Abstract
Laser-induced forward transfer is a versatile, non-contact, and nozzle-free printing technique which has demonstrated high potential for different printing applications with high resolution. In this article, three most widely used hydrogels in bioprinting (2% hyaluronic acid sodium salt, 1% methylcellulose, and 1% sodium alginate) were used to study laser printing processes. For this purpose, the authors applied a laser system based on a pulsed infrared laser (1064 nm wavelength, 8 ns pulse duration, 1 – 5 J/cm2 laser fluence, and 30 μm laser spot size). A high-speed shooting showed that the increase in fluence caused a sequential change in the transfer regimes: No transfer regime, optimal jetting regime with a single droplet transfer, high speed regime, turbulent regime, and plume regime. It was demonstrated that in the optimal jetting regime, which led to printing with single droplets, the size and volume of droplets transferred to the acceptor slide increased almost linearly with the increase of laser fluence. It was also shown that the maintenance of a stable temperature (±2°C) allowed for neglecting the temperature-induced viscosity change of hydrogels. It was determined that under room conditions (20°C, humidity 50%), the hydrogel layer, due to drying processes, decreased with a speed of about 8 μm/min, which could lead to a temporal variation of the transfer process parameters. The authors developed a practical algorithm that allowed quick configuration of the laser printing process on an applied experimental setup. The configuration is provided by the change of the easily tunable parameters: Laser pulse energy, laser spot size, the distance between the donor ribbon and acceptor plate, as well as the thickness of the hydrogel layer on the donor ribbon slide.
Keywords: LIFT, Laser-induced forward transfer, Hydrogel parameters, Optimal jetting regime, Jet and droplets parameters
1 Introduction and background
Laser-induced forward transfer (LIFT) is a digital printing technique that allows for the nozzle-free direct transfer of matter. This technology has been used (1) to print a wide range of biological materials, such as proteins[1,2] and DNA[3,4]; (2) to separate microorganisms[5]; (3) to print various types of cells to create three-dimensional (3D) bioequivalents of natural tissues[6-10]; and (4) to transfer stimulatory factors for cell differentiation[6-10].
For the printing process, one should prepare the ribbon (target, donor, or a source film). The ribbon usually consists of a glass slide on the surface, of which a nanolayer of laser-absorbing material (Au, Ti, etc.) is applied[8,11,12], which is then covered with a hydrogel-containing transferred objects.
Laser pulses pass through the uncoated transparent side of the ribbon and then focus on a layer that absorbs laser radiation. The partial absorption of the laser pulse energy by the absorbing layer leads to the rapid heating of its local area and of the adjacent thin layer of hydrogel, which leads to the formation of the high-pressure and high-temperature vapor bubble[13]. The rapid expansion of this vapored region leads to the formation of jets of various types[14] with the subsequent separation of one or more droplets and their transfer to the acceptor surface[15]. Finally, according to a predetermined design and with a high productivity of the process[16], a two-dimensional (2D)[17] and, if necessary, 3D[18] structure with the necessary concentration of transferred objects is fabricated from these microdroplets.
To become a truly universal method, laser bioprinting applying the LIFT technique must be an accurate technology that can guarantee the transfer of a strictly specified volume of hydrogel with minimal negative effects on living objects in the gel. The main difficulty in the work with this technology is the accurate selection of factors that significantly affect the process of laser transfer.
The characteristics of the laser printing process depend (Figure 1) on the (1) parameters of the laser impact (wavelength, laser pulse duration, focusing parameters, and energy); (2) ribbon factors (absorbing layer material, type of transferred objects, hydrogel parameters, and hydrogel layer thickness); and (3) external factors (experiment configuration, environmental factors, and transfer distance).
Figure 1.
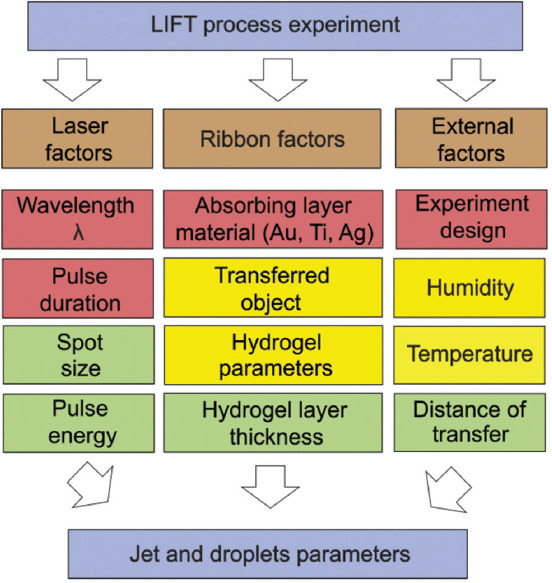
Factors affecting the laser-induced forward transfer printing process.
Red parameters, as a rule, are set by an experimental setup, and most often stay unchanged. Yellow parameters change, as a rule, when printed objects are changed. Green parameters are configured each time according to a particular experimental condition.
To implement the LIFT printing process, a laser system with a set of basic optomechanical components (3D stages and focusing optics) is required. The main component of the system is a laser source, which determines the main characteristics of the laser pulse. The most common, commercially available, and studied[19,20], laser sources have nanosecond pulse length[21-24] and a wavelength of λ = 1064 nm (near infrared range). Near-IR radiation is well absorbed by the metal absorbing layer, while remaining transparent to living tissues and cells[25,26]. It is also important that IR radiation, due to the low energy of quanta, practically does not affect the physicochemical characteristics of hydrogels[27] and does not damage cells[25]. It is worth noting that several studies Zhang et al.[28,29] used laser sources of the ultraviolet spectrum, and their radiation is well absorbed by the hydrogel material, which makes it possible to avoid using the absorbing layer. On the other hand, such short-wavelength laser radiation can change the physicochemical characteristics of hydrogels, and moreover, this radiation is well absorbed by living tissues and cells, which can be detrimental to them.
When choosing a laser source and an radiation optical focusing scheme, the following laser factors should be considered (Figure 1): Pulse duration, laser pulse energy, and laser spot size, since these parameters directly determine the energy density (fluence)[16,21,30,31], and peak laser power. However, the vapor bubble growth dynamics and all LIFT laser transfer process[19,21] depend on the pulse duration and absorbed fluence, which is, in turn, determined by the laser radiation absorption coefficient of the energy absorbing layer. The choice of material and thickness of the ribbon absorbing layer are associated with the need to provide the most efficient conversion of a laser pulse with a given wavelength λ into heat, while minimizing transmitted radiation, which can adversely affect biological objects contained in the hydrogel layer. It is also necessary to consider that, from a metal absorbing layer, laser irradiation can produce nanoparticles which are then transferred together with the hydrogel causing a negative effect on the transferred objects[13,32].
In addition to the parameters of laser exposure, the transfer process is also affected by the hydrogel parameters (viscosity, density, and surface tension)[16,33] and its thickness on the ribbon. The variation of these parameters allows changing the jetting dynamics and droplet formation[15] and achieving the optimal transfer regime with the formation of a stable jet without spraying[14], the formation of hydrogel droplets of the required volume[15], and the absence of unnecessary shock stresses on the transferred objects[13,34].
When transferring living objects using the LIFT technique, a major role is played by the controlled optimal “external factors” (Figure 1), such as: Temperature, humidity, and the composition of the gaseous medium ensuring the vital activity of the transferred objects, for example, various cell populations[6,23,35] or bacterial cultures[36]. During the printing process, it is necessary to provide “comfortable” conditions for the transferred objects, as well as to prevent changes in the parameters of ribbon and acceptor surfaces over time (for example, due to the drying of hydrogels).
For transferred objects, it is important to select the physicochemical characteristics of the hydrogel medium: The viscosity, surface tension, and the nature (polymer or inorganic). Furthermore, the presence of the additives (growth factors, proteins, etc.) in the gel helps to create the most favorable conditions for the metabolism of the transferred objects. In some cases, an important factor is also the geometric arrangement of droplets on the acceptor surface, which provides, in particular, the optimal overlap and droplet spacing of individual non-merging drops[37,38].
At present, a number of laser systems have been described in the scientific literature where LIFT printing is feasible[24,31]. However, it is worth noting that the selection of optimal parameters for a specific combination of laser-hydrogel microobjects actually represents a separate study. This is explained by the fact that the final result (high-quality pattern with the droplets of a given size) is determined by a combination of many interdependent factors (Figure 1). A change in one parameter may not lead to the desired effect due to the mutual influence of the parameters on each other. From this point of view, one of the actual challenges for LIFT technique is the development of the algorithm allowing for the selection of the optimal parameters for high-quality laser printing for each given laser system.
The purpose of this article is to develop an algorithm based on the determination of the operating ranges for laser fluence to achieve the optimal regime with the transfer of single microdroplets. The performed studies were carried out on the most common hydrogel materials for laser printing, such as hyaluronic acid sodium salt[39,40], methylcellulose[41], and sodium alginate[8,42,43].
2 Experiment
The main input parameters and working stages are presented in Figure 2. We studied the influence of various parameters on the printing process, as well as ways to optimize the experimental conditions to obtain the most stable and repeatable printing results.
Figure 2.
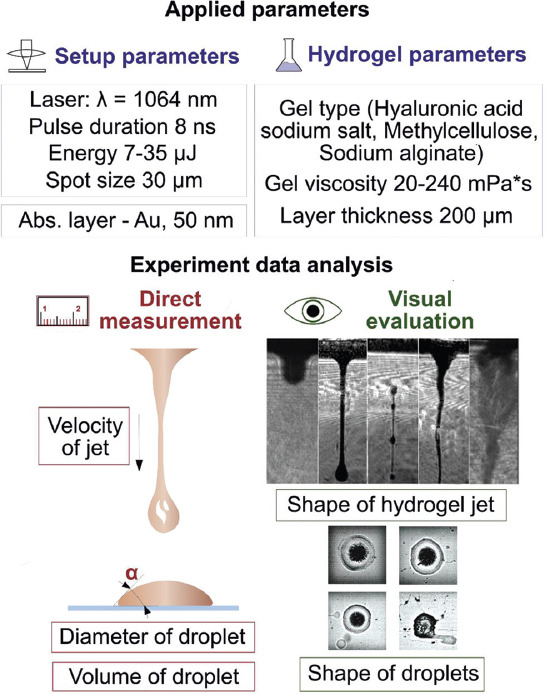
The main input parameters of the experiments and the scheme for evaluating the results.
2.1 Laser printing setup
The experimental setup used a pulsed fiber laser YLPM-1-4x200-20-20 (NTO “IRE-Polus,” Russia) with a wavelength of 1064 nm, with an approximate Gaussian intensity profile in the beam (m2 <1.3). Laser radiation was focused on an absorbing layer of the ribbon using a LscanH-10-1064 galvo scanning head (AtekoTM, Russia) with a F-theta SL-1064-110-160 lens (Ronar-Smith, Singapore) with a focal length of 160 mm. The system (Figure 3) formed a laser spot with diameter of 30 μm positioning it with an accuracy of several microns in the X-Y plane. The range of laser energies in the experiments ranged from 7 to 35 μJ with a pulse duration of 8 ns.
Figure 3.
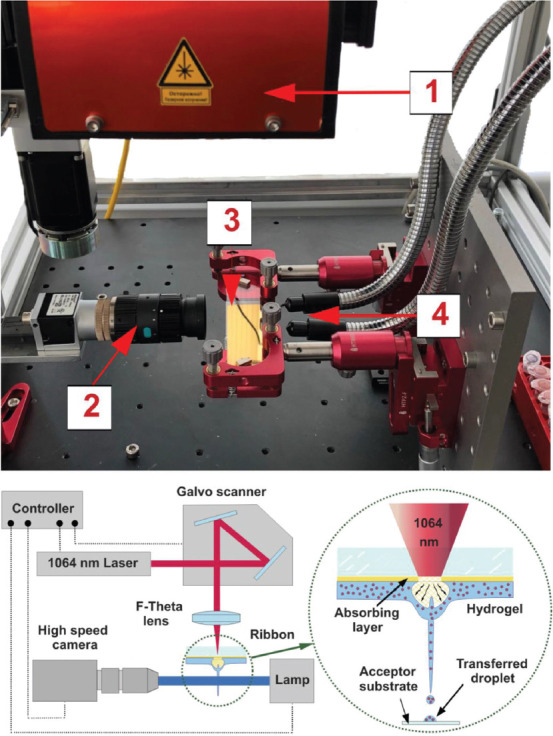
The image and scheme of the part of the experimental laser-induced forward transfer (LIFT) setup. On the top panel, (1) galvo scanner with F-theha lens, (2) digital camera, (3) ribbon, and (4) light source. The scheme of LIFT setup is shown on the bottom panel. The scheme of printing process is depicted in the circle.
The glass slides, the donor (ribbon) and acceptor, were mounted on the precision optical translators with the distance of 0.5 – 2 mm and were placed in the working plane parallel to the lens. Using a galvo scanning system, laser radiation was moved along the surface of the donor ribbon according to a predetermined pattern.
2.2 Ribbon preparation
For all printing presented experiments, 1-mm thick glass slides (25 × 75 mm) were purified by sequential exposure to isopropanol, deionized water, and acetone in an ultrasonic treatment for 30 s for each liquid, followed by purging with purified air. The slides were coated with a 50-nm thick gold layer by magnetron sputtering. The thickness of the deposited layers was controlled by a microinterferometer (Lomo Mii4, AO Lomo, Russia). A hydrogel layer 200 ± 20 μm thick was applied to the absorbing layer using blade-coater.
Importantly, we used a gel layer of relatively large thickness based on the following. The use of hydrogel layers with a thickness of more than 100 μm allows (1) a significant reduction of the effect of drying-induced hydrogel layer thickness change of the ribbon; (2) avoidance from strong shock stresses associated with the action of shock waves and a reduction of dynamic stresses during transfer[34]; (3) minimization of the negative effect of high temperature and nanoparticles produced by the absorbing layer[13,32,44]; and (4) an increase in system performance and the ability to transfer quite large objects, for example, cell spheroids.
2.3 Hydrogel viscosity and evaporation determination
For the experiments, the hydrogels were obtained by dissolving the powder of methylcellulose (Sigma-Aldrich – M0262), hyaluronic acid sodium salt (Contipro - HySilk), and sodium alginate (Sigma-Aldrich A-2033) in deionized water overnight with mass concentrations of 1%, 2%, and 1%, respectively.
The viscosity of the studied hydrogels was measured using a Kyoto Electronics Manufacturing EMS-1000 electromagnetically spinning viscometer. The measurements were carried out in the temperature range of 20 – 37°C, with exposure at each temperature point for 5 min. The drying intensity of the hydrogel layer on ribbon was estimated gravimetrically using Adventurer Pro AV114C (Ohaus, USA).
2.4 Measuring droplet sizes
The size of the transferred hydrogel microdroplets was measured using a direct optical microscope (Huvitz HRM-300). The volume of transferred droplets was calculated according to the contact angles and droplet diameter on the acceptor plate (Figure 2). The static contact angles of hydrogels were determined by the method of a sessile drop using an ACAM D-3 goniometer (APEX Instruments, India).
2.5 Visualization of jetting and measurement of jetting velocity
The printing and transfer processes of the material were visualized and investigated using a high-speed Fastcam SA-3 camera (Photron, Japan) (shutter 1/60000, FPS 10000-60000) with a long-focus microscopic lens providing a shooting field of up to 5 × 3.5 mm. The illumination was performed using a K150 Laboratory Illuminator (Optics Co, China).
3 Results and discussion
3.1 General observations
The LIFT process is based on local heating occurring during the interaction of a laser pulse with an absorbing layer, which leads to explosive boiling of water in a gel[13], while more intensive heating can lead to explosive boiling of the absorbing layer material[45]. As a result, a high-pressure bubble is formed[46-48], the expansion of which leads, depending on the initial parameters[15], to the formation of various types of jets[49], and the separation of one or more droplets and their subsequent transfer to an acceptor plate[15]. The processes associated with the formation and collapse of a gas bubble, as well as possible regimes of jet formation are represented in Figure 4.
Figure 4.
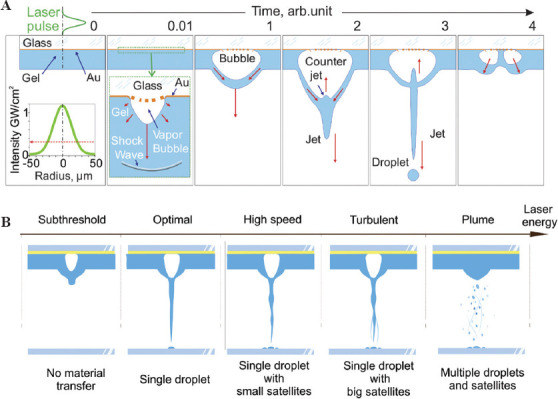
(A) The main stages of the jet and droplet formation during laser printing. Red arrows indicate the direction of fluid movement (B) changes in jetting regimes with the increase of laser energy.
At the first stage of the LIFT process (t = 0.01 a.u.), the metal energy absorbing layer, the nearest thin layers of the glass slide, and the hydrogel are heated to high temperatures due to the partial absorption of the laser pulse energy by the energy absorbing layer. In the applied laser printing regimes, the achieved temperature significantly exceeds the melting temperature of the material of the absorbing layer (for Au T≈1337 K)[13]. On reaching a temperature of ~ 0.95 from the critical temperature (for water Tc = 647 K), the water contained in the gel boils explosively[34], which converts the water into steam compressed to a high (around critical) pressure (Pc ~ 22.5 MPa). As a result, a rapidly expanding vapor bubble forms in the gel in the area of laser exposure.
At t = 1, the size of this bubble already exceeds the thickness of the gel. Due to the hydrogel viscosity, the presence of a solid surface of the donor plate and the limited thickness of the gel, this bubble becomes easier to expand along the optical axis, but not across it. All these factors generate a pressure gradient along the bubble wall leading to the gel flows from the periphery to the optical axe center of the bubble top. The interaction of these flows leads at t = 2 to the appearance of a jet and a counter jet.
As the bubble expands, the pressure inside it drops monotonically and its expansion at the stages t = 1 and t = 2 occurs by inertia. When the bubble reaches its maximum size, the pressure inside it decreases almost to zero and the bubble begins to compress (t = 3) due to the pressure difference outside (≈105 Pa)[48] and inside it. The bubble apex serves as the base of an elongated jet, from which a microdroplet detaches due to Rayleigh–Plateau instability. Depending on the selected regime, the jet can move back and be completely absorbed by the gel layer or it can separate and continue the independent movement toward the acceptor plate or it can break up into several fragments.
At stage t = 4, the microdroplet (not shown in the figure) continues to move toward the acceptor plate or has already reached its surface. Having collapsed, the bubble begins to expand again. Moreover, since its center is substituted with by fragments of the counter jet, its shape looks like a donut. The maximum speeds of the outer walls of this “donut” are directed at a certain angle to the optical axis. At sufficiently high laser energies, hydrogel splashing can occur in these directions.
3.2 Regimes of jet formation
In this part, we will discuss the regimes of jet formation in more detail. The energy of a laser pulse directly sets the amount of transferred energy which leads to the formation of a gas bubble[47]. The effect of the kinetic energy transferred to the jet/droplet on the printing process is well studied in the literature[14,15]. Figure 4B shows five basic laser transfer regimes that determine the amount of transferred material and printing quality[14,15,45]. At low values of laser energy (subthreshold regime) a small jet forms and does not transfer the material. When a certain energy threshold is overpassed (optimal regime), a part of the jet begins to separate and transfer in the form of a single droplet onto an acceptor plate. With a further increase in the laser pulse energy, the length and velocity of the transferring jet increase, which lead to the transfer, besides the main droplet, of small satellite droplets (high speed regime). On reaching the energies leading to turbulent jets or the formation of several jets simultaneously (turbulent regime), large satellite drops are transferred along with the main droplet. With a further increase in laser energy, the energy stored in the expanding bubble becomes so large that a rupture of the outer wall of the bubble (plume regime) occurs, leading to a chaotic transfer of hydrogel both in shape and volume.
The best printing quality achievable in laser transferring can only be realized in a relatively narrow range of laser energies corresponding to the optimal regime and leading to the transfer of a single droplet. It is worth noting that usually for describing the LIFT process, instead of the parameter “laser pulse energy,” the parameter “laser fluence” is used, which is determined by the ratio of laser pulse energy to laser spot size and measured in J/cm2. Laser fluence is a more universal parameter, since the main role in the transfer processes is played by the radiation energy density. In our experiments, when the energy changed from 7 to 25 μJ while maintaining a spot size of 30 μm, the fluence range was 1 – 5 J/cm2.
To assess the effect of laser fluence on the jetting regime and jetting velocity, we used a high-speed shooting of the printing process. The LIFT occurred between the donor ribbon and acceptor plate located at a distance of 1 mm.
Figure 5 shows that using hyaluronic acid sodium salt (2%) as an example, with small fluence (up to 1.7 J/cm2), the transfer process did not occur. With an increase in laser fluence, there was a transition to the optimal jetting regime with single droplet transferring (2.0 J/cm2), then to the high-speed regime (2.5 J/cm2), and, finally, to the turbulent regime (3.7 J/cm2).
Figure 5.
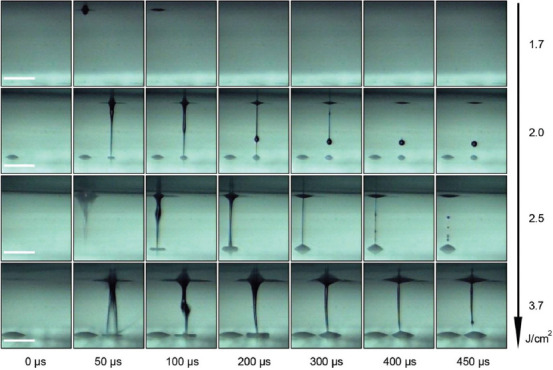
Time resolved images of the hyaluronic acid sodium salt (2%) transferring process for various laser fluences. Scale bar: 400 µ.
During the process of laser printing, the living objects transferred inside the jet practically do not experience any mechanical and thermal stresses. They experience significant dynamic stresses only at the initial moment of bubble and jet formation, as well as at the final moment during the impingement of a gel microdroplet with an acceptor plate (or landing)[50]. Hence, according to the calculations, at a jet speed of 50 m/s, the accelerations can reach 7 × 108 m/s2 at the initial moment and 6 × 109 m/s2 during the landing process. Caused by dramatic accelerations and fluid dynamics, mechanical stress can have a negative effect on transferred objects, for example, it can lead to severe deformation of cells, rupture of their membranes and subsequent cell deaths[50]. As shown in Wang and Chrisey[50], the acceleration at the final stage can be reduced by almost an order of magnitude to 7 × 108 m/s2 when the acceptor plate is coated with a 40-μm thick acceptor gel layer. Such a coating during landing significantly reduces dynamic stress, which leads to a decrease in mechanical damage of the cells and increases post-transfer cell viability up to 95%[51]. It is worth noting that with an increase in the thickness of the gel on the acceptor plate, dynamic stress will decrease. However, with a large thickness of the gel, negative effects associated with immersion of cells at a great depth can be observed, which are explained by a decrease in cell metabolism due to diffusion limitations[52].
The whole process, ending with droplet transfer, begins with the absorption of the laser pulse and rapid heating to high temperatures of the area of the absorbing layer and nearby thin layers of the donor glass plate and gel. In the case of short (nanosecond) pulses, when it can be assumed that heat spreads only in the direction of the optical axis, the increase in temperature under laser irradiation with fluence F0 will be:

where a is the absorption coefficient of laser radiation by a metal energy absorbing layer; ρEff, CPEff, and hEff are the effective density, heat capacity, and thickness of a thin heated gel/metal / glass layer. The hEff value is determined by the thermal diffusion length of the donor plate materials (fused silica) and gel: where τ is the time, at which the temperature reaches its maximum, which in most cases are equal to the laser pulse duration, D – is the thermal diffusivity[53]. Taking into account Dsilica = 5.8 × 10−7 m2/s and Dwater = 1.4 × 10−7 m2/s, at τ = 8 ns we get: hEff = 68 nm + 34 nm = 102 nm. Thus, all the main processes of heat transfer are associated with the heating of this thin layer.
3.3 Effect of laser pulse fluence on printed droplets
It is known that the key role in controlling the LIFT process is played by laser fluence[19,30]. Changing the laser fluence allows for controlling the diameter and volume of the transferred droplets, which determine the resolution and throughput of printing as well as the ability to transfer objects of various sizes from single cells to cell spheroids. Figure 6 shows the influence of laser fluence on the diameter and volume of droplets for different hydrogels.
Figure 6.
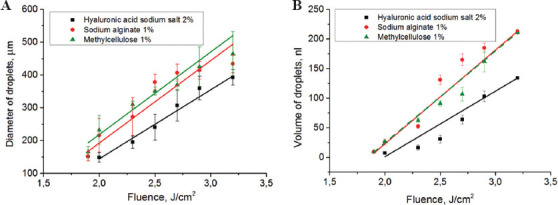
Relationship between laser fluence, diameter, and volume of transferred droplets. (A) Droplet diameters (inserted image illustrates the transferred droplets of 2% hyaluronic acid sodium salt, scale bar: 100 µm); (B) droplet volumes. For all relationships, linear trends are plotted with the error considered. The measurements were carried out in the optimal transfer regime.
The volumes of transferred droplets were calculated by measuring their contact angles on the acceptor plate, particularly, for the applied hydrogels the contact angles were: 2% hyaluronic acid sodium salt −21.5° ± 0.4, 1% sodium alginate −17.4° ± 0.3, and 1% methylcellulose −22.4° ± 0.6. From the data obtained, it follows that for the optimal jetting regime, the increase in the diameter and volume of the transferred droplets has linear-like relationship with laser fluence. In this case, the smallest droplets are obtained for hyaluronic acid-based hydrogel. For other two hydrogels, the obtained values of the diameter and volume of the transferred droplets are practically the same for most of the fluences applied.
For all the gels used in this work, various transfer regimes were defined depending on the change in laser pulse energy. It was realized by conducting a series of experiments with a high-speed shooting of transfer processes, where we the measured the resulting jet velocities, the diameters and volumes of the droplets formed on the acceptor plate. The measurement results for the all gel types are represented in Figure 7.
Figure 7.
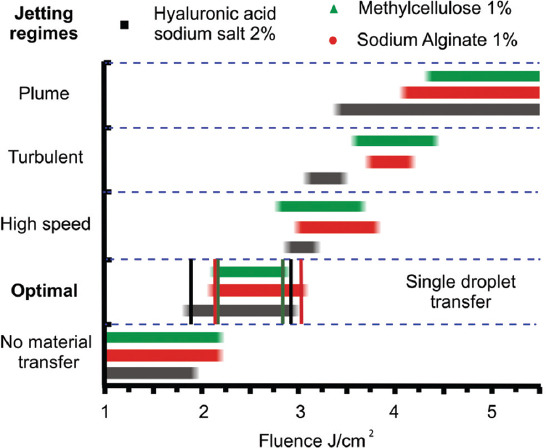
Relationship between laser-induced forward transfer regimes and laser fluences for three gel types. Vertical lines indicate the corresponding boundaries of the optimal range.
From Figure 7, it follows that for all the gels used in the experiment, the optimal laser fluence range for the formation of a single jet and stable transfer of hydrogel droplets is between 2 and 3 J/cm2. For optimal transfer regime, the widest fluence range is observed for hyaluronic acid sodium salt, while the narrowest fluence range is observed for methylcellulose. That is, the use of 2% solution of hyaluronic acid sodium salt provides the most stable transfer of gel droplets. In this case, small changes in the parameters of the hydrogel, the laser fluence, and the parameters of the absorbing layer of the plate will not approve the transition to other non-optimal printing regimes.
3.4 The environment-dependent parameters of the hydrogel
This section discusses the influence of external factors (experiment temperature and experiment time) on the viscosity of the hydrogel layer and its drying on the donor ribbon. The vast majority of articles pay insufficient attention to the drying-induced change of the hydrogel layer parameters during the experiment. When the hydrogel layer dries, the changes in its viscosity and thickness can significantly impact the process of laser transfer. The viscosity of the hydrogel layer directly determines (1) the required laser fluence to provide the optimal transfer regime, (2) the shape and size of the jet[54,55], as well as (3) the resulting droplet volume on the acceptor plate.
In our experiments, the viscosities of hydrogels were determined at temperatures ranging from 20 to 37 °C. These temperatures were chosen deliberately, since they covered the range from standard (room) temperature to standard cell culture temperatures. A temperature change had a similar effect on the viscosity of hydrogels based on sodium alginate and hyaluronic acid sodium salt: An increase in temperature by 4°C led to a decrease in viscosity by an average of 9%. In the case of methylcellulose, the viscosity of the solutions also decreased with an increase in temperature for every 4°C by approximately 14% (Table 1). Importantly, all the obtained viscosity values are suitable for LIFT: According to Hölzl et al.[56], LIFT technology utilizes hydrogels with a viscosity ranging from 1 to 300 mPa*s.
Table 1.
The relationship between the hydrogel viscosity and ambient temperature.
| Hydrogel | Viscosity (mPa*s) | ||||
|---|---|---|---|---|---|
| Temperature, °C | 20 | 24 | 28 | 32 | 37 |
| Methylcellulose 1% w/v | 231±12 | 194±11 | 170±12 | 133±11 | 104±13 |
| Sodium alginate 1% w/v | 76±3 | 68±2 | 61±3 | 56±4 | 50±5 |
| Hyaluronic acid sodium salt 2% w/v | 20±2 | 18±2 | 16±1 | 15±1 | 13±1 |
Table 1 shows that with a change in temperature in the range of 20–37° C, the viscosity of hydrogels can reduce by almost 2 times, which must be considered in experiments.
In addition to controlling the ambient temperature during the experiment, one should also monitor the drying of the hydrogel layer on the donor ribbon and printed drops on the acceptor plate, which is largely dependent on, besides the temperature, the air humidity. The high drying rate of the gel layer covered on the donor ribbon can lead to a change in its thickness and viscosity during the experiment, which will directly affect the printing regimes and landed droplet sizes. The rapid drying of the gel on an acceptor plate, in particular, leads to the change of gel droplet shapes resulting in the “coffee-ring” effect[21] observed within few minutes after printing.
In our experiments, at a relative humidity of 50%, an ambient temperature of 20°C, and with the use of relatively thick hydrogel layers (200 ± 20 μm) within 30 min after applying the gel layer to ribbon, a mass loss of more than 60% was observed (Table 2).
Table 2.
The time-dependent hydrogel mass changes.
| Hydrogel | Hydrogel layer weight (% of the initial) | ||||
|---|---|---|---|---|---|
| Time, min | 0 | 2 | 5 | 10 | 30 |
| Methylcellulose1% w/v | 100 | 92.2±0.4 | 82±2 | 67±4 | 35±4 |
| Sodium alginate 1% w/v | 100 | 91.6±0.4 | 81±2 | 68±3 | 32±4 |
| Hyaluronic acid sodium salt 2% w/v | 100 | 92.1±0.4 | 82±2 | 68±3 | 27±5 |
However, glycerol can be added to prevent the hydrogel from drying; for example, in one study [21] introduced 10% (v/v) of glycerol into all printing solutions. Nevertheless, it should be borne in mind that an increase in glycerol concentration above 10% (v/v) is detrimental for cell survival[21]. Furthermore, glycerol should be added to protein-containing solutions with caution, since glycerin is considered to be a denaturing agent[57]. Alternatively, in another study[58], the authors added methylcellulose to reduce the drying rate of the hydrogel layer.
Thus, to ensure they stable controlled laser printing, it is necessary to maintain a constant ambient temperature and consider the high drying rate of hydrogels (~ 8 μm/min) under standard conditions during the experiment (Table 2). For example, within 5 min, the mass of the hydrogel (and, therefore, its thickness on the donor ribbon) decreases by 20%, which is beyond the measurement error (10%). Therefore, to obtain droplets of the same size within the experiment, one should finish it in few minutes, or organize additional donor ribbon moisturizing, or introduce the drying-preventing agents into the hydrogel.
4 Decision tree for controlled hydrogel transfer
For more than 15 years, various printing methods for the transfer of biomaterials have been studied[56,59]. Regarding laser printing methods, they have the best resolution as well as provide cell survival during the transfer at the level of 95% or more[10,56]. One of the main difficulties in applying the LIFT technique for biological applications is the need for individual selection of a large number of parameters for the combination “hydrogel-transferred object”[59]. For example, two studies[14,15] on LIFT printing establish the detailed relationships between printing parameters, the regimes of hydrogel jet and droplet formation using sodium alginate in various concentrations as a model. Moreover, the authors of several research articles[15,60] mainly operate with the (1) Weber Number (We), which characterizes the ratio between inertial force and surface tension, and the (2) Ohnesorge number (Oh), which describes the process of droplet formation. These dimensionless quantities comprehensively characterize printing regimes:
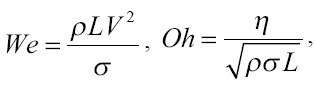
where ρ, hydrogel density; L, characteristic length which in many cases is determined by the spot size[14,60,61]; V, fluid gel speed; σ, surface tension; η, the shear viscosity of the gel.
In one study,[61] the authors suggested using the inverse number J = 1/Oh. It is shown that jets form well in the range of 0.86 ≤J ≤2.49 at F = 717 ± 45 mJ/cm2. With an increase in fluence, jet formation occurs well at a slightly higher viscosity (lower J).
Hydrogel parameters are very important for the jet formation. The characteristic time Δτ for the destruction of a cylindrical jet of radius (R) with the density (ρ) and surface tension σ as a result of Plateau–Rayleigh instability is[62]:
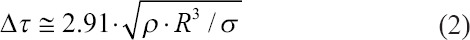
In the case of R = 50 μm, with σ = 73 × 10−3 N/m, we obtain Δτ = 120 μs with a radius of microdroplets of ~ 9 R[62]. It was established[54] that at a low gel viscosity, the gas bubble does not form and breaks at the initial stage. In this case, the lower working value of the viscosity at which the formation of the gel jet occurs decreases with decreasing spot size.
The We and Oh ranges for optimal printing regimes have already been described in the literature. The works[15,59] present an algorithm in the form of a decision tree that helps to find the optimal printing regimes for specific gels in terms of the We and Oh values. However, in practice, usually there is a task of transferring certain bioink with unique physicochemical characteristics at an already-made laser system. Moreover, there is also sometimes no possibility of a significant change in the viscosity and surface tension of the hydrogel and adapting them to the parameters known in the literature. The task of identifying the optimal regime for a given bioink is quite complicated, and it may require a separate study using high-speed shooting for the selection of We and Oh values[14,15,60] to ensure the optimal transfer regime. Therefore, we want to suggest a simplified decision tree based on our results. Following this decision tree, you can get a successful printing, changing the most accessible parameters and analyzing the results without using additional expensive equipment such as high-speed shooting.
From a practical point of view, the most optimal algorithm for producing “quality” droplets of a given size is based on the variation of easily changeable parameters of the laser, such as energy per pulse (E) and spot size (SS), as well as at the distance between the donor and acceptor (D). In some cases (for example, if very large or very small droplets are needed), the thickness of the hydrogel layer (T) on the donor ribbon can also be varied.
Figure 8 shows a decision tree, which allows for achieving an optimal printing regime by sequential selection of easily variable parameters for a particular experiment.
Figure 8.
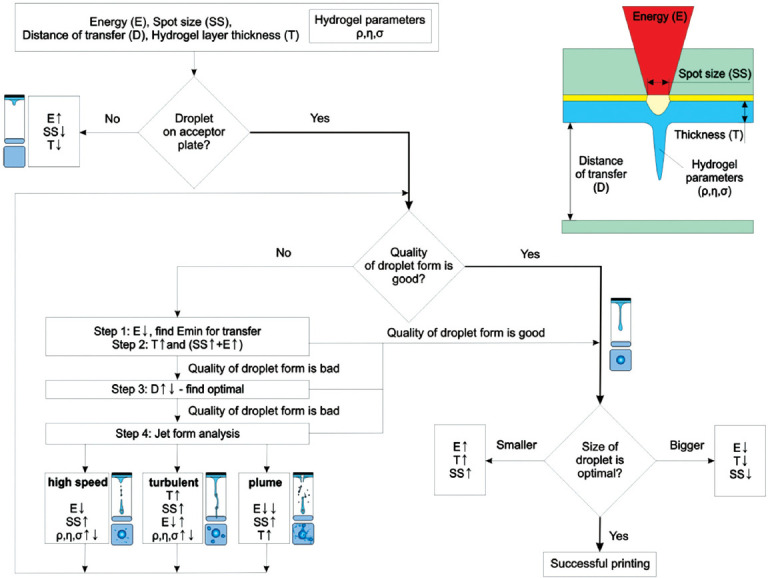
Decision tree for achieving a success story with laser-induced forward transfer. The symbol “↓” represents a decrease, “↑” an increase, and “↓ ↑” a variation.
The scheme shows an algorithm that allows for quick configuration of the laser printing process to achieve the optimal regime, which forms “quality” droplets from the bioink with the given parameters. Based on the technical specifications for the formation of 2D or 3D structures, the optimal diameter of hydrogel droplets is determined. Subsequently, the parameters of the experiment, namely, Energy (E), Distance (D), Thickness (T), and Spot size (SS) are set and a trial transfer is performed. Based on the results of our works[13,32,44,63,64] and the results of other authors[14,15,54], for a wide variety of hydrogels[24,65,66], the threshold fluence is 2 – 3 J/cm2 (nanosecond laser and Au absorbing layer). In practice, the starting values for the experiments can be: E = 15 – 20 μJ and SS = 30 μm for a laser with a wavelength of 1064 nm and a pulse duration of ~ 10 ns. Such values allow successful realization of laser transfer process with most hydrogel types.
If no hydrogel transfer toward acceptor plate occurs, one should gradually increase the laser pulse energy and reduce the spot diameter and gel layer thickness to initiate the transfer process. When the gel transfer is achieved, the next step assesses the “quality” of the droplet shape by its appearance. If the transfer is carried out with a droplet of an irregular shape or with several droplets (splashing), then it is necessary to gradually adjust the experimental parameters. First, it is necessary to find the minimum laser pulse energy (Step 1, Figure 8) sufficient for droplet transfer. Second, it is advisable to increase the thickness of the gel (Step 2, Figure 8), while raising the energy of the laser pulse and spot diameter to achieve the required droplet size.
If after the implementation of these steps the quality of the droplet is still poor, then this is probably due to suboptimal distance between the donor and acceptor slides. Usually, it occurs if instead of a single droplet, the acceptor plate is reached by the forming jet, which leads to its splashing. Therefore, in the next step, it is advisable to increase the distance between the donor and acceptor slides. However, it will be wrong to conclude that for the implementation of high-quality printing, a long distance is always necessary, since with its increase, the printing quality will deteriorate. This deterioration is associated with the horizontal velocity component of transferred droplets, which emerges due to hydrogel heterogeneity and no axial symmetry in absorbing layer heating. In some cases, one can encounter a regime when the jet breaks up into several droplets landing on the acceptor plate. In this case, as the distance increases, the shape of the resulting droplet may distort thus demonstrating a spraying effect[15]. Therefore, we believe that it is necessary to vary the entire range of distances between the donor and acceptor plates to detect the optimal transfer regime (Step 3, Figure 8). If the change of the distance D still does not yield a “quality” droplet shape, then it is necessary to analyze the operating regimes of jet formation. It can be performed using high-speed shooting or, if it is unavailable, by the analysis of the landed droplet images (Step 4, Figure 8). Further, depending on the registered transfer regimes (high-speed, turbulent, and plume), it is necessary to select parameters according to the mentioned algorithm. In addition, high-speed shooting can help determine the optimal distance D between the donor and acceptor slides.
On reaching the regime resulting in “quality” droplets, further it may be necessary to adjust their size to the required parameters of the experiment. At the first stage, for small adjustments, it will be sufficient to change the laser pulse energy. However, for significant adjustments, one should simultaneously change several parameters, since otherwise, optimal transfer regime will be lost.
5 Conclusion
We investigated laser printing processes using three most widely used hydrogels in bioprinting: 2% hyaluronic acid sodium salt, 1% methylcellulose, and 1% sodium alginate. For all gels, various transfer regimes (no transfer mode, optimal jetting mode with single droplet transfer, high speed mode, turbulent mode, and plume mode) were determined depending on the change in laser pulse energy. It was shown that in the optimal jetting regime, for all hydrogels, the size and volume of droplets increased almost linearly with increasing laser fluence. The effect of ambient temperature on the viscosity of hydrogels was also evaluated. It was shown that at room temperature (22 ± 2°C), this change can be neglected. For the experiments where no additional methods used for preventing ribbon hydrogel drying, we determined the time periods which provide a stable controlled laser printing and which allow for neglecting the drying-induced hydrogel thickness change. Moreover, we suggested a simple practical algorithm for quick setting up of the LIFT printing process to obtain “quality” bioink droplets with the required parameters on a given experimental setup. Furthermore, this setting is mainly provided by easily changing variable parameters, such as laser pulse energy, laser spot size, the distance between the donor ribbon and acceptor slide, as well as the thickness of the hydrogel layer on the donor ribbon.
Acknowledgments
This study was supported partially by the Russian Foundation Research (19-75-00108, 3D laser printing), Russian Foundation for Basic Research (18-32-20184, experimental setup and discussion of transfer mechanism) partially by the Ministry of Science and Higher Education within the State assignment FSRC “Crystallography and Photonics” RAS (lift process analysis), partially by the Russian academic excellence project ‘5-100’ (hydrogel support and analysis).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Serra P, Fernández-Pradas JM, Berthet FX, et al. Laser Direct Writing of Biomolecule Microarrays. Appl Phys A Mater Sci Process. 2004;79:949–952. DOI:10.1007/s00339-004-2577-2. [Google Scholar]
- 2.Zergioti I, Karaiskou A, Papazoglou DG, et al. Femtosecond Laser Microprinting of Biomaterials. Appl Phys Lett. 2005;86:1–3. DOI:10.1063/1.1906325. [Google Scholar]
- 3.Colina M, Serra P, Fernández-Pradas JM, et al. DNA Deposition through Laser Induced Forward Transfer. Biosens. Bioelectron. 2005;20(8):1638–1642. doi: 10.1016/j.bios.2004.08.047. DOI:10.1016/j.bios.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Serra P, Colina M, Fernández-Pradas JM, et al. Preparation of Functional DNA Microarrays through Laser-induced Forward Transfer. Appl Phys Lett. 2004;85:1639–1641. DOI:10.1063/1.1787614. [Google Scholar]
- 5.Cheptsov VS, Tsypina SI, Minaev NV, et al. New Microorganism Isolation Techniques with Emphasis on Laser Printing. Int J Bioprinting. 2019;5:1–12. doi: 10.18063/ijb.v5i1.165. DOI:10.18063/ijb.v5i1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch L, Deiwick A, Schlie S, et al. Skin Tissue Generation by Laser Cell Printing. Biotechnol. Bioeng. 2012;109:1855–1863. doi: 10.1002/bit.24455. DOI:10.1002/bit.24455. [DOI] [PubMed] [Google Scholar]
- 7.Gaebel R, Liu J, Koch L, et al. Patterning Human Stem Cells and Endothelial Cells with Laser Printing for Cardiac Regeneration. Biomaterials. 2011;32:9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. DOI:10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 8.Gaebel R, Kuhn S, Sorg H, et al. Laser printing of skin cells and human stem cells. Tissue Eng Part C Methods. 2009;16:847–854. doi: 10.1089/ten.TEC.2009.0397. DOI:10.1089/ten.tec.2009.0397. [DOI] [PubMed] [Google Scholar]
- 9.Mandrycky C, Wang Z, Kim K, et al. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016;34:422–434. doi: 10.1016/j.biotechadv.2015.12.011. DOI:10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch L, Kuhn S, Sorg H, et al. Laser Printing of Skin Cells and Human Stem Cells. Tissue Eng Part C Methods. 2010;16:847–854. doi: 10.1089/ten.TEC.2009.0397. DOI:10.1089/ten.tec.2009.0397. [DOI] [PubMed] [Google Scholar]
- 11.Catros S, Fricain JC, Guillotin B, et al. Laser-assisted Bioprinting for Creating on-demand Patterns of Human Osteoprogenitor Cells and Nano-hydroxyapatite. Biofabrication. 2011;3:025001. doi: 10.1088/1758-5082/3/2/025001. DOI:10.1088/1758-5082/3/2/025001. [DOI] [PubMed] [Google Scholar]
- 12.Hopp B, Smausz T, Antal Z, et al. Absorbing Film Assisted Laser Induced Forward Transfer of Fungi (Trichoderma conidia) J Appl Phys. 2004;96:3478–3481. DOI:10.1063/1.1782275. [Google Scholar]
- 13.Yusupov VI, Zhigarkov VS, Churbanova ES, et al. Laser-induced Transfer of Gel Microdroplets for Cell Printing. Quantum Electron. 2017;47:1158–1165. DOI:10.1070/qel16512. [Google Scholar]
- 14.Zhang Z, Xiong R, Mei R, et al. Time-Resolved Imaging Study of Jetting Dynamics during Laser Printing of Viscoelastic Alginate Solutions. Langmuir. 2015;31:6447–6456. doi: 10.1021/acs.langmuir.5b00919. DOI:10.1021/acs.langmuir.5b00919. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Xiong R, Corr DT, et al. Study of Impingement Types and Printing Quality during Laser Printing of Viscoelastic Alginate Solutions. Langmuir. 2016;32:3004–3014. doi: 10.1021/acs.langmuir.6b00220. DOI:10.1021/acs.langmuir.6b00220. [DOI] [PubMed] [Google Scholar]
- 16.Guillotin B, Souquet A, Catros S, et al. Laser Assisted Bioprinting of Engineered Tissue with High Cell Density and Microscale Organization. Biomaterials. 2010;31:7250–7256. doi: 10.1016/j.biomaterials.2010.05.055. DOI:10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 17.Gaebel R, Ma N, Liu J, et al. Patterning Human Stem Cells and Endothelial Cells with Laser Printing for Cardiac Regeneration. Biomaterials. 2011;32:9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. DOI:10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 18.Gruene M, Pflaum M, Hess C, et al. Laser Printing of Three-dimensional Multicellular Arrays for Studies of Cell-cell and Cell-environment Interactions. Tissue Eng Part C Methods. 2011;17:973–982. doi: 10.1089/ten.tec.2011.0185. DOI:10.1089/ten.tec.2011.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch L, Brandt O, Deiwick A, et al. Laser-assisted Bioprinting at Different Wavelengths and Pulse Durations with a Metal Dynamic Release Layer:A Parametric Study. Int J Bioprinting. 2017;3:42–53. doi: 10.18063/IJB.2017.01.001. DOI:10.18063/ijb.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorkio A, Koch L, Koivusalo L, et al. Human Stem Cell Based Corneal Tissue Mimicking Structures Using Laser-assisted 3D Bioprinting and Functional Bioinks. Biomaterials. 2018;171:57–71. doi: 10.1016/j.biomaterials.2018.04.034. DOI:10.1016/j.biomaterials.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Guillemot F, Souquet A, Catros S, et al. High-throughput Laser Printing of Cells and Biomaterials for Tissue Engineering. Acta Biomater. 2010;6:2494–2500. doi: 10.1016/j.actbio.2009.09.029. DOI:10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Catros S, Guillemot F, Nandakumar A, et al. Layer-by-Layer Tissue Microfabrication Supports Cell Proliferation In Vitro and In Vivo. Tissue Eng Part C Methods. 2011;18:62–70. doi: 10.1089/ten.TEC.2011.0382. DOI:10.1089/ten.tec.2011.0382. [DOI] [PubMed] [Google Scholar]
- 23.Michael S, Sorg H, Peck CT, et al. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS One. 2013;8:e57741. doi: 10.1371/journal.pone.0057741. DOI:10.1371/journal.pone.0057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antoshin AA, Churbanov SN, Minaev NV, et al. LIFT-Bioprinting, is it Worth it? Bioprinting. 2019;15:e00052. DOI:10.1016/j.bprint.2019 e00052. [Google Scholar]
- 25.Bashkatov AN, Genina EA, Kochubey VI, et al. Optical Properties of Human Skin Subcutaneous and Mucous Tissues in the Wavelength Range from 400 to 2000 nm. J Phys D Appl Physics. 2005;38:2543–2555. DOI:10.1088/0022-y3727/38/15/004. [Google Scholar]
- 26.Carvalho S, Gueiral N, Nogueira E, et al. Comparative Study of the Optical Properties of Colon Mucosa and Colon Precancerous Polyps between 400 and 1000 nm. Dynamics and Fluctuations in Biomedical Photonics. XIVXIV International Society for Optics and Photonics. 2017;10063:100631L. DOI:10.1117/12.2253023. [Google Scholar]
- 27.Pagès E, Rémy M, Kériquel V, et al. Creation of Highly Defined Mesenchymal Stem Cell Patterns in Three Dimensions by Laser-Assisted Bioprinting. J Nanotechnol Eng Med. 2015;6:021005. DOI:10.1115/1.4031217. [Google Scholar]
- 28.Zhang Z, Xu C, Xiong R, et al. Effects of Living Cells on the Bioink Printability during Laser Printing. Biomicrofluidics. 2017;11:034120. doi: 10.1063/1.4985652. DOI:10.1063/1.4985652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Chai W, Xiong R, et al. Printing-induced Cell Injury Evaluation during Laser Printing of 3T3 Mouse Fibroblasts. Biofabrication. 2017;9:025038. doi: 10.1088/1758-5090/aa6ed9. DOI:10.1088/1758-5090/aa6ed9. [DOI] [PubMed] [Google Scholar]
- 30.Unger C, Gruene M, Koch L, et al. Time-resolved Imaging of Hydrogel Printing Via Laser-induced Forward Transfer. Appl Phys A Mater Sci Process. 2011;103:271–277. DOI:10.1007/s00339-010-6030-4. [Google Scholar]
- 31.Desrus H, Chassagne B, Catros S, et al. Laser Assisted Bioprinting using a Femtosecond Laser with and without a Gold Transductive Layer:A Parametric Study Proceedings Volume. Optical Interactions with Tissue and Cells XXVII. 2016:9706. DOI:10.1117/12.2209087. [Google Scholar]
- 32.Cheptsov VS, Churbanova ES, Yusupov VI, et al. Laser Printing of Microbial Systems:Effect of Absorbing Metal Film. Lett Appl Microbiol. 2018;67:544–549. doi: 10.1111/lam.13074. DOI:10.1111/lam.13074. [DOI] [PubMed] [Google Scholar]
- 33.Riester D, Budde J, Gach C, et al. High Speed Photography of Laser Induced Forward Transfer (LIFT) of Single and Double-layered Transfer Layers for Single Cell Transfer. J Laser Micro Nanoeng. 2016;11:199–203. DOI:10.2961/jlmn.2016.02.0010. [Google Scholar]
- 34.Zarubin VP, Zhigarkov VS, Yusupov VI, et al. Physical Processes Affecting the Survival of Microbiological Systems in Laser Printing of Gel Droplets. Quantum Electron. 2019;49:1068–1073. DOI:10.1070/qel17081. [Google Scholar]
- 35.Tomasina S, Bodet C, Mota T, et al. Bioprinting Vasculature:Materials, Cells and Emergent Techniques. Materials (Basel) 2019;12:2701. doi: 10.3390/ma12172701. DOI:10.3390/ma12172701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young HD, Modi R, Bucaro M. Generation of Mesoscopic Patterns of Viable Escherichia coli by Ambient Laser Transfer. Biomaterials. 2002;23:161–166. doi: 10.1016/s0142-9612(01)00091-6. DOI:10.1016/s0142-9612(01)00091-6. [DOI] [PubMed] [Google Scholar]
- 37.Xiong R, Zhang Z, Huang Y. Identification of Optimal Printing Conditions for Laser Printing of Alginate Tubular Constructs. J Manuf Process. 2015;20:450–455. DOI:10.1016/j.jmapro.2015.06.023. [Google Scholar]
- 38.Palla-Papavlu A, Córdoba C, Patrascioiu A, et al. Deposition and Characterization of Lines Printed through Laser-induced Forward Transfer. Appl Phys A Mater Sci Process. 2013;110:751–755. DOI:10.1007/s00339-012-7279-6. [Google Scholar]
- 39.Pescosolido L, Miatto S, Di Meo C, et al. Injectable and In Situ Gelling Hydrogels for Modified Protein Release. Eur Biophys J. 2010;39:903–9. doi: 10.1007/s00249-009-0440-2. DOI:10.1007/s00249-009-0440-2. [DOI] [PubMed] [Google Scholar]
- 40.Ouyang L, Highley CB, Rodell CB, et al. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater Sci Eng. 2016;2:1743–1751. doi: 10.1021/acsbiomaterials.6b00158. DOI:10.1021/acsbiomaterials.6b00158. [DOI] [PubMed] [Google Scholar]
- 41.Cochis A, Bonetti L, Sorrentino R, et al. 3D Printing of Thermo-responsive Methylcellulose Hydrogels for Cell-sheet Engineering. Materials (Basel) 2018;11:1–14. doi: 10.3390/ma11040579. DOI:10.3390/ma11040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ovsianikov A, Gruene M, Pflaum M, et al. Laser Printing of Cells into 3D Scaffolds. Biofabrication. 2010;2:014104. doi: 10.1088/1758-5082/2/1/014104. DOI:10.1088/1758-5082/2/1/014104. [DOI] [PubMed] [Google Scholar]
- 43.Gruene M, Pflaum M, Deiwick A, et al. Adipogenic Differentiation of Laser-printed 3D Tissue Grafts Consisting of Human Adipose-derived Stem Cells. Biofabrication. 2011;3:015005. doi: 10.1088/1758-5082/3/1/015005. DOI:10.1088/1758-5082/3/1/015005. [DOI] [PubMed] [Google Scholar]
- 44.Yusupov VI, Gorlenko MV, Cheptsov VS, et al. Laser Engineering of Microbial Systems. Laser Phys Lett. 2018;15:015604. [Google Scholar]
- 45.Unger C, Koch J, Overmeyer L, et al. Time-resolved Studies of Femtosecond-laser Induced Melt Dynamics. Opt Express. 2012;20:24864. doi: 10.1364/OE.20.024864. DOI:10.1364/oe.20.024864. [DOI] [PubMed] [Google Scholar]
- 46.Hill C. Liquid-Phase Laser Induced Forward Transfer for Complex Organic Inks and Tissue Engineering. Ann Biomed Eng. 2016;45:84–99. doi: 10.1007/s10439-016-1617-3. DOI:10.1007/s10439-016-1617-3. [DOI] [PubMed] [Google Scholar]
- 47.Akhatov W, Lindau I, Topolnikov O, et al. Collapse and Rebound of a Laser-induced Cavitation Bubble. Phys Fluids. 2001;13:29–32. DOI:10.1063/1.1401810. [Google Scholar]
- 48.Mézel C, Hallo L, Souquet A, et al. Self-consistent Modeling of Jet Formation Process in the Nanosecond Laser Pulse Regime. Phys Plasmas. 2009;16:123112. DOI:10.1063/1.3276101. [Google Scholar]
- 49.Ali M, Pages E, Ducom A, et al. Controlling Laser-induced Jet Formation for Bioprinting Mesenchymal Stem Cells with High Viability and High Resolution. Biofabrication. 2014;6:045001. doi: 10.1088/1758-5082/6/4/045001. DOI:10.1088/1758-5082/6/4/045001. [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Chrisey DB. Study of Impact-Induced Mechanical Effects in Cell Direct. J Manuf Sci Eng. 2008;130:1–10. [Google Scholar]
- 51.Ringeisen BR, Pages E, Ducom A, et al. Laser Printing of Pluripotent Embryonal Carcinoma Cells. Tissue Eng. 2004;10:483–491. doi: 10.1089/107632704323061843. [DOI] [PubMed] [Google Scholar]
- 52.Kawecki F, Clafshenkel WP, Auger FA, et al. Self-assembled Human Osseous Cell Sheets as Living Biopapers for the Laser-assisted Bioprinting of Human Endothelial Cells. Biofabrication. 2018;10:035006. doi: 10.1088/1758-5090/aabd5b. DOI:10.1088/1758-5090/aabd5b. [DOI] [PubMed] [Google Scholar]
- 53.Vass C, Smausz T, Hopp B. Wet Etching of Fused Silica:A Multiplex Study. J Phys D Appl Phys. 2004;37:2449–2454. DOI:10.1088/0022-3727/37/17/018. [Google Scholar]
- 54.Gruene M, Unger C, Koch L, et al. Dispensing Pico to Nanolitre of a Natural Hydrogel by Laser-assisted Bioprinting. Biomed Eng Online. 2011;10:9–12. doi: 10.1186/1475-925X-10-19. DOI:10.1186/1475-925x-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown MS, Brasz CF, Ventikos Y, Arnold CB. Impulsively Actuated Jets from thin Liquid Films for High-resolution Printing Applications. J Fluid Mech. 2012;709:341–370. DOI:10.1017/jfm.2012.337. [Google Scholar]
- 56.Hölzl K, Lin S, Tytgat L, et al. Bioink Properties before, during and after 3D Bioprinting. Biofabrication. 2016;8:032002. doi: 10.1088/1758-5090/8/3/032002. DOI:10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 57.Ringeisen BR, Spargo BJ, Wu PK. Cell and Organ Printing. Springer Science and Business Media, Berlin. 2010 [Google Scholar]
- 58.Nasatto PL, Pignon F, Silveira JL, et al. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers (Basel) 2015;7:777–803. DOI:10.3390/polym7050777. [Google Scholar]
- 59.Shafiee A, Elham G, Ramesh H, et al. Physics of Bioprinting. Appl Phys Rev. 2019;6:021315. [Google Scholar]
- 60.McKinley GH, Renardy M. Wolfgang von Ohnesorge. Phys Fluids. 2011;23:127101. DOI:10.1063/1.3663616. [Google Scholar]
- 61.Yan J, Huang Y, Xu C, et al. Effects of Fluid Properties and Laser Fluence on Jet Formation during Laser Direct Writing of Glycerol Solution. J Appl Phys. 2012;112:083105. DOI:10.1063/1.4759344. [Google Scholar]
- 62.Bush JW. Rayleigh Instability. In:MIT Lecture Notes on Surface Tension, Lecture 5 (PDF) Massachusetts Institute of Technology. 2004 [Google Scholar]
- 63.Gorlenko M, Chutko EA, Churbanova ES, et al. Laser Microsampling of Soil Microbial Community. J Biol Eng. 2018;12:1–11. doi: 10.1186/s13036-018-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minaev NV, Chutko EA, Churbanova ES, et al. Laser Printing of Gel Microdrops with Living Cells and Microorganisms. KnE Energy Phys. 2018;2018:23–31. DOI:10.18502/ken.v3i3.2010. [Google Scholar]
- 65.Koch L, Deiwick A, Franke A, et al. Laser Bioprinting of Human Induced Pluripotent Stem Cells the Effect of Printing and Biomaterials on Cell Survival, Pluripotency, and Differentiation. Biofabrication. 2018;10:035005. doi: 10.1088/1758-5090/aab981. DOI:10.1088/1758-5090/aab981. [DOI] [PubMed] [Google Scholar]
- 66.Cheptsov VS, Tsypina SI, Minaev NV, et al. New Microorganism Isolation Techniques With Emphasis On Laser Printing. Int J Bioprint. 2019;5:165. doi: 10.18063/ijb.v5i1.165. DOI:10.18063/ijb.v5i1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]


