Abstract
Opioid receptors (ORs) convert extracellular messages to signaling events by coupling to the heterotrimeric G proteins, Gα•βγ. Classic pharmacological methods, such as [35S]GTPγS binding and inhibition of cyclic AMP production, allow for general opioid characterization, but they are subject to the varying endogenous Gα proteins in a given cell type. Bioluminescence resonance energy transfer (BRET) technology offers new insight by allowing the direct observation of Gα subunit–specific effects on opioid pharmacology. Using a Venus-tagged Gβγ and nanoluciferase-tagged truncated G protein receptor kinase 3, an increase in BRET signal correlated with OR activation mediated by a specific Gα protein. The magnitude of the BRET signal was normalized to the maximum response obtained with 10 µM 2-(3,4-dichlorophenyl)-N-methyl-N-[(1R,2R)-2-pyrrolidin-1-ylcyclohexyl]acetamide (U50,488) for the kappa OR (KOR). Opioids reached equilibrium with the KOR, and concentration-response curves were generated. Although the full agonists U50,488, salvinorin A, nalfurafine, and dynorphin peptides were equally efficacious regardless of the Gα subunit present, the concentration-response curves were leftward shifted when the KOR was signaling through Gαz compared with other Gαi/o subunits. In contrast, the Gα subunit distinctly affected both the efficacy and potency of partial kappa agonists, such as the benzomorphans, and the classic mu opioid antagonists, naloxone, naltrexone, and nalmefene. For example, (-)pentazocine had EC50 values of 7.3 and 110 nM and maximal stimulation values of 79% and 35% when the KOR signaled through Gαz and Gαi1, respectively. Together, these observations suggest KOR pharmacology varies based on the specific Gα subunit coupled to the KOR.
SIGNIFICANCE STATEMENT
Opioid receptors couple to various heterotrimeric Gαβγ proteins to convert extracellular cues to precise intracellular events. This paper focuses on how the various inhibitory Gα subunits influence the pharmacology of full and partial agonists at the kappa opioid receptor. Using a bioluminescent assay, the efficacy and potency of kappa opioids was determined. Opioid signaling was more potent through Gαz compared with other Gα proteins. These observations suggest that Gαz may impact opioid pharmacology and cellular physiology more than previously thought.
Introduction
Kappa opioid receptors (KORs), members of the classic seven-transmembrane G protein–coupled receptor (GPCR) family, transduce extracellular cues into intracellular signaling events through receptor-coupled heterotrimeric G proteins, Gα•βγ. When stimulated by an opioid, activated opioid receptors (ORs) initiate a conformational change in the Gα subunit (Rasmussen et al., 2011). This change allows for the binding of GTP to the Gα subunit, which in turn allows the activated Gα to dissociate from Gβγ and the OR (Kenakin, 2011). Gα and Gβγ can then independently associate with their downstream effectors. For example, Gα proteins interact with adenylyl cyclase (AC) to regulate intracellular cAMP concentrations. The signal is terminated when the GTP bound to the Gα subunit is hydrolyzed to GDP and Gα again associates with Gβγ and the OR.
There are four families of Gα proteins (Gαi, Gαs, Gαq, Gα13) that share features including a guanine nucleotide binding site, intrinsic GTPase activity, and lipid modifiers to facilitate localization to the plasma membrane (Wedegaertner et al., 1995; Syrovatkina et al., 2016; Hilger et al., 2018). Differences between the families are based on sequence homology, distinct downstream effectors, and toxin sensitivity (Glick et al., 1998; Milligan and Kostenis, 2006). KORs predominately couple to the Gαi class, comprising Gαi1, Gαi2, Gαi3, GαoA, GαoB, and Gαz. Since all members of this class inhibit AC activity and thus decrease cAMP levels, it is common to downplay the differences that exist within this Gα class. However, when individual Gα subunits within the inhibitory Gα class were knocked down by intracerebroventricular administration of Gα siRNA into the right ventricle of mice, differences in the activation profiles of various OR agonists were observed (Sánchez-Blázquez et al., 1999, 2001). Thus, the growing evidence that the unique GPCR•Gα interaction could influence signaling creates a much more complex picture than previously thought.
Current in vitro pharmacological methods, however, lack the specificity needed to distinguish contributions from unique Gα-mediated signaling. For example, it is common to correlate changes in cAMP levels to OR activation. This assay has several limitations. First, changes in cAMP are subject to amplification from many converging pathways including signaling from competing Gαs proteins (Yung et al., 1995). Additionally, cell lines may express up to nine isoforms of AC, to which the Gα proteins couple to varying degrees (Sadana and Dessauer, 2009). Thus, by simply measuring changes in cAMP levels in a given cell line, we are looking downstream of the OR and not accounting for the specific Gα protein. In contrast, the [35S]GTPγS binding assay measures the binding of nonhydrolyzable GTPγS to the Gα subunit, the initial step in GPCR signaling, and thus is not subject to influences from concurrent signaling pathways. However, this assay still fails to account for the specific Gα subunit coupled to the receptor or the pool of available Gα subunits in a given cell line (Traynor and Nahorski, 1995; Bidlack and Parkhill, 2004). Although these aforementioned assays have offered insight into the OR signaling mechanisms and opioid pharmacology, determining how the cellular environment of G proteins influences OR signaling will be useful in obtaining a more thorough understanding of opioid receptor signaling.
To address the limitations of prior methods, researchers have adapted a novel bioluminescence resonance energy transfer (BRET)–based assay. BRET monitors protein-protein interactions in live cells with the necessary sensitivity to view G protein signaling (Stoddart et al., 2015). Previous data from our laboratory using BRET to obtain the Gα-specific pharmacological profiles of buprenorphine and samidorphan showed that the KOR signaling was very sensitive to the Gα subunit (Bidlack et al., 2018). For instance, at the KOR, the 1:3 molar combination of buprenorphine:samidorphan generated maximal stimulation (Emax) values ranging from 22% signaling through Gαi2 to 85% when signaling through Gαz. In this study, we expanded upon this concept and determined how the different Gα subunits from the Gαi/o family influenced the pharmacology of full and partial agonists at the KOR.
Materials and Methods
Cell Culture, Plasmids, and Transfection.
Human embryonic kidney (HEK) 293T cells (ATCC, Manassas, VA) were cultured on poly-l-lysine (Millipore Sigma, Darmstadt, Germany)–coated 100-mm dishes in Dulbecco’s modified Eagle’s medium (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 1 mM sodium pyruvate, and 100 U/ml penicillin and streptomycin. Cells were maintained at 37°C in a 5% CO2 atmosphere. Prior to transfection, 4 × 106 cells were seeded onto a Matrigel-coated (Corning, Inc., Corning, NY) 60-mm dish in antibiotic-free medium and incubated for 4 hours at 37°C and 5% CO2, as previously described (Masuho et al., 2015a). The human KOR (cDNA Resource Center, Bloomsburg, PA), human Gα subunit of interest (cDNA Resource Center), Venus 156–239-Gβ1, Venus 1–155-Gγ2, or Venus 1–155-Gγ7, and myristic acid attachment peptide (mas) with the C terminus of G protein–coupled receptor kinase (GRK3ct) fused with nanoluciferase (nLuc) plasmids (masGRK3ct-nLuc; gifts from Dr. Kirill A. Martemyanov, The Scripps Research Institute Florida, Jupiter, FL) were transfected at a 1:2:1:1:1 ratio (ratio 1 = 0.42 μg of plasmid DNA) as previously described (Masuho et al., 2015a) using Lipofectamine LTX with PLUS reagent (Invitrogen, Carlsbad, CA) (Masuho et al., 2015a) in antibiotic-free Opti-MEM I Reduced Serum Media (GIBCO).
Measuring KOR Signaling Through Different Gα Subunits Using BRET.
BRET measurements between Venus-Gβ1γ2 or Venus-Gβ1γ7 and masGRK3ct-nLuc were performed to determine agonist-dependent activation of the Gα protein of interest in live HEK 293T cells. For each experiment performed, a separate transfection with Gα, Venus-Gβ1γ2, and masGRK3ct-nLuc was also performed to ensure the opioid of interest was not having an effect without the KOR expressed. Additionally, another transfection with the KOR, Venus-Gβ1γ2, and masGRK3ct-nLuc was performed to confirm that endogenous Gα proteins were not contributing to the BRET signal. Cells were prepared 16–20 hours posttransfection as previously described (Masuho et al., 2015a). BRET assays were performed at 25°C in 96-well flat bottom white plates (Greiner Bio-One North America, Inc., Monroe, NC) in a final volume of 100 µl/well. Approximately 75,000 transfected cells per well (25 µl) were incubated for 50 minutes or for varying times with or without opioids in BRET buffer [PBS (GIBCO) with 0.5 mM MgCl2 and 0.1% glucose]. Plates were read after addition of 25 µl 2× Nano-GloTM Luciferase Assay Substrate (Promega, Madison, WI) on a Flexstation 3 (Molecular Devices, San Jose, CA) at 535 and 475 nm. The BRET signal was calculated as emission of Venus at 535 nm divided by the emission of nLuc at 475 nm. Each assay was performed in duplicate and repeated with separate transfections at least three times. A baseline, with no opioid stimulation, was set as the minimum BRET value, and 10 µM 2-(3,4-dichlorophenyl)-N-methyl-N-[(1R,2R)-2-pyrrolidin-1-ylcyclohexyl]acetamide (U50,488), a full KOR agonist, was set as the maximal BRET signal. The mean baseline BRET ratio was subtracted from each experimental BRET ratio to obtain a ΔBRET ratio. All ΔBRET ratios were normalized to the 10 µM U50,488 ΔBRET ratio, which was set at 100%.
Data Analysis and Statistics.
Concentration-response curves were generated in SigmaPlot (version 11; Systat Software Inc., San Jose, CA), and Emax and EC50 values were calculated from a logistic-3 parameter curve fit of a log-probit plot. Data are expressed as the mean EC50 and Emax values ± S.D. from three or more independent experiments, performed in duplicate. The averages of the duplicates for each experiment were used to calculate the mean and S.D. values. The S.D. was computed from the n ≥ 3 independent experiments. Statistical significance between all Gα subunits was determined using one-way ANOVA with Holm-Sidak post hoc testing. Statistical significance between Gαi1 and Gαz EC50 and Emax values were determined using a two-tailed Student’s t test. All statistical analysis was performed in PRISM software (version 6.0; GraphPad Software Inc., La Jolla, CA).
Western Blotting.
Approximately 5 × 106 HEK 293T cells transfected with KOR, Gα subunit of interest, Venus-Gβ1γ2, and masGRK3ct-nLuc were scraped with PBS, containing Roche cOmplete, EDTA-free protease inhibitors (Roche, Indianapolis, IN). Cells were then lysed using a Dounce homogenizer (Dounce et al., 1955; DeCaprio and Kohl, 2019). Lysates were centrifuged at 18,000g for 20 minutes at 4°C. The soluble fraction was removed. Membrane proteins were extracted by resuspending the pellet in PBS containing EDTA-free protease inhibitors and 0.1% Triton-X 100, with gentle rocking at 4°C for 20 minutes. Samples were centrifuged again at 18,000g for 15 minutes at 4°C. The supernatant was collected, and protein content was determined using Pierce BCA assay kit according to manufacturer’s guidelines (Thermo Fisher Scientific, Rochester, NY). Total protein, 50 μg, in 2× Laemmli sample buffer (0.005% bromophenol blue, 4% SDS, 20% glycerol, 120 mM Tris-Cl, pH 6.8) with 5% β-mercaptoethanol (Karlsson et al., 1994) was heated at 100°C for 10 minutes. Samples were separated on a 4%–20% gradient polyacrylamide gel and transferred to a nitrocellulose membrane. Blots were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20, pH 7.4, for 60 minutes at room temperature and incubated overnight at 4°C with 1:1000 dilution of polyclonal rabbit anti-human Gα antibody against each Gα subunit (Cell Signaling Technologies, Dansvers, MA). Blots were washed for 5 minutes three times with Tris-buffered saline containing 0.1% Tween 20, pH 7.4, and incubated for 60 minutes with a 1:1000 dilution of goat anti-rabbit horseradish peroxidase secondary antibody (Cell Signaling Technologies). A BioRad ChemiDoc chemiluminescent imager (Hercules, CA) was used to image the protein.
Real-Time Quantitative Polymerase Chain Reaction Analysis.
Total RNA was extracted from either HEK 293A or CHO cells using E.N.Z.A. Total RNA kit following the manufacturer’s protocol (Omega bio-tek, Norcross, GA). cDNA was produced using ThermoScript RT-PCR Systems (Invitrogen). iTaq Universal SYBR Green (Bio-Rad) was used as a double strand DNA-specific dye. Species-specific primers were designed for each Gα subunit in both cell lines. The CHO cell line primers were as follows: Gαi1 [forward (fwd): GGAGGTTGAAGATAGACTTTGGAG, reverse (rev): TGCAGAATCATTGAGCTGGTACTC], Gαi2 (fwd: CTGAGGAACAAGGGATGCTGC, rev: GTTTTCACACGGGTCCGCA), Gαi3 (fwd: AGGCGTGATTAAACGGCTCT, rev: AGTGTGTCTCCACAATGCCT), GαO (fwd: GCCAAAGACGTGAAATTACTCC, rev: AGTATCCATGGCCCGGACGATGGC), and Gαz (fwd: AAGCTCTATGAGGATAACCAGACG, rev: TACGTGTTCTGACCCTTGTACTCT). The HEK 293 cell line primers were as follows: Gαi1 (fwd: GGAGGTTGAAGATAGACTTTGGTG, rev: TGCAGAATCATTAAGCTGGTACTC), Gαi2 (fwd: ACAACATCCTCAAGGGCTCAAG, rev: ATGCCAGAATCCCTCCAGAGT), Gαi3 (fwd: ATGGGACGGCTAAAGATTGACTT, rev: ATTGAGCTGATATTCCCTGGATCT), GαO (fwd: GGCATCGAATATGGTGATAAGG, rev: GTAGTATTTGGCAGAGTCGTTGAG), and Gαz (fwd: ACGACCTGAAACTCTACGAGGATA, rev: CTTGTACTCGGGAAAGCAGATG). Relative Gα transcript levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Opioid Alkaloids and Peptides.
The κ-selective agonist U50,488 methanesulfonate (Von Voigtlander and Lewis, 1982) and the κ-selective antagonist norbinaltorphimine (nor-BNI) (Portoghese et al., 1987) were obtained from Sigma-Aldrich (St. Louis, MO). Nalfurafine was obtained from the National Institute on Drug Abuse Division of Drug Supply and Analytical Services. Enadoline was obtained from Parke-Davis Pharmaceuticals (Cambridge, UK). Salvinorin A was purchased from ChromaDex Inc. (Irving, CA). The κ partial agonists (-)pentazocine hydrochloride (Archer et al., 1964), (-)cyclazocine hydrochloride (Archer et al., 1996), and nalmefene hydrochloride (Bart et al., 2005) were obtained from Dr. Mark Wentland (Rensselaer Polytechnic Institute, Troy, NY) (Wentland et al., 2009). ((±)-α-5,9-Dimethyl-2-(l-tetra-hydrofurfuryl)-2′-hydroxy-6,7-benzomorphan) hydrochloride (Mr 2033) was obtained from Boehringer Ingelheim (Germany). Naloxone and naltrexone were obtained from Dr. Mark Wentland (Rensselaer Polytechnic Institute, Troy, NY). Samidorphan was synthesized as previously described (Wentland et al., 2005). Dynorphin A (1–17) was purchased from AnaSpec, Inc. (Freemont, CA). Dynorphin A (1–13) and α-neo-endorphin were purchased from Bachem (Torrance, CA). Dynorphin B (1–13) was purchased from GenScript (Picataway, NJ).
Results
Using BRET Sensors to Study KOR Signaling.
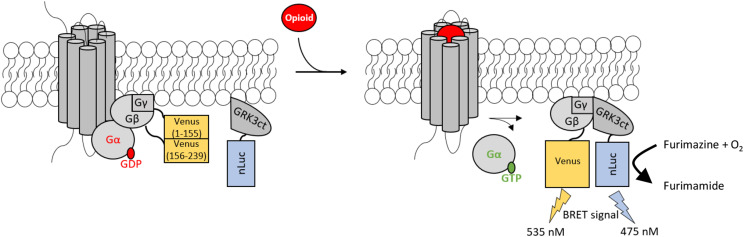
Since the focus of this study was to measure Gα subunit–specific activation of the KOR, we did not want to impede the KOR•Gα interaction by modifying either protein. Instead, correlating KOR activation to the release of free Gβγ was favored, similar to previous strategies (Donthamsetti et al., 2015; Masuho et al., 2015a,b). Since Gβγ functions as an obligate dimer, the BRET acceptor, Venus, was split with Venus 1–155 fused to Gγ2 or Gγ7 and Venus 156–239 fused to Gβ1 (Fig. 1). This ensured only a functional Venus (1–239) formed after Gβγ dimerization. Gβ1γ2-Venus was used for all experiments unless otherwise specified. The BRET donor, nLuc, was fused to the C-terminal end of a truncated form of the Gβγ’s downstream effector, G protein–coupled receptor kinase 3 (GRK3). The C-terminal domain of this protein only contains the pleckstrin homology domain, which is responsible for Gβγ binding (Lodowski et al., 2003); the central protein kinase domain has been removed. Consequently, increased receptor phosphorylation and subsequent desensitization did not influence KOR activation. Lastly, a mas sequence (MGSSKSKTSNS) precedes the GRK3ct construct, ensuring its localization to the plasma membrane. When activation of the KOR occurred, Gα was released from Gβγ. The free Gβγ-Venus coupled with its downstream effector, masGRK3ct-nLuc, allowing for nonradiative energy transfer between nLuc and Venus and for a BRET signal to be calculated (Fig. 1).
Fig. 1.
Overview of the BRET assay to measure KOR signaling through different Gα subunits. The KOR in its resting state is coupled with Gα•GDP and Gβγ. Venus is split between Gβ and Gγ; thus, a fully functional Venus forms only when Gβγ comes together as an obligate dimer. masGRK3ct-nLuc is tethered to the membrane in the resting state. When an opioid binds to the KOR, GDP is released from the expressed Gα subunit, and GTP binds. This binding causes dissociation of the Gαβγ heterotrimer. Gβγ will then interact with its downstream target, masGRK3ct-nLuc, thus bringing nLuc and Venus in close proximity to each other. When furimazine, the substrate for nLuc, is added, nonradiative energy transfer occurs between nLuc and Venus. The BRET signal is calculated as emission of Venus at 535 nm divided by the emission of nLuc at 475 nm, which correlates to activation of the KOR through the specific Gα subunit of interest.
Although BRET sensors have been used to monitor protein-protein interactions, proper controls are essential for Gα-specific data interpretation. The maximum possible BRET signal was obtained when no exogenous Gα subunit was expressed, allowing the expressed Gβ1γ2-Venus to couple to masGRK3ct-nLuc (Fig. 2A). Upon addition of 10 μM U50,488, there was no increase in the BRET ratio above baseline, signifying that endogenous Gα proteins did not affect KOR-mediated BRET signaling through an exogenously expressed Gα subunit. When Gα was expressed in excess, it served as a sink for the Gβγ and thus pulled free Gβγ away from GRK3 (Hollins et al., 2009; Donthamsetti et al., 2015). This allowed for a minimum BRET signal to be obtained (Fig. 2B). Thus, by capitalizing on the relative affinities of Gβγ to masGRK3ct-nLuc and Gα, the dynamic range of the system was determined. When the KOR, Gα subunit of interest, Gβ1γ2-Venus, and masGRK3ct-nLuc were all expressed, efficient coupling was observed as indicated by the low baseline BRET ratio (Fig. 2, C and E). Application of the κ-selective agonist, U50,488, increased the BRET ratio signifying activation; however, the stimulated ratio was well within the dynamic range of the system. Moreover, application of a κ-selective antagonist, nor-BNI, did not result in a significant increase in the BRET ratio (Fig. 2C). Lastly, to ensure the BRET ratio was a result of KOR•Gα of interest coupling, a Gα subunit from another class, Gαs, was overexpressed into the system. Again, a low baseline BRET ratio was obtained, signifying the Gβγ coupled to Gαs. Upon KOR activation with agonist U50,488, the BRET ratio remained unchanged, implying that KOR•Gαs coupling was not capable of signaling (Fig. 2D). The baseline BRET ratios across all experiments performed among the six different Gα subunits were not statistically different from each other (P ≥ 0.2, Fig. 2E). Similarly, the maximum U50,488-stimulated ratios were not statistically different among Gα subunits (P ≥ 0.2, Fig. 2E).
Fig. 2.
BRET experimental controls. (A) The maximum BRET signal was obtained when HEK 293T cells were transfected with KOR, Gβγ-Venus, and masGRK3ct-nLuc. Since there were no exogenous Gα subunits expressed, the Gβγ-Venus couples to masGRK3ct-nLuc, and a BRET ratio of 0.58 ± 0.037 was obtained. When 10 µM U50,488 was applied, there was no change in the BRET signal, signifying that the endogenous Gα proteins did not affect the signal. (B) The minimum BRET signal was obtained when Gβγ-Venus, masGRK3ct-nLuc, and a Gα subunit of interest were expressed. In this scenario, Gβγ-Venus coupled to the Gα subunit. Since HEK 293T cells did not endogenously express the KOR, when U50,488 was applied, there was no change from the baseline BRET signal. (C) Optimal assay conditions were obtained when KOR, Gβγ-Venus, masGRK3ct-nLuc, and Gα were expressed. In the baseline condition, Gβγ-Venus coupled to Gα, and thus there was a minimal BRET signal. When the agonist U50,488 was applied, the BRET ratio significantly increased (P = 0.003). Lastly, when the KOR antagonist nor-BNI was applied, the BRET ratio did not change from the baseline condition (P = 0.8). (D) Since KOR couples to the Gαi/o class of proteins, when Gαs was expressed with KOR, Gβγ-Venus, and masGRK3ct-nLuc, no signal was transmitted when 10 µM U50,488 was applied. Data are the mean BRET ratio from three independent experiments performed in duplicate ± S.D. (E) Baseline and U50,488-stimulated ratios for all experiments performed across the various Gα subunits. No statistically significant differences were observed between baseline and 10 µM U50,488–stimulated ratios between the Gα subunits. Data are mean BRET ratios ± S.D.
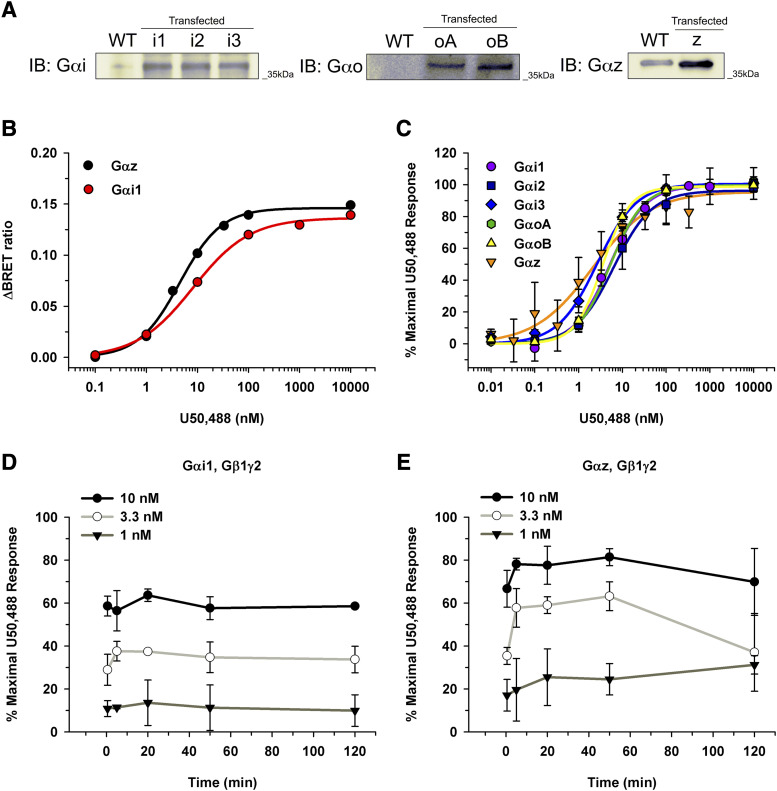
In the experimental system, the Gα subunit of interest was expressed in excess to minimize baseline Gβγ interacting with masGRK3ct-nLuc and ensure that the generated BRET signal was the result of desired Gα subunit coupling rather than endogenous Gα. To confirm expression of the Gα subunit in excess, Western blot analysis was performed against the individual Gα subunits, Gαi, Gαo, and Gαz (Fig. 3A). Although expression levels using different antibodies cannot be directly compared, the Gα subunits were expressed in excess compared with endogenous Gα proteins (Fig. 3A). Representative curves of raw BRET ratios for U50,488 when the KOR was signaling through either Gαi1 or Gαz are shown in Figure 3B. To control for subtle variation in expression level, a natural byproduct of transient transfections, all subsequent BRET data were normalized to the maximal BRET signal obtained with 10 µM of the full agonist, U50,488. To ensure 10 µM U50,488 produced a maximal BRET signal independent of the Gα subunit, concentration-response curves were generated illustrating the KOR signaling through each Gα subunit (Fig. 3C). Regardless of which Gα subunit the KOR coupled to, the efficacy of U50,488 did not significantly vary (Fig. 3C). The EC50 values ranged from 1.5 ± 0.85 nM through Gαz to 7.9 ± 3.3 nM through Gαi2 (Fig. 3C). In contrast to previous studies (Masuho et al., 2015b), nonsaturating concentrations of opioids were tested. A time course was generated for KOR activation to ensure adequate time for ligand-receptor interaction to reach equilibrium (Fig. 3, D and E). The BRET signal remained constant for a given U50,488 concentration when the KOR signaled through Gαi1 regardless of time (Fig. 3D). In contrast, when the KOR signaled through Gαz, a maximum BRET signal was obtained after 5 minutes and remained constant until the signal diminished at 120 minutes (Fig. 3E). Thus, we observed that 50 minutes allowed adequate time to obtain a maximal BRET signal at nonsaturating concentrations (Fig. 3, D and E). Subsequent data were normalized to values obtained with 10 µM U50,488 with a 50-minute incubation performed for each individual experiment.
Fig. 3.
Gα subunit expression level and U50,488 concentration-response and time-course experiments in HEK 293T cells. (A) To confirm overexpression of Gα subunits, Western blot analysis was performed on the individual Gα subunit transfections. Overexpression levels of Gαi1 (i1), Gαi2 (i2), Gαi3 (i3), GαoA (oA), GαoB (oB), and Gαz (z) were compared with the endogenous Gα levels in HEK 293T cells. Notably, HEK 293T cells did not express Gαo. (B) Representative concentration-response curves for U50,488 are shown illustrating raw BRET ratios for KOR•Gαz and KOR•Gαi1 after a 50-minute incubation. Ratios were calculated by subtracting opioid-induced BRET ratio from the baseline (no opioid) condition. (C) To control for variations in expression level, the BRET ratio from each experiment was normalized to 10 µM U50,488. Regardless of which Gα subunit the KOR was signaling through, U50,488 had similar Emax and EC50 values. (D and E) Time-course experiments with varying concentrations of U50,488 were performed for the KOR signaling through Gαi1 (D) and Gαz (E). Data are from three to six independent experiments performed in duplicate with mean values ± S.D. reported. IB, immunoblot; WT, wild type.
Dynorphin Peptide Signaling.
Dynorphin and its derivatives, dynorphin A (1–17), dynorphin A (1–13), dynorphin B (1–13), and α-neo-endorphin, are endogenous KOR peptides. Concentration-response curves were generated for each peptide, and the average Emax and EC50 values were determined (Table 1). The maximal efficacy was similar for each peptide regardless of which Gα subunit was coupled to the KOR. Interestingly, each dynorphin derivative tested was most potent when the KOR was signaling through Gαz compared with the other Gα subunits. For example, the EC50 values of dynorphin A (1–17) were 9.6 ± 2.7 and 52 ± 11 nM when the KOR was signaling through Gαz and Gαi2, respectively. A similar pattern was observed with the most potent signaling through Gαz for the truncated dynorphin A (1–13), producing an EC50 value of 0.85 ± 0.20 nM. Again, EC50 values of dynorphin B (1–13) ranged from 3.7 ± 2.3 nM when the KOR was signaling through Gαz compared with 28 ± 14 nM when the KOR was signaling through Gαi1. Continuing with that trend, α-neo-endorphin was most potent when the KOR signaled through Gαz, followed by Gαi3, GαoA, Gαi1, and GαoB, and finally least potent through Gαi2 (Table 1). Thus, although all of the dynorphin derivatives were efficacious regardless of which Gα subunit the KOR was signaling through, the potencies significantly varied depending on the Gα subunit.
TABLE 1.
Potency and efficacy of dynorphin peptides signaling through the KOR and different Gα subunits
Concentration-response curves were generated for the dynorphin peptides signaling through the KOR and various Gα subunits after 50-minute incubation. Although the Emax values for dynorphin A (1–17) were not significantly different regardless of the Gα subunit (P > 0.5), dynorphin A (1–17) had a statistically significant lower EC50 value when the KOR was signaling through Gαz compared with the other Gα subunits (P ≤ 0.05). Dynorphin A (1–13) was significantly more potent when the KOR signaled through Gαz compared with Gαi3 (P = 0.011). In addition, dynorphin A (1–13) was more efficacious through Gαz compared with either GαoA (P < 0.001) or GαoB (P < 0.05). Similarly, dynorphin B (1–13) was more potent when the KOR signaled through Gαz compared with Gαi1 (P < 0.01). Dynorphin B (1–13) was more efficacious through Gαz compared than GαoA (P = 0.01). Although α-neo-endorphin had a similar efficacy regardless of Gα subunit (P > 0.26), it was significantly more potent when the KOR signaled through Gαz compared with all other Gα subunits (P < 0.05). All values are means ± S.D.; measurements were performed in duplicate in three independent experiments.
| Gα | Dynorphin A (1–17) | Dynorphin A (1–13) | Dynorphin B (1–13) | α-Neo-Endorphin | ||||
|---|---|---|---|---|---|---|---|---|
| EC50 | Emax | EC50 | Emax | EC50 | Emax | EC50 | Emax | |
| nM | % | nM | % | nM | % | nM | % | |
| Gαi1 | 23 ± 4.4 | 97 ± 5.0 | 5.8 ± 1.7 | 95 ± 7.0 | 28 ± 14 | 97 ± 3.1 | 54 ± 9.8 | 100 ± 5.2 |
| Gαi2 | 52 ± 11 | 98 ± 3.1 | 6.1 ± 0.45 | 97 ± 1.4 | 6.2 ± 0.50 | 94 ± 4.2 | 110 ± 40 | 98 ± 13 |
| Gαi3 | 45 ± 0.93 | 99 ± 0.97 | 12 ± 7.1 | 96 ± 6.6 | 13 ± 5.5 | 100 ± 7.5 | 26 ± 9.4 | 100 ± 6.0 |
| GαoA | 30 ± 8.5 | 91 ± 5.5 | 5.8 ± 0.17 | 70 ± 6.6 | 5.7 ± 1.8 | 88 ± 2.7 | 45 ± 2.3 | 88 ± 5.5 |
| GαoB | 43 ± 7.7 | 97 ± 11 | 5.9 ± 2.7 | 84 ± 6.5 | 11 ± 2.3 | 92 ± 1.4 | 78 ± 9.5 | 91 ± 8.8 |
| Gαz | 9.6 ± 2.7 | 97 ± 6.8 | 0.85 ± 0.20 | 96 ± 3.3 | 3.7 ± 2.3 | 96 ± 6.1 | 6.0 ± 1.5 | 96 ± 4.5 |
Full KOR Agonist Signaling Through Different Gα Subunits.
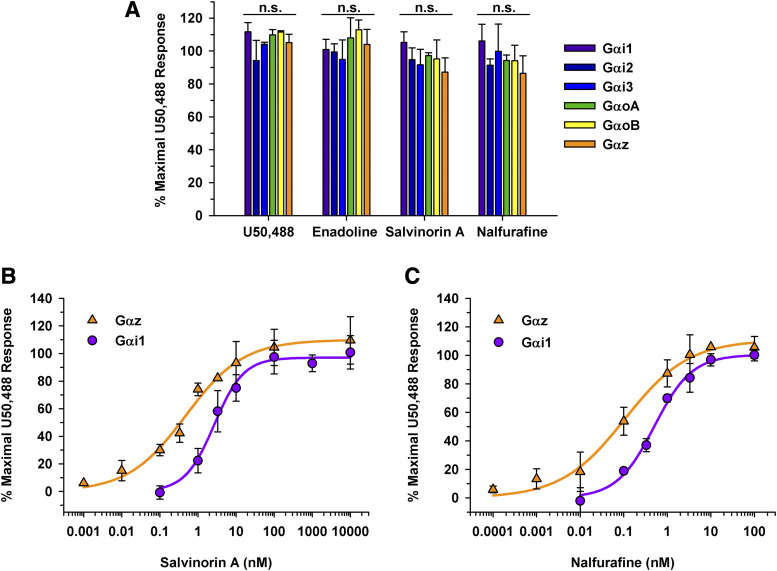
A distinct pattern emerged with dynorphin peptide signaling; however, it was unclear if opioid alkaloids would show a Gα subunit preference as well. To obtain initial opioid profiles, classically defined KOR full agonists, U50,488, enadoline, salvinorin A, and nalfurafine, at a saturating concentration of 10 µM were screened for KOR signaling through different Gα subunits (Fig. 4A). Data were normalized to separate samples containing 10 µM U50,488. Although these data are not Emax values as they were not obtained from a concentration-response curve, distinct signaling patterns were observed. For example, when a full agonist bound to the KOR, a maximum response was observed regardless of which Gα subunit the KOR was signaling through as seen with U50,488, enadoline, salvinorin A, and nalfurafine (Fig. 4A). This finding agreed with the results obtained for the dynorphin peptides (Table 1). Additionally, concentration-response curves were generated for the full KOR agonists, U50,488, salvinorin A, and nalfurafine for each Gα subunit (Table 2). Similar to the opioid peptides, salvinorin A had a similar efficacy regardless of which Gα subunit the KOR was signaling through (Table 2). However, salvinorin A was significantly more potent when the KOR was signaling through Gαz compared with any other Gα subunit. For clarity, concentration-response curves are shown for Gαi1 and Gαz resulting in EC50 values of 3.2 ± 0.83 nM and 0.36 ± 0.048, respectively (Fig. 4B, P = 0.0040). Representative concentration-response curves are shown for nalfurafine signaling through Gαi1 and Gαz (Fig. 4C). Nalfurafine was maximally efficacious regardless of which Gα subunit the KOR was signaling through (Emax values ranging from 95% ± 2.9% through GαoB to 110% ± 8.2% through Gαz). Again, nalfurafine was significantly more potent when KOR was signaling through Gαz compared with all other Gα subunits. Nevertheless, nalfurafine had EC50 values of less than 1 nM regardless of which Gα subunits were coupled to the KOR.
Fig. 4.
KOR full agonists signaling through the KOR and different Gα subunits. (A) Opioids were tested at a 10 µM final concentration with a 50-minute incubation in HEK 293T cells transiently expressing the KOR, Gα subunit of interest, Gβγ-Venus, and masGRK3ct-nLuc. No statistically significant (n.s.) differences were observed between the various Gα subunits for U50,488, enadoline, salvinorin A, or nalfurafine. Data are mean percentages of maximal stimulation ± S.D.; measurements were performed in duplicate in three independent experiments. (B) Concentration-response curves were generated for salvinorin A when the KOR was signaling through Gαi1 and Gαz. Salvinorin A was equally efficacious whether the KOR was signaling through Gαi1 (Emax values of 99% ± 13%) or Gαz (Emax value of 100% ± 11%) (P > 0.05). In contrast, salvinorin A was more potent when the KOR was signaling through Gαz with an EC50 value of 0.36 ± 0.048 nM vs. 3.2 ± 0.83 nM through Gαi1 (P < 0.01). (C) Nalfurafine had similar Emax values of 99% ± 1.1% and 110% ± 8.2% through Gαi1 and Gαz, respectively. Nalfurafine was more potent when the KOR was signaling through Gαz (EC50 value of 0.10 ± 0.050 nM) than through Gαi1 (EC50 value of 0.46 ± 0.0040 nM) (P < 0.01).
TABLE 2.
Full opioid agonists signaling through the KOR and different Gα subunits
Concentration-response curves were generated for U50,488, salvinorin A and nalfurafine, signaling through various Gα subunits after 50-minute incubation. Emax values for U50,488 were not significantly different between Gα subunits (P = 0.53). Although EC50 values varied slightly, the EC50 value for U50,488 signaling through Gαz was significantly different from Gαi1, Gαi2, and GαoA (P < 0.05). Emax values for salvinorin A did not vary between Gα subunits (P > 0.98); however, salvinorin A was significantly more potent when the KOR signaled through Gαz compared with all other Gα subunits (P < 0.01). Similarly, when the KOR was coupled to Gαz, nalfurafine was more potent compared with when the KOR was coupled to any other Gα subunits (P < 0.05). Nalfurafine was similarly efficacious regardless of the Gα subunit (P > 0.05). Data are means ± S.D.; measurements were performed in duplicate in three independent experiments.
| Gα | U50,488 | Salvinorin A | Nalfurafine | |||
|---|---|---|---|---|---|---|
| EC50 | Emax | EC50 | Emax | EC50 | Emax | |
| nM | % | nM | % | nM | % | |
| Gαi1 | 5.6 ± 1.3 | 99 ± 1.4 | 3.2 ± 0.83 | 99 ± 13 | 0.46 ± 0.0040 | 99 ± 1.1 |
| Gαi2 | 7.9 ± 3.3 | 99 ± 1.1 | 3.1 ± 0.67 | 98 ± 2.1 | 0.38 ± 0.10 | 99 ± 2.1 |
| Gαi3 | 2.6 ± 0.54 | 99 ± 1.0 | 2.2 ± 0.61 | 95 ± 5.6 | 0.27 ± 0.050 | 100 ± 7.9 |
| GαoA | 5.4 ± 0.96 | 99 ± 0.63 | 1.7 ± 0.36 | 100 ± 8.0 | 0.25 ± 0.038 | 99 ± 2.6 |
| GαoB | 3.5 ± 0.49 | 99 ± 0.31 | 1.6 ± 0.22 | 97 ± 2.8 | 0.37 ± 0.029 | 95 ± 2.9 |
| Gαz | 1.5 ± 0.85 | 96 ± 5.6 | 0.36 ± 0.048 | 100 ± 11 | 0.10 ± 0.050 | 110 ± 8.2 |
Partial KOR Agonist Signaling Through Different Gα Subunits.
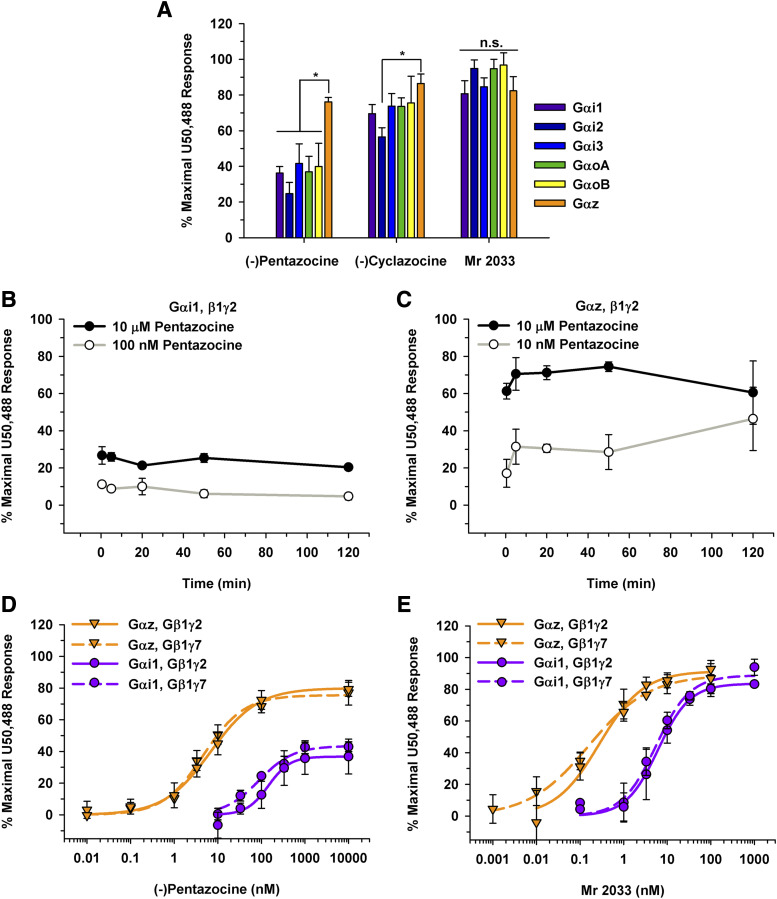
The benzomorphan partial agonists, (-)pentazocine, (-)cyclazocine, and Mr 2033, were also profiled at 10 µM (Fig. 5A). In contrast to full agonists, when a classically defined partial agonist bound to the KOR, the greatest activation was observed when the KOR signaled through Gαz compared with other Gα subunits (Fig. 5A). To ensure that the 50-minute time point allowed for equilibrium to be reached without confounding any results, a time course was performed for (-)pentazocine when the KOR was signaling through Gαi1 and Gαz (Fig. 5, B and C). Of note, the response obtained was not dependent on the incubation time. Concentration-response curves were then generated for (-)pentazocine signaling through Gαz and Gαi1 (Fig. 5D). The Emax value was greater when the KOR signaled through Gαz than Gαi1 (79% ± 6.4% vs. 35% ± 9.2%, respectively). Again, a leftward shift in the curve was observed when the KOR was signaling through Gαz compared with Gαi1 (Fig. 5D) with corresponding EC50 values of 7.3 ± 2.8 and 110 ± 17 nM, respectively, for (-)pentazocine. To investigate whether the Gγ subunit also affected the signaling, concentration-response curves were generated using Gβ1γ7. EC50 values of 85 ± 16 and 5.9 ± 3.5 nM were obtained when (-)pentazocine signaling through the KOR activated Gαi1 and Gαz, respectively (Fig. 5D). Of note, the EC50 values when (-)pentazocine was signaling through Gαi1•Gβ1γ7 or Gαi1•Gβ1γ2 were not significantly different (85 ± 16 nM vs. 110 ± 17 nM, respectively; P = 0.14). Similarly, EC50 values of (-)pentazocine were not significantly different when the KOR coupled with Gαz•Gβ1γ7 or Gαz•Gβ1γ2 (5.9 ± 3.5 nM vs. 7.3 ± 2.8 nM, respectively; P = 0.56).
Fig. 5.
KOR partial agonists signaling through the KOR and different Gα subunits. (A) Opioids were tested at a 10 µM final concentration with a 50-minute incubation in HEK 293T cells transiently expressing the KOR, Gα subunit of interest, Gβγ-Venus, and masGRK3ct-nLuc. For (-)pentazocine, the BRET response was greatest when the KOR signaled through Gαz (*P ≤ 0.01 compared with all other Gα subunits). For (-)cyclazocine, the BRET response was significantly higher when the KOR was signaling through Gαz compared with Gαi2 (*P < 0.01). No statistical differences (n.s.) were observed between the various Gα subunits for Mr 2033. (B and C) Time-course experiments with (-)pentazocine were performed over a range of concentrations for Gαi1 (B) and Gαz (C). No statistically significant differences were observed. (D) Concentration-response curves were generated for (-)pentazocine at the KOR signaling through Gαi1 and Gαz with both Gγ2 and Gγ7. The EC50 values for (-)pentazocine of 110 ± 17 and 7.3 ± 2.8 nM signaling through Gαi1•Gβ1γ2 and Gαz•Gβ1γ2, respectively, were statistically significant (P = 0.010). The Emax values were 79% ± 6.4% vs. 35% ± 9.2% signaling through Gαz and Gαi1, respectively (P = 0.002). The EC50 and Emax values were not significantly different when the KOR was signaling through Gβ1γ7 compared with Gβ1γ2 for either Gαi1 or Gαz (P > 0.1). (E) Mr 2033 concentration-response curves resulted in an EC50 value of 5.4 ± 2.8 nM when the KOR is signaling through Gαi1γ2. This value was different from the EC50 value of 0.31 ± 0.15 nM when signaling through Gαzγ2 (P = 0.02). Neither the EC50 value (5.2 ± 0.79 nM) nor the Emax value (89% ± 8.5%) was significantly different when the KOR was signaling through Gαi1•Gβ1γ7 compared with Gαi1•Gβ1γ2 (P > 0.6). Likewise, the concentration-response curve was similar when the KOR signaled through Gαz•Gβ1γ7 compared with when the KOR signaled through Gαz•Gβ1γ2, resulting in EC50 values of 0.19 ± 0.11 nM and 0.31 ± 0.15, respectively (P = 0.3). The Emax value for Mr 2033 was not different between Gαz•Gβ1γ2 and Gαz•Gβ1γ7 (94% ± 6.8% vs. 90% ± 8.2%, respectively; P = 0.7). Data are mean percentages of maximal stimulation ± S.D.; measurements were performed in duplicate in three to six independent experiments.
Concentration-response curves were generated for Mr 2033 signaling through the various Gα subunits. EC50 and Emax values were calculated (Fig. 5E; Table 3). Mr 2033 behaved as an efficacious partial agonist with a mean Emax value of 81% ± 3.2% when the KOR was signaling through Gαi1. In contrast, when the KOR was signaling through GαoB or Gαz, Mr 2033 behaved as a full agonist with Emax values of 94% ± 3.6% and 94% ± 6.8%, respectively (Table 3). Again, Mr 2033 was most potent when the KOR was signaling through Gαz compared with any other Gα subunit (Fig. 5E; Table 3). A comparison of Mr 2033 signaling through the KOR showed a leftward shift in the concentration-response curve when the KOR signaled through Gαz compared with Gαi1. The EC50 value for Mr 2033 was approximately 17-fold higher when signaling through Gαi1 than Gαz (5.4 ± 2.8 nM vs. 0.31 ± 0.15 nM, respectively). Moreover, to illustrate that the variation observed was not affected by the Gγ subunit, concentration-response curves were generated using Gβ1γ7. When the KOR coupled with Gαi1•Gβ1γ2 or Gαi1•Gβ1γ7, neither the EC50 values (5.4 ± 2.8 nM vs. 5.2 ± 0.79 nM, respectively) nor the Emax values (81% ± 3.2% vs. 89% ± 8.5%, respectively) were significantly different. Likewise, when the KOR coupled with Gαz•Gβ1γ7, Mr 2033 was similarly potent with an EC50 value of 0.19 ± 0.11 nM compared with 0.31 ± 0.15 nM when the KOR coupled with Gαz•Gβ1γ2 (Fig. 5E). Again, Emax values were similar regardless of the Gβγ subunit (90% ± 8.2% through Gαz•Gβ1γ7 vs. 94% ± 6.8% through Gαz•Gβ1γ2). Overall, Mr 2033 was more potent when the KOR signaled through Gαz than Gαi1 regardless of the Gβγ subunit.
TABLE 3.
Potency and efficacy of the benzomorphan Mr 2033 signaling through the KOR and different Gα subunits after a 50-minute incubation
Concentration-response curves were generated for Mr 2033, signaling through various Gα subunits. Mr 2033 has similar Emax values, except when the KOR signaled through GαoB compared with Gαi1 (P = 0.049). Mr 2033 was significantly more potent when the KOR signaled through Gαz compared with Gαi1, Gαi2, Gαi3, or GαoB (P < 0.020). Data are means ± S.D.; measurements were performed in duplicate in three to six independent experiments.
| Gα | EC50 | Emax |
|---|---|---|
| nM | % | |
| Gαi1 | 5.4 ± 2.8 | 81 ± 3.2 |
| Gαi2 | 13 ± 1.8 | 92 ± 5.7 |
| Gαi3 | 8.0 ± 3.9 | 87 ± 5.0 |
| GαoA | 3.7 ± 2.1 | 93 ± 3.4 |
| GαoB | 6.0 ± 0.49 | 94 ± 3.6 |
| Gαz | 0.31 ± 0.15 | 94 ± 6.8 |
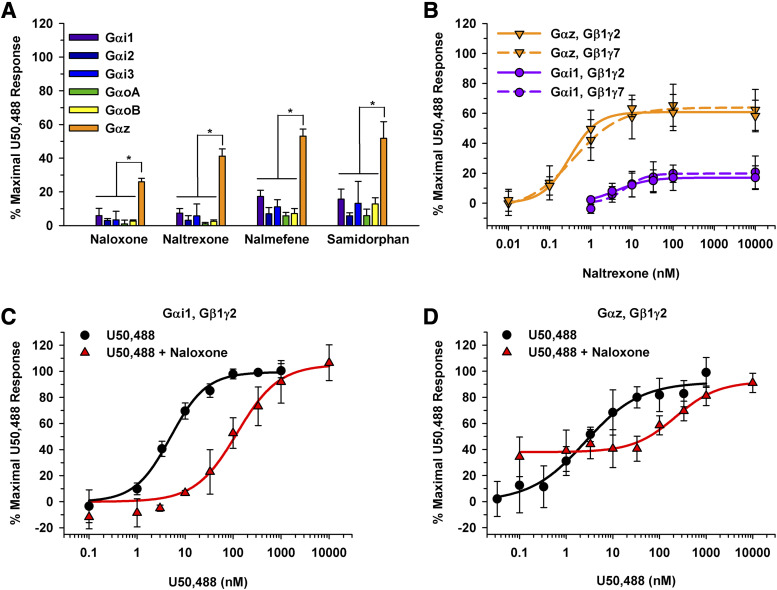
Mu Opioid Receptor Antagonists Signaling Through the KOR.
Since mu opioid receptor (MOR) antagonists, naloxone, naltrexone, nalmefene, and samidorphan, have partial activity at the KOR (Bart et al., 2005; Bidlack et al., 2018), a 10 µM compound screen was performed (Fig. 6A). Interestingly, as seen with partial agonists, maximal KOR signaling was attained when the KOR was coupled to the Gαz subunit, resulting in stimulation greater than 26% for each MOR antagonist. In contrast, naloxone produced less than 10% stimulation when the KOR signaled through other Gα subunits within the inhibitory class besides Gαz. A previous publication using this BRET assay reported that naloxone signaled through Gαi and Gαo (Masuho et al., 2015b). However, this previous report did not normalize the data to a full agonist to account for variability in transient transfections. Figure 6A shows that 10 µM of nalmefene and samidorphan stimulated KOR activation by 17% ± 3.7% and 16% ± 5.6% when the KOR signaled through Gαi1, respectively. Similarly, when the KOR signaled through Gαi3, 10 µM nalmefene stimulated the KOR to 11% ± 4.5%. In contrast, neither opioid activated the KOR by more than 10% when signaling through Gαi2, GαoA, or GαoB. Concentration-response curves were generated for naltrexone with the KOR coupled to Gαz and Gαi1 (Fig. 6B). Again, a leftward and upward shift in the curve was observed when KOR was signaling through Gαz compared with Gαi1 (Fig. 6B). When the KOR coupled to Gαz, naltrexone had an EC50 value of 0.32 ± 0.090 nM and an Emax value of 61% ± 8.8%. Emax and EC50 values could not be calculated for naltrexone signaling through KOR•Gαi1 due to the low stimulation. Gβ1γ7 had no effect on the potency or efficacy of naltrexone compared with Gβ1γ2 (Fig. 6B). The Emax and EC50 values were not statistically different when Gγ7 was present (64% ± 13% and 0.63 ± 0.30 nM, respectively) compared with Gγ2 (61% ± 8.8% and 0.32 ± 0.090 nM, respectively). Similarly, Emax and EC50 values could not be calculated for naltrexone signaling through KOR•Gαi1; however, the results appear consistent between the different Gγ subunits (Fig. 6B).
Fig. 6.
MOR antagonists signaling through the KOR and different Gα subunits. (A) Opioids were tested at a 10 µM final concentration with a 50-minute incubation in HEK 293T cells transiently expressing the KOR, Gα subunit of interest, Gβγ-Venus, and masGRK3ct-nLuc. For each of the four opioids, the BRET response was greatest when the KOR signaled through Gαz (*P ≤ 0.001 for Gαz compared with all other subunits). (B) Concentration-response curves were generated for naltrexone binding to the KOR and signaling through Gαi1 and Gαz with Gβ1γ2 or Gβ1γ7. Although Emax and EC50 values were not calculated for Gαi1 due to low stimulation, Gβ1γ2 or Gβ1γ7 did not significantly influence the Emax (61% ± 8.8% vs. 64% ± 13%, respectively; P = 0.7) or EC50 values (0.32 ± 0.090 nM vs. 0.63 ± 0.30 nM, respectively; P = 0.1) when the KOR was signaling through Gαz. (C and D) To demonstrate the pharmacological differences of naloxone when the KOR was signaling through Gαi1 (C) vs. Gαz (D), shifts in U50,488 concentration-response curves were observed. When the KOR was signaling through Gαi1 (C), the EC50 value for U50,488 shifted from 5.1 ± 1.1 to 120 ± 15 nM in the presence of 330 nM naloxone (P < 0.001). Similarly, 330 nM naloxone shifted the EC50 value from 1.7 ± 0.81 to 170 ± 45 nM when the KOR signaled through Gαz (D) (P < 0.001). The U50,488 concentration-response curve did not return to baseline and remained at approximately 34% due to naloxone acting as a partial agonist at KOR when the receptor signaled through Gαz (A). Data are mean percentages of maximal stimulation ± S.D.; measurements were performed in duplicate in three independent experiments.
To demonstrate how this assay can be used to measure antagonism and partial agonism, we sought to determine how 330 nM naloxone would shift the potency of U50,488 in the presence of the different Gα subunits. When the KOR was signaling through Gαi1, the U50,488 EC50 values had an approximate 23-fold shift from 5.1 ± 1.1 to 120 ± 15 nM in the presence of naloxone (Fig. 6C). This shift was expected, as naloxone had minimal activity and behaved as an antagonist when the KOR is signaling through Gαi1 (Fig. 6A). Similarly, the U50,488 concentration-response curve had a 100-fold rightward shift when the KOR was signaling through Gαz in the presence of naloxone, with an EC50 value of 170 ± 45 nM compared with 1.7 ± 0.81 nM without naloxone present (Fig. 6D). In contrast, naloxone behaved as a partial agonist and not strictly as a pure antagonist when signaling through Gαz. Subsequently, the U50,488 concentration-response curve never returned to baseline, as the 330 nM naloxone activated the KOR to approximately 34% stimulation. Thus, depending on which Gα subunit the KOR was coupled to, naloxone behaved as an antagonist (Gαi1) or partial agonist (Gαz) and shifted the U50,488 curve accordingly.
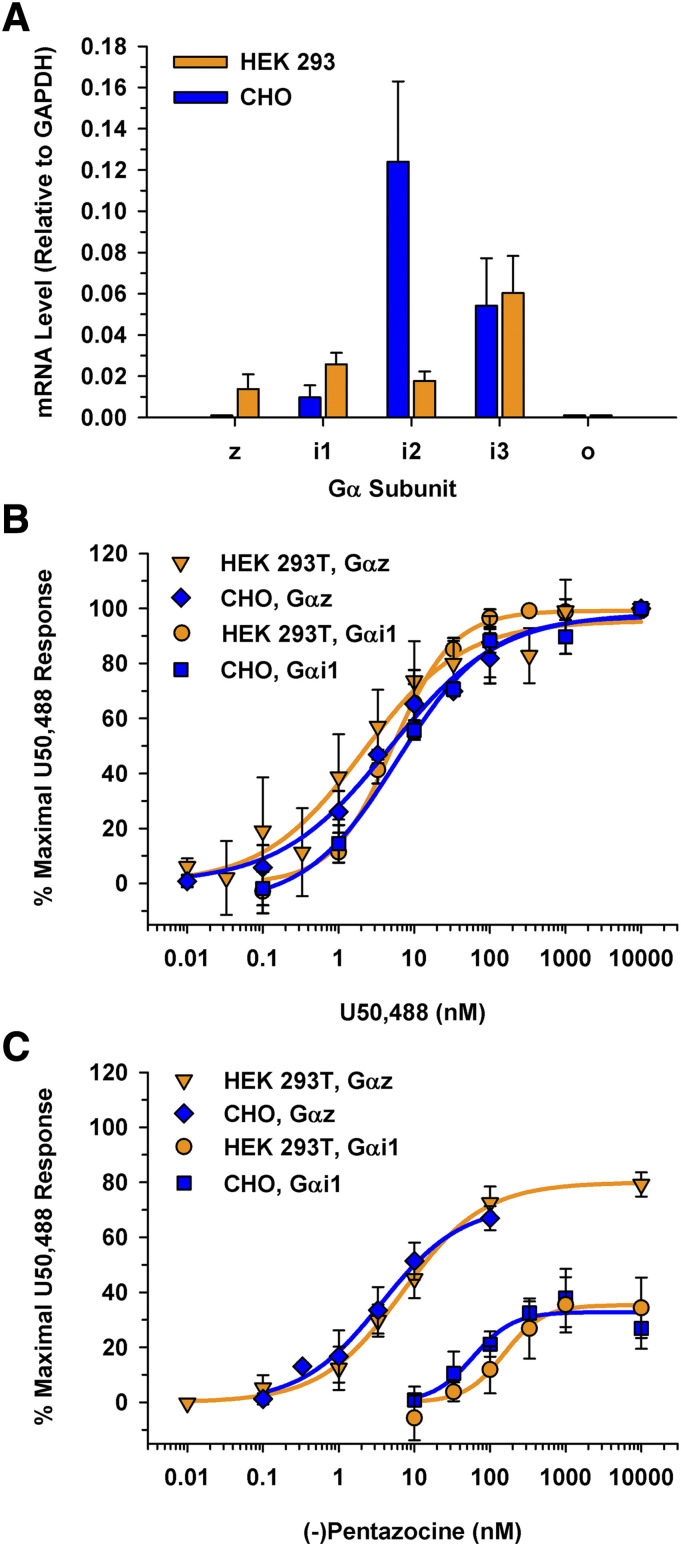
Endogenous Gα mRNA Expression Levels and Cell Line Translatability.
As previously discussed, many pharmacological assays are limited by the endogenous Gα proteins expressed in a given cell line. To determine if the potential expression of endogenous Gα proteins in two commonly used cell lines might influence an assay system, mRNA levels of Gαi/o/z were determined for HEK 293 and CHO cells using species-specific primers. Both cell lines expressed Gαi1, Gαi2, and Gαi3 (Fig. 7A); however, neither cell line expressed Gαo. Interestingly, HEK 293 cells expressed detectable levels of Gαz mRNA, which correlated with the expression of Gαz protein observed in the Western blot (Fig. 3A). In contrast, the CHO cells did not express Gαz mRNA endogenously (Fig. 7A). Clearly, these two cell lines expressed different Gα subunits. Thus, it is feasible that performing experiments in these cell lines, which do not express Gαo, does not accurately recapitulate KOR signaling in the brain where Gαo and Gαz are expressed (Jeong and Ikeda, 1998; Jiang and Bajpayee, 2009).
Fig. 7.
Comparing mRNA levels of endogenous Gα subunits and KOR signaling through Gαz and Gαi1 in both HEK 293T and CHO cells as measured with BRET. (A) Gα subunit mRNA profile in CHO and HEK 293 cells. Total mRNA was isolated from CHO and HEK 293 cell lines. Species-specific primers were used to determine relative mRNA levels to a GAPDH internal control. HEK 293 cells expressed the Gαz subunit transcript; CHO cells did not express detectable levels of Gαz. Gαo mRNA was not detected in either cell line. Data are the mean mRNA levels relative to GAPDH from three independent experiments performed in triplicate ± S.D. (B) U50,488 concentration-response curves were generated in HEK 293T and CHO cells after a 50-minute incubation. EC50 values were 7.3 ± 0.95 nM through Gαi1 and 5.0 ± 1.9 nM through Gαz in CHO cells compared with 5.6 ± 1.3 nM through Gαi1 and 1.5 ± 0.85 nM through Gαz in HEK 293T cells. (C) Concentration-response curves were generated for (-)pentazocine in HEK 293T and CHO cells after a 50-minute incubation. When the KOR was signaling through Gαi1, an Emax value of 31% ± 7.0% in CHO cells and of 35% ± 9.2% in HEK 293T cells (P = 0.57). KOR•Gαi1 signaling resulted in an EC50 value of 63 ± 24 nM in CHO cells and 110 ± 17 nM in HEK 293T cells (P = 0.023). (-)Pentazocine had similar Emax values when the KOR signaled through Gαz in both the CHO and HEK 293T cells (Emax values of 68% ± 6.6% and 79% ± 6.4%, respectively; P = 0.056). (-)Pentazocine had similar EC50 values when the KOR signaled through Gαz in CHO cells (EC50 value of 3.3 ± 1.1 nM) and HEK 293T cells (EC50 value of 7.3 ± 2.8 nM) (P = 0.051 between cell lines).
Furthermore, to demonstrate the translatability of this BRET assay, key experiments were repeated in the CHO cell line. By expressing an individual Gα subunit, this assay offers the advantage of not being dependent on the endogenous Gα proteins present within a cell line. As shown in Figure 7B, U50,488 had a similar potency in CHO cells as in HEK 293T cells when the KOR was signaling through Gαi1 (EC50 values of 7.3 ± 0.95 nM vs. 5.6 ± 1.3 nM, respectively) and through Gαz (EC50 values of 5.0 ± 1.9 nM vs. 1.5 ± 0.85 nM vs., respectively). The partial KOR agonist (-)pentazocine had similar efficacies in CHO and HEK 293T cells when the KOR was signaling through Gαi1 (Emax = 31% ± 7.0% and 35% ± 9.2%, respectively), or Gαz (Emax = 79% ± 6.4% and 68% ± 6.6%, respectively). Although (-)pentazocine was slightly more potent when signaling through Gαi1 expressed in CHO cells (EC50 = 63 ± 24 nM) than in HEK 293T cells (EC50 = 110 ± 17 nM), it was equipotent when signaling through Gαz in these cell lines (EC50 = 7.3 ± 2.8 nM for HEK 293T cells and 3.3 ± 1.1 nM for CHO cells). Overall, the functional activity profiles of U50,488 and (-)pentazocine signaling through Gαi1 or Gαz were largely unaffected by cell type.
Discussion
A BRET sensor technique was adapted to better understand Gα-specific KOR pharmacology. By fusing the BRET donor and acceptor proteins to the Gβγ subunit and a truncated form of its downstream effector, GRK3, respectively, the effects of individual Gα subunits could be observed unrestricted. In contrast to previous work (Masuho et al., 2015b), this BRET technology was used at nonsaturating opioid concentrations. By allowing the opioid and KOR to reach equilibrium, concentration-response curves were generated to calculate Emax and EC50 values. Thus, the first Gα-specific KOR pharmacology was observed. Although no significant differences were detected between Gα subunits when saturating concentrations of full agonists were bound to the KOR, a distinct pattern emerged when partial agonists were bound. For instance, when the KOR was signaling through Gαz, (-)pentazocine and naltrexone had higher Emax values compared with KOR signaling through Gαi1. Additionally, both concentration-response curves had a leftward shift when signaling through Gαz compared with Gαi1. Since Gβ1γ2 is ubiquitously expressed, we sought to determine if a more striatum-specific dimer, Gβ1γ7 (Betty et al., 1998), would also influence KOR pharmacology. Gγ2 and Gγ7 share 66% sequence similarity (Khan et al., 2013). In contrast to the Gα subunit, no differences in efficacy or potency were observed between Gγ2 and Gγ7 for (-)pentazocine, Mr 2033, and naltrexone. To demonstrate the utility of this assay, it was performed in both HEK 293T and CHO cell lines and resulted in similar findings. Most notably, all opioids tested were more potent when the KOR was signaling through Gαz regardless of cell line.
Traditional assays used to study OR signaling, such as [35S]GTPγS binding and cAMP levels after AC inhibition, often do not account for simultaneous signaling through various Gα subunits (Strange, 2010). Although these assays offer some insight into OR pharmacology, it is difficult to recapitulate the complexity of signaling due to cell line limitations, namely, the differential expression of specific Gα subunits and regulator of G protein signaling (RGS) proteins in a given cell line (Strange, 2010). For example, we determined that the relative expression of Gα subunits was different in two commonly used cell lines, HEK 293 and CHO. Notably, neither cell line expressed Gαo, the most abundant Gα subunit present in the brain (Gierschik et al., 1986). Additionally, only HEK 293 cells expressed Gαz. The mRNA distribution of Gα proteins within HEK 293 cells agree with previous findings (Atwood et al., 2011). Though it has been established that Gαz is widely expressed in the brain, particularly in regions that also express ORs, its signaling properties have been less studied than the other Gαi/o-class subunits (Fields, 1998; Glick et al., 1998). Thus, a more sensitive technique was necessary to parse out the unique signaling effects of each Gα subunit.
This novel approach to study Gα subunit–specific pharmacology may help corroborate in vitro assays with in vivo observations, thus allowing differences in agonist activation profiles to be studied. For example, intracerebroventricular administration of Gαz siRNA in mice resulted in reduced supraspinal antinociception after MOR-specific opioid administration. In contrast to other Gαi/o knockdown mice, the Gαz knockdown mice showed an impaired response to all tested opioid agonists in the 52°C warm-water tail withdrawal test (Sánchez-Blázquez et al., 1999). Furthermore, these researchers observed that the MOR agonists morphine and DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin) had a greater potency at the MOR in mouse periaqueductal gray slices when signaling through Gαz than Gαi2 in a [35S]GTPγS assay (Garzón et al., 1997). These studies suggest a prominent role for Gαz in vivo OR signaling, particularly in the context of analgesia. Although in vitro studies typically rely on Gαi/o-mediated OR signaling to predict in vivo observations, our findings further indicate the importance of understanding OR coupling to various Gα subunits and how the Gα subunits influence opioid pharmacology.
Taken together, these data indicate the importance for further study of Gαz signaling in regard to the OR. As mentioned earlier, Gαz is a member of the Gαi/o class; however, it shares the least sequence identity with the other members (Casey et al., 1990). Although Gαi/o-class subunits have a broader expression profile, Gαz has a more limited expression and is restricted primarily to the brain (Casey et al., 1990). Thus, cells that express Gαz may have a highly specialized function. Additionally, Gαz is pertussis toxin–insensitive, as it lacks the cysteine residue at the C terminus responsible for ADP-ribosylation (Ho and Wong, 1998). Although the BRET overexpression system used in this current study allowed the effects of individual Gα subunits to be observed, further study will be important to understand these signaling effects in a more physiologic context. By capitalizing on the differences in pertussis toxin sensitivity of Gαi/o and Gαz, endogenous Gαz signaling might be viewed in a physiologic environment, such as isolated primary neurons. Moreover, Gαz has a much slower intrinsic hydrolysis rate than other Gαi/o class members (Fields, 1998), which may account for some of the observed differences. Once Gαz signaling is initiated, the signaling may persist much longer than through other Gα subunits (Garzón et al., 2005). Since no exogenous RGS proteins (Hollinger and Hepler, 2002) were expressed in the current system, Gαz’s slower hydrolysis rate may be contributing to the increased signal of partial agonists. Using the same BRET technique described and additionally expressing exogenous RGS proteins, future experiments can better parse out the kinetics of Gα subunit–specific pharmacology in a more physiologic environment.
In summary, this paper demonstrated the unique pharmacological profile obtained when the KOR signals through Gαz compared with other inhibitory Gα proteins. Although we acknowledge the limitations of this overexpression system, it offers insight into the intricacies of KOR•Gα signaling.
Acknowledgments
We thank Dr. Kirill A. Martemyanov for the generous gift of the BRET sensors, Dr. David I. Yule for the use of his Felxstation 3 plate reader, Dr. Angela Glading and Harsha Swamy for their assistance with the Western blots, and Dr. Cesare Orlandi for useful discussions.
Abbreviations
- AC
adenylyl cyclase
- BRET
bioluminescence resonance energy transfer
- Emax
maximal stimulation
- fwd
forward
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GPCR
G protein–coupled receptor
- GRK3
G protein–coupled receptor kinase 3
- GRK3ct
G protein–coupled receptor kinase 3 C terminus
- HEK
human embryonic kidney
- KOR
kappa opioid receptor
- mas
myristic acid attachment peptide (MGSSKSKSTSNS)
- MOR
mu opioid receptor
- Mr 2033
((±)-α-5,9-dimethyl-2-(l-tetra-hydrofurfuryl)-2′-hydroxy-6,7-benzomorphan) hydrochloride
- nLuc
nanoluciferase
- nor-BNI
norbinaltorphimine
- OR
opioid receptor
- rev
reverse
- RGS
regulator of G protein signaling
- U50,488
2-(3,4-dichlorophenyl)-N-methyl-N-[(1R,2R)-2-pyrrolidin-1-ylcyclohexyl]acetamide
Authorship Contributions
Participated in research design: Barnett, Knapp, Bidlack.
Conducted experiments: Barnett, Knapp.
Performed data analysis: Barnett, Knapp.
Wrote or contributed to the writing of the manuscript: Barnett, Knapp, Bidlack.
Footnotes
This work was supported by National Institutes of Health National Institute of General Medical Sciences [Grant GM068411] (M.E.B.) and the National Institute on Drug Abuse [Grant DA046817] (J.M.B.). The J.R. Murlin Memorial Fund (M.E.B.) and the Margo Cleveland Fund (J.M.B.) also supported this research.
References
- Archer S, Albertson NF, Harris LS, Pierson AK, Bird JG. (1964) Pentazocine. Strong analgesics and analgesic antagonists in the benzomorphan series. J Med Chem 7:123–127. [DOI] [PubMed] [Google Scholar]
- Archer S, Glick SD, Bidlack JM. (1996) Cyclazocine revisited. Neurochem Res 21:1369–1373. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. (2011) Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, Kreek MJ. (2005) Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology 30:2254–2262. [DOI] [PubMed] [Google Scholar]
- Betty M, Harnish SW, Rhodes KJ, Cockett MI. (1998) Distribution of heterotrimeric G-protein beta and gamma subunits in the rat brain. Neuroscience 85:475–486. [DOI] [PubMed] [Google Scholar]
- Bidlack JM, Knapp BI, Deaver DR, Plotnikava M, Arnelle D, Wonsey AM, Fern Toh M, Pin SS, Namchuk MN. (2018) In vitro pharmacological characterization of buprenorphine, samidorphan, and combinations being developed as an adjunctive treatment of major depressive disorder. J Pharmacol Exp Ther 367:267–281. [DOI] [PubMed] [Google Scholar]
- Bidlack JM, Parkhill AL. (2004) Assay of G protein-coupled receptor activation of G proteins in native cell membranes using [35S]GTP gamma S binding. Methods Mol Biol 237:135–143. [DOI] [PubMed] [Google Scholar]
- Casey PJ, Fong HK, Simon MI, Gilman AG. (1990) Gz, a guanine nucleotide-binding protein with unique biochemical properties. J Biol Chem 265:2383–2390. [PubMed] [Google Scholar]
- DeCaprio J, Kohl TO. (2019) Using Dounce homogenization to lyse cells for immunoprecipitation. Cold Spring Harb Protoc 2019 (7) Available from: 10.1101/pdb.prot098574. [DOI] [PubMed] [Google Scholar]
- Donthamsetti P, Quejada JR, Javitch JA, Gurevich VV, Lambert NA. (2015) Using Bioluminescence resonance energy transfer (BRET) to characterize agonist-induced arrestin recruitment to modified and unmodified G protein-coupled receptors. Curr Protoc Pharmacol 70:2.14.1-2.14.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dounce AL, Witter RF, Monty KJ, Pate S, Cottone MA. (1955) A method for isolating intact mitochondria and nuclei from the same homogenate, and the influence of mitochondrial destruction on the properties of cell nuclei. J Biophys Biochem Cytol 1:139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields TA. (1998) Identification of a GTPase activating protein specific for the heterotrimeric G protein, Gz. Cell Signal 10:43–48. [DOI] [PubMed] [Google Scholar]
- Garzón J, García-España A, Sánchez-Blázquez P. (1997) Opioids binding mu and delta receptors exhibit diverse efficacy in the activation of Gi2 and G(x/z) transducer proteins in mouse periaqueductal gray matter. J Pharmacol Exp Ther 281:549–557. [PubMed] [Google Scholar]
- Garzón J, Rodríguez-Muñoz M, López-Fando A, Sánchez-Blázquez P. (2005) The RGSZ2 protein exists in a complex with mu-opioid receptors and regulates the desensitizing capacity of Gz proteins. Neuropsychopharmacology 30:1632–1648. [DOI] [PubMed] [Google Scholar]
- Gierschik P, Milligan G, Pines M, Goldsmith P, Codina J, Klee W, Spiegel A. (1986) Use of specific antibodies to quantitate the guanine nucleotide-binding protein Go in brain. Proc Natl Acad Sci USA 83:2258–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick JL, Meigs TE, Miron A, Casey PJ. (1998) RGSZ1, a Gz-selective regulator of G protein signaling whose action is sensitive to the phosphorylation state of Gzalpha. J Biol Chem 273:26008–26013. [DOI] [PubMed] [Google Scholar]
- Hilger D, Masureel M, Kobilka BK. (2018) Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol 25:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MK, Wong YH. (1998) Structure and function of the pertussis-toxin-insensitive Gz protein. Biol Signals Recept 7:80–89. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. (2002) Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev 54:527–559. [DOI] [PubMed] [Google Scholar]
- Hollins B, Kuravi S, Digby GJ, Lambert NA. (2009) The C-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell Signal 21:1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SW, Ikeda SR. (1998) G protein alpha subunit G alpha z couples neurotransmitter receptors to ion channels in sympathetic neurons. Neuron 21:1201–1212. [DOI] [PubMed] [Google Scholar]
- Jiang M, Bajpayee NS. (2009) Molecular mechanisms of go signaling. Neurosignals 17:23–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson JO, Ostwald K, Kåbjörn C, Andersson M. (1994) A method for protein assay in Laemmli buffer. Anal Biochem 219:144–146. [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336:296–302. [DOI] [PubMed] [Google Scholar]
- Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbé JC, Miller GJ, Hébert TE. (2013) The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev 65:545–577. [DOI] [PubMed] [Google Scholar]
- Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. (2003) Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science 300:1256–1262. [DOI] [PubMed] [Google Scholar]
- Masuho I, Martemyanov KA, Lambert NA. (2015a) Monitoring G protein activation in cells with BRET. Methods Mol Biol 1335:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuho I, Ostrovskaya O, Kramer GM, Jones CD, Xie K, Martemyanov KA. (2015b) Distinct profiles of functional discrimination among G proteins determine the actions of G protein-coupled receptors. Sci Signal 8:ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Kostenis E. (2006) Heterotrimeric G-proteins: a short history. Br J Pharmacol 147 (Suppl 1):S46–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese PS, Lipkowski AW, Takemori AE. (1987) Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci 40:1287–1292. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Gómez-Serranillos P, Garzón J. (2001) Agonists determine the pattern of G-protein activation in mu-opioid receptor-mediated supraspinal analgesia. Brain Res Bull 54:229–235. [DOI] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Rodríguez-Díaz M, DeAntonio I, Garzón J. (1999) Endomorphin-1 and endomorphin-2 show differences in their activation of mu opioid receptor-regulated G proteins in supraspinal antinociception in mice. J Pharmacol Exp Ther 291:12–18. [PubMed] [Google Scholar]
- Stoddart LA, Johnstone EKM, Wheal AJ, Goulding J, Robers MB, Machleidt T, Wood KV, Hill SJ, Pfleger KDG. (2015) Application of BRET to monitor ligand binding to GPCRs. Nat Methods 12:661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange PG. (2010) Use of the GTPγS ([35S]GTPγS and Eu-GTPγS) binding assay for analysis of ligand potency and efficacy at G protein-coupled receptors. Br J Pharmacol 161:1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrovatkina V, Alegre KO, Dey R, Huang XY. (2016) Regulation, signaling, and physiological functions of G-proteins. J Mol Biol 428:3850–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. (1995) Modulation by mu-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol 47:848–854. [DOI] [PubMed] [Google Scholar]
- Von Voigtlander PF, Lewis RA. (1982) U-50,488, a selective kappa opioid agonist: comparison to other reputed kappa agonists. Prog Neuropsychopharmacol Biol Psychiatry 6:467–470. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR. (1995) Lipid modifications of trimeric G proteins. J Biol Chem 270:503–506. [DOI] [PubMed] [Google Scholar]
- Wentland MP, Lou R, Lu Q, Bu Y, VanAlstine MA, Cohen DJ, Bidlack JM. (2009) Syntheses and opioid receptor binding properties of carboxamido-substituted opioids. Bioorg Med Chem Lett 19:203–208. [DOI] [PubMed] [Google Scholar]
- Wentland MP, Lu Q, Lou R, Bu Y, Knapp BI, Bidlack JM. (2005) Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett 15:2107–2110. [DOI] [PubMed] [Google Scholar]
- Yung LY, Tsim ST, Wong YH. (1995) Stimulation of cAMP accumulation by the cloned Xenopus melatonin receptor through Gi and Gz proteins. FEBS Lett 372:99–102. [DOI] [PubMed] [Google Scholar]