Abstract
Diabetes reduces the number and induces dysfunction in circulating endothelial progenitor cells (EPCs) by mechanisms that are still uncovered. This study aims to evaluate the number, viability, phenotype, and function of EPCs in dyslipidemic mice with early diabetes mellitus and EPC infiltration in the aortic valve in order to identify possible therapeutic targets in diabetes-associated cardiovascular disease. A streptozotocin-induced diabetic apolipoprotein E knock-out (ApoE−/−) mouse model was used to identify the early and progressive changes, at 4 or 7 days on atherogenic diet after the last streptozotocin or citrate buffer injection. Blood and aortic valves from diabetic or nondiabetic ApoE−/− animals were collected.
EPCs were identified as CD34 and vascular endothelial growth factor receptor 2 positive monocytes, and the expression levels of α4β1, αVβ3, αVβ5, β1, αLβ2, α5 integrins, and C-X-C chemokine receptor type 4 chemokine receptor on EPC surface were assessed by flow cytometry. The number of CD34 positive cells in the aortic valve, previously found to be recruited progenitor cells, was measured by fluorescence microscopy. Our results show that aortic valves from mice fed 7 days with atherogenic diet presented a significantly higher number of CD34 positive cells compared with mice fed only 4 days with the same diet, and diabetes reversed this finding. We also show a reduction of circulatory EPC numbers in diabetic mice caused by cell senescence and lower mobilization. Dyslipidemia induced EPC death through apoptosis regardless of the presence of diabetes, as shown by the higher percent of propidium iodide positive cells and higher cleaved caspase-3 levels. EPCs from diabetic mice expressed α4β1 and αVβ3 integrins at a lower level, while the rest of the integrins tested were unaffected by diabetes or diet. In conclusion, reduced EPC number and expression of α4β1 and αVβ3 integrins on EPCs at 4 and 7 days after diabetes induction in atherosclerosis-prone mice have resulted in lower recruitment of EPCs in the aortic valve.
Keywords: EPC, endothelial progenitor cells, integrins, recruitment, aortic valve
Introduction
Endothelial progenitor cells (EPCs) are a small fraction of circulatory monocytes involved in vascular repair and angiogenesis. According to the protocol by which they are separated, their number in healthy subjects was shown to vary between 0.1% and 0.01% of all monocytes1. When a cardiovascular event occurs, EPC peripheral numbers are transiently increased by granulocyte-macrophage colony-stimulating factor, stromal cell-derived factor 1 (SDF-1), vascular endothelial growth factor (VEGF), and erythropoietin-mediated bone marrow mobilization2. On the contrary, EPC counts are reduced in diabetes mellitus patients with cardiovascular events3, and this reduction is thought to be caused by reduced mobilization due to lower nitric oxide availability4, reduced proliferation and differentiation of bone marrow cells into EPCs5, and also lower SDF-1, VEGF, and erythropoietin levels which not only reduce EPC availability but also their homing2. SDF-1 is a chemokine whose actions are exerted through the C-X-C chemokine receptor type 4 (CXCR4) receptor expressed on all hematopoietic stem cells and involved in their homing and mobilization6.
Various integrins are of importance in EPC mobilization from bone marrow, adhesion to activated endothelial cells (ECs), platelets, and extracellular matrix. It is already well known that integrins are cell adhesion receptors that mainly bind to extracellular matrix ligands and cell-surface ligands. In humans, the integrin family is made of 24 transmembrane αβ heterodimers, formed from 18α and 8β subunits7, binding targets such as the fibronectin RDG motif, vitronectin, fibrinogen, the epitope GFOGER of collagen, laminin, vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule (ICAM)-1,-2,-3,-5, and others8. Integrins play roles not only in cell to cell and cell to extracellular matrix adhesion but also in cell shape change, migration, differentiation, proliferation, and apoptosis through inside-out and outside-in signaling9.
Integrins are involved in EPC homing and recruitment of circulatory cells as well as in interaction with the extracellular matrix. The dimeric α4β1 integrin (also called very late antigen-VLA-4) is a known ligand of VCAM-1 and fibronectin10 expressed on monocytes, lymphocytes, natural killer cells, eosinophils, neutrophils, and also on progenitor cells. This integrin dimer was involved in EPC recruitment by the activated endothelium as well as in EPC retention in the bone marrow11. In diabetes, protein kinase A (PKA)-mediated phosphorylation of α4β1 reduces EPC mobilization from the bone marrow12.
There is data supporting that the αVβ3 and αVβ5 integrins, promiscuous ligands for vitronectin, fibronectin, fibrinogen, osteopontin, and von Willebrand factor, are involved in EPC adherence to denuded vessels as the inhibition of αVβ3 and αVβ5 integrins blocks the re-endothelialization of denuded arteries13,14. Another integrin shown to be involved in the adhesion is the β2 integrin binding the receptor for advanced glycation end products at the surface of ECs15, while the αLβ2 integrin dimer binds ICAM-1 on activated ECs16.
Integrin levels are altered in pathology both with roles in healing mechanisms and as collateral targets. In models of shear stress, similar to that in atherosclerosis or valve stenosis, upregulation of the β1 and β3 integrins, facilitating EPC adhesion to the lesion site17, and EPC differentiation associated with lower CD34 and CD133 stem cell marker mRNA levels were observed18. In cultured human glomerular epithelial cells, the presence of high (25 mM) glucose upregulated α5 and αVβ3 integrins and downregulated α1, α2, and α3 integrins, leading to an overall lower collagen IV binding19.
However, due to the scarce circulatory presence of EPCs, their integrin expression profile was, to our knowledge only, evaluated after culturing, proliferation, and differentiation. In this study, we aimed to evaluate circulatory EPC number, viability, and integrin expression profile in the early stages of diabetes using a streptozotocin (STZ)-induced diabetic model on mice prone to atherosclerotic lesions. Moreover, since there are reports which suggest that in patients with aortic stenosis, valvular EC regeneration is impaired not only by increased senescence of valvular ECs but also by a reduced number and function of circulating EPCs20, we also aimed to evaluate the aortic valve homing and recruitment ability of EPCs in the context of progressive diabetic valvulopathy.
Materials and Methods
Animals
Apolipoprotein E knock-out (ApoE−/−) mice from the breeding colony of Taconi were bred in our facility at the Institute of Cellular Biology and Pathology (ICBP) “Nicolae Simionescu”, kept under a 12 h light:12 h dark cycle, with food and water ad libitum. All experimental protocols were approved by the Ethics Committee of ICBP “Nicolae Simionescu” and by the national authority in charge, ANSVSA.
Male, 12-weeks-old, ApoE−/− mice were injected intraperitoneally (i.p.) for five consecutive days with 55 mg/kg of body weight of STZ (Sigma-Aldrich, St. Louis, MO, USA) in citrate buffer (final citrate concentration 20.7 mM), pH 4.5, or with an equivalent volume of citrate buffer (CIT, pH 4.5) as recently described by Tucureanu et al21. After the last i.p. injection, the diet was switched from standard chow to atherogenic diet (standard chow supplemented with 1% cholesterol and 15% butter) for 4 days (STZ4 and CIT4 groups, 8 and 7 animals, respectively) or 7 days (STZ7 and CIT7 groups, 8 animals each) when the animals were sacrificed. Consequently, four experimental groups of mice were established: STZ4, STZ7, and appropriate controls such as CIT4 and CIT7. After profound surgical anesthesia was induced with a ketamine and xylazine mixture (100 mg/10 mg/kg body weight) via i.p. injection, blood was collected on 5 mM EDTA through a ventricular puncture, mice were perfused with phosphate-buffered saline (PBS; pH 7.2), and the aortic valves were collected from each animal by dissection.
The biochemical and echocardiographic parameters, as well as the aortic valve histology of these animals, were presented at length in our recent article21. This model of STZ-induced diabetes on a background of diet-induced atherosclerosis recapitulates the major aspects of valve pathology within 7 days of atherogenic diet after the last STZ injection.
Flow Cytometry Analysis of Circulating Endothelial Progenitor Cells
The mononuclear cell fractions were obtained from the whole blood of each mouse by density gradient centrifugation using Histopaque-1077 (density 1.077 g/ml; Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. The remaining erythrocytes were lysed by a 5-min incubation with ammonium-chloride-potassium (ACK) lysing buffer (Life Technologies, Waltham, MA, USA), and unspecific binding was blocked by washing with 2% fetal bovine serum (FBS) in PBS. Cells were numbered and aliquoted at 2 × 105/sample. All samples, except the autofluorescence and isotype control samples, were incubated with both anti-CD34-AlexaFluor488 (FAB65181G; R&D Systems, Minneapolis, MN, USA) and anti-VEGFR2 allophycocyanin (APC) (FAB4432A; R&D Systems, MN, USA) antibodies for the identification of EPCs as previously described by Georgescu et al22–24. In addition, each sample was also incubated with anti-CD133-phycoerythrin (PE) (BZ-141204; Biolegend, San Diego, CA, USA), anti-CXCR4-PE (FAB21651P-100; R&D Systems, MN, USA), anti-αLβ2 integrin (unconjugated ab13219; Abcam, Cambridge, UK), anti-α5 integrin-PE (103905; Biolegend, San Diego, CA, USA), anti-β1-PE (FAB2405P; R&D Systems, MN, USA), anti-α4β1 (unconjugated BZ-103705; Biolegend, San Diego, CA, USA), anti-αVβ3-PE (sc7312; Santa Cruz, Dallas, TX, USA), anti-caspase3 (unconjugated 9664; Cell Signaling Technology, Leiden, The Netherlands), anti-TRF2 (unconjugated MA141001; Thermo Fisher Scientific, Waltham, MA, USA), anti-αVβ5 (unconjugated sc81632; Santa Cruz, Dallas, TX, USA) antibodies, or propidium iodide. Where needed conjugated secondary antibodies such as goat anti-mouse-PE (ab7002; Abcam), goat anti-rat-PE (A10545; Invitrogen Waltham, MA, USA), and goat anti-rabbit-PE (F0110; R&D Systems, MN, USA) were also added after a wash. Samples were measured using a Gallios Beckman Coulter flow cytometer (ex: 488 nm, em: 525 nm BP for Alexa Fluor 488; em: 575 nm BP for PE; and ex: 635 nm, em: 660 nm BP for APC), and data were analyzed using Flowing Software 2 (Turku University, Finland). For the gating strategy see Supplemental Fig. 1.
Immunohistological Examination of Aortic Valve Leaflets
The heart specimens were cryoprotected in solutions containing increasing concentrations of glycerol (5%, 10%, 20%, and 50%), washed in 3% sucrose, snap-frozen in liquid nitrogen, and mounted in OCT compound (NEG-50, Thermo Scientific, Waltham, MA, USA). Serial cryostat sections, 5-µm thick (Leica CM1850, IL, USA), containing the three aortic valvular leaflets were collected on poly-l-lysine-treated slides. The sections were fixed in cold acetone for 20 min at −20 °C, washed, and incubated in 0.1% Sudan Black B in ethanol for 1 min for autofluorescence reduction. After blocking in 3% bovine serum albumin (BSA) for 30 min, the sections were incubated overnight at 4 °C with anti-CD34-AlexaFluor488 (FAB65181G; R&D Systems, MN, USA). The next day, the sections were incubated for 5 min with 0.4 µg/ml 4′,6-diamidino-2-phenylindole (DAPI); coverslips were mounted using ProLong Gold Antifade Mountant (Thermo Fisher Scientific, Waltham, MA, USA) and after curing the mountant for 24 h at 4 °C, the coverslips were sealed with acrylic nail polish. Bright-field images, as well as DAPI and CD34-AlexaFluor488 fluorescence images, were acquired with a fluorescence microscope (Olympus IX81, Shinjuku, Tokyo, Japan), using the same exposure for all sections. All cells, as indicated by the DAPI-stained nuclei and the CD34 positive cells were counted automatically using an ImageJ 1.48v Macro requiring the user to select the leaflet area from bright-field images. The CD34 positive cells (EPCs) were defined as regions of more than 500 contiguous pixels each having a fluorescence intensity of more than the mean intensity + 2 standard deviations of the pixels’ grayscale intensity in that image.
Results
ApoE−/− Mice with Diabetes and High-Fat Diet, a Model for Diabetic Dyslipidemia
In this experimental model, mean glycemia values for CIT control groups were 144.3 ± 5.2 mg/dl for CIT4 and 144.3 ± 5.2 mg/dl for CIT7, while for STZ groups, these were 219.1 ± 18.2 mg/dl for STZ4 and 271.8 ± 25.8 mg/dl for STZ7 as previously published21. Also, the duration of the atherogenic diet of 4 or 7 days significantly interacted with diabetes to increase plasma cholesterol and triglyceride concentration and induce early molecular and functional changes in aortic heart valves21.
Number and Viability of Circulating Endothelial Progenitor Cells Decline in ApoE−/− Mice with Diabetes and High-Fat Diet
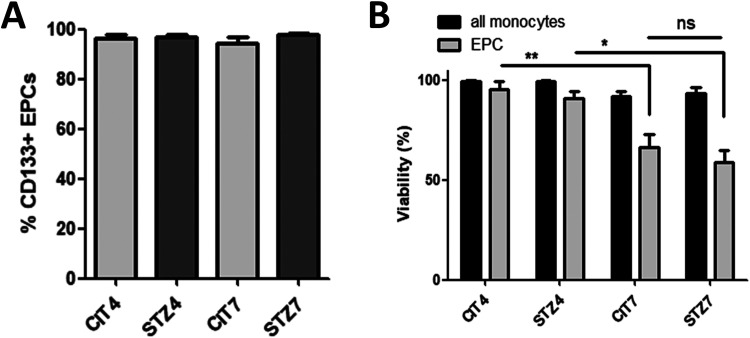
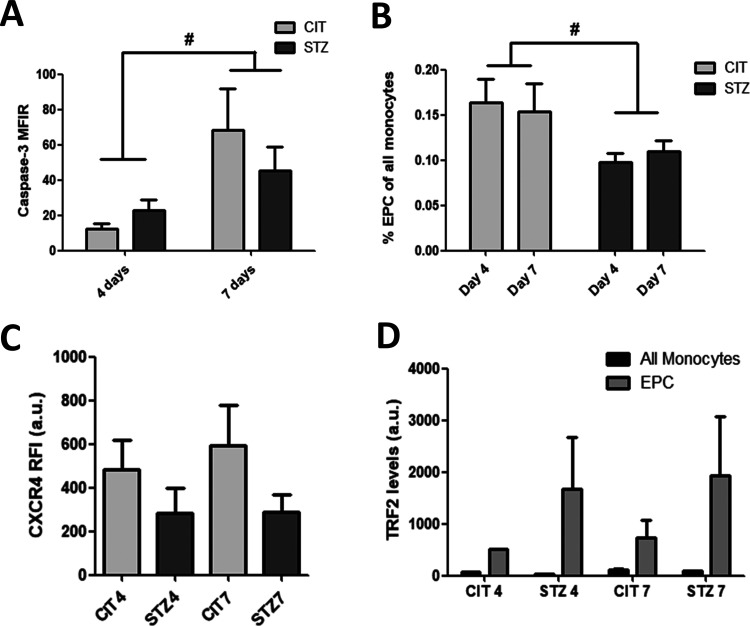
EPCs phenotype was tested by flow cytometry, where EPCs were identified as CD34/VEGFR double-positive monocyte-derived cells. Another marker for circulatory EPCs (early EPCs) described in the literature is the stem-cell marker CD133 and, as expected, the majority (more than 94% for all groups) of EPCs were also CD133 positive cells (see Fig. 1A). Further, the cell count and viability of EPCs from the atherogenic diet-fed control (CIT4 and CIT7 groups) and diabetic animals (STZ4 and STZ7 groups) were studied. EPCs’ viability, as measured by propidium iodide staining, dropped from 95.4% at 4 days to 66.5% at 7 days in the CIT groups (one-way analysis of variance [ANOVA], Bonferroni posttest, **P < 0.01) and from 87.4% to 64.3% in the STZ groups (one-way ANOVA, Bonferroni posttest, *P < 0.05; Fig. 1B), suggesting that the diet length is the main source of variance. This reduction in viability was potentially caused by apoptosis as shown by the higher cleaved Caspase 3 levels in EPCs from the 7-day groups, regardless of diabetes (two-way ANOVA, # P < 0.05; Fig. 2A). However, the cell death was not mirrored by the EPCs’ count, as diabetes alone, with no source of variation from diet’s length, reduced the number of circulatory EPCs to about two-thirds of the ones observed in citrate controls, even at 4 days after the last STZ injection (0.16 and 0.15% EPC cells in CIT groups vs 0.10 and 0.11% in STZ groups at 4 and 7 days, respectively) (two-way ANOVA, # P < 0.05, Fig. 2B). This is probably caused by a drop in EPC’s mobilization from bone marrow as suggested by a possible reduction in CXCR4 expression in cells from diabetic animals (our results showed a reduction by 42.1% at 4 days and by 50.8% at 7 days; however, these results were not statistically significant) (two-way ANOVA, P = 0.08; Fig. 2C). We also examined TRF2 levels, as indicative of cell senescence, and not only EPCs in all groups showed a higher level of this marker compared with other monocytes, but EPCs from diabetic animals had double TRF2 levels compared with the nondiabetic controls; however, no statistically significant modifications were seen between the treatment groups (see Fig. 2D).
Figure 1.
EPC phenotype and viability. (A) The vast majority of EPCs identified as CD34+/VEGFR+ cells were also CD133+ corresponding to the early EPC phenotype normally found in circulation. (B). Seven-day long atherogenic diet and not diabetes-induced EPCs cell death as measured by propidium iodide staining. EPC: endothelial progenitor cell; VEGFR: vascular endothelial growth factor receptor.
Figure 2.
EPC numbers and alterations. EPCs from atherogenic diet-fed animals suffered apoptosis, as shown by the elevated cleaved caspase-3 levels in STZ7 and CIT7 groups (A). However, circulatory EPC counts were reduced in diabetic animal groups (STZ4 and STZ7) compared with control groups (CIT4 and CIT7) (B) as diabetic EPCs show lower but not significantly modified levels of the chemotaxis-mediating CXCR4 (C) and the TRF2 levels seemed to be higher in EPCs from diabetic animals, but no statistical significance was noted among groups (D) (*P < 0.05, **P < 0.01 in one-way ANOVA and # P < 0.05 in two-way ANOVA). ANOVA: analysis of variance; CIT: citrate buffer; CXCR4,: C-X-C chemokine receptor type 4; EPC: endothelial progenitor cell; STZ: streptozotocin.
Expression of α4β1 and αVβ3 Integrins on Circulating Endothelial Progenitor Cells Diminishes in ApoE−/− Mice with Diabetes and High-Fat Diet
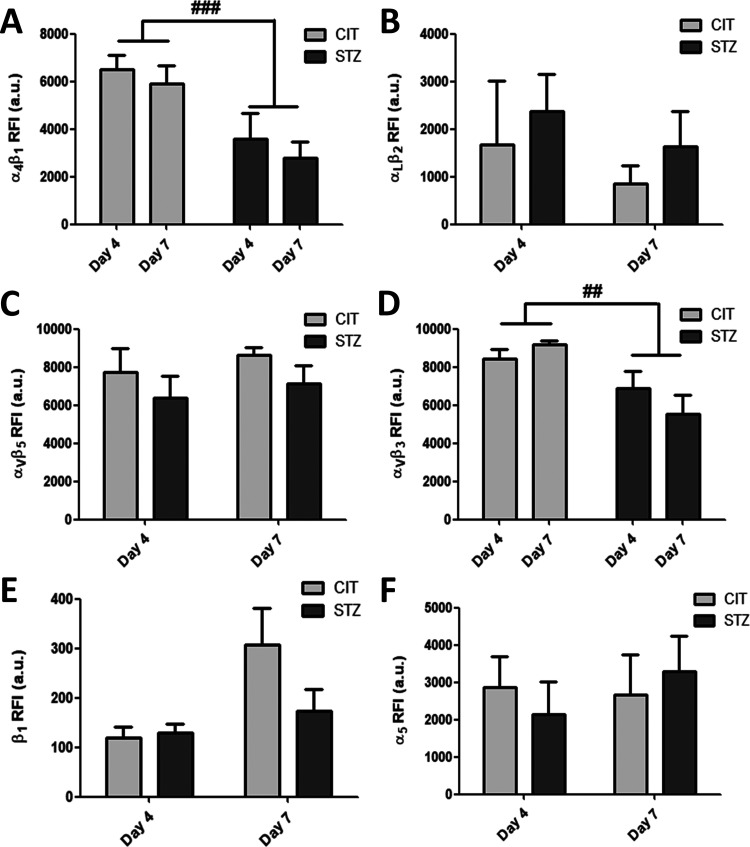
In our flow cytometry experiments, the α4β1 integrin expression level was significantly decreased in EPCs from diabetic animals (STZ4 and STZ7 groups compared with CIT4 and CIT7 groups) (two-way ANOVA, ### P < 0.001, Fig. 3A). In EPCs of diabetic origin, we also found a decrease in αVβ3 integrin expression level (two-way ANOVA, ## P < 0.01, Fig. 3D). Also, by applying flow cytometry analysis, we found no significant changes in the other integrins tested, α5 and β1 monomers and αVβ5 and αLβ2 integrins (Fig. 3B, C, E, F).
Figure 3.
Relative expression levels of integrins. Diabetes reduced the levels of α4β1 (A) and αVβ3 (D) integrins and had no significant effect on the relative expression level of αLβ2 (B) and αVβ5 (C) dimers and β1 (E) and α5 (F) monomers (two-way analysis of variance, ### P < 0.001, ## P < 0.01).
Recruitment of CD34 Positive Cells in Aortic Valve Leaflets Decreases in ApoE−/− Mice with Diabetes and High-Fat Diet
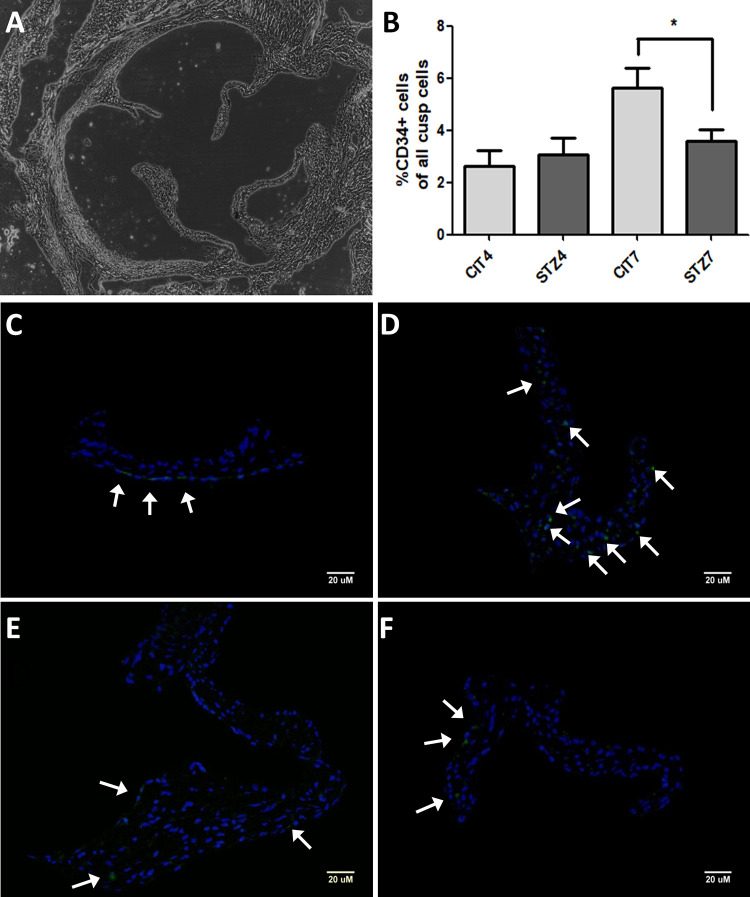
To evaluate whether if the altered integrin expression observed was associated with altered EPC recruitment in the aortic valve (Fig. 4A, C-F), we assayed the CD34 positive cell counts between investigated experimental groups (STZ4, CIT4 and STZ7, CIT7). We observed that the 7-day atherogenic diet led to a higher CD34 positive cell number in the control group (CIT7), while diabetes (STZ7 group) significantly reduced that number to values observed at earlier time points (CIT4 and STZ4 groups; one-way ANOVA, Bonferroni post-test, *P < 0.05; see Fig. 4B).
Figure 4.
EPC recruitment in the aortic valve. Bright-field image with whole aortic valve section (A). Fluorescence microscopy images from sections incubated with anti-CD34 antibodies (green) and 4′,6-diamidino-2-phenylindole (blue) showing representative microscopic fields for the CIT4 (C), CIT7 (D), STZ4 (E), and STZ7 (F) groups. Arrows show CD34+ cells. Bar graph showing percent CD34-possitive cells for the groups analyzed (B) (one-way analysis of variance, *P < 0.05). CIT: citrate buffer; EPC: endothelial progenitor cell; STZ: streptozotocin.
Discussions and Conclusions
Circulating progenitor cells are more affected in diabetes associated with atherosclerosis, but the mechanisms involved still need to be uncovered, especially in early diabetes25. The aim of the present study was to investigate the mechanisms involved in the dysfunction of circulating EPCs in early diabetes associated with severe atherosclerosis compared with severe atherosclerosis alone. Moreover, we evaluated the consequences of EPC dysfunction in their recruitment in aortic valve lesions. To this end, we used ApoE−/− mice with a high-fat diet to mimic severe atherosclerosis (CIT group) or ApoE−/− mice with high-fat diet and diabetes (STZ group), to mimic diabetes combined with atherosclerosis. The EPCs were identified and quantified by CD34 and VEGFR2 staining, and the expression levels of α4β1, αVβ3, αVβ5, β1, αLβ2, α5 integrins, and CXCR4 chemokine receptor on EPC surface were assessed at 4 or 7 days after the last STZ or CIT injection when the diet was switched from standard chow to atherogenic diet. Also, EPC recruitment in the aortic valves was measured by identifying CD34 positive cells in valve sections from the same four established experimental groups (STZ4, STZ7, CIT4, and CIT7), as CD34 positive valve cells were previously found to be recruited progenitor cells26.
Our data showed that the number and function of EPCs declined in ApoE−/− mice with diabetes and a high-fat diet. Also, the present results support the claims on the importance of a hypocaloric, polyunsaturated fat-rich diet, seeing how the 7 days atherogenic diet alone was able to reduce EPCs’ viability to two-thirds through apoptosis induction.
Regarding the function of EPCs, it was previously shown that EPCs from diabetic patients have normal adhesion to fibronectin and collagen but altered adhesion to activated human umbilical vein ECs27. Our results provide possible clues toward the mechanism involved in this previous finding: α4β1 integrin involved in adhesion to activated ECs is reduced in EPC from diabetic animals (STZ4 and STZ7 groups), but not RGD-binding integrin (α5β1) which bind fibronectin. Also, at both time points, EPCs from diabetic mice expressed αVβ3 integrin at a lower level, the rest of integrins being seemingly unaffected by diabetes superimposed on atherosclerosis or atherosclerosis alone.
The observed trend of diminution of CXCR4 expression levels in our study is in agreement with published data3 showing a 44% reduction in CXCR4 positive EPC cells in diabetic patients compared with healthy controls. Moreover, our data show that this modification occurs early in the progression of diabetes, CXCR4 levels being already reduced at 4 days after the last STZ injection (STZ4 group).
To the best of our knowledge, there have been no studies investigating the EPC phenotype and their contributions to aortic valve disease in diabetes associated with atherosclerosis or in atherosclerosis alone. In our experiments, aortic valves from mice fed 7 days with a high-fat diet showed an increased number of CD34 positive cells compared with animals on shorter diet lengths and their diabetic counterparts. In a previous article, we showed that at this time point, VCAM-1 and P-selectin are significantly increased in the aortic valve, a hallmark of endothelium activation21. This probably leads to EPC recruitment from the circulation in dyslipidemic controls (CIT4 and CIT7 groups) but, due to their lower number and lower VCAM-1 ligand, α4β1, EPCs from diabetic animals are recruited less efficiently.
Further studies are needed to elucidate the mechanisms leading to the decrease of EPCs numbers in diabetes associated with severe atherosclerosis and to investigate the role of EPCs in repairing early lesions associated with aortic valve disease.
Recently, Abplanalp et al. showed that PKA-mediated phosphorylation of α4β1 induced by high glucose plays a role in bone marrow retention of EPCs12. Thus, the reduced α4β1 integrin levels observed in circulatory EPCs may be caused by cells expressing lower levels of α4β1 being able to escape into the bloodstream while those expressing higher levels being retained in the bone marrow. These results point to potential therapeutic avenues: one route would be that of bone marrow PKA inhibition, which would help EPC mobilization, and another could be the i.v. administration of autologous or allogeneic EPCs modified to express higher α4β1 and αVβ3 levels to improve their adhesion at sites of vascular and valvular lesions. This latter route is currently under investigation in our group.
In conclusion, we show here that early stage diabetes superimposed on atherosclerosis induced alterations in EPC number, phenotype, and homing. These data indicate that functional disruption of α4β1 and αVβ3 integrins on EPCs may represent a potential therapeutic target for aortic valve disease by potentially influencing the reparative capacity of valvular ECs in severe atherosclerosis-associated diabetes mellitus.
Supplemental Material
Supplemental Material, suppl1 for Integrins α4β1 and αVβ3 are Reduced in Endothelial Progenitor Cells from Diabetic Dyslipidemic Mice and May Represent New Targets for Therapy in Aortic Valve Disease by Alexandru Filippi, Alina Constantin, Nicoleta Alexandru, Geanina Voicu, Cristina Ana Constantinescu, Daniela Rebleanu, Madalina Fenyo, Dan Simionescu, Agneta Simionescu, Ileana Manduteanu and Adriana Georgescu in Cell Transplantation
Footnotes
Ethical Approval: All experimental protocols were approved by the Ethics Committee of ICBP “Nicolae Simionescu” and by the national authority in charge, ANSVSA (authorization 304/10.10.2016).
Statement of Human and Animal Rights: All experimental procedures involving animals were conducted in accordance with national, European, and international legislation on the use of experimental animals in biomedical research and approved by the Ethics Committees from IBPC “Nicolae Simionescu” (accredited by the Order No. 789 from February 21, 2008, according to the national Law No. 206 from May 27, 2004).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by a grant from the Competitiveness Operational Program 2014-2020, Targeted therapies for aortic valve disease in diabetes, THERAVALDIS, ID P_37_298, MySMIS code: 104362, contract number 115/13.09.2016.
In addition, the authors declare that the research was conducted in the absence of any either commercial or financial relationships that could be construed as a potential conflict of interest.
ORCID iD: Alexandru Filippi  https://orcid.org/0000-0002-4948-5454
https://orcid.org/0000-0002-4948-5454
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bogoslovsky T, Maric D, Gong Y, Qu B, Yang K, Spatz M, Hallenbeck J, Diaz-Arrastia R. Preservation and enumeration of endothelial progenitor and endothelial cells from peripheral blood for clinical trials. Biomark Med. 2015;9(7):625–637. [DOI] [PubMed] [Google Scholar]
- 2. Wils J, Favre J, Bellien J. Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacol Ther. 2017;170:98–115. [DOI] [PubMed] [Google Scholar]
- 3. Antonio N, Fernandes R, Soares A, Soares F, Lopes A, Carvalheiro T, Paiva A, Pego GM, Providencia LA, Goncalves L, Ribeiro CF. Reduced levels of circulating endothelial progenitor cells in acute myocardial infarction patients with diabetes or pre-diabetes: accompanying the glycemic continuum. Cardiovasc Diabetol. 2014;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56(3):666–674. [DOI] [PubMed] [Google Scholar]
- 5. Tsukada S, Masuda H, Jung SY, Yun J, Kang S, Kim DY, Park JH, Ji ST, Kwon SM, Asahara T. Impaired development and dysfunction of endothelial progenitor cells in type 2 diabetic mice. Diabetes Metab. 2017;43(2):154–162. [DOI] [PubMed] [Google Scholar]
- 6. Sainz J, Sata M. CXCR4, a key modulator of vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27(2):263–265. [DOI] [PubMed] [Google Scholar]
- 7. Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4(4):E83–E90. [DOI] [PubMed] [Google Scholar]
- 10. Chan BM, Elices MJ, Murphy E, Hemler ME. Adhesion to vascular cell adhesion molecule 1 and fibronectin. Comparison of alpha 4 beta 1 (VLA-4) and alpha 4 beta 7 on the human B cell line JY. J Biol Chem. 1992;267(12):8366–8370. [PubMed] [Google Scholar]
- 11. Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, Gavin M, Goukassian DA, Yoon YS, Papayannopoulou T, Asahara T, et al. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203(1):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abplanalp WT, Conklin DJ, Cantor JM, Ginsberg MH, Wysoczynski M, Bhatnagar A, O’Toole TE. Enhanced integrin alpha4beta1-mediated adhesion contributes to a mobilization defect of endothelial progenitor cells in diabetes. Diabetes. 2016;65(11):3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caiado F, Dias S. Endothelial progenitor cells and integrins: adhesive needs. Fibrogenesis Tissue Repair. 2012;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kokubo T, Uchida H, Choi ET. Integrin alpha(v)beta(3) as a target in the prevention of neointimal hyperplasia. J Vasc Surg. 2007;45(Suppl A):A33–A38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayakawa K, Pham LD, Arai K, Lo EH. Reactive astrocytes promote adhesive interactions between brain endothelium and endothelial progenitor cells via HMGB1 and beta-2 integrin signaling. Stem Cell Res. 2014;12(2):531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verloop RE, Koolwijk P, van Zonneveld AJ, van Hinsbergh VW. Proteases and receptors in the recruitment of endothelial progenitor cells in neovascularization. Eur Cytokine Netw. 2009;20(4):207–219. [DOI] [PubMed] [Google Scholar]
- 17. Cui X, Zhang X, Guan X, Li H, Li X, Lu H, Cheng M. Shear stress augments the endothelial cell differentiation marker expression in late EPCs by upregulating integrins. Biochem Biophys Res Commun. 2012;425(2):419–425. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki Y, Yamamoto K, Ando J, Matsumoto K, Matsuda T. Arterial shear stress augments the differentiation of endothelial progenitor cells adhered to VEGF-bound surfaces. Biochem Biophys Res Commun. 2012;423(1):91–97. [DOI] [PubMed] [Google Scholar]
- 19. Kitsiou PV, Tzinia AK, Stetler-Stevenson WG, Michael AF, Fan WW, Zhou B, Tsilibary EC. Glucose-induced changes in integrins and matrix-related functions in cultured human glomerular epithelial cells. Am J Physiol Renal Physiol. 2003;284(4):F671–F679. [DOI] [PubMed] [Google Scholar]
- 20. Matsumoto Y, Adams V, Walther C, Kleinecke C, Brugger P, Linke A, Walther T, Mohr FW, Schuler G. Reduced number and function of endothelial progenitor cells in patients with aortic valve stenosis: a novel concept for valvular endothelial cell repair. Eur Heart J. 2009;30(3):346–355. [DOI] [PubMed] [Google Scholar]
- 21. Tucureanu MM, Filippi A, Alexandru N, Ana Constantinescu C, Ciortan L, Macarie R, Vadana M, Voicu G, Frunza S, Nistor D, Simionescu A, et al. Diabetes-induced early molecular and functional changes in aortic heart valves in a murine model of atherosclerosis. Diab Vasc Dis Res. 2019:16(6):562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Georgescu A, Alexandru N, Andrei E, Titorencu I, Dragan E, Tarziu C, Ghiorghe S, Badila E, Bartos D, Popov D. Circulating microparticles and endothelial progenitor cells in atherosclerosis: pharmacological effects of irbesartan. J Thromb Haemost. 2012;10(4):680–691. [DOI] [PubMed] [Google Scholar]
- 23. Georgescu A, Alexandru N, Andrei E, Dragan E, Cochior D, Dias S. Effects of transplanted circulating endothelial progenitor cells and platelet microparticles in atherosclerosis development. Biol Cell. 2016;108(8):219–243. [DOI] [PubMed] [Google Scholar]
- 24. Georgescu A, Alexandru N, Nemecz M, Titorencu I, Popov D. Irbesartan administration therapeutically influences circulating endothelial progenitor cell and microparticle mobilization by involvement of pro-inflammatory cytokines. Eur J Pharmacol. 2013;711(1–3):27–35. [DOI] [PubMed] [Google Scholar]
- 25. Fadini GP, Avogaro A. Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes Obes Metab. 2010;12(7):570–583. [DOI] [PubMed] [Google Scholar]
- 26. Gossl M, Khosla S, Zhang X, Higano N, Jordan KL, Loeffler D, Enriquez-Sarano M, Lennon RJ, McGregor U, Lerman LO, Lerman A, et al. Role of circulating osteogenic progenitor cells in calcific aortic stenosis. J Am Coll Cardiol. 2012;60(19):1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–2786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, suppl1 for Integrins α4β1 and αVβ3 are Reduced in Endothelial Progenitor Cells from Diabetic Dyslipidemic Mice and May Represent New Targets for Therapy in Aortic Valve Disease by Alexandru Filippi, Alina Constantin, Nicoleta Alexandru, Geanina Voicu, Cristina Ana Constantinescu, Daniela Rebleanu, Madalina Fenyo, Dan Simionescu, Agneta Simionescu, Ileana Manduteanu and Adriana Georgescu in Cell Transplantation