USA) was used for analysis of the pesticides. Chromatographic separation was carried out using an HP-5MS capillary column (30 m × 0.25 mm ID, 0.25 μm film thickness). The GC oven temperature was programmed as follows: initial temperature of 70 °C, hold at 70 °C for 2 min, increase the temperature to 300 °C at a rate of 20 °C/min, hold at 300 °C for 8 min. The injection port temperature was set at 150 °C, and helium was used as the carrier gas at a flow rate of 1.0 mL/min. Splitless injection was used for trace analysis of the residual pesticides. For the MS measurements, the ion source and interface temperatures were 300 and 280 °C, respectively, and the electron impact voltage was 70 eV. MS was performed by measuring the retention time of the total ion chromatogram obtained in full scan mode (m/z 50–500) then selecting the retention times, precursor ions, and product ions of the 66 pesticides (Table 1). To increase the selectivity, two product ions were selected. Collision energy values were obtained and multiple reaction monitoring conditions were set.
Table 1.
Optimum conditions for multiple reaction monitoring of GC–MS/MS analysis
| Pesticide | Retention time (min) | Precursor ion (m/z) | Product ion (m/z) | Collision energy (eV) |
|---|---|---|---|---|
| Dichlorvos | 6.70 | 109/185/185 | 79/93/109 | 6/12/16 |
| Methacrifos | 8.45 | 125/208/180 | 79/93/165 | 8/14/6 |
| Diphenyl0amine | 9.23 | 168/169/167 | 167/168/166 | 14/12/18 |
| Phorate | 9.65 | 121/231/260 | 65/129/75 | 10/22/7 |
| Dimethoate | 9.85 | 87/93/125 | 86/63/79 | 6/8/8 |
| BHC-gamma | 10.09 | 181/183/217 | 145/147/181 | 14/12/8 |
| Terbufos | 10.11 | 153/231/231 | 97/129/175 | 10/22/12 |
| Quintozene | 10.18 | 142/212/237 | 107/177/119 | 24/12/20 |
| Diazinon | 10.19 | 137/179/304 | 84/137/179 | 12/16/8 |
| Disulfoton | 10.29 | 88/153/142 | 60/97/81 | 6/10/12 |
| Etrimfos | 10.36 | 181/292/292 | 153/181/153 | 8/6/18 |
| Primicarb | 10.47 | 238/166/166 | 166/86/71 | 8/14/24 |
| Pentachloraniline | 10.60 | 263/265/230 | 192/194/195 | 18/18/10 |
| Chlorpyrifos-methyl | 10.74 | 125/286/286 | 79/93/271 | 6/20/12 |
| Vinclozolin | 10.72 | 212/200/214 | 172/147/174 | 12/14/22 |
| Heptachlor | 10.87 | 272/270/235 | 237/235/141 | 12/12/24 |
| Primiphos methyl | 10.99 | 233/290/276 | 151/125/244 | 8/20/8 |
| Fenitrothion | 11.00 | 277/277/260 | 260/109/125 | 6/16/12 |
| Pentachlorothioanisole | 11.10 | 296/244/294 | 263/174/261 | 12/28/12 |
| Fenthion | 11.19 | 278/245/279 | 156/97/81 | 18/12/16 |
| Chlorpyrifos | 11.21 | 197/286/314 | 169/258/258 | 12/8/12 |
| Aldrin | 11.23 | 257/261/263 | 222/191/193 | 12/30/30 |
| Triadimefon | 11.25 | 208/181/208 | 111/127/127 | 20/6/14 |
| Penconazole | 11.58 | 159/248/186 | 123/206/115 | 18/12/30 |
| Isofenphos | 11.61 | 213/255/255 | 121/121/185 | 14/22/10 |
| Chlorfenvinphos | 11.62 | 267/323/295 | 159/267/267 | 14/12/8 |
| Mecarbam | 11.63 | 159/160/131 | 131/132/86 | 6/8/12 |
| Oxychlordane | 11.64 | 149/115/115 | 85/51/87 | 8/22/10 |
| Triadimenol | 11.66 | 168/128/128 | 70/65/100 | 8/18/12 |
| Phenthoate | 11.66 | 274/246/125 | 121/121/79 | 10/6/8 |
| Heptachlor epoxide | 11.67 | 217/217/353 | 147/182/263 | 28/18/12 |
| Methidathion | 11.82 | 145/85/93 | 85/58/63 | 6/6/8 |
| Chinomethionat | 11.85 | 234/206/116 | 206/148/89 | 8/12/12 |
| Chlordane-trans | 11.86 | 375/373/371 | 266/266/264 | 18/20/20 |
| Endosulfan alpha | 11.99 | 241/195/243 | 206/159/208 | 10/6/10 |
| Chlordane-cis | 12.01 | 264/373/264 | 194/264/229 | 34/18/22 |
| Profenfos | 12.10 | 139/337/339 | 97/267/269 | 6/12/12 |
| p,p′-DDE | 12.15 | 246/248/316 | 176/176/246 | 28/28/18 |
| Myclobutanil | 12.20 | 179/150/152 | 125/123/125 | 14/16/8 |
| Kresoxim methyl | 12.21 | 116/206/206 | 89/116/131 | 14/6/10 |
| Flusilazole | 12.22 | 233/206/206 | 165/151/137 | 16/14/18 |
| Dieldrin | 12.24 | 261/263/271 | 191/193/241 | 30/30/8 |
| Endrin | 12.46 | 261/245/263 | 191/173/193 | 28/24/28 |
| Fensulfothion | 12.49 | 292/293/140 | 156/97/81 | 15/20/25 |
| Endosulfan beta | 12.54 | 241/195/195 | 206/159/125 | 12/8/22 |
| p,p′-DDD | 12.55 | 235/200/199 | 165/165/163 | 22/10/30 |
| Ethion | 12.57 | 125/153/231 | 97/97/129 | 6/10/22 |
| o,p-DDT | 12.65 | 235/237/235 | 165/165/199 | 22/22/12 |
| Trizaofos | 12.69 | 161/161/257 | 134/106/162 | 8/12/6 |
| Edifenphos | 12.87 | 173/201/201 | 109/109/173 | 8/14/6 |
| Propiconazole | 12.90 | 173/259/259 | 145/173/69 | 4/14/10 |
| Endosulfan sulfate | 12.94 | 272/195/195 | 237/159/125 | 12/8/22 |
| p,p′-DDT | 12.94 | 235/165/199 | 165/164/163 | 20/24/28 |
| Propagite | 13.03 | 135/201/173 | 107/81/107 | 12/8/22 |
| Bifenthrin | 13.32 | 181/166/182 | 165/165/166 | 24/14/24 |
| Phosmet | 13.38 | 160/160/133 | 77/133/77 | 22/10/12 |
| Fenpropathrin | 13.41 | 181/265/125 | 152/210/97 | 22/8/6 |
| keto Endrin | 13.41 | 243/317/317 | 173/281/245 | 24/8/14 |
| Phosalone | 13.72 | 182/182/367 | 111/75/182 | 14/28/6 |
| Pyriproxyfen | 13.72 | 136/136/226 | 79/96/186 | 20/10/12 |
| Fenarimol | 14.00 | 139/219/251 | 111/107/139 | 14/10/12 |
| Permethrin | 14.34 | 183/183/163 | 168/153/91 | 10/10/12 |
| Prochloraz | 14.50 | 180/310/308 | 138/70/70 | 10/12/12 |
| Fenbuconazole | 14.70 | 129/198/125 | 102/129/89 | 14/8/14 |
| Cypermethrin | 14.95 | 127/181/163 | 91/152/91 | 8/22/12 |
| Fenvalerate | 15.70 | 167/125/225 | 125/89/119 | 8/18/16 |
Sample preparation
The homogenized sample (10 g) was transferred to a shaking bottle, a solution of acetonitrile containing 1% formic acid (50 mL) was added, and the resulting mixture was shaken for 30 min. After this time, anhydrous magnesium sulfate (4 g) and sodium chloride (1 g, to increase the ionic strength and distribution efficiency) were added, and the mixture was shaken for 10 min prior to centrifugation for 10 min at 4000 rpm. The supernatant (25 mL) was then added to acetone containing 2% diethylene glycol (0.2 mL) and the solvent was evaporated to dryness. The resulting extract was redissolved in a mixture of acetone/hexane (2:8, v/v, 4 mL), which was subsequently loaded onto an SPE-florisil cartridge that was activated with hexane (5 mL) and acetone/hexane (2:8, v/v, 5 mL) for sample purification. It should be noted that florisil cartridges are the most widely used adsorbents for pigments, maintenance, and removal, and are often used to remove the fats and residues present in livestock products (Chae et al., 2013). The extract was then filtered through the cartridge with the addition of acetonitrile/hexane (2:8, v/v, 5 mL). The obtained eluent was concentrated to dryness under a flow of nitrogen gas, and then redissolved in acetonitrile/hexane (2:8, v/v, 1 mL) for GC–MS/MS analysis.
Method validation
To verify the applicability of the developed method for the target pesticides, representative samples of beef, pork, chicken, eggs, and milk were used to determine the recovery rate. Five replicates were prepared by adding the standard solution mixture to each of the five representative food samples at three concentrations, 5, 10, and 100 µg/kg. Validation was determined by measurement of the linearity, limit of detection (LOD), limit of quantification (LOQ), recovery, and reproducibility, according to the standard procedure for the preparation of test methods, the Codex Alimentarius Guideline, CAC/GL 40 (2003). The method selectivity was confirmed using the total ion peak areas of the pesticide standards in the blank solution and in each matrix. The linearity was calculated using the correlation coefficient (R2) of the calibration curves obtained using pesticide concentrations of 10, 20, 50, 100, 500, and 1000 µg/kg. The LOD and LOQ values were determined as 3 and 10 times the signal-to-noise ratio (S/N), respectively. The accuracy (recovery measurement) was calculated at three different pesticide concentrations (5, 10, and 100 µg/kg) for the five products (beef, pork, chicken, eggs, and milk) as the representative matrices. The precision was assessed using the relative standard deviation (RSD) of the recovery.
Results and discussion
Verification of the GC–MS/MS conditions
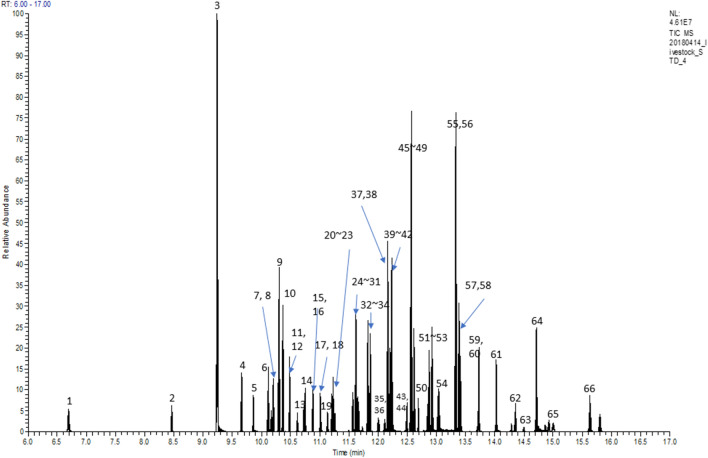
The GC–MS/MS conditions for determination of the pesticide residues present in the various matrices were optimized using an HP5MS separation column. The total ion chromatogram of the 66 pesticides is shown in Fig. 1, where the concentrations of the standard pesticides in acetone were 100 μg/mL, and the various peaks were observed between 6 and 16 min. The peak numbers and corresponding retention times are presented in Table 1, along with the precursor ions (m/z), product ions (m/z), and collision energies of the quantification and confirmatory transitions for the 66 pesticides.
Fig. 1.
Total ion chromatogram of 66 pesticides. The names of pesticides for the numbers indicated on the peaks are listed in Table 1
Specificity, LOD, and LOQ
To verify the specificity of the developed technique, the retention times and selected ions were confirmed. In terms of the pesticide retention times, only the selected precursor and product ions were detected in the samples to which the standard, blank, and mixed standard solutions were added. The lowest LODs were obtained as 0.4 µg/kg for beef and pork, and 0.2 µg/kg for chicken, eggs, and milk. The lowest LOQ was obtained as 0.74 µg/kg for the bifenthrin content in milk, and the highest LOQ was obtained as 23.1 µg/kg for the heptachlor epoxide content in pork (Table 2). Manav et al. (2019) reported LODs for permethrin in milk and endosulfan sulfate in dairy products of 0.40 and 0.48 µg/kg, respectively using GC–MS and the QuEChERS method, which are similar to those determined in this study. In contrast, some studies have reported relatively high LODs (5.2–14 µg/kg) and LOQs (1.5–44 µg/kg) (Hamadamin and Hassan, 2020) and relatively low LOQs (less than 5 ng/g) (Sapozhnikova, 2018) compared with this study. As demonstrated by several studies, multi-class pesticide analysis using the QuEChERS method and MS is a simple, excellent approach, with low LODs and LOQs and high recoveries.
Table 2.
Linearity, limit of detection (LOD) and limit of quantification (LOQ) in various matrices for 66 pesticides
| Pesticide | Linearity (R2) | LOD (μg/kg) | LOQ (μg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beef | Pork | Chicken | Eggs | Milk | Beef | Pork | Chicken | Eggs | Milk | ||
| Aldrin | 0.9986 | 1.91 | 2.31 | 1.04 | 0.79 | 1.63 | 6.30 | 7.61 | 3.43 | 2.62 | 5.39 |
| BHC-gamma | 0.9989 | 1.42 | 1.14 | 0.52 | 0.85 | 0.38 | 4.70 | 3.75 | 1.71 | 2.80 | 1.25 |
| Bifenthrin | 0.9999 | 0.35 | 0.78 | 0.37 | 0.43 | 0.22 | 1.16 | 2.58 | 1.23 | 1.41 | 0.74 |
| Chinomethionat | 0.9999 | 2.29 | 0.87 | 0.51 | 0.43 | 0.87 | 7.55 | 2.88 | 1.68 | 1.43 | 2.87 |
| Chlordane-cis | 0.9982 | 2.15 | 5.01 | 2.82 | 2.31 | 2.60 | 7.08 | 16.5 | 9.32 | 7.62 | 8.56 |
| Chlordane-trans | 0.9986 | 1.89 | 1.46 | 0.60 | 0.81 | 1.20 | 6.22 | 4.81 | 1.97 | 2.69 | 3.96 |
| Chlorfenvinphos | 0.9992 | 3.68 | 1.30 | 0.57 | 0.68 | 0.94 | 12.1 | 4.29 | 1.89 | 2.25 | 3.09 |
| Chlorpyrifos | 0.9996 | 2.99 | 2.44 | 0.80 | 0.81 | 0.66 | 9.88 | 8.05 | 2.64 | 2.69 | 2.16 |
| Chlorpyrifos-methyl | 0.9997 | 3.92 | 5.07 | 0.88 | 1.27 | 2.31 | 12.9 | 16.7 | 2.91 | 4.20 | 7.62 |
| Cypermethrin | 0.9968 | 1.01 | 2.18 | 1.10 | 0.78 | 1.89 | 3.33 | 7.20 | 3.64 | 2.58 | 6.24 |
| p,p′-DDD | 0.9999 | 0.87 | 0.67 | 0.23 | 0.48 | 0.40 | 2.85 | 2.20 | 0.75 | 1.60 | 1.33 |
| p,p′-DDE | 1.0000 | 0.94 | 1.08 | 0.64 | 0.61 | 0.89 | 3.09 | 3.55 | 2.13 | 2.02 | 2.94 |
| o,p-DDT | 0.9978 | 0.79 | 1.80 | 0.60 | 0.84 | 0.91 | 2.60 | 5.95 | 1.97 | 2.77 | 2.99 |
| p,p′-DDT | 0.9973 | 1.67 | 0.76 | 0.73 | 0.59 | 0.34 | 5.50 | 2.50 | 2.42 | 1.93 | 1.13 |
| Diazinon | 0.9998 | 0.56 | 1.51 | 5.57 | 0.42 | 1.08 | 1.85 | 4.97 | 18.4 | 1.38 | 3.55 |
| Dichlorvos | 0.9999 | 0.77 | 1.87 | 1.16 | 0.86 | 1.90 | 2.55 | 6.16 | 3.81 | 2.85 | 6.25 |
| Dieldrin | 0.9976 | 3.36 | 4.34 | 2.41 | 1.38 | 2.77 | 11.1 | 14.3 | 7.95 | 4.55 | 9.14 |
| Dimethoate | 0.9989 | 2.56 | 2.77 | 1.09 | 0.62 | 0.52 | 8.45 | 9.14 | 3.61 | 2.05 | 1.71 |
| Diphenylamine | 0.9999 | 0.96 | 0.36 | 0.29 | 0.30 | 0.33 | 3.15 | 1.20 | 0.96 | 1.00 | 1.10 |
| Disulfoton | 0.9998 | 0.49 | 0.85 | 0.60 | 0.35 | 0.68 | 1.63 | 2.81 | 1.98 | 1.15 | 2.25 |
| Edifenphos | 0.9996 | 0.73 | 1.33 | 0.69 | 0.65 | 0.52 | 2.40 | 4.39 | 2.29 | 2.15 | 1.71 |
| Endosulfan alpha | 0.9951 | 2.13 | 2.02 | 2.12 | 1.80 | 0.86 | 7.04 | 6.67 | 7.00 | 5.92 | 2.84 |
| Endosulfan beta | 0.9969 | 1.91 | 3.49 | 2.57 | 2.55 | 3.11 | 6.31 | 11.5 | 8.47 | 8.42 | 10.25 |
| Endosulfan sulfate | 0.9991 | 5.77 | 3.88 | 1.01 | 4.17 | 0.89 | 19.0 | 12.8 | 3.33 | 13.8 | 2.95 |
| Endrin | 0.9995 | 2.42 | 3.38 | 1.05 | 0.75 | 2.91 | 8.00 | 11.1 | 3.45 | 2.49 | 9.60 |
| Ethion | 0.9851 | 1.22 | 4.29 | 2.43 | 0.56 | 3.08 | 4.01 | 14.1 | 8.01 | 1.83 | 10.2 |
| Etrimfos | 0.9998 | 0.57 | 1.26 | 0.43 | 0.33 | 0.80 | 1.89 | 4.16 | 1.43 | 1.09 | 2.62 |
| Fenarimol | 0.9997 | 0.23 | 0.60 | 0.43 | 0.53 | 0.88 | 0.75 | 1.99 | 1.43 | 1.74 | 2.91 |
| Fenbuconazole | 0.9999 | 0.30 | 0.55 | 0.34 | 0.24 | 0.27 | 0.98 | 1.82 | 1.12 | 0.78 | 0.88 |
| Fenitrothion | 0.9998 | 2.27 | 1.42 | 0.69 | 1.31 | 0.37 | 7.50 | 4.69 | 2.28 | 4.33 | 1.22 |
| Fenpropathrin | 0.9999 | 2.91 | 1.02 | 0.96 | 0.63 | 0.76 | 9.62 | 3.37 | 3.17 | 2.08 | 2.52 |
| Fensulfothion | 0.9944 | 3.45 | 4.99 | 2.58 | 2.21 | 2.48 | 11.4 | 16.5 | 8.50 | 7.28 | 8.20 |
| Fenthion | 0.9989 | 1.39 | 1.23 | 0.73 | 0.56 | 1.02 | 4.59 | 4.07 | 2.41 | 1.86 | 3.38 |
| Fenvalerate | 0.9998 | 0.93 | 4.43 | 0.37 | 0.90 | 2.32 | 3.08 | 14.6 | 1.23 | 2.96 | 7.65 |
| Flusilazole | 0.9996 | 0.88 | 0.57 | 0.79 | 0.33 | 0.85 | 2.90 | 1.89 | 2.62 | 1.10 | 2.80 |
| Heptachlor | 0.9995 | 1.96 | 2.02 | 0.57 | 1.07 | 1.20 | 6.46 | 6.68 | 1.88 | 3.51 | 3.96 |
| Heptachlor epoxide | 0.9974 | 1.93 | 5.82 | 3.10 | 1.21 | 1.11 | 6.37 | 19.2 | 10.2 | 3.99 | 3.69 |
| Isofenphos | 0.9994 | 0.80 | 1.27 | 0.76 | 0.70 | 0.51 | 2.64 | 4.18 | 2.52 | 2.32 | 1.67 |
| keto Endrin | 0.9984 | 3.25 | 3.09 | 1.17 | 1.18 | 1.34 | 10.7 | 10.2 | 3.87 | 3.90 | 4.43 |
| Kresoxim methyl | 0.9997 | 1.20 | 0.87 | 0.38 | 0.69 | 0.59 | 3.95 | 2.86 | 1.26 | 2.28 | 1.94 |
| Mecarbam | 0.9988 | 2.25 | 3.44 | 4.34 | 0.71 | 1.78 | 7.42 | 11.4 | 14.3 | 2.35 | 5.89 |
| Methacrifos | 1.0000 | 0.96 | 2.53 | 1.16 | 0.96 | 1.34 | 3.17 | 8.35 | 3.81 | 3.15 | 4.43 |
| Methidathion | 0.9978 | 1.89 | 1.32 | 0.69 | 0.58 | 0.79 | 6.22 | 4.37 | 2.29 | 1.91 | 2.60 |
| Myclobutanil | 0.9998 | 1.10 | 0.68 | 0.65 | 0.46 | 1.02 | 3.63 | 2.23 | 2.14 | 1.53 | 3.35 |
| Oxychlordane | 0.9994 | 0.96 | 1.72 | 1.55 | 1.08 | 1.73 | 3.18 | 5.66 | 5.10 | 3.56 | 5.71 |
| Penconazole | 0.9999 | 0.77 | 2.53 | 0.66 | 0.76 | 1.42 | 2.53 | 8.36 | 2.17 | 2.50 | 4.69 |
| Pentachloraniline | 0.9996 | 0.72 | 1.16 | 1.03 | 0.67 | 1.64 | 2.37 | 3.83 | 3.41 | 2.21 | 5.43 |
| Pentachlorothioanisole | 0.9989 | 3.12 | 2.57 | 0.84 | 1.65 | 1.66 | 10.3 | 8.48 | 2.78 | 5.45 | 5.49 |
| Permethrin | 0.9999 | 1.88 | 1.98 | 2.30 | 2.31 | 0.63 | 6.20 | 6.53 | 7.58 | 7.62 | 2.08 |
| Phenthoate | 0.9980 | 1.10 | 0.94 | 1.05 | 0.53 | 0.74 | 3.64 | 3.11 | 3.46 | 1.74 | 2.46 |
| Phorate | 0.9998 | 0.86 | 0.46 | 0.39 | 0.44 | 0.95 | 2.83 | 1.53 | 1.28 | 1.46 | 3.14 |
| Phosalone | 0.9998 | 1.10 | 0.85 | 1.55 | 0.56 | 1.19 | 3.61 | 2.81 | 5.10 | 1.83 | 3.92 |
| Phosmet | 0.9999 | 3.48 | 1.62 | 0.70 | 0.45 | 0.49 | 11.5 | 5.36 | 2.29 | 1.47 | 1.61 |
| Primicarb | 0.9985 | 0.37 | 0.65 | 0.46 | 0.19 | 0.41 | 1.23 | 2.13 | 1.52 | 0.62 | 1.35 |
| Primiphos methyl | 0.9988 | 4.61 | 1.66 | 1.99 | 0.87 | 1.15 | 15.2 | 5.48 | 6.56 | 2.85 | 3.78 |
| Prochloraz | 0.9992 | 1.17 | 1.77 | 1.30 | 3.03 | 0.48 | 3.87 | 5.83 | 4.28 | 10.01 | 1.59 |
| Profenphos | 0.9926 | 0.68 | 2.20 | 2.68 | 1.38 | 0.62 | 2.25 | 7.26 | 8.8 | 4.57 | 2.03 |
| Propagite | 0.9972 | 2.14 | 3.20 | 3.21 | 2.33 | 4.91 | 7.05 | 10.6 | 10.6 | 7.70 | 16.2 |
| Propiconazole | 0.9959 | 3.89 | 7.01 | 1.78 | 2.19 | 0.79 | 12.9 | 23.1 | 5.87 | 7.21 | 2.60 |
| Pyriproxyfen | 0.9993 | 0.82 | 1.42 | 2.94 | 0.88 | 0.95 | 2.70 | 4.69 | 9.70 | 2.89 | 3.13 |
| Quintozene | 0.9979 | 3.25 | 3.87 | 2.16 | 1.02 | 1.08 | 10.7 | 12.8 | 7.11 | 3.36 | 3.57 |
| Terbufos | 0.9995 | 2.87 | 1.73 | 4.82 | 5.65 | 3.24 | 9.4 | 5.70 | 15.9 | 18.7 | 10.7 |
| Triadimefon | 0.9976 | 0.68 | 1.95 | 1.28 | 0.58 | 0.93 | 2.26 | 6.43 | 4.24 | 1.92 | 3.05 |
| Triadimenol | 0.9959 | 3.34 | 2.13 | 2.46 | 2.37 | 2.45 | 11.0 | 7.03 | 8.12 | 7.81 | 8.09 |
| Trizaofos | 0.9982 | 1.54 | 3.52 | 0.69 | 0.55 | 1.11 | 5.07 | 11.6 | 2.29 | 1.83 | 3.66 |
| Vinclozolin | 0.9982 | 0.91 | 1.26 | 0.95 | 1.07 | 1.19 | 3.00 | 4.15 | 3.14 | 3.54 | 3.94 |
Linearity
The matrix-matched calibration curve was used to reduce the matrix effect of the method. During substance analysis, the intensity of the instrumental response should show a linearity that is quantitatively proportional to the amount of residue in the sample. This linearity can be confirmed using an internal standard material or by the addition of a standard to the matrix. In this study, the linearity of the calibration curve obtained by GC–MS/MS was observed at concentrations of 2.5, 10, 50, 100, 500, and 1000 µg/L with 5 replicates. Chromatograms of the pesticides in each time period contain the MS information of each ion; thus, a calibration curve can be produced using the MS intensity at each concentration. The correlation coefficients (R2) for the 66 substances reached 0.99–1.0 (Table 2), indicating a satisfactory agreement with the level (R2 > 0.99) recommended by the International Commission on Food Standards (CAC/GL 40, 2003). These results indicate that the proposed method is suitable for the calculation of residual amounts of the examined pesticides in the samples of interest and over the concentration range employed herein.
Accuracy and precision
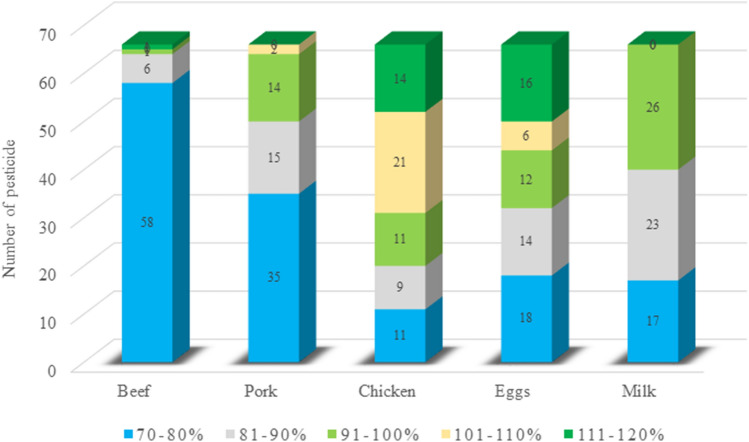
To verify the efficiency and reliability of the proposed analytical method, the recovery was used to determine the accuracy, while the RSD was used to obtain the precision. The standard pesticide solutions were added to each matrix at concentrations of 5, 10, and 100 µg/kg for five replicates to determine recovery and RSD. A high-concentration standard (100 µg/kg) was included to validate the method for pesticides with high Korean MRLs in livestock products such as endrin (1.0 mg/kg for poultry meat, 0.1 mg/kg for pig muscle), DDT (0.3 mg/kg for poultry meat, 0.1 mg/kg for eggs), chlorfenvinphos (0.2 mg/kg for cattle meat), and permethrin (0.1 mg/kg for milk). The results presented in Table 3 indicate that the pesticides showed similar tendencies for all samples, and the various pesticides were successfully recovered in all cases, likely due to the inclusion of 1% formic acid in the acetonitrile extraction solvent, i.e., the recovery was improved by the auxiliary role of the acid (AOAC, 2010; Codex, 2003; USFDA, 1999). In addition, a previous study showed that the recovery increased upon increasing the concentration from 5 to 10 µg/kg and then to 100 µg/kg due to a smaller matrix effect (Mastovska et al., 2005); however, no significant differences were observed herein when different concentrations were employed. The recoveries ranged from 70.1 to 118% for beef, 70.1 to 116% for pork, 70.0 to 120% for chicken, 70.1 to 120% for eggs, and 70.1 to 105% for milk, giving an overall recovery range of 70.0–120%. Figure 2 shows the distribution of the average recovery ranges of the pesticides for each product. The main recovery distribution was located differently for each product. Of the tested pesticides, 88% had recoveries of 70–80% for beef and 35% had recoveries of 81–90% for pork. The highest recovery rates were 91–100% for chicken, 101–110% for eggs, and 111–120% for milk, but the distribution rates were not significantly different.
Table 3.
Recovery and relative standard deviation (RSD) at three concentrations of pesticides in livestock products
| Pesticide | Fortified Concentration (mg/kg) | Beef | Pork | Chicken | Eggs | Milk | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||
| Aldrin | 0.005 | 71.6 | 10.1 | 70.8 | 8.79 | 76.7 | 13.2 | 87.6 | 10.0 | 85.7 | 11.4 |
| 0.01 | 72.3 | 3.73 | 70.9 | 6.57 | 85.5 | 6.65 | 85.6 | 16.3 | 95.5 | 8.05 | |
| 0.1 | 70.4 | 6.42 | 70.1 | 4.45 | 90.2 | 12.3 | 90.0 | 11.7 | 93.7 | 2.93 | |
| BHC-gamma | 0.005 | 72.7 | 16.7 | 70.6 | 6.99 | 75.4 | 15.9 | 95.1 | 1.26 | 92.2 | 8.69 |
| 0.01 | 72.0 | 3.90 | 70.5 | 6.30 | 77.8 | 9.09 | 85.0 | 11.0 | 88.7 | 4.31 | |
| 0.1 | 72.5 | 9.80 | 74.4 | 6.64 | 73.2 | 18.6 | 90.0 | 8.45 | 88.9 | 3.98 | |
| Bifenthrin | 0.005 | 75.1 | 8.26 | 82.3 | 4.83 | 89.4 | 4.96 | 116 | 4.68 | 90.6 | 5.76 |
| 0.01 | 79.9 | 5.88 | 76.2 | 6.57 | 90.0 | 3.90 | 111 | 13.1 | 86.1 | 3.81 | |
| 0.1 | 75.8 | 6.87 | 85.9 | 3.60 | 116 | 3.08 | 120 | 10.7 | 84.2 | 2.99 | |
| Chinomethionat | 0.005 | 71.2 | 8.44 | 72.0 | 10.0 | 103 | 7.86 | 73.0 | 3.61 | 95.1 | 9.34 |
| 0.01 | 75.8 | 4.85 | 70.1 | 9.41 | 105 | 6.90 | 71.1 | 6.43 | 95.3 | 3.67 | |
| 0.1 | 74.5 | 7.12 | 74.3 | 12.4 | 114 | 9.62 | 70.6 | 14.7 | 90.9 | 3.67 | |
| Chlordane-cis | 0.005 | 102 | 9.12 | 77.8 | 15.1 | 81.1 | 17.2 | 110 | 12.7 | 100 | 11.7 |
| 0.01 | 92.4 | 5.30 | 73.9 | 12.9 | 86.9 | 12.6 | 97.7 | 15.9 | 97.4 | 3.35 | |
| 0.1 | 71.4 | 6.16 | 74.3 | 17.1 | 105 | 7.18 | 77.1 | 19.5 | 94.7 | 5.90 | |
| Chlordane-trans | 0.005 | 74.6 | 10.8 | 78.2 | 14.1 | 85.9 | 14.2 | 74.9 | 2.43 | 73.3 | 12.3 |
| 0.01 | 72.5 | 5.31 | 77.3 | 12.1 | 89.2 | 9.88 | 71.5 | 11.4 | 76.9 | 12.2 | |
| 0.1 | 70.5 | 10.0 | 83.2 | 7.13 | 116 | 8.81 | 71.0 | 11.9 | 91.1 | 8.42 | |
| Chlorfenvinphos | 0.005 | 76.6 | 12.7 | 83.4 | 6.44 | 85.3 | 9.43 | 78.4 | 13.7 | 92.9 | 7.21 |
| 0.01 | 81.0 | 5.37 | 74.2 | 6.73 | 85.9 | 4.65 | 84.3 | 8.16 | 84.2 | 3.65 | |
| 0.1 | 70.6 | 8.56 | 86.8 | 3.78 | 85.4 | 8.56 | 87.5 | 13.3 | 95.9 | 3.10 | |
| Chlorpyrifos | 0.005 | 73.4 | 10.8 | 86.5 | 9.52 | 86.7 | 7.38 | 116 | 6.27 | 95.2 | 9.80 |
| 0.01 | 78.9 | 1.56 | 72.2 | 7.30 | 91.8 | 8.62 | 104 | 12.0 | 95.4 | 4.60 | |
| 0.1 | 74.7 | 19.1 | 81.5 | 2.76 | 103 | 5.07 | 111 | 10.9 | 97.2 | 3.34 | |
| Chlorpyrifos-methyl | 0.005 | 81.4 | 12.8 | 95.3 | 6.84 | 81.9 | 13.2 | 91.7 | 15.2 | 81.9 | 10.6 |
| 0.01 | 92.6 | 5.47 | 80.0 | 4.25 | 84.0 | 4.21 | 92.8 | 16.4 | 87.9 | 8.33 | |
| 0.1 | 75.0 | 9.02 | 97.8 | 2.79 | 86.7 | 14.7 | 99.1 | 8.36 | 95.8 | 1.81 | |
| Cypermethrin | 0.005 | 88.6 | 3.04 | 104 | 8.01 | 86.9 | 5.25 | 109 | 10.4 | 89.7 | 4.64 |
| 0.01 | 93.8 | 4.66 | 93.5 | 7.78 | 90.6 | 3.58 | 119 | 16.2 | 84.6 | 2.38 | |
| 0.1 | 77.9 | 7.29 | 101 | 3.91 | 109 | 4.99 | 120 | 9.76 | 92.2 | 5.20 | |
| p,p′-DDD | 0.005 | 72.7 | 14.3 | 81.2 | 3.91 | 87.3 | 9.25 | 114 | 2.93 | 93.5 | 5.96 |
| 0.01 | 71.4 | 5.03 | 74.7 | 7.19 | 89.2 | 8.52 | 114 | 13.4 | 89.1 | 3.77 | |
| 0.1 | 70.3 | 11.6 | 89.1 | 4.52 | 112 | 5.24 | 118 | 12.1 | 95.2 | 4.31 | |
| p,p′-DDE | 0.005 | 70.5 | 8.81 | 71.8 | 8.35 | 88.9 | 6.90 | 108 | 0.44 | 89.6 | 4.63 |
| 0.01 | 70.3 | 4.25 | 70.9 | 7.94 | 93.0 | 5.77 | 91.7 | 16.1 | 85.5 | 2.70 | |
| 0.1 | 70.2 | 6.96 | 71.1 | 13.9 | 118 | 3.81 | 94.9 | 13.0 | 72.6 | 3.73 | |
| o, p-DDT | 0.005 | 113 | 11.3 | 74.1 | 3.75 | 85.5 | 5.17 | 82.4 | 9.18 | 80.4 | 7.57 |
| 0.01 | 71.3 | 18.1 | 75.7 | 6.71 | 83.8 | 9.56 | 75.8 | 15.8 | 90.1 | 2.73 | |
| 0.1 | 70.2 | 16.3 | 77.1 | 7.66 | 103 | 1.55 | 74.9 | 8.90 | 79.2 | 3.72 | |
| p,p′-DDT | 0.005 | 70.7 | 14.7 | 75.7 | 5.15 | 73.8 | 6.02 | 102 | 19.2 | 74.9 | 5.57 |
| 0.01 | 75.9 | 8.64 | 78.6 | 6.60 | 71.3 | 3.25 | 106 | 4.92 | 70.1 | 5.01 | |
| 0.1 | 72.2 | 13.1 | 75.9 | 5.39 | 89.4 | 8.10 | 88.6 | 12.2 | 72.6 | 3.73 | |
| Diazinon | 0.005 | 72.0 | 12.7 | 71.3 | 7.86 | 72.0 | 12.7 | 99.4 | 3.99 | 71.3 | 15.4 |
| 0.01 | 76.6 | 2.41 | 70.6 | 8.41 | 76.6 | 2.41 | 88.2 | 10.8 | 73.4 | 5.54 | |
| 0.1 | 74.6 | 7.84 | 77.9 | 5.71 | 74.6 | 7.84 | 94.4 | 12.4 | 70.5 | 6.99 | |
| Dichlorvos | 0.005 | 103 | 13.1 | 76.5 | 19.8 | 73.9 | 3.04 | 77.5 | 11.9 | 73.5 | 13.1 |
| 0.01 | 110 | 4.92 | 74.4 | 11.8 | 72.1 | 8.02 | 70.9 | 9.74 | 71.9 | 5.19 | |
| 0.1 | 113 | 5.22 | 77.7 | 14.5 | 70.2 | 3.38 | 71.5 | 19.6 | 70.6 | 7.90 | |
| Dieldrin | 0.005 | 77.9 | 15.2 | 116 | 7.41 | 83.9 | 11.0 | 98.2 | 2.59 | 86.6 | 7.09 |
| 0.01 | 72.4 | 8.69 | 70.9 | 10.8 | 87.6 | 10.6 | 81.7 | 8.80 | 88.1 | 3.72 | |
| 0.1 | 76.2 | 7.99 | 74.8 | 7.28 | 102 | 3.75 | 85.1 | 10.6 | 81.4 | 3.17 | |
| Dimethoate | 0.005 | 79.5 | 15.0 | 87.4 | 8.86 | 90.3 | 7.16 | 91.5 | 15.2 | 76.8 | 8.85 |
| 0.01 | 90.4 | 5.61 | 86.8 | 6.90 | 82.9 | 11.2 | 104 | 13.7 | 70.9 | 6.08 | |
| 0.1 | 81.7 | 11.3 | 104 | 1.86 | 116 | 5.65 | 96.8 | 8.09 | 82.4 | 3.54 | |
| Diphenylamine | 0.005 | 113 | 11.5 | 70.5 | 17.7 | 107 | 13.1 | 84.3 | 3.41 | 80.9 | 11.8 |
| 0.01 | 111 | 3.33 | 112 | 9.94 | 114 | 11.4 | 75.7 | 4.71 | 94.5 | 10.9 | |
| 0.1 | 88.7 | 6.54 | 97.8 | 11.0 | 107 | 5.25 | 72.4 | 6.48 | 93.8 | 9.91 | |
| Disulfoton | 0.005 | 96.7 | 13.1 | 91.9 | 4.07 | 71.4 | 11.4 | 99.5 | 2.16 | 81.0 | 7.38 |
| 0.01 | 102 | 8.19 | 105 | 13.5 | 79.9 | 5.16 | 89.9 | 11.7 | 78.9 | 4.22 | |
| 0.1 | 86.8 | 8.09 | 79.7 | 13.2 | 73.3 | 8.91 | 77.1 | 9.07 | 77.2 | 5.68 | |
| Edifenphos | 0.005 | 73.0 | 15.3 | 84.3 | 8.68 | 75.1 | 4.58 | 77.1 | 9.02 | 75.5 | 6.40 |
| 0.01 | 82.0 | 4.13 | 75.9 | 13.1 | 75.8 | 6.13 | 70.5 | 10.6 | 71.9 | 3.86 | |
| 0.1 | 74.9 | 12.4 | 99.6 | 10.3 | 97.9 | 5.57 | 79.6 | 15.3 | 71.3 | 5.40 | |
| Endosulfan alpha | 0.005 | 70.9 | 15.8 | 77.2 | 18.5 | 91.6 | 9.83 | 102 | 3.44 | 97.8 | 10.5 |
| 0.01 | 72.8 | 12.0 | 72.5 | 12.7 | 97.7 | 13.4 | 98.0 | 12.3 | 96.3 | 3.49 | |
| 0.1 | 71.7 | 14.6 | 75.5 | 14.9 | 107 | 9.28 | 71.0 | 8.24 | 94.3 | 2.34 | |
| Endosulfan beta | 0.005 | 108 | 10.7 | 77.4 | 16.2 | 83.6 | 12.4 | 114 | 2.22 | 95.9 | 12.7 |
| 0.01 | 71.2 | 19.7 | 70.6 | 5.92 | 90.4 | 12.4 | 95.2 | 15.8 | 83.4 | 6.16 | |
| 0.1 | 71.6 | 10.7 | 79.4 | 2.83 | 109 | 2.94 | 114 | 8.18 | 95.5 | 4.54 | |
| Endosulfan sulfate | 0.005 | 74.4 | 12.3 | 84.1 | 6.82 | 71.7 | 4.12 | 73.8 | 8.69 | 99.9 | 10.9 |
| 0.01 | 70.5 | 6.40 | 77.6 | 10.1 | 80.5 | 4.23 | 86.5 | 18.1 | 91.7 | 4.17 | |
| 0.1 | 70.6 | 12.2 | 87.8 | 8.92 | 114 | 6.35 | 84.9 | 9.86 | 92.1 | 2.88 | |
| Endrin | 0.005 | 70.7 | 15.7 | 75.9 | 11.3 | 84.3 | 15.1 | 93.8 | 15.7 | 92.2 | 7.15 |
| 0.01 | 77.0 | 8.13 | 70.8 | 5.34 | 86.7 | 6.96 | 86.5 | 18.1 | 79.9 | 5.68 | |
| 0.1 | 71.7 | 12.9 | 77.0 | 5.69 | 99.2 | 4.27 | 84.9 | 9.86 | 79.5 | 2.71 | |
| Ethion | 0.005 | 89.8 | 11.7 | 74.3 | 19.9 | 76.2 | 10.6 | 115 | 0.23 | 92.9 | 11.5 |
| 0.01 | 78.1 | 11.9 | 71.5 | 17.8 | 77.8 | 12.0 | 110 | 0.52 | 84.1 | 5.41 | |
| 0.1 | 90.0 | 3.44 | 91.2 | 19.1 | 99.3 | 4.18 | 115 | 6.29 | 96.2 | 2.07 | |
| Etrimfos | 0.005 | 70.1 | 11.9 | 76.8 | 5.42 | 70.6 | 17.7 | 97.5 | 0.24 | 76.7 | 8.09 |
| 0.01 | 73.2 | 3.05 | 77.4 | 5.79 | 70.5 | 2.28 | 83.5 | 9.61 | 76.7 | 6.27 | |
| 0.1 | 72.5 | 9.04 | 74.8 | 4.10 | 70.2 | 7.20 | 88.0 | 6.32 | 76.3 | 4.67 | |
| Fenarimol | 0.005 | 75.6 | 10.8 | 85.4 | 7.38 | 80.6 | 14.2 | 111 | 11.9 | 93.4 | 4.09 |
| 0.01 | 84.3 | 6.30 | 81.3 | 6.91 | 88.0 | 2.07 | 111 | 12.0 | 94.9 | 2.10 | |
| 0.1 | 71.6 | 7.77 | 89.4 | 5.12 | 102 | 8.56 | 119 | 10.0 | 96.3 | 3.28 | |
| Fenbuconazole | 0.005 | 84.2 | 9.81 | 88.5 | 7.16 | 82.2 | 14.7 | 119 | 15.4 | 85.3 | 9.05 |
| 0.01 | 90.2 | 6.05 | 86.2 | 6.99 | 82.0 | 2.72 | 119 | 9.62 | 80.2 | 2.77 | |
| 0.1 | 76.8 | 6.88 | 95.0 | 4.22 | 108 | 9.73 | 117 | 12.3 | 87.2 | 1.55 | |
| Fenitrothion | 0.005 | 72.6 | 13.4 | 70.8 | 4.83 | 80.0 | 8.37 | 111 | 2.40 | 83.2 | 9.77 |
| 0.01 | 80.5 | 4.89 | 80.1 | 5.49 | 87.3 | 9.80 | 95.1 | 8.32 | 86.0 | 4.91 | |
| 0.1 | 71.0 | 11.4 | 90.7 | 6.26 | 100 | 8.56 | 72.4 | 18.0 | 85.4 | 5.59 | |
| Fenpropathrin | 0.005 | 79.0 | 7.11 | 82.3 | 4.42 | 89.0 | 5.91 | 110 | 8.43 | 90.2 | 3.95 |
| 0.01 | 83.8 | 4.76 | 79.4 | 7.48 | 88.9 | 7.12 | 113 | 11.2 | 88.4 | 3.41 | |
| 0.1 | 73.6 | 7.61 | 90.3 | 3.24 | 115 | 0.57 | 119 | 9.94 | 92.7 | 4.01 | |
| Fensulfothion | 0.005 | 73.7 | 8.67 | 72.3 | 18.7 | 75.7 | 9.16 | 73.8 | 12.2 | 72.4 | 16.6 |
| 0.01 | 71.3 | 11.7 | 71.3 | 7.77 | 82.8 | 10.7 | 78.5 | 5.63 | 75.9 | 11.5 | |
| 0.1 | 70.8 | 10.5 | 72.4 | 10.9 | 103 | 11.8 | 70.4 | 10.3 | 71.1 | 14.7 | |
| Fenthion | 0.005 | 72.8 | 10.3 | 72.0 | 7.33 | 76.6 | 6.90 | 108 | 2.52 | 93.0 | 9.64 |
| 0.01 | 72.6 | 4.02 | 71.1 | 4.74 | 76.6 | 9.12 | 87.4 | 12.1 | 83.6 | 4.05 | |
| 0.1 | 72.2 | 8.18 | 71.9 | 8.97 | 79.1 | 12.7 | 93.9 | 9.45 | 90.3 | 4.73 | |
| Fenvalerate | 0.005 | 87.2 | 8.51 | 99.4 | 2.76 | 88.1 | 6.90 | 111 | 15.6 | 98.4 | 5.29 |
| 0.01 | 89.9 | 5.29 | 91.9 | 7.08 | 87.6 | 5.19 | 107 | 14.5 | 91.8 | 2.92 | |
| 0.1 | 76.3 | 7.14 | 100 | 3.69 | 109 | 6.90 | 113 | 9.61 | 82.0 | 3.78 | |
| Flusilazole | 0.005 | 76.3 | 11.0 | 74.6 | 8.31 | 87.1 | 10.6 | 114 | 6.34 | 91.0 | 6.69 |
| 0.01 | 82.5 | 3.87 | 78.2 | 6.36 | 86.7 | 6.50 | 107 | 13.8 | 82.0 | 4.72 | |
| 0.1 | 70.2 | 9.68 | 80.3 | 4.51 | 116 | 6.50 | 113 | 11.9 | 89.0 | 1.92 | |
| Heptachlor | 0.005 | 71.9 | 15.0 | 74.5 | 6.95 | 73.3 | 1.73 | 92.6 | 2.55 | 89.4 | 9.89 |
| 0.01 | 72.1 | 3.70 | 75.5 | 7.26 | 77.9 | 8.80 | 80.7 | 10.4 | 83.6 | 9.75 | |
| 0.1 | 70.5 | 7.83 | 72.1 | 10.3 | 97.6 | 3.22 | 78.7 | 7.59 | 93.2 | 6.93 | |
| Heptachlor epoxide | 0.005 | 73.5 | 16.3 | 75.5 | 15.4 | 80.0 | 12.0 | 85.3 | 1.95 | 95.0 | 12.3 |
| 0.01 | 70.6 | 10.4 | 71.0 | 9.65 | 95.8 | 11.3 | 87.9 | 5.46 | 95.6 | 3.71 | |
| 0.1 | 72.0 | 6.90 | 79.3 | 3.61 | 102 | 8.02 | 101 | 7.50 | 94.6 | 1.22 | |
| Isofenphos | 0.005 | 76.0 | 6.87 | 80.2 | 6.66 | 85.0 | 9.72 | 96.0 | 3.46 | 93.6 | 7.31 |
| 0.01 | 77.1 | 4.21 | 72.6 | 7.13 | 83.9 | 6.18 | 87.6 | 11.0 | 89.8 | 4.43 | |
| 0.1 | 75.3 | 9.32 | 81.3 | 2.13 | 86.4 | 10.5 | 99.1 | 10.2 | 90.7 | 2.54 | |
| keto Endrin | 0.005 | 97.6 | 17.0 | 70.2 | 9.77 | 78.7 | 11.5 | 107 | 5.14 | 89.7 | 7.45 |
| 0.01 | 70.6 | 15.2 | 70.2 | 6.71 | 80.2 | 8.10 | 91.3 | 14.3 | 97.9 | 4.85 | |
| 0.1 | 71.3 | 10.9 | 71.8 | 14.4 | 109 | 2.07 | 97.2 | 11.3 | 95.4 | 3.43 | |
| Kresoxim methyl | 0.005 | 71.1 | 9.98 | 81.1 | 6.46 | 82.4 | 15.2 | 118 | 4.51 | 90.9 | 6.34 |
| 0.01 | 70.7 | 4.17 | 72.4 | 7.14 | 86.5 | 11.2 | 112 | 15.3 | 72.4 | 6.73 | |
| 0.1 | 70.8 | 7.64 | 72.7 | 11.4 | 106 | 9.76 | 113 | 15.7 | 87.8 | 5.70 | |
| Mecarbam | 0.005 | 81.0 | 9.76 | 94.1 | 15.7 | 89.8 | 6.61 | 112 | 3.00 | 98.7 | 11.7 |
| 0.01 | 76.0 | 2.82 | 82.5 | 19.9 | 85.3 | 14.8 | 110 | 2.88 | 93.0 | 6.21 | |
| 0.1 | 71.7 | 9.78 | 81.5 | 3.76 | 113 | 6.41 | 106 | 9.67 | 89.4 | 3.20 | |
| Methacrifos | 0.005 | 110 | 12.0 | 70.4 | 17.9 | 81.0 | 9.76 | 81.9 | 2.93 | 70.2 | 12.0 |
| 0.01 | 72.5 | 19.4 | 71.3 | 4.74 | 76.0 | 2.82 | 85.8 | 12.2 | 70.4 | 9.82 | |
| 0.1 | 72.5 | 19.1 | 76.5 | 8.05 | 71.7 | 9.78 | 84.7 | 5.89 | 73.2 | 11.1 | |
| Methidathion | 0.005 | 71.8 | 10.0 | 71.1 | 10.8 | 105 | 8.20 | 70.1 | 12.4 | 94.4 | 6.69 |
| 0.01 | 71.4 | 4.93 | 70.4 | 7.06 | 101 | 4.92 | 71.1 | 10.7 | 91.5 | 3.27 | |
| 0.1 | 75.7 | 7.83 | 71.8 | 7.90 | 120 | 9.98 | 71.5 | 13.7 | 88.0 | 2.56 | |
| Myclobutanil | 0.005 | 75.3 | 9.05 | 77.1 | 9.11 | 83.0 | 18.2 | 117 | 15.8 | 86.8 | 7.09 |
| 0.01 | 71.1 | 5.68 | 70.5 | 7.01 | 85.8 | 8.41 | 118 | 16.2 | 86.9 | 5.77 | |
| 0.1 | 71.2 | 7.40 | 77.6 | 8.19 | 112 | 8.03 | 115 | 13.4 | 92.3 | 4.62 | |
| Oxychlordane | 0.005 | 72.3 | 11.6 | 73.8 | 8.78 | 93.5 | 9.65 | 102 | 6.83 | 92.5 | 11.6 |
| 0.01 | 72.0 | 8.33 | 72.0 | 10.8 | 90.0 | 4.28 | 93.5 | 6.20 | 93.9 | 4.21 | |
| 0.1 | 72.3 | 9.81 | 75.5 | 5.06 | 102 | 10.4 | 105 | 10.5 | 91.2 | 3.77 | |
| Penconazole | 0.005 | 79.8 | 8.54 | 92.4 | 10.6 | 97.5 | 6.41 | 109 | 3.16 | 93.8 | 8.93 |
| 0.01 | 81.4 | 7.29 | 82.5 | 8.07 | 91.7 | 8.28 | 101 | 10.5 | 95.4 | 3.09 | |
| 0.1 | 72.7 | 7.94 | 93.2 | 3.12 | 118 | 4.12 | 110 | 10.3 | 88.5 | 3.03 | |
| Pentachloraniline | 0.005 | 71.3 | 9.68 | 75.9 | 4.57 | 82.6 | 13.7 | 84.0 | 0.33 | 89.1 | 11.5 |
| 0.01 | 70.2 | 2.78 | 71.6 | 8.05 | 86.5 | 2.78 | 81.3 | 10.6 | 76.7 | 4.73 | |
| 0.1 | 71.6 | 5.47 | 80.1 | 3.16 | 87.8 | 11.7 | 83.9 | 9.23 | 84.7 | 11.7 | |
| Pentachlorothioanisole | 0.005 | 70.1 | 19.2 | 72.1 | 3.15 | 85.2 | 2.55 | 90.8 | 9.53 | 82.4 | 5.68 |
| 0.01 | 70.6 | 11.7 | 71.4 | 4.40 | 87.0 | 5.37 | 83.6 | 14.0 | 92.0 | 5.98 | |
| 0.1 | 71.9 | 12.9 | 78.1 | 4.55 | 97.5 | 17.5 | 86.5 | 9.59 | 99.0 | 6.30 | |
| Permethrin | 0.005 | 77.2 | 4.96 | 72.8 | 4.09 | 94.4 | 4.56 | 117 | 7.87 | 81.9 | 9.70 |
| 0.01 | 73.2 | 6.01 | 72.2 | 8.39 | 93.7 | 9.63 | 111 | 11.2 | 76.1 | 2.05 | |
| 0.1 | 75.4 | 6.93 | 94.2 | 4.17 | 110 | 6.54 | 116 | 10.1 | 80.4 | 3.53 | |
| Phenthoate | 0.005 | 73.2 | 10.6 | 80.3 | 7.18 | 90.4 | 8.99 | 100 | 2.26 | 94.0 | 6.86 |
| 0.01 | 78.6 | 4.52 | 70.1 | 7.04 | 90.2 | 7.38 | 93.7 | 11.7 | 86.8 | 5.46 | |
| 0.1 | 75.4 | 7.54 | 79.4 | 1.94 | 88.4 | 7.84 | 94.6 | 10.8 | 91.5 | 4.74 | |
| Phorate | 0.005 | 86.8 | 17.3 | 70.3 | 10.8 | 88.4 | 15.8 | 93.8 | 2.75 | 86.6 | 8.16 |
| 0.01 | 110 | 5.27 | 84.9 | 3.96 | 118 | 7.47 | 87.3 | 11.8 | 80.9 | 6.75 | |
| 0.1 | 92.3 | 5.20 | 74.3 | 4.28 | 116 | 5.69 | 90.4 | 6.63 | 80.6 | 4.99 | |
| Phosalone | 0.005 | 79.6 | 13.4 | 91.3 | 3.28 | 83.7 | 11.8 | 105 | 11.0 | 83.1 | 4.47 |
| 0.01 | 84.6 | 4.40 | 83.2 | 7.35 | 88.5 | 6.03 | 93.4 | 12.0 | 90.7 | 6.96 | |
| 0.1 | 71.0 | 9.27 | 92.4 | 3.11 | 107 | 3.90 | 101 | 10.7 | 96.4 | 5.71 | |
| Phosmet | 0.005 | 75.3 | 13.2 | 87.6 | 6.97 | 73.4 | 9.46 | 93.2 | 19.8 | 90.1 | 2.91 |
| 0.01 | 82.8 | 7.43 | 84.1 | 8.68 | 74.4 | 5.84 | 83.7 | 10.6 | 85.2 | 3.14 | |
| 0.1 | 72.6 | 10.8 | 99.4 | 3.45 | 99.9 | 5.64 | 87.2 | 14.2 | 87.0 | 3.19 | |
| Primicarb | 0.005 | 72.4 | 10.5 | 76.9 | 9.61 | 76.1 | 15.4 | 101 | 9.37 | 73.3 | 12.6 |
| 0.01 | 76.2 | 3.87 | 71.1 | 6.77 | 72.0 | 1.69 | 92.0 | 8.83 | 71.3 | 7.73 | |
| 0.1 | 81.5 | 6.87 | 79.6 | 3.54 | 86.1 | 11.7 | 95.6 | 7.88 | 74.3 | 6.11 | |
| Primiphos methyl | 0.005 | 70.1 | 18.3 | 74.4 | 11.3 | 72.5 | 12.0 | 72.4 | 8.62 | 74.9 | 15.5 |
| 0.01 | 77.4 | 4.18 | 74.8 | 9.06 | 78.9 | 6.27 | 74.1 | 6.52 | 71.3 | 6.11 | |
| 0.1 | 72.4 | 11.6 | 83.6 | 5.50 | 85.8 | 10.2 | 72.7 | 16.6 | 72.5 | 3.74 | |
| Prochloraz | 0.005 | 77.1 | 10.0 | 81.7 | 7.64 | 80.1 | 12.8 | 115 | 9.44 | 79.5 | 9.51 |
| 0.01 | 82.9 | 6.66 | 79.0 | 8.18 | 77.8 | 5.30 | 96.3 | 11.8 | 74.0 | 6.81 | |
| 0.1 | 70.2 | 8.38 | 88.0 | 5.25 | 97.0 | 8.77 | 98.3 | 13.6 | 77.3 | 2.69 | |
| Profenphos | 0.005 | 118 | 9.12 | 92.4 | 15.9 | 118 | 9.12 | 80.2 | 10.6 | 71.1 | 19.7 |
| 0.01 | 94.9 | 6.01 | 76.0 | 16.9 | 94.9 | 6.01 | 91.2 | 11.2 | 71.0 | 13.7 | |
| 0.1 | 70.3 | 14.8 | 72.7 | 17.0 | 70.3 | 14.5 | 85.3 | 12.3 | 90.0 | 4.71 | |
| Propagite | 0.005 | 104 | 16.3 | 74.2 | 19.4 | 70.2 | 1.78 | 70.1 | 3.05 | 74.3 | 8.53 |
| 0.01 | 75.2 | 15.2 | 70.6 | 14.5 | 70.5 | 3.67 | 74.2 | 4.77 | 75.1 | 6.94 | |
| 0.1 | 72.6 | 6.96 | 73.3 | 4.24 | 70.0 | 4.60 | 71.2 | 2.98 | 77.2 | 3.16 | |
| Propiconazole | 0.005 | 71.6 | 16.1 | 85.9 | 19.9 | 71.6 | 16.1 | 107 | 14.8 | 92.8 | 10.9 |
| 0.01 | 109 | 6.59 | 77.8 | 15.4 | 109 | 6.59 | 84.2 | 15.2 | 70.9 | 5.02 | |
| 0.1 | 71.7 | 7.38 | 71.0 | 3.22 | 71.7 | 7.38 | 73.0 | 14.5 | 73.9 | 3.13 | |
| Pyriproxyfen | 0.005 | 84.9 | 10.2 | 86.7 | 5.13 | 74.1 | 15.1 | 112 | 17.1 | 87.9 | 8.26 |
| 0.01 | 85.2 | 7.02 | 80.1 | 6.85 | 83.7 | 6.06 | 113 | 14.5 | 91.6 | 5.18 | |
| 0.1 | 71.8 | 7.75 | 88.0 | 3.61 | 114 | 4.91 | 119 | 9.96 | 89.9 | 6.26 | |
| Quintozene | 0.005 | 102 | 14.2 | 74.0 | 6.62 | 77.2 | 15.2 | 81.4 | 2.16 | 88.0 | 7.68 |
| 0.01 | 99.2 | 4.33 | 75.5 | 7.47 | 79.6 | 5.55 | 77.0 | 13.6 | 87.4 | 5.60 | |
| 0.1 | 82.3 | 4.98 | 90.6 | 1.71 | 102 | 1.30 | 77.0 | 6.53 | 86.8 | 3.41 | |
| Terbufos | 0.005 | 79.3 | 15.0 | 112 | 10.1 | 79.3 | 15.0 | 81.4 | 18.4 | 82.2 | 11.4 |
| 0.01 | 77.3 | 18.2 | 83.0 | 5.20 | 77.3 | 18.2 | 104 | 7.62 | 105 | 11.2 | |
| 0.1 | 76.1 | 11.2 | 87.0 | 3.49 | 76.1 | 11.2 | 89.2 | 8.83 | 85.1 | 5.02 | |
| Triadimefon | 0.005 | 78.1 | 13.0 | 79.9 | 10.2 | 86.6 | 12.6 | 109 | 6.78 | 82.9 | 13.0 |
| 0.01 | 79.6 | 9.36 | 72.0 | 7.33 | 86.2 | 2.41 | 99.4 | 8.45 | 76.5 | 6.35 | |
| 0.1 | 71.2 | 7.80 | 84.1 | 4.13 | 109 | 7.23 | 106 | 10.7 | 87.3 | 3.08 | |
| Triadimenol | 0.005 | 83.3 | 15.1 | 99.7 | 11.9 | 86.7 | 5.41 | 105 | 6.69 | 72.2 | 4.37 |
| 0.01 | 73.9 | 1.79 | 71.7 | 10.9 | 72.6 | 12.5 | 115 | 4.99 | 73.1 | 4.31 | |
| 0.1 | 76.2 | 6.03 | 73.5 | 8.46 | 104 | 12.3 | 117 | 9.98 | 75.1 | 3.10 | |
| Trizaofos | 0.005 | 81.0 | 7.93 | 83.3 | 7.49 | 74.1 | 9.60 | 118 | 9.84 | 89.7 | 7.13 |
| 0.01 | 84.6 | 5.43 | 78.9 | 6.17 | 81.7 | 12.7 | 115 | 11.1 | 83.9 | 4.77 | |
| 0.1 | 71.4 | 6.89 | 88.1 | 3.71 | 101 | 7.41 | 118 | 10.9 | 93.7 | 4.27 | |
| Vinclozolin | 0.005 | 90.6 | 8.08 | 82.2 | 2.74 | 83.8 | 14.3 | 100 | 1.92 | 95.1 | 7.07 |
| 0.01 | 92.8 | 4.24 | 82.9 | 8.53 | 86.0 | 5.35 | 97.9 | 10.1 | 89.1 | 4.20 | |
| 0.1 | 79.9 | 8.10 | 95.2 | 1.94 | 96.3 | 6.47 | 99.2 | 9.03 | 85.3 | 3.04 | |
Fig. 2.
Distribution of the average recovery of pesticides in beef, pork, chicken, eggs, and milk
The method precision was then obtained by calculating the RSD of the pesticide recoveries from the beef, pork, chicken, egg, and milk samples. The RSD ranged from 1.56 to 19.7% for beef, 1.71 to 19.9% for pork, 0.57 to 19.9% for chicken, 0.23 to 19.8% for eggs, and 1.22 to 19.7% for milk (Table 3). These results confirm that for all samples, the RSD satisfied the CAC/GL 40 criteria of < 20% at concentrations of > 0.01 mg/kg and ≤ 0.1 mg/kg.
Monitoring of market samples
Monitoring was conducted for a total of 89 samples (14 beef, 15 pork, 15 chicken, 15 eggs, and 15 milk) collected from Seoul, Busan, and Incheon markets in Korea, and the contents of the 66 pesticides in these samples were simultaneously analyzed. Chlorpyrifos and fenitrothion in beef and chlorpyrifos in pork were detected at levels lower than the respective MRLs (chlorpyrifos: 1.0 mg/kg in cattle fat; 0.02 mg/kg in pig fat; fenitrothion 0.05 mg/kg in mammal fat). No other pesticides were found in any of the samples. In addition, 15 samples of lamb, the consumption of which has increased in Korea were also collected for monitoring; however, none of the 66 target pesticides were detected. These results were compared with Surma et al. (2014), who found organochlorine pesticides, DDT, BHC and its isomers in ham. Additionally, Rejczak and Tuzimski (2017) reported that low (ng/mL) levels of monuron, methabenzthiazuron, buturon, linuron, aziprotryne, bitertanol, and clofentezine were detected in natural milk samples. However, no pesticides were found in milk in this study. Since pesticides are ubiquitous in the environment and are commonly found as residues in livestock products, continuous pesticide monitoring is required, as is the development of improved methods to allow for the determination of low–concentration multi-residue pesticides in these matrices.
In conclusion, the simultaneous analysis of pesticides presented in this study indicated the potential of the method for the rapid monitoring of residual pesticides in livestock products, due to its short run time, inexpensive nature, simple procedure, and high efficiency. An efficient extraction method was developed using a florisil cartridge as an adsorbent to simultaneously analyze 66 pesticides, including organic phosphorus and chlorine, in a single experiment. The experimental steps were simple and resulted in low LODs and LOQs. The highly sensitive method satisfies the Codex’s criteria and is environmentally friendly with less solvent and waste than conventional approaches. The results of this study will help in establishing a continuous, precise and reliable monitoring system for livestock products in a fast and efficient manner. Expanding the simultaneous analysis of pesticide residues in livestock products will continue to receive focus in our future studies.
Acknowledgements
This study was supported by a Grant (18161MFDS014) from the Ministry of Food and Drug Safety, Korea.
Compliance with ethical standards
Conflict of interest
No potential conflicts of interest are reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hye Soon Kang, Email: cobaltblue@korea.kr.
MeeKyung Kim, Email: mkim@korea.kr.
Eun Jeong Kim, Email: hisclif@korea.kr.
Won-Jo Choe, Email: aragara06@korea.kr.
References
- Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003;86:412–431. doi: 10.1093/jaoac/86.2.412. [DOI] [PubMed] [Google Scholar]
- AOAC. Association of Official Analytical Communities. AOAC Official Method 2007.01, 18th ed. Arlington, VA, USA (2010)
- Bidari A, Ganjali MR, Norouzi P, Hosseini MRM, Assasi Y. Sample preparation method for the analysis of some organophosphorus pesticides residues in tomato by ultrasound-assisted solvent extraction followed by dispersive liquid-liquid micro extraction. Food Chem. 2011;126:1840–1844. doi: 10.1016/j.foodchem.2010.11.142. [DOI] [PubMed] [Google Scholar]
- Chae Y-S, Cho Y-J, Jang K-J, Kim J-W, Lee S-M, Chang M-I. Establishment of an analytical method for prometryne residues in clam using GC-MS. Korean J. Food Sci. Technol. 2013;45:531–536. doi: 10.9721/KJFST.2013.45.5.531. [DOI] [Google Scholar]
- Cho S-K, Abd El-Aty AM, Jeon H-R, Choi J-H, Shin H-C, Shim J-H. Comparison of different extraction methods for the simultaneous determination of pesticide residues in kiwi fruit using gas chromatography-mass spectrometry. Biomed. Chromatogr. 2008;22:727–735. doi: 10.1002/bmc.990. [DOI] [PubMed] [Google Scholar]
- Codex. Guidelines on good laboratory practice in pesticide residue analysis CAC/GL 40-1993, Revision 2003. Available from: http://www.fao.org/home/search/en/?q=Guidelines%20on%20good%20laboratory%20practice%20in%20pesticide%20residue%20analysis%20CAC%2FGL%2040-1993%2C%20Revision%202003. Accessed Feb. 1, 2003.
- Covaci A, Gheorghe A, Schepens P. Distribution of organochlorine pesticides, polychlorinated biphenyls and a-HCH enantiomers in pork tissues. Chemosphere. 2004;56:757–766. doi: 10.1016/j.chemosphere.2004.02.014. [DOI] [PubMed] [Google Scholar]
- da Costa Morais EH, Collins CH, Jardim ICSF. Pesticide determination in sweet peppers using QuEChERS and LC-MS/MS. Food Chem. 2018;249:77–83. doi: 10.1016/j.foodchem.2017.12.092. [DOI] [PubMed] [Google Scholar]
- Facco JF, Martins ML, Bernardi G, et al. Optimization and validation of a multi residues method for pesticides determination in maize using gas chromatography coupled to tandem mass spectrometry. Anal. Method. 2015;7:359–365. doi: 10.1039/C4AY01970A. [DOI] [Google Scholar]
- He Z, Wang L, Paeng Y, Luo M, Wang W, Liu X. Multiresidue analysis of over 200 pesticides in cereal using a QuEChERS and gas chromatography-tandem mass spectrometry based method. Food Chem. 2015;169:372–380. doi: 10.1016/j.foodchem.2014.07.102. [DOI] [PubMed] [Google Scholar]
- Hamadamin AY, Hassan KI. Gas chromatography-mass spectrometry based sensitive analytical approach to detect and quantify non-polar pesticides accumulated in the fat tissues of domestic animals. Saudi J. Biolog. Sci. 2020;27:887–893. doi: 10.1016/j.sjbs.2019.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercegová A, Dömötörová M, Matisová E. Sample preparation methods in the analysis of pesticide residues in baby food with subsequent chromatographic determination. J. Chromatogr. A. 2007;1153:54–73. doi: 10.1016/j.chroma.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou Q, Xiao J, Xie G. Determination of trace organophosphorus pesticides in water samples with TiO2 nanotube cartridge prior to GC-flame photometric detection. J. Sep. Sci. 2010;33:2184–2190. doi: 10.1002/jssc.201000147. [DOI] [PubMed] [Google Scholar]
- Hunter RE, Jr, Riederer AM, Ryan PB. Methods for the determination of organophosphorus and pyrethroid pesticides in food via gas chromatography with electron-capture detection. J. Agric. Food Chem. 2010;58:1396–1402. doi: 10.1021/jf9028859. [DOI] [PubMed] [Google Scholar]
- Juhler RK. Optimized method for the determination of organophosphorus pesticides in meat and fatty matrices. J. Chromatogr. A. 1997;786:145–153. doi: 10.1016/S0021-9673(97)00690-0. [DOI] [PubMed] [Google Scholar]
- Ko AY, Kim H, Jang J, Lee EH, Ju Y, Noh M, Kim S, Park S-W, Chang M-I, Rhee G-S. Development of an official analytical method for determination of phorate and its metabolites in Livestock using LC-MS/MS. J. Food Hyg. Saf. 2015;30:272–280. doi: 10.13103/JFHS.2015.30.3.272. [DOI] [Google Scholar]
- LeDoux M. Analytical methods applied t the determination of pesticides residues in food of animal origin. a review of the past two decades. J. Chromatogr. A 1218: 1021-1036 (2011) [DOI] [PubMed]
- Manav ÖG, Dinç-Zor S, Alpdoğan G. Optimization of a modified QuEChERS method by means of experimental design for multiresidue determination of pesticides in milk and dairy products by GC-MS. Microchemical J. 2019;144:124–129. doi: 10.1016/j.microc.2018.08.056. [DOI] [Google Scholar]
- Mastovska K, Lehotay SJ, Anastassiades M. Combination of analyte protectants to overcome matrix effects in routine GC analysis of pesticide residue in food matrix. Anal. Chem. 2005;77:8129–8137. doi: 10.1021/ac0515576. [DOI] [PubMed] [Google Scholar]
- MFDS. Ministry of Food and Drug Safety. Food Code. Available from: https://mfds.go.kr/eng/brd/m_15/view.do?seq=69982. Accessed Jan. 1, 2019.
- Molina-Ruiz JM, Cieslik E, Cieslik I, Walkowska I. Determination of pesticides residues in fish tissue by modified QuEChERS method and dual-d-SPE clean-up coupled to gas chromatography-mass spectrometry. Environ. Sci. Pollut. Res. Int. 2015;22:369–378. doi: 10.1007/s11356-014-3361-2. [DOI] [PubMed] [Google Scholar]
- Oh JH, Kwon CH, Jeon JS, Choi DM. Management of veterinary drugs residues in food. Korea J. Environ. Agric. 2009;28:310–325. doi: 10.5338/KJEA.2009.28.3.310. [DOI] [Google Scholar]
- Pagliuca G, Gazzotti T, Zironi E, Sticca P. Residue analysis of organophosphorus pesticides in animal matrices by dual column capillary gas chromatography with nitrogen-phosphorus detection. J. Chromatogr. A. 2005;1071:67–70. doi: 10.1016/j.chroma.2004.08.142. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhang J, Zhang Y, Li F, Han Y, Zou N, Xu H, Qian M, Pan C. Automated multi-plug filtration cleanup for liquid chromatographic-tandem mass spectrometric pesticide multi-residue analysis in representative crop commodities. J. Chromatogr. A. 2016;1462:19–26. doi: 10.1016/j.chroma.2016.07.073. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Kim SW, Na TW, Abd El-Aty AM, Lee YJ, Park JS, Ramadan A, Lee HS, Chung HS, Choi JH, Shin HC, Shim JH. QuEChERS method for the simultaneous quantification of phorate and its metabolites in porcine and chicken muscle and table eggs using ultra-high performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2016;39:2079–2086. doi: 10.1002/jssc.201600151. [DOI] [PubMed] [Google Scholar]
- Rejczak T, Tuzimski T. QuEChERS-based extraction with dispersive solid phase extraction clean-up using PSA and ZrO2 based sorbents for determination of pesticides in bovine milk samples by HPLC-DAD. Food Chem. 2017;217:225–233. doi: 10.1016/j.foodchem.2016.08.095. [DOI] [PubMed] [Google Scholar]
- Rimkus GG, Rummler M, Nausch I. Gel permeation chromatography-high performance liquid chromatography combination as an automated clean-up technique for the multiresidue analysis of fats. J. Chromatogr. A. 1996;737:9–14. doi: 10.1016/0021-9673(96)00020-9. [DOI] [PubMed] [Google Scholar]
- Samadi S, Sereshti H, Assadi Y. Ultra-preconcentration and determination of thirteen oragnophosphorus pesticides in water samples using solid-phase extraction followed by dispersive liquid-liquid microextraction and gas chromatography with flame photometric detection. J. Chromatogr. A. 2012;1219:61–65. doi: 10.1016/j.chroma.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Sapozhnikova Y. High-throughput analytical method for 265 pesticides and environmental contaminants in meats and poultry by fast low pressure gas chromatography and ultrahigh-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A. 2018;1572:203–211. doi: 10.1016/j.chroma.2018.08.025. [DOI] [PubMed] [Google Scholar]
- Surma MK, Sadowska-Rociek AB, Cieślik EJ. Evaluation of the QuEChERS method with GC-MS detection for the determination of organochlorine pesticides in food of animal origin. Food Anal. Methods. 2014;7:366–376. doi: 10.1007/s12161-013-9635-3. [DOI] [Google Scholar]
- Stachniuk A, Fornal E. Liquid chromatography-mass spectrometry in the analysis of pesticide residues in food. Food Anal. Methods. 2016;9:1654–1665. doi: 10.1007/s12161-015-0342-0. [DOI] [Google Scholar]
- USDA. Food Safety Inspection Service. Programs and Services. Residue Testing; National Residue Program. Available from: https://www.fsis.usda.gov/wps/portal/fsis/topics/data-collection-and-reports/chemistry/blue-books/ct_index. Accessed Jan. 1, 2019.
- USFDA. Pesticide Analytical Manual, Vol 1: Multi-residue method. 3rd ed. U.S. Food and Drug Administration, Silver Spring, MD, USA (1999)
- Xu X, Xu X, Han M, Qiu S, Hou X. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS. Food Chem. 2019;276:419–426. doi: 10.1016/j.foodchem.2018.10.051. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang H, Liu Y, Wang J, Zhang YC, Dong AJ, Zhao HT, Sun CH, Cui J. Multiresidue methods for determination of 88 pesticides in berry fruits using solid-phase extraction and gas chromatography-mass spectrometry: determination of 88 pesticides in berries using SPE and GC-MS. Food Chem. 2011;127:855–865. doi: 10.1016/j.foodchem.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang Y, Sun C, Yang S, He H. Simultaneous determination of organochlorine, organophosphorus, and pyrethroid pesticides in bee pollens by solid phase extraction cleanup followed by gas chromatography using electron-capture detector. Food Anal. Methods. 2013;6:1508–1514. doi: 10.1007/s12161-012-9539-7. [DOI] [Google Scholar]