A COVID-19 vaccine must first be proven to be safe and effective, but making billions of doses of high quality vaccine and using it equitably and accessibly may present an equal if not greater challenge to the global community.
Abstract
Over the past 9 mo, with 34 million infections and 1 million deaths, the COVID-19 pandemic has levied a grisly toll. Some countries, through political will and social organization, have successfully reduced the number of infections and deaths, but the global scale of loss reflects the difficulty of translating these approaches in other countries. An effective SARS-CoV-2 vaccine presents a technological solution to the failure of social and political ones. Vaccines are, however, not a silver bullet, but a safe, cost-effective, and globally applicable tool that will require a substantial effort—cooperation, commitment, time, and funding—to be effective.
Key unknowns in early vaccine development
An important question early in development is, Does infection provide immunity from reinfection? With classical vaccine–pathogen combinations, natural infection is followed by host-directed adaptive immune responses that first control infection and then eliminate it. Those same immune responses, neutralizing or functional antibodies, and various T cell subsets (CD4 and CD8) also protect the individual against reinfection. For instance, hepatitis A infection may manifest as asymptomatic infection or fulminant hepatitis, but once the infection is cleared, durable immunity against reinfection is present. These types of vaccines are more straightforward to develop. Consider the difficulty in developing an HIV vaccine: anti-HIV immune responses never successfully eliminate infection, and superinfection occurs despite those immune responses. There is a strong hypothesis that SARS-CoV-2 infection provides at least short-term protection against reinfection. To date, three nonhuman primate (NHP) studies have shown that SARS-CoV-2 infection protects against rechallenge (Chandrashekar et al., 2020).
The next unknown concerns specific immune responses thought to be protective. In the absence of vaccine-induced correlates of protection (Plotkin, 2020), convalescent responses are often used as a first-order estimate (Vabret et al., 2020). Often we assume that the protective immune response is antibody mediated, though cellular immune responses may play a critical role in shaping and maintaining the humoral immune response.
The third unknown is applicable animal models, a convenient screen for vaccine candidates. With HIV, only humans can be reliably infected, and the use of simian immunodeficiency virus (SIV) or HIV-SIV chimeric viruses has not correlated with human HIV vaccine trials. SARS-CoV-2 can infect transgenic mice, hamsters, ferrets, and NHP, and vaccines and neutralizing monoclonal antibodies have been shown to be effective in preventing infection and disease in at least 13 animal studies (Moore and Klasse, 2020).
The fourth unknown is safety. There are two hypothetical concerns raised by preclinical studies of SARS-CoV (SARS from 2002) vaccines. In vitro studies raised concerns about antibody-dependent enhancement. In several studies, predominantly in mice using whole inactivated vaccines and alum, there was protection against infection but subsequent, post-challenge eosinophilic infiltration of the lung. There was no clear explanation for vaccine-associated enhanced respiratory disease, but it was less consistently seen with other species (ferret and NHP), with other vaccines (viral vectored), with different adjuvants (more balanced Th1 vs. Th2), and with other coronavirus (e.g., Middle East Respiratory Syndrome) vaccines (Lambert et al., 2020; Zellweger et al., 2020). There is no evidence of antibody-dependent enhancement or vaccine-associated enhanced respiratory disease in the SARS-CoV-2 vaccines tested and reported to date in ferrets, hamsters, mice, or NHP, using whole inactivated vaccines, protein, viral vectored, RNA, or DNA vaccines and a variety of adjuvants including alum. However, this remains an area of concern and necessitates longer term follow-up of volunteers receiving SARS-CoV-2 vaccines.
Prove it, make it, use it
A SARS-CoV-2 vaccine must first be proven to reduce infection and/or disease, then made in quantity, and used in vaccination programs. This seemingly straightforward approach normally takes 5–10 yr, costs an estimated $500 million to $1.5 billion, and is associated with a high failure rate: 93% of vaccine candidates drop out between laboratory and licensure (Gouglas et al., 2018; Young et al., 2018). The once-in-a-century COVID-19 pandemic is not “normal,” and the unprecedented speed of vaccine development provides a new paradigm and new potential risks. Out of the >200 SARS-CoV-2 candidates in various stages of development (World Health Organization, 2020), >40 are in human clinical testing, and as of September 2020, 9 mo into the pandemic, 8 are in Phase III trials of efficacy and safety. Interim data on efficacy and short-term safety are expected by the end of 2020 or the beginning of 2021 (Le et al., 2020).
Proving it: Vaccine clinical development
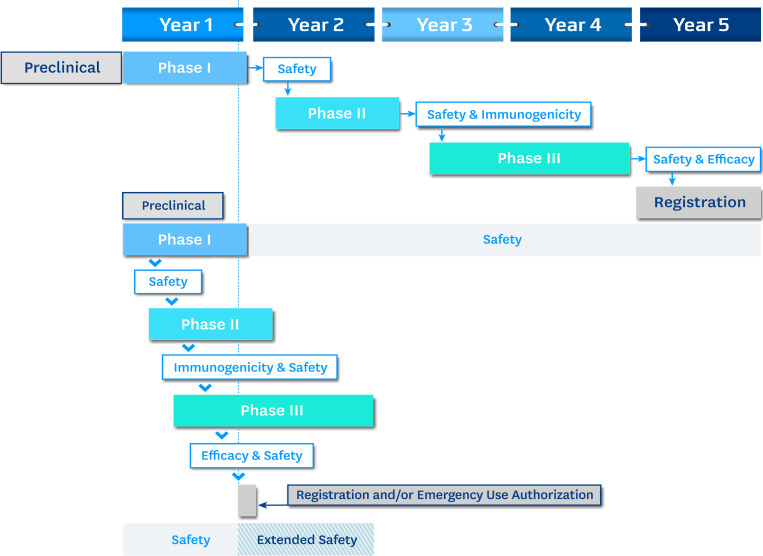
The progress of a vaccine in a 5-yr development cycle is shown schematically in Fig. 1. From antigen discovery through preclinical testing in animals, vaccine development can take months to years for optimization. Candidates are then advanced into human testing and divided into Phases I–III. While depicted sequentially, development is iterative and recursive, as companies want to ensure that candidates reaching large and expensive Phase III efficacy trials are de-risked and likely to succeed. Phase I looks primarily at safety and preliminary immunogenicity in ∼50 volunteers. Phase II trials look at immunogenicity and safety in the target population and typically enroll hundreds of volunteers. Success in Phase II allows a vaccine to enter Phase III, involving thousands of volunteers. If the Phase III trial demonstrates safety and efficacy, an application is made to the national regulatory authority (e.g., the US Food and Drug Administration [FDA]) for licensure and market authorization.
Figure 1.
Schematic of hypothetical 5-yr and 1-yr vaccine development timelines. Phase I human trials often have ~50 volunteers; Phase II trials have 200–500 volunteers; and Phase III trials involve thousands (often tens of thousands) of volunteers. As seen in the 1-yr development cycle, it is possible to telescope the phases, and it is further possible (subject to regulatory review) to combine clinical trial phases (Phase I/II or Phase II/III). Safety and efficacy remain the key elements in any licensure or emergency use decision.
The lower panel of Fig. 1 shows a hypothetical 12-mo timeline (Lurie et al., 2020) leading to emergency use authorization or registration. Expedited procedures accelerate, but do not circumvent, appropriate ethical, scientific, and regulatory review. Preclinical animal studies are done in parallel with Phase I (this applies to technologies that have prior use and safety in humans; DNA or chimpanzee adenovirus-vectored vaccines, for example). After receipt of the final dose of vaccine and review of initial safety data, Phase II is initiated, and Phase I continues in follow-up. After the final dose of vaccine in Phase II, review of safety and immunogenicity data will trigger enrollment in Phase III. The overlap or telescoping of Phases I–III allows considerable time savings. In general, however, the criteria for vaccine efficacy (VE) in the standard and expedited models remain the same, and the US FDA and World Health Organization (WHO) have suggested that VE should be >50% with a lower bound of the 95% confidence interval that exceeds 30% (a standard for vaccines in general). Companies and regulatory authorities have approached VE using an endpoint definition of “disease” (symptomatic, virologically confirmed COVID-19), though the US FDA also notes that confirmed SARS-CoV-2 infection might be considered. For standardization purposes, the FDA recommends that a virologically confirmed infection with at least one COVID-19–related symptom be considered (Food and Drug Administration, 2020).
Making it: Vaccine manufacturing
The 5–10–yr vaccine development timeline affords manufacturers the opportunity to de-risk by optimizing dose, schedule, process development, and immune responses to ensure safety and efficacy. Given the pace of vaccine development for COVID-19, de-risking comes from government funding. While the Coalition for Epidemic Preparedness Innovations funded some of the initial work on SARS-CoV-2 vaccines being pursued by Moderna, Inovio, Curevac, and AstraZeneca, the US government committed over $10 billion for vaccine development and manufacturing at risk. Operation Warp Speed has preordered hundreds of millions of doses of a variety of vaccines including those from AstraZeneca, Sanofi, Pfizer, Johnson & Johnson, Moderna, and Novavax (Slaoui and Hepburn, 2020).
The initial phase of manufacturing scale-up will be a key regulator of vaccine access initially. This could potentially be impacted by vaccine nationalism and the bilateral agreements that exist between manufacturers and high-income countries (HIC). Several companies have licensed or contracted vaccine production to other manufacturers—AstraZeneca with Serum Institute (India) and SK bioscience (Korea); Moderna with Lonza (Switzerland), Johnson & Johnson with Biological E (India); and Chinese Sinovac with Butantan (Brazil) and BioFarma (Indonesia). Hopefully the license and contract manufacturing arrangements will allow sufficient doses of vaccines to provide access to at risk populations.
Using it: The looming crisis in vaccine distribution and use
In the end, it is not vaccines but vaccination that will provide pandemic relief. The efforts of WHO, Gavi, and donors have accomplished remarkable feats in providing childhood vaccines globally—with the infrastructure, capacity building, and health system strengthening that it implies. But there have been gaps, and these are the lessons that we should remember as we implement COVID-19 vaccination worldwide, in adults as well as children. Rotavirus vaccine (RV) is an example. A highly effective vaccine against rotavirus diarrhea was approved in the US in 2007 and was recommended by WHO in 2009. By 2015, only 20% of the world’s children had received all three doses of RV (International Vaccine Access Center, 2016); 10 yr after its recommendation, in 2019, under 40% had received three doses. With premium vaccines, like rotavirus, pneumococcal conjugate, and HPV, the greatest number of unvaccinated children live in middle-, not low-, income countries. The RV implementation gap, a decade or more after approval of vaccines in HIC, cannot be allowed to happen with COVID-19.
The US National Academy of Sciences, Engineering and Medicine recognized the dangerous intersection of the vaccine nationalism of HIC and the pressing needs of low- and middle-income countries (Gayle et al., 2020), noting the work of Chinazzi et al. (2020) suggesting that exclusive purchase of the first 2 billion doses of vaccine by HIC without equitable global distribution will double the number of expected deaths. The US National Academy of Sciences, Engineering and Medicine endorsed COVAX as a potential solution. COVAX is an effort by the Coalition for Epidemic Preparedness Innovations, Gavi, and WHO to ensure that vaccine technology can be distributed worldwide (Kupferschmidt, 2020; Usher, 2020). 170 countries have expressed interest in COVAX, and $1.4 billion in funding has been pledged. The principles of COVAX are as follows: (1) global access; (2) impact and transparency; and (3) solidarity and collective ownership. COVAX should have 2 billion doses of WHO-prequalified SARS-CoV-2 vaccines by the end of 2021, which is roughly 20% of global need and sufficient to vaccinate the elderly and health care workers. How COVAX will be impacted by direct HIC–vaccine manufacturer agreements is unknown. For low- and middle-income countries, COVAX is critical to access; failure may very well lead to a critical implementation gap and unnecessary disease, death, and economic crisis. China has recently committed to COVAX; notable holdouts include the US and Russia.
Unanswered questions
The unprecedented speed of COVID-19 vaccine development will leave unanswered questions beyond manufacturing and delivery (Frederiksen et al., 2020). First, we have not optimized the dose and schedule for maximal magnitude and durability in target populations. Second, samples collected at baseline and after vaccination must be used to determine correlates of protection; correlates may vary based on the vaccine type, but will nonetheless be useful in reducing the time to optimization or in the development of improved vaccines (Plotkin, 2020). Third, the work of randomized clinical trials is clearly important in establishing safety and efficacy, but effectiveness trials and real world evidence will be critical in understanding how best to deploy the vaccines and in determination of “herd immunity” (Fontanet and Cauchemez, 2020). It is possible that different vaccines with similar efficacy may have differential herd protection related to the nature of immune responses induced by the specific vaccine. Fourth, questions around safety and longer-term adverse events will require continued follow-up of vaccine trial participants, and as the vaccines are introduced (Shah et al., 2020), will require strengthening of pharmaco-vigilance and vaccine adverse events reporting systems around the world.
It is, finally, difficult to assess the consequences of vaccine hesitancy, nationalism, imperialism, and geopolitics on the implementation and impact of a safe and effective SARS-CoV-2 vaccines.
Final thoughts
The COVID-19 pandemic is a defining moment, as science, politics, and social forces converge in this grim reminder of the power of contagion. Can we marshal the great capacities of new technologies, vaccine manufacturing, and globalization into a vaccine-enabled, accessible, and comprehensive solution in the fight against SARS-CoV-2, or will the global community fall victim to nationalistic pettiness, denialism, and inequity that will amplify global disparities, heighten the magnitude, and prolong the duration of this crisis?
Acknowledgments
Special thanks to Dr. Jean-Louis Excler for insightful comments and suggestions.
References
- Chandrashekar A., et al. Science. 2020 doi: 10.1126/science.abc4776. [DOI] [Google Scholar]
- Chinazzi M., et al. 2020. https://www.networkscienceinstitute.org/publications/estimating-the-effect-of-cooperative-versus-uncooperative-strategies-of-covid-19-vaccine-allocation-a-modeling-study
- Fontanet A., and Cauchemez S. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration 2020. https://www.fda.gov/media/139638/download
- Frederiksen L.S.F., et al. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle H., Foege W., Brown L., and Kahn B., editors. 2020. Framework for equitable allocation of COVID-19 vaccine. The National Academy Press, Washington, DC: 10.17226/25917 [DOI] [PubMed] [Google Scholar]
- Gouglas D., et al. Lancet Glob. Health. 2018 doi: 10.1016/S2214-109X(18)30346-2. [DOI] [Google Scholar]
- International Vaccine Access Center 2016. ScienceDaily. https://www.sciencedaily.com/releases/2016/05/160524144709.htm
- Kupferschmidt K. 2020. Science. 10.1126/science.369.6511.1553 [DOI] [Google Scholar]
- Lambert P.-H., et al. . 2020. Vaccine. 10.1016/j.vaccine.2020.05.064 [DOI] [Google Scholar]
- Le T.T., et al. Nat. Rev. Drug Discov. 2020 doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- Lurie N., et al. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2005630. [DOI] [Google Scholar]
- Moore J.P., and Klasse P.J. J. Virol. 2020 doi: 10.1128/JVI.01083-20. [DOI] [Google Scholar]
- Plotkin S.A. Vaccine. 2020 doi: 10.1016/j.vaccine.2019.10.046. [DOI] [Google Scholar]
- Shah A., et al. JAMA. 2020 doi: 10.1001/jama.2020.15725. [DOI] [Google Scholar]
- Slaoui M., and Hepburn M. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2027405. [DOI] [PubMed] [Google Scholar]
- Usher A.D. Lancet. 2020 doi: 10.1016/S0140-6736(20)31354-4. [DOI] [Google Scholar]
- Vabret N., et al. Immunity. 2020 doi: 10.1016/j.immuni.2020.05.002. [DOI] [Google Scholar]
- World Health Organization 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- Young R., et al. Gates Open Res. 2018 doi: 10.12688/gatesopenres.12817.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., et al. . 2020. Expert Rev. Vaccines. 10.1080/14760584.2020.1800463 [DOI] [PMC free article] [PubMed] [Google Scholar]