Highlights
-
•
Mesonephric-like adenocarcinomas (MLA) of the ovary are rare, require differentiation from other histiotypes.
-
•
True histiogenesis of MLA of ovary remains debated, possibly arise from mullerian lesions of the ovary.
-
•
MLA of ovary have differing genotypes from true mesonephric carcinomas of the cervix.
-
•
Case described reflects further evidence for mullerian origin of MLA of ovary, with endometriosis in affected ovary.
-
•
MLA of ovary can act aggressively, case described recurred and responded only partially to chemotherapy.
Keywords: Mesonephric-like adenocarcinoma, Ovary, Endometriosis, Mesonephros
Abstract
Introduction
Mesonephric-like adenocarcinoma (MLA) is a rare malignant gynecologic neoplasm occurring in the uterine corpus and ovary. The morphological and immunohistochemical characteristics of MLA closely resemble that of cervical mesonephric adenocarcinomas, but whether they share a common histogenesis remains unclear. Two main theories for histogenesis of MLAs include the origination of these neoplasms from mesonephric remnants, as is the case for cervical mesonephric adenocarcinoma, versus the differentiation along a mesonephric pathway from Mullerian lesions.
Case
A 67-year-old presented after a right salpingo-oophorectomy for a complex ovarian mass revealed a mesonephric-like adenocarcinoma of the ovary and endometriosis. She underwent a total abdominal hysterectomy, pelvic lymphadenectomy, and infra-colic omentectomy, and diagnosed with Stage IA mesonephric-like adenocarcinoma of the ovary. At 18 months post-operatively, the patient developed flank and abdominal pain and was found to have multiple sites of recurrent disease. She was referred to medical oncology for chemotherapy as she was not a candidate for surgical cytoreduction.
Discussion
This case demonstrates the aggressive nature of ovarian MLA and the need for a multidisciplinary approach when determining the treatment. In addition, this case provides further evidence to support the theory that at least a subset of MLAs arises from a Mullerian lesion which then differentiates down a mesonephric pathway.
1. Introduction
Mesonephric adenocarcinoma is a rare malignant gynecologic neoplasm that most commonly occurs in the uterine cervix and is assumed to arise from normal or hyperplastic mesonephric remnants (McFarland et al., 2016, Bagué et al., 2004). The exact incidence of these neoplasms outside the cervix is difficult to determine, as they were often previously misclassified as clear cell carcinomas and yolk sac tumors. Reports have begun to reliably describe these carcinomas in the uterine corpus, vagina, and ovary. Recently, McFarland et al. described a case series of 5 ovarian and 7 endometrial mesonephric-like adenocarcinomas (MLA) (McFarland et al., 2016). The terminology of “mesonephric-like adenocarcinoma” of the ovary is encouraged by these authors, as the true histogenesis of primary mesonephric adenocarcinoma of the ovary is not yet firmly established. The main theories for the histogenesis include origination of these neoplasms from mesonephric remnants, versus originating from Mullerian lesions, such as endometriosis, and differentiating down a mesonephric pathway.
Based on a systematic literature search of the databases PubMed, Scopus, and Cochrane Library using the search terms “mesonephric-like adenocarcinomas” AND “ovary” there has been a reported 12 cases of MLA in the ovary with 8 out of those 12 cases demonstrating coexistent Mullerian lesions (Table 1) (McFarland et al., 2016, McCluggage et al., 2018, Dundr, 2020). We present a case that provides potential evidence for the Mullerian origin of MLA, due to the presence of endometriosis in the same ovary as the MLA.
Table 1.
Review of the literature in table format.
| Case | Age | Associated Findings | FIGO stage | Chemotherapy | Radiation | Recurrence | Follow-up time (mo) |
|---|---|---|---|---|---|---|---|
| 1–5 (McFarland et al., 2016) | 42–72 | Endometriosis in 3 of 5 | IA(3 cases), IB(1 case), IIIC(1case) | not reported | not known | N (IA, IB cases) Y (IIIC case) | 18 (IA, IB cases) 56 (IIIC case) |
| 6 (Chapel, 2018) | 80 | Serous borderline tumor and low-grade serous carcinoma | not reported | carboplatin paclitaxel | N | N | 3 |
| 7 (McCluggage et al., 2018) | 61 | Serous borderline tumor (low-grade serous carcinoma in extraovarian tissues) | IIIA1 | carboplatin paclitaxel | N | not reported | not reported |
| 8 (McCluggage et al., 2018) | 66 | Borderline endometrioid adenofibroma | not reported | not reported | not reported | not reported | not reported |
| 9 (McCluggage et al., 2018) | 77 | Endometriosis; mixed serous and mucinous cystadenoma | not reported | not reported | not reported | not reported | not reported |
| 10 (McCluggage et al., 2018) | 50 | None | not reported | not reported | not reported | not reported | not reported |
| 11 (McCluggage et al., 2018) | 73 | Serous cystadenoma | not reported | not reported | not reported | not reported | not reported |
| 12 (Dundr, 2020) | 61 | Serous borderline tumor | IV | carboplatin paclitaxel bevacizumab | N | N | 12 |
| 13 (present case) | 67 | Endometriosis | IA | carboplatin paclitaxel bevacizumab | N | Y | 18 |
2. Case presentation
A 67-year-old was referred to gynecologic oncology by her general gynecologist after right salpingo-oophorectomy for a complex ovarian mass. The histopathology was reported and reviewed as a mesonephric like adenocarcinoma of the ovary. The patient had originally presented with pelvic “heaviness” and polyuria. Subsequent work-up revealed an 11 cm complex right adnexal mass on ultrasound and confirmed on MRI. Imaging was otherwise significant for an endometrial lining of 3.9 mm and no evidence of lymphadenopathy, ascites or carcinomatosis. Her pre-operative CA-125 was 31 and a pre-operative colonoscopy revealed no lesions or polyps. Her previous obstetric and gynecologic history included three vaginal births and two spontaneous abortions. She underwent menopause at age 54. She had a history of a left salpingo-oophorectomy for ovarian torsion at age 29 was benign.
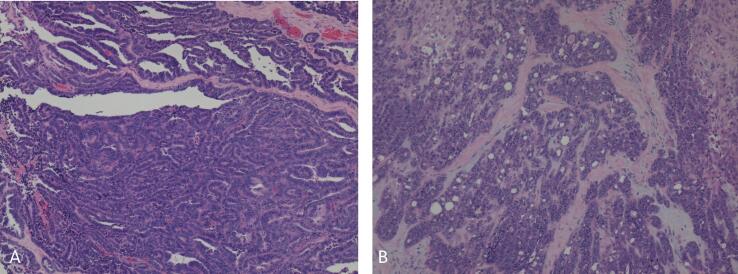
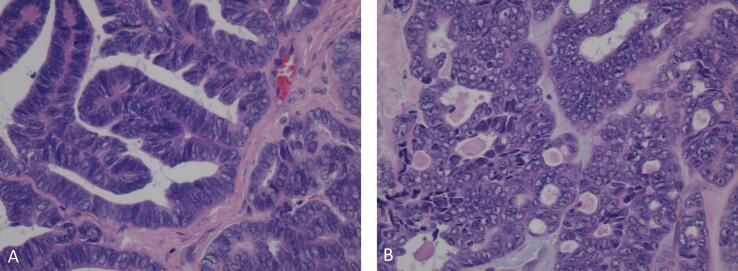
Histologically, the ovary showed an infiltrative tumor, along with endometriosis. The tumor showed a varying admixture of histologic patterns including tubular, glandular, papillary, corded and solid (Fig. 1). The cells featured scant cytoplasm, nuclear overlap, moderately atypical nuclei with vesicular changes, inconspicuous to variably prominent nucleoli and increased mitotic activity (Fig. 2). Tumors cells were strongly positive for CK7, PAX-8; focally positive for HNF1B, GATA-3, TTF1, CD10 and p16 while negative for ER, PR, WT-1 and calretinin. Additionally, tumor showed non aberrant p53 expression, retained expression of DNA mismatch repair proteins (MLH1, PMS2, MSH6, MSH2), PTEN, and ARID1A. With the morphology and supportive immunoprofile, diagnosis of an ovarian mesonephric-like adenocarcinoma was rendered.
Fig. 1.
Ovarian mesonephric-like adenocarcinoma showing, A. Glandular and papillary patterns, B. Tubular, trabecular and corded pattern with associated desmoplasia. (H&E stain, 10× magnification).
Fig. 2.
Ovarian mesonephric-like adenocarcinoma showing, A. Angulated glands lined by atypical columnar cells, B. Tubules with cells showing atypical angulated overlapping nuclei and containing intraluminal eosinophilic secretions. (H&E stain, 40× magnification).
The patient underwent a surgical staging with exploratory laparotomy, total abdominal hysterectomy, pelvic lymphadenectomy, infra-colic omentectomy and lysis of adhesions. She recovered well, with no post-operative complications. After tumor board and pathology review, the final diagnosis was Stage IA mesonephric adenocarcinoma of the ovary. After discussion at the multi-disciplinary tumor board, the decision was made for active surveillance.
The patient continued to follow for surveillance visits with no evidence of disease until about 18 months post-operatively when she began to report abdominal and flank pain. CT imaging displayed 2 pelvic masses - a soft tissue mass in the right hemi-pelvis immediately adjacent to the external iliac chain measuring 4.1 × 3.7 cm, possibly representing lymphadenopathy and a contiguous mass that was more central, measuring 2.9 × 2.3 cm. Her CA-125 which had previously been normal was now increased to 56 U/ml. A fine needle aspiration biopsy of the lesion was non-diagnostic, showing rare cellular atypia with blood and inflammation. The patient then began to experience bright red blood per rectum and a colonoscopy was urgently performed showing a non-obstructing friable medium-sized mass in the sigmoid colon. The endoscopic biopsy revealed colonic mucosa involved by metastatic carcinoma with morphologic features and immunoprofile (PAX-8 positive; CDX-2 and WT-1 negative) consistent with metastatic ovarian mesonephric-like adenocarcinoma. PET/CT scan was performed showing hypermetabolic hepatic capsular implants, pelvic peritoneal nodules and a large right pelvic mass abutting the sigmoid colon compatible with metastatic disease. The patient was not candidate for cytoreductive surgery due to multiple sites of disease. She was referred to medical oncology for chemotherapy.
PD-L1 and multigene panel next generation sequencing were performed on a sample of the recurrent tumor with no actionable mutation identified. She was noted to have PD-L1 score of 0, and found to have variants of unknown significance in the ATM gene (c.4303A > C (p.Lys145Gln) and PALB2 gene (c.693A > T (p.Lys231Asn). She was started on combination chemotherapy with carboplatin and paclitaxel. After the first three cycles of chemotherapy, her disease responded with decreased size of the hepatic capsular and peritoneal implants and decreased thickening of sigmoid colon wall. She continued the chemotherapy for total of nine cycles, until CT imaging revealed an increased size of the hepatic capsular implants. She was then switched to single agent bevacizumab and received a total of 14 cycles. To date she is tolerating the single agent therapy well. She continues to demonstrate stable disease clinically and on imaging and her CA-125 is normal.
3. Discussion
To the best of our knowledge this case represents the thirteenth reported case of ovarian MLA and the tenth case with a Mullerian associated lesion of the same ovary (McFarland et al., 2016, McCluggage et al., 2018, Dundr, 2020). As MLA is heterogeneous in its architecture, it is often misdiagnosed as other malignant neoplasms. To support the presence of MLA of the ovary, we described a tumor that contains an admixture of glands, tubules, cords, papillae, and solid growth patterns. Nuclear morphology included moderately atypical nuclei with prominent nucleoli, nuclear overlapping and nuclear vesicular changes. In addition, there was a distinctive immunophenotype of MLA which included wild-type P53, negative staining for ER and PR, and focally positive staining for PAX8, TTF-1, CD10, and GATA3 (Yano, 2019).
Ovarian MLA is an extremely rare neoplasm that has been reported in women between the ages of 50 to 73. The differential diagnosis of such a neoplasm includes female adnexal tumor of probable Wolffian origin (FATWO), endometroid adenocarcinoma and metastasis from primary thyroid carcinoma or primary colorectal adenocarcinoma (Chapel, 2018). In our case, the differential diagnosis was narrowed by the absence of a primary lesion of the thyroid or colorectal region at the initial presentation. The presence of GATA3 staining also points against primary thyroid carcinoma as GATA3 is a marker of mesonephric epithelium (Howitt, 2015). The absence of WT1 and calretinin and the positive staining for GATA3 described in this tumor weighed against a diagnosis of FATWO (Gupta et al., 2014). Lastly, the negative staining for ER and PR with a wild type p53 expression points in favor of MLA over endometrioid adenocarcinoma.
The diagnosis of either true mesonephric adenocarcinoma or MLA is challenging on biopsy materials and frozen sections as it shares many similar features to clear cell carcinoma, serous carcinoma, endometrial carcinoma and carcinosarcoma. However, true mesonephric adenocarcinoma and MLA will display limited pseudo-stratification a single layer off cuboidal lining and the presence of eosinophilic secretion in the lumen (Zhang, 2019). To reiterate, the terminology of ‘mesonephric-like’ adenocarcinoma is being used when describing tumors of primary ovarian or endometrial origin, as the etiology of these tumors outside of the cervix is only recently described and the histogenesis is not proven.
Evidence to support a mesonephric origin of MLA include the morphologic and immunophenotypic similarities to true mesonephric adenocarcinomas. Molecular analysis of 7 cases of MLA of the uterine corpus and ovary also showed similarities to ‘true’ mesonephric carcinomas as KRAS mutations were noted in all cases analyzed. However, 3 out of 7 cases harbored a PIK3CA mutations, which in is not characteristic of ‘true’ mesonephric adenocarcinoma (McCluggage et al., 2018, Mirkovic, 2018). Evidence to support a Mullerian origin include the absence of mesonephric remnants within the tumor. In the case of primary endometrial malignancies, the extensive involvement of the endometrium without myometrial involvement is seen in some cases. Another point in favor of the Mullerian origin of at least some of the cases of MLA is the presence of Mullerian lesions, such as endometriosis and adenofibromas in a majority of the MLA cases. Recently, two groups have published case reports of ovarian neoplasms with distinct areas of MLA and serous borderline tumor/low-grade grade carcinoma. Chapel et al used molecular analysis to determine that both components of the biphasic tumor contained the same driver mutation and 5 distinct copy number variants, thus were clonally related (Chapel, 2018). McCluggage et al expanded on the conclusions of Chapel by providing additional cases of ovarian MLA with associated Mullerian lesions of the same ovary (McCluggage et al., 2018). Together, the authors suggested that at least a subset of ovarian and uterine corpus MLAs are of Mullerian origin, differentiate along a mesonephric pathway and are probably closely related to endometroid carcinomas (McCluggage et al., 2018, Chapel, 2018).
Due to the limited number of cases with adequate clinical follow-up, the overall prognosis is difficult to determine. There is also no recommended or standard post-operative treatment of uterine or ovarian MLA. Among those cases reported, the most common post-operative treatment is combination chemotherapy with carboplatin plus paclitaxel. Although MLA has shown to be responsive to carboplatin plus paclitaxel in patients with late-stage disease, many cases have demonstrated disease progression despite post-operative treatment. Other forms of adjuvant treatment include chemoradiation or radiation therapy alone, but clinical response has been variable (Bagué et al., 2004, Montagut, 2003).
In summary, we described a rare case of a FIGO Stage IA ovarian MLA with coexisting endometriosis that subsequently developed metastasis detected at 18 months follow up. This case provides additional evidence that at least a subset of MLAs has a Mullerian origin which then may differentiate down a mesonephric pathway. This case also demonstrates the aggressive nature of ovarian MLA and that future studies are needed to determine the best postoperative adjuvant treatment recommendations.
CRediT authorship contribution statement
Kieran Seay: Visualization, Conceptualization, Investigation, Writing - original draft. Tracey Akanbi: Visualization, Investigation, Writing - original draft. Bethany Bustamante: Conceptualization, Writing - review & editing. Shweta Chaudhary: Formal analysis. Gary L. Goldberg: Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bagué S., Rodríguez I.M., Prat J. Malignant mesonephric tumors of the female genital tract: a clinicopathologic study of 9 cases. Am. J. Surg. Pathol. 2004;28(5):601–607. doi: 10.1097/00000478-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Chapel D.B. An ovarian adenocarcinoma with combined low-grade serous and mesonephric morphologies suggests a Müllerian origin for some mesonephric carcinomas. Int. J. Gynecol. Pathol. 2018;37(5):448–459. doi: 10.1097/PGP.0000000000000444. [DOI] [PubMed] [Google Scholar]
- Dundr P. Ovarian mesonephric-like adenocarcinoma arising in serous borderline tumor: a case report with complex morphological and molecular analysis. Diagn. Pathol. 2020;15(1):91. doi: 10.1186/s13000-020-01012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.K., Srinivasan R., Nijhawan R. Female adnexal tumor of probable Wolffian origin. Indian J. Pathol. Microbiol. 2014;57(4):620–622. doi: 10.4103/0377-4929.142703. [DOI] [PubMed] [Google Scholar]
- Howitt B.E. GATA3 is a sensitive and specific marker of benign and malignant mesonephric lesions in the lower female genital tract. Am. J. Surg. Pathol. 2015;39(10):1411–1419. doi: 10.1097/PAS.0000000000000471. [DOI] [PubMed] [Google Scholar]
- McCluggage W.G., Vosmikova H., Laco J. Ovarian combined low-grade serous and mesonephric-like adenocarcinoma: further evidence for a mullerian origin of mesonephric-like adenocarcinoma. Int. J. Gynecol. Pathol. 2018 doi: 10.1097/PGP.0000000000000573. [DOI] [PubMed] [Google Scholar]
- McFarland M., Quick C.M., McCluggage W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology. 2016;68(7):1013–1020. doi: 10.1111/his.12895. [DOI] [PubMed] [Google Scholar]
- Mirkovic J. Targeted genomic profiling reveals recurrent KRAS mutations in mesonephric-like adenocarcinomas of the female genital trac. Am. J. Surg. Pathol. 2018;42(2):227–233. doi: 10.1097/PAS.0000000000000958. [DOI] [PubMed] [Google Scholar]
- Montagut C. Activity of chemotherapy with carboplatin plus paclitaxel in a recurrent mesonephric adenocarcinoma of the uterine corpus. Gynecol. Oncol. 2003;90(2):458–461. doi: 10.1016/s0090-8258(03)00228-2. [DOI] [PubMed] [Google Scholar]
- Yano M. Coexistence of endometrial mesonephric-like adenocarcinoma and endometrioid carcinoma suggests a Müllerian duct lineage: a case report. Diagn. Pathol. 2019;14(1):54. doi: 10.1186/s13000-019-0830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Mesonephric adenocarcinoma of the uterine corpus: report of 2 cases and review of the literature. Int. J. Gynecol. Pathol. 2019;38(3):224–229. doi: 10.1097/PGP.0000000000000493. [DOI] [PubMed] [Google Scholar]