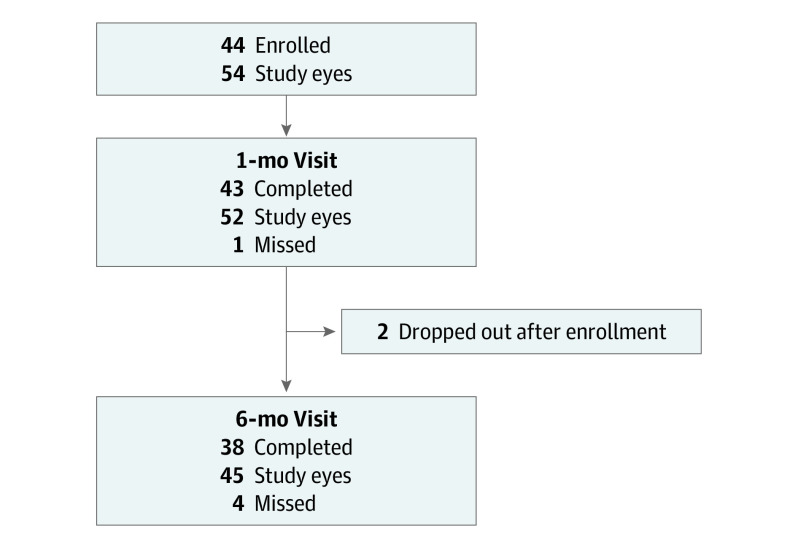
This cohort study used data from pediatric ophthalmology and neuro-ophthalmology clinics in the United States and Canada to assess the 6-month visual acuity in pediatric patients with optic neuritis.
Key Points
Question
What are the 6-month visual acuity (VA) outcomes of children with optic neuritis and is it feasible to enroll a sufficient number of participants for a randomized clinical trial?
Findings
Among 44 children enrolled over 22 months in this cohort study, 48% had isolated cases of optic neuritis, 52% had optic neuritis associated with neurologic autoimmune diagnoses, and the mean presenting distance high-contrast VA was 0.95 logMAR (approximately 20/200). VA improved by a mean of approximately 8 lines after 6 months.
Meaning
Relatively few children with optic neuritis were enrolled in this study over 2 years, which suggests that poor VA at presentation markedly improves in most children by 6 months after onset.
Abstract
Importance
Optic neuritis (ON) in children is uncommon. There are limited prospective data for visual acuity (VA) outcomes, associated diseases, and neuroimaging findings. Prospective data from a large sample would be useful for counseling families on treatment decisions and prognosis.
Objective
To prospectively study children with a first episode of ON, describe VA after 6 months, and ascertain the network’s (Pediatric Eye Disease Investigator Group and Neuro-Ophthalmology Research Disease Investigator Consortium) ability to enroll pediatric patients with ON prospectively.
Design, Setting, and Participants
This nonrandomized cohort study was conducted from September 20, 2016, to July 20, 2018, at 23 sites in the United States and Canada in pediatric ophthalmology or neuro-ophthalmology clinics. A total of 44 children (aged 3-15 years) presented with a first episode of ON (visual loss, pain on eye movements, or both) within 2 weeks of symptom onset and at least 1 of the following in the affected eye: a distance high-contrast VA (HCVA) deficit of at least 0.2 logMAR below age-based norms, diminished color vision, abnormal visual field, or optic disc swelling. Exclusion criteria included preexisting ocular abnormalities or a previous episode of ON.
Main Outcomes and Measures
Primary outcomes were monocular HCVA and low-contrast VA at 6 months. Secondary outcomes were neuroimaging, associated diagnoses, and antibodies for neuromyelitis optica and myelin oligodendrocyte glycoprotein.
Results
A total of 44 children (mean age [SD], 10.2 [3.5] years; 26 boys [59%]; 23 White individuals [52%]; 54 eyes) were enrolled in the study. Sixteen patients (36%) had bilateral ON. Magnetic resonance imaging revealed white matter lesions in 23 children (52%). Of these children, 8 had myelin oligodendrocyte glycoprotein–associated demyelination (18%), 7 had acute disseminated encephalomyelitis (16%), 5 had multiple sclerosis (11%), and 3 had neuromyelitis optica (7%). The baseline mean HCVA was 0.95 logMAR (20/200), which improved by a mean 0.76 logMAR (95% CI, 0.54-0.99; range, –0.70 to 1.80) to 0.12 logMAR (20/25) at 6 months. The baseline mean distance low-contrast VA was 1.49 logMAR (20/640) and improved by a mean 0.72 logMAR (95% CI, 0.54-0.89; range, –0.20 to 1.50) to 0.73 logMAR (20/100) at 6 months. Baseline HCVA was worse in younger participants (aged <10 years) with associated neurologic autoimmune diagnoses, white matter lesions, and in those of non-White race and non-Hispanic ethnicity. The data did not suggest a statistically significant association between baseline factors and improvement in HCVA.
Conclusions and Relevance
The study network did not reach its targeted enrollment of 100 pediatric patients with ON over 2 years. This indicates that future treatment trials may need to use different inclusion criteria or plan a longer enrollment period to account for the rarity of the disease. Despite poor VA at presentation, most children had marked improvement by 6 months. Associated neurologic autoimmune diagnoses were common. These findings can be used to counsel families about the disease.
Introduction
Although optic neuritis (ON) has been well studied in adults,1 there are limited prospective data on this condition in children.2 The Optic Neuritis Treatment Trial3 provided valuable knowledge regarding the cause, management, and prognosis of ON, as well as subsequent diagnosis of multiple sclerosis (MS) in affected adults. However, the Optic Neuritis Treatment Trial was performed in adults 18 to 46 years, and its findings and recommended treatment strategy cannot be generalized to pediatric patients.
Our understanding of the cause and natural history of pediatric ON, patient response to therapy, and overall prognosis is primarily based on case reports and retrospective case series.4,5,6,7,8,9 A survey involving a hypothetical treatment trial comparing intravenous corticosteroids, oral corticosteroids, and placebo for pediatric ON found that 98% of the 49 clinicians queried would enroll their pediatric patients in such a trial, thereby demonstrating the desire of practitioners for evidence-based management of pediatric ON.10 However, because clinical trials of uncommon diseases often have difficulty enrolling a sufficient number of participants, we launched a prospective pilot study of children with ON. The study had 2 primary aims: (1) to obtain estimates of clinically important outcomes in a prospectively enrolled cohort and (2) to determine the feasibility of sufficient enrollment for a randomized clinical treatment trial.
Methods
The study was conducted according to the tenets of the Declaration of Helsinki11 by the Pediatric Eye Disease Investigator Group and the Neuro-Ophthalmology Research Disease Investigator Consortium at 23 academic- and community-based clinical sites specializing in either pediatric or neuro-ophthalmology in North America. The study, protocol, and Health Insurance Portability and Accountability Act–compliant informed consent forms were approved by each site’s institutional review board. Each patient’s parent or guardian provided written informed consent. Children also provided written consent when applicable as determined by the local institutional review board. The full study protocol is available on the Pediatric Eye Disease Investigator Group website (eAppendix 1 in the Supplement).12 Families were compensated $50 per completed study visit. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients (aged 3-15 years at the time of enrollment) who presented with a first episode of ON in 1 or both previously unaffected eyes (based on clinical diagnosis) within 2 weeks of symptom onset were enrolled. To be considered a study eye with ON, affected eyes had to have visual loss, pain on eye movements, or both for less than or equal to 2 weeks and at least 1 of the following in the affected eye: a distance high-contrast visual acuity (HCVA) deficit at least 0.2 logMAR below age-based norms13,14 (eTable 1 in the Supplement), diminished color vision, abnormal visual field, or optic disc swelling. Participants were excluded if they had preexisting ocular abnormalities or a previous episode of ON. Additional eligibility and exclusion criteria are shown in eTable 1 in the Supplement. For unilateral cases, a relative afferent pupillary defect was required. Optic neuritis was deemed bilateral and simultaneous if present in both eyes at enrollment or if the contralateral eye developed ON within 1 month of enrollment. Not all participants who were classified as having bilateral ON contributed 2 study eyes given that inclusion of study eyes was based on additional eye-level eligibility criteria (as defined previously).
At enrollment, demographic and clinical information were collected during the patient examination and from the medical records. Monocular distance HCVA and low-contrast VA (LCVA) using 2.5% low-contrast letters were tested in the participant’s habitual refractive correction in each eye (right, then left) by a study-certified examiner using the electronic Amblyopia Treatment Study HOTV acuity protocol if the child was younger than 7 years,15 or the electronic Early Treatment Diabetic Retinopathy Study (ETDRS) acuity protocol if the child was older than 7 years.16 A cycloplegic refraction was performed at the enrollment examination (if not conducted within the past month) and HCVA testing was repeated in trial frames if the participant was found to have an uncorrected refractive error requiring optical correction (defined in eTable 1 in the Supplement). Results of anterior and posterior segment examinations were recorded.
Magnetic resonance imaging (MRI) of the brain with and without gadolinium was required within 2 weeks of symptom onset. If the MRI had not yet been performed at enrollment, the recommended technique included fat saturation for orbital images and short T1 inversion recovery sequences. The MRI images were collected and sent for review by a masked examiner (A.T.W.) to confirm the presence of optic nerve enhancement and associated white matter lesions (WMLs). The masked assessment was performed without demographic or clinical information. Enrolled participants with no evidence of optic nerve ON enhancement on MRI in at least 1 eye continued in the study, but their data were analyzed separately. If already performed by the enrolling site as part of the standard of care, the results of aquaporin-4 (AQP4) antibody testing for neuromyelitis optica (NMO) were collected. If blood or serum had been collected as part of the standard of care, the parents and child (if old enough to consent) were given the option to participate in a study to send their blood or serum to the Neuroimmunology Research Laboratory at Mayo Clinic in Rochester, Minnesota, to measure AQP4 antibodies as well as myelin oligodendrocyte glycoprotein (MOG) antibodies. A Clinical Laboratory Improvement Amendments–certified test for antibodies to MOG was not clinically available at the start of the present study.
Follow-up visits were conducted at 1 and 6 months postenrollment. At each study visit, monocular HCVA and LCVA were tested, and anterior and posterior ocular segments were examined. At the 1-month visit, a Tanner puberty stage questionnaire and a neurologic symptom questionnaire17 were administered, and study sites completed a neurological summary diagnosis form (eTable 2 in the Supplement), detailing the investigator’s diagnosis using standardized definitions18 (isolated ON, acute disseminated encephalomyelitis [ADEM], NMO spectrum, or MOG-positive ON). Cases initially classified as clinically isolated syndrome were combined for analysis with isolated ON. The cause was considered an associated neurological autoimmune diagnosis if the MRI showed evidence of WMLs. If the masked MRI reading or results of the AQP4 or MOG antibody tests yielded discrepant findings from the initial investigator’s diagnosis, the enrolling investigator’s initial diagnosis and enrollment data were reviewed by the study chairs, and the diagnoses were adjudicated to a final diagnosis.
Statistical Analyses
A convenience sample size of up to 100 patients was planned for recruitment over a 24-month period. Analyses of eye-level outcomes were adjusted for intereye correlation because some children contributed 2 study eyes. For 6-month outcomes, analyses were limited to data from patients who completed the visit within the prespecified analysis window (range, 76-272 days). The primary outcome was the change in study eye logMAR HCVA from baseline to 6 months. The mean change in logMAR HCVA and the corresponding 95% CI were calculated using a generalized linear model with robust variance estimation. We used an exchangeable correlation matrix to account for correlation between eyes from the same patient. The primary analysis was repeated, limited to eyes having a 6-month HCVA within the prespecified protocol window (range, 169-197 days after enrollment) as a sensitivity analysis. Secondary outcomes included the proportion of eyes with age-normal HCVA and monocular distance HCVA and LCVA scores at 6 months. The proportion of study eyes with age-normal HCVA was calculated using a binomial regression model that was adjusted for between-eye correlation.
Generalized linear models with robust variance estimation were used to determine whether there was any association between demographic and clinical factors at baseline and outcomes and either (1) distance HCVA scores at ON presentation adjusted for baseline age or (2) the change in HCVA from baseline to 6 months adjusted for baseline age and HCVA. We used an exchangeable correlation matrix to account for correlation between eyes on the same patient. We considered the following demographic and clinical factors: age at presentation (continuous), final diagnosis (nonisolated; ie, ADEM, NMO, or MS vs isolated ON), laterality of pediatric ON at presentation (unilateral vs bilateral), the presence (or absence) of WMLs on MRI, prepuberty stage (prepubertal vs not), and race/ethnicity (White non-Hispanic vs not White non-Hispanic).
No adjustment for multiple testing was performed because these analyses were considered exploratory. Analyses were conducted from January 13, 2020, to April 29, 2020, using SAS, version 9.4 (SAS Institute Inc). Further information regarding the statistical analyses may be found in eAppendix 2 of the Supplement.
Results
Baseline Characteristics
A total of 44 children (mean age [SD], 10.2 [3.5] years; 26 boys [59%]; 23 White individuals [52%]; 54 eyes) were enrolled between September 20, 2016, and July 20, 2018, at 23 sites (range, 1-15 participants per site). Sixteen (36%) had bilateral ON (10 of 16 contributed 2 study eyes). The mean (SD) age of patients with bilateral ON was 8.6 (2.9) years (quartiles, 5.8 and 10.4 years; range, 3.9-13.5 years). Twenty-eight (64%) had unilateral ON. The mean (SD) age of participants with unilateral ON was 11.2 (3.5) years (quartiles, 8.3 and 13.7 years; range, 4.8-16.0 years). Thirty-five (80%) patients were receiving corticosteroid treatment at enrollment, and 5 (11%) began receiving corticosteroids at the enrollment visit. Overall, 40 patients (91%) were treated with corticosteroids; 4 (9%) did not receive corticosteroids or other medications during the follow-up period. Additional demographic information, family history of disease, systemic symptoms, and other medications reported at enrollment are included in Table 1.
Table 1. Baseline Characteristics of Enrolled Patients.
| Characteristic | Overall, No. (%)a |
|---|---|
| No. | 44 (100) |
| Participant sex | |
| Male | 26 (59) |
| Female | 18 (41) |
| Age, y | |
| 3-6 | 10 (23) |
| 7-9 | 8 (18) |
| 10-12 | 16 (36) |
| ≥13 | 10 (23) |
| Mean (SD) | 10.2 (3.5) |
| Race/ethnicity (self-reported) | |
| White | 23 (52) |
| Black/African American | 6 (14) |
| Hispanic | 10 (23) |
| Asian | 4 (9) |
| Unknown/not reported | 1 (2) |
| Prepuberty status by Tanner questionnaire | |
| Yesb | 9 (20) |
| No | 28 (64) |
| Not done/unknown | 7 (16) |
| Systemic symptoms reported, yes | 23 (52) |
| Type of symptoms reported | |
| Headache | 19 (43) |
| Focal weakness | 7 (16) |
| Focal numbness | 2 (5) |
| Difficulty with coordination | 6 (14) |
| Loss of bowel or bladder control | 1 (2) |
| Nausea | 6 (14) |
| Vomiting | 5 (11) |
| Tinnitus | 3 (7) |
| Diplopia | 2 (5) |
| Neck stiffness/pain | 5 (11) |
| Altered mental status | 1 (2) |
| Medications at enrollment, yes | 14 (32) |
| Type of mediations reported | |
| ADHD medications | 2 (5) |
| Seizure medications | 2 (5) |
| Analgesics | 7 (16) |
| Otherc | 8 (18) |
| Family history of medical conditions (listed below), yes | 16 (36) |
| Type of medical conditions | |
| Optic neuritis | 3 (7) |
| Autoimmune conditions | 10 (23) |
| Cardiovascular disease | 7 (16) |
| Laterality at PON presentation | |
| Bilaterald | 16 (36) |
| Bilateral sequential | 1 (2) |
| Bilateral simultaneous | 15 (34) |
| Unilateral | 28 (64) |
| Corticosteroid treatment at time of enrollmente | |
| No | 4 (9) |
| Yes | 40 (91) |
| History of demyelination | |
| None | 37 (86) |
| Optic neuritis | 1 (2) |
| Multiple sclerosis | 1 (2) |
| ADEM | 3 (7) |
| Transverse myelitis | 1 (2) |
| Not reported, No. | 1 |
| AQP4 test resultf | |
| Negative | 14 (93) |
| Positive | 1 (7) |
| Not done/unknown, No. | 29 |
| MOG test resultf | |
| Negative | 6 (46) |
| Positive | 7 (54) |
| Not done/unknown, No. | 31 |
| Brain lesion(s) on MRIg | |
| Site assessment | |
| No | 21 (48) |
| Yes | 23 (52) |
| Independent masked assessment | |
| No | 20 (47) |
| Yes | 23 (52) |
| Unknown/unable to determine, No. | 1 |
Abbreviations: ADEM, acute disseminated encephalomyelitis; ADHD, attention-deficit/hyperactivity disorder; AQP4, aquaporin 4; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; PON, pediatric optic neuritis.
Values are expressed as No. (%) unless otherwise specified.
Prepuberty defined as Tanner questionnaire level 1 response = scrotum and penis size are the same as when young (if boy) or breasts are flat (if girl).
The type and number of participants with other medications at time of enrollment included the following: amphetamine/dextroamphetamine, mometasone, montelukast (n = 3), albuterol, cyproheptadine, melatonin (n = 2), ranitidine, loratadine, famotidine, pantoprazole, acetazolamide, cetirizine (n = 2), fluticasone, doxycycline, and idebenone.
Of those with bilateral optic neuritis at presentation, laterality was defined as simultaneous bilateral if both eyes developed an initial episode of acute optic neuritis within 1 month of each other; otherwise, participants were classified as having sequential bilateral optic neuritis.
Patients were classified as having corticosteroid treatment (oral or intravenous) at enrollment if corticosteroid use was either current or reported before enrollment or if corticosteroid treatment was prescribed at enrollment.
Results are based on analyses of blood samples collected from participants (or their parents) who consented to optional additional testing for AQP4 (n = 15) and MOG (n = 13) antibodies.
Based on MRI scan performed at enrollment (or within 2 weeks after enrollment).
At enrollment, biomicroscopic examination did not reveal anterior chamber reaction, subretinal fluid, prepapillary cells, or retinal exudates in any eye. A vitreous cellular reaction was seen in 1 eye (2%; 95% CI, 0%-13%); optic disc edema was seen in 41 eyes (75%; 95% CI, 60%-86%); and retinal hemorrhage was present in 2 eyes (4%; 95% CI, 1%-14%).
The MRI findings revealed cerebral WMLs (aside from ON enhancement) in 23 of 44 patients (52%) based on site interpretation of the scan and 23 (52%) based on masked reading of the MRI (Table 1). Of 44 MRIs reviewed, 34 (77%) had fat suppression images of the orbit. Of 39 patients with sufficient images of the orbit to evaluate optic nerve enhancement, 36 demonstrated definite optic nerve enhancement on the masked MRI evaluation (92%). Of the 15 (34%) enrollees who participated in the optional study for AQP4 testing, 1 (7%) (95% CI, 0%-32%) tested positive for AQP4 antibodies. Consent for optional MOG testing was obtained from 13 (30%) enrollees, of whom 7 (54%) (95% CI, 25%-81%) tested positive for MOG antibodies. Based on the combination of clinical findings, MRI review, and serological testing, the final 6-month diagnosis was isolated ON in 21 patients (48%). Other final 6-month diagnoses were MOG-associated demyelination (n = 8 [18%]), ADEM (n = 7 [16%]), MS (n = 5 [11%]), and NMO (n = 3 [7%]) (eTable 3 in the Supplement). In addition to the 7 patients (16%) who tested positive for MOG antibodies on the optional study assay, 1 additional patient (2%) was diagnosed with MOG-associated disease based on MOG testing ordered as part of their clinical care. The initial diagnoses made by the site and the reclassified diagnoses using the masked MRI reading and available antibody (AQP4 and MOG) tests are listed in eTable 3 in the Supplement. Four idiopathic cases (9%), 1 (2%) ADEM case, and 2 (5%) seronegative NMO cases were reclassified as MOG-positive ON.
Distance VA at Enrollment
The mean distance HCVA at enrollment in the 54 eyes with ON was 0.95 logMAR (20/200; 95% CI, 0.75-1.16); 28 eyes (52%; 95% CI, 38%-65%) had a distance HCVA of less than 20/200, and 16 eyes (30%; 95% CI, 18%-44%) had HCVA of less than 20/800 (Table 2). Only 11 of 54 eyes (20%; 95% CI, 11%-33%) had HCVA that was within the age-normal range at enrollment (Table 2). The mean distance LCVA in the 54 eyes was 1.49 logMAR (20/640; 95% CI, 1.36-1.61); 46 eyes (84%; 95% CI, 70%-92%) had distance LCVA less than 20/200, and 33 eyes (61%; 95% CI, 47%-74%) had LCVA less than 20/800.
Table 2. Distribution of Distance Visual Acuity Scores for Affected Eyes at Enrollment and 6 Months.
| Variable | VA, No. (%)a | |||
|---|---|---|---|---|
| High contrast | Low contrast | |||
| Enrollment (n = 54) | 6 mo (n = 45) | Enrollment (n = 54) | 6 mo (n = 42) | |
| VA in affected eyes, Snellen equivalent (No. of letters) | ||||
| Worse than 20/800 (0-2 letters)b | 16 (30) | 1 (2) | 33 (61) | 6 (14) |
| 20/250 to 20/800 (3-32) | 12 (22) | 1 (2) | 13 (24) | 3 (7) |
| 20/50 to 20/200 (33-67) | 10 (19) | 5 (11) | 6 (11) | 25 (60) |
| 20/20 to 20/40 (68-83) | 12 (22) | 22 (49) | 2 (4) | 8 (19) |
| Better than 20/20 (≥88) | 4 (7) | 16 (36) | 0 (0) | 0 (0) |
| Mean logMAR VA (95% CI)c | 0.95 (0.75 to 1.16) | 0.12 (−0.01 to 0.26) | 1.49 (1.36 to 1.61) | 0.73 (0.56 to 0.90) |
| Age-normal VA, No. (%) [95% CI]c | 11 (20) [11 to 33] | 34 (77) [61 to 88] | NA | NA |
| Change in VA from enrollment (logMAR lines) | ||||
| ≥13 Lines better | NA | 15 (33) | NA | 7 (17) |
| 9-12 Lines better | 6 (13) | 14 (33) | ||
| 5-8 Lines better | 4 (9) | 7 (17) | ||
| 2-4 Lines better | 9 (20) | 3 (7) | ||
| Within 1 line | 9 (20) | 10 (24) | ||
| ≥2 Lines worse | 2 (4) | 1 (2) | ||
| Mean change (95% CI), logMARc,d | 0.76 (0.54 to 0.99) | 0.72 (0.54 to 0.89) | ||
| Different test method used from enrollment | 7 (16) | 7 (17) | ||
Abbreviations: NA, not available; VA, visual acuity.
Values are expressed as No. (%) unless otherwise specified.
LogMAR converted to Snellen as follows: –0.2 = 20/12; –0.1 = 20/16; 0 = 20/20; 0.1 = 20/25; 0.2 = 20/32; 0.3 = 20/40; 0.4 = 20/50; 0.5 = 20/63; 0.6 = 20/80; 0.7 = 20/100; 0.8 = 20/125; 0.9 = 20/160; 1 = 20/200; 1.1 = 20/250; 1.2 = 20/320; 1.3 = 20/400; 1.4 = 20/500; 1.5 = 20/640; 1.6 = 20/800; 1.7 = <20/800.
Adjusted for intereye correlation.
Positive values indicate improvement in amblyopic-eye visual acuity.
Associations With Poor HCVA at Enrollment
At enrollment, factors associated with poor HCVA in study eyes included younger age (mean HCVA worse by 0.06 logMAR [95% CI, 0.00-0.12] per additional year of age), an associated neurologic autoimmune diagnosis in addition to ON (difference in logMAR, 0.68; 95% CI, 0.34-1.01), and the presence of brain lesions on MRI (difference in logMAR, 0.70; 95% CI, 0.36-1.04). In addition, patients with non-White race and non-Hispanic ethnicity had worse HCVA than their White and non-Hispanic counterparts (difference in logMAR, 0.61; 95% CI, 0.26-0.95) (Table 3). Laterality of pediatric ON and pubertal status were not associated with HCVA at enrollment.
Table 3. Change in Distance High-Contrast Visual Acuity From Enrollment to 6 Months by Baseline Subgroupsa.
| Variable | Enrollment | 6-Mo visit | |||||
|---|---|---|---|---|---|---|---|
| Eyes, No. | Mean (95% CI) | Eyes, No. | Mean (95% CI) | ||||
| HCVA logMAR score | Group difference for logMAR scoreb | HCVA logMAR score | Change from enrollment in logMARc,d | Group difference for change in logMARc | |||
| Overall | 54 | 0.95 (0.75 to 1.16) | NA | 45 | 0.12 (−0.01 to 0.26) | 0.76 (0.54 to 0.99) | NA |
| Age, y | −0.06 (−0.12 to −0.00)e | −0.005 (−0.045 to 0.034)e | |||||
| 3-6 | 14 | 1.2 (0.8 to 1.6) | NA | 13 | 0.1 (−0.2 to 0.4) | 1.13 (0.70 to 1.55) | NA |
| 7-9 | 9 | 1.1 (0.6 to 1.6) | 7 | −0.1 (−0.4 to 0.3) | 1.01 (0.47 to 1.55) | ||
| 10-12 | 20 | 0.8 (0.5 to 1.2) | 16 | 0.2 (0.0 to 0.5) | 0.44 (0.08 to 0.80) | ||
| ≥13 | 11 | 0.8 (0.3 to 1.2) | 9 | 0.1 (−0.2 to 0.3) | 0.68 (0.23 to 1.13) | ||
| Diagnosis | |||||||
| Isolated optic neuritis | 27 | 0.6 (0.3 to 0.8) | 1 [Reference] | 22 | −0.0 (−0.2 to 0.2) | 0.44 (0.15 to 0.72) | 1 [Reference] |
| Neurological association | 27 | 1.3 (1.1 to 1.6) | 0.68 (0.34 to 1.01) | 23 | 0.2 (0.1 to 0.4) | 1.06 (0.79 to 1.33) | −0.205 (−0.497 to 0.087) |
| Laterality | |||||||
| Bilateral | 26 | 1.1 (0.8 to 1.4) | 0.08 (−0.35 to 0.50) | 20 | 0.2 (−0.0 to 0.5) | 0.82 (0.45 to 1.20) | −0.189 (−0.523 to 0.145) |
| Unilateral | 28 | 0.9 (0.6 to 1.1) | 1 [Reference] | 25 | 0.1 (−0.1 to 0.2) | 0.73 (0.44 to 1.01) | 1 [Reference] |
| MRI lesions | |||||||
| Lesions present | 27 | 1.3 (1.1 to 1.6) | 0.70 (0.36 to 1.04) | 21 | 0.2 (0.0 to 0.4) | 1.07 (0.78 to 1.36) | −0.087 (−0.379 to 0.204) |
| None | 27 | 0.6 (0.3 to 0.8) | 1 [Reference] | 24 | 0.1 (−0.1 to 0.2) | 0.48 (0.20 to 0.76) | 1 [Reference] |
| Puberty statusf | |||||||
| Prepuberty | 12 | 0.8 (0.4 to 1.3) | −0.12 (−0.63 to 0.39) | 10 | −0.0 (−0.3 to 0.3) | 0.81 (0.28 to 1.35) | 0.159 (−0.203 to 0.520) |
| Puberty/postpuberty | 31 | 1.0 (0.7 to 1.2) | 1 [Reference] | 27 | 0.2 (−0.0 to 0.3) | 0.75 (0.46 to 1.05) | 1 [Reference] |
| Unknown/not reported | 11 | NA | NA | 8 | NA | NA | NA |
| White/non-Hispanic | |||||||
| No | 25 | 1.3 (1.1 to 1.6) | 0.61 (0.26 to 0.95) | 20 | 0.2 (0.0 to 0.4) | 1.06 (0.75 to 1.36) | −0.096 (−0.387 to 0.196) |
| Yes | 27 | 0.7 (0.4 to 0.9) | 1 [Reference] | 23 | 0.1 (−0.1 to 0.2) | 0.55 (0.27 to 0.84) | 1 [Reference] |
| Unknown/not reported | 2 | NA | NA | 2 | NA | NA | NA |
Abbreviations: MRI, magnetic resonance imaging; NA, not available.
CIs for mean distance visual acuity score (enrollment and 6-month visit) and change in visual acuity from enrollment to 6 months are adjusted for intereye correlation for participants who contributed both eyes.
With the exception of puberty status, group differences in mean distance visual acuity (logMAR) at enrollment were adjusted for age at enrollment.
Group differences in mean change (logMAR) in distance visual acuity from enrollment to the 6-month visit were adjusted for baseline covariates (continuous factors) of visual acuity and age (except for puberty status).
Distance visual acuity at 6 months was tested using a different method from enrollment for 7 eyes (16%).
Age at baseline was included as a continuous factor in the linear models; therefore, the estimate represents the difference in change in logMAR visual acuity for each additional year of age.
Group comparisons were not adjusted for baseline age due to collinearity between puberty and age at enrollment.
HCVA and LCVA at 6 Months
Visual acuity data were available for 37 participants (84%) who completed the 6-month follow-up visit (Figure 1). At 6 months, mean HCVA improved from baseline by 0.76 logMAR (approximately 8 lines on a standard ETDRS chart) (95% CI, 0.54-0.99 logMAR) to 0.12 logMAR (20/25; 95% CI, –0.01 to 0.26 logMAR), and 34 of 45 eyes (77%; 95% CI, 61%-88%) were within the age-normal range at 6 months (Table 2, Figure 2A). The sensitivity analysis, which was limited to patients returning within the prespecified protocol window, yielded similar results (40 eyes, 0.78 logMAR; 95% CI, 0.54-1.01 logMAR). After adjusting for baseline factors of age and HCVA, no associations between other baseline factors and 6-month HCVA were found (Table 3).
Figure 1. Patient Enrollment and Completed Visits.
Figure 2. Distance Visual Acuity (VA) at Enrollment and at 6 Months.
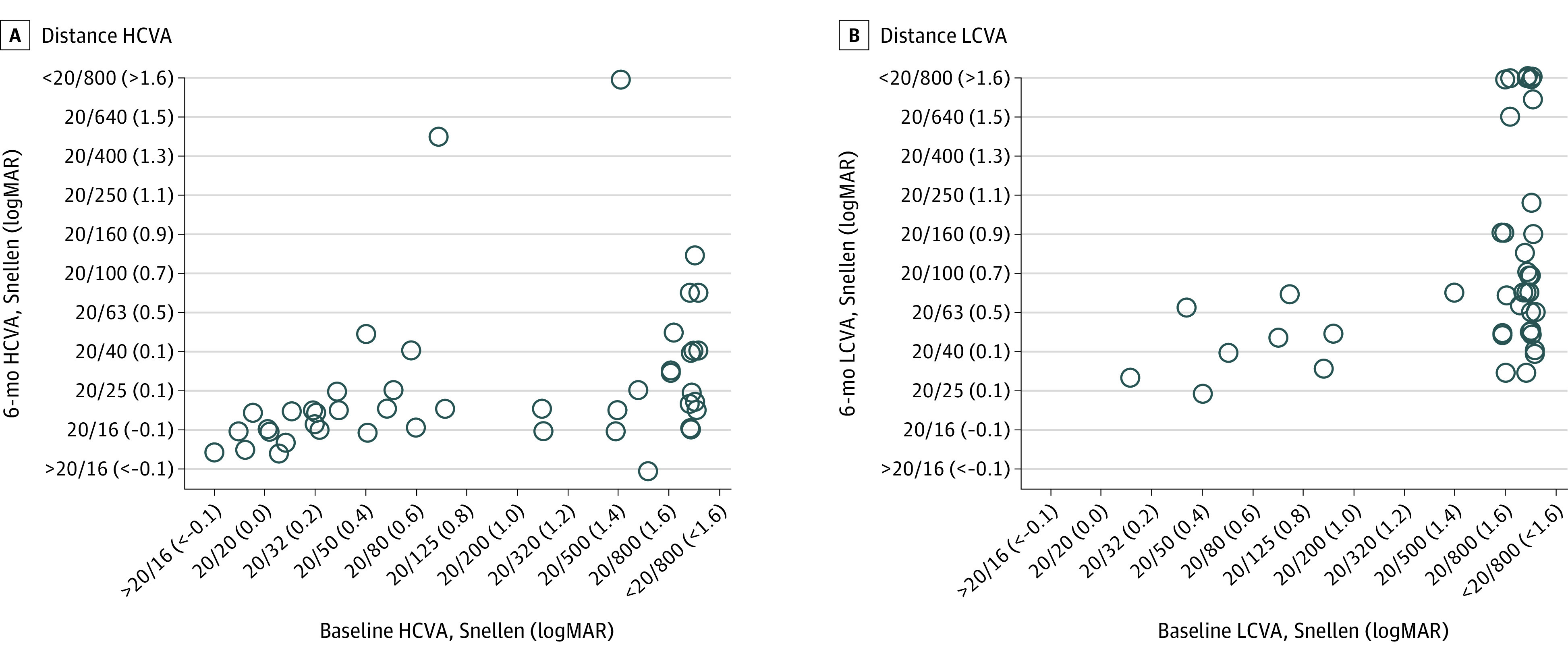
The circles represent the eyes evaluated in this study. A, Distance high-contrast VA (HCVA). The association between HCVA at the time of enrollment and at the 6-month visit for eyes (n = 45) of patients who completed the 6-month visit within the prespecified analysis window. HCVA improved from baseline to 6 months by a mean 0.76 logMAR and 77% of eyes were within normal range for age at the 6-month visit. B, Distance low-contrast VA. The association between LCVA at the time of enrollment and at the 6-month visit for eyes (n = 42) of patients who completed the 6-month visit within the prespecified analysis window. The LCVA improved from baseline to 6 months by a mean 0.72 logMAR.
At 6 months, the mean LCVA logMAR score improved from baseline by 0.72 logMAR (95% CI, 0.54-0.89 logMAR) to a mean of 0.73 logMAR (20/100; 95% CI, 0.56-0.90 logMAR) (Table 2, Figure 2B). At 6 months, LCVA was worse than 20/63 for 52% of study eyes.
Discussion
To our knowledge, the current study provides the largest amount of prospectively collected VA data on pediatric patients after the first episode of ON. Although we enrolled less than 50% of the target number of enrollees over the 2-year study period, the findings suggest that VA improved within 6 months of symptom onset. We found that 77% of patients with ON in this cohort recovered age-normal HCVA by 6 months after onset regardless of the VA at enrollment, with just 2 patients (4%) having VA worse than 20/200. Our results are consistent with those of a retrospective review of 29 children with ON by Bonhomme et al8 in which 89% of affected eyes had abnormal HCVA at baseline, whereas 96% of affected eyes had HCVA of 20/40 or better at follow-up. Wilejto et al6 described similar outcomes in 36 children who presented with acute ON. Eighty percent of the children were treated with intravenous corticosteroids, and 83% of affected eyes had normal VA at the final follow-up visit. A study of 44 children in the United Kingdom found recovery of HCVA to be better than 20/40 in 70% of children; 73% of these patients had documented treatment with corticosteroids.19 In a larger retrospective series of 46 children from Boston Children’s Hospital, 91% were treated with corticosteroids.9 At baseline, 50% of the worse-seeing affected eyes had HCVA worse than 20/200; at the 1-year follow-up, 81% of eyes had HCVA of 20/20 or better. Retrospective studies from India,20,21 Japan,22 and Korea23 also have reported excellent visual recovery in patients with ON.
Although HCVA improved in this study, at 6 months, LCVA was worse than 20/63 for 52% of study eyes, which is the typical LCVA of healthy children.24 The mean improvement of LCVA was similar to the mean improvement in HCVA (0.78 vs 0.72 logMAR). Although only 2 patients (1 eye each) who completed 6-month examinations had HCVA worse than 20/200, 8 patients (1 eye in each of 7 patients, 1 bilateral; total 9 eyes) had LCVA worse than 20/200. This finding is similar to that reported by previous retrospective studies that have shown good recovery of HCVA but less recovery of LCVA.25 Waldman et al26 compared LCVA in patients with a history of ON with pediatric patients who had a history of MS but no history of ON and with normal control participants. The authors found that children with a history of ON demonstrated markedly worse LCVA scores compared with normal control participants and patients with MS but no history of ON. The implications of abnormal LCVA for children, to our knowledge, have not been well studied, but in adults with demyelinating disease, poor LCVA has been found to correlate with poorer scores on vision-related quality of life instruments.27 Yeh et al25 studied the association between ON, optical coherence tomography parameters, and abnormal visual perception in children and found that contrast sensitivity measured using Pelli-Robson charts is a more sensitive measure of functional visual impairment in patients than variations in retinal nerve fiber layer thickness.
We found that concurrent associated neurologic autoimmune diagnoses, such as ADEM or NMO, the presence of lesions on MRI, and non-White race and non-Hispanic ethnicity were associated with worse HCVA at baseline after adjusting for age. Given the relatively small sample size and the lack of adjustment for multiple tests, these findings should be considered exploratory and interpreted with caution. Previous studies have suggested that older age at presentation and abnormal findings on MRI may be associated with worse visual outcomes.7 We found no statistically significant group differences in HCVA improvement from baseline to 6 months for any of the subgroup factors, including age or MRI findings. However, given the small sample size, this study had limited ability to evaluate differences in VA outcomes by baseline characteristics such as age, the presence of an associated neurologic autoimmune diagnosis, laterality, MRI lesions, or prepuberty status.
Despite the active participation of 23 sites, we recruited only 44 participants in 22 months with the stated enrollment criteria. Anecdotally, the most common barriers to recruitment included presentation later than the 2-week required window from symptom onset and age older than 16 years. Thus, recruitment for a randomized clinical treatment trial with the same eligibility criteria is not likely feasible in an investigator group of our size. Onset of symptoms within 2 weeks of enrollment may have been the biggest barrier to recruitment given the tertiary care setting of many sites. Nevertheless, the possibility of a pediatric ON treatment trial should not be completely abandoned; instead, a planning group may consider allowing a longer interval between symptom onset and enrollment, which may provide a larger sample size, and may consider whether enrolling such a cohort could answer the proposed study question.
Limitations
This study has limitations, including its small sample size and the diversity of neurological associations. Treatment timing and dosing were performed at the discretion of the treating physician, with nearly all children treated with corticosteroids. The current study cannot address whether corticosteroid treatment of childhood ON is more effective than any other treatment or no treatment. Despite these limitations, this is, to our knowledge, the first prospective study of outcomes of ON in children; it provides information regarding 6-month VA outcomes as well as data on the frequency of associated autoimmune neurologic diseases, such as ADEM, MS, and MOG positivity. Additionally, these data suggest a disagreement with the previous standard teaching28 that most pediatric ON cases are bilateral and neurologically isolated; neurological disease is frequently associated with this condition. Therefore, evaluation for associated neurological autoimmune disease is recommended for children with ON.
Conclusions
The findings of this study suggest that most children with ON who present with poor VA experience considerable improvement in VA by 6 months. This information can be used to counsel patients and their families about the disease. In addition, future treatment trials may need to use different inclusion criteria or modify the enrollment period because of the rarity of ON. The patients in this study will continue to be followed through November 2020. Future reports will present longer-term 2-year outcome data and detailed results for patients with MOG-positive ON. In addition, neuroimaging findings and results of optical coherence tomography testing will also be evaluated.
eAppendix 1. Study Protocol
eAppendix 2. Statistical Analysis Plan
eTable 1. Study Inclusion and Exclusion Criteria
eTable 2. Neurological Summary Diagnosis Worksheet
eTable 3. Diagnosis at Optic Neuritis Presentation
References
- 1.Liu GT, Volpe NJ, Galetta SL. Liu, Volpe and Galetta’s Neuro-Ophthalmology: Diagnosis and Management. Elsevier Saunders; 2019. [Google Scholar]
- 2.O’Mahony J, Marrie RA, Laporte A, et al. . Recovery from central nervous system acute demyelination in children. Pediatrics. 2015;136(1):e115-e123. doi: 10.1542/peds.2015-0028 [DOI] [PubMed] [Google Scholar]
- 3.Beck RW, Cleary PA, Anderson MM Jr, et al. ; The Optic Neuritis Study Group . A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326(9):581-588. doi: 10.1056/NEJM199202273260901 [DOI] [PubMed] [Google Scholar]
- 4.Lana-Peixoto MA, Andrade GC. The clinical profile of childhood optic neuritis. Arq Neuropsiquiatr. 2001;59(2-B):311-317. doi: 10.1590/S0004-282X2001000300001 [DOI] [PubMed] [Google Scholar]
- 5.Lucchinetti CF, Kiers L, O’Duffy A, et al. . Risk factors for developing multiple sclerosis after childhood optic neuritis. Neurology. 1997;49(5):1413-1418. doi: 10.1212/WNL.49.5.1413 [DOI] [PubMed] [Google Scholar]
- 6.Wilejto M, Shroff M, Buncic JR, Kennedy J, Goia C, Banwell B. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006;67(2):258-262. doi: 10.1212/01.wnl.0000224757.69746.fb [DOI] [PubMed] [Google Scholar]
- 7.Brady KM, Brar AS, Lee AG, Coats DK, Paysse EA, Steinkuller PG. Optic neuritis in children: clinical features and visual outcome. J AAPOS. 1999;3(2):98-103. doi: 10.1016/S1091-8531(99)70078-9 [DOI] [PubMed] [Google Scholar]
- 8.Bonhomme GR, Waldman AT, Balcer LJ, et al. . Pediatric optic neuritis: brain MRI abnormalities and risk of multiple sclerosis. Neurology. 2009;72(10):881-885. doi: 10.1212/01.wnl.0000344163.65326.48 [DOI] [PubMed] [Google Scholar]
- 9.Wan MJ, Adebona O, Benson LA, Gorman MP, Heidary G. Visual outcomes in pediatric optic neuritis. Am J Ophthalmol. 2014;158(3):503-7.e2. doi: 10.1016/j.ajo.2014.05.036 [DOI] [PubMed] [Google Scholar]
- 10.Waldman AT, Shumski MJ, Jerrehian M, Liu GT. Parent and medical professional willingness to enroll children in a hypothetical pediatric optic neuritis treatment trial. Front Neurol. 2011;2:75. doi: 10.3389/fneur.2011.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Jaeb Center for Health Research PEDIG public website. Accessed November 25, 2019. http://www.pedig.net
- 13.Pan Y, Tarczy-Hornoch K, Cotter SA, et al. ; Multi-Ethnic Pediatric Eye Disease Study Group . Visual acuity norms in pre-school children: the Multi-Ethnic Pediatric Eye Disease Study. Optom Vis Sci. 2009;86(6):607-612. doi: 10.1097/OPX.0b013e3181a76e55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drover JR, Felius J, Cheng CS, Morale SE, Wyatt L, Birch EE. Normative pediatric visual acuity using single surrounded HOTV optotypes on the Electronic Visual Acuity Tester following the Amblyopia Treatment Study protocol. J AAPOS. 2008;12(2):145-149. doi: 10.1016/j.jaapos.2007.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes JM, Beck RW, Repka MX, et al. ; Pediatric Eye Disease Investigator Group . The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol. 2001;119(9):1345-1353. doi: 10.1001/archopht.119.9.1345 [DOI] [PubMed] [Google Scholar]
- 16.Cotter SA, Chu RH, Chandler DL, et al. . Reliability of the electronic early treatment diabetic retinopathy study testing protocol in children 7 to <13 years old. Am J Ophthalmol. 2003;136(4):655-661. doi: 10.1016/S0002-9394(03)00388-X [DOI] [PubMed] [Google Scholar]
- 17.Waldman AT, Yeshokumar AK, Lavery A, et al. . Validation of a symptom-based questionnaire for pediatric CNS demyelinating diseases. J AAPOS. 2019;23(3):157.e1-157.e7. doi: 10.1016/j.jaapos.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Absoud M, Cummins C, Desai N, et al. . Childhood optic neuritis clinical features and outcome. Arch Dis Child. 2011;96(9):860-862. doi: 10.1136/adc.2009.175422 [DOI] [PubMed] [Google Scholar]
- 20.Ambika S, Padmalakshmi K, Venkatraman V, Noronha OV. Visual outcomes and clinical manifestations of pediatric optic neuritis in Indian population: an institutional study. J Neuroophthalmol. 2018;38(4):462-465. doi: 10.1097/WNO.0000000000000646 [DOI] [PubMed] [Google Scholar]
- 21.Khadse R, Ravindran M, Pawar N, Maharajan P, Rengappa R. Clinical profile and neuroimaging in pediatric optic neuritis in Indian population: a case series. Indian J Ophthalmol. 2017;65(3):242-245. doi: 10.4103/ijo.IJO_939_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizota A, Niimura M, Adachi-Usami E. Clinical characteristics of Japanese children with optic neuritis. Pediatr Neurol. 2004;31(1):42-45. doi: 10.1016/j.pediatrneurol.2003.11.011 [DOI] [PubMed] [Google Scholar]
- 23.Kim YM, Kim HY, Cho MJ, et al. . Optic neuritis in Korean children: low risk of subsequent multiple sclerosis. Pediatr Neurol. 2015;53(3):221-225. doi: 10.1016/j.pediatrneurol.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 24.Waldman AT, Lavery AM, Liu GW, et al. . High- and low-contrast letter acuity perception matures with age in normally sighted children. J Neuroophthalmol. 2020;40(2):148-156. doi: 10.1097/WNO.0000000000000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh EA, Marrie RA, Reginald YA, et al. ; Canadian Pediatric Demyelinating Disease Network . Functional-structural correlations in the afferent visual pathway in pediatric demyelination. Neurology. 2014;83(23):2147-2152. doi: 10.1212/WNL.0000000000001046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldman AT, Hiremath G, Avery RA, et al. . Monocular and binocular low-contrast visual acuity and optical coherence tomography in pediatric multiple sclerosis. Mult Scler Relat Disord. 2013;3(3):326-334. doi: 10.1016/j.msard.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schinzel J, Zimmermann H, Paul F, et al. . Relations of low contrast visual acuity, quality of life and multiple sclerosis functional composite: a cross-sectional analysis. BMC Neurol. 2014;14:31. doi: 10.1186/1471-2377-14-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy C, Carroll FD. Optic neuritis in children. Trans Am Acad Ophthalmol Otolaryngol. 1960;64:700-712. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Study Protocol
eAppendix 2. Statistical Analysis Plan
eTable 1. Study Inclusion and Exclusion Criteria
eTable 2. Neurological Summary Diagnosis Worksheet
eTable 3. Diagnosis at Optic Neuritis Presentation