Abstract
This cohort study evaluates the association of early trastuzumab interruption with clinical outcomes in patients with ERBB2-positive breast cancer.
Human epidermal growth factor receptor 2 (ERBB2)-targeted therapies such as trastuzumab improve disease-free and overall survival for patients with ERBB2-positive breast cancer (BCA).1 Cardiotoxic effects, characterized by reduced left ventricular ejection fraction (LVEF) or clinical heart failure (HF), are an important treatment-limiting adverse effect of ERBB2-targeted therapy and the most common indication for early trastuzumab interruption.2 Findings from the PHARE and PERSEPHONE trials on the noninferiority of 6- vs 12-month durations of trastuzumab are conflicting, thus the clinical significance of early trastuzumab interruption on BCA outcomes remains uncertain.3,4 Our primary objective was to evaluate the association of early trastuzumab interruption with BCA outcomes in clinical practice.
Methods
All patients with early-stage ERBB2-positive BCA treated with trastuzumab at Memorial Sloan Kettering Cancer Center (MSKCC) from 2004 to 2013 were identified. The study was approved by the MSKCC institutional review board and a waiver of written informed consent was granted because data were deidentified. Early interruption, defined as a 6-week or longer interval between scheduled trastuzumab doses,2 was ascertained. Indications for interruption were cardiac (ie, asymptomatic LVEF decline or HF) vs noncardiac. The primary outcome was recurrence-free survival (RFS), defined as time from start of trastuzumab to the date of invasive BCA recurrence or all-cause death. Survival was estimated using the Kaplan-Meier method with a 12-month landmark, given that treatment interruption occurs within this timeframe of initiating trastuzumab and to account for bias from patients with early interruption owing to disease recurrence. Multivariable Cox proportional hazards models evaluated associations between RFS and treatment interruption and treatment interruption by indication (no interruption, cardiac interruption, or noncardiac interruption) or cumulative trastuzumab dose (no interruption, ≤56 mg/kg, or >56 mg/kg), adjusting for age, anthracycline, cancer stage, estrogen/progesterone receptor status, hypertension, and diabetes. A dose cutoff of 56 mg/kg was chosen because this approximates 6 months of trastuzumab treatment. Statistical analyses were performed using STATA statistical software (version 16.1, StataCorp).
Results
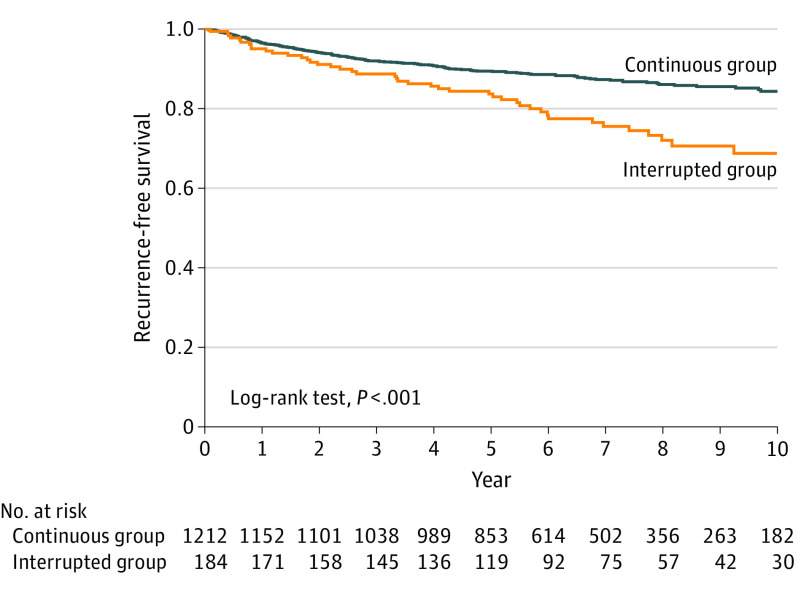
Among 1396 trastuzumab-treated patients (median [interquartile range] age, 51 [44-59] years), treatment interruption occurred in 184 (13%)–124 for cardiotoxic effects (92 with LVEF decline and 32 with HF) and 60 for noncardiac reasons. After median follow-up of 6.0 years, invasive BCA recurrence or death occurred in 44 (24%) of 184 in the interrupted group and 153 (13%) of 1212 in the continuous group (log-rank P < .001) (Figure). Patients with early trastuzumab interruption had a significantly increased hazard of invasive BCA recurrence or death (adjusted hazard ratio [HR], 1.56; 95% CI, 1.10-2.21) (Table). The adjusted HR for RFS was 1.47 (95% CI, 0.98-2.20) in the group with a cardiac interruption and 1.78 (95% CI, 1.03-3.08) in the group with a noncardiac interruption. Sixty (33%) patients in the interrupted group and none in the continuous group received a cumulative trastuzumab dose of 56 mg/kg or less. Trastuzumab interruption with a cumulative trastuzumab dose of 56 mg/kg or less was associated with reduced RFS with an adjusted HR of 1.96 (95% CI, 1.16-3.33).
Figure. Kaplan-Meier Plot of Recurrence-Free Survival According to Continuous vs Interrupted Trastuzumab.
Recurrence-free survival was assessed in a landmark analysis from 12 months after the start date of trastuzumab for the continuous and interrupted groups. The median cumulative dose of trastuzumab received was 108 (interquartile range [IQR], 104-110) mg/kg in the continuous group and 81 (IQR, 44-98) mg/kg in the interrupted group.
Table. Multivariable Cox Proportional Hazards Regression Models of Treatment Interruption and Recurrence-Free Survival.
| Variable | No. (%) | Unadjusted HR (95% CI) | P value | Adjusted HRa (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Total patients | Total events | |||||
| Model 1 | <.001 | .01 | ||||
| Trastuzumab interruption | ||||||
| No | 1212 (87) | 153 (78) | 1 [Reference] | 1 [Reference] | ||
| Yes | 184 (13) | 44 (22) | 1.94 (1.39-2.72) | 1.56 (1.10-2.21) | ||
| Model 2 | ||||||
| Trastuzumab interruption (by indication) | ||||||
| No | 1212 (87) | 153 (78) | 1 [Reference] | 1 [Reference] | ||
| Yes, cardiac | 124 (9) | 29 (15) | 1.83 (1.23-2.72) | .003 | 1.47 (0.98-2.20) | .07 |
| Yes, noncardiac | 60 (4) | 15 (8) | 2.21 (1.30-3.75) | .003 | 1.78 (1.03-3.08) | .04 |
| Model 3 | ||||||
| Trastuzumab interruption (by dose) | ||||||
| No | 1212 (87) | 153 (78) | 1 [Reference] | 1 [Reference] | ||
| Yes, ≤56 mg/kg | 60 (4) | 16 (8) | 2.24 (1.34-3.76) | .002 | 1.96 (1.16-3.33) | .01 |
| Yes, >56 mg/kg | 124 (9) | 28 (14) | 1.81 (1.21-2.70) | .004 | 1.39 (0.92-2.11) | .12 |
Abbreviation: HR, hazard ratio.
All models were adjusted for age, estrogen receptor status, progesterone receptor status, anthracycline exposure, cancer stage, hypertension, and diabetes.
Discussion
This study demonstrates that patients with early trastuzumab interruption had higher rates of BCA recurrence and death compared with patients receiving uninterrupted treatment. Importantly, RFS was reduced in patients with early treatment interruption who received a cumulative trastuzumab dose of 56 mg/kg or less (equivalent to ≤6 months of trastuzumab), suggesting that the total trastuzumab dose plays an important role in BCA outcomes. The most common cause for early interruption was cardiotoxic effects, accounting for 124 (67%) of 184 cases, highlighting the potential that such interruptions may adversely affect BCA outcomes. Given that most patients in this study were treated with anthracyclines, our findings may not be generalizable to patients receiving nonanthracycline treatment regimens. Recent studies suggest that asymptomatic LVEF declines can safely be treated without interrupting trastuzumab when close cardiac monitoring and appropriate cardiac medications are instituted.5,6 Given that current clinical trial data are insufficient to support shortened durations of trastuzumab, ongoing collaboration between cardiologists and oncologists is needed to minimize treatment interruptions and allow patients with asymptomatic LVEF decline to continue receiving ERBB2-targeted therapy.
References
- 1.Slamon D, Eiermann W, Robert N, et al. ; Breast Cancer International Research Group . Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-1283. doi: 10.1056/NEJMoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu AF, Yadav NU, Lung BY, et al. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res Treat. 2015;149(2):489-495. doi: 10.1007/s10549-014-3253-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earl HM, Hiller L, Vallier AL, et al. ; PERSEPHONE Steering Committee and Trial Investigators . 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393(10191):2599-2612. doi: 10.1016/S0140-6736(19)30650-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pivot X, Romieu G, Debled M, et al. ; PHARE trial investigators . 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393(10191):2591-2598. doi: 10.1016/S0140-6736(19)30653-1 [DOI] [PubMed] [Google Scholar]
- 5.Lynce F, Barac A, Geng X, et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175(3):595-603. doi: 10.1007/s10549-019-05191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leong D, Cosman T, Alhussein M, et al. Safety of continuing trastuzumab despite mild cardiotoxicity: a phase I trial. JACC: Cardiooncology. 2019;1(1):1-10. doi: 10.1016/j.jaccao.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]