Abstract
The plant YABBY transcription factors are key regulators in the lamina development of lateral organs. Orchid is one of the largest families in angiosperm and known for their unique floral morphology, reproductive biology, and diversified lifestyles. However, nothing is known about the role of YABBY genes in orchids, although biologists have never lost their fascination with orchids. In this study, a total of 54 YABBY genes, including 15 genes in CRC/DL, eight in INO, 17 in YAB2, and 14 in FIL clade, were identified from the eight orchid species. A sequence analysis showed that all protein sequences encoded by these YABBY genes share the highly conserved C2C2 zinc-finger domain and YABBY domain (a helix-loop-helix motif). A gene structure analysis showed that the number of exons is highly conserved in the same clades. The genes in YAB2 clade have six exons, and genes in CRC/DL, INO, and FIL have six or seven exons. A phylogenetic analysis showed all 54 orchid YABBY genes could be classified into four major clades, including CRC/DL, INO, FIL, and YAB2. Many of orchid species maintain more than one member in CRC/DL, FIL, and YAB2 clades, implying functional differentiation among these genes, which is supported by sequence diversification and differential expression. An expression analysis of Phalaenopsis YABBY genes revealed that members in the CRC/DL clade have concentrated expressions in the early floral development stage and gynostemium, the fused male and female reproductive organs. The expression of PeINO is consistent with the biological role it played in ovule integument morphogenesis. Transcripts of members in the FIL clade could be obviously detected at the early developmental stage of the flowers. The expression of three genes, PeYAB2, PeYAB3, and PeYAB4, in the YAB2 clade could be revealed both in vegetative and reproductive tissues, and PeYAB4 was transcribed at a relatively higher level than that of PeYAB2 and PeYAB3. Together, this comprehensive analysis provides the basic information for understanding the function of the YABBY gene in Orchidaceae.
Keywords: Orchidaceae, Phalaenopsis equestris, YABBY gene, genome-wide, expression pattern
1. Introduction
The small plant-specific YABBY gene family, belonging in the subfamily of the zinc-finger superfamily, plays important roles in the development of lateral organs [1], establishment of adaxial–abaxial polarity [2], leaf margin establishment [3], and stress response [4]. The members of which encode a class of transcription factors containing two conserved domains, which are a N-terminal Cys2 Cys2 zinc-finger motif and C-terminal helix-loop-helix YABBY domain [5,6]. Five subfamilies, including CRABS CLAW (CRC), FILAMENTOUS FLOWER (FIL)/YABBY3 (YAB3), INNER NO OUTER (INO), YABBY2 (YAB2), and YABBY5 (YAB5), are classified among extant angiosperms [7,8]. The genome of Arabidopsis thaliana encodes for six YABBY members (FIL, CRC, INO, YAB2, YAB3, and YAB5), and Orzya sativa has eight. It has been indicated that, before the diversification of the angiosperms, four gene duplication events have occurred in the YABBY gene family [9], leading to genes with both innovated and redundant functions.
Among the members of YABBY gene family, functional and expression characterizations suggest that CRC plays as a carpel development regulator across angiosperms [10,11] and nectaries in the core eudicots [12]. Arabidopsis INO is expressed limitedly in the abaxial domain of the outer integument and is important for the regulation of the outer integument development [13]. Conservation of the abaxial expression of INO orthologs in eudicots [14], eumagnoliids [15], and several basal angiosperm plants [16,17] has also documented. Members in the CRC and INO subfamilies have specified roles in reproductive organ development. Vegetative YABBY genes show functional redundancy during leaf development in Arabidopsis [18], as well as in other core eudicots [5] and monocots [19]. However, various expression patterns could also be observed in vegetative YABBY genes among monocot species. For example, maize ZYB9 and ZYB14 (FIL/YAB3-like genes) are expressed adaxially and may act a regulator in lateral outgrowth [19]. OsYABBY1, a YAB2-like gene from rice, is expressed in precursor cells that give rise to abaxial sclerenchyma in the leaves, the mestome sheath in the large vascular bundle, and sclerenchymatous cells in the palea and lemma of the flower and is thus suggested to determine the differentiation of certain cell types [20].
Containing more than 900 genera and 27,000 species [21], the Orchidaceae represents about 10% of the angiosperm plants and the largest family in species number. The Orchidaceae comprise five subfamilies, including Apostasioideae, Cypripedioideae, Vanilloideae, Orchidoideae, and Epidendroideae. They show a wide diversity of 70% epiphytic, 25% terrestrial, and 5% on various supports of vegetative growth and have successfully colonized almost every habitat on Earth [22]. Especially, they are known for their unique floral morphology and reproductive biology. Their flowers possess several reliable floral morphological synapomorphies, including a gynostemium, fused by the style and at least part of the androecium, and a highly evolved petal, the labellum [23]. In addition, many of the orchids show their mature pollen grains packaged as pollinia, and their ovary/ovule development is precisely triggered by the deposition of pollinia into the stigmatic cavity of gynostemium [24]. Evidence from flowering plants has shown that the YABBY gene family plays important roles in vegetative and reproductive developments. However, nothing is known about the role of YABBY genes in orchids, although biologists have never lost their fascination with orchids. Recently, several whole genomes of orchid species, including Apostasia shenzhnica (Apostasioideae) [25], Phalaenopsis equestris (Epidendroideae) [26], Dendrobium catenatum (Epidendroideae) [27], and Gastrodia elata (Epidendroideae) [28], have been sequenced. The completed assembly of the whole genome of these orchid species provides an opportunity for the systematic study of the orchid YABBY family. The aim of this study was to identify and compare YABBY genes at a genome-wide scale in orchids. In addition, the expression patterns of eight putative Phalaenopsis YABBY genes were analyzed using qRT-PCR in various tissues/organs.
2. Materials and Methods
2.1. Plant Materials
The species Phalaenopsis aphrodite subsp. formosana were collected from Chain-Port Orchids Nursery (Pingtung, Taiwan). All Phalaenopsis plants were grown in a glasshouse at National Cheng Kung University (NCKU) under natural light (photosynthetic photon flux density, 90 μmol m−2 s−1) and controlled temperature from 25 °C to 30 °C.
2.2. Sample Collection and RNA Preparation
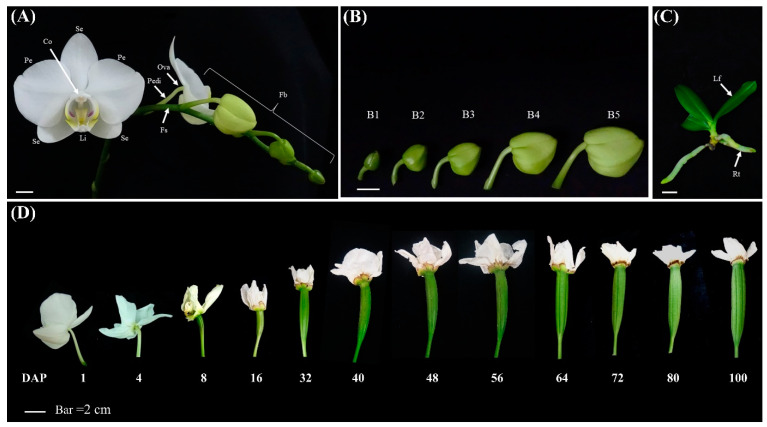
The flower buds were defined as B1 (0.5–1.0 cm), B2 (1.0–1.5 cm), B3 (1.5–2.0 cm), B4 (2.0–2.5 cm), and B5 (2.5–3.0 cm) stages (Figure 1A,B), accordingly [29]. The vegetative tissue (pedicel, floral stalk, leaf seedlings, and root seedlings) and floral organs (sepal, petal, labellum, and gynostemium) were collected (Figure 1A,C). Developing ovary and ovule from 0 to 100 days after pollination (DAP) were collected, as described by Chen et al. (Figure 1D) [30]. For total RNA preparation, all of Phalaenopsis orchid samples were quickly frozen in liquid nitrogen before extraction and stored at −80 °C for further use. Total RNA was extracted following the guanidium thiocyanate method described by O’Neill et al. [31].
Figure 1.
Morphology of Phalaenopsis aphrodite subsp. formosana. (A) Fully blooming flowers with floral buds and floral stalks in Phalaenopsis orchids. Scale bar = 1 cm. (B) Flower buds. Scale bar = 1 cm. (C) Leaf and root. Scale bar = 1 cm. (D) Developing ovary. Scale bar = 2 cm. Se, sepals; Pe, petals; Li, lip; Co, column (gynostemium); Pedi, pedicel; Fs, floral stalk; B1–B5, stage 1 to stage 5 floral buds; Lf, leaf; Rt, root; Ova, ovary; and DAP, days after pollination.
2.3. Identification of YABBY Genes in Orchids
To identify YABBY genes in orchids, the Hidden Markov Model (HMM) profile of the Pfam YABBY domain (PF04690) was performed, respectively, against predicted proteomes of P. equestris, D. catenatum, G. elata, and A. shenzhenica [25,26,27,28]. For a more comprehensive collection of YABBY genes from other orchid species, several ongoing whole-genome sequencing data of orchid species, including Vanilla shenzhenica (Vanilloideae), V. pompona, Platanthera zijinensis (Orchidoideae), and P. guangdongensis were further searched. All identified orchid YABBY sequences were further confirmed by the blastp program in the NCBI database (https://www.ncbi.nlm.nih.gov/) for checking both zinc-finger and YABBY domains existing in each sequence.
2.4. Sequence Alignment
Multiple sequences alignment of full-length YABBY protein sequences of the orchid and other plants was calculated by the Align X program provided in the software Vector NTI package (Invitrogen, Carlsbad, CA, USA, Version 10). To investigate the conservation of the C2C2 zinc-finger domain and YABBY domain in orchid YABBY proteins and rice, the online tool WEBLOGO (https://weblogo.berkeley.edu/logo.cgi) [32] was adopted, and the multiple sequence alignment results were used as the input file.
2.5. Gene Structure Analysis
The exon positions and sizes of identified orchid YABBY genes were acquired from the gff3 files (https://m.ensembl.org/info/website/upload/gff3.html). The identified YABBY genes were further compared to the assembled transcriptomic sequences. If the identified YABBY gene-coding sequence was inconsistent with the correspondent mRNA sequence, the correspondent mRNA sequence was mapped to the assembled genomic sequence, and the gene structure was edited manually. All of the exon numbers and lengths are listed in Table 1.
Table 1.
The gene structures of the YABBY genes in the exon region.
| Clades | Species | Gene Name | Exon 1 | Exon 2 | Exon 3 | Exon 4 | Exon 5 | Exon 6 | Exon 7 |
|---|---|---|---|---|---|---|---|---|---|
| CRC/DL | Apostasia shenzhenica | AshDL | 69 a | 129 | 94 | 49 | 76 | 93 | 93 |
| Dendrobium catenatum | DcaDL1 | 69 | 129 | 94 | 49 | 76 | 87 | 66 | |
| Gastrodia elata | GelDL1 | 69 | 129 | 94 | 49 | 76 | 87 | 66 | |
| Phalaenopsis equestris | PeDL1 | 69 | 129 | 94 | 49 | 76 | 87 | 66 | |
| Platanthera guangdongensis | PgDL1 | 69 | 129 | 97 | 49 | 76 | 87 | 66 | |
| Platanthera zijinensis | PzDL1 | 69 | 129 | 97 | 49 | 76 | 87 | 66 | |
| Vanilla planifolia | VpoDL1 | 69 | 129 | 94 | 49 | 76 | 87 | 66 | |
| Vanilla shenzhenica | VshDL1 | 69 | 129 | 94 | 49 | 76 | 87 | 66 | |
| Dendrobium catenatum | DcaDL2 | 69 | 132 | 103 | 49 | 76 | 84 | 72 | |
| Dendrobium catenatum | DcaDL3 | 69 | 120 | 82 | 49 | 76 | 102 | 66 | |
| Phalaenopsis equestris | PeDL2 | 69 | 132 | 103 | 49 | 76 | 87 | 75 | |
| Platanthera guangdongensis | PgDL2 | 72 | 141 | 106 | 49 | 76 | 84 | 69 | |
| Platanthera zijinensis | PzDL2 | 72 | 141 | 106 | 49 | 76 | 84 | 69 | |
| Vanilla planifolia | VpoDL2 | 69 | 114 | 82 | 49 | 76 | 60 | ||
| Vanilla shenzhenica | VshDL2 | 69 | 114 | 82 | 49 | 76 | 198 | ||
| FIL | Apostasia shenzhenica | AshFIL.1 | 102 | 168 | 124 | 49 | 76 | 78 | 78 |
| Apostasia shenzhenica | AshFIL.2 | 108 | 138 | 118 | 49 | 76 | 129 | ||
| Dendrobium catenatum | DcaFIL.1 | 105 | 159 | 130 | 49 | 76 | 75 | 75 | |
| Dendrobium catenatum | DcaFIL.2 | 102 | 159 | 127 | 49 | 76 | 75 | 75 | |
| Gastrodia elata | GelFIL.1 | 102 | 153 | 139 | 49 | 76 | 78 | 81 | |
| Gastrodia elata | GelFIL.2 | 99 | 159 | 112 | 49 | 76 | 75 | 78 | |
| Phalaenopsis equestris | PeFIL | 105 | 159 | 127 | 49 | 76 | 75 | 78 | |
| Phalaenopsis equestris | PeYAB1 | 102 | 159 | 127 | 49 | 76 | 75 | 75 | |
| Platanthera guangdongensis | PgFIL.1 | 105 | 159 | 139 | 49 | 76 | 75 | 75 | |
| Platanthera zijinensis | PzFIL.1 | 105 | 159 | 139 | 49 | 76 | 75 | 75 | |
| Vanilla planifolia | VpoFIL.1 | 105 | 159 | 130 | 49 | 76 | 75 | 84 | |
| Vanilla planifolia | VpoFIL.2 | 111 | 159 | 133 | 49 | 76 | 75 | 81 | |
| Vanilla shenzhenica | VshFIL.1 | 105 | 159 | 130 | 49 | 76 | 75 | 84 | |
| Vanilla shenzhenica | VshFIL.2 | 111 | 159 | 133 | 49 | 76 | 75 | 81 | |
| INO | Apostasia shenzhenica | AshINO | 90 | 81 | 115 | 49 | 76 | 99 | 75 |
| Dendrobium catenatum | DcaINO | 84 | 81 | 127 | 49 | 76 | 99 | 87 | |
| Gastrodia elata | GelINO | 87 | 81 | 136 | 49 | 76 | 105 | ||
| Phalaenopsis equestris | PeINO | 84 | 81 | 130 | 49 | 76 | 99 | 66 | |
| Platanthera guangdongensis | PgINO | 84 | 81 | 127 | 49 | 76 | 156 | ||
| Platanthera zijinensis | PzINO | 84 | 81 | 127 | 49 | 76 | 126 | ||
| Vanilla planifolia | VpoINO | 87 | 81 | 133 | 49 | 76 | 90 | 96 | |
| Vanilla shenzhenica | VshINO | 87 | 81 | 133 | 49 | 76 | 90 | 96 | |
| YAB2 | Apostasia shenzhenica | AshYAB2 | 69 | 123 | 121 | 49 | 76 | 111 | |
| Dendrobium catenatum | DcaYAB2 | 69 | 120 | 106 | 49 | 76 | 114 | ||
| Gastrodia elata | GelYAB2 | 66 | 123 | 103 | 49 | 76 | 117 | ||
| Phalaenopsis equestris | PeYAB2 | 69 | 126 | 118 | 49 | 76 | 114 | ||
| Phalaenopsis equestris | PeYAB4 | 69 | 120 | 118 | 49 | 76 | 69 | ||
| Platanthera guangdongensis | PgYAB2 | 84 | 117 | 118 | 49 | 76 | 36 | ||
| Platanthera zijinensis | PzYAM2 | 105 | 117 | 118 | 49 | 76 | 36 | ||
| Vanilla planifolia | VpoYAB2 | 69 | 120 | 85 | 49 | 76 | 114 | ||
| Vanilla shenzhenica | VshYAB2 | 69 | 120 | 85 | 49 | 76 | 114 | ||
| Apostasia shenzhenica | AshYAB3 | 78 | 120 | 88 | 49 | 76 | 111 | ||
| Dendrobium catenatum | DcaYAB3 | 78 | 120 | 124 | 49 | 76 | 48 | ||
| Phalaenopsis equestris | PeYAB3 | 78 | 120 | 124 | 49 | 76 | 81 | ||
| Platanthera guangdongensis | PgYAB3 | 78 | 123 | 133 | 49 | 76 | 108 | ||
| Platanthera zijinensis | PzYAM3.1 | 78 | 123 | 133 | 49 | 76 | 108 | ||
| Platanthera zijinensis | PzYAM3.2 | 78 | 123 | 133 | 49 | 76 | 108 | ||
| Vanilla planifolia | VpoYAB3 | 78 | 120 | 127 | 49 | 76 | 117 | ||
| Vanilla shenzhenica | VshYAB3 | 78 | 120 | 127 | 49 | 76 | 105 |
a The number represents the length of the exons in base pairs.
2.6. Phylogenetic Analysis
Before the phylogenetic analysis, a multiple sequence alignment of full-length YABBY proteins was performed by using ClustalW (Hinxton, Cambridgeshire, UK) with the default parameters. The phylogenetic trees were built using the MEGA6 Neighbor-Joining method with the Dayhoff model and pairwise deletion option parameters [33]. A bootstrap analysis was performed using 1000 iterations. The eudicots and monocots of YABBY proteins were collected from the NCBI database. All of the protein accession numbers are listed in Supplementary Table S1.
2.7. Real-Time RT-PCR
After RNA extraction, total RNA was treated with RNase-free DNase (NEB, Hertfordshire, UK) following the manufacturer’s protocols to remove contaminating genomic DNA. Reverse transcription to cDNA was synthesized using the Superscript II kit (Invitrogen, Carlsbad, CA, USA). Real-time RT-PCR was performed on the ABI 7500, Applied Biosystems System using SYBR GREEN PCR Master Mix (Applied Biosystems, Warrington, UK). PCR was performed with the following reaction conditions: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. All the raw data were analyzed with the Sequencing Detection System v1.2.3 (Applied Biosystems). PeActin4 (PACT4, AY134752) was used for normalization [34]. Mean and standard error were calculated from three biological and technical replicates. Primers used for real-time RT-PCR were designed by using Primer Express 3.0 (Thermo Fisher Scientific, Foster City, CA, USA) and listed in Supplementary Table S2.
3. Results and Discussion
3.1. Identification and Sequence Analyses of YABBY Genes from Orchidaceae
To identify the YABBY genes from orchids, the YABBY domain conserved sequence (PF04690) generated from the Pfam protein family database was used to search the orchid predicted proteomes. Eight members of YABBY family were identified in the genome of P. equestris, eight in D. catenatum, six in A. shenzenica, and five in G. elata. For a more comprehensive collection of YABBY genes from other orchid species, several ongoing whole-genome sequencing data of orchid species, including V. shenzhenica, V. pompona, P. zijinensis, and P. guangdongensis, were further searched. In two Vanilla species, seven genes of YABBY family were identified in their respective genomes. In addition, seven and six YABBY genes could be identified from P. zijinensis and P. guangdongensis, respectively. In total, 54 predicted YABBY genes were obtained from Orchidaceae.
Multiple sequence alignments were performed to generate sequence logos of both C2C2 and YABBY domains in eight orchid species and rice (Figure 2). The results showed that both the C2C2 zinc-finger and YABBY domain are highly conserved in rice and orchid plants (Figure 2). Additionally, the YABBY domain is more conserved than the C2C2 zinc-finger domain (Figure 2). We also compared the amino acid sequences encoded in orchid YABBY genes with two Arabidopsis YABBY proteins (AtCRC and AtINO) (Figure S1). As shown in Figure S1, we found not only cysteine residues at the expected positions but, also, that valine, leucine, proline, valine, and glycine (indicated by the triangle) at the zinc-finger domain are completely conserved among Arabidopsis and eight orchid species. In addition, the distributions of the conserved YABBY domain with a helix-loop-helix motif were significantly conserved in rice and eight orchid species (Figure 2 and Figure S2–S5).
Figure 2.
Conserved domains of orchid and rice YABBY protein sequences. (A) Sequence logo revealing the N-terminal conserved zinc-finger domain. Conserved cysteine residues in the zinc-finger domain are indicated with black asterisks. (B) Sequence logo showing the C-terminal conserved YABBY domain.
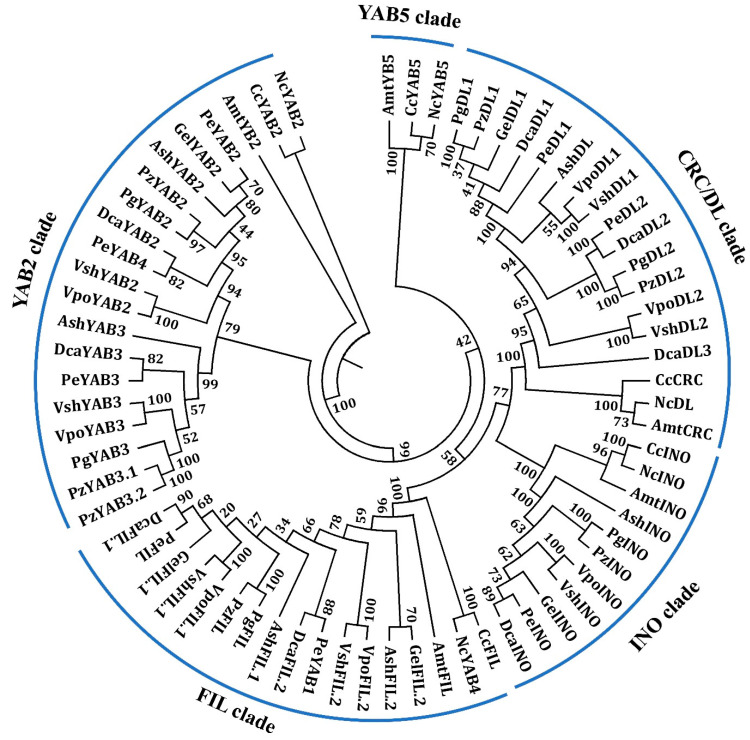
3.2. Phylogenetic Analysis of YABBY Genes Orchids among Orchid and Other Angiosperm Plants
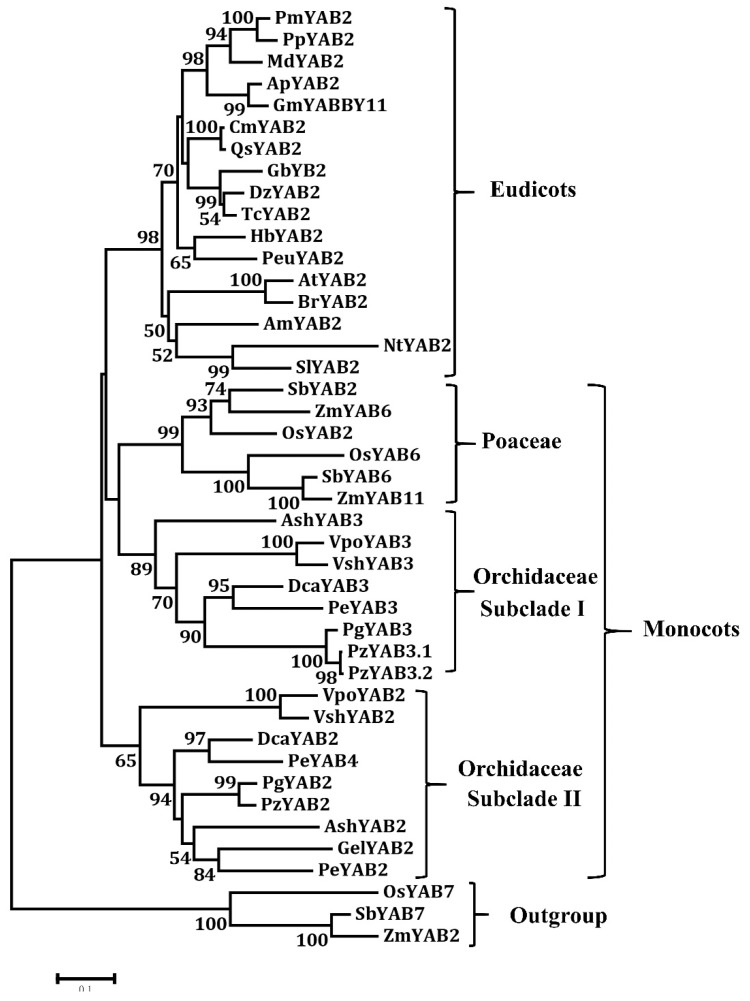
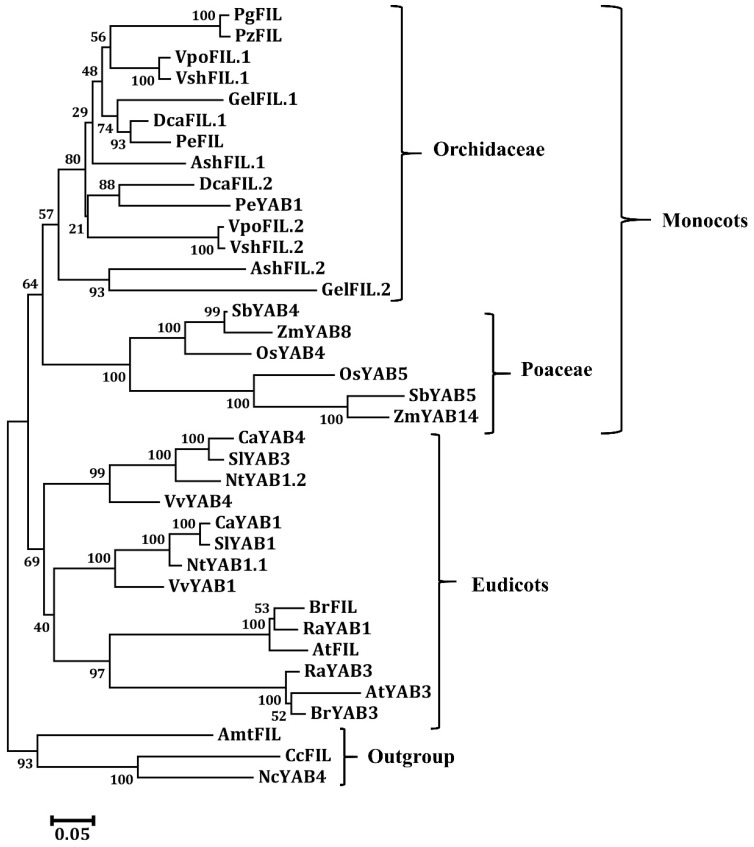
To analyze the evolutionary relationships among these orchid YABBY genes, we constructed a phylogenetic tree based on the full-length sequences of YABBY proteins together with YABBY proteins from Amborella trichopoda, Cabomba caroliniana, and Nymphaea colorata, the first diverging angiosperm lineages [17,35,36]. The phylogenetic tree indicated that the orchid YABBY proteins could be divided into four clades, named the CRC/DL, INO, FIL, and YAB2, with strong bootstrap value support (Figure 3). We did not find orchid gene-encoded proteins belonging to YAB5 clade (Figure 3). This result suggested that YAB5-related genes might be lost in Orchidaceae. Fifteen of orchid YABBY proteins (GelDL1, VpoDL2, VpoDL1, PeDL2, PeDL1, VshDL2, VshDL1, PgDL2, PgDL1, PzDL2, PzDL1, DcaDL3, DcaDL2, DcaDL1, and AshDL) were in the CRC/DL clade; eight proteins (GelINO, VpoINO, PeINO, VshINO, PgINO, PzINO, DcaINO, and AshINO) in the INO clade; fourteen (GelFIL.1, GelFIL.2, VpoFIL.1, VpoFIL.2, PeYAB1, PeFIL, VshFIL.1, VshFIL.2, PgFIL, PzFIL, DcaFIL.1, DcaFIL.2, AshFIL.1, and AshFIL.2) in the FIL clade; and seventeen (AshYAB2, AshYAB3, DcaYAB2, DcaYAB3, GelYAB2, PeYAB2, PeYAB3, PeYAB4, PgYAB2, PgYAB3, PzYAB2, PzYAM3.1, PzYAM3.2, VpoYAB2, VpoYAB3, VshYAB2, and VshYAB3) in the YAB2 clade (Figure 3).
Figure 3.
Phylogenetic tree of YABBY proteins from Amborella trichopoda, Cabomba caroliniana, Nymphaea colorata, and different types of orchids. The phylogenetic tree was constructed with the neighbor-joining (NJ) method in MEGA 6.0 software and was divided into five subgroups. A bootstrap analysis was conducted with 1000 replications. Gel, Gastrodia elata; Vpo, Vanilla planifolia; Pe, Phalaenopsis equestris; Vsh, Vanilla shenzhenica; Pg, Platanthera guangdongensis; Pz, Platanthera zijinensis; Dca, Dendrobium catenatum; Ash, Apostasia shenzhenica; Amt, Amborella trichopoda; Cc, Cabomba caroliniana; and Nc, Nymphaea colorata. The protein accession numbers for the related proteins are listed in Supplementary Table S1.
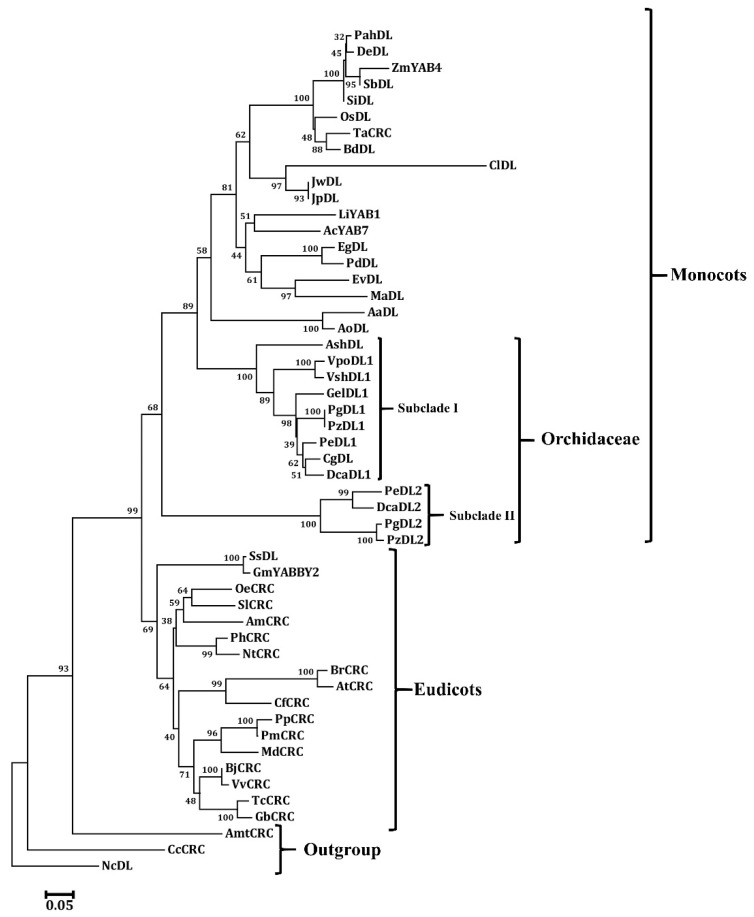
To further investigate the evolutionary relationships among orchid YABBY proteins, the YABBY protein sequences of eudicots and monocots were retrieved from the public database for further analysis. We found that each orchid species contains two members of the CRC/DL clade, except that Apostasia and Gastrodia, respectively, has only one and Dendrobium has three members (Table 1). Owing to that VpoDL2 and VshDL2 from Vanilla and DcaDL3 from Dendrobium have variable exon numbers and/or exon lengths (Table 1), these genes were excluded for further phylogenetic analyses. The phylogenetic analysis of CRC/DL showed that orchid CRC/DL could be divided into two subclades (Figure 4). Orchid subclade I is grouped with monocot CRC/DL members supported by high bootstrap values, and orchid subgroup II is positioned at the base of the monocots with moderate bootstrap values (Figure 4). This result suggests that a duplication event might occur at the most recent common ancestor of the monocots. Orchid CRC/DL-like proteins could be divided into subclade I and subclade II (Figure 4). In addition, most monocot and orchid plants, Apostasia and Gastrodin, might lose their members in subclade II (Figure 4). We also re-added VpoDL2 and VshDL2 for the phylogenetic analysis. The tree topology is similar to that shown in Figure 4, though the support of VpoDL2 and VshDL2 located in subclade II was not significant (Figure S6).
Figure 4.
Phylogenetic tree of the CRC/DL protein from the angiosperms. The phylogenetic tree was constructed with the neighbor-joining (NJ) method in MEGA 6.0 software (Phoenix, AZ, USA). A bootstrap analysis was conducted with 1000 replications. The protein accession numbers for the related proteins are listed in Supplementary Table S1.
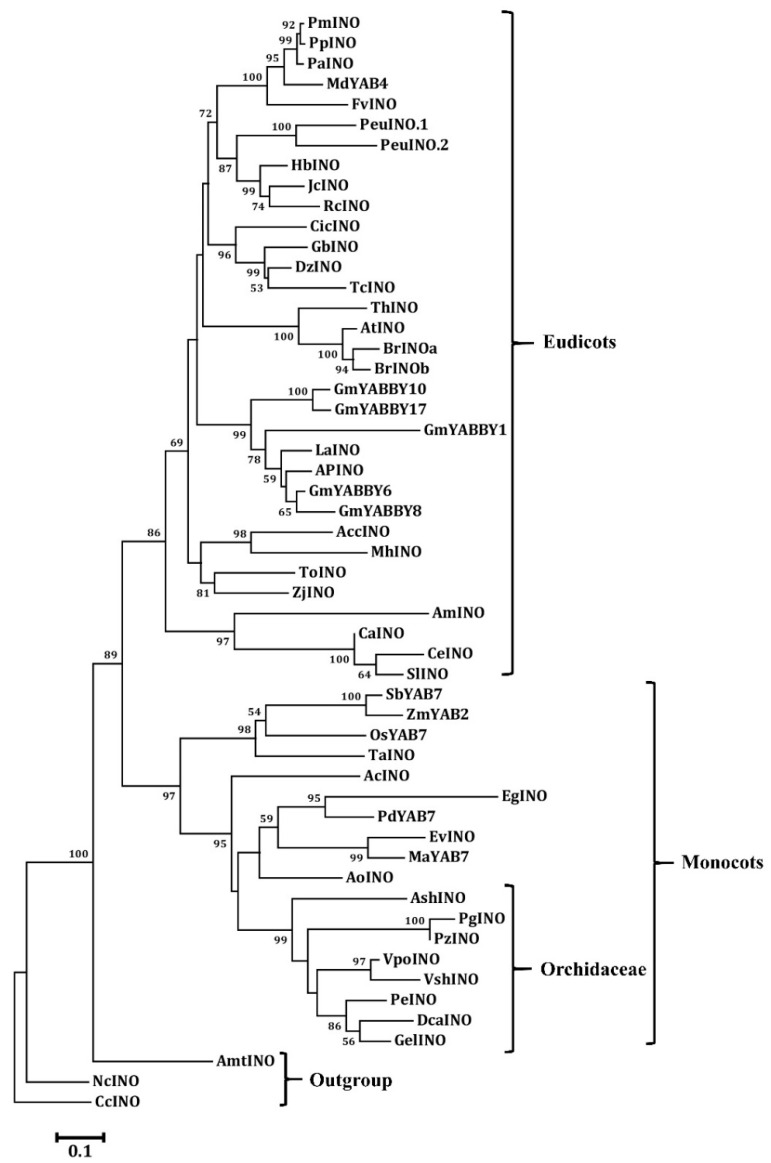
We found each orchid species contains one member of the INO clade as the other monocot and eudicot species has. All of the orchid INO members were grouped together, with very high bootstrap value support (Figure 5). It is possible that the group has not undergone expansion, and group members may perform biologically conserved functions.
Figure 5.
Phylogenetic tree of the INO protein from the angiosperms. The phylogenetic tree was constructed with the neighbor-joining (NJ) method in MEGA 6.0 software. A bootstrap analysis was conducted with 1000 replications. The protein accession numbers for the related proteins are listed in Supplementary Table S1.
The orchid genes in the YAB2 clade can be divided into two subclades (Figure 6). Members in the orchid subclade I are sister to the YAB2 members of Poaceae. Members in the Orchid subclade I and Poaceae are sister to those of eudicot plants (Figure 6). Relationships among the orchid subclade I, Poaceae, and eudicots are very robust in this analysis. These results suggest that these genes have similar functions involved in leaf development. Noticeably, neither eudicot nor monocot YAB2 members are grouped with members in the orchid subclade II (Figure 6), suggesting that YAB2 in orchid subclade II has particular functions in orchid plants. Interestingly, G. elata, a fully mycoheterotrophic plant without leaves [28], does not have members in subclade I (Figure 6). It is reasonable that G. elata lost YAB2 in subclade I, causing the defect of leaf development.
Figure 6.
Phylogenetic tree of the YAB2 protein from the angiosperms. The phylogenetic tree was constructed with the neighbor-joining (NJ) method in MEGA 6.0 software. A bootstrap analysis was conducted with 1000 replications. The protein accession numbers for the related proteins are listed in Supplementary Table S1.
It is noteworthy that FIL-like genes have undergone expansion in eudicots and monocots, and we found that each orchid species has two copies of FIL-like genes, except for the two species in Platanthera (Orchidoideae). The most parsimonious interpretation of this observation is that the two FIL-like genes were generated by the whole-genome duplication event that occurred at the most recent common ancestor of orchids [25], though the phylogenetic tree was not obviously indicated (Figure 7). The Platanthera species contain only one copy of FIL-like genes, suggesting that the other gene has been lost in the Platanthera genus or in the most recent common ancestor of Orchidoideae.
Figure 7.
Phylogenetic tree of the FIL protein from the angiosperms. The phylogenetic tree was constructed with the neighbor-joining (NJ) method in MEGA 6.0 software. A bootstrap analysis was conducted with 1000 replications. The protein accession numbers for the related proteins are listed in Supplementary Table S1.
3.3. Gene Structure Analysis of YABBY Genes in Orchids
To further investigate the phylogenetic relationship, we compared YABBY gene structures in eight orchid species. Previous reports described that the rice and Cabomba YABBY genes showed highly similar exon patterns [17,20]. In this study, we found that the orchids YABBY genes in the CRC/DL, INO, YAB2, and FIL clades have six to seven exons (Table 1). The fourth and fifth exons constituting YABBY domain have the exact same lengths (exon 4 and 49 bp and exon 5 and 76 bp, Table 1). In general, genes close to each other in the phylogenetic tree showed highly similar exon numbers and lengths. For example, most of genes in the CRC/DL, FIL, and INO clades have seven exons, and all genes in the YAB2 clade have six exons. The first exon length of most genes in the CRC/DL clade is 69 bp and is larger than 100 bp in the FIL clade members (Table 1). Many genes in the CRC/DL clade have six exon lengths 87 or 84 bp, and those in the FIL clade have 75 bp in six exons (Table 1).
3.4. Gene Expression Analysis in Phalaenopsis Orchid
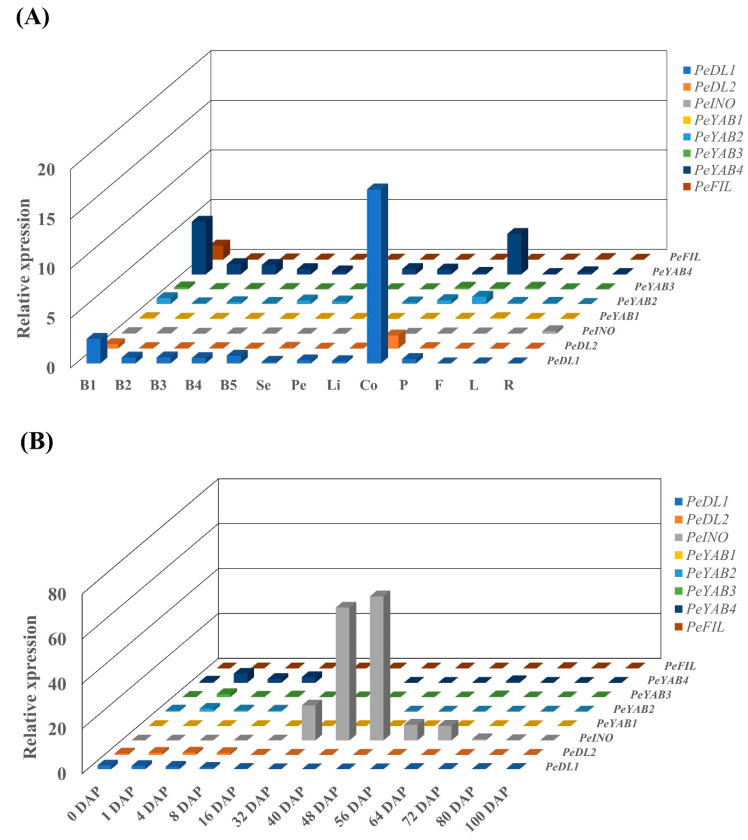
To explore and characterize the YABBY genes expression profiles in orchids, real-time RT-PCR was performed with use of the Phalaenopsis orchid as the model plant. The results showed that high expressions of PeDL1, PeDL2, and PeINO were detected at reproductive tissues. Both PeDL1 and PeDL2 transcripts could be significantly measured at stage 1 developing floral buds and gynostemium, and the expression of PeDL1 is much higher than that of PeDL2 in these two tissues/organs (Figure 8A and Figure S7). In addition, both of them could be expressed at early developmental stages of the ovule (0 to 8 days after pollination, DAP) (Figure 8B and Figure S8). Noticeably, a high expression of PeINO was specifically concentrated during 32 to 64 DAP of ovule development (Figure 8B and Figure S8). Phalaenopsis contains two genes, PeFIL and PeYAB1, in the FIL clade. Transcripts of PeFIL could be obviously detected at stage 1 developing floral buds. In general, PeFIL has higher expression levels than that of PeYAB1 in any organs/tissues. The expression of three genes, PeYAB2, PeYAB3, and PeYAB4, in the YAB2 clade revealed variable profiles. PeYAB4 was transcribed at a relatively higher level than that of PeYAB2 and PeYAB3, though all of them shared overlapping expressions at tissues/organs (Figure 8, Figures S7 and S8). PeYAB4 was majorly expressed at stage 1 developing floral buds, pedicel, and during 1 to 8 DAP of the developing ovule (Figure 8, Figures S7 and S8). These results suggest members in the YAB2 clade may have functions in both vegetative and reproductive tissues, and PeYAB4 might play a more important role than that of PeYAB2 and PeYAB3. To further define the expressions in Orchidaceae, the expression data for various tissues of Dendrobium and Apostasia, the FPKM values (flower bud, pollinium, gynostemium, seed, stem, and leaf) were downloaded from OrchidBase 4.0. (http://orchidbase.itps.ncku.edu.tw/est/home2012.aspx). The expression of orchid YABBY genes in reproductive tissues (flower bud, pollinium, and gynostemium) was higher than in vegetative tissues (stem, leaf, and root) (Figure S9). DcaYAB2, DcaYAB3, DcaDL1, DcaDL2, and DcaDL3 also showed higher expressions in gynostemium (Figure S9). A similar expression pattern was observed in PeYAB2, PeYAB3, PeDL1, and PeDL2 (Figure S9). A higher expression of orchid YABBY genes was observed in flower buds, with an exception of AshINO and DcaINO, which showed lower expressions in all the tissues (Figure S9).
Figure 8.
Expression patterns of PeYABBY-related genes in P. aphrodite subsp. formosana. (A) Expression patterns of PeYABBY-related genes in various organs. (B) Expression patterns of PeYABBY-related genes in various ovule developmental stages. B1–B5, stage 1 to stage 5 floral buds; Se, sepals; Pe, petals; Li, lip; Co, column (gynostemium); P, pedicel; F, floral stalk; L, leaf; R, root; and DAP, days after pollination.
4. Discussion
The YABBY transcription factors represent a small family in plants, whose members are characterized by two conserved domains: the C2C2 zinc-finger and YABBY with helix–loop–helix motif [37,38,39]. Five YABBY genes have been described in species A. trichopoda and N. colorata as representatives of two of the three orders of basal angiosperms (Amborellales and Nymphaeales, respectively), as well as in the species C. caroliniana, as a representative of the core eudicots. [17,35,36]. In the model eudicot plant Arabidopsis, six YABBY genes have been identified [13,37,40], and eight in the monocot representative—rice Oryza sativa [20]. The YABBY genes play important roles in regulating the development of lateral organs such as flowers, ovule, and leaves. In this study, five to eight YABBY genes were identified from each genome of eight orchid species. The number of YABBY genes that exist in each orchid genome could be comparable to that identified in the monocot and dicot species; an exception is the lack of YAB5-like genes in orchids.
The phylogenetic tree showed that the YABBY genes can be divided into five groups (CRC/DL, INO, FIL, YAB2, and YAB5), but orchid YABBY genes are only found in four clades and absent in the YAB5 clade, which exclusively comprises YABBY genes of basal angiosperms and eudicots. It has been reported that monocot species, including rice and pineapple, also do not contain YAB5-like genes [41]. De Almeida et al. [42] studied the comparative development of the androecial form across the Zingiberales and found one YABBY2 gene, which was also less homologous to YAB5. Based on this, they suggested that the duplication resulting in the separate YABBY2 and YABBY5 gene lineages most likely occurred after the divergence of monocots and eudicots [42]. This result suggested that the monocot plants might lose the genes in the YAB5 clade. The phylogenetic analysis also revealed that two CRC/DL paralogous genes were retained in many of the orchid species, and similar expression patterns of Phalaenopsis PeDL1 and PeDL2 were discovered that both genes majorly expressed in gynostemium. A gynostemium is a compound structure formed by stamens and pistils fused by filaments and style. Orchids have one, two, or three fertile abaxial stamens, which are invariably fused to the style [43]. The adaxial side of gynostemium is specialized to form a stigmatic cavity for providing an elaborate sticky space for accepting pollinium. Notably, only one DL/CRC-like gene was found in the genome of primitive A. shenzhenica, which had a gynostemium without stigmatic cavity formations. The retention of duplicated DL/CRC genes in the sister lineage to the Apostasioideae suggested an important role for DL/CRC genes in orchid gynostemium innovation.
Orchids are unusual among flowering plants in that, in many species, the ovule is not mature at the time of pollination. Pollination-regulated ovule initiation and development in Phalaenopsis has been characterized [24,30]. We found a high expression of PeINO is restricted during the process of ovule development at 32 to 64 DAP (Figure 8B). The period of PeINO transcribed nicely fits the process of ovule integument development [30], suggesting that PeINO plays a vital role involved in ovule integument morphogenesis. Consistent results were observed where the expression of INO-like genes is confined to the outer integument of the ovule in Arabidopsis and several basal angiosperms [13,16,17]. In this study, each of the orchid species examined only has one member in the INO clade. Together, the expression regulation and biological function of INO-like genes should be conserved in angiosperm plants.
Arabidopsis FIL, YAB2, and YAB3 are expressed in cotyledons, leaves, and flowers and redundantly control these lateral organ developments [38,44,45]. The FIL orthologs of TOB1-related YABBY genes play similar functions in flower developments in rice [46]. Our results indicated that most of the orchid species retain two FIL-like and two or three YAB2-like genes. The Phalaenopsis FIL-like gene PeFIL conferred an 18-fold expression level higher than that of PeYAB1 at the early developmental stage of floral buds (Figure S7), which suggests that the function of PeFIL and PeYAB1 is redundant, and PeFIL might be more important than PeYAB1 for floral development. The expression of YAB2-like genes could be detected both at reproductive and vegetative tissues, and PeYAB4 expression showed higher than that of the other two genes (Figure 8). It is worth noticing that G. elata only has one YAB2-like gene, GelYAB2, classified into the orchid YAB2 subclade II (Figure 6). This orchid species has highly reduced leaves, and systematics often refer to the plants as leafless [28]. The evidence provided here implied that orchid YAB2-like genes in subclade I might be related to the determination of lamina outgrowth of the leaves.
5. Conclusions
In this study, fifty-four YABBY genes were identified from eight orchid species and analyzed systematically in Orchidaceae. The orchid YABBY genes could be categorized into four major clades, including CRC/DL, INO, FIL, and YAB2. Many of the orchid species maintain more than one member in the CRC/DL, FIL, and YAB2 clades. Sequence and gene structure diversifications, as well as differential expressions of genes in these three clades, suggested functional differentiation among these genes. Our results represent a comprehensive genome-wide study of the YABBY gene family that opens the gate into further detailed studies on the gene function and evolution of YABBY genes in orchids.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/9/955/s1, Supplemental Figure S1: Conserved C2C2 zinc-finger and YABBY domains of YABBY proteins among A. thaliana and eight orchid species. Supplemental Figure S2: Multiple sequence alignment of the CRC/DL-related protein among eight orchid species. Supplemental Figure S3: Multiple sequence alignment of the INO-related protein among eight orchid species. Supplemental Figure S4: Multiple sequence alignment of YAB2-related protein among eight orchid species. Supplemental Figure S5: Multiple sequence alignment of the FIL-related protein among eight orchid species. Supplemental Figure S6: Phylogenetic tree of the CRC/DL protein from the angiosperms. Supplemental Figure S7: Quantitative real-time PCR analysis of the expressions of PeYABBY genes in P. aphrodite subsp. formosana at various organs. Supplemental Figure S8: Quantitative real-time PCR analysis of the expressions of PeYABBY genes in P. aphrodite subsp. formosana at various ovule developmental stages. Figure S9: Expression patterns of YABBY genes in different tissues of Apostasia, Dendrobium, and Phalaenopsis. Supplemental Table S1: YABBY-related genes in the phylogenetic tree and protein alignments. Supplementary Table S2: List of primers used in this work.
Author Contributions
Conceptualization, Y.-Y.C., Z.-J.L., and W.-C.T.; data curation, Y.-Y.C., Y.-Y.H., S.-B.C., D.Z., S.-R.L., Z.-J.L., and W.-C.T.; investigation, Y.-Y.C. and Y.-Y.H.; methodology, Y.-Y.C. and Y.-Y.H.; writing—original draft, Y.-Y.C.; and writing—review and editing, Z.-J.L. and W.-C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan (grants MOST 107-2313-B-006-002-MY3 and MOST 108-2622-B-006-006-CC1), Key Laboratory of National Forestry and Grassland Administration for Orchid Conservation and Utilization Construction Funds (nos. 115/118990050 and 115/KJG18016A), and National Key R&D Program of China (Grant No.2019YFD1000400).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Bowman J.L., Smyth D.R., Meyerowitz E.M. Genes directing flower development in Arabidopsis. Plant Cell. 1989;1:37–52. doi: 10.1105/tpc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumaran M.K., Bowman J.L., Sundaresan V. YABBY Polarity Genes Mediate the Repression of KNOX Homeobox Genes in Arabidopsis. Plant Cell. 2002;14:2761–2770. doi: 10.1105/tpc.004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finet C., Floyd S.K., Conway S.J., Zhong B., Scutt C.P., Bowman J.L., Zhong B. Evolution of the YABBY gene family in seed plants. Evol. Dev. 2016;18:116–126. doi: 10.1111/ede.12173. [DOI] [PubMed] [Google Scholar]
- 4.Zhao S.-P., Lu D., Yu T.-F., Ji Y.-J., Zheng W.-J., Zhang S.-X., Chai S.-C., Chen Z.-Y., Cui X.-Y. Genome-wide analysis of the YABBY family in soybean and functional identification of GmYABBY10 involvement in high salt and drought stresses. Plant Physiol. Biochem. 2017;119:132–146. doi: 10.1016/j.plaphy.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Golz J.F., Roccaro M., Kuzoff R., Hudson A. GRAMINIFOLIA promotes growth and polarity of Antirrhinum leaves. Development. 2004;131:3661–3670. doi: 10.1242/dev.01221. [DOI] [PubMed] [Google Scholar]
- 6.Kanaya E., Nakajima N., Okada K. Non-sequence-specific DNA Binding by the FILAMENTOUS FLOWER Protein from Arabidopsis thaliana Is Reduced by EDTA. J. Biol. Chem. 2002;277:11957–11964. doi: 10.1074/jbc.M108889200. [DOI] [PubMed] [Google Scholar]
- 7.Bowman J.L. The YABBY gene family and abaxial cell fate. Curr. Opin. Plant Biol. 2000;3:17–22. doi: 10.1016/S1369-5266(99)00035-7. [DOI] [PubMed] [Google Scholar]
- 8.Yamada T., Ito M., Kato M. YABBY2-Homologue Expression in Lateral Organs of Amborella trichopoda (Amborellaceae) Int. J. Plant Sci. 2004;165:917–924. doi: 10.1086/423793. [DOI] [Google Scholar]
- 9.Bartholmes C., Hidalgo O., Gleissberg S. Evolution of the YABBY gene family with emphasis on the basal eudicot Eschscholzia californica (Papaveraceae) Plant Biol. 2011;14:11–23. doi: 10.1111/j.1438-8677.2011.00486.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi T., Nagasawa N., Kawasaki S., Matsuoka M., Nagato Y., Hirano H.-Y. The YABBY gene drooping leaf regulates carpel specification and midrib development in Oryza sativa. Plant Cell. 2004;16:500–509. doi: 10.1105/tpc.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fourquin C., Vinauger-Douard M., Fogliani B., Dumas C., Scutt C.P. Evidence that CRABS CLAW and TOUSLED have conserved their roles in carpel development since the ancestor of the extant angiosperms. Proc. Natl. Acad. Sci. USA. 2005;102:4649–4654. doi: 10.1073/pnas.0409577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.-Y., Baum S.F., Oh S.-H., Jiang C.-Z., Chen J.-C., Bowman J.L. Recruitment of CRABS CLAW to promote nectary development within the eudicot clade. Development. 2005;132:5021–5032. doi: 10.1242/dev.02067. [DOI] [PubMed] [Google Scholar]
- 13.Villanueva J.M., Broadhvest J., Hauser B.A., Meister R.J., Schneitz K., Gasser C.S. INNER NO OUTER regulates abaxial- adaxial patterning in Arabidopsis ovules. Genes Dev. 1999;13:3160–3169. doi: 10.1101/gad.13.23.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAbee J.M., Kuzoff R.K., Gasser C.S. Mechanisms of Derived Unitegmy among Impatiens Species. Plant Cell. 2005;17:1674–1684. doi: 10.1105/tpc.104.029207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lora J., Hormaza J.I., Herrero M., Gasser C.S. Seedless fruits and the disruption of a conserved genetic pathway in angiosperm ovule development. Proc. Natl. Acad. Sci. USA. 2011;108:5461–5465. doi: 10.1073/pnas.1014514108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada T., Ito M., Kato M. Expression pattern of INNER NO OUTER homologue in Nymphaea (water lily family, Nymphaeaceae) Dev. Genes Evol. 2003;213:510–513. doi: 10.1007/s00427-003-0350-8. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T., Yokota S., Hirayama Y., Imaichi R., Kato M., Gasser C.S. Ancestral expression patterns and evolutionary diversification of YABBY genes in angiosperms. Plant J. 2011;67:26–36. doi: 10.1111/j.1365-313X.2011.04570.x. [DOI] [PubMed] [Google Scholar]
- 18.Eckardt N.A. YABBY Genes and the Development and Origin of Seed Plant Leaves. Plant Cell. 2010;22:2103. doi: 10.1105/tpc.110.220710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juarez M.T., Twigg R.W., Timmermans M.C.P. Specification of adaxial cell fate during maize leaf development. Development. 2004;131:4533–4544. doi: 10.1242/dev.01328. [DOI] [PubMed] [Google Scholar]
- 20.Toriba T., Harada K., Takamura A., Nakamura H., Ichikawa H., Suzaki T., Hirano H.-Y. Molecular characterization the YABBY gene family in Oryza sativa and expression analysis of OsYABBY1. Mol. Genet. Genom. 2007;277:457–468. doi: 10.1007/s00438-006-0202-0. [DOI] [PubMed] [Google Scholar]
- 21.Chase M.W., Cameron K.M., Freudenstein J.V., Pridgeon A.M., Salazar G., Berg C.V.D., Schuiteman A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015;177:151–174. doi: 10.1111/boj.12234. [DOI] [Google Scholar]
- 22.Atwood J.T. The size of the Orchidaceae and the systematic distribution of epiphytic orchids. Selbyana. 1986;9:171–186. [Google Scholar]
- 23.Tsai W.-C., Kuoh C.-S., Chuang M.-H., Chen W.-H., Chen H.-H. Four DEF-Like MADS Box Genes Displayed Distinct Floral Morphogenetic Roles in Phalaenopsis Orchid. Plant Cell Physiol. 2004;45:831–844. doi: 10.1093/pcp/pch095. [DOI] [PubMed] [Google Scholar]
- 24.Tsai W.-C., Hsiao Y.-Y., Pan Z.-J., Kuoh C.-S., Chen W.-H., Chen H.-H. The role of ethylene in orchid ovule development. Plant Sci. 2008;175:98–105. doi: 10.1016/j.plantsci.2008.02.011. [DOI] [Google Scholar]
- 25.Zhang G.-Q., Liu K.-W., Li Z., Lohaus R., Hsiao Y.-Y., Niu S.-C., Wang J.-Y., Lin Y.-C., Xu Q., Chen L.-J., et al. The Apostasia genome and the evolution of orchids. Nature. 2017;549:379–383. doi: 10.1038/nature23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai J., Liu X., Vanneste K., Proost S., Tsai W.-C., Liu K.-W., Chen L.-J., He Y., Xu Q., Bian C., et al. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet. 2014;47:65–72. doi: 10.1038/ng.3149. [DOI] [PubMed] [Google Scholar]
- 27.Zhang G.-Q., Xu Q., Bian C., Tsai W.-C., Yeh C.-M., Liu K.-W., Yoshida K., Zhang L.-S., Chang S.-B., Chen F., et al. The Dendrobium catenatum Lindl. genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2016;6:19029. doi: 10.1038/srep19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang C., Jin X., Liu J., Zhao X., Zhou J., Wang X., Wang D., Lai C., Xu W., Huang J., et al. The Gastrodia elata genome provides insights into plant adaptation to heterotrophy. Nat. Commun. 2018;9:1615. doi: 10.1038/s41467-018-03423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Z.-J., Chen Y.-Y., Du J.-S., Chen Y.-Y., Chung M.-C., Tsai W.-C., Wang C.-N., Chen H.-H. Flower development of Phalaenopsis orchid involves functionally divergent SEPALLATA-like genes. New Phytol. 2014;202:1024–1042. doi: 10.1111/nph.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.-Y., Lee P.-F., Hsiao Y.-Y., Wu W., Pan Z.-J., Liu Z.-J., Tsai W.-C. C- and D-class MADS-Box Genes from Phalaenopsis equestris (Orchidaceae) Display Functions in Gynostemium and Ovule Development. Plant Cell Physiol. 2012;53:1053–1067. doi: 10.1093/pcp/pcs048. [DOI] [PubMed] [Google Scholar]
- 31.O’Neill S.D., Nadeau J.A., Zhang X.S., Bui A.Q., Halevy A.H. Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell. 1993;5:419–432. doi: 10.1105/tpc.5.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crooks G.E., Hon G., Chandonia J.-M., Brenner S.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y.F., Chen Y.Y., Hsiao Y.Y., Shen C.Y., Hsu L.L., Yeh C.M., Mitsuda N., Ohme-Takagi M., Liu Z.J., Tsai W.C. Genome-wide identifcation and characterization of TCP genes involved in ovule development of Phalaenopsis equestris. J. Exp. Bot. 2016;67:5051–5066. doi: 10.1093/jxb/erw273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saarela J.M., Rai H.S., Doyle J.A., Endress P.K., Mathews S., Marchant A.D., Briggs B.G., Graham S.W. Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature. 2007;446:312–315. doi: 10.1038/nature05612. [DOI] [PubMed] [Google Scholar]
- 36.Angiosperm Phylogeny Group An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009;161:105–121. doi: 10.1111/j.1095-8339.2009.00996.x. [DOI] [Google Scholar]
- 37.Sawa S., Ito T., Shimura Y., Okada K. FILAMENTOUS FLOWER controls the formation and development of Arabidopsis inflorescences and floral meristems. Plant Cell. 1999;11:69–86. doi: 10.1105/tpc.11.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegfried K.R., Eshed Y., Baum S.F., Otsuga D., Drews G.N., Bowman J.L. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999;126:4117–4128. doi: 10.1242/dev.126.18.4117. [DOI] [PubMed] [Google Scholar]
- 39.Bowman J.L., Baum S.F., Eshed Y., Putterill J., Alvarez J. Current Topics in Developmental Biology. Volume 45. Elsevier BV; Amsterdam, The Netherlands: 1999. 4 Molecular Genetics of Gynoecium Development in Arabidopsis; pp. 155–205. [DOI] [PubMed] [Google Scholar]
- 40.Bowman J.L., Smyth D.R. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development. 1999;126:2387–2396. doi: 10.1242/dev.126.11.2387. [DOI] [PubMed] [Google Scholar]
- 41.Li Z., Li G., Cai M., Priyadarshani S., Aslam M., Zhou Q., Huang X., Wang X., Liu Y., Qin Y. Genome-Wide Analysis of the YABBY Transcription Factor Family in Pineapple and Functional Identification of AcYABBY4 Involvement in Salt Stress. Int. J. Mol. Sci. 2019;20:5863. doi: 10.3390/ijms20235863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Almeida A.M.R., Yockteng R., Schnable J.C., Alvarez-Buylla E.R., Freeling M., Specht C.D. Co-option of the polarity gene network shapes filament morphology in angiosperms. Sci. Rep. 2014;4:06194. doi: 10.1038/srep06194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudall P.J., Bateman R.M. Roles of synorganisation, zygomorphy and heterotopy in floral evolution: The gynostemium and labellum of orchids and other lilioid monocots. Biol. Rev. 2002;77:403–441. doi: 10.1017/S1464793102005936. [DOI] [PubMed] [Google Scholar]
- 44.Chen Q., Atkinson A., Otsuga D., Christensen T., Reynolds L., Drews G.N. The Arabidopsis FILAMENTOUS FLOWER gene is required for flower formation. Development. 1999;126:2715–2726. doi: 10.1242/dev.126.12.2715. [DOI] [PubMed] [Google Scholar]
- 45.Kumaran M.K., Ye D., Yang W.-C., Griffith M.E., Chaudhury A.M., Sundaresan V. Molecular cloning of ABNORMAL FLORAL ORGANS: A gene required for flower development in Arabidopsis. Sex. Plant Reprod. 1999;12:118–122. doi: 10.1007/s004970050180. [DOI] [Google Scholar]
- 46.Tanaka W., Toriba T., Hirano H. Three TOB1 -related YABBY genes are required to maintain proper function of the spikelet and branch meristems in rice. New Phytol. 2017;215:825–839. doi: 10.1111/nph.14617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.