Abstract
Vaccination uptake has decreased globally in recent years, with a subsequent rise of vaccine-preventable diseases. Travellers, immunocompromised patients (ICP), and healthcare workers (HCW) are groups at increased risk for (severe) infectious diseases due to their behaviour, health, or occupation, respectively. While targeted vaccination guidelines are available, vaccination uptake seems low. In this review, we give a comprehensive overview of determinants—based on the integrated change model—predicting vaccination uptake in these groups. In travellers, low perceived risk of infection and low awareness of vaccination recommendations contributed to low uptake. Additionally, ICP were often unaware of the recommended vaccinations. A physician’s recommendation is strongly correlated with higher uptake. Furthermore, ICP appeared to be mainly concerned about the risks of vaccination and fear of deterioration of their underlying disease. For HCW, perceived risk of (the severity of) infection for themselves and for their patients together with perceived benefits of vaccination contribute most to their vaccination behaviour. As the determinants that affect uptake are numerous and diverse, we argue that future studies and interventions should be based on multifactorial health behaviour models, especially for travellers and ICP as only a limited number of such studies is available yet.
Keywords: vaccination uptake, vaccine refusal, vaccine hesitancy, risk groups, immunocompromised, travellers, healthcare workers, health behaviour model, determinants
1. Introduction
Vaccinations have proven to play a major role in the prevention and control of many infectious diseases. However, in the twenty-first century, vaccination programs face multiple challenges [1]. The first one is the need for fast development of effective and safe vaccines for new (re-)emerging pathogens. The recent SARS-CoV-2 pandemic is an example in which a vaccination is highly desired and may reduce the enormous impact of the current pandemic. The second challenge in the field of vaccinology is the upcoming trend of vaccine hesitancy and declining vaccination uptake.
Vaccine hesitancy is recognised by the World Health Organization (WHO) to be one of the ten threats to global health [2]. Vaccination uptake is declining globally, resulting in a rise in outbreaks of vaccine-preventable diseases (VPD) [3]. For instance, measles cases have increased—up to 300 percent—over the past years [4]. Vaccine hesitancy has predominantly received attention in the light of parents rejecting the national immunization programs. However, low vaccination uptake among adult populations also raises concerns [5]. Adults are progressively at risk for infectious diseases because life expectancy increases [6], the incidence of chronic diseases that require immunosuppressive treatment rises [7], and international travel expands [8]. Other determinants will play a role in vaccination uptake in adult populations as compared to children.
Adults who are recommended to get vaccinated can be divided into several risk groups. Risk populations in this context are defined as groups of human individuals with an increased risk of acquiring a (severe) infection due to their behaviour, health, or occupation. To get a broad overview of determinants that play a role in the vaccination uptake among risk groups, this review will focus on three distinct risk groups which consult vaccination clinics frequently, namely: “travellers, immunocompromised patients (ICP) and healthcare workers (HCW)”.
Travellers comprise a risk population, as at their destinations they can be exposed to infectious diseases they have not encountered before. Traveller vaccination guidelines are available to protect this population. These guidelines do not only differ per destination but are also dependent on the activities the travellers will undertake and the duration of their stay. Additionally, the country of origin is of importance, because of the endemicity of infectious diseases and therefore natural exposure, and national immunization programs. Moreover, travellers who are not properly vaccinated for their trip are not only at risk for getting sick themselves, they can also create a public health concern for communicable diseases, as they could carry an infection back home to a naïve population [9].
ICP have an increased risk for serious illnesses caused by infectious diseases due to a diminished function of their immune system. The compromised state of their immune system can be induced by either an underlying disease or the treatment of a disease. As a consequence of fast-developing immunosuppressive therapies for e.g., auto-immune diseases and malignancies, ICP are a constantly growing population [7]. Therefore, optimal protection of this vulnerable group is of utmost importance.
HCW are another risk category for acquiring infectious diseases. Their occupation brings them in close contact with patients, that possibly carry an infectious disease. Furthermore, HCW are not only personally at risk, they may also put their—mostly vulnerable—patients at risk when they work while carrying an infection [10]. On top of that, HCW play an important role in providing their (immunocompromised or travelling) patients with information or recommendations regarding vaccinations.
Vaccination uptake varies between risk populations and there may be differences in determinants that play a role in this behaviour. To find general patterns each risk group will be studied separately. However, as travellers, ICP, and HCW are interrelated, we aim to learn from similarities and differences between these groups. If we understand risk populations’ motivations and concerns, we might be able to address these either separately or combined by effective interventions. To get a better overview of all determinants that have a possible impact on uptake, we classified these in a model of health behaviour change.
An abundance of behaviour change models are available that describe determinants affecting preventive health behaviour [11]. In 2003, the integrated change (I-Change) model was developed by de Vries et al. [12]. This model is derived from the attitude-social norm-self-efficacy (ASE) model and integrates several other models, among which are the often-used health belief model (HBM) and the theory of planned behaviour (TPB) (Supplementary Table S1). According to the I-Change model, vaccination behaviour is shaped by the intention to get vaccinated which is subject to barriers and facilitators. Intention is established by motivation, awareness, information, and predisposing determinants. As this I-Change model comprises a wide variety of determinants that are used by other studies, for example those based on the HBM and ASE model, we use this model as a conceptual framework.
With this comprehensive review, we aim to better understand determinants that play a role in the uptake of vaccinations in travellers, ICP, and HCW and explore similarities and differences in these three groups. Hereby, we aim to create a solid ground for the development of evidence-based interventions to increase vaccination uptake in the populations that need optimal prevention strategies for infectious diseases.
2. Methods
2.1. Search Strategy
We performed a systematic database search on 19 February 2020. We performed one search for all three risk groups (Supplementary File S1). For each risk groups we combined search terms for vaccination uptake and health behavioural models. We searched the following databases: Embase, Medline, Cinahl, Web of Science Core Collection, ERIC, PsychINFO, and SocINDEX. As determinants of vaccination uptake may vary over time, we limited our search to studies published during the last ten years (between 1 January 2010 and 1 January 2020). We excluded research papers written in another language than English. All records were retrieved into an EndNote database. Duplicates were removed and titles and abstracts were screened (by LD). Thereafter, papers were sorted in the three different groups and full texts articles were reviewed for suitability using inclusion and exclusion criteria (by L.D. and L.v.L.) using EndNote X9.
2.2. Study Selection
Studies were included if they met all of the following criteria: (1) at least 75% of the included respondents are either ICP (patients with autoimmune diseases, malignancies, HIV, asplenia and solid organ or stem cell transplantations) or travellers (including travellers visiting friends and relatives (VFR), short- and long-term business travellers) or HCW (including general practitioners (GPs), physicians and nurses working in a hospital); (2) addressing self-reported cognitive determinants that may explain vaccination uptake; and (3) being performed in Western countries (defined as Europe, North America, Australia, and New Zealand).
We excluded studies that focussed on: (1) children; (2) HCW who care for populations other than the ICP defined in our study (e.g., paediatricians, elderly home physicians) or who are not directly involved in the care for this group (e.g., pharmacists, dentists); (3) future healthcare workers (e.g., medicine or nursing students); (4) uptake of the national immunization programme (e.g., HPV vaccination); (5) hypothetical vaccinations (e.g., a HIV vaccine); (6) vaccinations administered in outbreak situations (e.g., H1N1 vaccine, Ebola vaccine); (7) other very specific target groups (e.g., Roma travellers, migrants, pregnant women; and (8) predisposing factors exclusively. We also excluded qualitative studies and non-peer reviewed articles such as conference abstracts.
In case any doubt or disagreement between the two researchers who performed the study selection (by L.D. and L.v.L.) arose, the specific papers were discussed in a plenary session with all co-authors.
2.3. Data Extraction
The following background characteristics from included studies were extracted: first author and year of publication; study design; enrolment period; enrolment site; sample size; study population; theoretical framework; and targeted outcome variables. Extracted data was collected in Microsoft Excel 2016 and the presence and impact of determinants were rated in separate sheets per study group (by L.D. and L.v.L.). Random samples were taken to check the data extraction and disagreements were discussed plenary with all co-authors. Furthermore, the quality of studies was assessed using the the AXIS tool [13], which is a screening tool specifically designed for cross-sectional studies, as those in our review, and includes 20 items relevant to this design. Scores 1–9 are rates as low, 10–14 as medium and 15–20 as high.
2.4. Labelling of Determinants
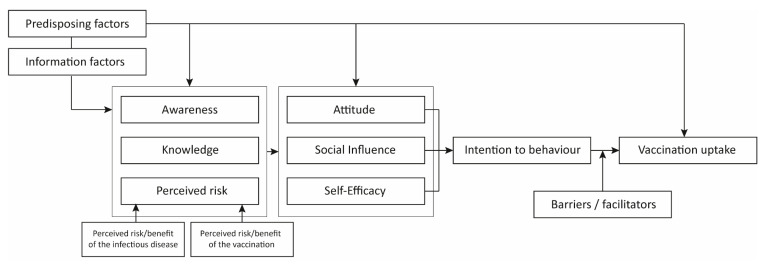
The I-Change model was used to organize all determinants that could explain vaccination uptake. A simplified version of this model is shown in Figure 1. The following concepts are used: (1) predisposing factors, including baseline characteristics of studied populations; (2) information factors, including information retrieved via media, social contacts and HCW; (3) awareness, of the infectious agent being present or a vaccine being available; (4) knowledge (either examined or self-evaluated), about the consequences of the infection, or about the efficacy and duration of protection of vaccination; (5a) perceived risk of the infection, which is divided into perceived severity of the disease and perceived susceptibility to get infected; (5b) perceived risk of vaccination, including vaccine-specific considerations such as fear of side-effects and trust in the effectiveness of the vaccine; (6) attitude, defined as a person’s disposition to respond favourably or unfavourably to vaccinations [14], often reflected by a person’s general believes about vaccinations; (7) social influence, which can be social norms imposed by family, friends or religion, but also recommendations from a healthcare professional or tour guide; (8) self-efficacy, defined as beliefs in one’s own capacity to perform certain behaviour [15]; (9) intention to behaviour, expressed by people before they perform the behaviour; (10) barriers and facilitators, that withhold individuals from or enable them to certain behaviour, such as time, costs, or accessibility.
Figure 1.
Simplified I-Change model summarizing the studied determinants that could predict vaccination uptake. We used a simplified version of the I-Change model applied to vaccination uptake. Uptake is shaped by the intention to get vaccinated which is subject to barriers and facilitators. Intention is established by motivation (attitude, social influence, and self-efficacy), awareness (awareness, knowledge, and perceived risk) and information and predisposing determinants. Predisposing factors include baseline characteristics of studied populations and influence awareness, motivation and uptake. Information factors include information retrieved via media, social contacts and healthcare workers.
3. Results
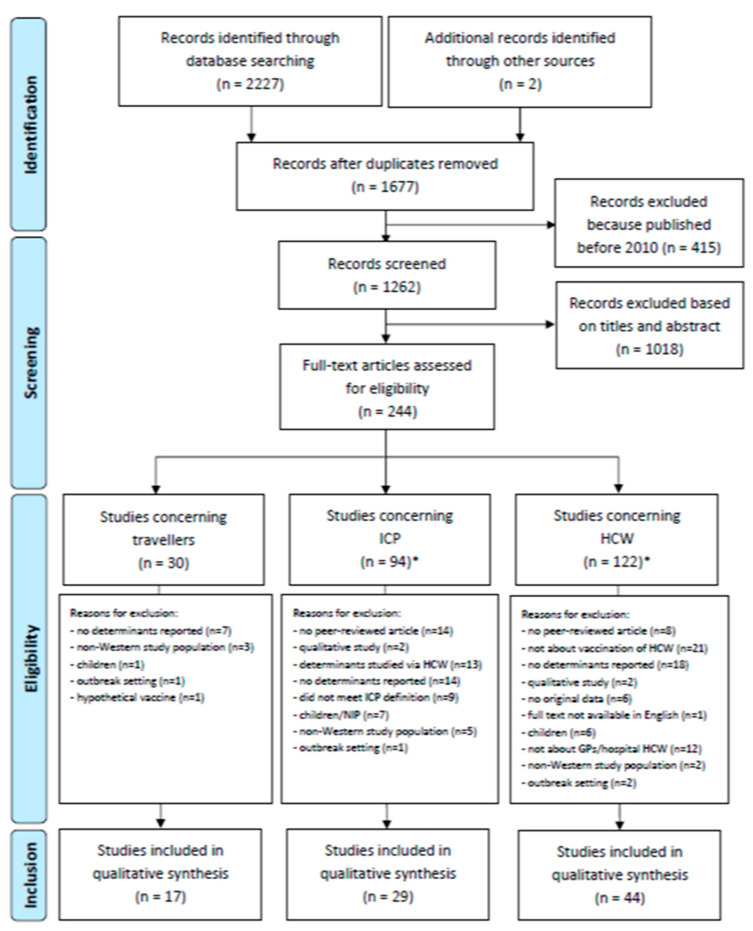
The literature search generated 2227 hits (Figure 2). After removing duplicates and excluding articles published before 2010, 1260 articles were available on the topic. These were screened based on title and abstract, resulting in 242 articles that were eligible for full-text assessment. These were divided into the three subgroups (some were included in more than one category): 30 for travellers, 95 for ICP, and 122 for HCW. Finally, 17, 29, and 44 articles were included in the data analysis for the three groups, respectively. The most common reason for exclusion was that no determinants (other than predisposing factors) were reported. Table 1 describes the characteristics and quality of included studies for travellers, ICP, and HCW. Determinants that play a role in vaccination uptake were retrieved from the articles and summarized in Table 2, Table 3 and Table 4 for travellers, ICP, and HCW respectively. The results of the quality assessment are presented in Supplementary Table S2.
Figure 2.
Flow diagram of study selection procedure. * n = 2 articles were included in both ICP and HCW.
Table 1.
Study characteristics of included studies for travellers, ICP and HCW.
| Study | Study Design | Enrolment Period | Enrolment Site | Sample Size | Study Population | Theoretical Framework | Outcome Measures * | Vaccination Coverage | Quality Score ** |
|---|---|---|---|---|---|---|---|---|---|
| Balaban, 2013 [16] | Pre- and post-travel surveys | 2009 | USA | 186 | American Hajj pilgrims | None | Seasonal influenza | - | Low |
| Barasheed, 2014 [17] | Cross-sectional survey | 2011–2012 | Mina (Mecca) | 966 | Australian Hajj pilgrims | None | Influenza | 62% | High |
| Duffy, 2013 [18] | Cross-sectional survey | 2007 (Aug.–Sept.) | United States | 1691 | American travellers to Asia | None | JE | 11% | Medium |
| Frew, 2017 [19] | Cross-sectional survey | 2015 (Feb.–March) | Ferry ports of 2 popular islands, Thailand | 1680 | Backpackers from Europe, Canada, Australia and New-Zealand (94%) | None (KAP) | HBV | 31% completed series | High |
| Goodman, 2014 [20] | Online cross-sectional survey | 2010 (Feb.) | UK | 302 | Travellers to the meningitis belt of Africa in last 3 years or planned to do so next 6 months | None | MenAWCY | 30% | Medium |
| Herbinger, 2011 [21] | Online cross-sectional survey | 2009 (Dec.) | Netherlands, Czech Republic, Spain, Sweden | 4203 | Travellers to countries of moderate or high prevalence for HBV in the last 5 years | None | HBV | 39% in the previous 5 years | Medium |
| Heywood, 2016 [22] | Online cross-sectional survey | 2014 (Aug.–Oct.) | Australia, Finland, Germany, Norway, Sweden, UK, Canada | 27,386 | Travellers (18–65 years) who travelled to HAV endemic countries in Africa, Asia, South/Central America in the last 3 years | None | HAV/HBV | 27% for 3-dose combined HAV and HBV and 37% for 2-dose monovalent HAV schedules | Medium |
| Igreja, 2019 [23] | Cross-sectional survey | 2019 (May–June) | Travel Clinic, Lisbon, Portugal | 55 | Portuguese travellers | None | Attitudes to vaccinations in general | - | Low |
| Lammert, 2016 [24] | Retrospective study | 2012–2014 | clinics from Global TravEpiNet, USA | 24,478 | International travellers who sought pre-travel health advice | None | Refusal rates of recommended vaccines and reasons | 25% refused one or more recommended vaccine(s) | High |
| Paudel, 2017 [25] | Prospective enhanced surveillance study | 2013 (Feb–2014 (Jan.) | Australia | 180 | confirmed cases of typhoid, paratyphoid, measles, HAV, HEV, chikungunya, malaria | None | Seeking pre-travel advice and uptake | 25% sought pre-travel advice and 16% got vaccinated | Medium |
| Pavli, 2019 [26] | Cross-sectional survey by email | 2015 (Nov.)–2016 (Mar.) | Greece | 231 | Greek (non-healthcare) students from 36 universities, planning to study abroad | None | Men, intention to vaccinate | 23% vaccinated, 15% intention | Medium |
| Pfeil, 2010 [27] | 2 cross-sectional surveys | 2009 (Jan.–Feb.), 2010 (Jan.) | Centre for Travel Health, Zurich, Switzerland | 623 | Travellers to a resource-limited destination | None (KAP) | Seasonal (and pandemic) influenza | 14% seasonal influenza | High |
| Selcuk, 2016 [28] | Cross-sectional survey | 2013 (July) | Istanbul Ataturk Airport, Istanbul, Turkey | 124 | Turkish travellers to Africa | None | Recommended for destination | 53% vaccinated pre-travel | Medium |
| Tan, 2017 [29] | Retrospective cohort study | 2012 (Jan.)–2013 (Dec.) | Mayo Clinic Travel and Tropical Medicine Clinic, Minnesota, USA | 2073 | Children and adults who sought pre-travel advice (19% VFR) | None | Documented receipt or positive serology or completion of series | 94% men in VFR, 12% rabies in VFR | High |
| Tashani, 2016 [30] | Cross-sectional survey | 2014–2015 | Immunization clinic, Sydney, Australia | 300 | Travellers (>18 year) planning to attend Hajj | None | Pneu and DTP when recommended | 17% pneu, 14% DTP | Medium |
| Wiemken, 2015 [31] | Cross-sectional study | 2013 (Nov.)–2014 (July) | University of Louisville, Travel Clinic, USA | 183 | American travellers before their consultation | TPB | Intention to get vaccinated | Not given | High |
| Yanni, 2010 [32] | Pre- and post-travel surveys | 2008 (June–Sept.) | Departure lounges at airports in New York, Chicago, Los Angeles, and San Francisco | 1301 (pre) (337 post) | American travellers who will travel to Asia | KAP | Influenza | 41% | High |
| Akin, 2016 [33] | Cross-sectional survey | 2015 (July–Sep.) | Daycare chemotherapy unit of Hacettepe University Cancer Institute, Ankara, Turkey | 229 | Adult patients with cancer receiving chemotherapy | None | Adult vaccination coverage (influenza, tetanus, hepatitis, pneu) | 54% were vaccinated at least once, only 9% after cancer diagnosis | Medium |
| Althoff, 2010 [34] | Nested influenza study (interview administered surveys) | 2006–2007 and 2007–2008 | 5 cities in the USA | 1462 | HIV+ women | HBM | Influenza | 55–57% of women reported vaccination (about 44% not vaccinated) | Medium |
| Battistella, 2019 [35] | Cross-sectional observational study | 2017 (Jan.–July) | 7 large dialysis services, Italy | 703 | Dialysis patients | None | Influenza | 58% adherence | High |
| Chehab, 2018 [36] | Cross-sectional study (in longitudinal cohort) | 2012 (Nov.)–2013 (Oct.) | Germany | 579 | SLE patients (48% on IS) | None | Influenza, tetanus, pneu, men and previous refusal | 45% influenza (last year); 65% tetanus; 32% pneu; 6% men | High |
| Chin-Yee, 2011 [37] | Cohort study (one time follow-up) | 2009 (Oct.)–2010 (Mar.) | Tertiary care cancer center, Canada | 129 | Patients with hematologic malignancies (92% chemotherapy, 76% in past 3 mo) | None | Seasonal influenza (and pandemic) | 57% seasonal influenza | Medium |
| Gagneux-Brunon, 2019 [38] | Cross-sectional survey | Unknown | France | 468 | HIV+ patients | None | Pneu, HAV, HBV, seasonal influenza | 30% IPD; 24% HAV; 64% HBV; 40% influenza | Low |
| Haroon, 2011 [39] | Cross-sectional survey (audit) | 2009 (Sept.) | Outpatient clinics, tertiary university hospital, Ireland | 110 | Rheumatology patients on IS | None | Seasonal influenza and pneu | 34% influenza; 11% pneu; 11% both | Medium |
| Harrison, 2017 [40] | Cross-sectional survey | 2015 (Aug.–June) | HIV out-patient department of the University Hospital of Vienna, Austria | 455 | HIV patients | None | Seasonal influenza | 12% influenza | Medium |
| Harrison, 2018 [41] | Cross-sectional survey | 2017 (July–Oct.) | Outpatient clinic, Medical University of Vienna, Austria | 490 | Inflammatory rheumatic disease patients on IS | None | Seasonal influenza | 25% influenza | Medium |
| Lachenal, 2010 [42] | Cross-sectional survey (standardized questionnaire) | 2008 (Jan.) | Centre Léon-Bérard, Lyon, France | 200 | Patients with haematological malignancies (hospitalized or at outpatient clinic) | None | Influenza | 26% | Medium |
| Loubet, 2015 [43] | Self-reported cross-sectional survey | 2013 (Summer) | AVNIR, a group of associations whose goal is to support ICP, France | 3653 | 79% autoimmune, 13% SOT, 8% treated for hematological malignancies. 85% on IS. | KAP | Influenza and pneu | 59% seasonal influenza and 49% pneu | Medium |
| Loubet, 2018 [44] | Self-reported cross-sectional survey | 2015 (Dec.)–2016 (March) | AFA, national association of patients with IBD, France | 199 | IBD patients (62% receiving IS) | KAP | Influenza and pneu | 34% influenza, 38% pneu | Medium |
| Malhi, 2015 [45] | Cross-sectional survey (self-reported, paper-based) | 2013 (Sept)–2014 (Jan.) | IBD Clinic or Endoscopy Suite at Mount Sinai Hospital, Toronto, Canada | 305 | IBD patients (53% using biologicals/steroids) | None | Influenza, pneu, HAV, HBV, VZV, men, HVZ, HPV | 61% influenza, 10% pneu, 61% HBV, 52% HAV, 26% VZV, 21% men, 5% HZV, 11% HPV | High |
| Miller, 2018 [46] | Cross-sectional survey | 2016 (June–Sept.) | 3 tertiary autologous and allogeneic HSCT centres, UK | 93 | HSCT patients (79% autologous) | adjusted HBM | Intention to receive seasonal influenza | 76% expressed high intent | High |
| Mouthon, 2010 [47] | Cross-sectional survey (standardized questionnaires) | 2006 and 2007 | Dept. Of Internal Medicine, Cochin Hospital, France | 177 | Patients with systemic sclerosis | None | Influenza | 39% (last year) | Medium |
| Narula, 2012 [48] | Cross-sectional survey | 2010 (May–Aug.) | McMaster University Medical Centre Digestive Disease Clinic, Canada | 250 | IBD patients (63% on IS) | None | Seasonal (and H1N1) influenza | 25% seasonal influenza | High |
| Nguyen, 2017 [49] | Cross-sectional survey with invitation RCT for new pneu vaccine) | 2014 (Oct.–Nov.) | Outpatients clinic of rheumatology at 2 hospitals in Graasten, Denmark | 192 | RA patients | None | Influenza and pneu | 59% seasonal influenza ever, 49% last year, 6% pneu | High |
| Poeppl, 2015 [50] | Cross-sectional survey | 2013 (July)–2013 (Oct.) | Outpatient departments of the General Hospital Vienna, Austria | 444 | Patients with malignancies (55% solid tumours, 22% haematological malignancy, and 17% had no diagnosed malignancy) | None | Influenza | 18% influenza last year | Medium |
| Price, 2019 [51] | Cross-sectional survey | 2014 (June–July) | Cancer center providing ambulatory care, USA | 703 | Patients (83%) (and caregivers and family (17%) of patients) treated for malignancies | None | Influenza | Patients 72%, caregivers 71% (last year) | Medium |
| Restivo, 2017 [52] | Prospective observational study | 2014 (Oct.)–2015 (April) | SOT Reference Center in Palermo, Sicilia, Italy | 82 | SOT recipients during hospital admission for transplantation | None | Influenza | 38% | Medium |
| Ruiz-Cuesta, 2016 [53] | Prospective observational study | 2012 (Jan.–March) | Reina Sofía University Hospital, Córdoba, Spain | 153 | IBD (50% UC, 50% CD) patients (>14 years old), 34% on biologicals/corticosteroids | None | HAV, HBV, VZV, MMR assessed by registry | 84% | Medium |
| Sadlier, 2015 [54] | Retrospective study, with provider-delivered survey | 2014 (Jan.–Feb.) | Tertiary university hospital in Ireland | 170 | Dermatology patients prescribed systemic IS | None | Influenza and pneu | 38% seasonal influenza last year, 21% pneu last 5 years, 18% both. | Medium |
| Sandler, 2016 [55] | Cross-sectional, telephone survey | 2013 (July–Sept.) | Memorial Medical Center in Chicago, USA | 102 | RA patients (85–91% taking IS) | None | Self-reported and EHR influenza, pneu, and HZV | 79% influenza last season, 54% pneu and 8% HZV | High |
| Savage, 2011 [56] | Retrospective audit | 2010 (Aug.–Oct.) | Outpatient dermatology clinics in Aberdeen RoyalInfirmary, Scotland | 87 | Immunocompromised dermatology patients | None | Influenza and pneu | 70% influenza (last year), 22% pneu | Medium |
| Struijk, 2015 [57] | Cross-sectional survey | Unknown | Renal Transplant Unit, Academic Medical Center, Amsterdam, NL | 526 | 77% renal transplant recipients (and their nephrologists) | KAB | Influenza, tetanus, pneumococci, HAV, HBV | 56% influenza, 15–30% tetanus, 0–5% pneu, 5–30% HAV, 10–20% HBV | High |
| Teich, 2011 [58] | Cross-sectional survey | 2009 (April–Sept.) | Germany | 203 | IBD patients who had not received vaccination counseling ≥1 year (54% on IS) | None | Vaccinations in general | 67% tetanus (<10 years), 21% pertussis, 28% seasonal influenza, 9% pneu | High |
| Urun, 2013 [59] | Cross-sectional survey (with face-to-face interviews) | 2012 (Jan.–March) | Medical Oncology Department of Ankara University Faculty of Medicine, Turkey | 359 | Patients with malignancies | None | Influenza and pneu | 17% influenza 4% pneumococcal | Medium |
| Waszczuk, 2018 [60] | Cross-sectional survey (self-completed) | Unknown | Wrowclaw, Poland | 195 | IBD patients (70% on IS) | None | Influenza, HBV and pneu | HBV 55%; Tdap 12%; HAV 7%; annual influenza 6%; VZV/HZV 3%, and pneu 2% | High |
| Wilckens, 2011 [61] | Cross-sectional survey | 2009 (April–Oct.) | IBD outpatients’ clinic, a tertiary referral center, Lueneburg, Germany | 102 | IBD patients (57% CD, 91% on IS) | None | Vaccinations in general | 19% influenza, 3% pneumoccous, 22% HBV, 5% VZV, 55% MMR, and 63% tetanus. Of those who had traveled, 9% HAV and 1% YF | High |
| Akan, 2016 [62] | Cross-sectional study | 2014 (June–Sept.) | family health care centres in Turkey | 596 | GPs | used, name not mentioned | Seasonal influenza | 27% | High |
| Asma, 2016 [63] | Cross-sectional study | 2015 (Jan.) | 6 university hospitals in Turkey | 642 | 177 (28%) physicians and 448 (71%) nurses | None | Seasonal influenza | 9% | Medium |
| Boey, 2018 [64] | Cross-sectional study | 2015 (Nov.–Dec.) | 13 hospitals and 14 nursing homes in Belgium | 5141 | 4506 hospital staff, 635 HCW nursing home staff. | HBM, HIM and ASE | Seasonal influenza | 2014: 62% (hospital) 2015: 65% (hospital) | High |
| Bonaccorsi, 2015 [65] | Cross-sectional study | 2010 (Oct.–Nov.) | Careggi University Teaching Hospital, Florance, Italy | 2576 | 10% physicians, 39% nurses, 23% students, 4% health care assistant, 15% other | None | Seasonal influenza | 18% | Medium |
| Castilla, 2013 [66] | Cross-sectional study | 2012 (Mar.–May) | PHC workers, Spain | 1956 | 47% GP, 10% paediatricians, 43% nurses | None | Seasonal influenza | 52–61% (2008–2011) | High |
| Ciftci, 2018 [67] | Cross-sectional study | 2015 (Sept.–Dec.) | University Hospital, Ankara, Turkey | 470 | Tertiary healthcare setting (18% physicians, 29% nurses, 11% assistants, 23% auxillary, 9% paramedics, 10% secretaries) | None | Seasonal influenza | 27% | High |
| Costantino, 2019 [68] | Cross sectional study | Influenza seasons 2016–2019 | University Hospital of Palermo, Italy | 1237 | Hospital HCW that had not received influenza vaccination | None | Seasonal influenza | 0% | High |
| Dedoukou, 2010 [69] | Cross-sectional study | 2018 (Oct.–Nov.) | 76 PHCs in Greece | 1617 | PHC: 35% physicians, 32% nurses, 23% paramedical/technical, 8% administrative | None | Seasonal influenza | 41% | Medium |
| deSante, 2010 [70] | Cross-sectional study | 2009 (Apr.) | 2 tertiary care hospitals in Pennsylvania, USA | 227 | House officers and attending physicians in emergency/internal medicine depts. | None | Seasonal influenza | 94% | Medium |
| Dominguez, 2013 [71] | Cross-sectional study | 2012 (Mar.–May) | PHC workers in 7 Spanish regions | 1749 | Familiy physician (47%), paediatrician (10%), nurses (43%). | None | Seasonal influenza | 51% | High |
| Durando, 2016 [72] | Cross-sectional study | 2013 (Oct.)–2014 (Apr.) | San Martino Teaching Hospital/Scientific Research Institute, Italy | 830 | HCW | None | Seasonal influenza | 26% | High |
| Ehrenstein, 2010 [73] | Cross-sectional study | 2006 (Feb.) | Tertiary care university hospital in Germany | 652 | HCW (physicians 36%, nurses 42%, administrators 22%) | None | Seasonal influenza | 34% | Medium |
| Giese, 2016 [74] | Cross-sectional study | 2013 | Ireland | 164 | HCW in a study group of Irish residents | None | Seasonal influenza | 28% | Medium |
| Gramegna, 2018 [75] | Cross-sectional study | 2016 | Italy | 144 | Italian Respiratory Society members | Seasonal influenza | 55% | Medium | |
| Gutknecht, 2016 [76] | Cross-sectional study | 2016 (Feb.–Mar.) | Poland | 77 | Physicians | None | Seasonal influenza | - | Low |
| Hagemeister, 2018 [77] | Cross-sectional study | 2015 (June-July) | University Hospital Würzburg, Germany | 677 | Physicians and nursing staff | None | Seasonal influenza | 55% | Medium |
| Harrison, 2016 [78] | Cross-sectional study | - | Vienna General Hospital, Austria | 116 | Nursing staff | None | HAV/HBV, DTP/Tdap, MMR, influenza, VZV, men, pneu | Seasonal influenza: 42%; Measles: 60% | Medium |
| Hopman, 2010 [79] | Cross-sectional study | 2008 (Nov.–Dec.) | All 8 University Medical Centers in NL | 1238 | HCW at medium and high risk for influenza | HBM, BIM, ASE | Seasonal influenza | 38% | Medium |
| Hulo, 2017 [80] | Cross-sectional study | 2014 | University Hospital Lille, France | 344 | HCW in the emergency departments and the IC units | None | Seasonal influenza | 18% | Medium |
| Johansen, 2012 [81] | Cross-sectional study | 2007 (May) | North and South Dakota | 155 | Randomly selected nurses (52% hospital, 13% clinic, 12% long term) | Triandis | Seasonal influenza | - | Medium |
| Kalemaki, 2020 [82] | Cross-sectional study | - | Crete, Greece | 260 | GPs | None | Seasonal influenza, measlesHBV, Tdap | Seasonal influenza 57%; Measles 26% HBV 68%; Tdap 47% | High |
| Karlsson, 2019 [83] | Cross-sectional study | - | Public hospitals in Finland | 2962 | Hospital personnel who may work with vaccinations (14% physicians) | None | Seasonal influenza | - | High |
| Kisic-Tepavcevic, 2017 [84] | Cross-sectional study | 2015 (Dec.) | Clinical Centre of Serbia, Belgrade, Serbia | 352 | HCW | None | HBV | 66% | High |
| Lehmann, 2015 [85] | Cross-sectional study | 2013 (Feb.–Apr.) | 20 hospitals in Belgium, Germany and NL | 1022 | 56% nurse, 15% physicians, 14% paramedics | None | Seasonal influenza | Total: 37%; Netherlands: 28%; Belgium: 53%; Germany: 36% | High |
| Maridor, 2017 [86] | Cross-sectional study | 2013 | 3 medium-sized, non-teaching hospitals, Switzerland | 252 | Nursing staff | None | Seasonal influenza | 58% | Medium |
| Napolitano, 2019 [87] | Cross-sectional study | 2018 (Sept.–Nov.) | 8 hospitals in Italy | 531 | Random sample of HCWs (29% physicians, 59% nurses) | None | HBV, influenza, MMR, VZV, pertussis | HBV: 98%; DTP: 91%; MMR: 64%; VZV: 59%; TBC: 50%; Influenza: 30%; Men C: 41% | High |
| Nowrouzi, 2014 [88] | Cross-sectional study | 2011 (Sept.–Nov.) | University of Toronto | 963 | Medical trainee’s (post graduate) | HBM | Seasonal (and pandemic) influenza | Seasonal influenza 69–76% (2008–2010) | High |
| Pielak, 2010 [89] | Cross-sectional study | 2005 (Apr.) | British Columbia, Canada | 719 | Immunization nurses of all health units and all physicians that administer vaccinations | TPB | Seasonal influenza | - | High |
| Prematunge, 2014 [90] | Cross-sectional study | 2010 (June) | Tertiary care hospital Ontario, Canada | 3275 | 35% nurse, 5% physician, 11% allied HCW’s, 22% administrative/clerical | None | Seasonal (and pandemic) influenza | Seasonal influenza: 74% | Medium |
| Quan, 2012 [91] | Retrospective cohort study | 2006–2011 | University of California Irvine Healthcare | 32,808 | all HCWs | None | Seasonal influenza | 44–92% (2007–2011) | Medium |
| Rabensteiner, 2018 [92] | Cross-sectional study | 2016 (Oct.–Dec.) | South Tyearolean Health Service, Italy | 4091 | 13% physicians, 20% administrative, 67% sanitary or executive non-medical staff | None | Seasonal influenza | 10% | High |
| Real, 2013 [93] | Cross-sectional study | - | Academic medical center in Lexington, USA | 318 | 80% clinical, 20% non-clinical | RPA | Seasonal influenza | 66% already received the vaccination or planned to get one soon | Medium |
| Rebmann, 2012 [94] | Cross-sectional study | 2011 (Apr.–June) | Saint Louis region, USA | 3188 | 54% non-hospital HCW, 46 % hospital HCW | None | Seasonal (and pandemic) influenza | 2010/11: 79% | High |
| Scatigna, 2017 [95] | Cross-sectional study | 2015 (Apr.–May) | San Salvatore Hospital, L’Aquila, Italy | 334 | Nurses 53%, physicians 23%, other 24% | None | HBV, influenza, MMR, VZV | - | Medium |
| Surtees, 2018 [96] | Cross-sectional study | 2016 | Tertiary referral hospital in Victoria, Australia | 1835 | HCW | None | Seasonal influenza | 97% | High |
| Taddei, 2014 [97] | Cross-sectional study | 2011 (June–Oct.) | 6 public hospitals in Florence, Italy | 436 | 59% nurses, 21% physicians, 13% nursing assistants, and 7% were midwives | None | MMR, VZV, Pertussis | 11% measles, 7% mumps, 17% rubella, 2% VZV, 7% pertussis | Medium |
| Tanguy, 2011 [98] | Cross-sectional study | 2009 (Nov.)–2010 (Feb.) | Tertiary care centre in Pays de la Loire Region, France | 532 | 24% medical staff, 65% nursing staff, 11% ancillary staff | None | Seasonal (and pandemic) influenza | 22% | Medium |
| Vallée-Tourange, 2018 [99] | Cross-sectional study | 2014 (June–July) | A single metropolitan hospital group, UK | 784 | 11% physicians, 36% nurses, 30% allied health professionals, 17% assistants | CME | Seasonal influenza | - | Medium |
| Verger, 2016 [100] | Cross-sectional study | 2014 (Apr.–July) | France | 1582 | GPs | None | Seasonal influenza, DTP, HBV | 72% influenza, 84% DTP, 86% HBV | High |
| Virseda, 2010 [101] | Cross-sectional study | 2009 (Dec.)–2010 (Jan.) | University Hospital 12 de Octubre, Madrid, Spain | 527 | HCW (23% physician, 29% nurse, 19% nursing assistant, 29% ancillary staff) | None | Seasonal (and pandemic) influenza | 50% | Medium |
| Wicker, 2010 [102] | Cross-sectional study | 2010 (Jan–May) | Frankfurt University Hospital, Germany | 1504 | Physicians 26%, nurses 35%, other HCW 23%, students 16% | None | Pertussis | 22% in last 10 years | Medium |
| Wilson, 2019 [103] | Cross-sectional study | Influenza seasons 2015–2017 | Southeast France | 1539 | 74% hospital nurses, 26% community nurses | None | Seasonal influenza | Both seasons: 24% at least one season: 34% | Medium |
| Wilson, 2020 [104] | Cross-sectional study | 2017–2018 | Southeast France | 1539 | 74% hospital nurses, 26% community nurses | None | Mandatory and recommended vaccines in France | 96% BCG, 73% DTP (<10 years), 61% HBV, 58% pertussis, 64% measles, 39% VZV, 27% seasonal influenza (last year) | Medium |
| Zhang, 2011 [105] and 2012 [106] | Cross-sectional study | 2010 (May-Oct.) | University Hospital London | 522 | Qualified nurses (79% working in hospital | None | Seasonal influenza | 36% | Medium |
* concerns vaccination uptake unless otherwise specified. ** Quality is assessed with the AXIS tool. A low score represents fulfillment of 1–9 out of 20 items, medium 10–14 and high 15–20 items (Exact scores are given in Supplementary Table S2). The following abbreviations are used (organized per column, in alphabetical order): Enrolment sites: USA = United States of America; UK = United Kingdom; NL = the Netherlands. Study populations: CD = Crohn’s Disease; GP = general practitioner; HCW = healthcare workers; HIV = human immunodefiency virus; HSCT = hematological stem cell transplantation; IBD = inflammatory bowel disease; ICP = immunocompromised patients; IS = immunosuppressive treatment; PHC = primary healthcare; RA = rheumatoid arthritis; SOT = solid organ transplantation; UC = colitis ulcerosa; VFR = travellers visiting friends and relatives. Theoretical frameworks: ASE = attitude, social influence and self-efficacy model; HBM = health belief model; KAP = knowledge, attitude, practice; HIM = the Health Intention Model; BIM = behavioral intention model; CME = Cognitive model of empowerment; RPA = risk perception attitude framework; Triandis = Triandis model of interpersonal behavior. Vaccinations: BCG = Bacillus Calmette-Guerin (vaccine for tuberculosis); DTP = diphtheria, tetanus, poliomyelitis; HAV = hepatitis A virus; HBV = hepatitis B virus; HZV = herpes zoster virus; JE = Japanese encephalitis; Men = meningococcal disease; menACWY = meningococcal serotype A, C, W and Y; MMR = measles, mumps, rubella; Pneu = pneumococcal disease; TBC = tuberculosis; Tdap = tetanus, diphtheria, acellular pertussis; VZV = varicella zoster virus, YF = yellow fever.
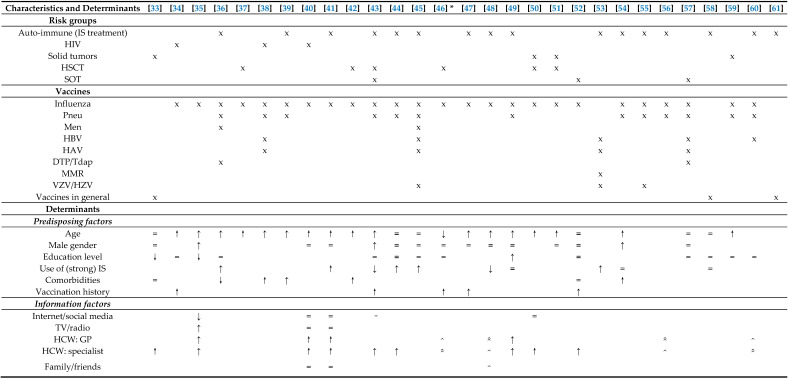
Table 2.
Overview of determinants of vaccination uptake in travellers.
The following symbols are used: x applicable; = no significant difference; ↑ significant positive association (tested by multivariate analysis); ↓ significant negative association (tested by multivariate analysis); ↑ significant positive association (tested by chi-square, univariate analysis or correlation coefficient); ↓ significant negative association (tested by chi-square, univariate analysis or correlation coefficient);  (double caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥50% of the population;
(double caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥50% of the population;  (double caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥50% of the population; ⌃ (caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥10% of the population; ⌄ (caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥10% of the population. * determinants were studied in relation to intention to be vaccinated instead of vaccination uptake. The following abbreviations are used (in alphabetical order): CD = Crohn’s Disease; DTP = diphtheria, tetanus, poliomyelitis; GP = general practitioner; HAV = hepatitis A virus; HBV = hepatitis B virus; HCW = healthcare workers; HIV = human immunodefiency virus; HSCT = hematological stem cell transplantation; HZV = herpes zoster virus; IBD = inflammatory bowel disease; IS = immunosuppressants; JE = Japanese encephalitis; Men = meningococcal disease; MMR = measles, mumps, rubella; Pneu = pneumococcal disease; Tdap = tetanus, diphtheria, acellular pertussis; SOT = solid organ transplantation; VFR = travellers visiting friends and relatives; VZV = varicella zoster virus; YF = yellow fever.
(double caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥50% of the population; ⌃ (caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥10% of the population; ⌄ (caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥10% of the population. * determinants were studied in relation to intention to be vaccinated instead of vaccination uptake. The following abbreviations are used (in alphabetical order): CD = Crohn’s Disease; DTP = diphtheria, tetanus, poliomyelitis; GP = general practitioner; HAV = hepatitis A virus; HBV = hepatitis B virus; HCW = healthcare workers; HIV = human immunodefiency virus; HSCT = hematological stem cell transplantation; HZV = herpes zoster virus; IBD = inflammatory bowel disease; IS = immunosuppressants; JE = Japanese encephalitis; Men = meningococcal disease; MMR = measles, mumps, rubella; Pneu = pneumococcal disease; Tdap = tetanus, diphtheria, acellular pertussis; SOT = solid organ transplantation; VFR = travellers visiting friends and relatives; VZV = varicella zoster virus; YF = yellow fever.
Table 3.
Overview of determinants of vaccination uptake in ICP.
The following symbols are used: x applicable; = no significant difference; ↑ significant positive association (tested by multivariate analysis); ↓ significant negative association (tested by multivariate analysis); ↑ significant positive association (tested by chi-square, univariate analysis or correlation coefficient); ↓ significant negative association (tested by chi-square, univariate analysis or correlation coefficient);  (double caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥50% of the population;
(double caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥50% of the population;  (double caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥50% of the population; ⌃ (caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥10% of the population; ⌄ (caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥10% of the population. The following abbreviations are used (in alphabetical order): CD = Crohn’s Disease; DTP = diphtheria, tetanus, poliomyelitis; GP = general practitioner; HAV = hepatitis A virus; HBV = hepatitis B virus; HCW = healthcare workers; HIV = human immunodefiency virus; HSCT = hematological stem cell transplantation; HZV = herpes zoster virus; IBD = inflammatory bowel disease; IS = immunosuppressants; JE = Japanese encephalitis; Men = meningococcal disease; MMR = measles, mumps, rubella; Pneu = pneumococcal disease; Tdap = tetanus, diphtheria, acellular pertussis; SOT = solid organ transplantation; VFR = travellers visiting friends and relatives. VZV = varicella zoster virus, YF = yellow fever.
(double caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥50% of the population; ⌃ (caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥10% of the population; ⌄ (caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥10% of the population. The following abbreviations are used (in alphabetical order): CD = Crohn’s Disease; DTP = diphtheria, tetanus, poliomyelitis; GP = general practitioner; HAV = hepatitis A virus; HBV = hepatitis B virus; HCW = healthcare workers; HIV = human immunodefiency virus; HSCT = hematological stem cell transplantation; HZV = herpes zoster virus; IBD = inflammatory bowel disease; IS = immunosuppressants; JE = Japanese encephalitis; Men = meningococcal disease; MMR = measles, mumps, rubella; Pneu = pneumococcal disease; Tdap = tetanus, diphtheria, acellular pertussis; SOT = solid organ transplantation; VFR = travellers visiting friends and relatives. VZV = varicella zoster virus, YF = yellow fever.
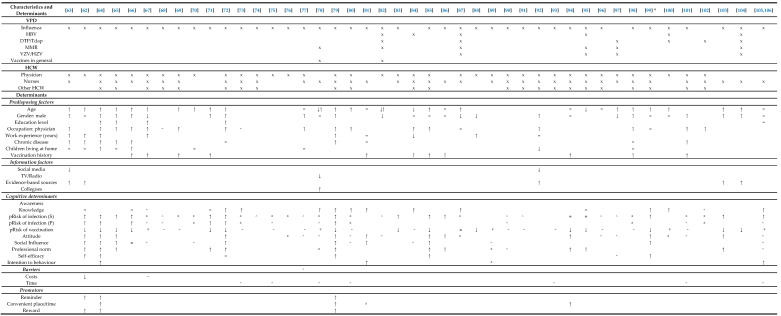
Table 4.
Overview of determinants of vaccination uptake in HCW.
* One scale (MoVac-flu scale) was used for following determinants: knowledge, attitude and self-efficacy. The following symbols are used: x applicable; = no significant difference; ↑ significant positive association (tested by multivariate analysis); ↓ significant negative association (tested by multivariate analysis); ↑ significant positive association (tested by chi-square, univariate analysis or correlation coefficient); ↓ significant negative association (tested by chi-square, univariate analysis or correlation coefficient); ↓↑ significant association, for one vaccine positive, for the other negative;  (double caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥50% of the population;
(double caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥50% of the population;  (double caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥50% of the population; ⌃ (caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥10% of the population; ⌄ (caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥10% of the population. pRisk = perceived risk. pRisk of infection (S/P): S = self; P = patient. The following abbreviations are used (in alphabetical order): CD = Crohn’s Disease; DTP = diphtheria, tetanus, poliomyelitis; GP = general practitioner; HAV = hepatitis A virus; HBV = hepatitis B virus; HCW = healthcare workers; HIV = human immunodefiency virus; HSCT = hematological stem cell transplantation; HZV = herpes zoster virus; IBD = inflammatory bowel disease; IS = immunosuppressants; JE = Japanese encephalitis; Men = meningococcal disease; MMR = measles, mumps, rubella; Pneu = pneumococcal disease; Tdap = tetanus, diphtheria, acellular pertussis; SOT = solid organ transplantation; VFR = travellers visiting friends and relatives. VZV = varicella zoster virus, YF = yellow fever.
(double caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥50% of the population; ⌃ (caret pointing upwards) significance was not tested, but determinant was positively linked to vaccination uptake in ≥10% of the population; ⌄ (caret pointing downwards) significance was not tested, but determinant was negatively linked to vaccination uptake in ≥10% of the population. pRisk = perceived risk. pRisk of infection (S/P): S = self; P = patient. The following abbreviations are used (in alphabetical order): CD = Crohn’s Disease; DTP = diphtheria, tetanus, poliomyelitis; GP = general practitioner; HAV = hepatitis A virus; HBV = hepatitis B virus; HCW = healthcare workers; HIV = human immunodefiency virus; HSCT = hematological stem cell transplantation; HZV = herpes zoster virus; IBD = inflammatory bowel disease; IS = immunosuppressants; JE = Japanese encephalitis; Men = meningococcal disease; MMR = measles, mumps, rubella; Pneu = pneumococcal disease; Tdap = tetanus, diphtheria, acellular pertussis; SOT = solid organ transplantation; VFR = travellers visiting friends and relatives. VZV = varicella zoster virus, YF = yellow fever.
3.1. Vaccination Uptake Among Travellers
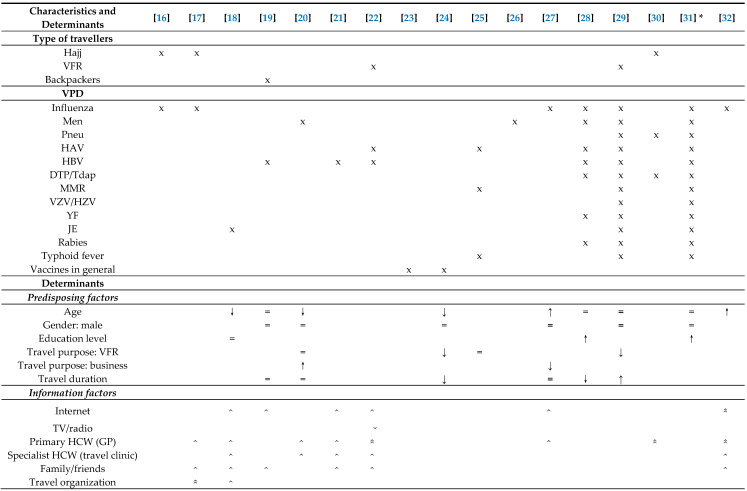
The 17 articles that studied determinants of vaccination uptake among travellers comprised 12 cross-sectional surveys, two pre- and post-travel surveys, and three retrospective studies of which one was based on confirmed cases of VPD (Table 1). Travellers that were studied originated from the USA (6 studies), Australia (4 studies), Europe (5 studies), or mixed continents (2 studies). Sample sizes ranged from 55 to 27,386 and comprised Hajj pilgrims in three studies, travellers to Africa in two studies and to Asia in two studies. Other studies had broader inclusion criteria. Three studies used KAP (knowledge-attitude-practices) surveys and one study mentioned a health behavioural model (theory of planned behaviour) as theoretical background for their study.
3.1.1. Predisposing Factors
Ten articles studied baseline characteristics of travellers that could be associated with vaccination uptake (Table 2). The vaccinations that were studied were diverse, most papers discussed vaccinations for influenza (n = 7), hepatitis B virus (HBV) (n = 6), hepatitis A virus (HAV) (n = 5) and meningococcal disease (n = 5). Regarding age, three papers reported that younger people had a higher uptake [18,20,24]. However, for influenza vaccination this was the opposite: older travellers were more likely to be vaccinated for seasonal influenza [27,32]. Gender was not a significant predictor of vaccination uptake in any of the studies. Education level was studied by three papers [18,27,31]. Two found this determinant to be positively associated with (intention to) obtaining recommended vaccinations [28,31]. Seven studies reported travel purpose in relation to vaccination uptake, but the results were diverse. One study concluded vaccination uptake was highest if the reason of travelling was business or backpacking [20]. However, work-related travel was associated with lower uptake in another study (OR = 0.39, (0.17–0.92)) [27]. Travellers visiting friends and relatives (VFR) had a lower uptake in two studies [24,29], but two other studies found no association [20,25]. Six papers studied the relation between travel duration and vaccination uptake. Two studies showed that uptake was significantly lower when people travelled longer [24,28], while one found that it was higher (for rabies only) [29] and three studies found no difference [19,20,27].
3.1.2. Information Factors
No clear relationship between information sources and vaccination uptake was reported. However, eight studies reported a role for the GP, of which three said that the GP was very influential [22,29,30,32].
3.1.3. Cognitive Determinants
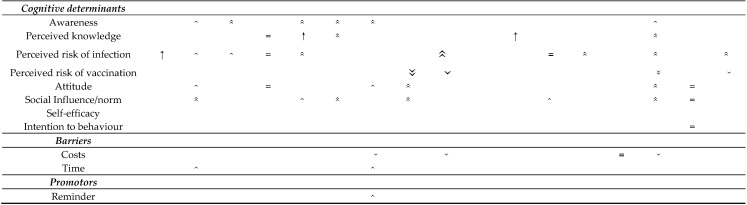
Of all the cognitive determinants studied, perceived risk of infection was most frequently described in relation to vaccination uptake (n = 10). Only one study found a significant positive relation (OR 1.74 (95% CI 1.14–2.62)) [16], and another five reported this factor to play a role in the majority of the study population. Although not often tested for significance, “not feeling at risk of the disease” was a common explanation of a lot of travellers for not receiving the recommended vaccinations. Perceived risk of vaccination was sparsely discussed (n = 4).
Social influence, which comprises mostly trust and recommendations of healthcare providers in this selection of studies, was reported in seven papers and was recognised as important by the majority of the study population in four papers.
Attitude was described in six papers, and was not found to be significant in two of them [19,31]; reliance on natural immunity was mentioned three times as a reason to reject vaccination [17,23,30]. Awareness was also discussed in six papers; although it was not tested for significance, 13–73% mentioned unawareness of the availability of the vaccination (or unawareness of the recommendation of the vaccination) as an important reason for non-uptake [17,18,20,21,22,30].
Five studies reported on knowledge of VPD; two found a significant positive relation between knowledge and vaccination uptake [20,26], one found no relation [19].
3.1.4. Barriers and Facilitators
Reported barriers could be classified in costs and lack of time. Costs were the most described; however, it played a modest role in explaining non-uptake and differed per vaccination. For instance, for influenza vaccination uptake costs were mentioned to play a role in less than 7% of travellers, while for HBV (12%), Japanese encephalitis (35%) and pneumococcal vaccination (38%) concerns about costs were much higher. In two papers lack of time was given as part of the explanation of non-uptake in more than 10% of the study population [17,22]. One paper described that 3–24% of travellers require a reminder to complete their vaccination series [22].
3.2. Vaccination Uptake among Immunocompromised Patients
Twenty-nine articles concerning ICP were included. Most of these studies were cross-sectional (n = 23), but four were prospective (with a follow-up moment) and two retrospective (Table 1). Studies were performed among European (n = 23), American (n = 3) and Canadian (n = 3) populations. Sixteen studies involved patients with auto-immune diseases, of which four studies focussed completely on patients with inflammatory bowel disease. The vaccination uptake of HIV patients was studied in three papers. Four papers studied populations with solid tumours, six papers studied patients who received haematological stem cell transplantation (HSCT) and three papers investigated patients who received a solid organ transplantation (SOT). Almost all papers addressed the influenza vaccination uptake (n = 25) and many also included the uptake of pneumococcal vaccinations (n = 13). Influenza vaccination rates varied from 6–79% and pneumococcal vaccination rates from 2–54%. Lowest rates were reported in Polish inflammatory bowel disease (IBD) patients [60] and highest in American rheumatic patients [55]. In ICP, health behaviour models were cited slightly more than in the travellers population. Two studies were based on the (HBM) and another three studies used KAP surveys.
3.2.1. Predisposing Factors
Most studies (17 out of 24 that studied age) found a positive association between age and vaccination uptake (Table 3). Especially for influenza vaccination, older patients tend to be more compliant with vaccination guidelines in the studied year. Only in one study a negative association was found (OR 0.02, 95% CI (0.01–0.57)) [46]. Most studies report that gender and education level are not significant predictors of vaccination uptake in ICP, with a few exceptions. Three studies showed in a multivariate analysis that males had a higher uptake. Two studies showed a negative association between uptake and education level, while one showed a positive association. In five studies, the use of strong immunosuppressive medication was positively associated with vaccination uptake, whereas in two studies the association was negative and in three there was no association. Generally, ICP with comorbidities in their medical history tend to have a higher uptake in four [38,39,42,54] out of seven studies. One study reported a negative association [42] and two found no significant difference [33,52]. All five papers that included vaccination history (for the same or another vaccination), concluded that there was a positive association between vaccination uptake in the past and current uptake [34,43,46,47,52].
3.2.2. Information Factors
Thirteen studies investigated where ICP retrieve their information from. In general, gathering information from online media sources was somewhat associated with a lower vaccination uptake, while receiving information from HCW resulted in a higher uptake [35,41].
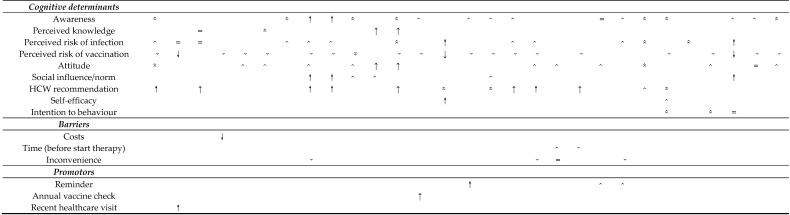
3.2.3. Cognitive Determinants
Perceived risk of vaccination was the most frequently mentioned cognitive determinant, being discussed in 21 of the 29 articles. In all three papers that tested for significance, a negative correlation with vaccination uptake was found, meaning that a higher perceived risk of a vaccine results in a lower uptake. But also that a lower perceived risk, reflected for example by trust in the effectivity of this specific vaccine, increases the uptake. Fear for side-effects or deterioration of their disease caused by the vaccination were mentioned often. Another concern that was often expressed was the doubt of effectivity of vaccination, due to either the immunogenicity of the vaccine or due to the compromised state of the patients’ immune system. Distrust was reported more often for influenza than for other vaccinations [55].
Awareness of either the availability of or the indication for a vaccination was also widely discussed (n = 17). While only found to be significantly correlated twice, this determinant played a role in the majority of the study population in seven papers. Because ICP often mention vaccination not being proposed as a reason for non-uptake, this determinant is related to the information factors, knowledge, and HCW recommendation.
Attitude, covering the attitude to vaccinations in general, was mentioned in 14 studies and was found to be positively correlated twice in multivariate analysis. The effect of a favourable attitude to vaccinations in general was larger on uptake of influenza (adjusted odds ratio (aOR) 3.4 (95% confidence interval (CI) 1.2–9.5)) than on uptake of pneumococcal vaccination (aOR 1.7 [95% CI 0.8–3.5]) [44]. Perceived risk of infection was mentioned equally often as attitude (n = 14) and was also positively associated with uptake, in two of the four studies that tested for significance [46,59].
Although knowledge was only addressed in four papers, in two out of the three articles that tested for significance a positive correlation was found. Recommendation of an HCW was studied in 12 out of the 29 papers and a significant correlation was found in all eight papers that performed statistical analysis. In addition, a frequently reported reason for not being vaccinated was that vaccination was not offered or recommended, which we included under awareness.
Self-efficacy was reported in two papers. One reported that more than 10% of unvaccinated ICP were unsure of how to arrange to receive the vaccines [56], while another reported that patients who find it easier to attend a GP for vaccination, have a higher intention to get vaccinated (p < 0.001) [46]. Regarding intention to behaviour, one high-quality study expressed that 80% of their IBD study population expressed to be willing to receive all of the recommended vaccinations, while only 9% had ever received a pneumococcal vaccination and only 28% was vaccinated against influenza at the time of participation in the study [58]. In another study with 17% influenza and 4% pneumococcal vaccination uptake, the intention to be vaccinated next year was also high and not significantly different between the vaccinated (89%) and unvaccinated group (80%) [59].
3.2.4. Barriers and Facilitators
Cost was only mentioned as a barrier in one paper that found a significant negative correlation with uptake [36]. Lack of time (n = 2) and the inconvenience of another appointment (n = 4) were more often given as reasons for declining vaccination.
3.3. Vaccination Uptake among Healthcare Workers
In HCW, influenza vaccination uptake is most widely studied. In 35 articles out of the 44, seasonal influenza vaccination was the only vaccine studied, with uptake varying between 9% [63] to 97% (mandatory policy) [96]. Most studies were conducted in Italy (n = 8), followed by France (n = 5) and the USA (n = 5). All but one were designed as cross-sectional surveys, with sample sizes ranging from 77 [76] to 32,808 [91]. Seven studies mentioned the use of a theoretical model for their study, which includes the HBM [88], the TPB [89], the risk perception attitude framework [93], the Triandis model of interpersonal behaviour [81], the cognitive model of empowerment [99] or mixtures of different models [64,79] (Table 1).
3.3.1. Predisposing Factors
Thirty-six articles studied at least one predisposing factor in relation to vaccination uptake (Table 4). Of the 30 articles that studied age, 22 found that older healthcare workers had a significantly higher uptake. On the other hand, in the case of hepatitis B [84,95] and measles [78,82], younger HCW’s had higher compliance. In the 27 papers that studied gender, being male was associated with higher vaccination uptake in 13 studies. Five papers mentioned a significantly higher uptake in women, one for rubella only [97], and another for hepatitis B only [82]. Occupation was studied in relation to vaccination uptake in 18 articles. Sixteen papers showed that physicians had a significantly higher uptake than other HCW. This also complies with the significant positive association between education level and uptake that was found in five papers. Presence of a chronic disease resulted in significantly higher uptake in seven studies. In three other studies investigating this factor, no association was found. Having children at home was studied in nine papers, but six found no significant role for this factor in vaccination uptake. Good vaccine compliance in the past turned out to be an excellent predictor of uptake in all 11 studies investigating this factor.
3.3.2. Information Factors
The role of information sources in vaccination uptake was studied in six articles. When information was gathered from evidence-based sources, uptake was significantly higher in all five studies that investigated this source. On the other hand, uptake was lower when information was retrieved from social media, television, or radio [63,92]. Only one study found that gaining information from colleagues was associated with a higher uptake [78].
3.3.3. Cognitive Determinants
Perceived risk was the most frequently described determinant in HCWs. More specifically, perceived personal risk of infection reflects the perceived risk to contract the VPD, including the perceived susceptibility to get infected and the perceived severity of the disease if contracted. In 33 out of 35 papers mentioning perceived risk of infection, a significant positive relation was found between this determinant and vaccination uptake (n = 13), or these reasons were mentioned in a considerable part of the study group (n = 20). Furthermore, in 18 papers a high perceived risk to infect patients was given as a reason for vaccination uptake. Perceived risk (vs. benefit) of vaccination was mentioned in 34 papers. Fifteen studies reported a significant negative relation between perceived risk and uptake, indicating that high perceived risk or low perceived benefit of the vaccination resulted in lower uptake. Additionally, five papers mentioned that this determinant played a role in the majority of the study population. Adequate knowledge of recommendations, effectiveness, and side-effects of vaccinations was significantly positively associated with uptake in 11 papers; in four studies, no significant association was found. Attitude towards vaccination was studied in 22 articles. In half of them, a significant positive association with vaccination uptake was found. Social influence (encouragement of colleagues, managers, family) was analysed in almost half of the studies (n = 15). In only one study no association was found [66], but the others showed either a significant (n = 8) or considerable (n = 6) positive relation with vaccination uptake. Specific for HCW are the social arguments ‘I got vaccinated because it’s my duty as an HCW’ or ‘as an HCW, I have a role in the prevention of epidemics/spread of diseases’, that we collected under the term ‘professional norms’. This determinant was positively associated with uptake in all 15 studies focusing on this factor; in seven out of 11 studies that tested for significance, this factor remained a strong predictor for uptake in multivariate analysis.
3.3.4. Barriers and Facilitators
In comparison with the previous determinants, barriers and facilitators are relatively less studied. Of the barriers, time-related factors were mentioned most frequently and played a considerable role (>10%) in hindering uptake in seven studies. Costs turned out to be no barrier. The fact that the vaccines were free of charge even appeared to be a reason for uptake in two studies [62,67]. On the other hand, facilitators stimulating uptake were getting a reminder (n = 3), convenient time/place of distribution (n = 4), and getting a reward (n = 3). However, in none of the studies were the potential rewards specified.
4. Discussion and Conclusions
Our review of the currently available literature shows that there are clear differences in determinants that play a role in vaccination uptake in travellers, ICP, and HCW. For travellers, low perceived risk of infection and low awareness of vaccination recommendations are most accountable for low uptake. For ICP, awareness of the indication of vaccination plays an important role, together with receiving vaccination recommendations from their treating physician. ICP have a high perceived risk of vaccination, due to not only fear for general side-effects but also concerns about potential consequences for their illness. For HCW, perceived risk of (the severity of) infection for themselves and for their patients together with perceived benefits of vaccination contribute most to their vaccination behaviour.
Regarding predisposing factors, there is a clear positive relationship between age and influenza vaccination uptake in all risk groups. This could be explained by the additional indication older people have for influenza vaccination. However, for other vaccinations, this relationship is either inverted or non-existent. Higher vaccination uptake was seen in males in HCW and ICP, which could be associated with the fact that females worry more about vaccine safety and efficacy than males [107]. Indeed, more side-effects are reported by females, while on the other hand, from a biological perspective, females typically mount higher antibody responses [107]. Although we did not find a clear relationship between education level and vaccination uptake in the risk groups, in HCW the uptake was markedly higher in physicians compared to other HCW. Overall, vaccination history seems to be an excellent universal predictor of future vaccination uptake, probably due to unaltered cognitive determinants.
Regarding cognitive determinants, the greatest diversity between risk groups was found in awareness. In ICP, almost two-thirds of the studies mentioned limited awareness, compared to one-third in travellers and none in HCW. With their education and occupation, it seems quite obvious that HCW are aware of the opportunities and indications for vaccinations. The fact that ICP seem less aware than travellers might have to do with travellers taking an active decision to go abroad realizing that they have to prepare themselves, while patients get passively diagnosed with a disease, and are more dependant of the HCW for information provision. In all groups, HCW as a source of information has a positive effect on uptake. The strong relationship between HCW recommendations and vaccination uptake in ICP (reaching odds ratios up to 53 [52] and 187 [44]), underline the importance of positive attitudes towards vaccination in HCW themselves [100,108].
In general, knowledge has a positive influence on uptake in all risk groups. However, since several studies showed no relation between knowledge and uptake [19,35,62,66,71,95], improving education alone will probably not be sufficient to increase uptake. In all groups, the perceived susceptibility and severity of diseases on one hand and the perceived effectiveness and risks of vaccinations on the other hand are important determinants predicting uptake. Especially ICP and HCW express concerns about the safety and effectiveness of vaccines particularly for influenza vaccination [38,44]. And although the effectiveness of influenza vaccination varies with the coverage of circulating strains each year, another part of the perceived lack of effectiveness could also be explained by the lack of protection for other common cold viruses that can cause influenza-like symptoms [109]. Travelers seem to have low risk perceptions for the diseases they could be vaccinated for as well as for the potential negative effects of vaccination. Despite the high morbidity and mortality of some VPD such as yellow fever, hepatitis B, and influenza, in all risk groups, some participants stated they preferred natural immunization or were against vaccinations in general. Remarkably, attitudes differ for specific vaccinations, for instance, people tend to have a more positive attitude towards pneumococcal vaccination in comparison to the seasonal influenza vaccination [55]. Interestingly, the mistrust of ICP and HCW towards the vaccinations produced by the pharmaceutical industry seems disproportionate to therapeutics manufactured by the same pharmaceutical companies [40,50,72,78]. Here, the difference between prevention and treatment might play a role, where the latter provides a more direct and visible effect. Another possible reason for the negative general attitude towards vaccination, also described in decision making for childhood vaccinations [110], is the increasing tendency for self-empowerment towards personal health decisions. In this view, individuals stand up against imposed policies and want to make their own decisions, which could also be judged by peers as independent and smart decision making [110,111]. At the same time, sources that are being used to make personal health decisions, such as the internet, contain a lot of negative stories [112].
Practical barriers and facilitators play a limited role in vaccination uptake compared to the other determinants. In all three groups, a reminder is an important facilitator and (lack of) time an important barrier. Especially for HCW, this factor is interesting. Physicians report this factor most frequently [73]. They do not only experience lack of time to get vaccinated, they also feel that lack of time impedes their duty to recommend vaccinations to their patients [113]. Again, as HCW recommendations are strongly positively associated with uptake, not only in the other risk groups, but also for HCW themselves (by colleagues for example) [66,80], removing this barrier can result in achieving optimal care for all groups.
Only 16 of the 90 articles that were analysed in this review were based on a health behaviour model. Many of those found determinants which contributed to vaccination uptake to a greater or lesser extent [46,64,79,93,99]. Interventions that focus on a single determinant, such as knowledge, repeatedly proved to be ineffective in the past [66], while multifactorial cognitive intervention strategies are effective to improve uptake [114,115]. Therefore, all determinants that play a role have to be taken into account. Predisposing factors could be used to target specific subgroups and personalize uptake strategies [93]. Facilitators and barriers could be added or taken away to increase vaccination uptake. But, most importantly, interventions need to address cognitive determinants. Interventions that increase awareness and risk perception of infectious diseases are more effective than those decreasing risk perceptions of vaccination by providing scientific information [116]. Social norms can be influenced in the case of hierarchical relationships, for instance, the employer will have an effect on the vaccination decision of HCW and HCW will impact ICP’s decisions. Therefore, multifactorial interventions are needed that address the most important cognitive determinants. As these include awareness and risk perceptions, reminders and incidence data could help. Reminders for travellers could be disseminated in general media before holidays, while for ICP patient associations and HCW could play a role. To improve risk perceptions for the infections, cases of vaccine-preventable diseases should be made public. To decrease risk perceptions of negative effects of vaccinations (e.g., adverse events) new studies should compare the number of influenza-like illnesses in vaccinated and non-vaccinated groups. Furthermore, social norms can be included by making the decisions of vaccination uptake public. For example, in HCW trials have been implemented to test the effects of providing a pin that vaccinated HCW may wear that is saying “deliberately vaccinated”, which could affect both colleagues and patients [117].
Vaccination decisions of travellers and ICP are less well studied than those of HCW. Additionally, data on uptake of vaccinations other than influenza are limited. As the available data show large differences in determinants predicting uptake of influenza versus other vaccinations, further studies are required regarding the uptake of recommended vaccinations for diseases other than influenza. Reaching a more comprehensive understanding of vaccination uptake in different risk groups for the different vaccinations that are indicated, interventions can be developed based on evidence. Moreover, this understanding could help with the implementation of new vaccines for certain risk groups, for instance when a novel SARS-CoV-2 vaccine will be recommended for HCW.
A number of limitations have to be taken into account when interpreting the results of this review. First, articles were only included if they discussed any cognitive determinants that were possibly related to vaccination uptake. This resulted in the exclusion of papers that looked only, although thoroughly, into predisposing factors. Secondly, there was a high level of heterogeneity in the determinants reported, as studies used various health behaviour models as a framework for their studies, and many did not even use a model but just reported results of questionnaires with either open-ended or multiple-choice questions. Furthermore, the influence of determinants on vaccination uptake was measured with different statistical analyses, which also contributed to the high heterogeneity of the data. Therefore, we choose to report the significance and direction of the association, instead of the magnitude. In addition, we choose to compare three different risk groups that we think are important, thereby we could not discuss all determinants in depth. Finally, included studies were based on self-reported vaccination behaviour. Therefore, we have to take into account a certain level of social desirability and recall bias.
To our knowledge, this is the first review that provides a comprehensive overview of health behavioural determinants explaining vaccination uptake in three different risk groups, namely travellers, ICP, and HCW. We showed that there is a large diversity of determinants that affect uptake to a greater or lesser extent. Therefore, we argue that future studies and interventions should be based on multifactorial health behaviour models, especially for travellers and ICP as only a limited number of such studies is available yet.
Acknowledgments
The authors wish to thank Sabrina Meertens-Gunput from the Erasmus MC Medical Library for developing and updating the search strategies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-393X/8/3/480/s1, Table S1: Health behaviour theories on the basis of the I-Change model; Table S2: Quality assessment of included articles, Supplementary File S1: Search strings databases.
Author Contributions
L.D.: conceptualization and design; acquisition and analysis; data interpretation; writing of manuscript. L.v.L.: analysis and data interpretation; writing of the manuscript. E.v.G.: conceptualization and design; revision of the manuscript. H.V.: conceptualization and design; data interpretation; revision of the manuscript. M.G.: conceptualization and design; data interpretation; revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Poland G.A., Whitaker J.A., Poland C.M., Ovsyannikova I.G., Kennedy R.B. Vaccinology in the third millennium: Scientific and social challenges. Curr. Opin. Virol. 2016;17:116–125. doi: 10.1016/j.coviro.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Ten Threats to Global Health in 2019. [(accessed on 19 May 2020)]; Available online: https://www.who.int/news-room/feature-stories/ten-threats-to-global-health-in-2019.
- 3.Phadke V.K., Bednarczyk R.A., Salmon D.A., Omer S.B. Association between vaccine refusal and vaccine-preventable diseases in the united states: A review of measles and pertussis. JAMA. 2016;315:1149–1158. doi: 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO New Measles Surveillance Data for 2019. [(accessed on 6 February 2020)]; Available online: https://www.who.int/immunization/newsroom/measles-data-2019/en/
- 5.Kohn M., Schaffner W. Vaccinating adults with chronic disease: We can do better. Vaccine. 2017;35:3431–3432. doi: 10.1016/j.vaccine.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 6.WHO Life Expectancy and Healthy Life Expectancy. [(accessed on 23 June 2020)]; Available online: https://apps.who.int/gho/data/view.main.SDG2016LEXREGv?lang=en.
- 7.Harpaz R., Dahl R., Dooling K. The prevalence of immunocompromised adults: United States, 2013. Open Forum Infect. Dis. 2016;3:1439. doi: 10.1093/ofid/ofw172.1141. [DOI] [Google Scholar]
- 8.UNWTO International Tourism Highlights. [(accessed on 23 June 2020)]; Available online: https://www.e-unwto.org/doi/pdf/10.18111/9789284421152.
- 9.Gautret P., Botelho-Nevers E., Brouqui P., Parola P. The spread of vaccine-preventable diseases by international travellers: A public-health concern. Clin. Microbiol. Infect. 2012;18(Suppl. 5):77–84. doi: 10.1111/j.1469-0691.2012.03940.x. [DOI] [PubMed] [Google Scholar]
- 10.Haviari S., Bénet T., Saadatian-Elahi M., André P., Loulergue P., Vanhems P. Vaccination of healthcare workers: A review. Hum. Vaccines Immunother. 2015;11:2522–2537. doi: 10.1080/21645515.2015.1082014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angus K., Carnis G., Purves R., Bryce S., MacDonald L., Gordon R. Systematic Literature Review to Examine the Evidence for the Effectiveness of Interventions that Use Theories and Models of Behaviour Change: Towards the Prevention and Control of Communicable Diseases. European Centre for Disease Prevention and Control; Stockholm, Sweden: 2013. [Google Scholar]
- 12.Knops-Dullens T., de Vries N., de Vries H. Reasons for non-attendance in cervical cancer screening programmes: An application of the integrated model for behavioural change. Eur. J. Cancer Prev. 2007;16:436–445. doi: 10.1097/01.cej.0000236250.71113.7c. [DOI] [PubMed] [Google Scholar]
- 13.Downes M.J., Brennan M.L., Williams H.C., Dean R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (axis) BMJ Open. 2016;6:e011458. doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ajzen I. The Theory of Planned Behavior In Organizational Behavior and Human Decision Processes. Volume 50. Academic Press, Inc.; Cambridge, MA, USA: 1991. pp. 179–211. [Google Scholar]
- 15.Bandura A. Social Cognitive Theory. [(accessed on 31 December 2019)]; Available online: https://www.sciencedirect.com/topics/immunology-and-microbiology/social-cognitive-theory.
- 16.Balaban V., Stauffer W., Hammad A., Afgarshe M., Abd-Alla M., Ahmed Q., Memish Z., Saba J., Harton E., Palumbo G., et al. Predictors of protective behaviors among american travelers to the 2009 hajj. J. Epidemiol. Global Health. 2013;3:187–196. doi: 10.1016/j.jegh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barasheed O., Rashid H., Heron L., Ridda I., Haworth E., Nguyen-Van-Tam J., Dwyer D.E., Booy R. Influenza vaccination among australian hajj pilgrims: Uptake, attitudes, and barriers. J. Travel Med. 2014;21:384–390. doi: 10.1111/jtm.12146. [DOI] [PubMed] [Google Scholar]
- 18.Duffy M.R., Reed C., Edelson P.J., Blumensaadt S., Crocker K., Griggs A., Biggerstaff B.J., Delorey M.J., Hayes E.B., Fischer M. A survey of us travelers to Asia to assess compliance with recommendations for the use of japanese encephalitis vaccine. J. Travel Med. 2013;20:165–170. doi: 10.1111/jtm.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frew G., McGeorge E., Grant S., de Wildt G. Hepatitis b: A cross-sectional survey of knowledge, attitudes and practices amongst backpackers in thailand. Travel Med. Infect. Dis. 2017;15:57–62. doi: 10.1016/j.tmaid.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Goodman A.L., Masuet-Aumatell C., Halbert J., Zuckerman J.N. Awareness of meningococcal disease among travelers from the united kingdom to the meningitis belt in africa. Am. J. Trop. Med. Hyg. 2014;91:281–286. doi: 10.4269/ajtmh.13-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbinger K.H., Nothdurft H.D., Prymula R. Online survey: Knowledge about risks, prevention and consequences of infections with hbv among travellers from four european countries. Curr. Med. Res. Opin. 2011;27:489–496. doi: 10.1185/03007995.2010.546392. [DOI] [PubMed] [Google Scholar]
- 22.Heywood A.E., Nothdurft H., Tessier D., Moodley M., Rombo L., Marano C., De Moerlooze L. Pre-travel advice, attitudes and hepatitis a and b vaccination rates among travellers from seven countries†. J. Travel Med. 2016;24:taw069. doi: 10.1093/jtm/taw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igreja R., Barros K., Teodósio R. Attitudes on vaccination among portuguese travelers and brazilian migrants: A pilot study in portugal. Acta Med. Saliniana. 2019;49:1–3. [Google Scholar]
- 24.Lammert S.M., Rao S.R., Jentes E.S., Fairley J.K., Erskine S., Walker A.T., Hagmann S.H., Sotir M.J., Ryan E.T., LaRocque R.C. Refusal of recommended travel-related vaccines among U.S. International travellers in global travepinet. J. Travel Med. 2016;24:taw075. doi: 10.1093/jtm/taw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paudel P., Raina C., Zwar N., Seale H., Worth H., Sheikh M., Heywood A.E. Risk activities and pre-travel health seeking practices of notified cases of imported infectious diseases in Australia. J. Travel Med. 2017;24:tax044. doi: 10.1093/jtm/tax044. [DOI] [PubMed] [Google Scholar]
- 26.Pavli A., Katerelos P., Maltezou H.C. Meningococcal disease awareness and meningoccocal vaccination among Greek students planning to travel abroad. Int. J. Adolesc. Med. Health. 2019;31 doi: 10.1515/ijamh-2017-0016. [DOI] [PubMed] [Google Scholar]
- 27.Pfeil A., Mütsch M., Hatz C., Szucs T.D. A cross-sectional survey to evaluate knowledge, attitudes and practices (kap) regarding seasonal influenza vaccination among european travellers to resource-limited destinations. BMC Public Health. 2010;10:402. doi: 10.1186/1471-2458-10-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selcuk E.B., Kayabas U., Binbasioglu H., Otlu B., Bayindir Y., Bozdogan B., Karatas M. Travel health attitudes among turkish business travellers to african countries. Travel Med. Infect. Dis. 2016;14:614–620. doi: 10.1016/j.tmaid.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Tan E.M., Njeru J.W., Jacobson D.J., Wilson P.M., Chun F., Marcelin J.R., Springer D.J., Wieland M.L., Sia I.G. Pre-travel health care utilization among travelers who visit friends and relatives. Int. J. Travel. Med. Global Health. 2017;5:53–59. doi: 10.15171/ijtmgh.2017.11. [DOI] [Google Scholar]
- 30.Tashani M., Alfelali M., Azeem M.I., Fatema F.N., Barasheed O., Alqahtani A.S., Tekin H., Rashid H., Booy R. Barriers of vaccinations against serious bacterial infections among australian hajj pilgrims. Postgrad. Med. 2016;128:541–547. doi: 10.1080/00325481.2016.1191956. [DOI] [PubMed] [Google Scholar]
- 31.Wiemken T.L., Carrico R.M., Kelley R.R., Binford L.E., Peyrani P., Ford K.D., Welch V., Ramirez J.A. Understanding why low-risk patients accept vaccines: A socio-behavioral approach. BMC Res. Notes. 2015;8:813. doi: 10.1186/s13104-015-1816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanni E.A., Marano N., Han P., Edelson P.J., Blumensaadt S., Becker M., Dwyer S., Crocker K., Daley T., Davis X., et al. Knowledge, attitudes, and practices of us travelers to asia regarding seasonal influenza and h5n1 avian influenza prevention measures. J. Travel Med. 2010;17:374–381. doi: 10.1111/j.1708-8305.2010.00458.x. [DOI] [PubMed] [Google Scholar]
- 33.Akin S., Dizdar O., Ozisik L., Tanriover M.D., Kamisli S., Erman M., Hayran M. Vaccination attitudes among patients with cancer receiving chemotherapy. UHOD Uluslar. Hematol. Onkol. Derg. 2016;26:167–172. doi: 10.4999/uhod.161317. [DOI] [Google Scholar]
- 34.Althoff K.N., Anastos K., Nelson K.E., Celentano D.D., Sharp G.B., Greenblatt R.M., French A.L., Diamond D.J., Holman S., Young M., et al. Predictors of reported influenza vaccination in hiv-infected women in the united states, 2006–2007 and 2007–2008 seasons. Prev. Med. 2010;50:223–229. doi: 10.1016/j.ypmed.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battistella C., Quattrin R., Celotto D., d’Angelo M., Fabbro E., Brusaferro S., Agodi A., Astengo M., Baldo V., Baldovin T., et al. Factors predicting influenza vaccination adherence among patients in dialysis: An italian survey. Hum. Vaccines Immunother. 2019;15:2434–2439. doi: 10.1080/21645515.2019.1588005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chehab G., Richter J.G., Brinks R., Fischer-Betz R., Winkler-Rohlfing B., Schneider M. Vaccination coverage in systemic lupus erythematosus-a cross-sectional analysis of the german long-term study (lula cohort) Rheumatology. 2018;57:1439–1447. doi: 10.1093/rheumatology/key120. [DOI] [PubMed] [Google Scholar]
- 37.Chin-Yee B.H., Monkman K., Hussain Z., Minuk L.A. Attitudes toward vaccination for pandemic h1n1 and seasonal influenza in patients with hematologic malignancies. J. Supportive Oncol. 2011;9:156–160. doi: 10.1016/j.suponc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Gagneux-Brunon A., Fresard A., Lucht F., Botelho-Nevers E. Vaccine coverage in plwh: Disparities and potential impact of vaccine hesitancy. Hum. Vaccines Immunother. 2019;15:305–306. doi: 10.1080/21645515.2018.1534517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haroon M., Adeeb F., Eltahir A., Harney S. The uptake of influenza and pneumococcal vaccination among immunocompromised patients attending rheumatology outpatient clinics. Jt. Bone Spine. 2011;78:374–377. doi: 10.1016/j.jbspin.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Harrison N., Poeppl W., Herkner H., Tillhof K.D., Grabmeier-Pfistershammer K., Rieger A., Forstner C., Burgmann H., Lagler H. Predictors for and coverage of influenza vaccination among HIV-positive patients: A cross-sectional survey. HIV Med. 2017;18:500–506. doi: 10.1111/hiv.12483. [DOI] [PubMed] [Google Scholar]
- 41.Harrison N., Poeppl W., Miksch M., Machold K., Kiener H., Aletaha D., Smolen J.S., Forstner C., Burgmann H., Lagler H. Predictors for influenza vaccine acceptance among patients with inflammatory rheumatic diseases. Vaccine. 2018;36:4875–4879. doi: 10.1016/j.vaccine.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 42.Lachenal F., Sebban C., Duruisseaux M., Biron P., Blay J.Y., Ghesquières H. Influenza vaccination in patients with haematologic malignancies: Analysis of practices in 200 patients in a single center. Bull. Cancer. 2010;97:E33–E36. doi: 10.1684/bdc.2010.1079. [DOI] [PubMed] [Google Scholar]
- 43.Loubet P., Kernéis S., Groh M., Loulergue P., Blanche P., Verger P., Launay O. Attitude, knowledge and factors associated with influenza and pneumococcal vaccine uptake in a large cohort of patients with secondary immune deficiency. Vaccine. 2015;33:3703–3708. doi: 10.1016/j.vaccine.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Loubet P., Verger P., Abitbol V., Peyrin-Biroulet L., Launay O. Pneumococcal and influenza vaccine uptake in adults with inflammatory bowel disease in france: Results from a web-based study. Dig. Liver Dis. 2018;50:563–567. doi: 10.1016/j.dld.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 45.Malhi G., Rumman A., Thanabalan R., Croitoru K., Silverberg M.S., Hillary Steinhart A., Nguyen G.C. Vaccination in inflammatory bowel disease patients: Attitudes, knowledge, and uptake. J. Crohn’s Colitis. 2015;9:439–444. doi: 10.1093/ecco-jcc/jjv064. [DOI] [PubMed] [Google Scholar]
- 46.Miller P.D.E., Forster A.S., De Silva T.I., Leonard H., Anthias C., Mayhew M., Klammer M., Paskar S., Hurst E., Peggs K., et al. Sociodemographic and psychological determinants of influenza vaccine intention among recipients of autologous and allogeneic haematopoietic stem cell transplant: A cross-sectional survey of uk transplant recipients using a modified health belief model. BMJ Open. 2018;8:e021222. doi: 10.1136/bmjopen-2017-021222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mouthon L., Mestre C., Bérezné A., Poiraudeau S., Marchand C., Guilpain P., Guillevin L., Launay O. Low influenza vaccination rate among patients with systemic sclerosis. Rheumatology. 2010;49:600–606. doi: 10.1093/rheumatology/kep440. [DOI] [PubMed] [Google Scholar]
- 48.Narula N., Dhillon A.S., Chauhan U., Marshall J.K. An audit of influenza vaccination status in adults with inflammatory bowel disease. Can. J. Gastroenterol. 2012;26:593–596. doi: 10.1155/2012/158362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen M.T.T., Lindegaard H., Hendricks O., Friis-Møller N. Factors associated with influenza and pneumococcal vaccine uptake among rheumatoid arthritis patients in denmark invited to participate in a pneumococcal vaccine trial (immunovax_ra) Scand. J. Rheumatol. 2017;46:446–453. doi: 10.1080/03009742.2016.1242774. [DOI] [PubMed] [Google Scholar]
- 50.Poeppl W., Lagler H., Raderer M., Sperr W.R., Zielinski C., Herkner H., Burgmann H. Influenza vaccination perception and coverage among patients with malignant disease. Vaccine. 2015;33:1682–1687. doi: 10.1016/j.vaccine.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 51.Price S.A., Podczervinski S., MacLeod K., Helbert L., Pergam S.A. Understanding influenza vaccination rates and reasons for refusal in caregivers and household contacts of cancer patients. Am. J. Infect. Control. 2019;47:468–470. doi: 10.1016/j.ajic.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Restivo V., Vizzini G., Mularoni A., Di Benedetto C., Gioè S.M., Vitale F. Determinants of influenza vaccination among solid organ transplant recipients attending sicilian reference center. Hum. Vaccines Immunother. 2017;13:346–350. doi: 10.1080/21645515.2017.1264792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruiz-Cuesta P., González-Alayón C., Jurado-García J., Iglesias-Flores E.M., Barranco-Quintana J.L., García-García L., Salgueiro-Rodríguez I.M., Benitez-Cantero J.M., García-Sánchez V. Adherence to a predefined vaccination program in patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2016;39:385–392. doi: 10.1016/j.gastrohep.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Sadlier M., Sadlier C., Alani A., Ahmad K., Bergin C., Ramsay B. Poor adherence to vaccination guidelines in dermatology patients on immunosuppressive therapies: An issue that needs addressing. Br. J. Dermatol. 2015;173:288–289. doi: 10.1111/bjd.13543. [DOI] [PubMed] [Google Scholar]
- 55.Sandler D.S., Ruderman E.M., Brown T., Lee J.Y., Mixon A., Liss D.T., Baker D.W. Understanding vaccination rates and attitudes among patients with rheumatoid arthritis. Am. J. Manag. Care. 2016;22:161–167. [PubMed] [Google Scholar]
- 56.Savage J., Muller F., Ormerod A.D. Awareness and uptake of recommended vaccines among immunosuppressed patients. J. R. Coll. Phys. Edinb. 2011;41:202–205. doi: 10.4997/JRCPE.2011.303. [DOI] [PubMed] [Google Scholar]
- 57.Struijk G.H., Lammers A.J.J., Brinkman R.J., Lombarts M.J.M.H., van Vugt M., van der Pant K.A.M.I., ten Berge I.J.M., Bemelman F.J. Immunization after renal transplantation: Current clinical practice. Transplant. Infect. Dis. 2015;17:192–200. doi: 10.1111/tid.12368. [DOI] [PubMed] [Google Scholar]
- 58.Teich N., Klugmann T., Tiedemann A., Holler B., Mössner J., Liebetrau A., Schiefke I. Vaccination coverage in immunosuppressed patients: Results of a regional health services research study. Dtsch. Arztebl. Int. 2011;108:105–111. doi: 10.3238/arztebl.2011.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urun Y., Akbulut H., Demirkazik A., Cay Senler F., Utkan G., Onur H., Icli F. Perception about influenza and pneumococcal vaccines and vaccination coverage among patients with malignancies and their family members. J. Balk. Union Oncol. 2013;18:511–515. [PubMed] [Google Scholar]
- 60.Waszczuk K., Waszczuk E., Szenborn L. Can we better protect patients with inflammatory bowel disease against infections—Patient attitude and personal immunization knowledge. Acta Gastro Enterol. Belg. 2018;81:257–261. [PubMed] [Google Scholar]
- 61.Wilckens V., Kannengiesser K., Hoxhold K., Frenkel C., Kucharzik T., Maaser C. The immunization status of patients with ibd is alarmingly poor before the introduction of specific guidelines. Scand. J. Gastroenterol. 2011;46:855–861. doi: 10.3109/00365521.2011.574734. [DOI] [PubMed] [Google Scholar]
- 62.Akan H., Yavuz E., Yayla M.E., Külbay H., Kaspar E., Zahmacioğlu O., Badur S. Factors affecting uptake of influenza vaccination among family physicians. Vaccine. 2016;34:1712–1718. doi: 10.1016/j.vaccine.2016.01.057. [DOI] [PubMed] [Google Scholar]
- 63.Asma S., Akan H., Uysal Y., Poçan A.G., Sucakli M.H., Yengil E., Gereklioğlu Ç., Korur A., Başhan İ., Erdogan A.F., et al. Factors effecting influenza vaccination uptake among health care workers: A multi-center cross-sectional study. BMC Infect. Dis. 2016;16:192. doi: 10.1186/s12879-016-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boey L., Bral C., Roelants M., De Schryver A., Godderis L., Hoppenbrouwers K., Vandermeulen C. Attitudes, believes, determinants and organisational barriers behind the low seasonal influenza vaccination uptake in healthcare workers—A cross-sectional survey. Vaccine. 2018;36:3351–3358. doi: 10.1016/j.vaccine.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 65.Bonaccorsi G., Santomauro F., Porchia B.R., Niccolai G., Pellegrino E., Bonanni P., Lorini C. Beliefs and opinions of health care workers and students regarding influenza and influenza vaccination in tuscany, central italy. Vaccines. 2015;3:137–147. doi: 10.3390/vaccines3010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castilla J., Martínez-Baz I., Godoy P., Toledo D., Astray J., García S., Mayoral J.M., Martín V., González-Candelas F., Guevara M., et al. Trends in influenza vaccine coverage among primary healthcare workers in spain, 2008–2011. Prev Med. 2013;57:206–211. doi: 10.1016/j.ypmed.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 67.Çiftci F., Şen E., Demir N., Çiftci O., Erol S., Kayacan O. Beliefs, attitudes, and activities of healthcare personnel about influenza and pneumococcal vaccines. Hum. Vaccines Immunother. 2018;14:111–117. doi: 10.1080/21645515.2017.1387703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Costantino C., Ledda C., Genovese C., Contrino E., Vitale E., Maida C.M., Squeri R., Vitale F., Rapisarda V. Immunization status against measles of health-care workers operating at three sicilian university hospitals: An observational study. Vaccines. 2019;7:175. doi: 10.3390/vaccines7040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dedoukou X., Nikolopoulos G., Maragos A., Giannoulidou S., Maltezou H.C. Attitudes towards vaccination against seasonal influenza of health-care workers in primary health-care settings in greece. Vaccine. 2010;28:5931–5933. doi: 10.1016/j.vaccine.2010.06.108. [DOI] [PubMed] [Google Scholar]
- 70.deSante J.E., Caplan A., Shofer F., Behrman A.J. Physician attitudes towards influenza immunization and vaccine mandates. Vaccine. 2010;28:2517–2521. doi: 10.1016/j.vaccine.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 71.Domínguez A., Godoy P., Castilla J., Soldevila N., Toledo D., Astray J., Mayoral J.M., Tamames S., García-Gutiérrez S., González-Candelas F., et al. Knowledge of and attitudes to influenza vaccination in healthy primary healthcare workers in spain, 2011–2012. PLoS ONE. 2013;8:e81200. doi: 10.1371/journal.pone.0081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durando P., Alicino C., Dini G., Barberis I., Bagnasco A.M., Iudici R., Zanini M., Martini M., Toletone A., Paganino C., et al. Determinants of adherence to seasonal influenza vaccination among healthcare workers from an italian region: Results from a cross-sectional study. BMJ Open. 2016;6:e010779. doi: 10.1136/bmjopen-2015-010779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ehrenstein B.P., Hanses F., Blaas S., Mandraka F., Audebert F., Salzberger B. Perceived risks of adverse effects and influenza vaccination: A survey of hospital employees. Eur. J. Public Health. 2010;20:495–499. doi: 10.1093/eurpub/ckp227. [DOI] [PubMed] [Google Scholar]
- 74.Giese C., Mereckiene J., Danis K., O’Donnell J., O’Flanagan D., Cotter S. Low vaccination coverage for seasonal influenza and pneumococcal disease among adults at-risk and health care workers in ireland, 2013: The key role of gps in recommending vaccination. Vaccine. 2016;34:3657–3662. doi: 10.1016/j.vaccine.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 75.Gramegna A., Dellafiore S., Contarini M., Blasi F., Aliberti S., Tosatto R., Mantero M. Knowledge and attitudes on influenza vaccination among italian physicians specialized in respiratory infections: An italian respiratory society (sip/irs) web-based survey. J. Prev. Med. Hyg. 2018;59:E128–E131. [PMC free article] [PubMed] [Google Scholar]
- 76.Gutknecht P., Winiarski T., Trzeciak B.G., Molisz A., Pietrzykowska M., Nowicka-Sauer K., Siebert J. Opinions and behavior of family doctors concerning vaccinating against influenza. Fam. Med. Prim. Care Rev. 2016;18:241–243. doi: 10.5114/fmpcr/63462. [DOI] [Google Scholar]
- 77.Hagemeister M., Stock N., Ludwig T., Heuschmann P., Vogel U. Self-reported influenza vaccination rates and attitudes towards vaccination among health care workers: Results of a survey in a german university hospital. Public Health. 2018;154:102–109. doi: 10.1016/j.puhe.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 78.Harrison N., Brand A., Forstner C., Tobudic S., Burgmann K., Burgmann H. Knowledge, risk perception and attitudes toward vaccination among austrian health care workers: A cross-sectional study. Hum. Vaccines Immunother. 2016;12:2459–2463. doi: 10.1080/21645515.2016.1168959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hopman C.E., Riphagen-Dalhuisen J., Looijmans-van den Akker I., Frijstein G., Van der Geest-Blankert A.D.J., Danhof-Pont M.B., De Jager H.J., Bos A.A., Smeets E., De Vries M.J.T., et al. Determination of factors required to increase uptake of influenza vaccination among hospital-based healthcare workers. J. Hosp. Infect. 2011;77:327–331. doi: 10.1016/j.jhin.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 80.Hulo S., Nuvoli A., Sobaszek A., Salembier-trichard A. Knowledge and attitudes towards influenza vaccination of health care workers in emergency services. Vaccine. 2017;35:205–207. doi: 10.1016/j.vaccine.2016.11.086. [DOI] [PubMed] [Google Scholar]
- 81.Johansen L.J., Stenvig T., Wey H. The decision to receive influenza vaccination among nurses in north and south dakota. Public Health Nurs. 2012;29:116–125. doi: 10.1111/j.1525-1446.2011.00966.x. [DOI] [PubMed] [Google Scholar]
- 82.Kalemaki D., Karakonstantis S., Galanakis E., Lionis C. Vaccination coverage of general practitioners: A cross-sectional study from greece. Public Health. 2020;181:110–113. doi: 10.1016/j.puhe.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 83.Karlsson L.C., Lewandowsky S., Antfolk J., Salo P., Lindfelt M., Oksanen T., Kivimäki M., Soveri A. The association between vaccination confidence, vaccination behavior, and willingness to recommend vaccines among finnish healthcare workers. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0224330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kisic-Tepavcevic D., Kanazir M., Gazibara T., Maric G., Makismovic N., Loncarevic G., Pekmezovic T. Predictors of hepatitis B vaccination status in healthcare workers in Belgrade, Serbia, December 2015. Eurosurveillance. 2017;22:30515. doi: 10.2807/1560-7917.ES.2017.22.16.30515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lehmann B.A., Ruiter R.A.C., van Dam D., Wicker S., Kok G. Sociocognitive predictors of the intention of healthcare workers to receive the influenza vaccine in belgian, dutch and german hospital settings. J. Hosp. Infect. 2015;89:202–209. doi: 10.1016/j.jhin.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 86.Maridor M., Ruch S., Bangerter A., Emery V. Skepticism toward emerging infectious diseases and influenza vaccination intentions in nurses. J. Health Commun. 2017;22:386–394. doi: 10.1080/10810730.2017.1296509. [DOI] [PubMed] [Google Scholar]
- 87.Napolitano F., Bianco A., D’Alessandro A., Papadopoli R., Angelillo I.F. Healthcare workers’ knowledge, beliefs, and coverage regarding vaccinations in critical care units in italy. Vaccine. 2019;37:6900–6906. doi: 10.1016/j.vaccine.2019.09.053. [DOI] [PubMed] [Google Scholar]
- 88.Nowrouzi-Kia B., McGeer A. External cues to action and influenza vaccination among post-graduate trainee physicians in Toronto, Canada. Vaccine. 2014;32:3830–3834. doi: 10.1016/j.vaccine.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 89.Pielak K.L., McIntyre C.C., Tu A.W., Remple V.P., Halperin B., Buxton J.A. Identifying attitudes, beliefs and reported practices of nurses and doctors as immunization providers. J. Adv. Nurs. 2010;66:1602–1611. doi: 10.1111/j.1365-2648.2010.05326.x. [DOI] [PubMed] [Google Scholar]
- 90.Prematunge C., Corace K., McCarthy A., Nair R.C., Roth V., Suh K.N., Garber G. Qualitative motivators and barriers to pandemic vs. Seasonal influenza vaccination among healthcare workers: A content analysis. Vaccine. 2014;32:7128–7134. doi: 10.1016/j.vaccine.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 91.Quan K., Tehrani D.M., Dickey L., Spiritus E., Hizon D., Heck K., Samuelson P., Kornhauser E., Zeitany R., Mancia S., et al. Voluntary to mandatory: Evolution of strategies and attitudes toward influenza vaccination of healthcare personnel. Infect. Control Hosp. Epidemiol. 2012;33:63–70. doi: 10.1086/663210. [DOI] [PubMed] [Google Scholar]
- 92.Rabensteiner A., Buja A., Regele D., Fischer M., Baldo V. Healthcare worker’s attitude to seasonal influenza vaccination in the south tyrolean province of italy: Barriers and facilitators. Vaccine. 2018;36:535–544. doi: 10.1016/j.vaccine.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 93.Real K., Kim S., Conigliaro J. Using a validated health promotion tool to improve patient safety and increase health care personnel influenza vaccination rates. Am. J. Infect. Control. 2013;41:691–696. doi: 10.1016/j.ajic.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 94.Rebmann T., Wright K.S., Anthony J., Knaup R.C., Peters E.B. Seasonal influenza vaccine compliance among hospital-based and nonhospital-based healthcare workers. Infect. Control Hosp. Epidemiol. 2012;33:243–249. doi: 10.1086/664057. [DOI] [PubMed] [Google Scholar]
- 95.Scatigna M., Fabiani L., Micolucci G., Santilli F., Mormile P., Giuliani A.R. Attitudinal variables and a possible mediating mechanism for vaccination practice in health care workers of a local hospital in l’aquila (Italy) Hum. Vaccines Immunother. 2017;13:198–205. doi: 10.1080/21645515.2016.1225638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Surtees T.C., Teh B.W., Slavin M.A., Worth L.J. Factors contributing to declination of annual influenza vaccination by healthcare workers caring for cancer patients: An australian experience. Vaccine. 2018;36:1804–1807. doi: 10.1016/j.vaccine.2018.02.098. [DOI] [PubMed] [Google Scholar]
- 97.Taddei C., Ceccherini V., Niccolai G., Porchia B.R., Boccalini S., Levi M., Tiscione E., Santini M.G., Baretti S., Bonanni P., et al. Attitude toward immunization and risk perception of measles, rubella, mumps, varicella, and pertussis in health care workers working in 6 hospitals of florence, italy 2011. Hum. Vaccin Immunother. 2014;10:2612–2622. doi: 10.4161/21645515.2014.970879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tanguy M., Boyeau C., Pean S., Marijon E., Delhumeau A., Fanello S. Acceptance of seasonal and pandemic a (h1n1) 2009 influenza vaccination by healthcare workers in a french teaching hospital. Vaccine. 2011;29:4190–4194. doi: 10.1016/j.vaccine.2011.03.107. [DOI] [PubMed] [Google Scholar]
- 99.Vallée-Tourangeau G., Promberger M., Moon K., Wheelock A., Sirota M., Norton C., Sevdalis N. Motors of influenza vaccination uptake and vaccination advocacy in healthcare workers: Development and validation of two short scales. Vaccine. 2018;36:6540–6545. doi: 10.1016/j.vaccine.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 100.Verger P., Fressard L., Collange F., Gautier A., Jestin C., Launay O., Raude J., Pulcini C., Peretti-Watel P. Vaccine hesitancy among general practitioners and its determinants during controversies: A national cross-sectional survey in france. EBioMedicine. 2015;2:891–897. doi: 10.1016/j.ebiom.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vírseda S., Restrepo M.A., Arranz E., Magán-Tapia P., Fernández-Ruiz M., de la Cámara A.G., Aguado J.M., López-Medrano F. Seasonal and pandemic a (h1n1) 2009 influenza vaccination coverage and attitudes among health-care workers in a spanish university hospital. Vaccine. 2010;28:4751–4757. doi: 10.1016/j.vaccine.2010.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wicker S., Zielen S., Rose M.A. Obstacles in the motivation of health care workers for pertussis vaccination. Procedia Vaccinol. 2010;2:104–106. doi: 10.1016/j.provac.2010.03.019. [DOI] [Google Scholar]
- 103.Wilson R., Scronias D., Zaytseva A., Ferry M.A., Chamboredon P., Dubé E., Verger P. Seasonal influenza self-vaccination behaviours and attitudes among nurses in southeastern france. Hum. Vaccines Immunother. 2019;15:2423–2433. doi: 10.1080/21645515.2019.1587274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilson R., Zaytseva A., Bocquier A., Nokri A., Fressard L., Chamboredon P., Carbonaro C., Bernardi S., Dubé E., Verger P. Vaccine hesitancy and self-vaccination behaviors among nurses in southeastern france. Vaccine. 2020;38:1144–1151. doi: 10.1016/j.vaccine.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J., While A.E., Norman I.J. Nurses’ knowledge and risk perception towards seasonal influenza and vaccination and their vaccination behaviours: A cross-sectional survey. Int. J. Nurs. Stud. 2011;48:1281–1289. doi: 10.1016/j.ijnurstu.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J., While A.E., Norman I.J. Seasonal influenza vaccination knowledge, risk perception, health beliefs and vaccination behaviours of nurses. Epidemiol. Infect. 2012;140:1569–1577. doi: 10.1017/S0950268811002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Flanagan K.L., Fink A.L., Plebanski M., Klein S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 108.ECDC . Vaccine Hesitancy among Healthcare Workers and Their Patients in Europe—A Qualitative Study. ECDC; Stockholm, Sweden: 2015. [Google Scholar]
- 109.Van Beek J., Veenhoven R.H., Bruin J.P., van Boxtel R.A.J., de Lange M.M.A., Meijer A., Sanders E.A.M., Rots N.Y., Luytjes W. Influenza-like illness incidence is not reduced by influenza vaccination in a cohort of older adults, despite effectively reducing laboratory-confirmed influenza virus infections. J. Infect. Dis. 2017;216:415–424. doi: 10.1093/infdis/jix268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hobson-West P. ‘Trusting blindly can be the biggest risk of all’: Organised resistance to childhood vaccination in the UK. Sociol. Health Illn. 2007;29:198–215. doi: 10.1111/j.1467-9566.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 111.Poltorak M., Leach M., Fairhead J., Cassell J. ‘Mmr talk’ and vaccination choices: An ethnographic study in Brighton. Soc. Sci Med. 2005;61:709–719. doi: 10.1016/j.socscimed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 112.Stahl J.P., Cohen R., Denis F., Gaudelus J., Martinot A., Lery T., Lepetit H. The impact of the web and social networks on vaccination. New challenges and opportunities offered to fight against vaccine hesitancy. Méd. Maladies Infect. 2016;46:117–122. doi: 10.1016/j.medmal.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 113.Mollema L., Staal J.M., van Steenbergen J.E., Paulussen T.G.W.M., de Melker H.E. An exploratory qualitative assessment of factors influencing childhood vaccine providers’ intention to recommend immunization in the netherlands. BMC Public Health. 2012;12:128. doi: 10.1186/1471-2458-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heinrich-Morrison K., McLellan S., McGinnes U., Carroll B., Watson K., Bass P., Worth L.J., Cheng A.C. An effective strategy for influenza vaccination of healthcare workers in australia: Experience at a large health service without a mandatory policy. BMC Infect. Dis. 2015;15:42. doi: 10.1186/s12879-015-0765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jarrett C., Wilson R., O’Leary M., Eckersberger E., Larson H.J. Strategies for addressing vaccine hesitancy—A systematic review. Vaccine. 2015;33:4180–4190. doi: 10.1016/j.vaccine.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 116.Horne Z., Powell D., Hummel J.E., Holyoak K.J. Countering antivaccination attitudes. Proc. Natl. Acad. Sci. USA. 2015;112:10321–10324. doi: 10.1073/pnas.1504019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Riphagen-Dalhuisen J., Burgerhof J.G., Frijstein G., van der Geest-Blankert A.D., Danhof-Pont M.B., de Jager H.J., Bos A.A., Smeets E.E., de Vries M.J., Gallee P.M., et al. Hospital-based cluster randomised controlled trial to assess effects of a multi-faceted programme on influenza vaccine coverage among hospital healthcare workers and nosocomial influenza in The Netherlands, 2009 to 2011. Eurosurveillance. 2013;18:20512. doi: 10.2807/1560-7917.ES2013.18.26.20512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.