Summary
We report findings from a new survey of US public attitudes toward human-animal chimeric embryo (HACE) research, designed to compare with recently reported Japanese survey data. We find that 59% of the US public can personally accept the process of injecting human induced pluripotent stem cells into genetically modified swine embryos and having human tissues produced in a pig's body transplanted into a human. This is greater acceptance than in Japan, and there is even strong acceptance among those with strong religious affiliations and who self-identify as conservatives. We argue that strong public support for HACE research, as well as the emerging literature suggesting that humanization of research animals is very unlikely, should compel the NIH to lift its current moratorium on HACE research.
Keywords: human-animal chimeric embryo, ethics and policy, US public attitudes, blastocyst complementation, gene editing
Highlights
-
•
430 US participants in a national survey on human-animal chimeric embryo research
-
•
83% of respondents can personally accept research on human-animal chimeric embryos
-
•
Self-identified political conservatives accept human-animal chimera research
-
•
Individuals opposed to animal research also accept human-animal chimera research
In this report, Crane and colleagues report findings on US public attitudes toward human-animal chimeric embryo research. A majority of survey respondents personally accept the process of injecting human stem cells into genetically modified swine embryos and transplanting human tissues produced in a pig's body. Strong public support should compel the NIH to lift the moratorium on human-animal chimeric embryo research.
Introduction
In the fall of 2015, the National Institutes of Health (NIH) issued notice NOT-OD-15-158, effectively placing a moratorium on funding research, “in which human pluripotent cells are introduced into non-human vertebrate animal pre-gastrulation stage embryos while the Agency considers a possible policy revision in this area.” The purpose of this moratorium was for the NIH to undertake a deliberative process in an effort to study the state of the science, as well as the ethical, legal, and social implications of human-animal chimeric embryos (HACEs). But other countries are now re-examining their policies toward HACE research (Foong, 2019). In March 2019, Japan's Ministry of Education, Culture, Sports, Science, and Technology lifted the country's ban on conducting research on HACEs beyond 14 days after the introduction of human pluripotent stem cells or the appearance of the primitive streak (Sawai et al., 2019). Four months later, Hiromitsu Nakauchi of the University of Tokyo and Stanford University received permission from the Japanese government to create human-animal embryos to be transplanted into surrogates (Lanese, 2019). In July 2019, a group at the Salk Institute in San Diego announced that, with partners in Spain and China, they had created embryos containing both human and monkey cells (Lanese, 2019).
In 2016, the NIH released notice NOT-OD-16-128, requesting public comment on the proposed changes to the Guidelines for Human Stem Cell Research. The Administrative Procedures Act of 1946 (Pub. L. No. 79-404) requires that federal rulemaking engage the public through public notice and comment. The public comment mechanism reflects an interest in considering public views when making science policy (Kolber, 2009). It is clear that the majority of those who commented in response to NOT-OD-16-128 objected to HACE research (National Institutes of Health). But the degree to which these responses are reflective of the general public were unknown.
In Japan—leading up to the change in government policy—Japanese researchers surveyed the public and identified the overall levels of support as well as the factors contributing to support for HACE research (Sawai et al., 2017a). To better inform policy debates in the United States, we replicated the Japanese study with a sample of Americans. Similar to the Japanese public, we find broad public acceptance for the injection of human induced pluripotent stem cells (iPSCs) into genetically modified swine embryos. We also find evidence of resistance to HACE research—with significantly lower support from individuals who object to animal research generally. Based on these findings, as well as mounting evidence that HACE research can be conducted without causing animal humanization, we argue that the NIH should lift its moratorium and replace it with strict guidelines for ethically sound research.
Results
Analysis of Public Attitudes toward HACE Research in the United States
In July 2018 and June 2020, 430 survey participants, from 48 states, were recruited via Amazon's Mechanical Turk service and completed a survey hosted on the Qualtrics online platform. The demographics of the study participants are reported in Table S1. The survey replicated and adapted the Sawai and colleagues study of Japanese public attitudes on HACE research (Sawai et al., 2017a). While underpowered and not sufficiently diverse to generalize to the American population as a whole, the sample remains national and we gain confidence in our results as they are consistent with other recent data on American public attitudes toward HACE research (Kantor, 2017; Pew Research Center, 2018).
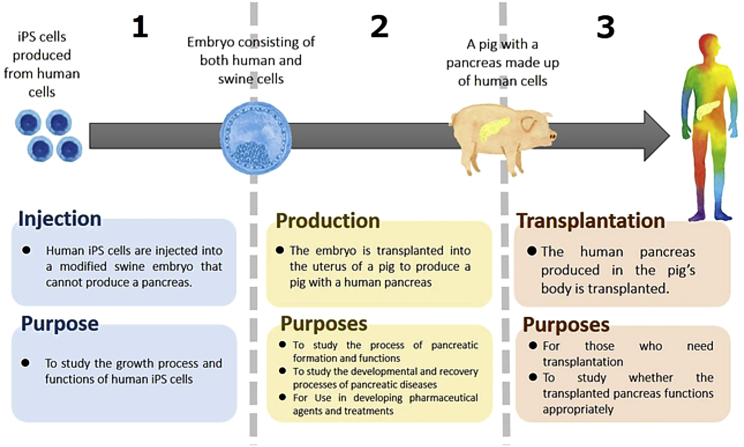
Using the pancreas as an example organ, all participants were shown Figure 1 to demonstrate the progressive steps of HACE research. Participants were then asked, “What steps of this research are you willing to accept according to your personal feelings?”
-
•
Step 1, Injection: human iPSCs are injected into a modified swine embryo that cannot produce a pancreas.
-
•
Step 2, Production: the embryo is transplanted into the uterus of a pig to produce a pig with a human pancreas.
-
•
Step 3, Transplantation: the human pancreas produced in the pig's body is transplanted into a human.
-
•
None: participants were also able to note if none the steps were acceptable to them.
Figure 1.
Illustration of the Three Progressive Steps in HACE Research
Image that was shown to survey participants to (adapted from Sawai et al., 2017a).
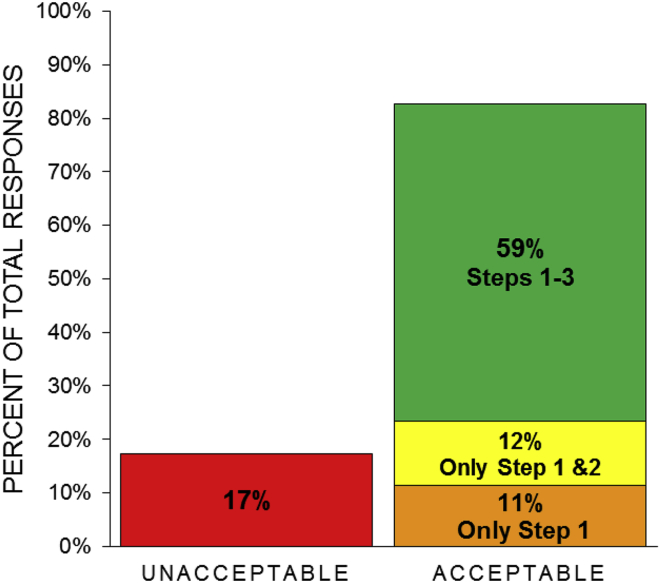
This approach allowed us to determine the level of acceptance for each stage of the research, as well as acceptance for chimerism in other organ and tissue systems. Across the 430 participants in the survey, there was broad acceptance for at least some HACE research, with 83% of the participants in our sample accepting at least injection of human iPSCs into genetically modified swine embryos (step 1), 71% accepting production of a pig with a human pancreas (step 2), and 59% accepting clinical transplantation of the HACE-generated pancreas (step 3; Figure 2).
Figure 2.
American Public Acceptance of the Three Steps of HACE Research
Graph of the proportion of total survey participants when asked the question: “What steps of this research are you willing to accept according to your personal feelings?”
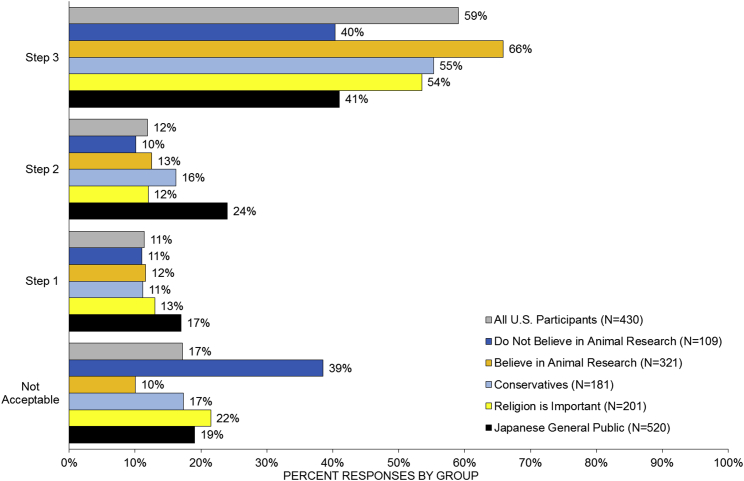
Although general support for HACE research is overwhelmingly positive, we were able to identify subpopulations of Americans who do not personally accept HACE research. Statistical analysis finds that there are significant and substantively meaningful differences in support between participants who (in a separate question) said they support the use of animals for research and those who do not (Figure 3; Table S2). We also found significant differences between conservative and liberal participants, and those who acknowledge the importance of religion in their daily life.
Figure 3.
Acceptance of the Three Steps of HACE Research across Different Sub-groups of American Participants and in the Japanese General Population
Each horizontal bar depicts the percentage of survey participants who supported up to that step in HACE research. See also Table S2.
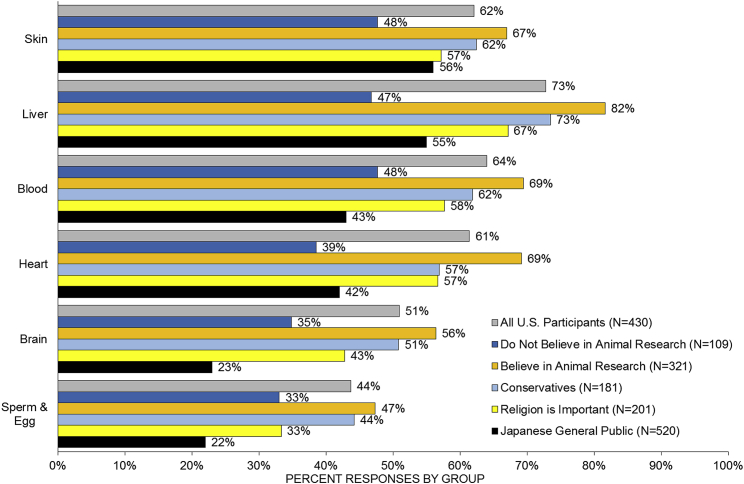
Broken down further, we identified variable levels of acceptance of the contribution of human cells within specific organ/tissue systems. Specifically, Americans were less likely to personally accept the presence of human cells in sperm/egg (44%) and brain (51%), relative to heart (61%), blood (64%), liver (73%), and skin (62%). Ordered logistic regression identified significant differences with individuals opposed to animal research in all organ tissue systems (Figure 4; Table S3). Individuals with a religious affiliation were less likely to accept human cells in sperm/egg, brain, and blood (Table S3).
Figure 4.
Acceptance of the Contribution of Human Cells to Organ/Tissue Systems across Different Sub-groups of American Participants and in the Japanese General Population
Percentage of survey participants who chose “can accept” or “somewhat can accept” when asked the question: “According to your personal feelings, can you accept the presence of human iPS cells in the following pig organs?” For each of six organs—liver, brain, sperm/egg, skin, blood, and heart—participants were asked to choose “I can accept it,” “I can somewhat accept it,” “I somewhat cannot accept it,” or “I cannot accept it.” See also Table S3.
Although the within-cultural differences between these communities are important, our study allows cross-national comparison with the Japanese results (Sawai et al., 2017a). Figure 4 demonstrates that even the groups with lower acceptance in the United States are still more accepting than the Japanese general public. For instance, while only 43% of the Japanese public can accept human iPSCs in a pig's bloodstream, it is accepted by 62% of US conservatives and 48% of US participants who are not favorable to animal research generally (Table S3). These groups are similarly more supportive than the Japanese public of the use of iPSCs in pig's sperm/egg and brain. It is clear that, compared with Japan, there is wide acceptance of HACE research in the United States.
Discussion
Research involving HACEs has the potential to answer significant questions in human developmental biology and be a source of human organs and tissues that can be used to combat the donor organ shortage across the globe. Our new survey data suggest the American public sees the potential of HACE research and is ready to accept it in a variety of forms.
These results are in support of findings by two separate nationally representative surveys in which a majority of respondents were in favor of HACE research (Kantor, 2017; Pew Research Center, 2018). The present study is unique in that we have provided greater detail on the stages of HACE research for organ production and transplantation and identified personal acceptance for each of these stages. In our study, we identified 11% of respondents whose personal acceptance of HACE research does not extend beyond the injection of human cells into a pig embryo (step 1), which may suggest a hesitancy toward applications of this technology. Our study was also able to examine how support for HACE research varies across multiple dimensions, with opposition to HACE research in individuals self-described as generally opposing animal research, supporting previous results (Kantor, 2017; Pew Research Center, 2018), as well as in individuals self-described as religious and politically conservative.
The present study was also able to identify personal acceptance of human cells contributing to individual organ/tissue systems. Although personal acceptance of human cells within specific organ/tissue systems is framed in the context of off-target effects from the production of a human pancreas in a pig, these data can be used as a guidepost for policy regarding targeted generation of human organ/tissue systems.
Another unique feature of this survey was the ability to directly compare cross-culturally, suggesting that, even more so than the Japanese public, the American public is ready to accept HACE research. It should be noted that no study, to date, has determined participant comprehension of the concept of HACE research. Future research should examine in greater detail how lay subjects understand HACE research, even when presented in detail as we did in this survey.
As public acceptance of HACE research in both the United States and Japan is emerging, the Japanese government has recently taken the next step to approve studies that involve generating human-animal chimeras (Cyranoski, 2019). Japan and other countries such as China are continuing to pursue such work, and there is an increase in the number of publications with a focus on multiple tissue systems integrating with gene-editing technologies (Crane et al., 2019a).
Despite the NIH moratorium, American researchers have nevertheless found ways to collaborate with international partners. The promise of this research line, and the strong support of the public for more of it, suggests that these work-around arrangements are likely to increase. Science will not sit idle. The NIH will face significant difficulties in maintaining a strict ethics framework when the research is funded and governed by foreign countries.
Working around the NIH moratorium, however, can lead to problematic ethical and policy issues. Our data, for instance, confirm ethical analysis suggesting that there is strong opposition from those who are generally skeptical about the necessity of animal research (Moy, 2017). Our study also identifies specific opposition to the contribution of human cells to the brain and sperm/egg of pigs. As the International Society for Stem Cell Research has recommended, HACE research should be undertaken only within clear ethical frameworks and with strict oversight, particularly regarding the contribution of human cells to the brain and reproductive organs. The present results serve to help shape those recommendations and policies, by better clarifying the differential public support for various aspects of the research.
Our literature review of more than 60 scholarly articles exploring the ethics of HACE research identified three primary concerns unique to human chimeric research: (1) infringement upon the natural order/“playing God”; (2) violations of human dignity; and (3) the potential humanization and resulting moral and legal status of the chimeric animal. These issues have been discussed at length elsewhere (Greely, 2011; Palacios-González, 2015; Streiffer, 2019) and should all be addressed when developing a new policy.
Of particular concern among respondents in the current study is the contribution of human cells in the brain of the pigs and the potential neurological humanization of the animals involved in HACE research (Crane et al., 2019b; Sawai et al., 2017b). Assuming humanization is possible, it would require researchers to contend with both the moral status of the resulting chimeric individual and the consequences of blurred species lines, including potential moral confusion (Baylis and Robert, 2007; Hübner, 2018). Some ethicists recommend a precautionary approach, but others argue that partially humanized animals should simply be treated commensurately with a moral worth derived from the animal's cognitive capacity (Porsdam Mann et al., 2019).
But how likely is it that humanization (however defined) will actually occur? Our recent review of 150 peer-reviewed transplantation studies found that, while a relatively high degree of human-animal neurological chimerism has been observed in multiple studies, there was no evidence to suggest that integration of human neurons within the non-human animal results in humanization (Crane et al., 2019b). Thus, although humanization could theoretically be an issue and should remain a focal point of ethical and legal concern when developing a new policy, HACE research currently being conducted has observed very limited contribution of human cells to a non-human animal brain in preterm human-animal chimeras. It has been previously proposed that, if HACE research should be allowed to resume, investigators monitor the extent to which human cells contribute to a non-human animal brain in a stepwise approach (Crane et al., 2019b) under careful ethical guidelines, to limit the likelihood that “human cells in an animal” will result in “humanization” of the sort that has generated so much ethical concern.
The Path Forward
In light of the strong public acceptance of HACE research, the path forward should be an eventual lifting of the moratorium on HACE research. In its place, the NIH should develop policy that adequately addresses ethical challenges such as animal welfare, human dignity, and neurological humanization. This policy can build on existing guidelines set forth by national and international research societies, current policies of foreign governments, and the well-developed ethics literature in this area, while also considering stakeholder input, including public survey data, to ensure concerns over the contribution to the brain and reproductive organs are addressed. Pursing such a path would garner not only the support of wide swaths of the scientific research community, but that of the vast majority of the American public as well.
Experimental Procedures
Survey Design
To facilitate cross-national comparison with support for HACE research in Japan, this study translated survey questions from Sawai and colleagues (Sawai et al., 2017a) and adapted questions on demographics for the US population (Supplemental Experimental Procedures). Our survey was conducted using the online survey platform Qualtrics. Qualtrics has been used in many fields, including bioethics (Wangmo and Provoost, 2017) and neuroethics (Cancer et al., 2018). After reading an informed consent page (UMN IRB Approved, study 00001760), participants read a brief page of information describing iPSCs. The information was translated from similar text used in the Sawai survey. Participants then answered a series of 10 questions related to their support of, and concerns about, HACE research. Following this battery of questions, participants then answered a series of background and demographic questions.
Recruitment of Survey Participants
The study includes two waves of data collection. In July 2018, 227 participants were recruited via Amazon's Mechanical Turk service to participate in our survey hosted on the Qualtrics online platform. To increase our sample size and provide a greater generalization to the American public, an additional survey was conducted in June 2020 and data from 203 participants were combined with those of the 227 participants for a total of 430 participants. We present here the results from analysis of the combined dataset including both waves of respondents. When analyzed independently, the results from the 2018 data and from the 2020 data analysis yield substantively similar conclusions and do not alter the main conclusions of this report. Amazon Mechanical Turk is “a web service that provides an on-demand, scalable, human workforce to complete tasks” (Amazon Mechanical Turk, 2014). Researchers advertise their studies on Mechanical Turk, and participants choose only those studies that interest them. Participants are paid for completing the studies. Payment is transferred directly to the participants' credit cards immediately after the completion of a study. We paid participants $1.00 for completion of the survey. No personally identifying information was collected. Mechanical Turk is regularly utilized by researchers in multiple disciplines to recruit participants to complete online research tasks (Amazon Mechanical Turk, 2014; Buhrmester et al., 2011; Paolacci et al., 2010; Sheehan and Pittman, 2016). A Human Intelligence Task “is a single, self-contained task a Requester creates on Mechanical Turk” (Sheehan and Pittman, 2016). We recognize that participants recruited via Mechanical Turk are not representative of the US public (Mortensen et al., 2018; Walters et al., 2018). Mechanical Turk samples, including ours, are typically younger, more educated, more liberal, and less racially diverse than the US general population (Berinsky et al., 2012; Paolacci and Chandler, 2014; Shapiro et al., 2013).
Ensuring Data Quality
In total, 743 surveys were logged through the Mechanical Turk service. Prior to analysis, survey responses were removed if participants completed less than 97% of the survey questions and if the duration of the survey was less than 100 s. This first pass removed 73 surveys. Ten surveys were then removed due to duplicate individual Mechanical Turk identification numbers. Concern about subjects' compliance with survey instructions are of special interest with online surveys because subjects cannot be monitored while engaged in the online task. To address this issue, psychologists have developed “attention filters” designed to ascertain whether subjects are in fact following instructions and paying attention to the material being presented to them online. In this study, we employed a modified version of the filter developed by psychologist Oppenheimer and colleagues (Oppenheimer et al., 2009). The design of the attention filter question was such that users who did not read carefully would see, in large font, a headline reading “Background Questions on Sources for News” as well as another large, bold question: “From which of these sources have you received information in the past month?” A series of check-box options was provided (e.g., local newspaper, local TV news). Subjects reading carefully, however, were instructed to check only the “magazine” box and to type “654” into the text box provided. The results presented in this article are based only on the “attentive” subjects, i.e., those subjects who were paying attention. Here, 135 of 620 (22%) did not complete the attention filter properly and were excluded from the analysis reported in the article. Finally, surveys in which responses on political ideology were absent were removed, leaving 430 surveys for analysis. Re-running the models reported below with the excluded participants included does not change the substantive results.
Analytic Methods
All statistical analysis was carried out using the program Stata, version 16.1. In addition to summary statistics and basic difference in means test, analysis utilized ordered logistic regression models. This is appropriate because our outcome variables take on values of 0, 1, 2, 3, and so on, i.e., they are non-continuous and they are ordered. Multicollinearity was assessed using the collin package in Stata. The mean VIF was 1.10 for the independent variables included in this model, suggesting no severe multicollinearity issues with the model.
Data and Code Availability
The complete dataset (Table S4), captions for Table S4, and complete survey are available in the Supplemental Information.
Author Contributions
Conceptualization, A.T.C., F.X.S., T.S., T.H., M.F., and W.C.L.; Methodology, A.T.C., F.X.S., J.L.B., J.P.V., and W.C.L.; Formal Analysis, A.T.C., F.X.S., J.L.B., and M.R.E.; Writing – Original Draft, A.T.C., F.X.S., W.C., and W.C.L.; Writing – Review & Editing, A.T.C., F.X.S., J.L.B., W.C., M.R.E., J.P.V., T.S., T.H., M.F., and W.C.L.
Conflicts of Interest
W.C.L. serves as a consultant for Saneron, is chief scientific officer for Regenevida, and is a founder of Metselex. All other authors declare no competing interests.
Acknowledgments
For helpful research assistance, we thank Jennifer Novo and Sydney Diekmann. The study was funded with internal University of Minnesota Law School faculty grant funding (to F.X.S.) and the University of Minnesota Brain Sciences Fund (to W.C.L.).
Published: October 1, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.08.018.
Contributor Information
Andrew T. Crane, Email: atcrane@umn.edu.
Francis X. Shen, Email: fxshen@umn.edu.
Supplemental Information
References
- Amazon Mechanical Turk . amazon.com; 2014. Amazon Mechanical Turk Requester User Interface Guide. [Google Scholar]
- Baylis F., Robert J.S. Part-human chimeras: worrying the facts, probing the ethics. Am. J. Bioeth. 2007;7:41–45. doi: 10.1080/15265160701290397. [DOI] [PubMed] [Google Scholar]
- Berinsky A.J., Huber G.A., Lenz G.S. Evaluating online labor markets for experimental research: Amazon.com’s Mechanical Turk. Polit. Anal. 2012;20:351–368. [Google Scholar]
- Buhrmester M.D., Kwang T.N., Gosling S.D. Amazon’s Mechanical Turk. Perspect. Psychol. Sci. 2011;6:3–5. doi: 10.1177/1745691610393980. [DOI] [PubMed] [Google Scholar]
- Cancer A., Schulz P.J., Castaldi S., Antonietti A. Neuroethical issues in cognitive enhancement: the undergraduates’ point of view. J. Cogn. Enhanc. 2018;2:323–330. [Google Scholar]
- Crane A.T., Aravalli R.N., Asakura A., Grande A.W., Krishna V.D., Carlson D.F., Cheeran M.C.-J., Danczyk G., Dutton J.R., Hackett P.B. Interspecies organogenesis for human transplantation. Cell Transplant. 2019;28:1091–1105. doi: 10.1177/0963689719845351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane A.T., Voth J.P., Shen F.X., Low W.C. Concise Review: human-animal neurological chimeras: humanized animals or human cells in an animal? Stem Cells. 2019;37:444–452. doi: 10.1002/stem.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D. Japan approves first human-animal embryo experiments. Nature. 2019 doi: 10.1038/d41586-019-02275-3. [DOI] [PubMed] [Google Scholar]
- Foong P. Human-animal chimeras: should other countries follow Japan’s lead? BioNews. 2019 [Google Scholar]
- Greely H.T. Human/Nonhuman chimeras: assessing the issues. In: Beauchamp T., Frey R., editors. The Oxford Handbook of Animal Ethics. Oxford University Press; 2011. pp. 671–698. [Google Scholar]
- Hübner D. Human-animal chimeras and hybrids: an ethical paradox behind moral confusion? J. Med. Philos. 2018;43:187–210. doi: 10.1093/jmp/jhx036. [DOI] [PubMed] [Google Scholar]
- Kantor J. Public support in the U.S. for human-animal chimera research: results of a representative cross-sectional survey of 1,058 adults. Stem Cells Transl. Med. 2017;6:1442–1444. doi: 10.1002/sctm.16-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber M. Rulemaking without rules: an emperical study of direct final rulemaking. Albany Lab Rev. 2009;72:37. [Google Scholar]
- Lanese N. First human-monkey chimeras developed in China. The Scientist Magazine. 2019 [Google Scholar]
- Mortensen K., Alcalá M.G., French M.T., Hu T. Self-reported health status differs for Amazon’s Mechanical Turk respondents compared with nationally representative surveys. Med. Care. 2018;56:211–215. doi: 10.1097/MLR.0000000000000871. [DOI] [PubMed] [Google Scholar]
- Moy A. Why the moratorium on human-animal chimera research should not be lifted. Linacre Q. 2017;84:226–231. doi: 10.1080/00243639.2017.1293931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health Public comments on “Proposed changes to the NIH guidelines for human stem ell research and the proposed scope of an NIH Steering Committee’s consideration of certain human-animal chimera research”. 2016. https://grants.nih.gov/grants/rfi/responses_57.cfm
- Oppenheimer D.M., Meyvis T., Davidenko N. Instructional manipulation checks: detecting satisficing to increase statistical power. J. Exp. Soc. Psychol. 2009;45:867–872. [Google Scholar]
- Palacios-González C. Human dignity and the creation of human–nonhuman chimeras. Med. Health Care Philos. 2015;18:487–499. doi: 10.1007/s11019-015-9644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolacci G., Chandler J. Inside the Turk: understanding Mechanical Turk as a participant pool. Curr. Dir. Psychol. Sci. 2014;23:184–188. [Google Scholar]
- Paolacci G., Chandler J., Ipeirotis P.G. Running experiments on Amazon Mechanical Turk. Judgment and Decision Making. 2010;5:411–419. [Google Scholar]
- Pew Research Center . Pew Research Center; 2018. Most Americans Accept Genetic Engineering of Animals that Benefits Human Health, but Many Oppose Other Uses. [Google Scholar]
- Porsdam Mann S., Sun R., Hermerén G. A framework for the ethical assessment of chimeric animal research involving human neural tissue. BMC Med. Ethics. 2019;20:10. doi: 10.1186/s12910-019-0345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Hatta T., Fujita M. Public attitudes in Japan towards human-animal chimeric embryo research using human induced pluripotent stem cells. Regen. Med. 2017;12:233–248. doi: 10.2217/rme-2016-0171. [DOI] [PubMed] [Google Scholar]
- Sawai T., Hatta T., Fujita M. The Japanese generally accept human-animal chimeric embryo research but are concerned about human cells contributing to brain and gametes. Stem Cells Transl. Med. 2017;6:1749–1750. doi: 10.1002/sctm.17-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Hatta T., Fujita M. Japan significantly relaxes its human-animal chimeric embryo research regulations. Cell Stem Cell. 2019;24:513–514. doi: 10.1016/j.stem.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Shapiro D.N., Chandler J., Mueller P.A. Using Mechanical Turk to study clinical populations. Clin. Psychol. Sci. 2013;1:213–220. [Google Scholar]
- Sheehan K.B., Pittman M. US: Melvin & Leigh, Publishers; 2016. Amazon’s Mechanical Turk for Academics: The HIT Handbook for Social Science Research. [Google Scholar]
- Streiffer R. Stanford Encyclopedia of Philosophy Archive; 2019. Human/Non-Human Chimeras. [Google Scholar]
- Walters K., Christakis D.A., Wright D.R. Are Mechanical Turk worker samples representative of health status and health behaviors in the U.S.? PLoS One. 2018;13:e0198835. doi: 10.1371/journal.pone.0198835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangmo T., Provoost V. The use of empirical research in bioethics: a survey of researchers in twelve European countries. BMC Med. Ethics. 2017;18:79. doi: 10.1186/s12910-017-0239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete dataset (Table S4), captions for Table S4, and complete survey are available in the Supplemental Information.