Abstract
COVID-19 is a pandemic viral infection caused by a novel coronavirus, SARS-CoV2, which is a global concern of the twenty-first century for its rapid spreading in a short period. Apart from its known acute respiratory involvements, the CNS manifestations of COVID-19 are common. These neurological symptoms are diverse and could range from mild nonspecific or specific symptoms such as the loss of various sensory perceptions, the worrying autoimmune Guillain–Barré syndrome, to the life-threatening acute disseminated encephalomyelitis, and the CNS-mediated respiratory distress. An autopsy report documented the presence of SARS-CoV2 in brain tissues of a COVID-19 patient. However, there is no definite conclusion on the mechanisms of SARS-CoV2 neuroinvasion. These proposed mechanisms include the direct viral invasion, the systemic blood circulation, or the distribution of infected immune cells. Concerning these different neuropathophysiologies, COVID-19 patients who are presenting with either the early-onset, multiple, and severe CNS symptoms or rapid respiratory deterioration should be suspected for the direct viral neuroinvasion, and appropriate management options should be considered. This article reviews the neurological manifestations, the proposed neuroinvasive mechanisms, and the potential neurological sequelae of SARS-CoV2.
Keywords: COVID-19, SARS-CoV2, Neuroinvasion, Severe acute respiratory syndrome, Angiotensin-converting enzyme 2 receptor
Introduction
The pandemics of COVID-19 affect global citizens and drastically alter our living norms. The causative virus for the disease is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), a positive-stranded RNA virus that belongs to the Coronaviridae family [1]. Other known coronaviruses in this family are the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV), which cause severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), respectively [2, 3].
Major clinical manifestations of the coronavirus-induced diseases are related to the pathologies of the upper and lower respiratory tract and the hyperactive immune responses that can be fatal independent from the direct impacts of the causative viruses [2]. Despite its variation by locations and changing over time, the case fatality rate, ranging from 0.2% in Germany to 7.7% in Italy, is relatively low [4]. However, the rapid spreading of COVID-19 cumulates the alarming global death toll [4–8]. Apart from the dominant respiratory involvements, there are reports of various clinical manifestations in multiple organs, including the cardiovascular, urological, musculoskeletal, and neurological symptoms [5, 7, 9].
The frequency of neurological manifestations was up to one-quarter in hospitalized COVID-19 patients [10]. Many of them reported nonspecific symptoms such as dizziness, headache, and confusion, while some had the alteration of consciousness, seizure, and other nerve dysfunctions [11]. A recent autopsy identified the SARS-CoV2 in neural and capillary endothelial cells in the frontal lobe of a COVID-19 patient [12]. These findings confirm that direct SARS-CoV2 neuroinvasion could also contribute to the overall presentations of COVID-19.
Several studies reported diverse neurological presentations and various clinical outcomes from a variety of treatment options, including immunotherapy, intravenous immunoglobulin, corticosteroid, and antiviral agents [13, 14]. However, proper clinical assessment, treatment, and management strategy cannot be established without a good understanding of SARS-CoV2 neuropathophysiologic mechanisms, which are scarcely explored in the previous studies [15–17]. This article reviews the neurological manifestations, the potential clinical sequelae, the proposed mechanisms of neuropathophysiology, and the situation that direct neuroinvasion of SARS-CoV2 should be considered.
Neurological Manifestations of COVID-19
A Chinese retrospective study of 214 patients, who had the laboratory-confirmed of SARS-CoV2 infection, reported the nervous system symptoms in 78 cases (36.4%), 45.5% in severe and 30.2% in nonsevere COVID-19 [10]. Among these patients, 53 cases had CNS symptoms, including dizziness, headache, impaired consciousness, acute cerebrovascular disease, ataxia, and epilepsy. In comparison, 19 cases showed peripheral nervous systems symptoms such as nerve pain and the reduction in taste, smell, and visual perceptions [10]. The group from University College London Queen Square Institute of Neurology recently reported five significant categories of neurological presentations in COVID-19, i.e., (i) encephalopathy with delirium/psychosis and no MRI or CSF abnormalities, (ii) inflammatory CNS syndromes including encephalitis and acute disseminated encephalomyelitis, (iii) ischemic strokes, (iv) peripheral neurological disorders including Guillain–Barré syndrome and brachial plexopathy, and (v) miscellaneous central disorders [13]. Therefore, the neurological manifestations of COVID-19 are diverse and not uncommon [14, 16].
The mental state alterations or the encephalopathy in COVID-19 could be either the systemic consequences of hyperactive immunologic responses or the direct neuroinvasion of SARS-CoV2, as earlier mentioned in a recent autopsy report [15, 18, 19]. Studies of other coronaviruses reported the SARS-CoV and MERS-CoV RNA in the brain tissues, particularly in the neurons, of SARS and MERS patients [2, 20–22]. Despite the limited evidence in SARS-CoV2, the shared structures and the infection pathways of these coronaviruses are likely to contribute to their comparable neuropathophysiologies [23, 24]. The neurons and glial cells are known for their expressions of the angiotensin-converting enzyme 2 (ACE2) receptor, the target of interactions between SARS-CoV2 and the host cells [25, 26]. The combination of direct viral neuroinvasion and the sequelae of systemic hyperactive immune responses could contribute to the neurological manifestations of COVID-19 [23].
Apart from the neurological symptoms, the viral neuroinvasion could partly lead to the respiratory distress in COVID-19, as referred to the evidence of respiratory symptoms in SARS patients from the combined viral-induced impacts on the medullary cardiorespiratory center of the brainstem and the mechano- and chemoreceptors in their respiratory tract cells [27–30]. Although the clinical respiratory distress from pulmonary and neurological pathologies is not precisely similar, the proof of SARS-CoV2’s RNA in human nervous tissues supports the possible contribution of SARS-CoV2 neuroinvasion and the respiratory depression [12, 31].
The Proposed Neuroinvasive Mechanisms of SARS-CoV2
Together with other coronaviruses, SARS-CoV2 potentially invades the nervous tissues through several mechanisms. The oronasal inoculation of coronaviruses in pig models reported the infections in the respiratory tracts, small intestine, peripheral nerve, and the retrograde propagation of viruses into the medullary neurons of the brainstem [32, 33]. SARS-CoV rapidly infected the brain of the human ACE2 transgenic mice via the olfactory bulbs and caused rapid neuronal death without evidence of immune responses [34]. MERS-CoV could enter the thalamus and brainstem via the olfactory nerve [3]. Animal models reported the coronavirus infections in the eye structures with the induced inflammation of the conjunctiva, retina, and optic nerves [35]. Some coronaviruses navigate across the nerve synapses after the oronasal inoculation in the animal models [32, 33]. All these pieces of evidence support the possible mechanism of SARS-CoV2 direct neuroinvasion through the olfactory nerve, possibly across the cribriform plate of the ethmoid bone, and the retrograde dissemination into the CNS tissues [23].
With active SARS-CoV2 infection, the systemic circulation could distribute and enable the virus to enter the cerebral blood flow. The interactions between the SARS-CoV2 spike proteins and their endothelial ACE2 receptors could enhance the compromised blood-brain, endothelial, and nasal epithelial barrier [36, 37]. The inflammation also causes sluggish blood flow, which facilitates these interactions and augments the viral neuroinvasion [20, 23, 38]. Systemic circulation could then be an entering passage of SARS-CoV2 into the CNS.
The in vitro studies of human and rat neuronal cell lines demonstrated their susceptibility to coronaviruses infection [39, 40]. The nucleic acids and RNA of SARS-CoV were detected in both alveolar pneumocytes and the macrophages of SARS patients [2, 41]. Without the direct invasion or the distribution through the systemic circulation, these infected leukocytes can infiltrate into brain tissues through the glial-lymphatic or glymphatic system and serve as the reservoir for the viral delivery in CNS [42, 43].
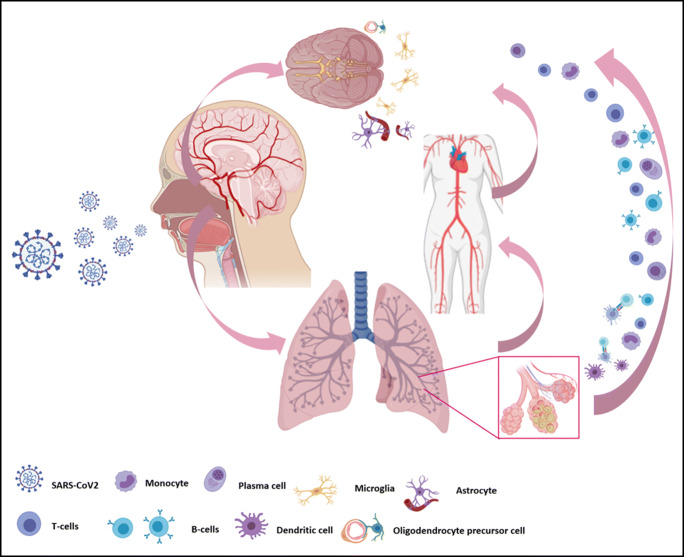
Although there is no concluded mechanism for SARS-CoV2 neuroinvasion, the virus had already made its presence in the brain tissue of a COVID-19 patient [12]. This possibility should be aware of while assessing the neurological manifestations of COVID-19. Figure 1 summarizes the proposed mechanisms of SARS-CoV2 neuroinvasion.
Fig. 1.
Proposed mechanism for SARS-CoV2 neuroinvasion. (i) SARS-CoV2 can directly infect the olfactory nerve and retrogradely disseminate across the cribriform plate of the ethmoid bone into the central nervous system (CNS). (ii) The systemic circulation could distribute and enable the virus to enter the cerebral blood flow. (iii) The infected leukocytes can also infiltrate into brain tissues through the glial-lymphatic system and serve as the reservoir for the viral delivery in the CNS
The Neurological Sequelae of COVID-19
The dominant characteristics of COVID-19 are the excessive host immune responses to the SARS-CoV2 invasion, potentially up to the degree of the fatal “cytokine storm” in severe cases [44]. The disproportion of pro-inflammatory chemokines and cytokines to the interferon-mediated anti-inflammatory responses is prominent in the susceptible hosts with compromised metabolic backgrounds [28, 42, 45–47]. Even without the evidence of CNS viral invasion, the immunologic hyperactivation can induce severe brain inflammation, as shown in the CT and MRI reports of hemorrhagic necrotizing encephalopathy of a COVID-19 female [19]. The sterile inflammation of nervous tissues could already produce the clinical symptoms of neuritis, meningitis, and encephalitis, which eventually lead to reactive gliosis, neuronal dysfunction, and neuronal death [12, 22, 48]. Several immunological consequences, such as the disseminated intravascular coagulation or the endothelial rupture in cerebral capillaries with the accompanied bleeding, can be fatal [23].
The early COVID-19 cases reported the loss of perceptions such as smell, taste, and vision, which are partially the symptoms of inflammatory-induced nerve dysfunctions [23, 49, 50]. Five Italian COVID-19 patients developed the autoimmune Guillain–Barré syndrome after 5 to 10 days following the onset of SARS-CoV2 infection [51]. With these hyperactive immunologic sequelae of SARS-CoV2, the range of COVID-19 neurological manifestations is diverse.
Cumulative animal studies established the detrimental impacts of direct coronavirus invasion in the nervous tissues with the resulting inflammation, degeneration, and death of neurons and glial cells [52–55]. The processes of viral replication within host cells could disrupt the neuronal functions with various clinical manifestations, including seizure, convulsion, loss of consciousness, and ataxia [56, 57]. Interestingly, the laboratory testing in a COVID-19 patient with neurological signs was positive for SARC-CoV2 in the cerebrospinal fluid but was negative for the virus in the nasopharyngeal swab [57]. These findings support the likelihood of SARS-CoV2 direct neuroinvasion independent from the primary respiratory inoculation. The direct viral neuroinvasion should be suspected in COVID-19 patients with either early-onset, multiple, severe CNS symptoms, or rapid clinical deterioration despite the appropriate supportive measures.
Specific Considerations for Neuroinvasion in COVID-19
With the probable SARS-CoV2 neuroinvasion, it is worth considering the specific treatment options in addition to the current COVID-19 management protocol. Apart from the antiviral strategy, there were animal studies on the possibility of modulating CNS excitatory pathways, i.e., the glutamate homeostasis [58–60]. A known N-methyl-d-aspartate (NDMA) receptor antagonist, memantine, improved the symptoms, reduced motor disabilities, and the viral replication in coronavirus-infected mice [58]. Other NMDA receptor blockers, including dizocilpine, agmatine sulfate, and ifenprodil, also reduced the neuronal death, the intraocular pressure (IOP), the reactive gliosis, and the neurodegeneration in the ZIKA-infected mice [59]. A glutamine antagonist, 6-diazo-5-oxo-l-norleucine, reduced CNS leukocyte migration, prevented inflammation, paralysis, and death in mice [60]. With the worsening prognosis of SARS-CoV2 neuroinvasion, these options are probably reserved for compassionate considerations.
Conclusion
COVID-19 is a global concern of the twenty-first century by its rapid spreading to every continent in a short period. Apart from the known respiratory involvements, the CNS manifestations of COVID-19 are common. The neurological symptoms are diverse and could range from mild symptoms such as the loss of various sensory perceptions, the worrying autoimmune Guillain–Barré syndrome, to the life-threatening progressive encephalopathy, and the CNS-mediated respiratory distress. An autopsy report documented the presence of SARS-CoV2 in the brain tissues of COVID-19 patients. However, there is no definite conclusion on its probable mechanisms of neuroinvasion. These proposed mechanisms include the direct viral invasion, the systemic blood circulation, or the distribution of infected immune cells. Concerning these different neuropathophysiologies, COVID-19 patients who are presenting with either the early-onset, multiple, and severe CNS symptoms or rapid respiratory deterioration should be suspected for the direct viral neuroinvasion. At the same time, appropriate management options and specific attention are warranted.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malik YS, Sircar S, Bhat S, Sharun K, Dhama K, Dadar M, Tiwari R, Chaicumpa W. Emerging novel coronavirus (2019-nCoV)—current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;40(1):68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, Gibson-Corley KN, Meyerholz DK, McCray PB., Jr Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazzerini M, Putoto G. COVID-19 in Italy: momentous decisions and many uncertainties. Lancet Glob Health. 2020;8(5):e641–e642. doi: 10.1016/S2214-109X(20)30110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia Ja YT, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection——a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Chen H, Tang K, Guo Y (2020) Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 10.1016/j.jinf.2020.02.028 [DOI] [PMC free article] [PubMed]
- 10.Mao L, Wang M, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D et al (2020) Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv:2020.2002.2022.20026500. 10.1101/2020.02.22.20026500
- 11.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M (2020) Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol: [published online ahead of print, 2020 Apr 2021]. 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed]
- 13.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, Jayaseelan DL, Kumar G et al (2020) The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain J Neurol. 10.1093/brain/awaa240 [DOI] [PMC free article] [PubMed]
- 14.Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921–105921. doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koralnik IJ, Tyler KL. COVID-19: a global threat to the nervous system. Ann Neurol. 2020;88(1):1–11. doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S et al (2020) Neurological associations of COVID-19. Lancet Neurol. 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed]
- 18.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;296:201187–20E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913–8921. doi: 10.1007/978-81-322-1777-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, Li Z, Deng P, Zhang J, Zhong N, Ding Y, Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41(8):1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 24.Chong Ng Kee Kwong K, Mehta PR, Shukla G, Mehta AR. COVID-19, SARS and MERS: a neurological perspective. J Clin Neurosci. 2020;77:13–16. doi: 10.1016/j.jocn.2020.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palasca O, Santos A, Stolte C, Gorodkin J, Jensen LJ. TISSUES 2.0: an integrative web resource on mammalian tissue expression. Database (Oxford) 2018;2018:bay028. doi: 10.1093/database/bay028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107(6):1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Bai WZ, Hashikawa T. Response to commentary on:“The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients”. J Med Virol. 2020;2024:707–709. doi: 10.1002/jmv.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980;193(2):467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- 30.Hadziefendic S, Haxhiu MA. CNS innervation of vagal preganglionic neurons controlling peripheral airways: a transneuronal labeling study using pseudorabies virus. J Auton Nerv Syst. 1999;76(2–3):135–145. doi: 10.1016/s0165-1838(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 31.Turtle L (2020) Respiratory failure alone does not suggest central nervous system invasion by SARS-CoV-2. J Med Virol published online ahead of print, 2020 Apr 2024. 10.1002/jmv.25828 [DOI] [PubMed]
- 32.Mengeling WL, Boothe AD, Ritchie AE. Characteristics of a coronavirus (strain 67N) of pigs. Am J Vet Res. 1972;33(2):297–308. [PubMed] [Google Scholar]
- 33.Andries K, Pensaert MB. Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am J Vet Res. 1980;41(9):1372–1378. [PubMed] [Google Scholar]
- 34.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/jvi.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;2(28(3)):391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desforges M, Le Coupanec A, Brison É, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96. doi: 10.1007/978-81-322-1777-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H et al (2020) Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med: [Epub ahead of print 6 May 2020]. 10.7326/m20-2003 [DOI] [PMC free article] [PubMed]
- 38.Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13(4):379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita M, Yamate M, Li G-M, Ikuta K. Susceptibility of human and rat neural cell lines to infection by SARS-coronavirus. Biochem Biophys Res Commun. 2005;334(1):79–85. doi: 10.1016/j.bbrc.2005.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan JF, Chan KH, Choi GK, To KK. Tse H, Cai JP, Yeung ML, Cheng VC, Chen H, Che XY, Lau SK, Woo PC, Yuen KY. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J Infect Dis. 2013;207(11):1743–1752. doi: 10.1093/infdis/jit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholls JM, Butany J, Poon LL, Chan KH, Beh SL, Poutanen S, Peiris JM, Wong M. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3(2):e27. doi: 10.1371/journal.pmed.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desforges M, Miletti TC, Gagnon M, Talbot PJ. Activation of human monocytes after infection by human coronavirus 229E. Virus Res. 2007;130(1–2):228–240. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127(9):3210–3219. doi: 10.1172/jci90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endocrinology TLDa COVID-19: underlying metabolic health in the spotlight. Lancet Diabetes Endocrinol. 2020;8:457. doi: 10.1016/S2213-8587(20)30164-9. [DOI] [Google Scholar]
- 46.Gasmi A, Noor S, Tippairote T, Dadar M, Menzel A, Bjørklund G (2020) Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol 108409. 10.1016/j.clim.2020.108409 [DOI] [PMC free article] [PubMed]
- 47.Spiegel M, Schneider K, Weber F, Weidmann M, Hufert FT. Interaction of severe acute respiratory syndrome-associated coronavirus with dendritic cells. J Gen Virol. 2006;87(7):1953–1960. doi: 10.1099/vir.0.81624-0. [DOI] [PubMed] [Google Scholar]
- 48.Desforges M, Favreau DJ, Brison É, Desjardins J, Meessen-Pinard M, Jacomy H, Talbot PJ (2013) Human coronaviruses: respiratory pathogens revisited as infectious neuroinvasive, neurotropic, and neurovirulent agents
- 49.Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, Xin N, Huang Z et al (2020) Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol:bjophthalmol-2020-316304. 10.1136/bjophthalmol-2020-316304 [DOI] [PMC free article] [PubMed]
- 50.Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C, Herman P, Manley GT et al (2020) Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 10.1016/S1473-3099(20)30293-0 [DOI] [PMC free article] [PubMed]
- 51.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, Baldanti F, Daturi R, Postorino P, Cavallini A, Micieli G. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, Talbot PJ. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conde G, Pájaro LDQ, Marzola IDQ, Villegas YR, Salazar LRM. Neurotropism of SARS-CoV 2: mechanisms and manifestations. J Neurol Sci. 2020;412:116824. doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host–virus stand-off. Nat Rev Microbiol. 2006;4(2):121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desforges M, Le Coupanec A, Brison É, Meessen-Pinard M, Talbot PJ. Human respiratory coronaviruses: neuroinvasive, neurotropic and potentially neurovirulent pathogens. Virologie. 2014;18(1):5–16. doi: 10.1684/vir.2014.0544. [DOI] [PubMed] [Google Scholar]
- 56.Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;18:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94(May 2020):55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brison E, Jacomy H, Desforges M, Talbot PJ. Novel treatment with neuroprotective and antiviral properties against a neuroinvasive human respiratory virus. J Virol. 2014;88(3):1548–1563. doi: 10.1128/JVI.02972-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costa VV, Del Sarto JL, Rocha RF, Silva FR, Doria JG, Olmo IG, Marques RE, Queiroz-Junior CM, Foureaux G, Araújo JMS. N-methyl-d-aspartate (NMDA) receptor blockade prevents neuronal death induced by Zika virus infection. MBio. 2017;8(2):e00350–e00317. doi: 10.1128/mBio.00350-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manivannan S, Baxter VK, Schultz KL, Slusher BS, Griffin DE. Protective effects of glutamine antagonist 6-diazo-5-oxo-l-norleucine in mice with alphavirus encephalomyelitis. J Virol. 2016;90(20):9251–9262. doi: 10.1128/JVI.01045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]