Abstract
This study aimed to improve the antimicrobial activity of natural extracts against oral bacteria by synergistic combination and nanoencapsulation. Among five natural antimicrobials: clove oil, thymol, naringin, naringenin, and licorice, clove oil and thymol were selected by comparing the antimicrobial activities against Streptococcus mutans and Streptococcus sobrinus before and after nanoencapsulation. The combination of clove oil and thymol (CLTY) was nanoencapsulated using chitosan and poly-γ-glutamic acid. While free CLTY showed additive and synergistic antimicrobial activity against S. mutans and S. sobrinus, respectively, CLTY nanoparticles (NPs) exhibited synergistic activity against both strains in a time-kill kinetic assay. CLTY NPs significantly decreased the growth of salivary S. mutans during testing, compared with free CLTY in the mouth rinse test. These results indicate that nanoencapsulation can significantly increase the synergistic antimicrobial activity of CLTY and maintain its antimicrobial activity in oral cavities for a longer time.
Keywords: Clove oil, Thymol, Synergistic antimicrobial effect, Nanoencapsulation, Oral bacteria
Introduction
Dental caries and periodontal disease are major oral health problems that are mainly initiated by dental plaque, known as bacterial communities, on the surface of teeth (Loesche, 2007). The major causative agents of these oral diseases are cariogenic bacteria, such as Streptococcus mutans and Streptococcus sobrinus (Ekstrand et al., 1997; Loesche, 2007). Antibiotics and antimicrobial agents such as hydrogen peroxide, fluorides, chlorhexidine, and penicillin are used to effectively inhibit the growth of oral pathogenic bacteria and are now included in oral care products (Bidault et al., 2007). However, concerns exist among consumers regarding their side effects, such as tooth staining, diarrhea, taste perception, tooth discoloration, and vomiting. These concerns have increased the demand for safe antimicrobials (Claydon et al., 2006; Van Strydonck et al., 2005). Moreover, due to the repeated use of antibiotics and increase in microbial resistance, natural antimicrobial agents against oral bacteria that are safe enough to be used in food are growing in popularity and have received much attention for their use against oral bacteria (Soares et al., 2011; Wang et al., 2007).
In dentistry, several herbal extracts such as garlic (Allium sativum), clove (Eugenia caryophyllata), aloe vera (Aloe barbadensis Miller), neem (Azadiracta indica), cinnamon (Cinnamon zeylanicum), and thyme (Thymus vulgaris) have been used for the treatment of oral infections (Fani and Kohanteb, 2017; Pandita et al., 2014). Licorice (Glycyrrhiza glabra) showed potent antimicrobial activity in disk diffusion tests and favorable minimum inhibitory concentration (MIC) values against S. mutans and Lactobacillus acidophilus (Ajagannanavar et al., 2014). Furthermore, grape seed extract has been shown to possess significant antimicrobial properties against pathogenic oral bacteria, with its antimicrobial activity being attributed to its main active ingredients, naringin and naringenin (Lee et al., 2009; Tsui et al., 2008; Yue et al., 2018). However, these natural antimicrobials have shown weaker activity and lower stability profiles compared to those of chemical antimicrobial agents. Moreover, natural ingredients, despite their obvious safety benefits, have unique flavors and tastes and therefore their application in the oral health care industry has been limited.
Natural antimicrobials have been shown to act together to more effectively inhibit the growth of pathogenic bacteria, compared to when single antimicrobials were used. This is thought to occur as a result of a synergistic effect when more than one natural antimicrobial is used (Pei et al., 2009). The synergistic combination of herbal extracts, including Tribulus terrestris, Capsella bursa-pastoris, and Glycyrrhiza glabra exhibited higher antibacterial activity against six oral pathogens compared with those of the individual extracts (Soleimanpour et al., 2015). The MIC in the combination of honey and propolis against oral bacteria was less than their individual MICs, indicating the synergistic antimicrobial effect (Eslami et al., 2016). In addition, various natural antimicrobials such as clove oil, silibinin, apigenin, and Ficus carica extract exhibited synergistic antimicrobial activity in association with antibiotics against oral bacteria (Cha et al., 2016; Jeong et al., 2009; Lee et al., 2011; Moon et al., 2011).
Nanoencapsulation of antimicrobials has been investigated to ensure that the antimicrobials fully exert their inherent biological properties, in addition to increasing their stability profiles (Prakash et al., 2018; Rai et al., 2017). Nano-sized particles have the potential to increase the water solubility (or dispersibility) and the contact with pathogenic bacteria, resulting in improved antimicrobial activity. Additionally, through nanoencapsulation, sensitive antimicrobials could be protected against oxidative environments such as oxygen, heat, and light during food processing and storage. Furthermore, their shelf life could also be extended (Bahrami et al., 2020; Prakash et al., 2018). Thus, nanoencapsulation technology may be used to alleviate the problems of natural antimicrobials. Moreover, as the synergistic combination was encapsulated together at the desired concentration in the same particles, the synergistic biological activity could be increased by simultaneous release, while maintaining the desired concentration (Windbergs et al., 2013). However, relatively little progress has been made on the natural preparations for oral health by combination and nanoencapsulation of synergistic antimicrobials.
Chitosan (CS) has been used as a wall material for many bioactive substance due to its nontoxic, biodegradable, and biocompatible properties. Poly-γ-glutamic acid (PGA) is a naturally-occurring anionic polymer composed of D- and l-glutamic acid linked by amide bonds (Lin et al., 2005). Particularly, CS/PGA nanoparticles (NPs) are spontaneously obtained through ionic gelation interaction between the positively charged amino groups of CS and the negatively charged carboxyl group of PGA in a mild conditions without using toxic solvent and heating process (Buescher and Margaritis, 2007). CS/PGA nanoencapsulation was reported to sustain release of catechin and increase its paracellular transport (Tang et al., 2013). In a previous study, we found that CS/PGA nanoencapsulation improved the solubility, stability, and cellular uptake of resveratrol (Jeon et al., 2016) and the antimicrobial activity of rosemary extract in vitro and food model (Lee et al., 2019). Thus, nanoencapsulation using CS and PGA may be suitable for nanodelivery system for oral health care.
The objective of this study was to improve natural antimicrobial properties through synergistic combination and nanoencapsulation methodology. Five natural antimicrobials, clove oil, thymol, naringin, naringenin, and licorice, were nanoencapsulated using CS and poly-γ-glutamic acid (PGA). By comparing the antimicrobial activities of nanoencapsulated and non-nanoencapsulated free form, clove oil and thymol were selected. The synergistic antimicrobial activity of the nanoencapsulated clove oil and thymol were investigated in time-kill studies and mouth rinse tests against oral bacteria.
Materials and methods
Materials
Clove oil and thymol were purchased from Newdia Co. (Seoul, Korea) and Neumond Co. (Raisting, Germany), respectively. Naringin and naringenin were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Licorice was obtained from CS chemical Co. Ltd. (Seoul, Korea). CS (water soluble, 24 cps, 95% deacetylated) and PGA were purchased from Kittolife Co. (Seoul, Korea) and BioLeaders Corp. (Daejeon, Korea), respectively. Ethanol (99% fermented ethanol), used in the mouth rinse test, was purchased from Korea ethanol supplies Co. (Seoul, Korea). All other reagents were of analytical grade.
Preparation of CS/PGA NPs
NPs entrapping each of the five natural antimicrobials (clove oil, thymol, naringin, naringenin, and licorice) were prepared by ionic gelation using CS and PGA (Liu et al., 2013). The stock solutions of clove oil, thymol, naringin, and naringenin were prepared using absolute ethanol and diluted with distilled water at a concentration of 5.0 mg/mL in 25% ethanol, respectively. The stock solutions of licorice was dissolved in distilled water and mixed with ethanol at a concentration of 5.0 mg/mL in 25% ethanol. CS and PGA were dissolved in distilled water at 4.0 mg/mL, respectively. Antimicrobial solution (2.0 mL) was premixed with 7 mL of CS solution. The NPs were prepared by adding 1.0 mL of PGA solution dropwise into the premixed solution using a pipette tip under magnetic stirring at 1000 rpm for 10 min. The clove oil and thymol-loaded NPs were prepared by the same method; however, the concentration and volume of each solution was different; 2.0 mL of clove oil solution (5.0 mg/mL), 2.0 mL of thymol solution (5.0 mg/mL), 4.0 mL of CS solution (7.0 mg/mL), and 2.0 mL of PGA solution (2.0 mg/mL). Blank NPs were prepared using the same procedure without adding any antimicrobials. The final concentration of ethanol in all NP dispersions was 5%.
Physicochemical properties of NPs
The mean particle size and polydispersity index (PDI) of antimicrobial NPs was measured by dynamic light scattering using Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, Worcestershire, UK). The measurements were performed at multiple narrow modes at 25 ± 1 °C. Samples were analyzed in triplicate, at the least, for each treatment.
The EE of NPs were indirectly determined by measuring the amount of unencapsulated free total phenolic content within NPs using a 3-k-Da filter device (Amicon Ultra-4 Centrifugal Filter, Millipore, Billerica, MA, USA). The total phenolic content in filtered solution was analyzed after centrifuging the NP dispersion at 7000×g (VS-24SMTi, Vision Scientific, Bucheon, Korea) for 30 min (Lee et al., 2019).
The total phenolic content was determined using the method of Anesini et al. (2008) with some modification. Approximately, 0.1 mL of the sample was reacted with 0.5 mL of 0.2 N Folin-Ciocalteu’s reagent for 3 min and then 0.4 mL of sodium carbonate (7.5%, w/v) was added and incubated at room temperature for 30 min. Absorbance was read at 765 nm on a spectrophotometer (DU 650, Beckman Coulter Inc., Fullerton, CA, USA) and the total phenolic content was calculated using gallic acid as a standard. The EEs of the NPs were calculated by the following equation: EE (%) = ((total phenolic content − total phenolic content in filtered solution)/total phenolic content) × 100 (Lee et al., 2019).
Determination of antimicrobial activity
Bacterial strain and growth conditions
The antimicrobial assays were carried out with S. mutans (Korean Culture Center of Microorganisms, KCCM 40105) and S. sobrinus (KCCM 11898). All cultures were stored at − 80 °C in brain heart infusion broth (BHIB, Difco, Detroit, MI, USA) with 87% glycerol. Working cultures were maintained in BHIB for 24 h at 37 °C and adjusted to the required concentration of 106 colony forming units (CFU)/mL.
Minimum inhibitory concentration (MIC)
The effect of combination and nanoencapsulation on the MIC values of antimicrobials was determined using 96-well microtiter plates (Fu et al., 2007; Koo et al., 2000). The wells containing stock solution in the first row were serially diluted two-fold with BHIB along each column. Next, 100 μL of BHIB containing 106 CFU/mL of the bacterial strain was added to each well. The positive controls contained BHIB inoculated with the bacterial strain and 5% ethanol without any antimicrobial agent in the place of sample. The negative controls contained the antimicrobial agent and BHIB only. The well plate was incubated at 37 °C for 24 h and the MIC was defined as the lowest concentration of antimicrobial agent that inhibited visible growth after incubation.
Fractional inhibitory concentration (FIC) index
The synergistic antimicrobial effect in combination with clove oil and thymol was determined by the checkerboard method, using 96-well microtiter plates to obtain the FIC index (Schelz et al., 2006). Clove oil and thymol were diluted two-fold with BHIB along the x- and y-axes, respectively. All wells had a total volume of 100 μL, comprising 50 μL of each dilution. Subsequently, 100 μL of BHIB containing 106 CFU/mL of the bacterial strain was added to each well. After 24 h incubation at 37 °C, the MIC of each solution alone, and in combination, was determined, as described above. The FIC indices were determined using the following Eq. (1):
| 1 |
This method defines synergism, additivity, indifference, and antagonism by the FIC index of ≤ 0.5, 0.5–1.0, 1.0–4.0, and > 4.0, respectively.
In order to determine the FIC index for nanoencapsulated clove oil and thymol combination (CLTY), clove oil NPs, thymol NPs, and CLTY NPs were prepared with various concentration of clove oil, thymol and CLTY, respectively and used as samples in the checkerboard method described above.
Time-kill assay
A time-kill assay was performed according to a modified method from Koo et al. (Koo et al., 2002) The final concentration of clove oil and thymol was 1/8 × MIC. BHIB containing 107 CFU/mL of S. mutans ATCC 25175, or S. sobrinus ATCC 27351, was incubated at 37 °C for 48 h. Samples were removed at 0, 3, 6, 24, and 48 h. Counts were performed by serially diluting samples 10-fold in PBS and spreading 50 μL volumes onto BHI agar. Plates were incubated at 37 °C for 48 h and the number of colonies were counted.
Mouth rinse test
Approval for the study was obtained from the Hanyang University Institutional Review Board, and all subjects provided informed consent prior to participation. The effect of nanoencapsulation of CLTY was evaluated by a mouth rinse test according to a modified method from Arunakul et al. (2011). A total of 18 healthy individuals, 1 male and 17 females, between 24 and 29 years in age (mean age: 26.2 years), were selected. All subjects were non-smokers with no current caries activity, periodontal disease, or other oral pathologies.
For the mouth rinse test, the subjects were randomly divided into three groups: (1) control (5% ethanol solution), (2) non-nanoencapsulated free CLTY, and (3) nanoencapsulated CLTY. Regardless of nanoencapsulation, both concentrations of CLTY were 0.125 mg/mL. The ethanol concentration of all three samples was fixed at 5% to eliminate the interference from ethanol.
The subjects brushed their teeth immediately after lunch and pre-experimental saliva was collected 2 h later. The subjects rinsed their mouths for 1 min with 10 mL of the test mouth rinse solution, or the control solution. Saliva samples were collected at 30, 60, and 90 min after mouth rinsing from each subject. All saliva samples were serially diluted ten-fold in PBS, and inoculated on Mitis Salivarius Sucrose Bacitracin Agar (MSBA, MBcell, Los Angeles, CA, USA). The plates were then incubated at 37 °C for 48 h and the number of colonies were counted.
Statistical analysis
All experiments were performed in triplicate and data are presented as mean ± standard deviation (SD). Statistical analysis was conducted using the Statistical Package for Social Science software (SPSS, Version 21.0, SPSS Inc., Chicago, IL, USA). The statistical analysis to evaluate the differences among multiple groups was carried out by one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test (p < 0.05).
Results and discussion
Effect of five natural antimicrobials against S. mutans and S. sobrinus
Based on several antimicrobial studies that have investigated the buccal cavity (Ajagannanavar et al., 2014; Botelho et al., 2007; Gafner et al., 2011; Lee et al., 2009; Moon et al., 2011; Tsui et al., 2008; Yue et al., 2018), clove oil, thymol, naringin, naringenin, and licorice were selected as antimicrobials against oral bacteria. Their antimicrobial activities were determined by an MIC assay (Table 1). The MIC value of clove oil, thymol, and licorice against S. mutans and S. sobrinus was 0.5 mg/mL, and that of naringin and naringenin was 1.0 mg/mL. These results indicate that the antimicrobial activity of clove oil, thymol, and licorice against S. mutans and S. sobrinus is higher than that of naringin and naringenin.
Table 1.
Minimum inhibitory concentration (MIC, mg/mL) values and fractional inhibitory concentration (FIC) index of natural antimicrobials against S. mutans and S. sobrinus, before and after nanoencapsulation
| Antimicrobials | S. mutans ATCC 25175 | S. sobrinus ATCC 27351 | |||
|---|---|---|---|---|---|
| Free | NPs | Free | NPs | ||
| MIC | Clove oil | 0.5 | 0.5 | 0.5 | 0.5 |
| Thymol | 0.5 | 0.5 | 0.5 | 0.5 | |
| Naringin | 1.0 | 1.0 | 1.0 | 1.0 | |
| Naringenin | 1.0 | 1.0 | 1.0 | 1.0 | |
| Licorice | 0.5 | > 1.0 | 0.5 | > 1.0 | |
| FIC | Clove oil + Thymol | 0.75 (A)a | 0.5 (S) | 0.5 (S) | 0.5 (S) |
aFIC index are interpreted as synergy (S, FIC ≤ 0.5), additive (A, 0.5 < FIC ≤ 1), indifference (I, 1 < FIC ≤ 4), or antagonism (AN, FIC > 4)
Particle properties and MIC values of natural antimicrobial-loaded NPs
Five NPs that entrapped clove oil, thymol, naringin, naringenin, and licorice were prepared by ionic gelation of soluble CS and PGA. CS and PGA nanoencapsulation was reported to produce NPs with high solubility and uniform particle size (Jeon et al., 2016; Kim et al., 2016; Lee et al., 2019). Therefore, CS/PGA NPs are proposed here as appropriate nanocarriers for antimicrobials. All the NPs formulated in this study ranged from 237 to 375 nm in diameter (Table 2). Their PDIs were lower than 0.3, indicating a homogeneous and monodisperse system. Their EEs ranged from 52.1 to 56.7%, with no significant difference between samples (data not shown).
Table 2.
Physical properties of natural antimicrobial-loaded nanoparticles
| Particle size (nm) | Polydispersity index | |
|---|---|---|
| Clove oil NP | 374.9 ± 29.6b | 0.17 ± 0.05b |
| Thymol NP | 371.2 ± 29.6b | 0.16 ± 0.05b |
| Naringin NP | 318.5 ± 24.7c | 0.03 ± 0.02c |
| Naringenin NP | 287.9 ± 10.3d | 0.12 ± 0.05b |
| Licorice NP | 236.6 ± 26.3e | 0.38 ± 0.08a |
| Clove oil + Thymol NP | 483.2 ± 21.7a | 0.18 ± 0.03b |
a−fMeans with different letters are significantly different at p < 0.05
To investigate the effect of nanoencapsulation on the antimicrobial activities of clove oil, thymol, naringin, naringenin, and licorice, their MIC values against S. mutans and S. sobrinus were determined after nanoencapsulation (Table 1). Blank NPs without any antimicrobial agent were used for MIC assays to evaluate the influence of the wall materials on the antimicrobial activities of NPs. However, blank NPs did not showed antimicrobial activity in MIC assays (data not shown). The MIC values of clove oil, thymol, naringin, and naringenin against S. mutans and S. sobrinus were not affected by nanoencapsulation. However, MIC values of licorice showed an increase after nanoencapsulation. These results may be explained by the difference in solubility of nanoencapsulated antimicrobials. It has been reported that the antimicrobial activity of poorly soluble antimicrobials is improved by nanoencapsulation as their water solubility, or dispersibility, is increased by their larger surface areas, which arises from the fact they are nano-sized particles (Bhawana et al., 2011; Lee et al., 2017). However, as the nanoencapsulated water-soluble substance must be released from within the capsule, any activity could take time and this can result in a reduction of initial activity. Therefore, the MIC values of water soluble licorice were increased after nanoencapsulation, resulting in decreased antimicrobial activity. Nevertheless, as MIC values of the poorly soluble clove oil, thymol, naringin, and naringenin were not affected by nanoencapsulation, it was not confirmed that their antimicrobial activity was increased from the results of the MIC assay in this study. This result may be due to the fact that MIC determination was not sensitive enough to clearly indicate the effect of nanoencapsulation (Lee et al., 2019). Moreover, among the five natural antimicrobials, the antimicrobial activities of clove oil and thymol were the highest in MIC tests and were not reduced by nanoencapsulation. Thus, CLTY was selected for further studies.
Effect of combination and nanoencapsulation on the in vitro antimicrobial activity of CLTY
To evaluate the effect of combination and nanoencapsulation on the antimicrobial activity of CLTY, after CLTY-loaded NPs were prepared using CS and PGA, the particle properties and antimicrobial activities were evaluated. The average particle size of CLTY-loaded NP was 483 nm, which is approximately 30% larger than that of clove oil or thymol NPs (Table 2). The EE of CLTY-loaded NP (58.7%) was slightly higher than those of single antimicrobial-loaded NPs with no significant difference (data not shown, p < 0.05). Although the particle size is increased, their PDI still remained below 0.3, indicating that CLTY NPs had a relatively narrow size distribution.
To numerically determinate the synergistic antimicrobial effects of CLTY, their FIC index was determined against S. mutans and S. sobrinus, before and after nanoencapsulation (Table 1). The FIC scale revealed synergistic and additive effects against S. sobrinus and S. mutans, respectively, before nanoencapsulation. However, the nanoencapsulated CLTY displayed synergistic activity against both S. sobrinus and S. mutans. This showed that non-nanoencapsulated CLTY exhibits partial synergistic antimicrobial activity, whereas nanoencapsulated CLTY is found to have synergistic effects against both S. sobrinus and S. mutans. This result can be explained by inferring that synergistic combinations increase the probability of synergy when present together within the same NP (Windbergs et al., 2013).
With regards to the effect of combination and nanoencapsulation on the antimicrobial activity of CLTY over time against S. mutans and S. sobrinus by the time-kill assay (Fig. 1A, B), clove oil and thymol as single agents, non-nanoencapsulated free and nanoencapsulated CLTY, were evaluated at concentrations of 1/8 × MIC for each strain. Blank NPs without any antimicrobial agent did not showed antimicrobial activity in time kill assays (data not shown). With the combination compared with colony count at 24 h incubation of the most active agent alone, synergy and antagonism in the time-kill assay were defined as greater than 2 log10 decrease and 2 log10 increase in colony count, respectively (Eliopoulos and Eliopoulos, 1988). Addition (or indifference) was defined as a less than 2 log10 change in colony count at 24 h with the combination, compared to the most active agent alone (Eliopoulos and Eliopoulos, 1988). Free CLTY and CLTY NPs showed additive and synergistic antimicrobial activity against S. mutans, respectively. In addition, both free CLTY and CLTY NPs showed significantly higher antimicrobial activity after 3 h than that of free clove oil or thymol. However, the significant difference between the antimicrobial activity of free CLTY and CLTY NPs was not observed at 24 h, but only at 48 h incubation. Regardless of nanoencapsulation, after 24 h, both free CLTY and CLTY NPs resulted in a greater than 2 log reduction in colony count compared to that by free clove oil or thymol, indicating a synergistic effect against S. sobrinus. In addition, CLTY NPs showed significantly higher antimicrobial activity against S. sobrinus at 24 h incubation than that of free CLTY. These results are similar to the results of the FIC index and indicate that the synergistic antimicrobial activity of CLTY is significantly improved by nanoencapsulation.
Fig. 1.
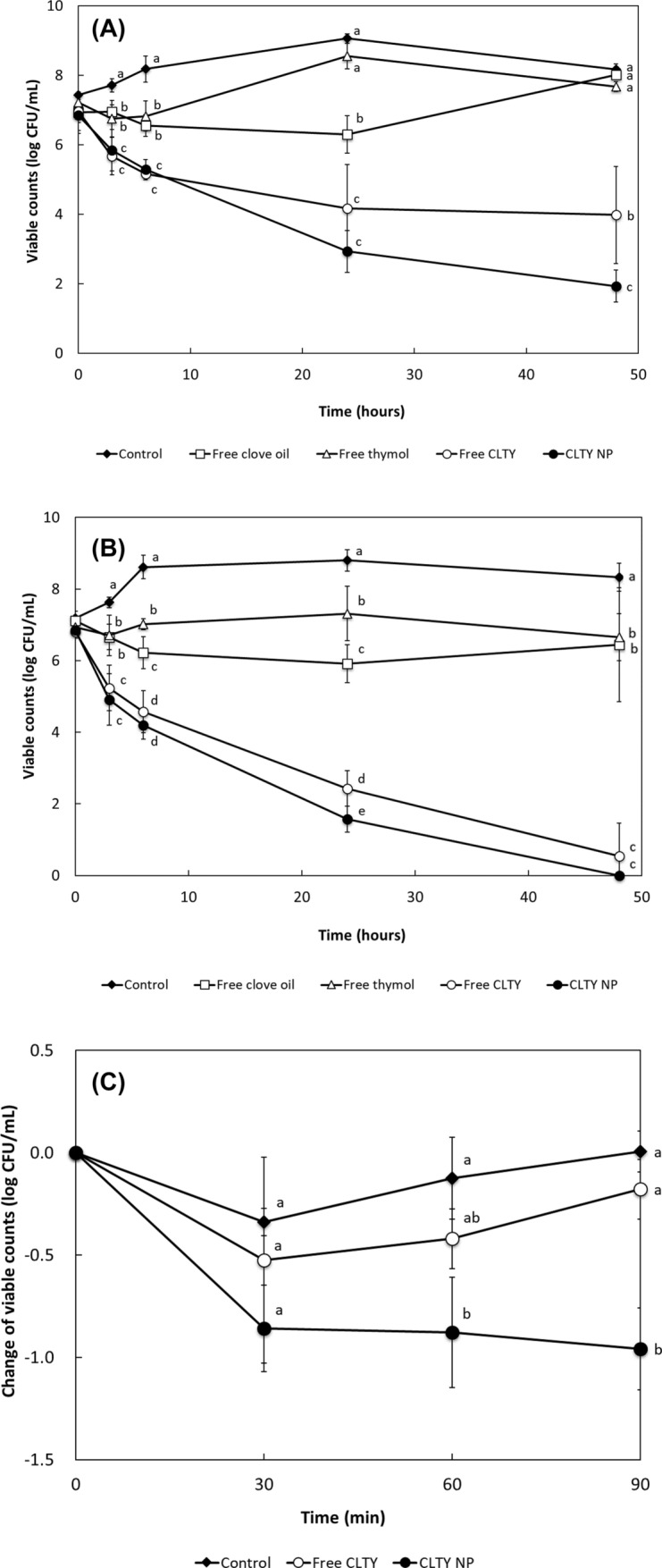
The effect of combination and nanoencapsulation on the antimicrobial activity of clove oil and thymol by the time-kill assay against S. mutans (A) and S. sobrinus (B) and the mouth rinse test (C). a−eMeans with different letters indicate significant difference between groups (p < 0.05)
Effect of nanoencapsulation on the in vivo antimicrobial activity of CLTY
The effect of nanoencapsulation on the in vivo antimicrobial activity of CLTY against S. mutans was evaluated by a mouth rinse test (Fig. 1C). S. mutans has been reported to be more prevalent than S. sobrinus in dental plaque samples (de Carvalho et al., 2006). The number of S. mutans in the control and free CLTY slightly decreased at 30 min and then re-increased. Although the number of S. mutans in free CLTY group was lower than that in the control group, there was no significant difference. However, CLTY NPs significantly decreased the growth of salivary S. mutans at all three sampling times, compared to the control and free CLTY groups. These results indicate that the CLTY NPs can maintain their antimicrobial activity in oral cavities for a longer time than the free combination.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03933069).
Compliance with ethical standards
Conflict of interest
None of the authors of this study has any financial interest or conflict with industries or parties.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ji-Soo Lee, Email: iamjisoo@hanyang.ac.kr.
Ye Seul Choi, Email: 01190706013@hanmail.net.
Hyeon Gyu Lee, Email: hyeonlee@hanyang.ac.kr.
References
- Ajagannanavar SL, Battur H, Shamarao S, Sivakumar V, Patil PU, Shanavas P. Effect of aqueous and alcoholic licorice (Glycyrrhiza glabra) root extract against streptococcus mutans and lactobacillus acidophilus in comparison to chlorhexidine: an in vitro study. J. Int. Oral Health. 2014;6:29–34. [PMC free article] [PubMed] [Google Scholar]
- Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J. Agr. Food Chem. 2008;56:9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- Arunakul M, Thaweboon B, Thaweboon S, Asvanund Y, Charoenchaikorn K. Efficacy of xylitol and fluoride mouthrinses on salivary mutans streptococci. Asian Pac. J. Trop. Biomed. 2011;1:488–490. doi: 10.1016/S2221-1691(11)60106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami A, Delshadi R, Assadpour E, Jafari SM, Williams L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface Sci. 2020;278:102140. doi: 10.1016/j.cis.2020.102140. [DOI] [PubMed] [Google Scholar]
- Bhawana, Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J. Agr. Food Chem. 59: 2056-2061 (2011) [DOI] [PubMed]
- Bidault PD, Chandad F, Grenier D. Risk of bacterial resistance associated with systemic antibiotic therapy in periodontology. J. Can. Den. Assoc. 2007;73:721–725. [PubMed] [Google Scholar]
- Botelho M, Nogueira N, Bastos G, Fonseca S, Lemos T, Matos F, Montenegro D, Heukelbach J, Rao V, Brito G. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz. J. Med. Biol. Res. 2007;40:349–356. doi: 10.1590/S0100-879X2007000300010. [DOI] [PubMed] [Google Scholar]
- Buescher JM, Margaritis A. Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit. Rev. Biotechnol. 2007;27:1–19. doi: 10.1080/07388550601166458. [DOI] [PubMed] [Google Scholar]
- Cha S-M, Kim G-U, Cha J-D. Synergistic antimicrobial activity of apigenin against oral pathogens. Int. J. Eng. Res. Sci. 2016;2:27–37. [Google Scholar]
- Claydon N, Addy M, Adams G, Smith S, Bosma M, North M, Moran J. A comparison of two chlorhexidine gel brushing regimens and a conventional toothpaste brushing regimen for the development of tooth staining over a 6-week period. Int. J. Dent. Hyg. 2006;4:183–188. doi: 10.1111/j.1601-5037.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- de Carvalho FG, Silva DS, Hebling J, Spolidorio LC, Spolidorio DMP. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch. Oral Biol. 2006;51:1024–1028. doi: 10.1016/j.archoralbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Ekstrand K, Bruun G, Bruun M. Plaque and gingival status as indicators for caries progression on approximal surfaces. Caries research. 1997;32:41–45. doi: 10.1159/000016428. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G, Eliopoulos C. Antibiotic combinations: should they be tested? Clin. Microbiol. Rev. 1988;1:139–156. doi: 10.1128/CMR.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami H, Ariamanesh N, Ariamanesh A, Kafil HS. Synergistic effect of honey and Azarian propolis on oral microorganisms: an in vitro study. J. Adv. Oral Res. 2016;7:31–36. [Google Scholar]
- Fani M, Kohanteb J. In vitro antimicrobial activity of Thymus vulgaris essential oil against major oral pathogens. J. Evidence-Based Complementary Altern. Med. 2017;22:660–666. doi: 10.1177/2156587217700772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S, Efferth T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 2007;21:989–994. doi: 10.1002/ptr.2179. [DOI] [PubMed] [Google Scholar]
- Gafner S, Bergeron C, Villinski JR, Godejohann M, Kessler P, Cardellina JH, Ferreira D, Feghali K, Grenier D. Isoflavonoids and coumarins from Glycyrrhiza uralensis: antibacterial activity against oral pathogens and conversion of isoflavans into isoflavan-quinones during purification. J. Nat. Prod. 2011;74:2514–2519. doi: 10.1021/np2004775. [DOI] [PubMed] [Google Scholar]
- Jeon YO, Lee J-S, Lee HG. Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and γ-poly (glutamic acid) Colloids Surf. B Biointerfaces. 2016;147:224–233. doi: 10.1016/j.colsurfb.2016.07.062. [DOI] [PubMed] [Google Scholar]
- Jeong M-R, Kim H-Y, Cha J-D. Antimicrobial activity of methanol extract from Ficus carica leaves against oral bacteria. J. Bacteriol. Virol. 2009;39:97–102. doi: 10.4167/jbv.2009.39.2.97. [DOI] [Google Scholar]
- Kim ES, Lee J-S, Lee HG. Nanoencapsulation of red ginseng extracts using chitosan with polyglutamic acid or fucoidan for improving antithrombotic activities. J. Agr. Food Chem. 2016;64:4765–4771. doi: 10.1021/acs.jafc.6b00911. [DOI] [PubMed] [Google Scholar]
- Koo H, Rosalen PL, Cury JA, Ambrosano GM, Murata RM, Yatsuda R, Ikegaki M, Alencar SM, Park YK. Effect of a New Variety of Apis mellifera Propolis on Mutans Streptococci. Curr. Microbiol. 2000;41:192–196. doi: 10.1007/s0028400101170. [DOI] [PubMed] [Google Scholar]
- Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 2002;46:1302–1309. doi: 10.1128/AAC.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-B, Ha Y-M, Shin S-H, Je K-M, Kim S-R, Choi J-S, Choi I-S. Antimicrobial activity of test dentifrice product containing grapefruit seed extract and processed sulfur solution against oral pathogens. J. Life Sci. 2009;19:956–962. doi: 10.5352/JLS.2009.19.7.956. [DOI] [Google Scholar]
- Lee J-S, Kim ES, Lee HG. Improving the water solubility and antimicrobial activity of silymarin by nanoencapsulation. Colloids Surf. B Biointerfaces. 2017;154:171–177. doi: 10.1016/j.colsurfb.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lee J-S, Kim ES, Lee HG. Preparation, characterization, and food application of rosemary extract-loaded antimicrobial nanoparticle dispersions. LWT. 2019;101:138–144. doi: 10.1016/j.lwt.2018.10.072. [DOI] [Google Scholar]
- Lee Y-S, Jang K, Cha J-D. Synergistic antibacterial effect between silibinin and antibiotics in oral bacteria. J. Biomed. Biotechnol. 2011;2012:1–7. doi: 10.1155/2012/618081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-H, Chung C-K, Chen C-T, Liang H-F, Chen S-C, Sung H-W. Preparation of nanoparticles composed of chitosan/poly-γ-glutamic acid and evaluation of their permeability through Caco-2 cells. Biomacromolecules. 2005;6:1104–1112. doi: 10.1021/bm049312a. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sun Y, Xu Y, Feng H, Fu S, Tang J, Liu W, Sun D, Jiang H, Xu S. Preparation and evaluation of lysozyme-loaded nanoparticles coated with poly-γ-glutamic acid and chitosan. Int. J. Biol. Macromol. 2013;59:201–207. doi: 10.1016/j.ijbiomac.2013.04.065. [DOI] [PubMed] [Google Scholar]
- Moon S-E, Kim H-Y, Cha J-D. Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch. Oral Biol. 2011;56:907–916. doi: 10.1016/j.archoralbio.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Pandita V, Patthi B, Singla A, Singh S, Malhi R, Vashishtha V. Dentistry meets nature-role of herbs in periodontal care: a systematic review. J. Indian Assoc. Public Health Dent. 2014;12:148. doi: 10.4103/2319-5932.144784. [DOI] [Google Scholar]
- Pei Rs, Zhou F, Ji Bp, Xu J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 74: M379-M383 (2009) [DOI] [PubMed]
- Prakash B, Kujur A, Yadav A, Kumar A, Singh PP, Dubey N. Nanoencapsulation: an efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control. 2018;89:1–11. doi: 10.1016/j.foodcont.2018.01.018. [DOI] [Google Scholar]
- Rai M, Paralikar P, Jogee P, Agarkar G, Ingle AP, Derita M, Zacchino S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: emerging trends and future perspectives. Int. J. Pharm. 2017;519:67–78. doi: 10.1016/j.ijpharm.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Schelz Z, Molnar J, Hohmann J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia. 2006;77:279–285. doi: 10.1016/j.fitote.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Soares LFJ, do Carmo F, de Almeida BV, Monteiro L, Rodrigues C, Cabral L, de Sousa V. Preparation and evaluation of lidocaine hydrochloride in cyclodextrin inclusion complexes for development of stable gel in association with chlorhexidine gluconate for urogenital use. Int. J. Nanomedicine. 6: 1143-1154 (2011) [DOI] [PMC free article] [PubMed]
- Soleimanpour S, Sedighinia FS, Afshar AS, Zarif R, Ghazvini K. Antibacterial activity of Tribulus terrestris and its synergistic effect with Capsella bursa-pastoris and Glycyrrhiza glabra against oral pathogens: an in vitro study. Avicenna J. Phytomed. 2015;5:210. [PMC free article] [PubMed] [Google Scholar]
- Tang D-W, Yu S-H, Ho Y-C, Huang B-Q, Tsai G-J, Hsieh H-Y, Sung H-W, Mi F-L. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocolloids. 2013;30:33–41. doi: 10.1016/j.foodhyd.2012.04.014. [DOI] [Google Scholar]
- Tsui V, Wong R, Rabie ABM. The inhibitory effects of naringin on the growth of periodontal pathogens in vitro. Phytother. Res. 2008;22:401–406. doi: 10.1002/ptr.2338. [DOI] [PubMed] [Google Scholar]
- Van Strydonck D, Timmerman M, Van Der Velden U, Van Der Weijden G. Plaque inhibition of two commercially available chlorhexidine mouth rinses. Clin. Periodontol. 2005;32:305–309. doi: 10.1111/j.1600-051X.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- Wang CS, Arnold RR, Trope M, Teixeira FB. Clinical efficiency of 2% chlorhexidine gel in reducing intracanal bacteria. J. Endod. 2007;33:1283–1289. doi: 10.1016/j.joen.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Windbergs M, Zhao Y, Heyman J, Weitz DA. Biodegradable core–shell carriers for simultaneous encapsulation of synergistic actives. J. Am. Chem. Soc. 2013;135:7933–7937. doi: 10.1021/ja401422r. [DOI] [PubMed] [Google Scholar]
- Yue J, Yang H, Liu S, Song F, Guo J, Huang C. Influence of naringenin on the biofilm formation of Streptococcus mutans. Int. J. Dent. 2018;76:24–31. doi: 10.1016/j.jdent.2018.04.013. [DOI] [PubMed] [Google Scholar]


