Abstract
Aim
To investigate the protective effects and possible mechanisms of action of resina draconis (RD) on acute liver injury and liver regeneration after 2/3 partial hepatectomy (PH) in mice.
Methods
2/3 PH was used to induce acute liver injury. Mice were divided into three groups: sham, vehicle + 2/3 PH, and RD + 2/3 PH. Resina draconis was administered intragastrically after 2/3 PH into the RD + 2/3 PH group, and the same volume of vehicle (1% sodium carboxymethyl cellulose) was injected into the vehicle + 2/3 PH group and sham group mice. The index of liver to body weight (ILBW) and proliferating cell nuclear antigen (PCNA) were assayed to evaluate liver regeneration. Blood and liver tissues were collected for serological and western blotting analysis.
Results
Resina draconis protected against 2/3 PH-induced acute severe liver injury and promoted liver regeneration as shown by significantly increased ILBW compared with that of controls. 2/3 PH increased serum AST and ALT levels, which were significantly decreased by RD treatment, while 2/3 PH decreased serum TP and ALB, which were increased by RD treatment. In the RD + 2/3 PH group, PCNA expression was significantly increased compared with the 2/3 PH group. Further, hepatocyte growth factor (HGF), TNFα, and EGFR levels were increased in the RD group at postoperative days 2 and 4 compared with the those in the 2/3 PH group.
Conclusion
Our results suggest that RD ameliorates acute hepatic injury and promotes liver cell proliferation, liver weight restoration, and liver function after 2/3 PH, probably via HGF, TNFα, and EGFR signaling.
1. Introduction
The liver plays a central role in detoxification and protein synthesis and produces digestive biochemicals. It also possesses the unique ability to regenerate after surgical resection or injury to maintain these essential functions [1–4]. The clinical application of partial hepatectomy (PH) or living donor liver transplantation (LDLT) for the treatment of primary or metastatic liver tumors and end-stage liver disease depends on effective liver regeneration [5–7]. Optimizing liver regeneration would have great clinical potential in improving outcomes from liver disease. Two-thirds (2/3) PH is a well-validated experimental model of liver regeneration that has been extensively used to study the underlying molecular and cellular mechanisms [8–12].
Growth factors such as TGFα, EGF, and HGF are highly expressed after PH and are thought to be important in regulating hepatocyte activation, proliferation, migration, and survival during liver regeneration [13–15]. Hepatocyte growth factor (HGF) is the major hepatocyte mitogen [16], and HGF is upregulated after PH. HGF initiates hepatocyte DNA synthesis and stimulates hepatocyte growth [16, 17]. Overexpression of HGF also increases hepatocyte proliferation and accelerates liver regeneration after PH [18]. Mice lacking c-MET, the HGF receptor, show delayed liver regeneration after PH, with hepatocytes showing S-phase entry defects [19].
TNFα is an important regulator of the “priming phase” of liver regeneration, and blocking TNFα signaling in rats prior to PH significantly reduces the proliferation of hepatocytes and nonparenchymal liver cells [20, 21]. TNFα, together with its downstream effector IL-6, initiates hepatocyte transition from G0 to G1 phase of the cell cycle [21–23]. TNFα also activates transcription factors like STAT3 and NF-κB, which in turn promote the expression of cell cycle regulator genes such as cyclin D [24, 25].
The EGF receptor (EGFR) also plays a vital role in liver regeneration, since liver regeneration is not impaired in mice lacking TGFα because other EGFR ligands compensate for the absence of TGFα [14, 26]. EGFR is expressed at high levels in the adult liver and regulates liver development, function, and regeneration [13]. Transgenic mice lacking EGFR have a higher mortality rate after PH, with surviving mice showing increased hepatocyte proliferation due to low cyclin D1 expression. The EGFR pathway regulates hepatocyte proliferation and efficient liver regeneration after PH [27, 28].
Resina draconis (RD), a bright resin extracted from Dracaena cochinchinensis (Lour.) S. C. Chen, is a Chinese Herbal medicine with a well-established safety and bioactivity record [29, 30]. RD contributes to skin repair and can promote the development of transplanted epidermis, enhance the capillary proliferation [31], and promote the growth of fetal rat skin fibroblasts [32]. It can also improve wound healing [33] and has been used for the treatment of skin diseases [34, 35]. Further, RD can facilitate blood circulation and improve platelet function [36, 37]. RD has anti-inflammatory effects [38] and antitumor activities [39, 40]. Of these multiple activities, RD strongly promotes tissue recovery after injury. However, the effects of RD on liver regeneration after extended hepatectomy have not been investigated. Here, we explored the effect and possible mechanisms of RD treatment on liver regeneration in 2/3 PH mice. The involvement of HGF, TNFα, and EGFR signaling in RD-stimulated liver regeneration was also investigated.
2. Materials and Methods
2.1. Preparation of Drugs
Resina draconis powder (Xishuangbanna Rainforest Pharmaceutical Co., Ltd., Jinghong, China) was dispersed in 1% sodium carboxymethyl cellulose (Na-CMC) at 10 mg/mL, 20 mg/mL, and 40 mg/mL before intragastric injection into mice.
2.2. Animals
One-hundred forty-four 6-8 week-old male C57BL/6 mice weighing between 20 and 25 g were used. Animals were divided into three groups (n = 8 in each group): sham, vehicle + 2/3 PH, and RD + 2/3 PH. The RD + 2/3 PH group received intragastric injections of 0.2 mL RD solution at doses of 0.1 g/kg, 0.2 g/kg, or 0.4 g/kg body weight once daily for 10 days after 2/3 PH surgery. All experiments were conducted under the institutional guidelines of the Animal Ethics Committee, Kunming Medical University.
2.3. Surgical Procedure and Anesthesia
2/3 PH was performed in the vehicle + 2/3 PH and RD + 2/3 PH groups using a slight modification of the methods developed by Harkness [41, 42]. Briefly, after general anesthesia, the left lateral, left median, and right median lobes were resected. Laparotomy was performed in the sham group without ligation and 2/3 PH.
2.4. Liver Tissue Collection
On days 0, 2, 4, 6, 8, and 10 after 2/3 PH, animals in each group were weighed and sacrificed humanly, liver tissues were collected and weighed, and the index of liver to body weight (ILBW) recorded.
2.5. Serum Parameters
Peripheral blood was collected and centrifuged. Serum alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), albumin (ALB), and total protein (TP) were measured in U/L with a serum multiple biochemical analyzer (Diamond Diagnostics Inc., Holliston, USA).
2.6. Western Blotting
Liver tissue proteins were prepared as previously described [43]. Western blotting was performed using antibodies targeting PCNA (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), β-actin (Sigma Aldrich, St. Louis, MO, USA), HGF (Abcam, Cambridge, UK), EGFR (Abcam), and TNF-α (Abcam). Super Signal West Dura Extended Duration Substrate (Pierce, Thermo Fisher Scientific, Waltham, MA, USA) was used to visualize antibody-antigen complexes.
2.7. Data Analysis
Three-way ANOVA was applied to analyze BW, ILBW, TP, ALB, ALT, ALP, and AST using the factors 2/3 PH operation, RD treatment, and time after injury. Tukey's post hoc tests were applied as appropriate. Student's t test was used to analyze data between two groups. All values were expressed as mean ± SEM, and a p value <0.05 was considered statistically significant.
3. Results
3.1. Resina Draconis Treatment Promotes Liver Weight Recovery after 2/3 PH
To address the effect of RD on general mouse health after PH, mouse body weights were monitored after 2/3 PH. The body weights of mice reduced on days 2 and 4 after 2/3 PH followed by weight gain on day 6. RD treatment at different doses induced faster and longer-lasting weight gain than vehicle group over 10 days (Figure 1(a)).
Figure 1.
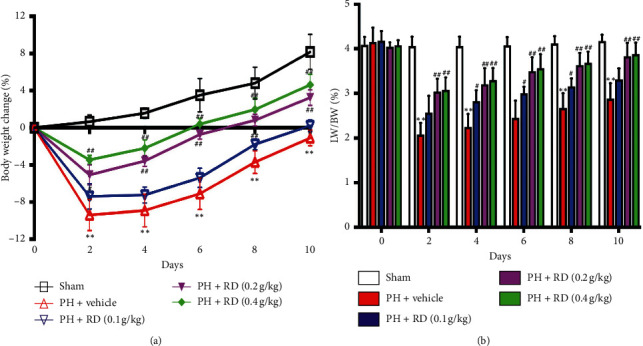
Resina draconis increases body weight and index of liver to body weight (ILBW) after 2/3 PH. (a) RD treatment at different doses increases body weight gain over time in mice receiving 2/3 PH. Results are represented as mean ± SEM of percentage change in body weight versus original body weight before 2/3 PH. n = 8 mice/time point. ∗∗p < 0.01, vehicle + 2/3 PH group vs sham group; ##p < 0.01, RD + 2/3 PH group vs vehicle + 2/3 PH group; #p < 0.05, RD vehicle + group vs vehicle + 2/3 PH group. (b) RD treatment increases ILBW over time in mice receiving 2/3 PH. Data are expressed as mean ± SEM. n = 8 mice/time point. ∗∗p < 0.01, vehicle + 2/3 PH group vs sham group; ##p < 0.01, RD + 2/3 PH group vs vehicle + 2/3 PH group; #p < 0.05, RD + 2/3 PH group vs vehicle + 2/3 PH group.
The ILBW from days 2 to 10 was measured after 2/3 PH to address the effect of RD on liver recovery. The ILBW was significantly higher on days 2, 4, 6, 8, and 10 in RD-treated mice versus vehicle controls (Figure 1(b)). Of the different RD concentrations tested, the 0.2 g/kg and 0.4 g/kg doses were most effective, with no significant difference between the 0.4 g/kg treatment and 0.2 g/kg treatment. Therefore, 0.2 g/kg was used in subsequent experiments. These data indicate that RD treatment promotes liver recovery.
Resina draconis treatment prevents decreases in serum total protein (TP) and albumin (ALB) after 2/3 PH.
Given that RD treatment promoted liver regeneration, we further measured changes in serum total protein (TP) and albumin (ALB). TP and ALB levels were attenuated in both the 2/3 PH group and the RD-treated 2/3 PH group on days 2, 4, 6, 8, and 10 after PH. However, TP and ALB levels were significantly higher in the RD-treated 2/3 PH group compared with the vehicle + 2/3 PH group (p < 0.01) (Figures 2(a) and 2(b)). Furthermore, there was no unequivocal changes in the RD-treated 2/3 PH group and the sham group on days 4, 6, 8, and 10 after PH.
Figure 2.

Resina draconis treatment prevents loss of serum TP and ALB after 2/3 PH. (a) RD treatment increases serum TP over time in mice receiving 2/3 PH. Results are represented as mean ± SEM. n = 8 mice/time point. ∗∗p < 0.01, vehicle + 2/3 PH group vs sham group; ##p < 0.01, RD + 2/3 PH group vs vehicle + 2/3 PH group. (b) RD treatment increases serum ALB over time in mice receiving 2/3 PH. Data are expressed as mean ± SEM. n = 8 mice/time point. ∗∗p < 0.01, vehicle + 2/3 PH group vs sham group; ##p < 0.01, RD + 2/3 PH group vs vehicle + 2/3 PH group; #p < 0.05, RD group vs 2/3 PH group.
3.2. Resina Draconis Treatment Restores Liver Function after 2/3 PH
Serum AST, ALT, and ALP levels are biomarkers of liver function. Serum AST and ALT levels significantly increased in both the 2/3 PH and RD groups on days 2, 4, 6, 8, and 10 after PH. However, the levels were significantly different in the 2/3 PH group and the RD group (p < 0.01) (Figures 3(a) and 3(b)). ALP levels also significantly increased in the 2/3 PH and RD groups on days 2, 4, 6, and 8 after PH. However, the levels were significantly different in the 2/3 PH group and the RD group on days 2 and 4 (p < 0.01) (Figure 3(c)). Interestingly, the ALP and ALT levels in the RD group and vehicle group showed no significant difference on days 6, 8, and 10 after PH. These results indicate that RD treatment not only enhances liver regeneration after 2/3 PH but also restores 2/3 PH-induced loss of liver function as measured by liver function biomarkers.
Figure 3.
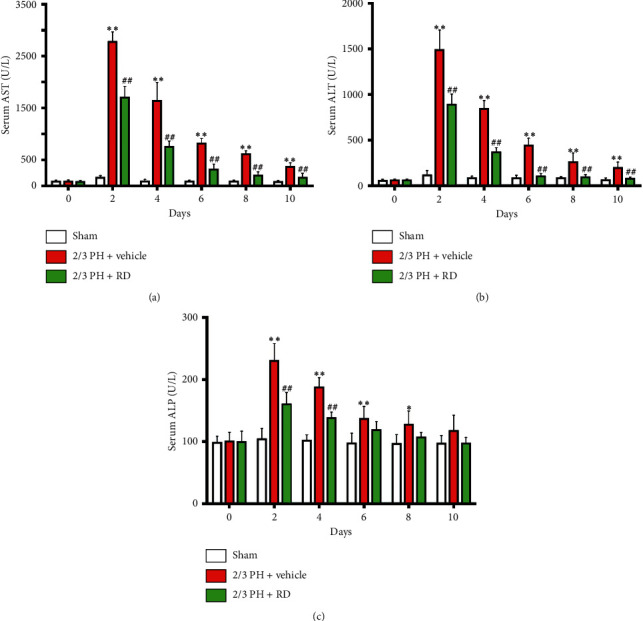
Resina draconis treatment prevents loss of liver function after 2/3 PH. (a) Serum AST levels were significantly increased in both the 2/3 PH group and the RD group on days 2, 4, 6, 8, and 10 following 2/3 PH. Results are represented as mean ± SEM. n = 8 mice/time point. ∗∗p < 0.01, vehicle + 2/3 PH group vs sham group; ##p < 0.01, RD + 2/3 PH group vs vehicle + 2/3 PH group. (b) Serum ALT levels were significantly increased in both the 2/3 PH group and the RD group on days 2, 4, 6, 8, and 10 following 2/3 PH. Results are represented as mean ± SEM. n = 8 mice/time point. ∗∗p < 0.01, vehicle + 2/3 PH group vs sham group; ##p < 0.01, RD + PH group vs vehicle + 2/3 PH group. (c) Serum ALP levels were significantly increased in both the 2/3 PH group and the RD group on day 2, 4, 6, and 8 following 2/3 PH. Results are represented as mean ± SEM. n = 8 mice/time point. ∗∗p < 0.01, vehicle + 2/3 PH group vs sham group; ∗p < 0.05, vehicle + 2/3 PH group vs sham group; ##p < 0.01, RD + 2/3 PH group vs vehicle + 2/3 PH group.
3.3. Resina Draconis Treatment Enhances Hepatocyte Proliferation after 2/3 PH
Liver regeneration is closely related to hepatocyte proliferation. As RD promoted liver recovery, we hypothesized that RD enhanced hepatocyte proliferation. To address this hypothesis, we compared proliferating cell nuclear antigen (PCNA) levels in liver tissues between the different groups. PCNA expression was significantly higher on days 2, 4, and 6 in the RD-treated group compared with the 2/3 PH group (Figures 4(a) and 4(b)), with no observable change in PCNA expressed observed in the sham group.
Figure 4.
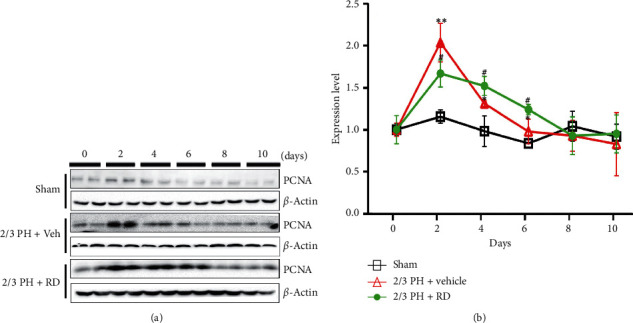
Resina draconis treatment enhances hepatocyte proliferation after 2/3 PH. (a). Representative western blotting analysis of PCNA protein in mouse livers after 2/3 PH and sham operation. RD treatment increases PCNA expression after 2/3 PH. (b). Quantification of PCNA normalized to β-actin. Data are expressed as mean ± SEM. n = 5. ∗∗p < 0.01, 2/3 PH group vs sham group; ∗p < 0.05, 2/3 PH group vs sham group; ##p < 0.01, RD + 2/3 PH group vs vehicle + 2/3 PH group; #p < 0.05, RD + 2/3 PH group vs vehicle + 2/3 PH group.
3.4. Resina Draconis Treatment Induces HGF and Increases EGFR and TNFα after 2/3 PH
HGF, EGFR, and TNFα are important growth factors that stimulate hepatocyte mitogenesis in liver regeneration [44, 45]. To explore the possible mechanism by which RD promotes liver regeneration after 2/3 PH, HGF, EGFR, and TNFα expressions were measured in liver tissue by western blotting. In the vehicle + 2/3 PH group and RD + 2/3 vehicle group, HGF, EGFR, and TNFα increased significantly on day 2 after 2/3 PH and decreased progressively to preoperative levels by the end of the observation period. However, the levels of HGF (Figures 5(a) and 5(b)), EGFR (Figures 5(c) and 5(d)), and TNFα (Figures 5(e) and 5(f)) were significantly higher on day 2 and day 4 after 2/3 PH (p < 0.05) in the RD + 2/3 PH group than in vehicle + 2/3 PH group and sham group. Further, trends in these increases continued to day 6 and day 8.
Figure 5.
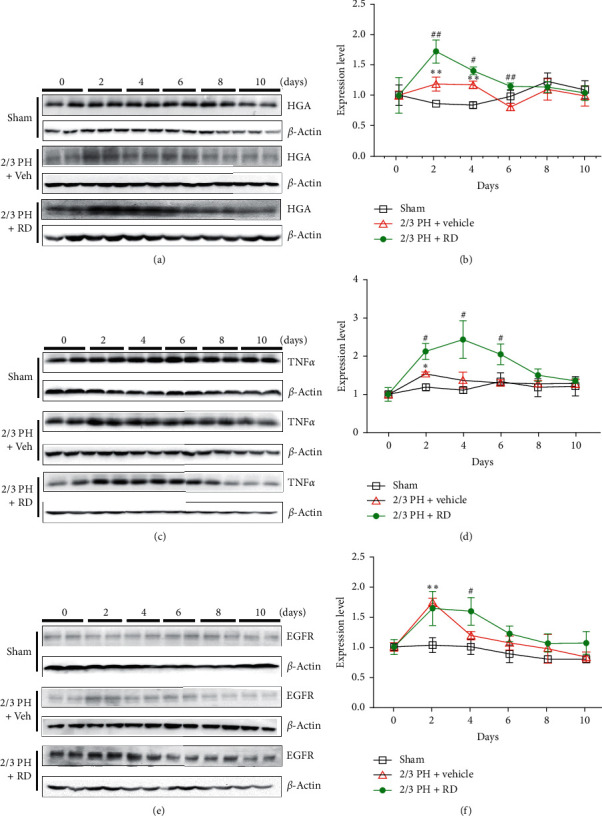
Resina draconis treatment affects HGF, EGFR, and TNFα levels after 2/3 PH. (a) and (b). Representative western blotting analysis of HGF protein in mouse livers after 2/3 PH and sham operations. RD treatment increases the HGF expression after 2/3 PH. Quantifications were normalized to β-actin. (c) and (d). Representative western blotting analysis of HGF protein in mouse livers after 2/3 PH and sham operations. RD treatment increases TNFα expression after 2/3 PH. Quantifications were normalized to β-actin. (e) and (f). Representative western blotting analysis of EGFR protein in mouse livers after 2/3 PH and sham operations. RD treatment increases the TNFα expression after 2/3 PH. Quantifications were normalized to β-actin. All the data are expressed as mean ± SEM. n = 5. ∗∗p < 0.01, vehicle + 2/3 PH group vs sham group; ∗p < 0.05, vehicle + 2/3 PH group vs sham group; #p < 0.05, RD + 2/3 PH group vs 2/3 PH group.
4. Discussion
Despite clinical advances, liver diseases remain a severe health problem worldwide. Liver resection is the primary treatment modality for liver cancer and other serious liver diseases [46–52]. Liver regeneration plays a vital role in recovery after resection. Acute liver failure after hepatectomy is a main cause of postoperative mortality and morbidity [53–57]. How to protect against postoperative liver injury and promote liver regeneration remains an urgent unmet clinical need. RD has been used for many years to promote wound healing [32, 58] and as an anticoagulant [59–61]. Several studies have shown that RD has hemostatic and anti-inflammatory properties and promotes blood circulation and skin repair [62–65]. Here, for the first time, we provide evidence that RD treatment is beneficial to liver regeneration and protects against liver injury after 2/3 PH. Furthermore, RD-stimulated HGF, EGFR, and TNFα signaling during liver regeneration after 2/3 PH. These findings offer a new avenue for protecting against postoperative liver failure and controlling liver regeneration after liver resection.
The exact mechanisms of liver regeneration after 2/3 PH are complicated and have not been fully defined, although a number of growth factors are implicated in the process. HGF, EGFR, and TNFα are potential initiators of liver regeneration and increase immediately and are maintained after 2/3 PH. Here, we found that the expressions of HGF, EGFR, and TNFα increased significantly after 2/3 PH compared with those of controls on days 2 and 4 after 2/3 PH and were significantly higher in the RD-treated 2/3 PH group than in the vehicle-treated 2/3 PH group. Expression of these growth factors is a key step to initial hepatocyte proliferation and improving liver regeneration and functional recovery. The promitogenic effect of RD-induced expression of these factors was further supported by the observation of higher expression of proliferating cell nuclear antigen (PCNA) in RD-treated mice.
Serum AST and ALT liver function biomarkers are inversely related to the severity of liver damage [66–68]. We found that both ALT and AST levels were significantly higher in the 2/3 PH group mice than in the sham group mice on day 2 and day 4 after 2/3 PH and that RD treatment reversed these changes. TB and ALB are markers of liver synthetic function [69–72]. Our study revealed that TB and ALB levels were higher in the RD-treated 2/3 PH group on day 2 and day 4 after the operation than in vehicle-treated 2/3 PH. Interestingly, RD-treated not only reduced the liver function biomarker levels compared with the vehicle-treated PH group but also it recovered levels to sham over the study period. These results strongly indicate that RD treatment protects against acute liver damage and stimulates liver regeneration after 2/3 PH.
Taken together, our results confirm that intragastric injection of RD after 2/3 PH prevents acute liver failure and promotes liver regeneration. HGF, EGFR, and TNFα are involved in this process. These results indicate that RD may be useful in the treatment of acute liver injury.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81960666 and 81860254, W.Y.H.), the National Natural Science Foundation of Yunnan (2019FA033, R.P.Z.), the Program for Innovative Research Team in Science and Technology in Yunnan Province (2019HC008), and Yunnan Provincial Science and Technology Department (202005AF150043). The support provided by Yunnan Key Laboratory of Stem Cell and Regenerative Medicine (M. Z. and W.Y.H.) is also appreciated.
Abbreviations
- RD:
Resina draconis
- PH:
Partial hepatectomy
- ILBW:
Index of liver to body weight
- PCNA:
Proliferating cell nuclear antigen
- AST:
Aspartate transaminase
- ALT:
Alanine transaminase
- TP:
Total protein
- ALB:
Albumin
- HGF:
Hepatocyte growth factor
- TNFα:
Tumor necrosis factor
- EGF:
Epidermal growth factor
- LDLT:
Living donor liver transplantation
- NF-κB:
Nuclear factor kappa B.
Contributor Information
Rong-ping Zhang, Email: zhrpkm@163.com.
Hu Wei-yan, Email: huweiyan2004@163.com.
Data Availability
The data used to support the study findings are available within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Zhi-yong He, Kai-han Lou, and Jia-hui Zhao performed the research; Wei-yan Hu and Rong-ping Zhang designed the research; Wei-yan Hu and Zhi-yong He analyzed the data and wrote the paper; Ming Zhang revised the manuscript; Lang-chun Zhang, Ju Li and Hao-fei Yu prepared the figures.
Supplementary Materials
This section includes graphical abstract.
References
- 1.Cienfuegos J. A., Rotellar F., Baixauli J., et al. Liver regeneration—the best kept secret: a model of tissue injury response. Revista Española de Enfermedades Digestivas. 2014;106(3):171–194. [PubMed] [Google Scholar]
- 2.Fausto N., Campbell J. S., Riehle K. J. Liver regeneration. Hepatology. 2000;43(2):S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 3.Gentile J. M., Avila L., Grace J. T. Liver regeneration. old and new concepts. The American Journal of Surgery. 1970;120(1):2–6. doi: 10.1016/s0002-9610(70)80132-5. [DOI] [PubMed] [Google Scholar]
- 4.Riehle K. J., Dan Y. Y., Campbell J. S., Fausto N. New concepts in liver regeneration. Journal of Gastroenterology and Hepatology. 2011;26(1):203–212. doi: 10.1111/j.1440-1746.2010.06539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cieslar-Pobuda A., Wiechec E. Research on liver regeneration as an answer to the shortage of donors for liver transplantation. Hepatology Research. 2014;44(9):944–946. doi: 10.1111/hepr.12265. [DOI] [PubMed] [Google Scholar]
- 6.Olthoff K. M., Emond J. C., Shearon T. H., et al. Liver regeneration after living donor transplantation: adult-to-adult living donor liver transplantation cohort study. Liver Transplantation. 2015;21(1):79–88. doi: 10.1002/lt.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagano D., Spada M., Parikh V., et al. Liver regeneration after liver resection: clinical aspects and correlation with infective complications. World Journal of Gastroenterology. 2014;20(22):6953–6960. doi: 10.3748/wjg.v20.i22.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucher N. L., Weir G. C. Insulin, glucagon, liver regeneration, and dna synthesis. Metabolism. 1976;25(11):1423–1425. doi: 10.1016/s0026-0495(76)80156-4. [DOI] [PubMed] [Google Scholar]
- 9.Kandilis A. N., Koskinas J., Vlachos I., et al. Liver regeneration: immunohistochemichal study of intrinsic hepatic innervation after partial hepatectomy in rats. BMC Gastroenterology. 2014;14(1):p. 202. doi: 10.1186/s12876-014-0202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzirogiannis K. N., Kourentzi K. T., Zyga S., et al. Effect of 5-HT 7 receptor blockade on liver regeneration after 60–70% partial hepatectomy. BMC Gastroenterology. 2014;14(1):p. 201. doi: 10.1186/s12876-014-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf J. H., Bhatti T. R., Fouraschen S., et al. Heat shock protein 70 is required for optimal liver regeneration after partial hepatectomy in mice. Liver Transplantation. 2014;20(3):376–385. doi: 10.1002/lt.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu T., Huang J., Wu S., et al. Deficiency of periostin impairs liver regeneration in mice after partial hepatectomy. Matrix Biology. 2018;66:81–92. doi: 10.1016/j.matbio.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Marti U., Burwen S. J., Jones A. L. Biological effects of epidermal growth factor, with emphasis on the gastrointestinal tract and liver: an update. Hepatology. 1989;9(1):126–138. doi: 10.1002/hep.1840090122. [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos G. K. Advances in liver regeneration. Expert Review of Gastroenterology & Hepatology. 2014;8(8):897–907. doi: 10.1586/17474124.2014.934358. [DOI] [PubMed] [Google Scholar]
- 15.Michalopoulos G. K., Khan Z. Liver regeneration, growth factors, and amphiregulin. Gastroenterology. 2005;128(2):503–506. doi: 10.1053/j.gastro.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imamura R., Matsumoto K. Hepatocyte growth factor in physiology and infectious diseases. Cytokine. 2017;98:97–106. doi: 10.1016/j.cyto.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Huh C. G., Factor V. M., Sanchez A., et al. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proceedings of the National Academy of Sciences. 2004;101(13):4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao X. F., Jin S. Z., Sun L., et al. Therapeutic effects of hepatocyte growth factor-overexpressing dental pulp stem cells on liver cirrhosis in a rat model. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-14995-5.15812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paranjpe S., Bowen W. C., Bell A. W., et al. Cell cycle effects resulting from inhibition of hepatocyte growth factor and its receptor c-Met in regenerating rat livers by RNA interference. Hepatology. 2017;45(6):1471–1477. doi: 10.1002/hep.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argast G. M., Campbell J. S., Brooling J. T., Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. Journal of Biological Chemistry. 2004;279(33):34530–34536. doi: 10.1074/jbc.m405703200. [DOI] [PubMed] [Google Scholar]
- 21.Sun J., Yang Z., Shi X. C., et al. G0S2a1 (G0/G1 switch gene 2a1) is downregulated by TNF-alpha in grass carp (Ctenopharyngodon idellus) hepatocytes through PPARalpha inhibition. Gene. 2018;641:1–7. doi: 10.1016/j.gene.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Cressman D. E., Greenbaum L. E., DeAngelis R. A., et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274(5291):1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 23.Ledda-Columbano G. M., Curto M., Piga R., et al. In vivo hepatocyte proliferation is inducible through a TNF and IL-6-independent pathway. Oncogene. 1998;17(8):1039–1044. doi: 10.1038/sj.onc.1202018. [DOI] [PubMed] [Google Scholar]
- 24.Li W., Liang X., Kellendonk C., Poli V., Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. Journal of Biological Chemistry. 2002;277(32):28411–28417. doi: 10.1074/jbc.m202807200. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y., Kirillova I., Peschon J. J., Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proceedings of the National Academy of Sciences. 1997;94(4):1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalopoulos G. K. Principles of liver regeneration and growth homeostasis. Comprehensive Physiology. 2013;3(1):485–513. doi: 10.1002/cphy.c120014. [DOI] [PubMed] [Google Scholar]
- 27.Bhushan B., Chavan H., Borude P., et al. Dual role of epidermal growth factor receptor in liver injury and regeneration after acetaminophen overdose in mice. Toxicological Sciences. 2017;155(2):363–378. doi: 10.1093/toxsci/kfw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H., Shi J. H., Jiang H., et al. ZBTB20 regulates EGFR expression and hepatocyte proliferation in mouse liver regeneration. Cell Death & Disease. 2018;9(5):p. 462. doi: 10.1038/s41419-018-0514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruck M. Dragon’s blood. A glance into the history of pharmacognosy. «Bulletin de la Société des Sciences Médicales du Grand Duché de Luxembourg. 1999;1:96–101. [PubMed] [Google Scholar]
- 30.Fan L. L., Tu P. F., He J. X., Chen H. B., Cai S. Q. Microscopical study of original plant of Chinese drug “dragon’s blood” dracaena cochinchinensis and distribution and constituents detection of its resin. Zhongguo Zhong Yao Za Zhi. 2008;33(10):1112–1117. [PubMed] [Google Scholar]
- 31.Liu A. J., Zhu Y. H., Zhao Z. X. Effect of resina draconis is in the repair of skin defect treated with tissue engineering skin. Guangzhou University of Chinese Medicine. 2009;26:260–262. [Google Scholar]
- 32.Liu A. J., Huang J. T., Zhang L. Q. Growth of fetal mouse skin filbrobasts stimulated by effect locus of resina draconis. Journal of Sichuan Traditional Chinese Medicine. 2014;28:46–48. [Google Scholar]
- 33.Namjoyan F., Kiashi F., Moosavi Z. B., Saffari F., Makhmalzadeh B. S. Efficacy of dragon’s blood cream on wound healing: a randomized, double-blind, placebo-controlled clinical trial. Journal of Traditional and Complementary Medicine. 2016;6:37–40. doi: 10.1016/j.jtcme.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J. S. Application of dragon’s blood in treating surface wounds and burns. Journal of Harbin Medical University. 2001;21:p. 70. [Google Scholar]
- 35.Lu X. P. 20 cases of alopecia areata treatment efficacy blood capsules. Jilin Medical Journal. 2009;30:p. 1391. [Google Scholar]
- 36.Nian X., Yu J. L., Yan L. Dragon’s blood extract has antithrombotic properties, affecting platelet aggregation functions and anticoagulation activities. Journal of Ethnopharmacology. 2011;135:510–514. doi: 10.1016/j.jep.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 37.Sun J., Liu J.-N., Fan B. Phenolic constituents, pharmacological activities, quality control, and metabolism of dracaena species: a review. Journal of Ethnopharmacology. 2019;244 doi: 10.1016/j.jep.2019.112138. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y. D., Zhang P., Yu H. P. Anti-helicobacter pylori and thrombin inhibitory components from Chinese dragon’s blood Dracaena cochinchinensis. Journal of Natural Products. 2007;70:1570–1577. doi: 10.1021/np070260v. [DOI] [PubMed] [Google Scholar]
- 39.Wen F., Zhao X., Zhao Y., Lu Z., Guo Q. The anticancer effects of resina draconis extract on cholangiocarcinoma. Tumour Biology. 2016;37(11):15203–15210. doi: 10.1007/s13277-016-5393-3. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y.-N., Yang A.-L., Pang D.-R., et al. Anti-tumor activity of HIS-4,a biflavonoid from resina draconis,on human hepatoma HepG2 and SK-HEP-1 cells. Zhongguo Zhong Yao Za Zhi. 2019;44(7):1442–1449. doi: 10.19540/j.cnki.cjcmm.20190218.001. [DOI] [PubMed] [Google Scholar]
- 41.Harkness R. D. Changes in the liver of the rat after partial hepatectomy. The Journal of Physiology. 1952;117(3):267–277. doi: 10.1113/jphysiol.1952.sp004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harkness R. D. Collagen in regenerating liver of the rat. The Journal of Physiology. 1952;117(3):257–266. doi: 10.1113/jphysiol.1952.sp004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou K., Yang M., Duan E., et al. Rosmarinic acid stimulates liver regeneration through the mTOR pathway. Phytomedicine. 2016;23(13):1574–1582. doi: 10.1016/j.phymed.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Blindenbacher A., Wang X., Langer I., et al. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 2003;38(3):674–682. doi: 10.1053/jhep.2003.50378. [DOI] [PubMed] [Google Scholar]
- 45.Quetier I., Brezillon N., Duriez M., et al. Hepatitis B virus HBx protein impairs liver regeneration through enhanced expression of IL-6 in transgenic mice. The Journal of Physiology. 2013;59(2):285–291. doi: 10.1016/j.jhep.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Aussilhou B., Doufle G., Hubert C., et al. Extended liver resection for polycystic liver disease can challenge liver transplantation. Annals of Surgery. 2010;252(5):735–743. doi: 10.1097/SLA.0b013e3181fb8dc4. [DOI] [PubMed] [Google Scholar]
- 47.Belda T., Montalva E. M., Lopez-Andujar R., et al. Role of resection surgery in breast cancer liver metastases: experience over the last 10 years in a reference hospital. Cirugía Española. 2010;88(3):167–173. doi: 10.1016/j.ciresp.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Bueno A., Rotellar F., Benito A., et al. Laparoscopic limited liver resection decreases morbidity irrespective of the hepatic segment resected. HPB. 2014;16(4):320–326. doi: 10.1111/hpb.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehrl D., Rothaug K., Hempel D., Rau H. G. Importance of liver resection in case of hepatic breast cancer metastases. Hepato-Gastroenterology. 2013;60(128):2026–2033. [PubMed] [Google Scholar]
- 50.Marin C., Robles R., Fuster M., Parrilla P. Laparoscopic liver resection of a desmoplastic nested spindle cell tumor of the liver. The American Surgeon. 2010;76(9):E184–E185. [PubMed] [Google Scholar]
- 51.McAteer J. P., Goldin A. B., Healey P. J., Gow K. W. Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatric Transplantation. 2013;17(8):744–750. doi: 10.1111/petr.12144. [DOI] [PubMed] [Google Scholar]
- 52.Vigano L., Capussotti L., De Rosa G., et al. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2013;258(5):731–740. doi: 10.1097/SLA.0b013e3182a6183e. [DOI] [PubMed] [Google Scholar]
- 53.Colle I., Verhelst X., Vanlander A., et al. Pathophysiology and management of post resection liver failure. Acta Chirurgica Belgica. 2013;113(3):155–161. doi: 10.1080/00015458.2013.11680904. [DOI] [PubMed] [Google Scholar]
- 54.Hirashita T., Ohta M., Iwashita Y., et al. Risk factors of liver failure after right-sided hepatectomy. The American Journal of Surgery. 2013;206(3):374–379. doi: 10.1016/j.amjsurg.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Roberts K. J., Bharathy K. G., Lodge J. P. Kinetics of liver function tests after a hepatectomy for colorectal liver metastases predict post-operative liver failure as defined by the international study group for liver surgery. HPB. 2013;15(5):345–351. doi: 10.1111/j.1477-2574.2012.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vibert E., Pittau G., Gelli M., et al. Actual incidence and long-term consequences of posthepatectomy liver failure after hepatectomy for colorectal liver metastases. Surgery. 2014;155(1):94–105. doi: 10.1016/j.surg.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 57.Yu D. C., Chen W. B., Jiang C. P., Ding Y. T. Risk assessment in patients undergoing liver resection. Hepatobiliary & Pancreatic Diseases International. 2013;12(5):473–479. doi: 10.1016/s1499-3872(13)60075-2. [DOI] [PubMed] [Google Scholar]
- 58.Pieters L., De Bruyne T., Van Poel B., et al. In vivo wound healing activity of dragon’s blood (croton spp.), a traditional south American drug, and its constituents. Phytomedicine. 1995;2(1):17–22. doi: 10.1016/S0944-7113(11)80043-7. [DOI] [PubMed] [Google Scholar]
- 59.Gupta D., Bleakley B., Gupta R. K. Dragon’s blood: botany, chemistry and therapeutic uses. Journal of Ethnopharmacology. 2008;115(3):361–380. doi: 10.1016/j.jep.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 60.Pieters L., De Bruyne T., Claeys M., et al. Isolation of a dihydrobenzofuran lignan from south American dragon’s blood (croton spp.) as an inhibitor of cell proliferation. Journal of Natural Products. 1993;56(6):899–906. doi: 10.1021/np50096a013. [DOI] [PubMed] [Google Scholar]
- 61.Xin N., Li Y. J., Li X., et al. Dragon’s blood may have radioprotective effects in radiation-induced rat brain injury. Radiation Research. 2012;178(1):75–85. doi: 10.1667/rr2739.1. [DOI] [PubMed] [Google Scholar]
- 62.Fan J. Y., Yi T., Sze-To C. M., et al. A systematic review of the botanical, phytochemical and pharmacological profile of Dracaena cochinchinensis, a plant source of the ethnomedicine “dragon’s blood”. Molecules. 2014;19(7):10650–10669. doi: 10.3390/molecules190710650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li N., Ma Z., Li M., Xing Y., Hou Y. Natural potential therapeutic agents of neurodegenerative diseases from the traditional herbal medicine Chinese dragon’s blood. Journal of Ethnopharmacology. 2014;152(3):508–521. doi: 10.1016/j.jep.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 64.Li Y. S., Wang J. X., Jia M. M., et al. Dragon’s blood inhibits chronic inflammatory and neuropathic pain responses by blocking the synthesis and release of substance P in rats. Journal of Pharmacological Sciences. 2012;118(1):43–54. doi: 10.1254/jphs.11160fp. [DOI] [PubMed] [Google Scholar]
- 65.Rao V. S., Gurgel L. A., Lima-Junior R. C., et al. Dragon’s blood from Croton urucurana (baill.) attenuates visceral nociception in mice. Journal of Ethnopharmacology. 2007;113(2):357–360. doi: 10.1016/j.jep.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 66.Avci B., Bahadir A., Tuncel O. K., Bilgici B. Influence of alpha-tocopherol and alpha-lipoic acid on bisphenol-A-induced oxidative damage in liver and ovarian tissue of rats. Toxicology and Industrial Health. 2016;32 doi: 10.1177/0748233714563433. [DOI] [PubMed] [Google Scholar]
- 67.Gurgen S., Yucel A., Karakus A., et al. Usage of whey protein may cause liver damage via inflammatory and apoptotic responses. Human & Experimental Toxicology. 2015;34 doi: 10.1177/0960327114556787. [DOI] [PubMed] [Google Scholar]
- 68.Henrique Da Silva G., Barros P. P., Silva Goncalves G. M., Landi M. A. Hepatoprotective effect of Lycopodium clavatum 30CH on experimental model of paracetamol-induced liver damage in rats. Homeopathy. 2015;104(1):29–35. doi: 10.1016/j.homp.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Akuyam S. A., Uchenna O. K., Adamu A., et al. Liver function tests profile in cancer patients on cytotoxic chemotherapy: a preliminary report. Nigerian Postgraduate Medical Journal. 2011;18(1):34–33. [PubMed] [Google Scholar]
- 70.Li J. X., Wu H., Huang J. W., Zeng Y. The influence on liver function after transcatheter arterial chemoembolization combined with percutaneous radiofrequency ablation in patients with hepatocellular carcinoma. Journal of the Formosan Medical Association. 2012;111(9):510–515. doi: 10.1016/j.jfma.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z., Chen X. P., Liang Z. Q., et al. Therapeutic effect of autologous bone marrow stem cell transplantation in the treatment of severe liver damage. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30(12):2762–2764. [PubMed] [Google Scholar]
- 72.Zhu L., Yang Z. C., Li A., Cheng D. C. Protective effect of early enteral feeding on postburn impairment of liver function and its mechanism in rats. World Journal of Gastroenterology. 2000;6(1):79–83. doi: 10.3748/wjg.v6.i1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This section includes graphical abstract.
Data Availability Statement
The data used to support the study findings are available within the article.


