Abstract
Ultrasound has emerged as a novel tool for clinical applications, particularly in the context of regenerative medicine. Due to its unique physico-mechanical properties, low-intensity ultrasound (LIUS) has been approved for accelerated fracture healing and for the treatment of established non-union, but its utility has extended beyond tissue engineering to other fields, including cell regeneration. Cells and tissues respond to acoustic ultrasound by switching on genetic repair circuits, triggering a cascade of molecular signals that promote cell proliferation, adhesion, migration, differentiation, and extracellular matrix production. LIUS also induces angiogenesis and tissue regeneration and has anti-inflammatory and anti-degenerative effects. Accordingly, the potential application of ultrasound for tissue repair/regeneration has been tested in several studies as a stand-alone treatment and, more recently, as an adjunct to cell-based therapies. For example, ultrasound has been proposed to improve stem cell homing to target tissues due to its ability to create a transitional and local gradient of cytokines and chemokines. In this review, we provide an overview of the many applications of ultrasound in clinical medicine, with a focus on its value as an adjunct to cell-based interventions. Finally, we discuss the various preclinical and clinical studies that have investigated the potential of ultrasound for regenerative medicine.
Keywords: ultrasound, LIUS, regenerative medicine, stem cells, proliferation, differentiation, migration, clinical trials
1. Introduction
Cell-based therapies that exploit our ever-expanding knowledge of disease processes and stem cell biology have the potential to address and improve a great number of conditions for which no cure or effective treatment is currently available. In the context of stem cell-based treatments, unfortunately, the majority of clinical trials to date show only modest functional improvements in damaged tissues. There are likely many reasons for this, including the difficulty in selecting the optimal cell population and the challenges in guiding the transplanted cells to the target tissue and ensuring their subsequent survival such that they can exert their regenerative actions [1,2]. Driven by these constraints, a number of strategies are being tested to improve the delivery/homing process of stem cells to target tissues, and to enhance engraftment, cell viability and regenerative capabilities [1]. Along this line, a novel strategy that has great potential to improve both homing and the regenerative capacity of stem cells is the use of ultrasound.
Ultrasound is defined as mechanical acoustic waves having a frequency above the upper limit of human hearing. Ultrasound technology has been used for more than fifty years in clinical practice, principally as a safe and non-invasive diagnostic tool, which currently allows for their use as a combined method for drug and gene delivery and therapeutic monitoring [3]. It has become an established and widely used intervention in biomedicine. Therapeutic ultrasound was born in the field of physical therapy, and comprises a group of heterogeneous ultrasound modalities that can be generally classed as being of “high-” or “low-intensity”, depending on whether their objective is to destroy tissue (e.g., kidney stone ablation) or to stimulate physiological processes (e.g., bone fracture repair), respectively [4]. Extracorporeal high-intensity focused ultrasound (HIFU) is an encouraging method for the non-invasive thermal or mechanical ablation of benign and malignant tissue [5]. Low-intensity pulsed ultrasound (LIPUS) was approved by the US Food and Drug Administration in 1994 for the accelerated healing of fresh fractures, and was soon thereafter approved for the treatment of established non-union [6]. A variation of LIPUS is the administration of ultrasound continuously rather than pulsed (100% duty cycle or pulsed ratio), which is known as continuous low-intensity ultrasound (cLIUS). In this context, low-intensity ultrasound (LIUS) would include both pulsed and continuous ultrasound. The success of LIUS in the field of bone regeneration has led to the development of new applications in the regeneration of soft tissue, including cartilage, tendons, and ligaments. Indeed, tissues/organs and also cells can respond positively to LIUS stimulation, resulting in improvements in cell properties and tissue regenerative capacities. Furthermore, LIUS can be used as an adjunct treatment to mesenchymal stem/stromal cell (MSC)-based therapies to bolster repair/regeneration [7,8]. Another variation of therapeutic ultrasound that has been tested to improve the success of cell therapy is pulsed focused ultrasound (pFUS), which is characterized by high-intensity localized pulses for short times to avoid harming the target tissue [9]. This modality has been shown to improve stem cell homing to target tissues [10], likely through its ability to locally upregulate cytokines and chemokines and create a chemotactic gradient for cell migration. Finally, while not considered conventional ultrasound, shock waves are acoustic pressure waves with the capacity to deliver mechanical forces to tissues, and are approved for kidney stone lithotripsy and for physical therapy, typically musculoskeletal applications [11,12]. Table 1 describes the various ultrasound modalities currently in use. Briefly, physical parameters of ultrasound comprise frequency, intensity, duty cycle, and pulse repetition frequency (number of pulses transmitted per second). Frequency is the number of times per second that a particle completes a compression and rarefaction cycle. Intensity is the time-average power transfer per area given in watts per square centimeter (W/cm2) and include the measure of spatial and temporal intensity, spatial average-temporal average (SATA). Duty cycle represents the ratio of time that the transducer is “on”.
Table 1.
Methodologies of therapeutic ultrasound and shock wave application.
| Parameters | Applications | ||
|---|---|---|---|
| Therapeutic Ultrasound (<5 MHz) |
Traditional | 0.1–3 W/cm2 SATA, frequency 1–3 MHz | tendonitis, osteoarthritis and pain relief |
| LIPUS | 30–100 mW/cm2 SATA, frequency 1.5 MHz, 1 kHz, duty cycle of 20% | Bone fracture healing, soft tissue regeneration, anti-inflammatory effects… | |
| cLIUS | 30–100 mW/cm2 SATA, frequency 1.5 MHz, 1 kHz, duty cycle of 100% | ||
| pFUS | 133 W/cm2 SATA, frequency 1 MHz, 5 Hz, duty cycle of 5% | Chemoattractant local and temporal gradient for homing process | |
| HIFU | 400–10,000 W/cm2 SATA, frequency 0.8–4 MHz | non-invasive thermal or mechanical ablation of benign and malignant tissue | |
| Shock waves | 300–3000 pulses, energy 0.05–0.12 mJ/mm2 |
kidney stone lithotripsy, physical therapy | |
SATA: spatial average-temporal average; LIPUS: Low intense pulsed ultrasound; cLIUS: continuous low-intensity ultrasound; pFUS: pulsed focused ultrasound; HIFU: high-intensity focused ultrasound.
The aim of this review is to describe the potential clinical applications of this emerging technology in the context of cell and tissue repair, and the status of clinical trials using ultrasound for regenerative medicine.
2. Biological Effects of Ultrasound
A wide variety of cell types, including osteoblasts [13,14,15], chondroblasts [16], periodontal ligament fibroblasts [17,18,19,20,21], as well as MSC, induced pluripotent stem cell (iPSC), embryonic stem cell (ESC) [22,23], and endothelial cells [24], have been explored for their response to LIUS [25]. The ultrasound-induced changes observed in cells in vitro have also been examined in vivo.
LIUS exerts diverse effects in different tissues. In general, LIUS induces mechanical stimulation enhancing proliferation, differentiation, and maturation of many cell types, and stimulates the specific cell differentiation of MSC [26], improving osteoblast maturation. Interestingly, LIUS has also been reported to increase the viability, proliferation [27], and differentiation of stem cells [28], having a double-edged effect that may promote cell differentiation or the maintenance of stemness.
2.1. Proliferation and Viability
The ability of LIUS to induce proliferation in a broad range of cell types is well recognized. LIPUS stimulates the proliferation of adult stem cells in vitro. For example, human hematopoietic stem cells (HSC) show enhanced proliferation following LIPUS stimulationand maintain the expression of the progenitor cell markers CD34 and CD14 [28]. Similarly, LIUS boosts cell proliferation and colony-forming efficiency of human MSC while preserving their multipotency and karyotype. Mechanistically, it has been proposed that ultrasound upregulates cyclins, which modulate the cell cycle through the activation of extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathways [29,30,31]. Additionally, LIPUS was shown to promote fibroblast proliferation via activation of integrin receptors and the Rho/ROCK/ERK signaling pathway [32]. LIPUS also stimulates the proliferation of induced pluripotent stem cell-derived neural crest stem cells (iPSC–NCSC) [33]. The proliferative capacity of other cell types, including osteoblasts [34] and epithelial cells [35], is also enhanced by LIUS, indicating that this is likely a general phenomenon.
Several studies have reported that LIPUS stimulates cell proliferation without impacting on cell viability [27,28] or apoptosis [36], establishing it as a safe tool to improve the properties of stem cells. Indeed, some studies have reported that ultrasound also enhances cell viability, as shown for human alveolar bone-derived MSC [37] and iPSC–NCSC [33]. Likewise, cLIUS seems to have a protective effect on MSC during chondrogenic differentiation, as revealed by an increase in cell viability in parallel with a decrease in apoptosis in three-dimensional (3D) alginate cultures. In this setting, cLIUS was shown to upregulate anti-apoptotic genes including Bcl-2 (B-cell lymphoma 2) and PCNA (proliferating cell nuclear antigen), which counteracted the induction of proapoptotic genes including P53 and Bax (Bcl-2 associated X-protein) stimulated by the addition of transforming growth factor beta 1 (TGβ-1) and 3D culture [38,39]. However, it has also been described that LIUS can induce apoptosis under specific conditions (usually higher intensities) and in specific cell types. These latter findings make LIUS a potential treatment for some cancers [40,41].
2.2. Adhesion
As mentioned, LIPUS stimulation increases the abundance of cell adhesion-related proteins such as integrins [42], fibronectin, and paxillin, and induces the formation of focal adhesions in MSC. Accordingly, LIUS could trigger the activation of cell adhesion processes through integrin signaling [43,44].
2.3. Extracellular Matrix Production
Largely related to the processes of chondrogenic and osteogenic differentiation, LIUS stimulation can induce the expression of extracellular matrix proteins not only in stem cells, but also in other cell types such as chondrocytes.
LIPUS-stimulated MSC show an increase in hydroxyapatite content and in the release of type I collagen and osteopontin, and this seems to be additive to the use of osteogenic differentiation medium [45]. Human nucleus pulposus cells are a promising cell therapy source for intervertebral disc degeneration, and LIPUS promotes the production of type II collagen, aggrecan, and Sox9 in these cells in vitro. In addition, LIPUS increases the secretion of TIMP-1 (tissue inhibitor of metalloproteinases 1) and reduces MMP-3 (matrix metalloproteinase-3) expression, which represents a positive balance for extracellular matrix (ECM) production. Mechanistically, this seems to involve the activation of the FAK/PI3K/Akt signaling pathway [46]. Similarly, LIUS stimulation increases the production of type II collagen and integrin β1 and reduces MMP-13 expression in rabbit chondrocytes via the integrin/p38 MAPK signaling pathway [47]. In human chondrocytes (C-28/I2 cell line), LIUS was found to induce the phosphorylation of other MAPK, JNK, and ERK signal transducers. The mechanotransduction pathways activated by LIUS seem to involve integrins and stretch-activated channels, and also MAPKs (JNK and ERK pathways) [48]. Thus, LIUS stimulation triggers integrins to mediate mechanotransduction and associated biochemical signaling through the cell membrane and downstream pathways, such as MAPK and PI3K/Akt [47].
2.4. Migration
MSC migration in vitro can be enhanced by LIPUS, and this phenomenon has been associated with increases in CXCR4, integrin-1β [49] and CCR-2 [42] expression, and also with cytoskeletal rearrangements involving the focal adhesion kinase (FAK)-ERK1/2 signaling pathway [50]. LIPUS treatment increases the migration of periodontal ligament stem cells (PDLSC) concomitant with an upregulation of TWIST1 and SDF-1, suggesting the involvement of the SDF1/CXCR4 signaling axis [51].
LIUS promotes the migration of other cell types such as osteoblasts [34], epithelial cells, and keratinocytes. In the latter example, LIUS-induced migration occurs through activation of the PI3K/AKT and MAPK c-Jun N-terminal kinase (JNK) pathways [52], whereas LIUS-induced migration of epithelial cells is associated with increased expression levels of adhesion-related genes, including integrin [35].
LIPUS stimulation of melanoma cells induces Rac activation through integrin-mediated cell-matrix adhesions, which promotes cell migration. Motility mechanisms stimulated by LIUS are dependent on the mechanosensitive focal adhesion protein vinculin and both FAK and Rab5 [53].
2.5. Homing
Ultrasound has great potential to enhance the recruitment and migration of stem cells to target tissue [50]. In the context of bone fractures, LIPUS induced the recruitment of MSC to the injury site, which resulted in improved callus formation and microarchitecture likely mediated by SDF-1 [54].
Pulsed focused ultrasound (pFUS) is an ultrasound modality that uses higher intensities and shorter times to stimulate tissues and generate a transitional gradient through the local upregulation of cytokines and chemokines, facilitating stem cell homing. Burks et al. studied the mechanism of action of pFUS in mouse hamstring muscles, finding an upregulation of cytokines, growth factors, and cell adhesion molecules without changes to the integrity of the tissue [55]. These molecules included SDF-1α, IL-1α, IL-1β, MCP-1, IFNγ, MIP-1α, GM-CSF, VEGF, FGF, HGF, PLGF, ICAM-1, and VCAM-1. The created cytokine gradient improved MSC homing and retention in the muscle [55]. In a mouse model of critical limb ischemia, the combination of pFUS and MSC had a superior therapeutic efficacy to the use of MSC alone. Specifically, pFUS stimulation increased MSC homing four-fold, resulting in an increase in vascular density, a reduction of the fibrotic area, and induced the expression of VEGF and IL-10 by MSCs [56].
2.6. Differentation
One of the most relevant features of LIPUS is its ability to stimulate cell differentiation. LIPUS can promote and accelerate cell differentiation and function in several ways, including increasing signal transduction and the synthesis of growth factors, inducing gene expression, stimulating enzymes in response to heat energy, enhancing metabolism, bolstering the number of blood vessels at the fracture site, enhancing mineralization and maturation, and inhibiting apoptosis and autophagy [57,58,59,60].
Interestingly, in the context of adult stem cells, LIPUS-mediated stimulation of HSC was found to enhance burst-forming unit-erythroid colony formation (of early erythroid progenitors) [28]. However, with respect to potential clinical applications, the majority of differentiation studies with LIUS have been performed with MSC or their derivatives under specific conditions of differentiation. For MSC cultured in the specific induction medium, LIPUS enhances the expression of osteogenic [31,37,45,59,61,62,63,64], chondrogenic [64,65,66,67,68,69,70] and adipogenic [59,71] markers, and also hepatic [72] and neural [36] lineages and astrocytes [73].
For instance, Cui et al. reported the beneficial effects of LIPUS on chondrogenic differentiation in bone marrow-derived MSC [74]. Besides, LIPUS enhanced TGFβ1-mediated chondrocyte differentiation of MSC by increasing the expression of chondrogenic genes and extracellular matrix proteins (type II collagen, aggrecan and SRY-related high mobility group-box gene 9 [SOX9] genes) [69,70], likely involving the integrin-mTOR signaling pathway [75] and the inhibition of autophagy [76].
In addition, in the presence of osteogenic differentiation medium, LIPUS enhanced the expression of osteogenic markers (type I collagen, runt-related transcription factor 2, osteopontin, osteocalcin, and heat shock proteins 70 and 90) in MSC [37,45]. The enhancement of osteogenic differentiation by LIPUS could be explained by the activation of the bone morphogenic protein (BMP) signaling pathway and the upregulation of heat shock proteins [77]. LIPUS also induces in vitro differentiation of other progenitor cells including osteoblasts [78].
It is evident that once MSCs are directed towards a certain fate, with the use of defined medium, LIPUS seems to enhance the differentiation towards that lineage. Interestingly, cells in soft and typically non-proliferating or low-proliferating tissues, such as cardiac and neural tissue, also respond to LIPUS.
LIPUS stimulation also triggers cardiac-related gene and protein expression and the production of binucleated cells in cardiac mesangioblasts by mimicking the effects of 5-azacytidne, a well-known inducer of cardiac differentiation [49]. LIUS treatment improves cardiomyocyte differentiation in murine embryonic stem cells (ESC) by reducing spontaneous differentiation. LIPUS stimulation of ESC-derived embryoid bodies induces cardiac gene expression (cardiac troponin T and α and β myosin heavy chains) and enhances the beating rates of putative cardiomyocytes [22].
Besides, differentiation of MSC to the neuronal lineage is also enhanced by LIPUS through the induction of neural markers and neurotrophic factors [36,79]. With regards to pluripotent stem cells, LIPUS induces neural differentiation in vitro in iPSC-NCSC [33,80], which has the potential for the regeneration of injured nerves. In this case, FAK-ERK1/2 was the proposed signaling pathway for the induction of proliferation and differentiation of iPSC-NCSC [81]. Yang et al. evaluated the effects of LIPUS on iPSC-NCSC and found beneficial effects in the context of cell viability, proliferation, and neural differentiation for peripheral nerve tissue engineering [33].
Finally, LIPUS also plays a role on cell stemness. Some authors including Kusuyama et al. propose a role for LIPUS in stemness maintenance [82], highlighting again the double-edged effect that LIPUS can induce, likely depending on the cell type and the pathophysiological context of the tissue.
2.7. Regenerative Effects
The ability of LIPUS to stimulate cell differentiation and accelerate tissue repair has been extensively exploited in the tissue-engineering field. Tissue engineering encounters many challenges, especially with regards to restoring adequate mechanical strength, which is correlated with matrix production by the engineered tissues. In a recent study using a rabbit spinal cord injury model, LIPUS was found to enhance cell expansion, differentiation, and matrix production by different cells [36]. Likewise, LIPUS stimulation led to better structural formation in the context of new osteogenic and chondrogenic tissue formation, and integration of the newly formed tissues and original bone [36].
Bone growth and repair are under the control of biochemical and mechanical signals. LIPUS stimulation seems not only useful for long bones, but may also be used to accelerate bone regeneration of non-critical calvaria defects, as confirmed in a study with rats [83]. Similarly, a study performed in beagles showed that periodontitis might be ameliorated by LIPUS, as revealed by the acceleration of new alveolar bone formation, with a prospective for promoting periodontal tissue repair [84].
Several studies have also explored the combination of LIPUS and MSC infusion [7]. In rat bone defect models, co-treatment with MSCs and LIPUS was found to improve fracture healing for delayed union or non-union [8], and to enhance cartilage repair and subchondral reconstitution [85]. LIPUS was also shown to enhance spinal fusion in a rabbit posterior spinal fusion model using MSC-derived osteogenic cells [86]. Similarly, the combination of LIPUS and iPCS-NCSC administration was shown to promote the regeneration and reconstruction of rat transected sciatic nerves [87] and was beneficial for nerve injury-induced erectile dysfunction [88], pointing to LIPUS as a regenerative tool in the context of peripheral nerve regeneration.
2.8. Angiogenic Effects
Moreover, LIPUS increases angiogenesis and local blood perfusion [89]. The therapeutic angiogenic effects of LIPUS have been reported in endothelial cells, chick chorioallantoic membrane, and in a rat hind limb ischemia model [90,91,92,93]. Also, a very recent study combined LIPUS and collagen and collagen/hyaluronan scaffolds to enhance angiogenesis in cocultures of human adipose derived stem cells and endothelial cells [94].
LIPUS therapy triggers multiple angiogenic pathways, and has been shown to effectively increase capillary density and regional myocardial blood flow, and normalize ischemia-induced myocardial dysfunction without any adverse effects [95]. Interestingly, the aforementioned study showed that LIPUS therapy ameliorates post-myocardial infarction left ventricular remodeling in mice, with pivotal roles played by the mechanotransduction system, including β1-integrin and caveolin-1 and associated downstream pathways [89]. A β-1integrin signaling pathway was also proposed by Bernal et al. to explain the mechanotransduction induced by LIPUS on cardiac mesoangioblasts and the enhancement of cardiac lineage differentiation [49].
2.9. Anti-Inflammatory Effects
LIPUS has been recently reported to have biologic relevance in the context of inflammation. LIPUS treatment was shown to alleviate the lipopolysaccharide-induced inflammatory response in murine RAW264.7 macrophages by increasing the activation of caveolin-1 [95], and also improved the general status of coxsackievirus-B3-infected mice including their ventricular function by suppressing CD68+ infiltration in the heart [96,97]. Related to inflammation, LIPUS was found to inhibit the inflammatory pathways activated during aseptic joint loosening [97], suggesting an anti-inflammatory role for LIPUS in the orthopedic context. In a similar line, LIPUS was shown to inhibit the production of mature IL1β in a murine model of synovial inflammation [98], and to reduce histological damage and lesion size in the joints of mice with systemic autoimmunity [99]. These studies, overall, suggest an important role of LIPUS not only as a regenerative stimulator but also as an anti-inflammatory treatment, and constitute the basis for a new therapeutic strategy for joint inflammatory diseases. Indeed, LIPUS is suggested to act on both anti-inflammatory and cell differentiation pathways to enhance the outcome of tissue engineering treatments of periodontitis by suppressing inflammation and increasing osteogenic differentiation [100]. Moreover, LIPUS has been proposed as a treatment option for autoimmune diseases [101].
2.10. Anti-Degenerative Effects
LIPUS might also function to protect against degenerative processes in neural tissue. It has been shown LIPUS stimulation increases the protein levels of BDNF, GDNF, VEGF, and GLUT1 in rat brain astrocytes, which have an important role in the growth and survival of developing neurons by secreting neurotrophic factors [102]. In this case, an integrin inhibitor was found to attenuate LIPUS-induced neurotrophic factor expression, suggesting that neurotrophic factor protein levels may be promoted by LIPUS through integrin receptor signaling, consistent with the model proposed in the cardiac context [49]. In addition, LIPUS stimulation protected cells against aluminum toxicity and reduced cerebral damage in terms of myelin loss and apoptosis [102]. Considering that some of these factors have emerged as major molecular players in the regulation of neural circuit development and function, LIPUS may play a beneficial role in neurodegenerative diseases [73].
Regarding the central nervous system, it has been shown that LIPUS protects retinal ganglion cells from apoptosis [103]. Application of LIPUS following nerve surgery may promote nerve regeneration and improve functional outcomes through a variety of mechanisms, including an increase of neurotrophic factors, Schwann cell activation, cellular signaling activation, and induction of mitosis [88].
The most frequently used ultrasound parameters to improve the properties of cells and the regenerative capacity of tissues are shown in Table 2.
Table 2.
More frequent ultrasound parameters used for regenerative effects. The most common ones are highlighted in bold.
| Biological Effects | Ultrasound Parameters | References |
|---|---|---|
| Proliferation and viability | frequency 1/1.5 MHz; 30/50/100 mW/cm2 SATA; 20%/100% duty cycle; 10/20/30 min/day | [28,30,32,33,34,36,39,41] |
| Adhesion | frequency 1.5 MHz; 30/100 mW/cm2 SATA; 20%/100% duty cycle; 10/20 min/day | [42,43,44] |
| Extracellular matrix production | frequency 1/1.5/3 MHz; 30/200 mW/cm2 SATA; 20% duty cycle; 15/20 min/day for 5/7 consecutive days | [45,46,47,48] |
| Migration | frequency 1/1.5/3 MHz; 30/160/240 mW/cm2 SATA; 20%/100% duty cycle; 15/20 min/day | [34,35,42,51,53] |
| Homing | LIPUS: frequency 1.5 MHz; 30 mW/cm2 SATA; 20% duty cycle; 20 min/day for 3 days pFUS: 133 mW/cm2 SATA; frequency 1 MHz; 1/5 Hz; 5% duty cycle |
[54,55,56] |
| Differentiation | frequency 1/1.5/2 MHz; 30/50/150/200/300/500 mW/cm2 SATA; 20% duty cycle; 10/20/30 min/day | [28,36,45,62,64,67,72,77,81] |
| Regenerative effects | frequency 1.5/1.6 MHz; 30/50/90 mW/cm2 SATA; 20% duty cycle; 20 min/day | [8,36,83,84,86] |
| Angiogenic effects | frequency 1/1875 MHz; 15/25 mW/cm2 SATA; 20% duty cycle; 20 min/day | [89,91,94,95] |
| Anti-inflammatory effects | frequency 1.5/3 MHz; 30/200 mW/cm2 SATA; 20% duty cycle; 15/20 min/day | [95,97,99,101] |
| Anti-degenerative effects | frequency 1 MHz; 50/110 mW/cm2 SATA; 20%/50% duty cycle; 10/15 min/day | [73,102,103] |
3. Mechanism of Action
Cell behavior (quiescence, proliferation, migration, differentiation…) can be regulated by chemical (cytokines, hormones, growth factors...) and physical (components of the extracellular matrix, mechanical stimuli, forces...) factors, and also by their relationship with other cells. Belonging to the physical factors, ultrasound is a mechanical signal that can be sensed by stem cells and other cell types such as chondrocytes, epithelial cells, and osteoblasts [29,78]. Ultrasound are able to induce biological effects trough thermal and non-thermal mechanism as mechanical stress, cavitation or gas body activation. LIUS has demonstrated the potential to exert physical force on cells [104]. However, the biophysical mechanisms for their therapeutic action remains unknown [4]. Is better known their safety. The use of low intensity ultrasound induce negligible thermal effects on cells or on living tissues, and there is no microbubbles formation or cavitation [105,106]. The activation of cell mechanoreceptors by ultrasound translates mechanical signals into biochemical responses in the cell resulting in the modulation of biological events linked to survival and growth, among others [107].
The above examples serve to illustrate that the different in vitro LIUS stimulation methods in several cell models seem to converge on similar and shared signaling pathways (Figure 1). Cells “sense” ultrasound mechanical stimulation through transmembrane proteins such as integrins and ion channels [48,108]. Mechanotransduction processes sense environmental mechanical signals and translate them into biochemical responses of the cells. This mechanism requires cell–matrix or cell–cell adhesion plus contractility of the actin cytoskeleton to sense and respond to changes. Integrins act as physical linkages of the cell actin cytoskeleton with the ECM, and mediate attachment and migration of cells [107]. The activation of integrins triggers the recruitment of different cytoskeletal adaptor proteins (talin, vinculin, paxillin…) that bind to the actin cytoskeleton, resulting in the clustering of focal adhesions [109]. LIUS stimulation activates integrins, inducing the formation of stress fibers and focal adhesions. This engagement stimulates signaling cascades that converge on the Rho family of small GTPases, which are powerful modulators of actin cytoskeletal rearrangements and cell migration. Briefly, RhoA activates Rho kinase (ROCK), which in turn promotes myosin II activity [49,107]. Downstream of Rho/ROCK, LIUS stimulates ERK/MAPK signaling. Focal adhesion assembly can, in turn, activate other downstream signaling pathways, including PI3K/Akt [110].
Figure 1.
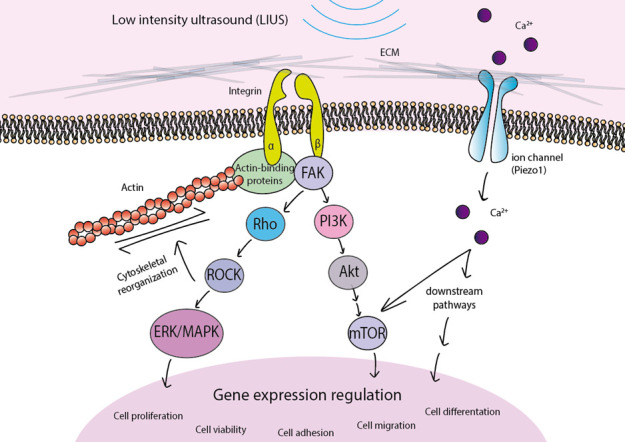
Stimulation of cells by low-intensity ultrasound activates mechanoreceptors including integrins and Piezo1. The subsequent phosphorylation of signaling proteins activates diverse pathways such as Rho/ROCK/ERK/MAPK and PI3K/Akt/mTOR, which ultimately govern different cellular responses including cell proliferation, viability, adhesion, migration or differentiation.
In addition to integrins, other proteins have been described as mechanoreceptors for LIUS stimulation. A study performed on chondrocytes proposed that LIUS stimulation might be mediated via stretch-activated ion channels and integrins and, subsequently, through JNK and ERK pathway activation [48]. More recently, Piezo mechanosensitive ion channels have been implicated in transducing the LIPUS response in cells [106,111,112], with Piezo1 proposed as an important regulator of MSC fate by determining BMP2 expression [113] and differentiation potential. It has also been described that Piezo1 can increase Ca2+ and activate Akt/mammalian target of rapamycin (mTOR) signaling pathways [114].
Cells use mechanoreceptors to sense LIUS, resulting in the activation of important downstream signaling cascades that regulate gene expression to affect fundamental cell functions including cell proliferation, cell viability, adhesion, migration, and differentiation.
4. Therapeutic Applications of Ultrasound
Diverse ultrasound procedures have been developed in the last decades to induce tissue regeneration. These procedures can be performed using a cell-based strategy with different cell types (e.g., MSC, progenitor cells…) or cell-free (e.g., physical or chemical stimulators, viral or non-viral vectors…). However, as discussed earlier, there is evidence that a combination of different strategies has the highest beneficial potential. A range of ultrasound-based interventions has been successfully tested in preclinical studies, and some of them have advanced to clinical trials with humans.
4.1. Search Strategy
We performed a systematic literature review of clinicaltrials.gov and PubMed. Selected studies met the following inclusion criteria: (1) Preclinical studies carried out in animals using diverse ultrasound procedures; (2) Clinical trial studies in any phase of clinical development, but especially in phase II/III testing therapeutic ultrasound efficacy; (3) The study design has a control group and one experimental arm using therapeutic ultrasound; (4) The primary outcome for the preclinical or clinical studies selected was increased tissue healing or improved disease control; (5) The studies selected are original research articles and had appropriate Institutional Review Board approval and informed consent procedures for humans or appropriate local Institutional Care and Animal Use Committee approval for animals. (See Supplementary File S1: Database files for complete data literature generated).
4.2. Preclinical Studies with Ultrasound
Preclinical studies are necessary to assess the safety and efficacy of ultrasound, and to validate the positive effects of therapies for the promotion of healing. Diverse approaches have been used to explore this, including in vitro studies and animal models, and to better understand the pathways involved in regeneration. The bulk of preclinical studies have assessed bone or cartilage regeneration, and relatively few studies have examined tissue remodeling in other systems or pathologies, including muscular or cardiovascular pathologies.
4.2.1. Preclinical Studies in Bone Healing
The search with the possible combinations of keywords; "LIPUS", “animal model” and “bone regeneration"; generated 119 entries in PubMed (June 2020), of which 58 are associated with the use of LIPUS in bone remodeling, 7 are reviews and the remainder use shock waves to stimulate bone healing (Table 3).
Table 3.
Preclinical studies carried out in different animal models for bone healing (LIPUS or shock wave treatment alone, or combined with other therapies, e.g., mesenchymal stem/stromal cell (MSC), grafts or chemical compounds).
| Mouse | Rat | Rabbit | Others | Total | |
|---|---|---|---|---|---|
| LIPUS | |||||
| 1990–1999 | 0 | 1 | 1 | 0 | 2 |
| 2000–2009 | 2 | 5 | 4 | 3 | 14 |
| 2010–2020 | 5 | 23 | 13 | 1 | 42 |
| Shock Wave | - | - | - | - | 31 |
The first preclinical studies of LIPUS in animal models were published in the 1990s, showing that LIPUS applied for 20 minutes daily was useful for bone repair and new bone formation in rabbits [115]. Other studies focused on treatment combinations and demonstrated that fracture healing can be enhanced by combining MSC with LIPUS, which promotes new bone formation and accelerates bone remodeling in rat models [116]. During the next decade, several other preclinical models were used such as mouse, rat, or rabbit, and these studies increased in number in the ‘10s (i.e., 2010–2020).
4.2.2. Preclinical Studies in Other Tissues
Beyond bone repair, other studies on tissue regeneration focused mainly on muscular, nerve, and dental regeneration. A search with the possible combinations of keywords "LIPUS", “animal model” and different “tissue regeneration" options generated 37 entries in PubMed (June 2020), and 19 of these articles described shock waves as ultrasound therapy (Table 4).
Table 4.
Preclinical studies carried out in different animal models for other tissue healing (LIPUS or shock wave treatment alone or combined with other therapies, e.g., MSC, grafts, or chemical compounds).
| Mouse | Rat | Rabbit | Other | Total | |
|---|---|---|---|---|---|
| LIPUS | 16 | ||||
| 1990–1999 | 1 | 13 | 0 | 2 | |
| Shock Wave | - | - | - | - | 19 |
Focusing on LIPUS therapy, six studies examined muscular regeneration, mainly in rats. The most recent work was published in 2019, and investigated the therapeutic effect of LIPUS in a urinary incontinence rat model [117]. The authors found that LIPUS restored bladder capacity and activated satellite cell myodifferentiation. Five studies assessed nerve regeneration in rats. Sato et al. (2016) described a possible novel therapy for inferior alveolar nerve injury, frequently caused by trauma or surgery, using a daily treatment LIPUS protocol [118]. More recently, Xia et al. (2019) described the effects of LIPUS combined with iPSC-NCSC, perfluorotributylamine, and growth differentiation factor 5 for the repair of peripheral nerve injury [81]. Five studies were found on dental regeneration, describing a regenerative effect of LIPUS to repair dentin-pulp complex injury in rats [119]. Finally, one study evaluated the use of LIPUS in wound healing [120] or other tissue regeneration.
4.3. Clinical Trials with Ultrasound
Ultrasound was traditionally used in rehabilitation medicine as an imaging modality to assess the musculoskeletal system. As a therapy, numerous clinical trials have been performed during the last few years. A general search in clinicaltrials.gov using “ultrasound” and “regeneration” resulted in 52 matches, but only 6 of them are related to the use of ultrasound for tissue remodeling or healing (Table 5). All of them are interventional studies, with a randomized design and in a non-determined phase of this study.
Table 5.
Clinical studies carried out for tissue healing with different ultrasound devices.
| NCT | Condition | Treatment | Outcome |
|---|---|---|---|
| NCT03705039 | Knee osteoarthritis | Ultrasound (1 mW/cm2, frequency 1 MHz, 1 kHz, ratio 1:4, 10 min) |
Synovial fluid and cartilage thickness |
| NCT03147313 | Peripheral nerve injury | Shock wave (300 or 500 pulses, frequency 3 Hz, energy 0.1 mJ/mm2) |
regeneration of peripheral nerve injuries |
| NCT04123782 | Muscle injury | Focus Extracorporeal shock wave (3000 impulses at 0.12 mJ/mm2) | Muscle injuries recovery |
| NCT02042066 | Hypertension | Shock wave (0.09–0.1 mJ/mm2) |
Increase tissue perfusion |
| NCT02800200 | Carpal tunnel syndrome | Plasma and Shock wave (100 MPa, 10 μs). |
Regeneration of peripheral neuropathy |
| NCT03986359 | Erectile dysfunction | Shock wave (0.05 mJ/mm2, 3000 pulses) |
Increases erection hardness score |
The first clinical study with ultrasound was in 2014 for hypertension (NCT02042066). The study evaluated the safety and efficacy of shock waves for the treatment of resistant hypertension, and the results were unpublished. However, none of the clinical trials have published their results yet.
A more specific search to select only “low-intensity pulse ultrasound” as therapeutic choice, resulted in 22 matches, of which 10 focused on bone healing (Table 6). The first clinical trial performed using LIPUS as a treatment for bone healing was published in 2007 (NCT00423956). The study enrolled 72 subjects randomized into two arms, and the procedure was to induce tooth root resorption by ultrasound or by sham control. The same group previously published a study with twelve female subjects where the use of LIPUS during orthodontic treatment enhanced the repair of root resorption [121]. They also observed an increase in pulp vascularity in the pulp, according to a published report on new blood-vessel formation by ultrasound [122].
Table 6.
Clinical studies carried out for tissue healing with different LIPUS therapies. (LIPUS parameters 30–100 mW/cm2, frequency 1.5 MHz, 1 kHz, duty cycle of 20%).
| NCT Number | Conditions | Interventions | Outcome |
|---|---|---|---|
| NCT00423956 | Root resorption | LIPUS | Induced inflammatory root resorption |
| NCT00744861 | Lumbar degenerative disc disease | LIPUS | Posterolateral fusion success |
| NCT00931749 | Knee osteoarthritis | LIPUS | Knee cartilage thickness and volume |
| NCT01623804 | Knee osteoarthritis | LIPUS | Pain and physical function |
| NCT02253212 | Glioblastoma | LIPUS + Drug | Blood–brain barrier opening |
| NCT02034409 | Osteoarthritis | LIPUS | Symptoms reduction |
| NCT02383160 | Fractures | LIPUS | Time to union of scaphoid non-unions |
| NCT00667849 | Tibial fractures | LIPUS | Healing of tibial fractures |
| NCT02872922 | Diabetes mellitus | LIPUS | Changes arterial endothelial function |
| NCT03251807 | Malocclusion | LIPUS | Dentoskeletal changes |
| NCT03119961 | Brain diseases | LIPUS | Blood–brain barrier opening |
| NCT03347084 | Brain diseases | LIFUP | Improvements in cognitive functioning |
| NCT03329482 | Low back pain | LIPUS | Pain intensity of patients |
| NCT03744026 | Glioblastoma | LIPUS + Drug | Blood–brain barrier opening |
| NCT03679507 | Osteoarthritis in the knee | LIPUS | Pain intensity measure |
| NCT03657056 | Temporal lobe epilepsy | LIFUP | Changes of the blood-oxygenation level |
| NCT03717922 | Brain diseases | LIFUP | Auditory verbal learning |
| NCT04021420 | Metastatic melanoma | LIPUS + Drug | Blood–brain barrier opening |
| NCT04131387 | Female stress urinary incontinence | LIPUS | Urinary incontinence questionaire |
| NCT03868293 | Drug resistant epilepsy | FOCCUS LIPUS | Reducing seizure frequency |
| NCT04406337 | Osteoarthritis | LIPUS | Visual analog scale (Pain) |
| NCT04339972 | Healthy adults | LIFUP | Analgesia |
LIFUP: Low-Intensity Focus Ultrasound Pulsation.
Several other clinical trials have tested LIPUS to treat osteoarthritis, fractures, or bone degenerative diseases, with all examining tissue remodeling. Indeed, a clinical practice guideline has been recently published for the use of this therapy in bone healing [123]. One of the main clinical trials reporting on bone remodeling is the TRUST Study (NCT00667849), which is the only trial with results to date [124,125]. This trial enrolled 501 patients in a multi-center randomized study; patients were allocated to receive daily LIPUS or sham on their tibial fracture. The aim of the study was to evaluate healing, and the results were negative for the use of LIPUS to improve functional recovery. By contrast, Zhou et al. [126] published a meta-analysis on the use of LIPUS for knee osteoarthritis and found a beneficial effect of LIPUS on pain relief and functional knee recovery.
The latest development in LIPUS technology concerns the application of the therapy in a focused manner (LIFUP, “Low-Intensity Focus Ultrasound Pulsation” or FLIPUS, “Focused Low-Intensity Pulsed Ultrasound”). Clinical trials have tested the effect of this type of LIPUS in different therapeutic scenarios for example, to open the blood–brain barrier in patients for delivery of therapeutic compounds in cancer (NCT03744026, NCT04021420) or brain diseases (NCT03119961). Furthermore, there are clinical trials examining the effects of ultrasound on arterial endothelial function, implying beneficial anti-inflammatory and vasodilatory effects, for example in patients with diabetes (NCT02872922). The group involved in this study previously published results in healthy volunteers, and they found that continuous and pulsed therapeutic 1-MHz ultrasound waveforms improved endothelial function, providing anti-inflammatory vascular effects [127].
In summary, there are a number of varied ongoing clinical trials with different types of ultrasound that may offer a new therapeutic approach for many diseases as a main or adjuvant treatment to enhance cell therapy and/or promote tissue healing, for instance, directing MSC differentiation to osteoblasts for bone formation [116], stimulating dental regeneration [18], or even for treatment of spinal cord injuries [36]. Accordingly, LIPUS could significantly increase the level of stem cell differentiation [25] and could be used as a novel cell therapy strategy. However, many of the preclinical trials developed to date have not been tested on humans, so it remains unknown whether they will clinically effective.
5. Limitations
Finally, it is important to discuss the current limitations of LIPUS as a biostimulator in clinical therapy. It is quite striking that some studies support the use of LIPUS to differentiate MSC toward an osteogenic fate [64], whereas other studies report chondrogenic differentiation [68]. These discrepancies may arise because of the different LIPUS stimulation settings [9]. LIPUS is a physical phenomenon with some characteristic features within a specific range, and it will be necessary to establish exactly the type of stimulation that is performed. Indeed, intensity-related effects have been reported; for example, 30 and 90 mW/cm2 of LIPUS at frequencies of 1–3 MHz were the more effective for cellular differentiation responses and in vitro cell biostimulation [25]. These authors showed that 20, 30, 40, and 50 mW/cm2 were suitable for the differentiation of bone marrow-derived MSC, with these intensities promoting differentiation through the up-regulation of HSP70 and HSP90 expression, activation of the BMP signaling pathway, and inhibiting autophagy [76,77]. However, another study indicated that alkaline phosphatase activity, a marker of osteoblastic cell differentiation, was enhanced at 125 mW/cm2 during osteoblast differentiation [128].
Further studies are required to clarify these discrepancies and to determine the best parameters of LIPUS stimulation as a new therapy.
6. Conclusions
Although ultrasound has shown great potential for the treatment of different conditions, there is a critical need for further studies and the design of standardized protocols (with established parameters of use). This is fundamental for understanding the mechanisms involved for regenerative medicine. While ultrasound continues to be extensively studied, there remains a paucity of human studies. Clinical trials mainly concern the bone field and an improvement in therapeutic treatment of certain diseases. The extensive number of preclinical studies should lead to a better understanding of how ultrasound improves stem cell therapy for the treatment of different conditions or diseases.
Acknowledgments
We thank Kenneth McCreath for critical reading of the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/9/1086/s1, File S1: Database files for complete data literature generated.
Author Contributions
B.d.L.: Conceptualization, Original draft preparation, Review—editing, L.M.P.: Original draft preparation, Review—editing, A.B.: Original draft preparation, Review—editing and B.G.G.: conceptualization, Supervision, Review—editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia, Innovación y Universidades, grant number SAF2015-67911-R.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Baldari S., Di Rocco G., Piccoli M., Pozzobon M., Muraca M., Toietta G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017;18:2087. doi: 10.3390/ijms18102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reisman M., Adams K.T. Stem cell therapy: A look at current research, regulations, and remaining hurdles. P T A Peer Rev. J. Formul. Manag. 2014;39:846–857. [PMC free article] [PubMed] [Google Scholar]
- 3.Sennoga C.A., Kanbar E., Auboire L., Dujardin P.A., Fouan D., Escoffre J.M., Bouakaz A. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Expert Opin. Drug Deliv. 2017;14:1031–1043. doi: 10.1080/17425247.2017.1266328. [DOI] [PubMed] [Google Scholar]
- 4.Miller D.L., Smith N.B., Bailey M.R., Czarnota G.J., Hynynen K., Makin I.R., Bioeffects Committee of the American Institute of Ultrasound in Medicine Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2012;31:623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siedek F., Yeo S.Y., Heijman E., Grinstein O., Bratke G., Heneweer C., Puesken M., Persigehl T., Maintz D., Grull H. Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MR-HIFU): Technical Background and Overview of Current Clinical Applications (Part 1) RoFo Fortschr. Geb. Rontgenstrahlen Nukl. 2019;191:522–530. doi: 10.1055/a-0817-5645. [DOI] [PubMed] [Google Scholar]
- 6.Rubin C., Bolander M., Ryaby J.P., Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. J. Bone Jt. Surg. Am. Vol. 2001;83:259–270. doi: 10.2106/00004623-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Li L., Wu S., Liu Z., Zhuo Z., Tan K., Xia H., Zhuo L., Deng X., Gao Y., Xu Y. Ultrasound-Targeted Microbubble Destruction Improves the Migration and Homing of Mesenchymal Stem Cells after Myocardial Infarction by Upregulating SDF-1/CXCR4: A Pilot Study. Stem Cells Int. 2015;2015:691310. doi: 10.1155/2015/691310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung W.H., Chin W.C., Wei F.Y., Li G., Leung K.S. Applications of exogenous mesenchymal stem cells and low intensity pulsed ultrasound enhance fracture healing in rat model. Ultrasound Med. Biol. 2013;39:117–125. doi: 10.1016/j.ultrasmedbio.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Liu D.D., Ullah M., Concepcion W., Dahl J.J., Thakor A.S. The role of ultrasound in enhancing mesenchymal stromal cell-based therapies. Stem Cells Transl. Med. 2020;9:850–866. doi: 10.1002/sctm.19-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziadloo A., Burks S.R., Gold E.M., Lewis B.K., Chaudhry A., Merino M.J., Frenkel V., Frank J.A. Enhanced homing permeability and retention of bone marrow stromal cells by noninvasive pulsed focused ultrasound. Stem Cells. 2012;30:1216–1227. doi: 10.1002/stem.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haake M., Buch M., Schoellner C., Goebel F., Vogel M., Mueller I., Hausdorf J., Zamzow K., Schade-Brittinger C., Mueller H.H. Extracorporeal shock wave therapy for plantar fasciitis: Randomised controlled multicentre trial. BMJ. 2003;327:75. doi: 10.1136/bmj.327.7406.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stania M., Juras G., Chmielewska D., Polak A., Kucio C., Krol P. Extracorporeal Shock Wave Therapy for Achilles Tendinopathy. Biomed Res. Int. 2019;2019:3086910. doi: 10.1155/2019/3086910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L., Yang Z., Zhang H., Chen W., Chen M., Zhu Z. Low-intensity pulsed ultrasound regulates proliferation and differentiation of osteoblasts through osteocytes. Biochem. Biophys. Res. Commun. 2012;418:296–300. doi: 10.1016/j.bbrc.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Gleizal A., Ferreira S., Lavandier B., Simon B., Beziat J.L., Bera J.C. The impact of low intensity pulsed ultrasound on mouse skull bone osteoblast cultures. Rev. Stomatol. Chir. Maxillo Faciale. 2010;111:280–285. doi: 10.1016/j.stomax.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Wu L., Lin L., Qin Y.X. Enhancement of cell ingrowth, proliferation, and early differentiation in a three-dimensional silicon carbide scaffold using low-intensity pulsed ultrasound. Tissue Eng. Part A. 2015;21:53–61. doi: 10.1089/ten.tea.2013.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda K., Takayama T., Suzuki N., Shimada K., Otsuka K., Ito K. Effects of low-intensity pulsed ultrasound on the differentiation of C2C12 cells. Life Sci. 2006;79:1936–1943. doi: 10.1016/j.lfs.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Kusuyama J., Nakamura T., Ohnishi T., Eiraku N., Noguchi K., Matsuguchi T. Low-Intensity Pulsed Ultrasound (LIPUS) Promotes BMP9-Induced Osteogenesis and Suppresses Inflammatory Responses in Human Periodontal Ligament-Derived Stem Cells. J. Orthop. Trauma. 2017;31:S4. doi: 10.1097/01.bot.0000520897.92470.70. [DOI] [PubMed] [Google Scholar]
- 18.Gao Q., Walmsley A.D., Cooper P.R., Scheven B.A. Ultrasound Stimulation of Different Dental Stem Cell Populations: Role of Mitogen-activated Protein Kinase Signaling. J. Endod. 2016;42:425–431. doi: 10.1016/j.joen.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Hu B., Zhang Y., Zhou J., Li J., Deng F., Wang Z., Song J. Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PLoS ONE. 2014;9:e95168. doi: 10.1371/journal.pone.0095168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren L., Yang Z., Song J., Wang Z., Deng F., Li W. Involvement of p38 MAPK pathway in low intensity pulsed ultrasound induced osteogenic differentiation of human periodontal ligament cells. Ultrasonics. 2013;53:686–690. doi: 10.1016/j.ultras.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Inubushi T., Tanaka E., Rego E.B., Kitagawa M., Kawazoe A., Ohta A., Okada H., Koolstra J.H., Miyauchi M., Takata T., et al. Effects of ultrasound on the proliferation and differentiation of cementoblast lineage cells. J. Periodontol. 2008;79:1984–1990. doi: 10.1902/jop.2008.080081. [DOI] [PubMed] [Google Scholar]
- 22.Teo A., Morshedi A., Wang J.C., Zhou Y., Lim M. Enhancement of Cardiomyogenesis in Murine Stem Cells by Low-Intensity Ultrasound. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2017;36:1693–1706. doi: 10.7863/ultra.16.12042. [DOI] [PubMed] [Google Scholar]
- 23.Appleford M.R., Oh S., Cole J.A., Protivinsky J., Ong J.L. Ultrasound effect on osteoblast precursor cells in trabecular calcium phosphate scaffolds. Biomaterials. 2007;28:4788–4794. doi: 10.1016/j.biomaterials.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He R., Chen J., Jiang J., Liu B., Liang D., Zhou W., Chen W., Wang Y. Synergies of accelerating differentiation of bone marrow mesenchymal stem cells induced by low intensity pulsed ultrasound, osteogenic and endothelial inductive agent. Artif. Cells Nanomed. Biotechnol. 2019;47:674–684. doi: 10.1080/21691401.2019.1576704. [DOI] [PubMed] [Google Scholar]
- 25.Amini A., Chien S., Bayat M. Impact of Ultrasound Therapy on Stem Cell Differentiation, A Systematic Review. Curr Stem Cell Res Ther. 2020;15:462–472. doi: 10.2174/1574888X15666200225124934. [DOI] [PubMed] [Google Scholar]
- 26.Angle S.R., Sena K., Sumner D.R., Virdi A.S. Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low-intensity pulsed ultrasound. Ultrasonics. 2011;51:281–288. doi: 10.1016/j.ultras.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Yoon J.H., Roh E.Y., Shin S., Jung N.H., Song E.Y., Lee D.S., Han K.S., Kim J.S., Kim B.J., Jeon H.W., et al. Introducing pulsed low-intensity ultrasound to culturing human umbilical cord-derived mesenchymal stem cells. Biotechnol. Lett. 2009;31:329–335. doi: 10.1007/s10529-008-9872-5. [DOI] [PubMed] [Google Scholar]
- 28.Xu P., Gul-Uludag H., Ang W.T., Yang X., Huang M., Marquez-Curtis L., McGann L., Janowska-Wieczorek A., Xing J., Swanson E., et al. Low-intensity pulsed ultrasound-mediated stimulation of hematopoietic stem/progenitor cell viability, proliferation and differentiation in vitro. Biotechnol. Lett. 2012;34:1965–1973. doi: 10.1007/s10529-012-0984-6. [DOI] [PubMed] [Google Scholar]
- 29.Budhiraja G., Sahu N., Subramanian A. Low-Intensity Ultrasound Upregulates the Expression of Cyclin-D1 and Promotes Cellular Proliferation in Human Mesenchymal Stem Cells. Biotechnol. J. 2018;13:e1700382. doi: 10.1002/biot.201700382. [DOI] [PubMed] [Google Scholar]
- 30.Ling L., Wei T., He L., Wang Y., Wang Y., Feng X., Zhang W., Xiong Z. Low-intensity pulsed ultrasound activates ERK1/2 and PI3K-Akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells. Cell Prolif. 2017;50 doi: 10.1111/cpr.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carina V., Costa V., Raimondi L., Pagani S., Sartori M., Figallo E., Setti S., Alessandro R., Fini M., Giavaresi G. Effect of low-intensity pulsed ultrasound on osteogenic human mesenchymal stem cells commitment in a new bone scaffold. J. Appl. Biomater. Funct. Mater. 2017;15:e215–e222. doi: 10.5301/jabfm.5000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou S., Schmelz A., Seufferlein T., Li Y., Zhao J., Bachem M.G. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J. Biol. Chem. 2004;279:54463–54469. doi: 10.1074/jbc.M404786200. [DOI] [PubMed] [Google Scholar]
- 33.Lv Y., Zhao P., Chen G., Sha Y., Yang L. Effects of low-intensity pulsed ultrasound on cell viability, proliferation and neural differentiation of induced pluripotent stem cells-derived neural crest stem cells. Biotechnol. Lett. 2013;35:2201–2212. doi: 10.1007/s10529-013-1313-4. [DOI] [PubMed] [Google Scholar]
- 34.Man J., Shelton R.M., Cooper P.R., Landini G., Scheven B.A. Low intensity ultrasound stimulates osteoblast migration at different frequencies. J. Bone Miner. Metab. 2012;30:602–607. doi: 10.1007/s00774-012-0368-y. [DOI] [PubMed] [Google Scholar]
- 35.Iwanabe Y., Masaki C., Tamura A., Tsuka S., Mukaibo T., Kondo Y., Hosokawa R. The effect of low-intensity pulsed ultrasound on wound healing using scratch assay in epithelial cells. J. Prosthodont. Res. 2016;60:308–314. doi: 10.1016/j.jpor.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Ning G.Z., Song W.Y., Xu H., Zhu R.S., Wu Q.L., Wu Y., Zhu S.B., Li J.Q., Wang M., Qu Z.G., et al. Bone marrow mesenchymal stem cells stimulated with low-intensity pulsed ultrasound: Better choice of transplantation treatment for spinal cord injury: Treatment for SCI by LIPUS-BMSCs transplantation. CNS Neurosci. Ther. 2019;25:496–508. doi: 10.1111/cns.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim K., Kim J., Seonwoo H., Park S.H., Choung P.H., Chung J.H. In vitro effects of low-intensity pulsed ultrasound stimulation on the osteogenic differentiation of human alveolar bone-derived mesenchymal stem cells for tooth tissue engineering. Biomed Res. Int. 2013;2013:269724. doi: 10.1155/2013/269724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H.J., Choi B.H., Min B.H., Park S.R. Low-intensity ultrasound inhibits apoptosis and enhances viability of human mesenchymal stem cells in three-dimensional alginate culture during chondrogenic differentiation. Tissue Eng. 2007;13:1049–1057. doi: 10.1089/ten.2006.0346. [DOI] [PubMed] [Google Scholar]
- 39.Park S.R., Choi B.H., Min B.H. Low-Intensity Ultrasound (LIUS) as an Innovative Tool for Chondrogenesis of Mesenchymal Stem Cells (MSCs) Organogenesis. 2007;3:74–78. doi: 10.4161/org.3.2.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feril L.B., Jr., Kondo T., Cui Z.G., Tabuchi Y., Zhao Q.L., Ando H., Misaki T., Yoshikawa H., Umemura S. Apoptosis induced by the sonomechanical effects of low intensity pulsed ultrasound in a human leukemia cell line. Cancer Lett. 2005;221:145–152. doi: 10.1016/j.canlet.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 41.Shi M., Liu B., Liu G., Wang P., Yang M., Li Y., Zhou J. Low intensity-pulsed ultrasound induced apoptosis of human hepatocellular carcinoma cells in vitro. Ultrasonics. 2016;64:43–53. doi: 10.1016/j.ultras.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Xiao W., Xu Q., Zhu Z., Li L., Chen W. Different performances of CXCR4, integrin-1beta and CCR-2 in bone marrow stromal cells (BMSCs) migration by low-intensity pulsed ultrasound stimulation. Biomed. Technik. Biomed. Eng. 2017;62:89–95. doi: 10.1515/bmt-2015-0166. [DOI] [PubMed] [Google Scholar]
- 43.Roper J., Harrison A., Bass M.D. Induction of adhesion-dependent signals using low-intensity ultrasound. J. Vis. Exp. 2012;63:e4024. doi: 10.3791/4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi W.H., Choi B.H., Min B.H., Park S.R. Low-intensity ultrasound increased colony forming unit-fibroblasts of mesenchymal stem cells during primary culture. Tissue Eng. Part C Methods. 2011;17:517–526. doi: 10.1089/ten.tec.2010.0231. [DOI] [PubMed] [Google Scholar]
- 45.Costa V., Carina V., Fontana S., De Luca A., Monteleone F., Pagani S., Sartori M., Setti S., Faldini C., Alessandro R., et al. Osteogenic commitment and differentiation of human mesenchymal stem cells by low-intensity pulsed ultrasound stimulation. J. Cell. Physiol. 2018;233:1558–1573. doi: 10.1002/jcp.26058. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X., Hu Z., Hao J., Shen J. Low Intensity Pulsed Ultrasound Promotes the Extracellular Matrix Synthesis of Degenerative Human Nucleus Pulposus Cells Through FAK/PI3K/Akt Pathway. Spine. 2016;41:E248–E254. doi: 10.1097/BRS.0000000000001220. [DOI] [PubMed] [Google Scholar]
- 47.Xia P., Ren S., Lin Q., Cheng K., Shen S., Gao M., Li X. Low-Intensity Pulsed Ultrasound Affects Chondrocyte Extracellular Matrix Production via an Integrin-Mediated p38 MAPK Signaling Pathway. Ultrasound Med. Biol. 2015;41:1690–1700. doi: 10.1016/j.ultrasmedbio.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Choi B.H., Choi M.H., Kwak M.G., Min B.H., Woo Z.H., Park S.R. Mechanotransduction pathways of low-intensity ultrasound in C-28/I2 human chondrocyte cell line. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2007;221:527–535. doi: 10.1243/09544119JEIM201. [DOI] [PubMed] [Google Scholar]
- 49.Bernal A., Perez L.M., De Lucas B., Martin N.S., Kadow-Romacker A., Plaza G., Raum K., Galvez B.G. Low-Intensity Pulsed Ultrasound Improves the Functional Properties of Cardiac Mesoangioblasts. Stem Cell Rev. Rep. 2015;11:852–865. doi: 10.1007/s12015-015-9608-6. [DOI] [PubMed] [Google Scholar]
- 50.Chen J., Jiang J., Wang W., Qin J., Chen J., Chen W., Wang Y. Low intensity pulsed ultrasound promotes the migration of bone marrow- derived mesenchymal stem cells via activating FAK-ERK1/2 signalling pathway. Artif. Cells Nanomed. Biotechnol. 2019;47:3603–3613. doi: 10.1080/21691401.2019.1657878. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Li J., Qiu Y., Hu B., Chen J., Fu T., Zhou P., Song J. Low intensity pulsed ultrasound promotes periodontal ligament stem cell migration through TWIST1mediated SDF1 expression. Int. J. Mol. Med. 2018;42:322–330. doi: 10.3892/ijmm.2018.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leng X., Shang J., Gao D., Wu J. Low-intensity pulsed ultrasound promotes proliferation and migration of HaCaT keratinocytes through the PI3K/AKT and JNK pathways. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2018;51:e7862. doi: 10.1590/1414-431x20187862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atherton P., Lausecker F., Harrison A., Ballestrem C. Low-intensity pulsed ultrasound promotes cell motility through vinculin-controlled Rac1 GTPase activity. J. Cell Sci. 2017;130:2277–2291. doi: 10.1242/jcs.192781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei F.Y., Leung K.S., Li G., Qin J., Chow S.K., Huang S., Sun M.H., Qin L., Cheung W.H. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS ONE. 2014;9:e106722. doi: 10.1371/journal.pone.0106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burks S.R., Ziadloo A., Hancock H.A., Chaudhry A., Dean D.D., Lewis B.K., Frenkel V., Frank J.A. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS ONE. 2011;6:e24730. doi: 10.1371/journal.pone.0024730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tebebi P.A., Kim S.J., Williams R.A., Milo B., Frenkel V., Burks S.R., Frank J.A. Improving the therapeutic efficacy of mesenchymal stromal cells to restore perfusion in critical limb ischemia through pulsed focused ultrasound. Sci. Rep. 2017;7:41550. doi: 10.1038/srep41550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto K., Shimo T., Kurio N., Okui T., Ibaragi S., Kunisada Y., Obata K., Masui M., Pai P., Horikiri Y., et al. Low-intensity pulsed ultrasound stimulation promotes osteoblast differentiation through hedgehog signaling. J. Cell. Biochem. 2018;119:4352–4360. doi: 10.1002/jcb.26418. [DOI] [PubMed] [Google Scholar]
- 58.Harrison A., Lin S., Pounder N., Mikuni-Takagaki Y. Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics. 2016;70:45–52. doi: 10.1016/j.ultras.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Kusuyama J., Bandow K., Shamoto M., Kakimoto K., Ohnishi T., Matsuguchi T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J. Biol. Chem. 2014;289:10330–10344. doi: 10.1074/jbc.M113.546382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ying Z.M., Lin T., Yan S.G. Low-intensity pulsed ultrasound therapy: A potential strategy to stimulate tendon-bone junction healing. J. Zhejiang Univ. Sci. B. 2012;13:955–963. doi: 10.1631/jzus.B1200129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.An Y., Song Y., Wang Z., Wang J., Wu G., Zhu G., Chen L. Effect of low-intensity pulsed ultrasound on the biological behaviors of bone marrow mesenchymal stem cells on titanium with different surface topographies. Am. J. Transl. Res. 2018;10:67–76. [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou X., Castro N.J., Zhu W., Cui H., Aliabouzar M., Sarkar K., Zhang L.G. Improved Human Bone Marrow Mesenchymal Stem Cell Osteogenesis in 3D Bioprinted Tissue Scaffolds with Low Intensity Pulsed Ultrasound Stimulation. Sci. Rep. 2016;6:32876. doi: 10.1038/srep32876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu C.Y., Tsai T.L., Vanderby R., Jr., Bradica G., Lou S.L., Li W.J. Osteoblastogenesis of Mesenchymal Stem Cells in 3-D Culture Enhanced by Low-Intensity Pulsed Ultrasound through Soluble Receptor Activator of Nuclear Factor Kappa B Ligand. Ultrasound Med. Biol. 2015;41:1842–1852. doi: 10.1016/j.ultrasmedbio.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Lai C.H., Chen S.C., Chiu L.H., Yang C.B., Tsai Y.H., Zuo C.S., Chang W.H., Lai W.F. Effects of low-intensity pulsed ultrasound, dexamethasone/TGF-beta1 and/or BMP-2 on the transcriptional expression of genes in human mesenchymal stem cells: Chondrogenic vs. osteogenic differentiation. Ultrasound Med. Biol. 2010;36:1022–1033. doi: 10.1016/j.ultrasmedbio.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Aliabouzar M., Lee S.J., Zhou X., Zhang G.L., Sarkar K. Effects of scaffold microstructure and low intensity pulsed ultrasound on chondrogenic differentiation of human mesenchymal stem cells. Biotechnol. Bioeng. 2018;115:495–506. doi: 10.1002/bit.26480. [DOI] [PubMed] [Google Scholar]
- 66.Aliabouzar M., Zhang L.G., Sarkar K. Lipid Coated Microbubbles and Low Intensity Pulsed Ultrasound Enhance Chondrogenesis of Human Mesenchymal Stem Cells in 3D Printed Scaffolds. Sci. Rep. 2016;6:37728. doi: 10.1038/srep37728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui J.H., Park S.R., Park K., Choi B.H., Min B.H. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 2007;13:351–360. doi: 10.1089/ten.2006.0080. [DOI] [PubMed] [Google Scholar]
- 68.Lee H.J., Choi B.H., Min B.H., Son Y.S., Park S.R. Low-intensity ultrasound stimulation enhances chondrogenic differentiation in alginate culture of mesenchymal stem cells. Artif. Organs. 2006;30:707–715. doi: 10.1111/j.1525-1594.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 69.Schumann D., Kujat R., Zellner J., Angele M.K., Nerlich M., Mayr E., Angele P. Treatment of human mesenchymal stem cells with pulsed low intensity ultrasound enhances the chondrogenic phenotype in vitro. Biorheology. 2006;43:431–443. [PubMed] [Google Scholar]
- 70.Ebisawa K., Hata K., Okada K., Kimata K., Ueda M., Torii S., Watanabe H. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:921–929. doi: 10.1089/1076327041348437. [DOI] [PubMed] [Google Scholar]
- 71.Fu N., Yang X., Ba K., Fu Y., Wei X., Yue Y., Li G., Yao Y., Chen J., Cai X., et al. Low-intensity pulsed ultrasound induced enhanced adipogenesis of adipose-derived stem cells. Cell Prolif. 2013;46:312–319. doi: 10.1111/cpr.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li F., Liu Y., Cai Y., Li X., Bai M., Sun T., Du L. Ultrasound Irradiation Combined with Hepatocyte Growth Factor Accelerate the Hepatic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Ultrasound Med. Biol. 2018;44:1044–1052. doi: 10.1016/j.ultrasmedbio.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Liu S.H., Lai Y.L., Chen B.L., Yang F.Y. Ultrasound Enhances the Expression of Brain-Derived Neurotrophic Factor in Astrocyte Through Activation of TrkB-Akt and Calcium-CaMK Signaling Pathways. Cereb. Cortex. 2017;27:3152–3160. doi: 10.1093/cercor/bhw169. [DOI] [PubMed] [Google Scholar]
- 74.Cui J.H., Park K., Park S.R., Min B.H. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: An in vivo study. Tissue Eng. 2006;12:75–82. doi: 10.1089/ten.2006.12.75. [DOI] [PubMed] [Google Scholar]
- 75.Xia P., Wang X., Qu Y., Lin Q., Cheng K., Gao M., Ren S., Zhang T., Li X. TGF-beta1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res. Ther. 2017;8:281. doi: 10.1186/s13287-017-0733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X., Lin Q., Zhang T., Wang X., Cheng K., Gao M., Xia P., Li X. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res. Ther. 2019;10:41. doi: 10.1186/s13287-019-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z., Ma Y., Guo S., He Y., Bai G., Zhang W. Low-intensity pulsed ultrasound stimulation facilitates in vitro osteogenic differentiation of human adipose-derived stem cells via up-regulation of heat shock protein (HSP)70, HSP90, and bone morphogenetic protein (BMP) signaling pathway. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu S., Kawahara Y., Manabe T., Ogawa K., Matsumoto M., Sasaki A., Yuge L. Low-intensity pulsed ultrasound accelerates osteoblast differentiation and promotes bone formation in an osteoporosis rat model. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2009;76:99–107. doi: 10.1159/000209387. [DOI] [PubMed] [Google Scholar]
- 79.Cho S.E., Kim Y.M., Jeong J.S., Seo Y.K. The effect of ultrasound for increasing neural differentiation in hBM-MSCs and inducing neurogenesis in ischemic stroke model. Life Sci. 2016;165:35–42. doi: 10.1016/j.lfs.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 80.Xia B., Zou Y., Xu Z., Lv Y. Gene expression profiling analysis of the effects of low-intensity pulsed ultrasound on induced pluripotent stem cell-derived neural crest stem cells. Biotechnol. Appl. Biochem. 2017;64:927–937. doi: 10.1002/bab.1554. [DOI] [PubMed] [Google Scholar]
- 81.Xia B., Chen G., Zou Y., Yang L., Pan J., Lv Y. Low-intensity pulsed ultrasound combination with induced pluripotent stem cells-derived neural crest stem cells and growth differentiation factor 5 promotes sciatic nerve regeneration and functional recovery. J. Tissue Eng. Regen. Med. 2019;13:625–636. doi: 10.1002/term.2823. [DOI] [PubMed] [Google Scholar]
- 82.Kusuyama J., Hwan Seong C., Ohnishi T., Bandow K., Matsuguchi T. 10. Low-Intensity Pulsed Ultrasound (LIPUS) Stimulation Helps to Maintain the Differentiation Potency of Mesenchymal Stem Cells by Induction in Nanog Protein Transcript Levels and Phosphorylation. J. Orthop. Trauma. 2016;30:S4–S5. doi: 10.1097/01.bot.0000489983.17459.0b. [DOI] [PubMed] [Google Scholar]
- 83.Hasuike A., Sato S., Udagawa A., Ando K., Arai Y., Ito K. In vivo bone regenerative effect of low-intensity pulsed ultrasound in rat calvarial defects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011;111:e12–e20. doi: 10.1016/j.tripleo.2010.09.061. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y., Qiu Y., Li J., Zhao C., Song J. Low-intensity pulsed ultrasound promotes alveolar bone regeneration in a periodontal injury model. Ultrasonics. 2018;90:166–172. doi: 10.1016/j.ultras.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 85.Yamaguchi S., Aoyama T., Ito A., Nagai M., Iijima H., Tajino J., Zhang X., Wataru K., Kuroki H. Effect of Low-Intensity Pulsed Ultrasound after Mesenchymal Stromal Cell Injection to Treat Osteochondral Defects: An In Vivo Study. Ultrasound Med. Biol. 2016;42:2903–2913. doi: 10.1016/j.ultrasmedbio.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 86.Hui C.F., Chan C.W., Yeung H.Y., Lee K.M., Qin L., Li G., Leung K.S., Hu Y.Y., Cheng J.C. Low-intensity pulsed ultrasound enhances posterior spinal fusion implanted with mesenchymal stem cells-calcium phosphate composite without bone grafting. Spine. 2011;36:1010–1016. doi: 10.1097/BRS.0b013e318205c5f5. [DOI] [PubMed] [Google Scholar]
- 87.Lv Y., Nan P., Chen G., Sha Y., Xia B., Yang L. In vivo repair of rat transected sciatic nerve by low-intensity pulsed ultrasound and induced pluripotent stem cells-derived neural crest stem cells. Biotechnol. Lett. 2015;37:2497–2506. doi: 10.1007/s10529-015-1939-5. [DOI] [PubMed] [Google Scholar]
- 88.Peng D.Y., Reed-Maldonado A.B., Lin G.T., Xia S.J., Lue T.F. Low-intensity pulsed ultrasound for regenerating peripheral nerves: Potential for penile nerve. Asian J. Androl. 2020;22:335–341. doi: 10.4103/aja.aja_95_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shindo T., Ito K., Ogata T., Hatanaka K., Kurosawa R., Eguchi K., Kagaya Y., Hanawa K., Aizawa K., Shiroto T., et al. Low-Intensity Pulsed Ultrasound Enhances Angiogenesis and Ameliorates Left Ventricular Dysfunction in a Mouse Model of Acute Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2016;36:1220–1229. doi: 10.1161/ATVBAHA.115.306477. [DOI] [PubMed] [Google Scholar]
- 90.Higashi Y., Azuma N., Takeishi Y., Minamino T., Kihara Y., Node K., Sata M., Fukumoto Y., Origasa H., Matsuo H., et al. Effect of a Low-Intensity Pulsed Ultrasound Device, SX-1001, on Clinical Symptoms in Buerger Disease With Limb Ischemia. Int. Heart J. 2015;56:632–638. doi: 10.1536/ihj.15-191. [DOI] [PubMed] [Google Scholar]
- 91.Ramli R., Reher P., Harris M., Meghji S. The effect of ultrasound on angiogenesis: An in vivo study using the chick chorioallantoic membrane. Int. J. Oral Maxillofac. Implant. 2009;24:591–596. [PubMed] [Google Scholar]
- 92.Mizrahi N., Seliktar D., Kimmel E. Ultrasound-induced angiogenic response in endothelial cells. Ultrasound Med. Biol. 2007;33:1818–1829. doi: 10.1016/j.ultrasmedbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Barzelai S., Sharabani-Yosef O., Holbova R., Castel D., Walden R., Engelberg S., Scheinowitz M. Low-intensity ultrasound induces angiogenesis in rat hind-limb ischemia. Ultrasound Med. Biol. 2006;32:139–145. doi: 10.1016/j.ultrasmedbio.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 94.Kang P.L., Huang H.H., Chen T., Ju K.C., Kuo S.M. Angiogenesis-promoting effect of LIPUS on hADSCs and HUVECs cultured on collagen/hyaluronan scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;102:22–33. doi: 10.1016/j.msec.2019.04.045. [DOI] [PubMed] [Google Scholar]
- 95.Hanawa K., Ito K., Aizawa K., Shindo T., Nishimiya K., Hasebe Y., Tuburaya R., Hasegawa H., Yasuda S., Kanai H., et al. Low-intensity pulsed ultrasound induces angiogenesis and ameliorates left ventricular dysfunction in a porcine model of chronic myocardial ischemia. PLoS ONE. 2014;9:e104863. doi: 10.1371/journal.pone.0104863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng C., Wu S.M., Lian H., Lin Y.Z., Zhuang R., Thapa S., Chen Q.Z., Chen Y.F., Lin J.F. Low-intensity pulsed ultrasound attenuates cardiac inflammation of CVB3-induced viral myocarditis via regulation of caveolin-1 and MAPK pathways. J. Cell. Mol. Med. 2019;23:1963–1975. doi: 10.1111/jcmm.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao X., Zhao G., Shi Z., Zhou C., Chen Y., Hu B., Yan S. Low-intensity pulsed ultrasound (LIPUS) prevents periprosthetic inflammatory loosening through FBXL2-TRAF6 ubiquitination pathway. Sci. Rep. 2017;7:45779. doi: 10.1038/srep45779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang B., Chen H., Ouyang J., Xie Y., Chen L., Tan Q., Du X., Su N., Ni Z., Chen L. SQSTM1-dependent autophagic degradation of PKM2 inhibits the production of mature IL1B/IL-1beta and contributes to LIPUS-mediated anti-inflammatory effect. Autophagy. 2020;16:1262–1278. doi: 10.1080/15548627.2019.1664705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakamura T., Fujihara S., Yamamoto-Nagata K., Katsura T., Inubushi T., Tanaka E. Low-intensity pulsed ultrasound reduces the inflammatory activity of synovitis. Ann. Biomed. Eng. 2011;39:2964–2971. doi: 10.1007/s10439-011-0408-0. [DOI] [PubMed] [Google Scholar]
- 100.Li H., Deng Y., Tan M., Feng G., Kuang Y., Li J., Song J. Low-intensity pulsed ultrasound upregulates osteogenesis under inflammatory conditions in periodontal ligament stem cells through unfolded protein response. Stem Cell Res. Ther. 2020;11:215. doi: 10.1186/s13287-020-01732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato M., Kuroda S., Mansjur K.Q., Khaliunaa G., Nagata K., Horiuchi S., Inubushi T., Yamamura Y., Azuma M., Tanaka E. Low-intensity pulsed ultrasound rescues insufficient salivary secretion in autoimmune sialadenitis. Arthritis Res. Ther. 2015;17:278. doi: 10.1186/s13075-015-0798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang F.Y., Lu W.W., Lin W.T., Chang C.W., Huang S.L. Enhancement of Neurotrophic Factors in Astrocyte for Neuroprotective Effects in Brain Disorders Using Low-intensity Pulsed Ultrasound Stimulation. Brain Stimul. 2015;8:465–473. doi: 10.1016/j.brs.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 103.Zhou J.X., Liu Y.J., Chen X., Zhang X., Xu J., Yang K., Wang D., Lin S., Ye J. Low-Intensity Pulsed Ultrasound Protects Retinal Ganglion Cell From Optic Nerve Injury Induced Apoptosis via Yes Associated Protein. Front. Cell. Neurosci. 2018;12:160. doi: 10.3389/fncel.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Padilla F., Puts R., Vico L., Raum K. Stimulation of bone repair with ultrasound: A review of the possible mechanic effects. Ultrasonics. 2014;54:1125–1145. doi: 10.1016/j.ultras.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 105.Sato M., Nagata K., Kuroda S., Horiuchi S., Nakamura T., Karima M., Inubushi T., Tanaka E. Low-intensity pulsed ultrasound activates integrin-mediated mechanotransduction pathway in synovial cells. Ann. Biomed. Eng. 2014;42:2156–2163. doi: 10.1007/s10439-014-1081-x. [DOI] [PubMed] [Google Scholar]
- 106.Qiu Z., Guo J., Kala S., Zhu J., Xian Q., Qiu W., Li G., Zhu T., Meng L., Zhang R., et al. The Mechanosensitive Ion Channel Piezo1 Significantly Mediates In Vitro Ultrasonic Stimulation of Neurons. iScience. 2019;21:448–457. doi: 10.1016/j.isci.2019.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Lucas B., Perez L.M., Galvez B.G. Importance and regulation of adult stem cell migration. J. Cell. Mol. Med. 2018;22:746–754. doi: 10.1111/jcmm.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi B.H., Kim K.H., Karmacharya M.B., Min B.-H., Park S.R. Low-Intensity Ultrasound in Stem Cells and Tissue Engineering. Cell Mol. Biol. Imaging Stem Cells. 2014:45–65. doi: 10.1002/9781118285602.ch3. [DOI] [Google Scholar]
- 109.Wu C. Focal adhesion: A focal point in current cell biology and molecular medicine. Cell Adhes. Migr. 2007;1:13–18. doi: 10.4161/cam.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takeuchi R., Ryo A., Komitsu N., Mikuni-Takagaki Y., Fukui A., Takagi Y., Shiraishi T., Morishita S., Yamazaki Y., Kumagai K., et al. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: A basic science study. Arthritis Res. Ther. 2008;10:R77. doi: 10.1186/ar2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prieto M.L., Firouzi K., Khuri-Yakub B.T., Maduke M. Activation of Piezo1 but Not NaV1.2 Channels by Ultrasound at 43 MHz. Ultrasound Med. Biol. 2018;44:1217–1232. doi: 10.1016/j.ultrasmedbio.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao Q., Cooper P.R., Walmsley A.D., Scheven B.A. Role of Piezo Channels in Ultrasound-stimulated Dental Stem Cells. J. Endod. 2017;43:1130–1136. doi: 10.1016/j.joen.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 113.Sugimoto A., Miyazaki A., Kawarabayashi K., Shono M., Akazawa Y., Hasegawa T., Ueda-Yamaguchi K., Kitamura T., Yoshizaki K., Fukumoto S., et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci. Rep. 2017;7:17696. doi: 10.1038/s41598-017-18089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han Y., Liu C., Zhang D., Men H., Huo L., Geng Q., Wang S., Gao Y., Zhang W., Zhang Y., et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int. J. Oncol. 2019;55:629–644. doi: 10.3892/ijo.2019.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pilla A.A., Mont M.A., Nasser P.R., Khan S.A., Figueiredo M., Kaufman J.J., Siffert R.S. Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit. J. Orthop. Trauma. 1990;4:246–253. doi: 10.1097/00005131-199004030-00002. [DOI] [PubMed] [Google Scholar]
- 116.Garg P., Mazur M.M., Buck A.C., Wandtke M.E., Liu J., Ebraheim N.A. Prospective Review of Mesenchymal Stem Cells Differentiation into Osteoblasts. Orthop. Surg. 2017;9:13–19. doi: 10.1111/os.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang B., Li M., Lei H., Xu Y., Li H., Gao Z., Guan R., Xin Z. Low Intensity Pulsed Ultrasound Influences the Myogenic Differentiation of Muscle Satellite Cells in a Stress Urinary Incontinence Rat Model. Urology. 2019;123:297.e1–297.e8. doi: 10.1016/j.urology.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 118.Sato M., Motoyoshi M., Shinoda M., Iwata K., Shimizu N. Low-intensity pulsed ultrasound accelerates nerve regeneration following inferior alveolar nerve transection in rats. Eur. J. Oral Sci. 2016;124:246–250. doi: 10.1111/eos.12271. [DOI] [PubMed] [Google Scholar]
- 119.Wang F., Li Y., Yang Z., Lu K., Zuo J., Zhou Z. Effect of Low-Intensity Pulsed Ultrasound on a Rat Model of Dentin-Dental Pulp Injury and Repair. Ultrasound Med. Biol. 2017;43:163–175. doi: 10.1016/j.ultrasmedbio.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 120.Maeda T., Masaki C., Kanao M., Kondo Y., Ohta A., Nakamoto T., Hosokawa R. Low-intensity pulsed ultrasound enhances palatal mucosa wound healing in rats. J. Prosthodont. Res. 2013;57:93–98. doi: 10.1016/j.jpor.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 121.El-Bialy T., El-Shamy I., Graber T.M. Repair of orthodontically induced root resorption by ultrasound in humans. Am. J. Orthod. Dentofac. Orthop. 2004;126:186–193. doi: 10.1016/j.ajodo.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 122.Young S.R., Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med. Biol. 1990;16:261–269. doi: 10.1016/0301-5629(90)90005-W. [DOI] [PubMed] [Google Scholar]
- 123.Poolman R.W., Agoritsas T., Siemieniuk R.A., Harris I.A., Schipper I.B., Mollon B., Smith M., Albin A., Nador S., Sasges W., et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: A clinical practice guideline. BMJ. 2017;356:j576. doi: 10.1136/bmj.j576. [DOI] [PubMed] [Google Scholar]
- 124.Tarride J.E., Hopkins R.B., Blackhouse G., Burke N., Bhandari M., Johal H., Guyatt G.H., Busse J.W. Low-intensity pulsed ultrasound for treatment of tibial fractures: An economic evaluation of the TRUST study. Bone Jt. J. 2017;99-B:1526–1532. doi: 10.1302/0301-620X.99B11.BJJ-2017-0737. [DOI] [PubMed] [Google Scholar]
- 125.TRUST Investigators writing group. Busse J.W., Bhandari M., Einhorn T.A., Schemitsch E., Heckman J.D., Tornetta P., III, Leung K.S., Heels-Ansdell D., Makosso-Kallyth S., et al. Re-evaluation of low intensity pulsed ultrasound in treatment of tibial fractures (TRUST): Randomized clinical trial. BMJ. 2016;355:i5351. doi: 10.1136/bmj.i5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou X.Y., Zhang X.X., Yu G.Y., Zhang Z.C., Wang F., Yang Y.L., Li M., Wei X.Z. Effects of Low-Intensity Pulsed Ultrasound on Knee Osteoarthritis: A Meta-Analysis of Randomized Clinical Trials. Biomed Res. Int. 2018;2018:7469197. doi: 10.1155/2018/7469197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cruz J.M., Hauck M., Cardoso Pereira A.P., Moraes M.B., Martins C.N., da Silva Paulitsch F., Plentz R.D., Peres W., Vargas da Silva A.M., Signori L.U. Effects of Different Therapeutic Ultrasound Waveforms on Endothelial Function in Healthy Volunteers: A Randomized Clinical Trial. Ultrasound Med. Biol. 2016;42:471–480. doi: 10.1016/j.ultrasmedbio.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 128.Yang K.H., Parvizi J., Wang S.J., Lewallen D.G., Kinnick R.R., Greenleaf J.F., Bolander M.E. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1996;14:802–809. doi: 10.1002/jor.1100140518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


