Abstract
The gastrointestinal tract hosts the natural reservoir of microbiota since birth. The microbiota includes various bacteria that establish a progressively mutual relationship with the host. Of note, the composition of gut microbiota is rather individual-specific and, normally, depends on both the host genotype and environmental factors. The study of the bacterial profile in the gut demonstrates that dominant and minor phyla are present in the gastrointestinal tract with bacterial density gradually increasing in oro-aboral direction. The cross-talk between bacteria and host within the gut strongly contributes to the host metabolism, to structural and protective functions. Dysbiosis can develop following aging, diseases, inflammatory status, and antibiotic therapy. Growing evidences show a possible link between the microbiota and Familial Mediterranean Fever (FMF), through a shift of the relative abundance in microbial species. To which extent such perturbations of the microbiota are relevant in driving the phenotypic manifestations of FMF with respect to genetic background, remains to be further investigated.
Keywords: amyloidosis, colchicine, inflammasome, interleukin-1b, MEFV
1. Introduction
The gut develops as a natural ecosystem hosting a complex polymicrobial community, referred to as microbiota. The microbiota can undergo major changes during healthy status and diseases [1,2]. The resident symbiotic microorganisms have progressively adapted to several factors, i.e., local environment, the host immune responses, antibiotic therapies, and several other conditions [3,4,5]. In the human gut, there are thousands of different microbial species [6], possibly conditioning health and disease. Examples include inflammatory bowel disease [7], irritable bowel syndrome [8], periodontal disease [9], atherosclerosis [10], rheumatoid arthritis [11], diabetes mellitus [12], obesity [13], allergy [14], and colonic cancer [15].
The exact role of intestinal microbiota in other conditions characterized by recurrent genetically-driven auto-inflammatory diseases is still to be comprehensively determined. Familial Mediterranean Fever (FMF) is an example of monogenic autoinflammatory disease due to MEFV gene pathogenic variants that lead to a dysfunctional hyperactive state of the pyrin protein eliciting proinflammatory cytokine release and pyroptosis (cell death).
Studies focusing on human twins [16], murine quantitative trait loci [17], and genome-wide associations [18], suggest that the host genomic profile can shape the composition of gut microbiota. This step becomes mainly host genotype-dependent [19,20]. The knowledge of mechanisms linking the genome with the abundance and the composition of gut microbiota, however, deserves further study. A main interfering factor is the role played by gene-environment interactions [21]. This topic is of great interest, since gut microbiota are essential for human health and in maintaining a systemic homeostasis. In fact, several pathological conditions have been linked to variations in the amount and/or composition of the microbiota. Instead, the therapeutic manipulation of gut microbiota is a possibly relevant and innovative tool, in particular during diseases characterized by a chronic inflammatory status. Based on genetic (i.e., monogenic disease) and phenotypic characteristics, FMF patients can represent an interesting model to assess the role of genome and gene-environment interactions, in modifying the composition of gut microbiota. A comprehensive view of these dynamics could be useful in the management and prevention of acute attacks and complications in FMF patients.
Thus, it appears of great relevance to comprehensively review the major features of gut microbiota in the human gastrointestinal tract, highlighting the emerging relationship between variations in the relative abundance of microbial species and FMF.
2. Familial Mediterranean Fever
FMF is a rare monogenic autoinflammatory disease which belongs to the group of “periodic fever syndromes.” The estimated number of subjects with FMF in the world is 150,000, making FMF the most common autoinflammatory disease worldwide [22]. FMF occurs more often in individuals of Jewish, Armenian, Turkish, North African, and Arab descent, and living in the Mediterranean basin. Cases of FMF also occur in different populations such as Greeks, Italian, and even Japanese [23,24]. Many patients with FMF describe their first attack in early childhood, i.e., before the ages of 10 (65%) and 20 years (90%). Depending on genetic penetrance and phenotypic characteristics, however, the initial attack can occur in subjects aged older than 50 years [23] with extremely delayed onset represented by one case diagnosed at the age of 86 [25].
FMF is mainly diagnosed for its typical clinical presentation, combined with ethnic origin, family history, and genetic assessment [22,26,27,28,29]. Genetic testing can help but it is challenging, since 377 different MEFV variants have been reported thus far (Infevers: an online database for autoinflammatory mutations. Available at https://infevers.umai-montpellier.fr/ accessed 26.08.2020) [30,31,32,33]. Whereas some MEFV variants appear as clearly pathogenic, many variants are common in the general population and some others have still an unknown significance in causing the disease [34]. In line with such difficulties in classification of MEFV variants, recent efforts have tried to develop novel classification tools based on machine learning, which led to improvement in MEFV variants classification [30,35,36]. On chromosome 16 (16p13.3), the MEFV gene (made of 10 exons) encodes for a 781-amino-acid ~95kDa protein named pyrin (also referred to as “marenostrin,” TRIM20), a pattern recognition receptor (PRRs) [37,38,39,40,41]. Pyrin is part of the complex molecular platforms involved in the response of the innate immune system and related cells, originally designed as first-line, fast response to components of pathogenic bacteria. Cells involved in the innate immune system response are monocytes, macrophages, dendritic cells, and neutrophils (myeloid lineage), which express a variety of PRRs. PRRs, in turn, detect pathogen-associated molecular patterns (PAMPs). The family of Toll-like receptors (TLRs) are membrane-bound PRRs sensing PAMPs in the extracellular milieu and in different types of intracellular endosomes [42]. TLR activation is associated to the expression of proinflammatory factors, which induce cytokine release, i.e., NF-κB. Cytosolic pathogen recognition sensors are the family of nucleotide-binding domain leucine-rich repeat (NLR) proteins, namely NLRP1, NLRP3, NLRP7, and NLRC4, the protein absent in melanoma 2 (AIM2), and pyrin [43]. These cytosolic sensors detect pathogens and endogenous danger-associated molecular patterns (DAMPs) which trigger the intracellular formation of multiprotein complexes, i.e., inflammasomes [44].
A common feature of inflammasomes is their capability to mediate the activation of caspase-1 and, subsequently, to promote the release of the proinflammatory cytokines IL-1β and IL-18. Another step is represented by the activation of inflammatory cell death via pyroptosis, with cellular swelling and lysis, at variance with apoptosis. Pyroptosis requires the caspase-1-mediated cleavage of gasdermin D (GSDMD), translocation of the fragment N-terminal pore-forming domain to the cellular membrane, and release of pro-inflammatory cytokines [45,46]. Notably, the direct binding of lipopolysaccharide (LPS) to caspase-4 and 5 in human cells (caspase-11 in mouse cells) will also result in caspase oligomerization, cleavage of GSDMD, and pyroptosis. In general, pyroptosis appears to amplify the defending immune responses following infections [45].
In a normal situation, pyrin senses the inactivation of the small RhoA guanosine triphosphatase (RhoA GTPase), triggered by bacterial toxin, and this step leads to the activation of numerous signal transduction pathways resulting in the formation of a multi-protein complex (inflammasome). Pyrin binds to several effector proteins, such as the serine/threonine-protein kinases PKN1 and PKN2 and actin-binding proteins. RhoA activation is associated to PKN-mediated phosphorylation-dependent pyrin inhibition. The inflammasome also contains the bridging molecule ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and the protease caspase-1 [38,44,47,48] (Figure 1).
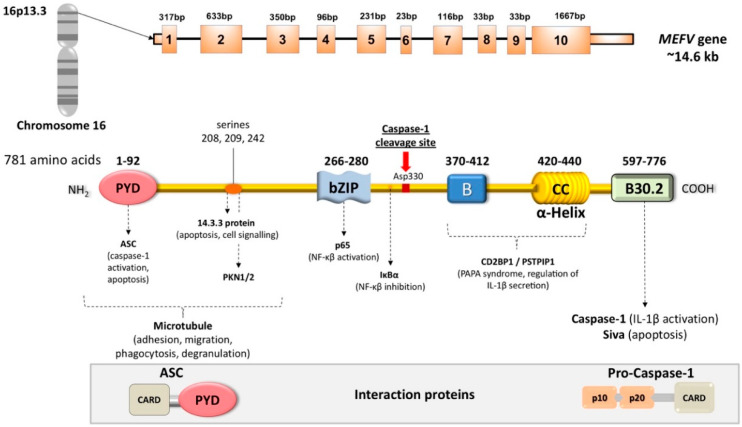
Figure 1.
Schematic structure of MEFV gene and encoded pyrin (marenostrin) protein. The MEFV gene encodes for the pyrin protein (781 amino acids). The most common mutations in Familial Mediterranean Fever (FMF) are in exon 10 encoding the B30.2 domain. The most important interaction partners appear below the pyrin structure. ASC and Pro-Caspase 1 are also drawn. Pyrin structure includes five different domains, each one responsible for protein-protein interaction, and each domain has a role in the regulation of innate response. From left to right, PYRIN (PYD) domain (residues 1–92) interacts with ASC (apoptosis-associated speck-like protein containing a CARD—caspase-recruitment domain). bZIP transcription factor basic domain (residues 266–280) interacts with the p65 subunit (transcription factor p65) of NF-κB, and IκB-α. The B-box zinc finger domain (residues 375–412) and α-helical (CC, coiled-coil) domain (residues 420–440) likely influence the oligomerization of pyrin, and interact with the PAPA protein (also named PSTPIP1, proline serine threonine phosphatase-interacting protein, also known as CD2BP1 involved in the organization of the cytoskeleton) and the regulation of IL-1β secretion. The B30.2 domain (PRYSPRY) (residues 597–776) is the most important, and interacts with caspase-1 and the proapoptotic protein Siva. Further pyrin interactions include binding to microtubules (starting from the N-terminal to bZIP), interaction with 14.3.3 (14-3-3 protein), and with the PKN1/2 (serine-threonine kinases PKN1 and PKN2) at the three serine residues 208, 209, 242 between PYD and bZIP. The position of Asp330 between bZIP and the B-box indicates the caspase-1 cleavage site. Mutations in the B30.2 domain tend to be transmitted in an autosomal-recessive fashion. Mutations in exons 2, 3 and 5 generally exhibit autosomal-dominant pattern of inheritance [24,34,35,36,41,43,48,49,50,51,52,53,54,55,56,57,58].
The activated inflammasome will then govern the steps of pyroptosis, i.e., a pro-inflammatory cell death mode which relies on innate immune response by myeloid cell lineage with release of pro-inflammatory cytokines IL-β1 and IL-18 [45,47,59]. Mutations in the MEFV gene are associated with impaired function of pyrin, which becomes insensitive to the microtubule control of this process [41]. Consequently, raised serum levels of IL-1β and increased inflammation will occur (Figure 2A,B).
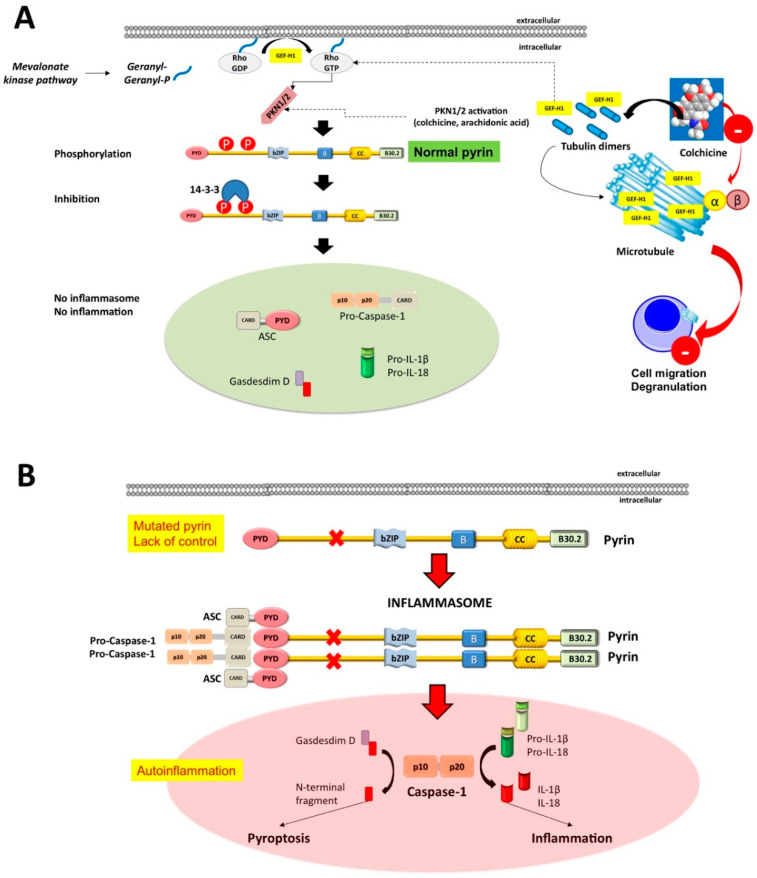
Figure 2.
Mechanisms underlying the assembly of the pyrin inflammasome. (A) With a normally functioning pyrin (i.e., when pyrin is non-mutated), the mevalonate kinase pathway provides geranyl-geranyl phosphate and, together with release of GEF-H1 (increased by colchicine acting as the inhibitor of the microtubule polymerization), activates RhoA. PKN1 and PKN2 are effector kinases of RhoA mediating the phosphorylation of pyrin and binding to the inhibitory proteins 14-3-3. Inhibition of pyrin can increase with agents activating PKN1/2 or following the release of GEF-H1 (i.e., colchicine). (B) If the pyrin phosphorylation decreases, (i.e., in FMF patients lacking the control by pyrin/marenostrin caused by pathogenic MEFV variants), with low GEF-H1 or defective function of the MVK-pathway, the activation of PKN1/2 also decreases. This step results in pyrin inflammasome activation and release of mature IL-1β and IL-18. The plasma membrane pore-forming N-terminal fragment of gasdermin D facilitates IL-1β and IL-18 release. Appropriate stimuli can also lead to the assembly of the inflammasome. The first step is the PYD-PYD homotypic interaction of ASC resulting in oligomerization into ASC specks. Pro-caspase-1 is recruited because of CARD-CARD interaction with ASC. This step anticipates the auto-cleavage of pro-caspase-1 into active caspase-1 tetramers (p10/p20) governing the transformation of pro-IL-1/18 into mature IL-1/18. The pyroptosis mediated by Gasdermin D also contributes to cytoplasmic enrichment with IL-1/18, and further reinforces the inflammatory pathway. Colchicine inhibits the polymerization of intracellular β-tubulin by forming colchicine-tubulin complexes via contact of A and C rings with the C domain of the tubulin β-subunit. These supramolecular interactions block the dockage of tubulin into the (+) ends of microtubules (cytoskeleton), thus preventing inflammasome activation in neutrophils and monocytes. The colchicine-dependent inhibition of tubulin also efficiently affects the migration and degranulation of white blood cells [43,50].
On the clinical ground, FMF consists of periodic recurrent febrile attacks, serositis at multiple body sites manifesting with pain (abdomen, joints, chest), erysipelas-like dermatitis (meaning limited erythematous skin rash), myalgia, arthralgia, and acute pericarditis. Depending on MEFV variants involved, symptoms may appear during the pediatric age, with episodes of pain and fever usually resolving within 2–3 days (Figure 3).
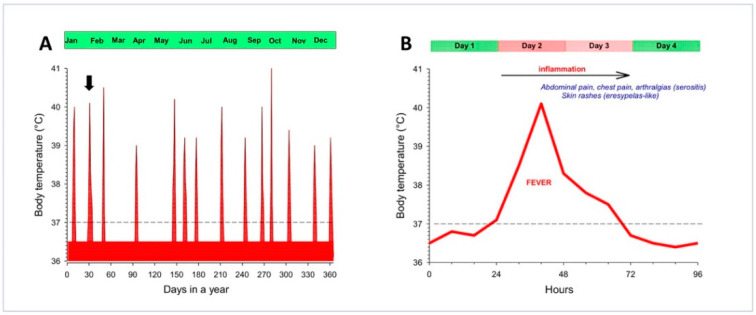
Figure 3.
Schematic appearance of time-dependent changes of body temperature in Familial Mediterranean Fever (FMF) before starting the treatment with colchicine. The profile refers to a typical case belonging to a cluster of families identified in the region of Apulia, Italy [24]. (A) Frequency of febrile attacks in a year. The dotted horizontal line is placed at 37 °C (cut-off value). In between attacks, the temperature has been conventionally set at 36.5 °C. This patient reported a total of 14 attacks in a year. The black arrow indicates the febrile attack described in panel B. (B) The single febrile attack lasts about 48 h and is associated with a major auto-inflammatory status and symptoms. Adapted from Portincasa et al. Familial Mediterranean fever: a fascinating model of inherited auto inflammatory disorder. Eur J Clin Invest 43, 1314-1327 (2013) with permission from John Wiley & Sons Ltd. [24].
Acute attacks of FMF produce elevation of serum indices of inflammation, including a raised count of white blood cells (especially neutrophils), increased levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fibrinogen, and serum amyloid A (SAA) protein. Depending on MEFV variants and intensity of attacks, long-term complications may include secondary (AA) amyloidosis leading to asymptomatic proteinuria, nephrotic syndrome and end-stage kidney disease, small bowel obstruction due to recurrent attacks of peritonitis and adhesions, and even infertility, especially in female patients due to fallopian tube obstruction [24,34,60]. The M694V variant, located in exon 10 of the MEFV gene, causes the most severe disease, in patients either homozygous or compound heterozygous for M694V [61,62]. The same is true for M694I and M680I variants, while R761H (or E148Q/R761H) has lesser penetrance and causes milder symptoms [24,35]. The FMF therapy focuses on preventing acute attacks, and minimizing subclinical inflammation in between attacks. In the most clinically-evident cases, the appropriate therapy will also prevent development and progression of amyloidosis. All guidelines suggest colchicine as the initial treatment of FMF, to be started as soon as a diagnosis of FMF is established and continued indefinitely. In addition, colchicine is effective as a prophylactic treatment for FMF attacks, and for this purpose, all patients should start with colchicine, regardless of the frequency and intensity of attacks [24,28,63,64] (Figure 2A and Figure 4). A subgroup of patients defined as resistant or intolerant to colchicine needs treatment with alternative biological agents targeting IL-1 inhibition. The human immunoglobulin (IgG) antibody canakinumab targets IL-1β and is effective in FMF [49,51,65,66,67,68] (Figure 4 and Figure 5).
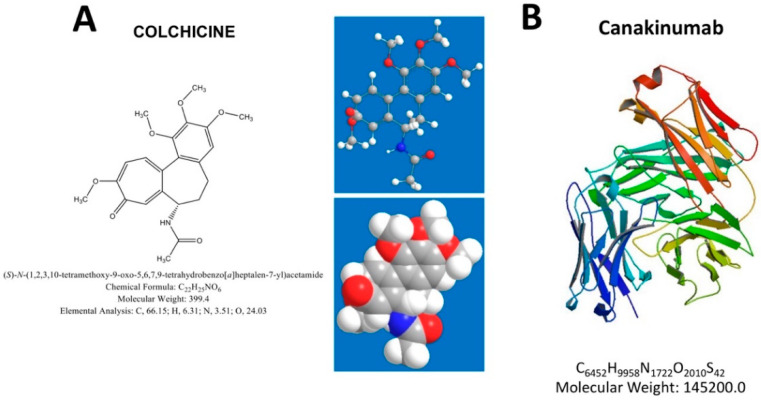
Figure 4.
Therapeutic agents effective in Familial Mediterranean Fever (FMF). (A) Colchicine: chemical structure, IUPAC names, chemical formula, molecular weight and three-dimensional (3D) structures. (B) Canakinumab: 3D structure, chemical formula and molecular weight.
Figure 5.
Mechanism of action of biologic agent canakinumab in FMF. Canakinumab is a fully human selective anti-IL-1β monoclonal antibody and binds human IL-1β. Subsequent binding of IL-1β to the IL-1R is inhibited with prevention of intracellular signal transduction and further proinflammatory events. Abbreviations: IL, interleukin; IL-1R-I, interleukin-1 receptor, type1; IL-1RAcP, IL-1 receptor accessory protein. Adapted from [49,50,51].
3. Gut Microbiota
Gut microbiota consists in a huge collection of microbes in the human gut. The human microbiota varies according to birth mode, age [69], diet and lifestyle, and is essential in preserving the integrity of the mucosal barrier [70]. Furthermore, gut microbiota protects the intestinal epithelial cells and contributes to the immune system [71] producing antimicrobial peptides and immunoglobulins [72,73,74].
3.1. Development of the Gastrointestinal Microbiota
The interplay between microbiota and the gut begins very early in human life.
The in utero environment is not sterile, with a maternal-fetal transmission of microbiota occurring early, during pregnancy [75,76].
The genome of the infant may affect gut microbial colonization but, on the other hand, the composition of gut microbiota depends on a number of maternal factors acting before birth (dietary habits, gestational age, smoking, obesity, antibiotic therapy during pregnancy) [5,77]. At birth, the newborn acquires further bacteria from the mother and the external environment [78,79]. Facultative aerobes anticipate the increase in strict anaerobes [80]. Bifidobacteria are more prevalent during breast- or formula feeding [81,82,83]. Feeding with solid food in the infant is followed by changes in the composition of gut microbiota, with features resembling those of the adult [84]. According to this scenario, the gut microbiota stabilizes after the first 12 months of life.
3.2. Characterization of the Gut Microbiota
Most of the gut bacterial species cannot be cultivated [81,85,86,87,88], while culture-independent molecular approaches provide information on composition and diversity of the gut microbiota [89,90,91,92,93]. Techniques include sequencing and phylogenetic micro-arrays [94,95], metagenomic assembly, annotation and comparison [3], genome-resolved metagenomics. Metaproteomics, metabolomics and metatranscriptomics require methodologies that are more sophisticated. The Human Microbiome Project (HMP) employed the 16S and metagenomic profiling to investigate the microbial communities from multiple body sites of healthy individuals, the relationships between disease occurrence and microbiota, and to identify a standardized dataset [96,97]. The human intestinal microbiota contains about 3.3 million microbial genes, and this amount is about 150 times greater than the human genome [98].
3.3. Main Features of Bacterial Communities in the Human Intestine
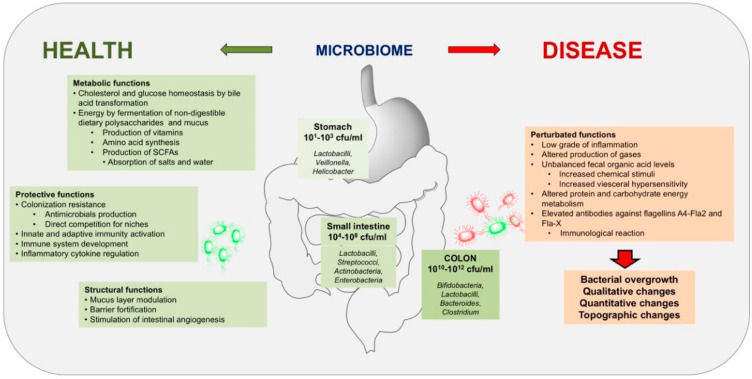
During health, the dominant phyla are Bacteroides and Firmicutes [99,100] with a minor presence of Spirochaetes, Proteobacteria, Verrucomicrobia, Actinobacteria, Lentisphaerae and Fusobacteria [19,94]. Microbial density increases in oro-aboral direction, raising from 104–108 cells in the jejunum-ileum to 1010–1012 cells at the level of the colon and in feces [3,101]. Different microbiota ecosystem changes depend on pH in the intestinal lumen, redox potential, type and amount of nutrients, gastrointestinal motility and secretions [3] (Figure 6).
Figure 6.
Distribution and function of microbiota in human intestine during health or disease. The bacterial community is the core component of microbiota in the gastrointestinal tract and there are eukaryotic microbes. In healthy subjects, microbial density and types vary across the gastrointestinal tract, with density increasing dramatically in oro-aboral direction. Microbial composition also changes. The crosstalk between bacteria and host contribute to maintain physiological metabolic, protective, and structural functions. In disease, shift of relative abundance in microbial species or quantitative variations of microbiota occur, generating chronic low-grade inflammation, atypical gases and fecal levels of organic acids, impaired metabolism of proteins and carbohydrates and immunological responses [2,102,103,104,105,106].
In the jejunum predominate few species of bacteria (i.e., Enterococci, Lactobacilli, oral Streptococci and other gram-positive aerobic or rare facultative anaerobes from the oropharynx), with concentrations reaching 104 CFU/mL of jejunal content. The microbiota at this level is sensitive to and controlled by physiological factors, including the presence and type of bile acids, the intraluminal pH, pancreatic secretion and gastrointestinal motility [101]. The ileum becomes a more favorable environment for microbial growth where enterobacteria and other coliforms reach a concentration of 109 CFU/mL [101]. Again, peristalsis in the small intestine and appropriate gastric acid secretion prevent qualitative, quantitative and/or topographic changes of the intestinal microbiota, namely the bacterial overgrowth [107]. The colon hosts up to 1012 CFU/mL of numerous bacterial species, primarily anaerobes (Bacteroides, Clostridium, Bifidobacteria, Lactobacilli) [108]. In the feces, anaerobic bacteria predominate with a great diversity (range 3000 to 5000 species) [109]. About 90% of the fecal mass is made of bacteria and optimal temperature and viscosity can facilitate bacterial growth. The human gut hosts three bacterial enterotypes, not country- or continent-specific. These clusters extract energy and produce vitamins differently [110].
3.4. Functions of the Intestinal Microbiota
The gut microbiota is essential for the metabolism, but also for protective and structural activities.
Metabolic functions include extraction of energy from indigestible dietary polysaccharides and release of vitamins not produced by the human host (vitamin B complex and vitamin K) [111]. In addition, bacteria produce short-chain fatty acids (SCFAs: acetate, propionate and butyrate) and amino acids. In the colonic lumen, the microbiota greatly contributes to the biotransformation of primary to secondary bile acids during their ongoing enterohepatic re-circulation [105]. In turn, in the small intestine, bile acids are important to avoid a significant bacterial colonization [112,113]. Shift towards different patterns of bile acids and bile acid pool may affect cholesterol and glucose homeostasis [114], as well as other metabolic pathways [115,116]. Gases and SCFAs [117] derive from the intraluminal fermentation of undigested carbohydrates by bacteria. SCFAs, in addition, protect the colonic mucosa [117]. Finally, SCFAs influence the lipogenesis in adipose tissue and cholesterol synthesis [118], and have hypocholesterolemic action by inhibiting liver cholesterol synthesis [117,119]. In obese individuals, intestinal microbiota harvests energy more efficiently from carbohydrates otherwise non-digestible. Mechanisms include increased production of SCFA and decreased intestinal expression of Fiaf (fasting induced adipose factor), with higher availability of fatty acids to the liver and adipose tissue [99,120,121,122,123].
The intestinal microbiota has a protective role due to a prevention of the gut colonization lumen by pathogens, the contribution in the development of the host immune system (B cell development [124], regulatory T cells, T helper type 1, 2 and 17 cells [125]) and a modulation of inflammatory cytokines. The microbial community prevents infections by pathogens via production of antimicrobial peptides, but also directly competes for metabolic niches [126]. SCFAs per se may modulate the immune system regulating the inflammatory responses [127,128].
The structural activity is mainly secondary to the interactions between the microbiota and the mucus layer which acts as a barrier to inflammatory molecules [129]. The SCFA butyrate improves the colonic defensive border [130]. Further beneficial effects derive from the strong immune-activating properties of microbiota and components such as LPS, peptidoglycans, superantigens, bacterial DNA and heat shock proteins (HSPs).
3.5. The Microbiota and the Brain-Gut Axis
The brain-gut axis is a bidirectional pathway involving immune cells and neural pathways [131]. More extensively, the “microbiota-gut-brain axis” is a communication system connecting the intestinal lumen and the brain through immune, endocrine and neuronal pathways. This complex interplay could affect mood, behavior and perception [132,133,134]. The central (CNS) and the autonomous (ANS) nervous system, the neuro-immune and neuro-endocrine systems, the enteric nervous system, and the gut microbiota are all involved in this scenario, where gastrointestinal function can modulate brain signaling [134]. Indeed, gut microbiota can influence anxiety, stress response [135,136,137] and memory function [138]. In animals exposed to infection or affected by inflammation, the gut microbiota modulates the system via a neural protection [138,139,140] and can modulate behavior [141]. Brain development could be regulated via neuronal circuits involved in motor control and anxiety behavior [136]. Indeed, gut bacteria has been reported to interact in a concentration-dependent manner with the brain derived neurotrophic factor (BDNF), a neurotrophin involved in neuronal growth, with modulation of cognitive and emotional behavior [135,136,137]. In addition, a shift in the relative abundance in microbial species or exposure to specific commensal bacteria can interfere with the hypothalamic-pituitary-adrenal (HPA) axis. This pathway influences stress response predisposing to altered mood or behavioral disorders [133,135,142,143]. Microbiota–vagus nerve interaction is also able to affect the interaction between immune, visceral signals and the CNS [140,141,142,144].
4. Gene-Bacteria Interplay and Composition of Gut Microbiota in FMF Patients
Autoinflammatory genes, such as MEFV, drive an exaggerated innate immune response to various signals in vitro, including microbial products [55]. In parallel, the NOD2/CARD15 gene is a major susceptibility gene for Crohn’s disease, a chronic, recurrent inflammatory bowel disease (IBD). Similar to the MEFV gene, the NOD2/CARD15 gene is localized to chromosome 16 [145]. Both MEFV and NOD2/CARD15 genes encode similar superfamily proteins, acting as intracellular pattern recognition receptors [146], and likely both regulates cytokine processing, cell apoptosis and inflammation. Patients with Crohn’s disease are carriers of mutated proteins, which sense bacterial products and activate the innate immune response [147]. NOD2/CARD15 mutations were not associated to an increased susceptibility to develop FMF. However, in a cohort of 103 FMF children, subjects with NOD2/CARD15 mutations had a higher rate of acute scrotum attacks, erysipelas-like erythema, a trend towards higher rates of resistance to colchicine, and a more severe disease, as compared to those without mutations [148].
Xu et al. found that pyrin is a specific immune sensor for bacterial modifications of Rho GTPases, and responds to Clostridium difficile, a frequent cause of nosocomial diarrhea. Pyrin does not directly recognize the microbial products but can detect pathogen virulence activity [38]. This finding is relevant for a full comprehension of FMF pathogenesis. On this way, colchicine is the principle therapy for FMF-patients, aimed to prevent acute attacks and complications secondary to chronic inflammation [149]. Colchicine is a fat-soluble alkaloid binding to β-tubulin, hindering its polarization and therefore inhibiting neutrophil chemotaxis and reducing the expression of adhesion molecules. Through these mechanisms, colchicine prevents febrile attacks and controls inflammation in FMF patients. However, 5–10% of FMF patients are non-responders to colchicine [150], possibly due to concomitant diseases (e.g., IBD or vasculitis) [151,152] or to occult infections triggering a reduced drug effectiveness [153,154].
MEFV variants are mainly represented by missense mutations in the C-terminal half of the pyrin protein [34,35,36,155]. In homozygous mutant mice expressing a truncated pyrin, the bacterial endotoxin lipopolysaccharide (LPS) induced increased fever and lethality. The mutant pyrin was less effective than the wild-type pyrin in binding to ASC and inhibiting caspase 1 and IL-1β production. Thus, one possibility is that FMF patients become more responsive to transient bacteremia and bacterial pathogens and LPS release, and therefore to systemic inflammatory response [156].
In FMF patients, the presence of a concomitant Helicobacter pylori (HP) infection is linked with more severe and more frequent febrile attacks. Of note, HP eradication was associated with beneficial effects (i.e., reduced febrile attacks and cytokine levels) [157,158].
Small intestinal bacterial overgrowth (SIBO), is a condition characterized by the increase of microorganisms in the small bowel exceeding 105 CFU/mL [159,160] and increased bacterial fermentation of a non-adsorbable carbohydrate substrate [161]. The occurrence of SIBO could exacerbate the FMF phenotypic expression. SIBO may generate variable clinical features, ranging from the absolute absence of symptoms to a classical malabsorption syndrome, with dyspepsia, abdominal distension and diarrhea with or without colicky pain, eventually modified by meals and evacuation of stools. The malabsorption and the altered intestinal microbiota might facilitate, in patients with SIBO, the diffusion of products deriving from bacterial metabolism through the blood stream, thus contributing to the dissemination of pathogen associated molecular patterns (PAMPs) [162,163]. This condition might also interfere with a physiological intestinal permeability [164] as well as with the bioavailability of drugs [165]. SIBO could be responsible of unresponsiveness to colchicine, while SIBO decontamination therapy with rifaximin, a non-absorbable antibiotic, contributed to a decrease in FMF attacks [166]. Therefore, SIBO-derived bacterial antigen production or release may promote the secretion of inflammatory cytokines, such as IL-1β, and may sustain a chronic or occult inflammation, leading to an FMF phenotype apparently unresponsive (or hyporesponsive) to colchicine.
Full characterization of gut microbiota in FMF patients is required. Major difficulties derive from phenotypic variations and gene-environment interactions. FMF patients, as compared with healthy subjects, might exhibit a different composition in gut microbiota [167,168]. In principle, the profile of microbial products and metabolites in the human metabolome from FMF patients (in particular the specific profile of long chain fatty acids) might become a marker for the disease [169]. Similarly, increased blood levels of short chain fatty acids appear in the acute phase of the disease, as a consequence of active inflammation [170].
In a series of 19 FMF patients explored during an attack, as compared with healthy controls, a poorer microbiota with loss of diversity has been described, with major shifts in bacterial populations within the Firmicutes, Bacteroidetes and Proteobacteria phyla (i.e., as compared with controls, a lower proportion of Prevotellaceae, Dialister and Prevotella; increase in Porphyromonadaceae, Phascolarctobacterium, aecalibacterium and arabacteroides). In the same subjects, during remission, the amount of Ruminococcus, Megasphaera, Enterobacteriaceae and Acidaminococcaceae was higher than in controls. Conversely, Roseburia was reduced. Thus, host genes may dictate the host-microbiota interaction, with a microbiota profile specific for FMF, and with the most diverse gut bacterial community observed during remission [168]. Additionally, a combined analysis of mutations in the MEFV gene and gut bacterial diversity suggested that the described depletion of total numbers of bacteria, loss of diversity and major shifts in bacterial populations depended on the allele carrier status of the host [168].
Different results derive from a more recent study on 41 FMF patients. Data from this series showed specific variations in gut microbiota linked with FMF but, more specifically, a decrease in α-diversity and a significantly altered microbiota composition, with several operational taxonomic units (OUT, i.e., cluster of similar sequence variants of the 16S rDNA marker gene sequence used to distinguish bacteria at the genus level) belonging to the order Clostridiales [167]. Variations with the study of Khachatryan et al. [168] might depend on the different statistical method employed for the analysis (multivariate analysis), a lower number of enrolled subjects in the previous study and the different country of origin of patients [167].
Moreover, Pepoyan et al. [171] observed that M694V/V726A pyrin mutations leading to FMF disease may contribute to gender-specific differences in microbial community structure in FMF patients, although this study analyzed a small number of subjects.
The autoinflammatory state per se can play a critical role in the determination of microbiota variations observed in FMF patients. Armenian FMF patients showed an elevated systemic reactivity against gut microbiota. Inflammatory alterations were also present in the absence of acute attacks, with increased levels of IgG antibodies against commensal microbiota (i.e., Bacteroides, Parabacteroides, Escherichia and Enteroccocus antigens) [172]. Another study found a specific association between the presence of AA amyloidosis (i.e., subjects with a complicated disease) and two operational taxonomic units belonging to Clostridiales [167]. This difference does not appear to be attributable to the use of colchicine, the most common drug employed in FMF patients, since this drug, in vitro, does not seem to be able to affect the gut microbiota [173]. Additionally, the oral administration of colchicine in subjects with FMF is not able to normalize the altered profile of microbial long chain fatty acids, microbial products circulating in the systemic metabolome [174]. Conversely, as suggested in other diseases linked with chronic inflammation, it is possible that the appearance of amyloidosis can depend on changes in the gut microbiota [167,175,176,177], independently from genetic factors. Studies exploring gut microbiota in FMF patients report discrepant results [167,168]. Evidence points to a dominant role of environmental factors over host genetics [21]. FMF patients display further variations in the microbiota linked with the presence of AA amyloidosis [167], i.e., when the most severe form of FMF occurs.
Alimov et al. [178] investigated the role of bile acid analogues (BAA) in activating the pyrin inflammasome. Both BAA473, and less potently BAA485, led to IL-18 release from peripheral blood mononuclear cells (PBMCs). Furthermore, BAA473 induced secretion of IL-18 from a human colonic adenocarcinoma cell line and the basolateral side of a human intestinal organoid. Finally, ASC and pyrin were required for IL-1β and IL-18 secretion and colchicine blocked BAA473-mediated inflammasome activation confirming the specific role of pyrin in the process. Increasing evidence is accumulating on the role of the gut microbiota on bile acid bioconversion with interindividual variations driving susceptibility to infections, altered metabolism and immune response [179,180,181,182]. Thus, genetic factors can represent one of several variables determining the gut microbiota profile in subjects with FMF.
5. The Role of Environmental Factors and the Determination of Phenotype
Despite the genetic origin, environmental factors might influence the prevalence of different FMF phenotypes. As previously shown, one factor might be the living country [183,184], possibly depending on the combinations of genetics with country-specific factors as dietary habits, deprivation, living conditions and contamination of environmental matrices. These observations are relevant, in particular, in the determination of individual susceptibility to amyloidosis [183].
An analysis on FMF patients from 14 countries demonstrated that the living country, rather than the MEFV genotype, was the major factor determining an increased risk of amyloidosis [183]. Furthermore, a comparison between Turkish children with FMF living in Turkey or in Germany showed a more severe course of the disease in those living in Turkey, pointing to the environment as a strong influencer of the FMF phenotype [184]. In this scenario, environmental factors affecting gut microbiota could have a role in determining onset and severity of complications in the context of a monogenic disease of the innate inflammatory pathway. Gut microbiota might also influence the evolution of AA amyloidosis, the most severe complication of FMF [167].
Finally, it is possible that different levels of basal state activation of pyrin, dependent on the MEFV genotype, could subtly influence the intestinal homeostasis in the gut conferring an interindividual diverse risk to develop chronic inflammation. In this light, microbiota metabolites are capable of modulating other inflammasomes [185].
6. Possible Therapeutic Implications
The link between gut microbiota, FMF acute attacks and FMF complications (i.e., AA amyloidosis), together with evidences pointing to an environmental modulation of gut microbiota, could allow novel therapeutic strategies in FMF patients. One evidence is that a specific probiotic therapy may induce a normalization of serum C-reactive protein (CRP) in FMF patients with high CRP levels during remission [186,187]. This approach appeared to restore the integrity and functionality of the gut microbiota [188].
In particular, Lactobacillus acidophilus INMIA 9602 Er-2 strain 317/402, a probiotic strain isolated from feces of a healthy newborn infant [189], produces a small anti-microbial peptide (bacteriocin acidocin LCHV), with a broad spectrum of activity against human pathogens, including methicillin-resistant Staphylococcus aureus and Clostridium difficile [190]. The strain’s clinically proven positive effects have been confirmed in different studies, also including FMF patients [191]. Interestingly, Lactobacillus acidophilus INMIA 9602 Er-2 strain 317/402 was able to reduce not only Enterobacteriaceae, thus bacterial-related intestinal dysbiosis, but also the relative abundance of Candida albicans, which is increased in FMF-patients. At the moment there is no evidence on the presence of an altered microbiota composition conditioning the resistance to colchicine in patients with FMF. We recently tested a combination of eight bacterial strains (Bifidobacterium breve DSM24732®, Streptococcus thermophilus DSM24731®, Bifidobacterium infantis DSM24737®, Bifidobacterium longum DSM24736®, Lactobacillus acidophilus DSM24735®, Lactobacillus paracasei DSM24733®, Lactobacillus plantarum DSM24730®, Lactobacillus delbrueckii ssp. bulgaricus DSM24734) at a concentration of 450 billion bacteria as the De Simone Formulation and available under the trademark Vivomixx® in Europe and Visbiome® in the US. Our preliminary results suggest that this combination, given during the inter-critical period, might improve symptoms in the subgroup of FMF patients carrying MEFV variants associated with more severe disease, and partially resistant to colchicine. However, well-designed, large, comprehensive, prospective and definitive studies are missing on the effects of probiotics in FMF patients to prevent the attacks, to reduce symptoms, to ameliorate the efficacy of colchicine and to prevent complications (i.e., amyloidosis).
7. Conclusions
The microbiota has an essential role in the host gut and is sensitive to genetic and environmental changes in both health and disease. FMF, as a model of rare inherited monogenic autoinflammatory disease, offers a background of periodic inflammatory changes, with a major involvement of the innate immunity. The microbiota is highly sensitive to such inflammatory changes. In addition, it might govern specific autoinflammatory responses in FMF. FMF symptoms might be sensitive as well, and this emerging topic deserves more attention as a model of environment-genetic interaction. Gut microbiota is likely a key factor in determining the FMF phenotype. In FMF patients, the microbiota abundance and its composition could depend on both genetic and environmental factors with the genome, although it plays a minor role. On the other hand, environmental variables could be critical in shaping the disease severity and complications onset (i.e., AA amyloidosis) in the long term. Further studies need to explore gene–environment interactions in FMF patients. Moreover, possible beneficial effects deriving from external manipulation of gut microbiota require additional investigation on how specific probiotic treatments could improve symptoms and microbiota growth, without reducing the beneficial effects of main therapeutic options in FMF patients.
Acknowledgments
We thank Ben-Chetrit, Tom LaMont and Mo Lamkanfi for helpful and scientific discussion. We acknowledge the technical expertise of Paola De Benedictis, Rosa De Venuto and Domenica Di Palo.
Author Contributions
Conceptualization, A.D.C., A.S. and P.P.; literature review, A.D.C. and L.B.; writing—original draft preparation, A.D.C.; writing—review and editing, A.S., D.Q.H.W. and P.P.; supervision, P.P.; funding acquisition, A.S. and P.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are recipient of the grant Fever Apuliae from Regione Puglia n. 289/26 June 2020 (A.D.C., A.S., L.B. and P.P.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kuramitsu H.K., He X., Lux R., Anderson M.H., Shi W. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 2007;71:653–670. doi: 10.1128/MMBR.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonfrate L., Tack J., Grattagliano I., Cuomo R., Portincasa P. Microbiota in health and irritable bowel syndrome: Current knowledge, perspectives and therapeutic options. Scand. J. Gastroenterol. 2013;48:995–1009. doi: 10.3109/00365521.2013.799220. [DOI] [PubMed] [Google Scholar]
- 3.Gerritsen J., Smidt H., Rijkers G.T., de Vos W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011;6:209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young V.B. The intestinal microbiota in health and disease. Curr. Opin. Gastroenterol. 2012;28:63. doi: 10.1097/MOG.0b013e32834d61e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penders J., Thijs C., Vink C., Stelma F.F., Snijders B., Kummeling I., van den Brandt P.A., Stobberingh E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 6.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S., Zhao W., Lan P., Mou X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell. 2020 doi: 10.1007/s13238-020-00745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mari A., Abu Baker F., Mahamid M., Sbeit W., Khoury T. The Evolving Role of Gut Microbiota in the Management of Irritable Bowel Syndrome: An Overview of the Current Knowledge. J. Clin. Med. 2020;9:685. doi: 10.3390/jcm9030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis M.A., Diaz P.I., Van Dyke T.E. The role of the microbiota in periodontal disease. Periodontol 2000. 2000;83:14–25. doi: 10.1111/prd.12296. [DOI] [PubMed] [Google Scholar]
- 10.Pieczynska M.D., Yang Y., Petrykowski S., Horbanczuk O.K., Atanasov A.G., Horbanczuk J.O. Gut Microbiota and Its Metabolites in Atherosclerosis Development. Molecules. 2020;25:594. doi: 10.3390/molecules25030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes-Castillo Z., Valdes-Miramontes E., Llamas-Covarrubias M., Munoz-Valle J.F. Troublesome friends within us: The role of gut microbiota on rheumatoid arthritis etiopathogenesis and its clinical and therapeutic relevance. Clin. Exp. Med. 2020 doi: 10.1007/s10238-020-00647-y. [DOI] [PubMed] [Google Scholar]
- 12.Zhu T., Goodarzi M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020;9:83–93. doi: 10.1007/s13668-020-00307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crovesy L., Masterson D., Rosado E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020 doi: 10.1038/s41430-020-0607-6. [DOI] [PubMed] [Google Scholar]
- 14.Aguilera A.C., Dagher I.A., Kloepfer K.M. Role of the Microbiome in Allergic Disease Development. Curr. Allergy Asthma Rep. 2020;20:44. doi: 10.1007/s11882-020-00944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Ghazaleh N., Chua W.J., Gopalan V. Intestinal microbiota and its association with colon cancer and red/processed meat consumption. J. Gastroenterol. Hepatol. 2020 doi: 10.1111/jgh.15042. [DOI] [PubMed] [Google Scholar]
- 16.Goodrich J.K., Davenport E.R., Beaumont M., Jackson M.A., Knight R., Ober C., Spector T.D., Bell J.T., Clark A.G., Ley R.E. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe. 2016;19:731–743. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKnite A.M., Perez-Munoz M.E., Lu L., Williams E.G., Brewer S., Andreux P.A., Bastiaansen J.W., Wang X., Kachman S.D., Auwerx J., et al. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PLoS ONE. 2012;7:e39191. doi: 10.1371/journal.pone.0039191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes D.A., Bacigalupe R., Wang J., Ruhlemann M.C., Tito R.Y., Falony G., Joossens M., Vieira-Silva S., Henckaerts L., Rymenans L., et al. Genome-wide associations of human gut microbiome variation and implications for causal inference analyses. Nat. Microbiol. 2020;5:1079–1087. doi: 10.1038/s41564-020-0743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoetendal E.G., Akkermans A.D.L., Akkermans van-Vliet W.M., de Visser J.A., de Vos W.M. The host genotype affects the bacterial community in the human gastrointestinal tract. Microb. Ecol. Health Dis. 2001;13:129–134. doi: 10.3402/mehd.v13i3.8013. [DOI] [Google Scholar]
- 20.Stewart J.A., Chadwick V.S., Murray A. Investigations into the influence of host genetics on the predominant eubacteria in the faecal microflora of children. J. Med. Microbiol. 2005;54:1239–1242. doi: 10.1099/jmm.0.46189-0. [DOI] [PubMed] [Google Scholar]
- 21.Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., Costea P.I., Godneva A., Kalka I.N., Bar N., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 22.Ozen S., Bilginer Y. A clinical guide to autoinflammatory diseases: Familial Mediterranean fever and next-of-kin. Nat. Rev. Rheumatol. 2014;10:135–147. doi: 10.1038/nrrheum.2013.174. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Chetrit E., Touitou I. Familial mediterranean Fever in the world. Arthritis Rheum. 2009;61:1447–1453. doi: 10.1002/art.24458. [DOI] [PubMed] [Google Scholar]
- 24.Bonfrate L., Scaccianoce G., Palasciano G., Ben-Chetrit E., Portincasa P. A novel cluster of patients with Familial Mediterranean Fever (FMF) in southern Italy. Eur. J. Clin. Investig. 2017;47:622–629. doi: 10.1111/eci.12783. [DOI] [PubMed] [Google Scholar]
- 25.Ricci P., Stella A., Settimo E., Passerini F., Minerva F., Belfiore A., Palmieri V.O., Pugliese S., Scaccianoce G., Portincasa P. The grandfather’s fever. Clin. Rheumatol. 2019;39:585–594. doi: 10.1007/s10067-019-04741-9. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Chetrit E., Levy M. Familial Mediterranean fever. Lancet. 1998;351:659–664. doi: 10.1016/S0140-6736(97)09408-7. [DOI] [PubMed] [Google Scholar]
- 27.Livneh A., Langevitz P., Zemer D., Zaks N., Kees S., Lidar T., Migdal A., Padeh S., Pras M. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997;40:1879–1885. doi: 10.1002/art.1780401023. [DOI] [PubMed] [Google Scholar]
- 28.Ozen S., Demirkaya E., Erer B., Livneh A., Ben-Chetrit E., Giancane G., Ozdogan H., Abu I., Gattorno M., Hawkins P.N., et al. EULAR recommendations for the management of familial Mediterranean fever. Ann. Rheum. Dis. 2016;75:644–651. doi: 10.1136/annrheumdis-2015-208690. [DOI] [PubMed] [Google Scholar]
- 29.Gattorno M., Hofer M., Federici S., Vanoni F., Bovis F., Aksentijevich I., Anton J., Arostegui J.I., Barron K., Ben-Cherit E., et al. Classification criteria for autoinflammatory recurrent fevers. Ann. Rheum. Dis. 2019;78:1025–1032. doi: 10.1136/annrheumdis-2019-215048. [DOI] [PubMed] [Google Scholar]
- 30.Van Gijn M.E., Ceccherini I., Shinar Y., Carbo E.C., Slofstra M., Arostegui J.I., Sarrabay G., Rowczenio D., Omoyimni E., Balci-Peynircioglu B., et al. New workflow for classification of genetic variants’ pathogenicity applied to hereditary recurrent fevers by the International Study Group for Systemic Autoinflammatory Diseases (INSAID) J. Med. Genet. 2018;55:530–537. doi: 10.1136/jmedgenet-2017-105216. [DOI] [PubMed] [Google Scholar]
- 31.Milhavet F., Cuisset L., Hoffman H.M., Slim R., El-Shanti H., Aksentijevich I., Lesage S., Waterham H., Wise C., Sarrauste de Menthiere C., et al. The infevers autoinflammatory mutation online registry: Update with new genes and functions. Hum. Mutat. 2008;29:803–808. doi: 10.1002/humu.20720. [DOI] [PubMed] [Google Scholar]
- 32.Touitou I., Lesage S., McDermott M., Cuisset L., Hoffman H., Dode C., Shoham N., Aganna E., Hugot J.P., Wise C., et al. Infevers: An evolving mutation database for auto-inflammatory syndromes. Hum. Mutat. 2004;24:194–198. doi: 10.1002/humu.20080. [DOI] [PubMed] [Google Scholar]
- 33.De Menthiere C.S., Terriere S., Pugnere D., Ruiz M., Demaille J., Touitou I. INFEVERS: The Registry for FMF and hereditary inflammatory disorders mutations. Nucleic Acids Res. 2003;31:282–285. doi: 10.1093/nar/gkg031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Gorp H., Huang L., Saavedra P., Vuylsteke M., Asaoka T., Prencipe G., Insalaco A., Ogunjimi B., Jeyaratnam J., Cataldo I., et al. Blood-based test for diagnosis and functional subtyping of familial Mediterranean fever. Ann. Rheum. Dis. 2020;79:960–968. doi: 10.1136/annrheumdis-2019-216701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stella A., Cortellessa F., Scaccianoce G., Pivetta B., Settimo E., Portincasa P. Familial Mediterranean fever: Breaking all the (genetic) rules. Rheumatology. 2019;58:463–467. doi: 10.1093/rheumatology/key328. [DOI] [PubMed] [Google Scholar]
- 36.Accetturo M., D’Uggento A.M., Portincasa P., Stella A. Improvement of MEFV gene variants classification to aid treatment decision making in familial Mediterranean fever. Rheumatology. 2020;59:754–761. doi: 10.1093/rheumatology/kez332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park Y.H., Wood G., Kastner D.L., Chae J.J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 2016;17:914–921. doi: 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H., Yang J., Gao W., Li L., Li P., Zhang L., Gong Y.N., Peng X., Xi J.J., Chen S., et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 39.Masters S.L., Lagou V., Jeru I., Baker P.J., Van Eyck L., Parry D.A., Lawless D., De Nardo D., Garcia-Perez J.E., Dagley L.F., et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci. Transl. Med. 2016;8:332ra45. doi: 10.1126/scitranslmed.aaf1471. [DOI] [PubMed] [Google Scholar]
- 40.Gao W., Yang J., Liu W., Wang Y., Shao F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc. Natl. Acad. Sci. USA. 2016;113:E4857–E4866. doi: 10.1073/pnas.1601700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Gorp H., Saavedra P.H., de Vasconcelos N.M., Van Opdenbosch N., Vande Walle L., Matusiak M., Prencipe G., Insalaco A., Van Hauwermeiren F., Demon D., et al. Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation. Proc. Natl. Acad. Sci. USA. 2016;113:14384–14389. doi: 10.1073/pnas.1613156113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 43.Schnappauf O., Chae J.J., Kastner D.L., Aksentijevich I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019;10:1745. doi: 10.3389/fimmu.2019.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 45.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 46.Walle L.V., Lamkanfi M. Pyroptosis. Curr. Biol. 2016;26:R568–R572. doi: 10.1016/j.cub.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 47.Broz P., Dixit V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 48.Portincasa P., Scaccianoce G., Palasciano G. Familial mediterranean fever: A fascinating model of inherited autoinflammatory disorder. Eur. J. Clin. Investig. 2013;43:1314–1327. doi: 10.1111/eci.12170. [DOI] [PubMed] [Google Scholar]
- 49.Grattagliano I., Bonfrate L., Ruggiero V., Scaccianoce G., Palasciano G., Portincasa P. Novel therapeutics for the treatment of familial Mediterranean fever: From colchicine to biologics. Clin. Pharmacol. Ther. 2014;95:89–97. doi: 10.1038/clpt.2013.148. [DOI] [PubMed] [Google Scholar]
- 50.Portincasa P. Colchicine, Biologic Agents and More for the Treatment of Familial Mediterranean Fever. The Old, the New, and the Rare. Curr. Med. Chem. 2016;23:60–86. doi: 10.2174/0929867323666151117121706. [DOI] [PubMed] [Google Scholar]
- 51.Wang D., Bonfrate L., de Bari O., Wang T., Portincasa P. Familial Mediterranean Fever: From Pathogenesis to Treatment. J. Genet. Syndr. Gene Ther. 2014;5:1000248. [Google Scholar]
- 52.Chae J.J., Aksentijevich I., Kastner D.L. Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. Br. J. Haematol. 2009;146:467–478. doi: 10.1111/j.1365-2141.2009.07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papin S., Cuenin S., Agostini L., Martinon F., Werner S., Beer H.D., Grutter C., Grutter M., Tschopp J. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ. 2007;14:1457–1466. doi: 10.1038/sj.cdd.4402142. [DOI] [PubMed] [Google Scholar]
- 54.Richards N., Schaner P., Diaz A., Stuckey J., Shelden E., Wadhwa A., Gumucio D.L. Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J. Biol. Chem. 2001;276:39320–39329. doi: 10.1074/jbc.M104730200. [DOI] [PubMed] [Google Scholar]
- 55.Shoham N.G., Centola M., Mansfield E., Hull K.M., Wood G., Wise C.A., Kastner D.L. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl. Acad. Sci. USA. 2003;100:13501–13506. doi: 10.1073/pnas.2135380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balci-Peynircioglu B., Waite A.L., Hu C., Richards N., Staubach-Grosse A., Yilmaz E., Gumucio D.L. Pyrin, product of the MEFV locus, interacts with the proapoptotic protein, Siva. J. Cell. Physiol. 2008;216:595–602. doi: 10.1002/jcp.21435. [DOI] [PubMed] [Google Scholar]
- 57.Kanneganti A., Malireddi R.K.S., Saavedra P.H.V., Vande Walle L., Van Gorp H., Kambara H., Tillman H., Vogel P., Luo H.R., Xavier R.J., et al. GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean Fever. J. Exp. Med. 2018;215:1519–1529. doi: 10.1084/jem.20172060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Gorp H., Van Opdenbosch N., Lamkanfi M. Inflammasome-Dependent Cytokines at the Crossroads of Health and Autoinflammatory Disease. Cold Spring Harb. Perspect. Biol. 2019;11:a028563. doi: 10.1101/cshperspect.a028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kayagaki N., Stowe I.B., Lee B.L., O’Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q.T., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 60.Chae J.J., Wood G., Masters S.L., Richard K., Park G., Smith B.J., Kastner D.L. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc. Natl. Acad. Sci. USA. 2006;103:9982–9987. doi: 10.1073/pnas.0602081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giaglis S., Papadopoulos V., Kambas K., Doumas M., Tsironidou V., Rafail S., Kartalis G., Speletas M., Ritis K. MEFV alterations and population genetics analysis in a large cohort of Greek patients with familial Mediterranean fever. Clin. Genet. 2007;71:458–467. doi: 10.1111/j.1399-0004.2007.00789.x. [DOI] [PubMed] [Google Scholar]
- 62.Mattit H., Joma M., Al-Cheikh S., El-Khateeb M., Medlej-Hashim M., Salem N., Delague V., Megarbane A. Familial Mediterranean fever in the Syrian population: Gene mutation frequencies, carrier rates and phenotype-genotype correlation. Eur. J. Med. Genet. 2006;49:481–486. doi: 10.1016/j.ejmg.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Goldfinger S.E. Colchicine for familial Mediterranean fever. N. Engl. J. Med. 1972;287:1302. doi: 10.1056/NEJM197212212872514. [DOI] [PubMed] [Google Scholar]
- 64.Dinarello C.A., Wolff S.M., Goldfinger S.E., Dale D.C., Alling D.W. Colchicine therapy for familial mediterranean fever. A double-blind trial. N. Engl. J. Med. 1974;291:934–937. doi: 10.1056/NEJM197410312911804. [DOI] [PubMed] [Google Scholar]
- 65.Gul A., Ozdogan H., Erer B., Ugurlu S., Kasapcopur O., Davis N., Sevgi S. Efficacy and safety of canakinumab in adolescents and adults with colchicine-resistant familial Mediterranean fever. Arthritis Res. Ther. 2015;17:243. doi: 10.1186/s13075-015-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brik R., Butbul-Aviel Y., Lubin S., Dayan E.B., Rachmilewitz-Minei T., Tseng L., Hashkes P.J. Canakinumab for the treatment of children with colchicine-resistant familial Mediterranean fever: A 6-month open-label, single-arm pilot study. Arthritis Rheumatol. 2014;66:3241–3243. doi: 10.1002/art.38777. [DOI] [PubMed] [Google Scholar]
- 67.Van der Hilst J., Moutschen M., Messiaen P.E., Lauwerys B.R., Vanderschueren S. Efficacy of anti-IL-1 treatment in familial Mediterranean fever: A systematic review of the literature. Biol. Targets Ther. 2016;10:75–80. doi: 10.2147/BTT.S102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Benedetti F., Gattorno M., Anton J., Ben-Chetrit E., Frenkel J., Hoffman H.M., Kone-Paut I., Lachmann H.J., Ozen S., Simon A., et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N. Engl. J. Med. 2018;378:1908–1919. doi: 10.1056/NEJMoa1706314. [DOI] [PubMed] [Google Scholar]
- 69.Maynard C., Weinkove D. Biochemistry and Cell Biology of Ageing: Part I Biomedical Science. Springer; Berlin/Heidelberg, Germany: 2018. The gut microbiota and ageing; pp. 351–371. [DOI] [PubMed] [Google Scholar]
- 70.Jakobsson H.E., Rodriguez-Pineiro A.M., Schutte A., Ermund A., Boysen P., Bemark M., Sommer F., Backhed F., Hansson G.C., Johansson M.E. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16:164–177. doi: 10.15252/embr.201439263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar Reddy D. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 74.Britanova L., Diefenbach A. Interplay of innate lymphoid cells and the microbiota. Immunol. Rev. 2017;279:36–51. doi: 10.1111/imr.12580. [DOI] [PubMed] [Google Scholar]
- 75.Chu D.M., Meyer K.M., Prince A.L., Aagaard K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes. 2016;7:459–470. doi: 10.1080/19490976.2016.1241357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vandenplas Y., Carnielli V.P., Ksiazyk J., Luna M.S., Migacheva N., Mosselmans J.M., Picaud J.C., Possner M., Singhal A., Wabitsch M. Factors affecting early-life intestinal microbiota development. Nutrition. 2020;78:110812. doi: 10.1016/j.nut.2020.110812. [DOI] [PubMed] [Google Scholar]
- 77.Palmer C., Bik E.M., DiGiulio D.B., Relman D.A., Brown P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanz Y. Gut microbiota and probiotics in maternal and infant health. Am. J. Clin. Nutr. 2011;94:2000S–2005S. doi: 10.3945/ajcn.110.001172. [DOI] [PubMed] [Google Scholar]
- 79.Cheng J., Palva A.M., de Vos W.M., Satokari R. Between Pathogenicity and Commensalism. Springer; Berlin/Heidelberg, Germany: 2011. Contribution of the intestinal microbiota to human health: From birth to 100 years of age; pp. 323–346. [DOI] [PubMed] [Google Scholar]
- 80.Bezirtzoglou E. The intestinal microflora during the first weeks of life. Anaerobe. 1997;3:173–177. doi: 10.1006/anae.1997.0102. [DOI] [PubMed] [Google Scholar]
- 81.Adlerberth I., Wold A. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229–238. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 82.Roger L.C., Costabile A., Holland D.T., Hoyles L., McCartney A.L. Examination of faecal Bifidobacterium populations in breast-and formula-fed infants during the first 18 months of life. Microbiology. 2010;156:3329–3341. doi: 10.1099/mic.0.043224-0. [DOI] [PubMed] [Google Scholar]
- 83.Roger L.C., McCartney A.L. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology. 2010;156:3317–3328. doi: 10.1099/mic.0.041913-0. [DOI] [PubMed] [Google Scholar]
- 84.Koenig J.E., Spor A., Scalfone N., Fricker A.D., Stombaugh J., Knight R., Angenent L.T., Ley R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA. 2011;108:4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rumney C.J., Rowland I.R. In vivo and in vitro models of the human colonic flora. Crit. Rev. Food Sci. Nutr. 1992;31:299–331. doi: 10.1080/10408399209527575. [DOI] [PubMed] [Google Scholar]
- 86.Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. Gut microbiota in health and disease. Physiol. Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 87.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rajilić-Stojanović M., Smidt H., De Vos W.M. Diversity of the human gastrointestinal tract microbiota revisited. Environ. Microbiol. 2007;9:2125–2136. doi: 10.1111/j.1462-2920.2007.01369.x. [DOI] [PubMed] [Google Scholar]
- 89.Egert M., De Graaf A.A., Maathuis A., De Waard P., Plugge C.M., Smidt H., Deutz N.E., Dijkema C., De Vos W.M., Venema K. Identification of glucose-fermenting bacteria present in an in vitro model of the human intestine by RNA-stable isotope probing. FEMS Microbiol. Ecol. 2007;60:126–135. doi: 10.1111/j.1574-6941.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 90.Ben-Amor K., Heilig H., Smidt H., Vaughan E.E., Abee T., de Vos W.M. Genetic diversity of viable, injured, and dead fecal bacteria assessed by fluorescence-activated cell sorting and 16S rRNA gene analysis. Appl. Environ. Microbiol. 2005;71:4679–4689. doi: 10.1128/AEM.71.8.4679-4689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rinttilä T., Kassinen A., Malinen E., Krogius L., Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004;97:1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 92.Amann R., Fuchs B.M. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat. Rev. Microbiol. 2008;6:339–348. doi: 10.1038/nrmicro1888. [DOI] [PubMed] [Google Scholar]
- 93.Lee K.S., Caughey B. A simplified recipe for prions. Proc. Natl. Acad. Sci. USA. 2007;104:9551–9552. doi: 10.1073/pnas.0703910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zoetendal E., Rajilić-Stojanović M., De Vos W. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57:1605–1615. doi: 10.1136/gut.2007.133603. [DOI] [PubMed] [Google Scholar]
- 95.Van den Bogert B., de Vos W.M., Zoetendal E.G., Kleerebezem M. Microarray analysis and barcoded pyrosequencing provide consistent microbial profiles depending on the source of human intestinal samples. Appl. Environ. Microbiol. 2011;77:2071–2080. doi: 10.1128/AEM.02477-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Human Microbiome Jumpstart Reference Strains Consortium A catalog of reference genomes from the human microbiome. Science. 2010;328:994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peterson J., Garges S., Giovanni M., McInnes P., Wang L., Schloss J.A., Bonazzi V., McEwen J.E., Wetterstrand K.A., Deal C., et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mariat D., Firmesse O., Levenez F., Guimarăes V., Sokol H., Doré J., Corthier G., Furet J. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 101.Booijink C.C., Zoetendal E.G., Kleerebezem M., De Vos W.M. Microbial communities in the human small intestine: Coupling diversity to metagenomics. Future Microbiol. 2007;2:285–295. doi: 10.2217/17460913.2.3.285. [DOI] [PubMed] [Google Scholar]
- 102.Altomare D.F., Bonfrate L., Krawczyk M., Lammert F., Caputi-Jambrenghi O., Rizzi S., Vacca M., Portincasa P. The inulin hydrogen breath test predicts the quality of colonic preparation. Surg. Endosc. 2014;28:1579–1587. doi: 10.1007/s00464-013-3354-0. [DOI] [PubMed] [Google Scholar]
- 103.Bonfrate L., Di Palo D.M., Celano G., Albert A., Vitellio P., De Angelis M., Gobbetti M., Portincasa P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020;50:e13201. doi: 10.1111/eci.13201. [DOI] [PubMed] [Google Scholar]
- 104.De Angelis M., Garruti G., Minervini F., Bonfrate L., Portincasa P., Gobbetti M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019;26:3567–3583. doi: 10.2174/0929867324666170428103848. [DOI] [PubMed] [Google Scholar]
- 105.Di Ciaula A., Garruti G., Lunardi Baccetto R., Molina-Molina E., Bonfrate L., Wang D.Q., Portincasa P. Bile Acid Physiology. Ann. Hepatol. 2017;16:4–14. doi: 10.5604/01.3001.0010.5493. [DOI] [PubMed] [Google Scholar]
- 106.Portincasa P., Bonfrate L., de Bari O., Lembo A., Ballou S. Irritable bowel syndrome and diet. Gastroenterol. Rep. 2017;5:11–19. doi: 10.1093/gastro/gow047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quigley E.M. Microflora modulation of motility. J. Neurogastroenterol. Motil. 2011;17:140. doi: 10.5056/jnm.2011.17.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guarner F., Malagelada J.R. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 109.Dethlefsen L., Huse S., Sogin M.L., Relman D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arumugam M., Raes J., Pelletier E., Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.M., et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mans J.J., von Lackum K., Dorsey C., Willis S., Wallet S.M., Baker H.V., Lamont R.J., Handfield M. The degree of microbiome complexity influences the epithelial response to infection. BMC Genom. 2009;10:380. doi: 10.1186/1471-2164-10-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Begley M., Gahan C.G.M., Hill C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 113.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 115.Garruti G., Di Ciaula A., Wang H.H., Wang D.Q., Portincasa P. Cross-Talk Between Bile Acids and Gastro-Intestinal and Thermogenic Hormones: Clues from Bariatric Surgery. Ann. Hepatol. 2017;16:S68–S82. doi: 10.5604/01.3001.0010.5499. [DOI] [PubMed] [Google Scholar]
- 116.Garruti G., Wang D.Q., Di Ciaula A., Portincasa P. Cholecystectomy: A way forward and back to metabolic syndrome? Lab. Invest. 2018;98:4–6. doi: 10.1038/labinvest.2017.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wong J.M.W., de Souza R., Kendall C.W.C., Emam A., Jenkins D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 118.Hijova E., Chmelarova A. Short chain fatty acids and colonic health. Bratisl. Med. J. 2007;108:354–358. [PubMed] [Google Scholar]
- 119.Chen W.-J.L., Anderson J.W., Jennings D. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Exp. Biol. Med. 1984;175:215–218. doi: 10.3181/00379727-175-41791. [DOI] [PubMed] [Google Scholar]
- 120.Machado M.V., Cortez-Pinto H. Gut microbiota and nonalcoholic fatty liver disease. Ann. Hepatol. 2012;11:440–449. doi: 10.1016/S1665-2681(19)31457-7. [DOI] [PubMed] [Google Scholar]
- 121.Tilg H., Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Manco M., Putignani L., Bottazzo G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010;31:817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 123.Serino M., Luche E., Gres S., Baylac A., Berge M., Cenac C., Waget A., Klopp P., Iacovoni J., Klopp C., et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543–553. doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hapfelmeier S., Lawson M.A., Slack E., Kirundi J.K., Stoel M., Heikenwalder M., Cahenzli J., Velykoredko Y., Balmer M.L., Endt K., et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prakash S., Rodes L., Coussa-Charley M., Tomaro-Duchesneau C. Gut microbiota: Next frontier in understanding human health and development of biotherapeutics. Biol. Targets Ther. 2011;5:71–86. doi: 10.2147/BTT.S19099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lührs H., Gerke T., Müller J., Melcher R., Schauber J., Boxberger F., Scheppach W., Menzel T. Butyrate inhibits NF-κB activation in lamina propria macrophages of patients with ulcerative colitis. Scand. J. Gastroenterol. 2002;37:458–466. doi: 10.1080/003655202317316105. [DOI] [PubMed] [Google Scholar]
- 128.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., Schilter H.C., Rolph M.S., Mackay F., Artis D., et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kleessen B., Blaut M. Modulation of gut mucosal biofilms. Br. J. Nutr. 2005;93:S35–S40. doi: 10.1079/BJN20041346. [DOI] [PubMed] [Google Scholar]
- 130.Hamer H.M., Jonkers D., Venema K., Vanhoutvin S., Troost F., Brummer R.J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 131.Drossman D.A. Gastrointestinal illness and the biopsychosocial model. Psychosom. Med. 1998;60:258–267. doi: 10.1097/00006842-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 132.Rhee S.H., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009;6:306. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Forsythe P., Kunze W.A. Voices from within: Gut microbes and the CNS. Cell. Mol. Life Sci. 2013;70:55–69. doi: 10.1007/s00018-012-1028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Mahony S.M., Dinan T.G., Cryan J.F. The gut microbiota as a key regulator of visceral pain. Pain. 2017;158:S19–S28. doi: 10.1097/j.pain.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 135.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heijtz R.D., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neufeld K., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23:255-e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 138.Gareau M.G., Wine E., Rodrigues D.M., Cho J.H., Whary M.T., Philpott D.J., MacQueen G., Sherman P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 139.Bercik P., Verdu E.F., Foster J.A., Macri J., Potter M., Huang X., Malinowski P., Jackson W., Blennerhassett P., Neufeld K.A. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 140.Bercik P., Park A., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P., Fahnestock M., Moine D. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Forsythe P., Kunze W.A., Bienenstock J. On communication between gut microbes and the brain. Curr. Opin. Gastroenterol. 2012;28:557–562. doi: 10.1097/MOG.0b013e3283572ffa. [DOI] [PubMed] [Google Scholar]
- 143.Dinan T.G., Quigley E.M., Ahmed S.M., Scully P., O’Brien S., O’Mahony L., O’Mahony S., Shanahan F., Keeling P.N. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: Plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 144.Goehler L.E., Gaykema R.P., Opitz N., Reddaway R., Badr N., Lyte M. Activation in vagal afferents and central autonomic pathways: Early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 145.Hugot J.-P., Chamaillard M., Zouali H., Lesage S., Cézard J.-P., Belaiche J., Almer S., Tysk C., O’Morain C.A., Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 146.Ogura Y., Inohara N., Benito A., Chen F.F., Yamaoka S., Núñez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 147.Ogura Y., Bonen D.K., Inohara N., Nicolae D.L., Chen F.F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R.H., et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 148.Berkun Y., Karban A., Padeh S., Pras E., Shinar Y., Lidar M., Livneh A., Bujanover Y. NOD2/CARD15 gene mutations in patients with familial Mediterranean fever. Semin. Arthritis Rheum. 2012;42:84–88. doi: 10.1016/j.semarthrit.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 149.Egritas O., Dalgic B. Infantile colitis as a novel presentation of familial Mediterranean fever responding to colchicine therapy. J. Paediatr. Gastroenterol. Nutr. 2011;53:102–105. doi: 10.1097/MPG.0b013e31820cfab1. [DOI] [PubMed] [Google Scholar]
- 150.Roldan R., Ruiz A.M., Miranda M.D., Collantes E. Anakinra: New therapeutic approach in children with Familial Mediterranean Fever resistant to colchicine. Jt. Bone Spine. 2008;75:504–505. doi: 10.1016/j.jbspin.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 151.Lachmann H.J., Sengul B., Yavuzsen T.U., Booth D.R., Booth S.E., Bybee A., Gallimore J.R., Soyturk M., Akar S., Tunca M., et al. Clinical and subclinical inflammation in patients with familial Mediterranean fever and in heterozygous carriers of MEFV mutations. Rheumatology. 2006;45:746–750. doi: 10.1093/rheumatology/kei279. [DOI] [PubMed] [Google Scholar]
- 152.Ozen S., Kone-Paut I., Gül A. Colchicine resistance and intolerance in familial mediterranean fever: Definition, causes, and alternative treatments. Semin. Arthritis Rheum. 2017;47:115–120. doi: 10.1016/j.semarthrit.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 153.Cattan D. MEFV mutation carriers and diseases other than familial Mediterranean fever: Proved and non-proved associations; putative biological advantage. Curr. Drug Targets. Inflamm. Allergy. 2005;4:105–112. doi: 10.2174/1568010053622948. [DOI] [PubMed] [Google Scholar]
- 154.Cerquaglia C., Diaco M., Nucera G., La Regina M., Montalto M., Manna R. Pharmacological and clinical basis of treatment of Familial Mediterranean Fever (FMF) with colchicine or analogues: An update. Curr. Drug Targets. Inflamm. Allergy. 2005;4:117–124. doi: 10.2174/1568010053622984. [DOI] [PubMed] [Google Scholar]
- 155.Portincasa P., Stella A., Bagnulo R., Ruggiero V., Bonfrate L., Castorani L., Scaccianoce G., Resta N., Palasciano G. Genotypical/phenotypical characterization of a novel cluster of patients with familial Mediterranean fever (FMF) in Apulia and Lucania in Southern Italy. Eur. J. Clin. Investig. 2013;43:72. [Google Scholar]
- 156.Chae J.J., Komarow H.D., Cheng J., Wood G., Raben N., Liu P.P., Kastner D.L. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell. 2003;11:591–604. doi: 10.1016/S1097-2765(03)00056-X. [DOI] [PubMed] [Google Scholar]
- 157.Demirturk L., Ozel A.M., Cekem K., Yazgan Y., Gultepe M. Co-existence of Helicobacter pylori infection in patients with Familial Mediterranean Fever (FMF) and the effect of Helicobacter pylori on the frequency and severity of FMF attacks. Dig. Liver Dis. 2005;37:153–158. doi: 10.1016/j.dld.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 158.Ozel A.M., Demirturk L., Aydogdu A., Gultepe M., Yazgan Y., Imirzalioglu N., Gurbuz A.K., Narin Y. Effect of Helicobacter pylori infection and eradication therapy on interleukin-6 levels in patients with Familial Mediterranean Fever. Int. J. Clin. Pract. 2008;62:754–761. doi: 10.1111/j.1742-1241.2006.01098.x. [DOI] [PubMed] [Google Scholar]
- 159.Ghoshal U.C., Shukla R., Ghoshal U. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome: A Bridge between Functional Organic Dichotomy. Gut Liver. 2017;11:196–208. doi: 10.5009/gnl16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ponziani F.R., Gerardi V., Gasbarrini A. Diagnosis and treatment of small intestinal bacterial overgrowth. Expert Rev. Gastroenterol. Hepatol. 2016;10:215–227. doi: 10.1586/17474124.2016.1110017. [DOI] [PubMed] [Google Scholar]
- 161.Gasbarrini A., Corazza G.R., Gasbarrini G., Montalto M., Di Stefano M., Basilisco G., Parodi A., Usai-Satta P., Vernia P., Anania C., et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009;29(Suppl. S1):1–49. doi: 10.1111/j.1365-2036.2009.03951.x. [DOI] [PubMed] [Google Scholar]
- 162.Balzan S., de Almeida Quadros C., de Cleva R., Zilberstein B., Cecconello I. Bacterial translocation: Overview of mechanisms and clinical impact. J. Gastroenterol. Hepatol. 2007;22:464–471. doi: 10.1111/j.1440-1746.2007.04933.x. [DOI] [PubMed] [Google Scholar]
- 163.Quigley E.M.M. Small intestinal bacterial overgrowth: What it is and what it is not. Curr. Opin. Gastroenterol. 2014;30:141–146. doi: 10.1097/MOG.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 164.Di Palo D.M., Garruti G., Di Ciaula A., Molina-Molina E., Shanmugam H., De Angelis M., Portincasa P. Increased Colonic Permeability and Lifestyles as Contributing Factors to Obesity and Liver Steatosis. Nutrients. 2020;12:564. doi: 10.3390/nu12020564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Scarpignato C., Gatta L. Commentary: Towards an effective and safe treatment of small intestine bacterial overgrowth. Aliment. Pharmacol. Ther. 2013;38:1409–1410. doi: 10.1111/apt.12531. [DOI] [PubMed] [Google Scholar]
- 166.Verrecchia E., Sicignano L.L., La Regina M., Nucera G., Patisso I., Cerrito L., Montalto M., Gasbarrini A., Manna R. Small Intestinal Bacterial Overgrowth Affects the Responsiveness to Colchicine in Familial Mediterranean Fever. Mediat. Inflamm. 2017;2017:7461426. doi: 10.1155/2017/7461426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deshayes S., Fellahi S., Bastard J.P., Launay J.M., Callebert J., Fraisse T., Buob D., Boffa J.J., Giurgea I., Dupont C., et al. Specific changes in faecal microbiota are associated with familial Mediterranean fever. Ann. Rheum. Dis. 2019;78:1398–1404. doi: 10.1136/annrheumdis-2019-215258. [DOI] [PubMed] [Google Scholar]
- 168.Khachatryan Z.A., Ktsoyan Z.A., Manukyan G.P., Kelly D., Ghazaryan K.A., Aminov R.I. Predominant role of host genetics in controlling the composition of gut microbiota. PLoS ONE. 2008;3:e3064. doi: 10.1371/journal.pone.0003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ktsoyan Z.A., Beloborodova N.V., Sedrakyan A.M., Osipov G.A., Khachatryan Z.A., Kelly D., Manukyan G.P., Arakelova K.A., Hovhannisyan A.I., Olenin A.Y., et al. Profiles of Microbial Fatty Acids in the Human Metabolome are Disease-Specific. Front. Microbiol. 2010;1:148. doi: 10.3389/fmicb.2010.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ktsoyan Z.A., Mkrtchyan M.S., Zakharyan M.K., Mnatsakanyan A.A., Arakelova K.A., Gevorgyan Z.U., Sedrakyan A.M., Hovhannisyan A.I., Arakelyan A.A., Aminov R.I. Systemic Concentrations of Short Chain Fatty Acids Are Elevated in Salmonellosis and Exacerbation of Familial Mediterranean Fever. Front. Microbiol. 2016;7:776. doi: 10.3389/fmicb.2016.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pepoyan A., Balayan M., Manvelyan A., Galstyan L., Pepoyan S., Petrosyan S., Tsaturyan V., Kamiya S., Torok T., Chikindas M. Probiotic Lactobacillus acidophilus Strain INMIA 9602 Er 317/402 Administration Reduces the Numbers of Candida albicans and Abundance of Enterobacteria in the Gut Microbiota of Familial Mediterranean Fever Patients. Front. Immunol. 2018;9:1426. doi: 10.3389/fimmu.2018.01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Manukyan G.P., Ghazaryan K.A., Ktsoyan Z.A., Khachatryan Z.A., Arakelova K.A., Kelly D., Grant G., Aminov R.I. Elevated systemic antibodies towards commensal gut microbiota in autoinflammatory condition. PLoS ONE. 2008;3:e3172. doi: 10.1371/journal.pone.0003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ktsoyan Z.A., Beloborodova N.V., Sedrakyan A.M., Osipov G.A., Khachatryan Z.A., Manukyan G.P., Arakelova K.A., Hovhannisyan A.I., Arakelyan A.A., Ghazaryan K.A., et al. Management of familial Mediterranean fever by colchicine does not normalize the altered profile of microbial long chain fatty acids in the human metabolome. Front. Cell. Infect. Microbiol. 2013;3:2. doi: 10.3389/fcimb.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Posautz A., Loncaric I., Westermark P. Is there a connection between the microbiome and AA amyloidosis? First hints from the European brown hare (Lepus europaeus) Amyloid Int. J. Exp. Clin. Investig. Off. J. Int. Soc. Amyloidosis. 2019;26:119–120. doi: 10.1080/13506129.2019.1593130. [DOI] [PubMed] [Google Scholar]
- 176.Chen Y., Fang L., Chen S., Zhou H., Fan Y., Lin L., Li J., Xu J., Chen Y., Ma Y., et al. Gut Microbiome Alterations Precede Cerebral Amyloidosis and Microglial Pathology in a Mouse Model of Alzheimer’s Disease. Biomed. Res. Int. 2020;2020:8456596. doi: 10.1155/2020/8456596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim M.S., Kim Y., Choi H., Kim W., Park S., Lee D., Kim D.K., Kim H.J., Choi H., Hyun D.W., et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut. 2020;69:283–294. doi: 10.1136/gutjnl-2018-317431. [DOI] [PubMed] [Google Scholar]
- 178.Alimov I., Menon S., Cochran N., Maher R., Wang Q., Alford J., Concannon J.B., Yang Z., Harrington E., Llamas L., et al. Bile acid analogues are activators of pyrin inflammasome. J. Biol. Chem. 2019;294:3359–3366. doi: 10.1074/jbc.RA118.005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Theriot C.M., Petri W.A., Jr. Role of Microbiota-Derived Bile Acids in Enteric Infections. Cell. 2020;181:1452–1454. doi: 10.1016/j.cell.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Song X., Sun X., Oh S.F., Wu M., Zhang Y., Zheng W., Geva-Zatorsky N., Jupp R., Mathis D., Benoist C., et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature. 2020;577:410–415. doi: 10.1038/s41586-019-1865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang Y., Gao X., Zhang X., Xiao Y., Huang J., Yu D., Li X., Hu H., Ge T., Li D., et al. Gut Microbiota Dysbiosis Is Associated with Altered Bile Acid Metabolism in Infantile Cholestasis. mSystems. 2019;4:e00463-19. doi: 10.1128/mSystems.00463-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Michonneau D., Latis E., Curis E., Dubouchet L., Ramamoorthy S., Ingram B., de Latour R.P., Robin M., de Fontbrune F.S., Chevret S., et al. Metabolomics analysis of human acute graft-versus-host disease reveals changes in host and microbiota-derived metabolites. Nat. Commun. 2019;10:5695. doi: 10.1038/s41467-019-13498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Touitou I., Sarkisian T., Medlej-Hashim M., Tunca M., Livneh A., Cattan D., Yalcinkaya F., Ozen S., Majeed H., Ozdogan H., et al. Country as the primary risk factor for renal amyloidosis in familial Mediterranean fever. Arthritis Rheum. 2007;56:1706–1712. doi: 10.1002/art.22507. [DOI] [PubMed] [Google Scholar]
- 184.Ozen S., Aktay N., Lainka E., Duzova A., Bakkaloglu A., Kallinich T. Disease severity in children and adolescents with familial Mediterranean fever: A comparative study to explore environmental effects on a monogenic disease. Ann. Rheum. Dis. 2009;68:246–248. doi: 10.1136/ard.2008.092031. [DOI] [PubMed] [Google Scholar]
- 185.Levy M., Thaiss C.A., Zeevi D., Dohnalova L., Zilberman-Schapira G., Mahdi J.A., David E., Savidor A., Korem T., Herzig Y., et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. 2015;163:1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Balayan M., Manvelyan A., Marutyan S., Isajanyan M., Tsaturyan V., Pepoyan A., Marotta F., Torok T. Impact of Lactobacillus Acidophilus INMIA 9602 Er-2 and Escherichia coli M-17 on some clinical blood characteristics of familial mediterranean fever disease patients from the armenian cohort. Int. J. Probiotics Prebiotics. 2015;10:91–95. [Google Scholar]
- 187.Pepoian A.Z., Arutunian N., Grigorian A., Tsaturian V.V., Manvelian A.M., Dilnian E., Balaian M.A., Torok T. The Certain Clinical Characteristics of Blood in Patients with Family Mediterranean Fever of Armenian Population. Klin. Lab. Diagn. 2015;60:46–47. [PubMed] [Google Scholar]
- 188.Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr. Rev. 2015;73(Suppl. S1):32–40. doi: 10.1093/nutrit/nuv039. [DOI] [PubMed] [Google Scholar]
- 189.Yerzinkyan L.A. Biological Properties of Some Isolates of Lactic Acid Bacteria. Yerevan Acad. Sci. Armen. 1971 [Google Scholar]
- 190.Mkrtchyan H., Gibbons S., Heidelberger S., Zloh M., Limaki H.K. Purification, characterisation and identification of acidocin LCHV, an antimicrobial peptide produced by Lactobacillus acidophilus nv Er 317/402 strain Narine. Int. J. Antimicrob. Agents. 2010;35:255–260. doi: 10.1016/j.ijantimicag.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 191.Pepoyan A.Z., Balayan M.H., Manvelyan A.M., Mamikonyan V., Isajanyan M., Tsaturyan V.V., Kamiya S., Netrebov V., Chikindas M.L. Lactobacillus acidophilus INMIA 9602 Er-2 strain 317/402 probiotic regulates growth of commensal Escherichia coli in gut microbiota of familial Mediterranean fever disease subjects. Lett. Appl. Microbiol. 2017;64:254–260. doi: 10.1111/lam.12722. [DOI] [PubMed] [Google Scholar]