Abstract
Background and Aims: Data from clinical trials suggest that biological drugs may improve the outcomes in Crohn’s disease (CD) by reducing the need for surgery or hospitalization. The aim of this study is to evaluate the time-trends of the use of biological drugs and other treatments for CD, and its relationship with outcomes in Catalonia. Materials and Methods: All patients with CD included in the Catalan Health Surveillance System (containing data on a population of more than 7.5 million) from 2011 to 2017 were identified. The exposures to different treatments for inflammatory bowel disease were retrieved from electronic invoicing records. Results: Between 2011 and 2017, the use of salicylates, corticosteroids and immunosuppressive treatment fell from 28.8% to 17.1%, 15.8% to 13.7%, and 32.9% to 29.6%, respectively (p < 0.001). Biological treatment use rose from 15.0% to 18.7% (p < 0.001). Ostomy rates per 1000 patients/year fell from 13.2 in 2011 to 9.8 in 2017 (p = 0.003), and surgical resection rates from 24.1 to 18.0 (p < 0.001). The rate of CD-related hospitalizations per 1000 patients/year also fell, from 92.7 to 72.2 (p < 0.001). Conclusions: Biological drug use rose from 15.0% to 18.7% between 2011 and 2017. During this period, we observed an improvement in the outcomes of CD patients.
Keywords: epidemiology, inflammatory bowel disease, Crohn’s disease
1. Introduction
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) that causes gastrointestinal tract inflammation [1]. Its prevalence and incidence are increasing: currently, its prevalence ranges between 28 and 322 per 100,000 inhabitants in Western Europe. The incidence and prevalence are especially high in Western Europe and North America [2,3,4].
CD has a variable clinical course, with the alternation of periods of remission and flares of active disease. The annual hospitalization rate approaches 20%; furthermore, about 50% of patients require surgery within 10 years of diagnosis [5]. Biological treatment (anti-tumor necrosis factor, anti-integrin and anti-interleukin 12/23) has changed the management of IBD, improving clinical and endoscopic remission rates [6] and reducing the need for surgery in clinical trials. The subanalysis of the SONIC trial suggested that the use of infliximab plus azathioprine correlated with fewer disease complications and fewer surgical procedures [7]. The ACCENT I trial and the CHARM trial showed a reduction in surgical procedures in the groups treated with infliximab and with adalimumab when compared to the groups receiving placebo [8,9]. Finally, Sandborn et al. recently showed that treatment with ustekinumab reduced surgery rates at two years by 30%–50% compared to placebo [10]. However, the data on the effects of biological drugs on outcomes in clinical practice are limited and controversial [11,12,13,14,15].
The primary aim of the present study was to trace the time-trends of the use of different CD treatments (and especially biologics) in Catalonia from 2011 to 2017 and to evaluate its correlation with disease outcomes such as surgery and hospitalization.
2. Material and Methods
All patients with CD included in the Catalan Health Surveillance System (CHSS) [16] between 2011 and 2017 were identified, according to their ICD-9-CM codes (the list of ICD-9 codes used is appended as Supplementary Table S1). The CHSS is a population-based healthcare register including data on more than 7.5 million individuals. The CHSS database has a unique personal identification number. The database and the baseline patients’ data have been described in detail in previous articles [17].
The exposures to different IBD treatments were retrieved from the electronic invoicing records for the same period. IBD treatments included biological drugs (infliximab, adalimumab, golimumab, vedolizumab and ustekinumab), immunosuppressive agents (azathioprine, 6-mercaptopurine and methotrexate), corticosteroids and salicylates. A patient was considered to have received a particular biological drug during a specific year if at least one prescription of the biologic was dispensed during this period. The list of active principles of the treatments studied is appended as Supplementary Table S2.
Data on the number of surgical procedures and the hospitalization rate of CD patients (scheduled and unscheduled) were also obtained from the CHSS and expressed both per 1000 patients/year and per 100,000 inhabitants. All-cause hospitalization was analyzed. Hospitalization related to CD, infections or neoplasms was also evaluated. ICD-9-CM codes for the different surgical procedures are detailed in Supplementary Table S3.
2.1. Statistical Methods
To calculate the annual rate per patient/year for surgical procedures and for the drugs used, we estimated the time at risk for each patient in each of the periods. Exposure periods were initiated on January 1st (or at the date of diagnosis for incident patients) and ended on December 31st of each year (or with the death of the patient). The sum of the drugs invoiced in the period was used as a numerator. The denominator was the number of patients/year. The statistical significance of the global variation of rates during this time period was calculated using generalized linear models. Pearson correlation coefficients were calculated between the rates of performing surgical procedures and the percentages of patients treated with each group of drugs. A p value of 0.05 or lower was considered as significant. The statistical analysis was carried out using the statistical package R, version 3.4.3. The study was performed and reported according to the STROBE Statement [18].
2.2. Ethical Issues
The research used retrospective anonymized data from the CHSS. No personal data were used, and all the patients data were encrypted, so that no personal identification would be retrievable or traceable to the original source from the working database.
The study complied with the ethical guidelines of the Declaration of Helsinki. The study was revised and approved by the local ethics committee of the Hospital Universitari Parc Taulí in Sabadell (CEIC 2018/625 on date 15 October 2018). Given the retrospective nature of the study, the fact that no personal data were available, and the impossibility of obtaining informed consent for the whole study population, the ethics committee waived the need for informed consent.
3. Results
Baseline characteristics of the patients have been described in detail in a previous article [17].
3.1. Treatment Time Trends in the Use of Drugs
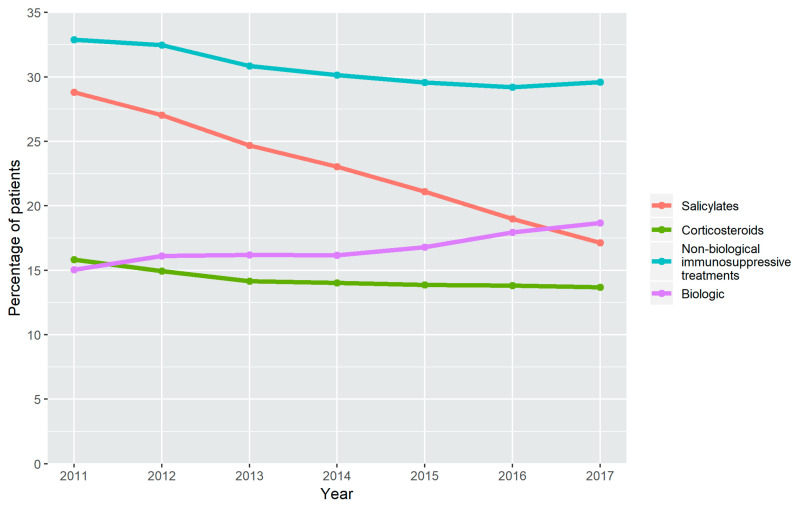
The percentage of patients treated with salicylates decreased markedly and continuously from 28.8% in 2011 to 17.1% in 2017 (p < 0.001). Systemic corticosteroid treatment has also fallen steadily from 15.8% in 2011 to 13.7% in 2017 (p < 0.001). The non-biological immunosuppressive treatments showed a smaller reduction, from 32.9% in 2011 to 29.6% in 2017 (p < 0.001). Biological treatments increased from 15.0% in 2011 to 18.7% in 2017 (p < 0.001) (Figure 1 and Supplementary Table S4).
Figure 1.
Treatment time trend. Percentage of the treatments used for CD from 2011 to 2017.
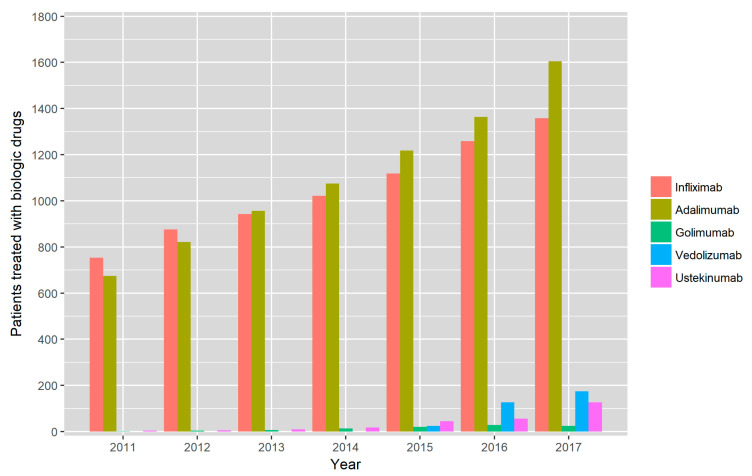
With regard to the different biological treatments (Figure 2 and Supplementary Table S5); infliximab was the most used drug in 2011 with 754 patients (8.2% of all CD patients) rising to 1,358 patients in 2017, although the percentage remained stable (8.2%), Adalimumab use increased from 674 patients in 2011 (7.3%) to 1604 patients in 2017 (9.7%). Few patients were treated with golimumab (a maximum of 29 patients in 2016, 0.2%). Ustekinumab was used off-label from 2011 to 2016 with a maximum of 56 patients (0.4%); its use rose to 127 patients (0.8%) in 2017, when it was licensed for CD treatment. Vedolizumab was used first in 2015, when it was administered to 25 patients (0.2%), and its use augmented to 174 patients (1.1%) in 2017.
Figure 2.
Absolute number of patients treated with biologic drugs from 2011 to 2017.
3.2. Surgical Procedures
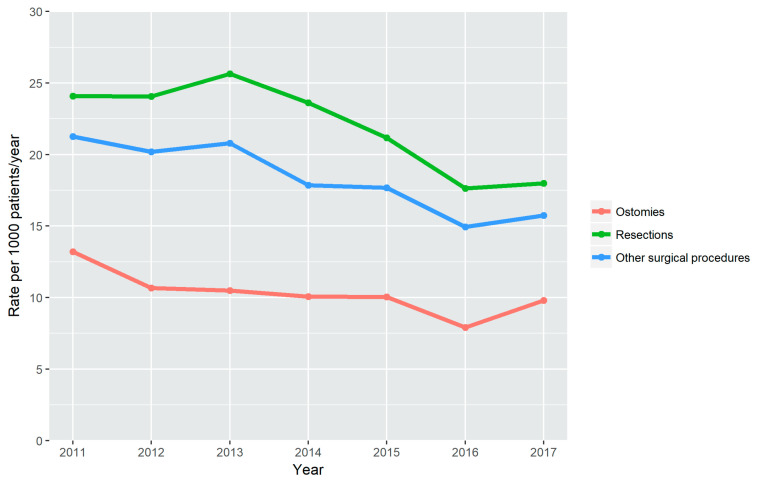
The absolute number of ostomies and resections increased slightly from 2011 to 2017, from 113 to 152 and from 206 to 279, respectively (Supplementary Table S6). However, as the prevalence of CD in this period of time increased markedly, the rate per 1000 patients/year decreased significantly for ostomies (from 13.2 in 2011 to 9.8 in 2017 (p = 0.003)), and resections (from 24.1 in 2011 to 18.0 in 2017 (p < 0.001)) (Figure 3).
Figure 3.
Surgical procedures. Rate per 1000 patients/year from 2011 to 2017.
The other surgical procedures showed a similar trend. The absolute number increased from 182 in 2011 to 244 in 2017; the rate per 1000 patients/year, however, declined from 21.3 in 2011 to 15.7 in 2017 (p < 0.001) (Figure 3).
There was a positive correlation between the use of salicylates, systemic corticosteroids and non-biological immunosuppressive treatments and surgical procedures. By contrast; biological agents were negatively correlated with surgical procedures. All correlations (except those between corticosteroids and bowel resection) reached statistical significance with a p value lower than 0.05 (Supplementary Table S7).
3.3. Hospital Admissions
The number of CD patients requiring hospitalization due to any cause rose from 2334 in 2011 to 4520 in 2017. Unscheduled hospitalization due to any cause increased from 1316 cases in 2011 to 2560 cases in 2017. Corresponding rates per 1000 patients/year also increased slightly from 272.8 in 2011 to 291.6 in 2017 (p < 0.001) and from 153.8 in 2011 to 165.1 in 2017 (p < 0.001), respectively (Supplementary Table S8 and Supplementary Figure S1).
Regarding specific causes of this hospitalization; CD-related hospitalization decreased from 92.7 per 1000 patients/year in 2011 to 72.2 per 1000 patients/year in 2017 (p < 0.001). The rate of infection-related hospitalization rose from 10.9 to 16.1 (p < 0.001). Finally, the rate of hospitalization due to cancer remained relatively stable, with slight variations over the period from 17.8 to 19.0, and a maximum value of 22 in 2016 (p = 0.02 for the trend) (Supplementary Table S8 and Figure S2).
Rates of CD-related hospitalization with respect to the general Catalan population increased from 10.56 per 100,000 inhabitants/year in 2011 to 14.8 per 100,000 inhabitants/year in 2017 (p < 0.001) (Supplementary Table S9).
With regard to the correlation with different treatments; there was a positive correlation between CD-related hospitalization and use of salicylates, corticosteroids and non-biological immunosuppressive treatments. The use of biological treatment showed a negative correlation (Supplementary Table S7).
4. Discussion
The present study shows that the use of biological drugs increased steadily between 2011 and 2017 in CD patients in Catalonia. Despite this increase, however, only 18.7% of patients were receiving these drugs in 2017. By contrast, the use of salicylates decreased markedly, and treatment with corticosteroids and non-biological immunosuppressive treatments also fell slightly. These changes in drug use correlated with a progressive decrease in the rate of surgeries. Rates of hospitalization due to CD complications also decreased. Therefore, our study suggests that the increase in the use of biological drugs was associated to a reduction in the need for surgery and hospitalization due to complications, although causality cannot be concluded. These data from a large population-based health register of clinical practice, along with the data recently published by Rahman A, et al. [19] in a population-based study from Ontario between 2003 and 2014 (Canada), corroborate previous data obtained in the clinical trials reported above [7,8,9,10].
The observed prevalence of the use of biological drugs (18.7%) is lower than those reported in other western countries. Yu et al. observed a far greater increase in the use of biologic treatment in CD (from 21.8% to 43.8%) in a recent study performed in the United States (US) between 2007 and 2015 [20]. Regarding western European studies, a population-based study in Denmark showed that 23.5% of CD patients were receiving biological treatment in 2012 [11]; in Norway, the use of anti-TNF in CD patients ranged from 20.9% to 31.4% in 2012 [21]; and a French study in 2014 reported rates of anti-TNF use of 33.8% in monotherapy, and 18.3% in combination with a non-biological immunosuppressive treatment [22]. Eastern European figures were much lower; for example, a Hungarian population-based study between 2011 and 2013 reported that only 8.5% of CD patients were receiving biological treatment [23]. These variable rates are probably related to differences in healthcare policies between countries, and highlight the fact that the optimal rates of biological prescription remain unclear. In a prospective population-based study, Vegh et al. showed that surgery and hospitalization rates were much higher in eastern European countries than in western Europe in 2011 [24]. According to our data, it could be hypothesized that this fact might be due, at least in part, to the differences in the use of biological drugs.
Nevertheless, the trends of surgical requirements after the introduction of biological treatment are still unclear. In our study, the rates of surgery procedures fell steadily. Both the ostomy rate and the rate of intestinal resections decreased by 25% between 2011 and 2017: from 13.2 to 9.8 per 1000 patients/year and from 24.1 to 18 per 1000 patients/year, respectively. These rates are similar to those reported by other studies, suggesting that surgical rates in IBD have decreased since the introduction of biological treatment [11,12,25,26,27,28]. In 2013, a meta-analysis of population-based studies demonstrated a reduction of around 25% in surgery rates for CD over the last six decades, from 16.3% and 33.3% at the 1st and 5th years after diagnosis in 1995 to 12.4% and 24.5% in 2000 [29]. More recent retrospective population-based studies have also revealed falls in surgery rates, with reductions of 27% in Poland between 2012 and 2014 [13], and 30% in Québec between 1996 and 2007 [30]. However, the data are heterogeneous and other studies reported that the rate of surgeries remained unchanged or even increased despite biological treatment [31]; in a retrospective study in the US, Lazarev et al. reported that the annual rate of small bowel resection did not change (1.6% between 1995 and 1998 vs. 1.9% between 1999 and 2001 vs. 1.6% between 2002 and 2004 vs. 1.9% between 2004 and 2007) [32]. In a recent retrospective study in Québec, Verdon et al. demonstrated that surgery in CD had risen from 8% in 2010 to 15% in 2015 [14]. Finally, some studies suggest that, although overall surgery rates were decreasing, this was not the case of all types of surgery; Malarcher et al., for instance, showed a reduction in small bowel resection (from 4.9% in 2003 to 3.9% in 2013) whereas colorectal resection and fistula surgery remained stable [33].
Although the rate of CD-related hospitalizations decreased from 92.7 per 1000 patients/year in 2011 to 72.2 in 2017, the total hospitalization rates showed a slight increase. In addition, annual rates per 100,000 inhabitants of CD-related hospitalization rose from 10.6 in 2011 to 14.08 per 100,000 inhabitants/year in 2017, mainly due to the near doubling of the prevalence of the disease [17]. These figures are lower than those presented by King et al. in a worldwide study using the OECD database (Organization for Economic Co-operation and Development), which reported an increase for CD-related hospitalization in Spain with an average annual rate of 23.8 per 100,000 inhabitants/year in the years 2010 to 2015 [34]. Possible explanations for the discrepancy include interregional differences, or differences in the ways hospitalization is reported in Spain’s regional health systems.
Variability in the reported trends and rates of hospitalization in CD occur also in other settings, with significant geographical variations. In a European study based on the European Crohn’s and Colitis Organization EpiCom cohort, Burish et al. demonstrated that 20% of CD patients required CD-related hospitalization in western Europe and 16% in eastern Europe in 2010 [35]. Recently, Murthy et al. showed in a Canadian population-based study between 1995 and 2012 that the use of infliximab did not reduce hospitalization rates (OR 1.06, 95% CI 0.811 to 1.39); they explain that this results from a misguided use of this drug in CD patients [36]. Other studies have recorded an increase in hospitalization rates. In a prospective population-based cohort from Hungary between 2000 and 2010, Golovics et al. observed high rates of CD-related hospitalization, especially during the first-year after the diagnosis, with a probability of 13.6% at one-year follow-up, 23.9% at three years, and 29.8% at five years [37]. Additionally, Sonnenberg et al. found an increase in the hospitalization trend between 1970 and 2004, and attributed this increase to the progressive population aging, with an increase in hospitalization of CD patients in the over-65 age group [15].
Regarding the causes of hospitalization, we observed a 48% increase in the rates of hospitalization due to infectious disease from 2011 to 2017. It is probable that the overall increase in hospitalization is attributable to the rise in infections. This increase might be explained by the use of non-biological immunosuppressive treatments and biological treatments, especially in the elderly population [38]. Shen et al. reported a 4-year infection rate of 43% in elderly vs. 31.6% in young patients receiving biological treatment. Furthermore, the rate of hospitalization due to anti-TNF side effects was 25% in the elderly, compared to 0% in younger patients [39]. We have not analyzed the effect of age on each of the individual causes of hospital admission.
The study has some limitations. First, the CHSS database includes only patients who use the public health system; conceivably, some patients with mild disease might not have been recorded. However, the public system is used by over 80% of the population of Catalonia. The rate of use is therefore much higher, as the 20% of non-users also includes healthy individuals. Furthermore, as CD is a chronic disease and pharmacological (and especially biological) treatments are relatively expensive, most patients request financial support from the public system, which offers universal coverage: in very expensive treatments such as biologics, public prescription approaches 100%.
The data provided by this study and by previous research provides insight into epidemiology and health outcomes of CD that will be useful for future care organization. In addition, the results suggest that biologic treatments may improve outcomes at population levels. As stated, the rate of biological drug use in this study is lower than in other western European countries. The outcomes, however, do not seem to be worse. Although the data of this study showed a low (and decreasing) rate of negative outcomes despite moderate use of biological treatment, the ideal prescription rate for these drugs, in relation to the best achievable outcomes, remains unknown. Future studies should address the heterogeneity in the indication of these drugs and its effect on outcomes.
In conclusion, our study shows that around 20% of CD patients in our country currently receive biological drugs and that this proportion is increasing at a rate of approximately 1% per year. Although causality cannot be proven, this change correlates with a significant decrease in the rates of surgery and CD-related hospitalizations.
Acknowledgments
We thank Michael Maudsley for his help with the English.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/9/2896/s1, Table S1: List of ICD-9 diagnoses for CD, Table S2: List of drugs used in CD and ATC codes, Table S3: ICD-9 codes for surgical procedures, Table S4: Use of drugs in CD from 2011 to 2017. Absolute number and percentage of CD patients treated, Table S5: Biologic treatment. Percentages are calculated by dividing the number of patients receiving the drug by the total number of patients with CD, Table S6: Surgical and diagnostic procedures in CD from 2011 to 2017. Absolute number and rate per 1000 patients/year, Table S7: Pearson correlation coefficient (p value) between pharmacological treatment and surgical procedures and CD-related hospitalization, Table S8: Hospitalization. Absolute number and rate per 1000 patients/year, Table S9: Hospitalization. Absolute number and rate per 100,000 inhabitants/year, Figure S1: Total CD patients hospitalized due to any cause either for scheduled and for emergency admissions. Rate per 1000 inhabitants/year, Figure S2: CD patients hospitalized for CD cause, infections and neoplasm.
Author Contributions
E.B., E.V. and X.C. designed the study, analyzed data and wrote the manuscript. X.C., E.B., E.V., L.M., M.C., C.P., L.P.L., M.G., P.G.-I., A.V. and M.V. critically reviewed the text and provided important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Albert Villoria has served as a speaker and consultant for MSD and Abbvie. Luigi Melcarne has received fees from Sandoz. Mercedes Vergara has given lectures for MSD, Abbott, Gilead, and Intercept, and has received fees from Intercept for advisory services. Xavier Calvet has received grants for research from Abbott, MSD, and Vifor, and fees for advisory services form Abbott, MSD, Takeda and Vifor. He has also given lectures for Abbott, MSD, Takeda, Shire and Allergan. Eduard Brunet, Emili Vela, Montserrat Clèries, Caridad Pontes, Laura Patricia Llovet and Marta Gallach and Pilar Garcia have no conflicts of interest to declare.
References
- 1.Abraham C., Cho J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burisch J., Kiudelis G., Kupcinskas L., Kievit H.A.L., Andersen K.W., Andersen V., Salupere R., Pedersen N., Kjeldsen J., D’Incà R., et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: An Epi-IBD study. Gut. 2019;68:423–433. doi: 10.1136/gutjnl-2017-315568. [DOI] [PubMed] [Google Scholar]
- 3.Pajares J.M., Gisbert J.P., Pajares J.M., Gisbert J.P. Epidemiology of inflammatory bowel disease in Spain. A systematic review. Rev. Esp. Enferm. Dig. 2001;93:9–20. [PubMed] [Google Scholar]
- 4.Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C., Chan F.K., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 5.Golovics P.A., Mandel M.D., Lovasz B.D., Lakatos P.L. Inflammatory bowel disease course in Crohn’s disease: Is the natural history changing? World J. Gastroenterol. 2014;20:3198–3207. doi: 10.3748/wjg.v20.i12.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutgeerts P., Van Assche G., Sandborn W.J., Douglas W.C., Geboes K., Colombel J.F., Reinisch W., Kumar A., Lazar A., Camez A., et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: Data from the EXTEND trial. Gastroenterology. 2012;142:1102–1111. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 7.Colombel J.F., Reinisch W., Mantzaris G.J., Kornbluth A., Rutgeerts P., Tang K.L., Oortwijn A., Bevelander G.S., Cornillie F.J., Sandborn W.J. Randomised clinical trial: Deep remission in biologic and immunomodulator naïve patients with Crohn’s disease—A SONIC post hoc analysis. Aliment. Pharmacol. Ther. 2015;41:734–746. doi: 10.1111/apt.13139. [DOI] [PubMed] [Google Scholar]
- 8.Hanauer S.B., Feagan B.G., Lichtenstein G.R., Mayer L.F., Schreiber S., Colombel J.F., Rachmilewitz D., Wolf D.C., Olson A., Bao A., et al. Manteniance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 9.Colombel J., Sandborn W.J., Rutgeerts P., Enns R., Hanauer S.B., Panaccione R., Schreiber S., Byczkowski D., Li J., Kent J.D., et al. Adalimumab for Maintenance of Clinical Response and Remission in Patients with Crohn’s Disease: The CHARM Trial. Gastroenterology. 2006;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn W.J., Sands B.E., Gasink C., Yeager B., Jacobstein D., Gao L.L., Daly K., Ghosh S., Rutgeerts P., Hanauer S.B., et al. Sa1743 Reduced Rates of Crohn’s- Related Surgeries; Hospitalizations and Alternate Biologic Initiation with Ustekinumab in the Im-Uniti Study Through 2 Years. Gastroenterology. 2018;154:S-377–S-378. doi: 10.1016/S0016-5085(18)31567-1. [DOI] [Google Scholar]
- 11.Vester-Andersen M.K., Prosberg M.V., Jess T., Andersson M., Bengtsson B.G., Blixt T., Munkholm P., Bendtsen F., Vind I. Disease course and surgery rates in inflammatory bowel disease: A population-based, 7-year follow-up study in the era of immunomodulating therapy. Am. J. Gastroenterol. 2014;109:705–714. doi: 10.1038/ajg.2014.45. [DOI] [PubMed] [Google Scholar]
- 12.Rungoe C., Langholz E., Andersson M., Basit S., Nielsen N.M., Wohlfahrt J., Jess T. Changes in medical treatment and surgery rates in inflammatory bowel disease: A nationwide cohort study 1979–2011. Gut. 2014;63:1607–1616. doi: 10.1136/gutjnl-2013-305607. [DOI] [PubMed] [Google Scholar]
- 13.Holko P., Kawalec P., Pilc A. Impact of biologic treatment of Crohn’s disease on the rate of surgeries and other healthcare resources: An analysis of a nationwide database from Poland. Front. Pharmacol. 2018;9:621. doi: 10.3389/fphar.2018.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdon C. Surgery and hospitalisations rates in inflammatory bowel disease patients in the Québec provincial database from 1996; Proceedings of the 14th Congress of ECCO; Copenhagen, Denmark. 6–9 March 2019. [Google Scholar]
- 15.Sonnenberg A. Hospitalization for inflammatory bowel disease in the United States between 1970 and 2004. J. Clin. Gastroenterol. 2009;43:297–300. doi: 10.1097/MCG.0b013e31816244a0. [DOI] [PubMed] [Google Scholar]
- 16.Agència de Qualitat i Avaluació Sanitàries de Catalunya. Departament de Salut. Generalitat de Catalunya. Observatori del Sistema de Salut de Catalunya Desigualtats Socioeconòmiques en la Salut i la Utilització dels Serveis Sanitaris públics en la Població de Catalunya. Observatori sobre els efectes de la crisi en la salut de la població. Barcelona. [(accessed on 27 October 2017)];2017 Available online: http//observatorisalut.gencat.cat/web/.content/minisite/observatorisalut/ossc_crisi_salut/Fitxers_crisi/Salut_crisi_informe_2016.pdf; http://observatorisalut.gencat.cat.
- 17.Brunet E., Roig-Ramos C., Vela E., Clèries M., Melcarne L., Villòria A., Pontes C., Calvet X. Prevalence, incidence and mortality of inflammatory bowel disease in Catalonia. A population-based analysis. Ann. Med. 2018;50:613–619. doi: 10.1080/07853890.2018.1523550. [DOI] [PubMed] [Google Scholar]
- 18.The PLOS Medicine Editors Observational studies: Getting clear about transparency. PLoS Med. 2014;11:e1001711. doi: 10.1371/journal.pmed.1001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman A., Jairath V., Feagan B.G., Khanna R., Shariff S.Z., Allen B.N., Jenkyn K.B., Vinden C., Jeyarajah J., Mosli M., et al. Declining hospitalisation and surgical intervention rates in patients with Crohn’s disease: A population-based cohort. Aliment. Pharmacol. Ther. 2019;50:1086–1093. doi: 10.1111/apt.15511. [DOI] [PubMed] [Google Scholar]
- 20.Yu H., MacIsaac D., Wong J.J., Sellers Z.M., Wren A.A., Bensen R., Kin C., Park K.T. Market Share and Costs of Biologic Therapies for Inflammatory Bowel Disease in the United States. Aliment. Pharmacol. Ther. 2018;47:364–370. doi: 10.1111/apt.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lirhus S.S., Høivik M.L., Moum B., Melberg H.O. Regional differences in anti-TNF-α therapy and surgery in the treatment of inflammatory bowel disease patients: A Norwegian nationwide cohort study. Scand. J. Gastroenterol. 2018;53:952–957. doi: 10.1080/00365521.2018.1495258. [DOI] [PubMed] [Google Scholar]
- 22.Kirchgesner J., Lemaitre M., Rudnichi A., Racine A., Zureik M., Carbonnel F., Dray-Spira R. Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: Analysis of the French administrative health databases 2009–2014. Aliment. Pharmacol. Ther. 2017;45:37–49. doi: 10.1111/apt.13835. [DOI] [PubMed] [Google Scholar]
- 23.Kurti Z., Vegh Z., Golovics P.A., Fadgyas-Freyler P., Gecse K.B., Gonczi L., Gimesi-Orszagh J., Lovasz B.D., Lakatos P.L. Nationwide prevalence and drug treatment practices of inflammatory bowel diseases in Hungary: A population-based study based on the National Health Insurance Fund database. Dig. Liver Dis. 2016;48:1302–1307. doi: 10.1016/j.dld.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Vegh Z., Burisch J., Pedersen N., Kaimakliotis I., Duricova D., Bortlik M., Vinding K.K., Avnstrøm S., Olsen J., Nielsen K.R., et al. Treatment Steps, Surgery, and Hospitalization Rates During the First Year of Follow-up in Patients with Inflammatory Bowel Diseases from the 2011 ECCO-Epicom Inception Cohort. J. Crohn’s Colitis. 2015;9:747–753. doi: 10.1093/ecco-jcc/jjv099. [DOI] [PubMed] [Google Scholar]
- 25.Annese V., Duricova D., Gower-Rousseau C., Jess T., Langholz E. Impact of New Treatments on Hospitalisation, Surgery, Infection, and Mortality in IBD: A Focus Paper by the Epidemiology Committee of ECCO. J. Crohn’s Colitis. 2016;10:216–225. doi: 10.1093/ecco-jcc/jjv190. [DOI] [PubMed] [Google Scholar]
- 26.Papamichael K., Chachu K.A., Vajravelu R.K., Vaughn B.P., Ni J., Osterman M.T., Cheifetz A.S. Improved Long-term Outcomes of Patients with Inflammatory Bowel Disease Receiving Proactive Compared with Reactive Monitoring of Serum Concentrations of Infliximab. Clin. Gastroenterol. Hepatol. 2017;15:1580–1588.e3. doi: 10.1016/j.cgh.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernstein C.N., Loftus E.V., Ng S.C., Lakatos P.L., Moum B. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622–629. doi: 10.1136/gutjnl-2011-301397. [DOI] [PubMed] [Google Scholar]
- 28.Mao E.J., Hazlewood G.S., Kaplan G.G., Peyrin-Biroulet L., Ananthakrishnan A.N. Systematic review with meta-analysis: Comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2017;45:3–13. doi: 10.1111/apt.13847. [DOI] [PubMed] [Google Scholar]
- 29.Frolkis A.D., Dykeman J., Negrón M.E., Debruyn J., Jette N., Fiest K.M., Frolkis T., Barkema H.W., Rioux K.P., Panaccione R., et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 30.Leombruno J.P., Nguyen G.C., Grootendorst P., Juurlink D., Einarson T. Hospitalization and surgical rates in patients with Crohn’s disease treated with infliximab: A matched analysis. Pharmacoepidemiol. Drug Saf. 2011;20:838–848. doi: 10.1002/pds.2132. [DOI] [PubMed] [Google Scholar]
- 31.Sokol H., Seksik P., Cosnes J. Complications and surgery in the inflammatory bowel diseases biological era. Curr. Opin. Gastroenterol. 2014;30:378–384. doi: 10.1097/MOG.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 32.Lazarev M., Saul M., Kip K.E., Regueiro M., Schraut W.H., Ullman T. Small bowel resection rates in Crohnʼs disease and the indication for surgery over time: Experience from a large tertiary care center. Inflamm. Bowel Dis. 2009;16:830–835. doi: 10.1002/ibd.21118. [DOI] [PubMed] [Google Scholar]
- 33.Malarcher C.A., Wheaton A.G., Liu Y., Greenlund S.F., Greenlund S.J., Lu H., Croft J.B. Hospitalizations for Crohn’s Disease—United States, 2003–2013. MMWR Morb. Mortal. Wkly. Rep. 2017;66:377–381. doi: 10.15585/mmwr.mm6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King J.A., Underwood F.E., Panaccione N., Quan J., Windsor W., Kotze P.G., Ng S.C., Ghosh S., Lakatos P.L., Jess T., et al. Trends in hospitalisation rates for inflammatory bowel disease in western versus newly industrialised countries: A population-based study of countries in the Organisation for Economic Co-operation and Development. Lancet Gastroenterol. Hepatol. 2019;4:287–295. doi: 10.1016/S2468-1253(19)30013-5. [DOI] [PubMed] [Google Scholar]
- 35.Burisch J., Pedersen N., Cukovic-Cavka S., Turk N., Kaimakliotis I., Duricova D., Shonová O., Vind I., Avnstrøm S., Thorsgaard N., et al. Initial disease course and treatment in an inflammatory bowel disease inception cohort in Europe: The ECCO-EpiCom cohort. Inflamm. Bowel Dis. 2014;20:36–46. doi: 10.1097/01.MIB.0000436277.13917.c4. [DOI] [PubMed] [Google Scholar]
- 36.Murthy S.K., Begum J., Benchimol E.I., Bernstein C.N., Kaplan G.G., McCurdy J.D., Singh H., Targownik L., Taljaard M. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut. 2020;69:274–282. doi: 10.1136/gutjnl-2019-318440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golovics P.A., Lakatos L., Mandel M.D., Lovasz B.D., Vegh Z., Kurti Z., Szita I., Kiss L.S., Pandur T., Lakatos P.L. Prevalence and predictors of hospitalization in Crohn’s disease in a prospective population-based inception cohort from 2000–2012. World J. Gastroenterol. 2017;21:7272–7280. doi: 10.3748/wjg.v21.i23.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakatos P.L., David G., Pandur T., Erdelyi Z., Mester G., Balogh M., Szipocs I., Molnar C., Komaromi E., Kiss L.S., et al. IBD in the elderly population: Results from a population-based study in Western Hungary, 1977–2008. J. Crohn’s Colitis. 2011;5:5–13. doi: 10.1016/j.crohns.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Shen H., Lipka S., Katz S. Increased hospitalizations in elderly with inflammatory bowel disease on anti-tumor necrosis factor therapy but not increased infections: A community practice experience. J. Crohn’s Colitis. 2014;8:898–899. doi: 10.1016/j.crohns.2013.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.